Repetitive Compressive Loading Downregulates Mitochondria Function and Upregulates the Cartilage Matrix Degrading Enzyme MMP-13 Through the Coactivation of NAD-Dependent Sirtuin 1 and Runx2 in Osteoarthritic Chondrocytes
Abstract
1. Introduction
2. Results
2.1. Repetitive Compressive Loading of the 3D Cell Culture Construct
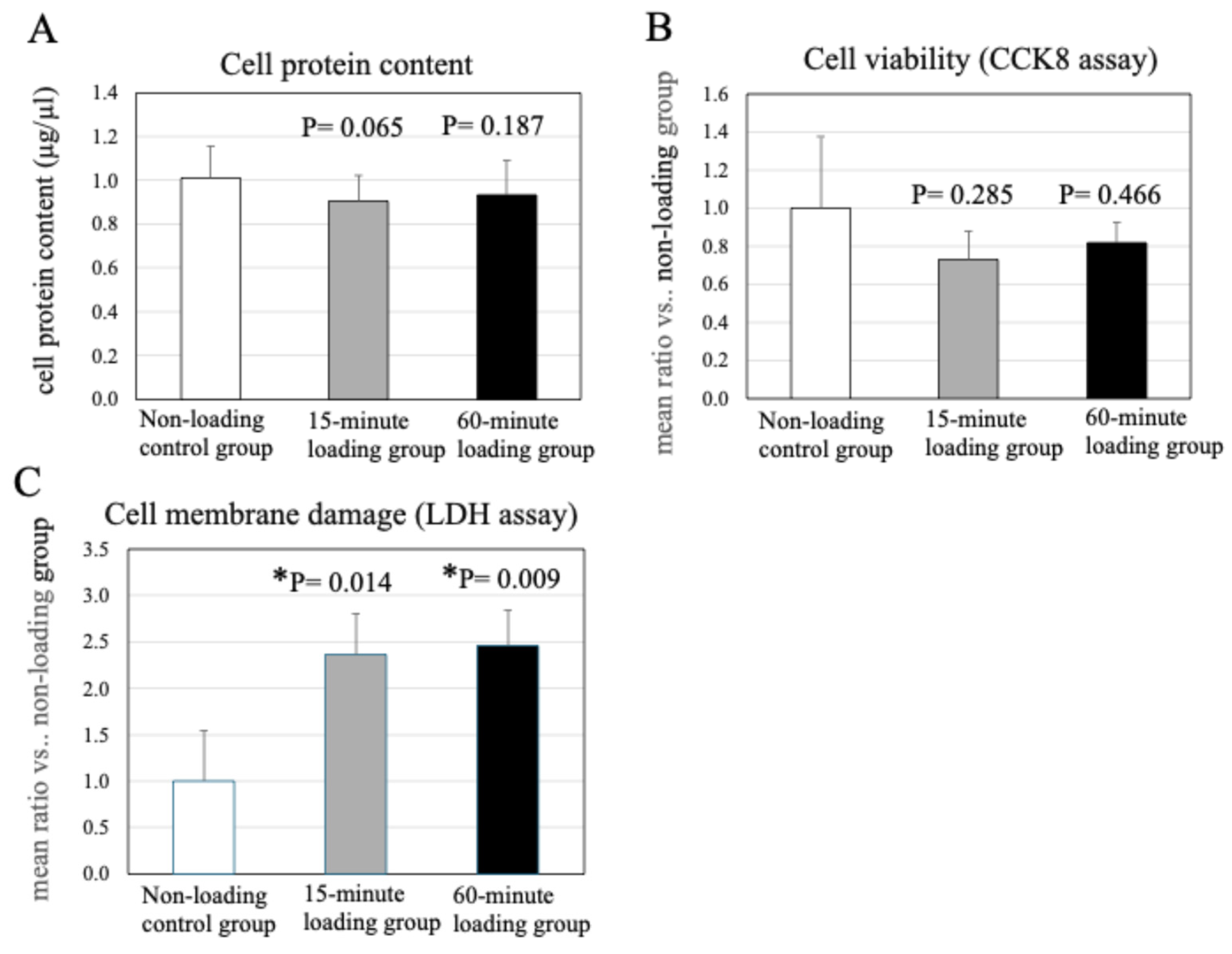
2.2. Effect of Physiologic and Repetitive Compressive Loading on Cell Protein Content in a 3D Cell-Culture Construct After Mechanical Loading
2.3. Effects of Physiologic and Repetitive Compressive Loading on Chondrocyte Viability
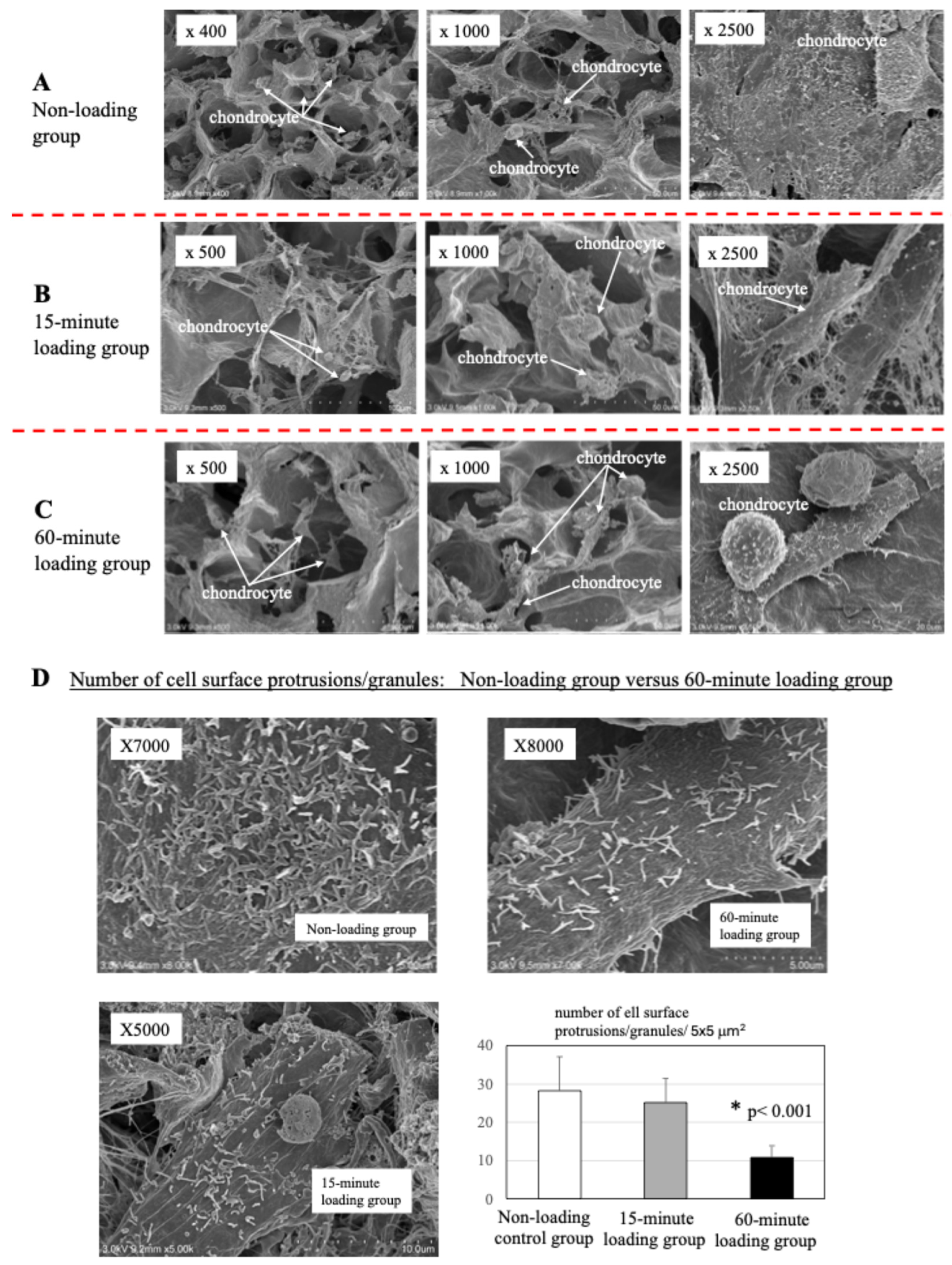
2.4. Scanning Electron Microscopy
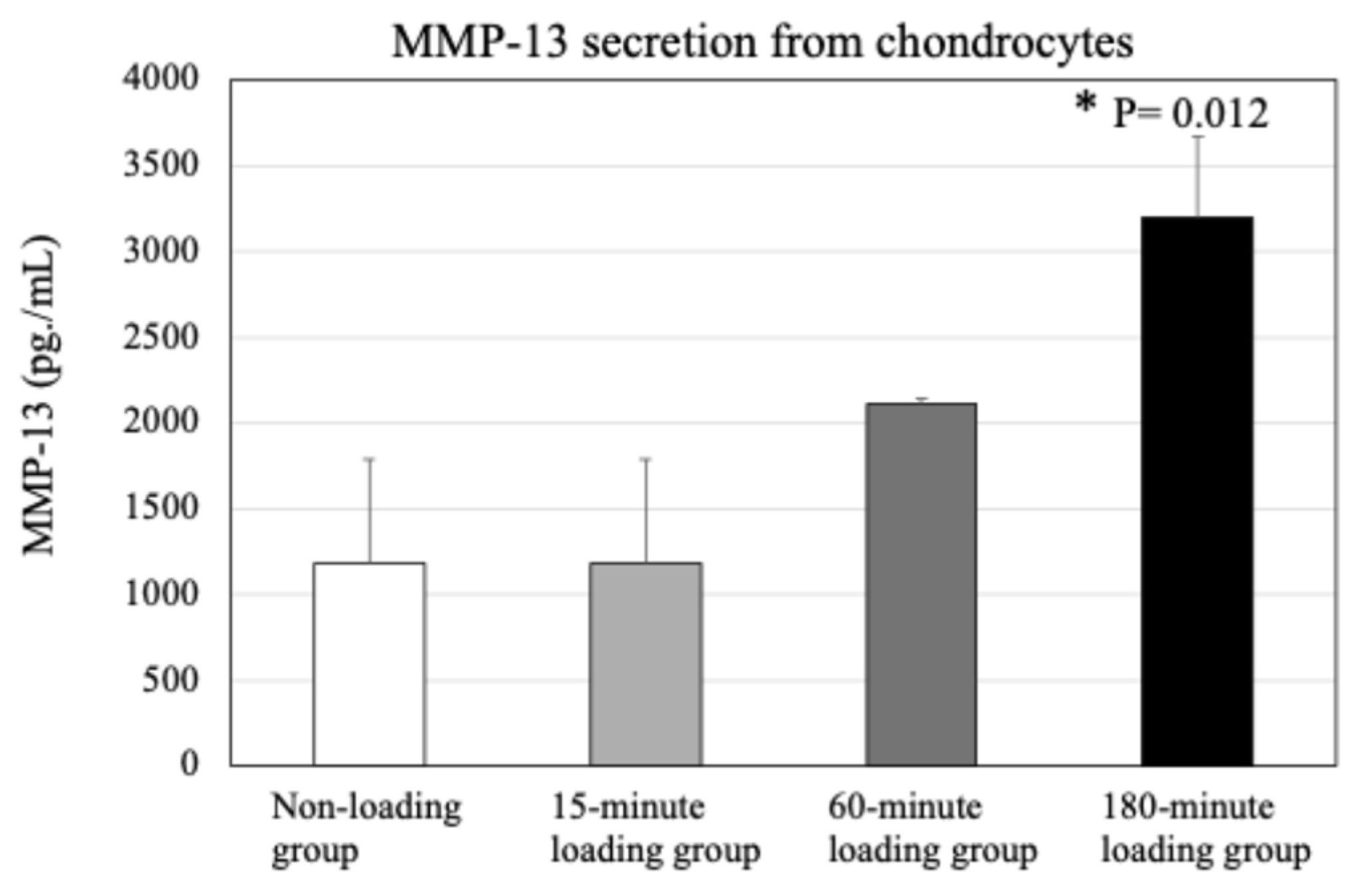
2.5. Effect of Physiologic and Repetitive Compressive Loading on Chondrocyte Activities, MMP-13 Production by Chondrocytes
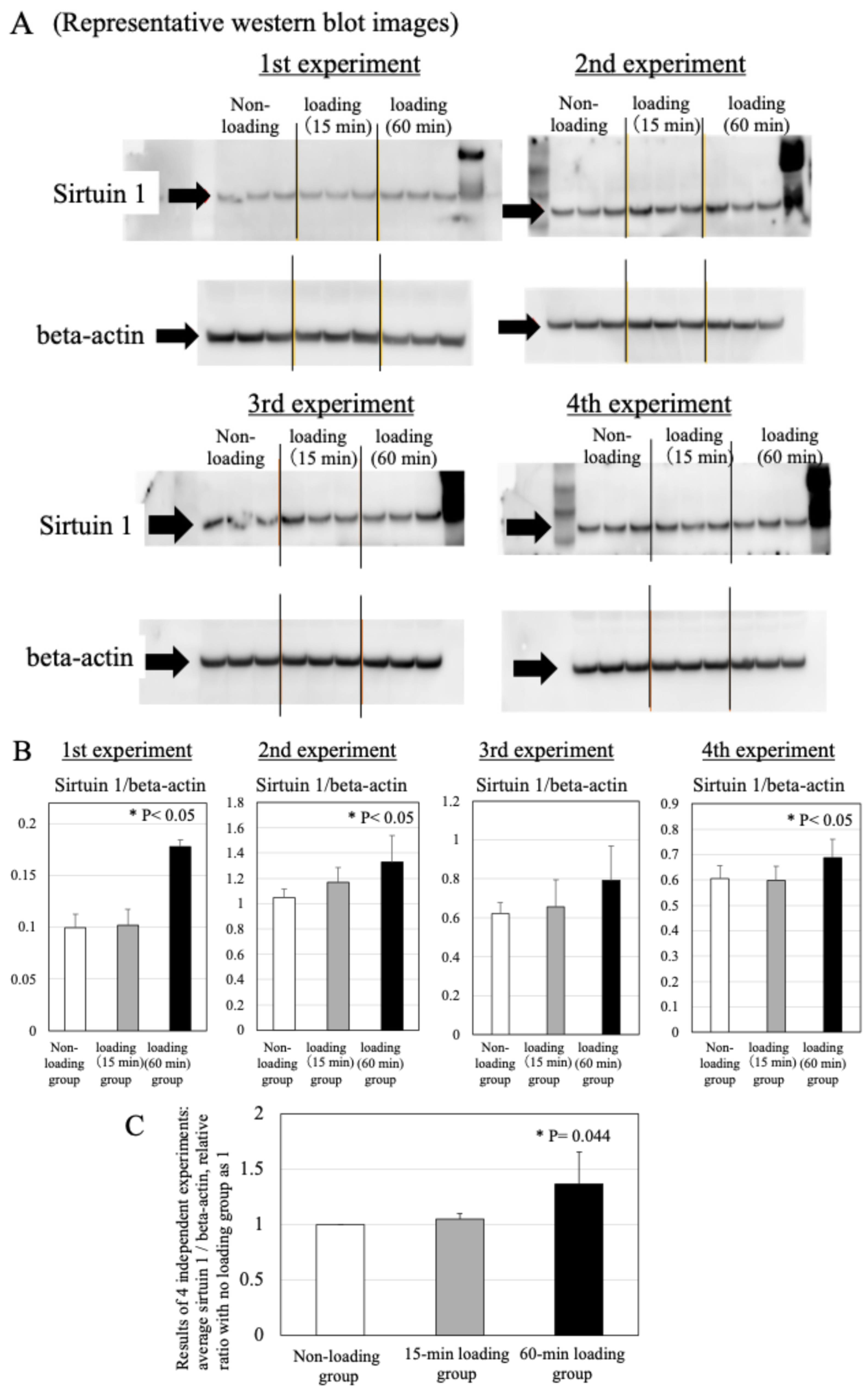
2.6. Effects of Physiologic and Repetitive Compressive Loading on the Expression of Sirtuin 1 in Chondrocytes
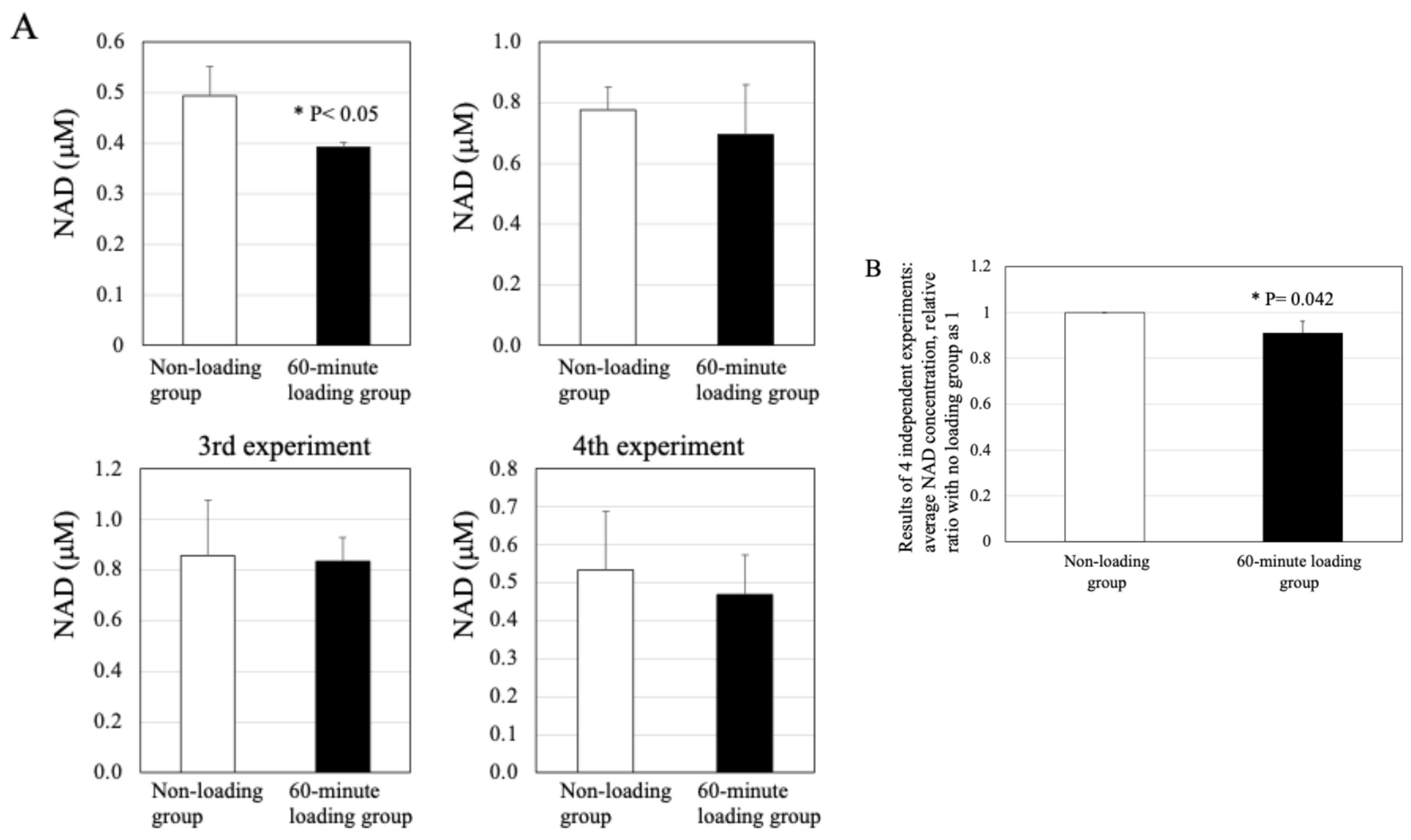
2.7. Effect of Physiologic and Repetitive Compressive Loading on the Level of NAD in Chondrocytes
2.8. Effects of Physiologic and Repetitive Compressive Loading on the Expression of Runx2 in Chondrocytes
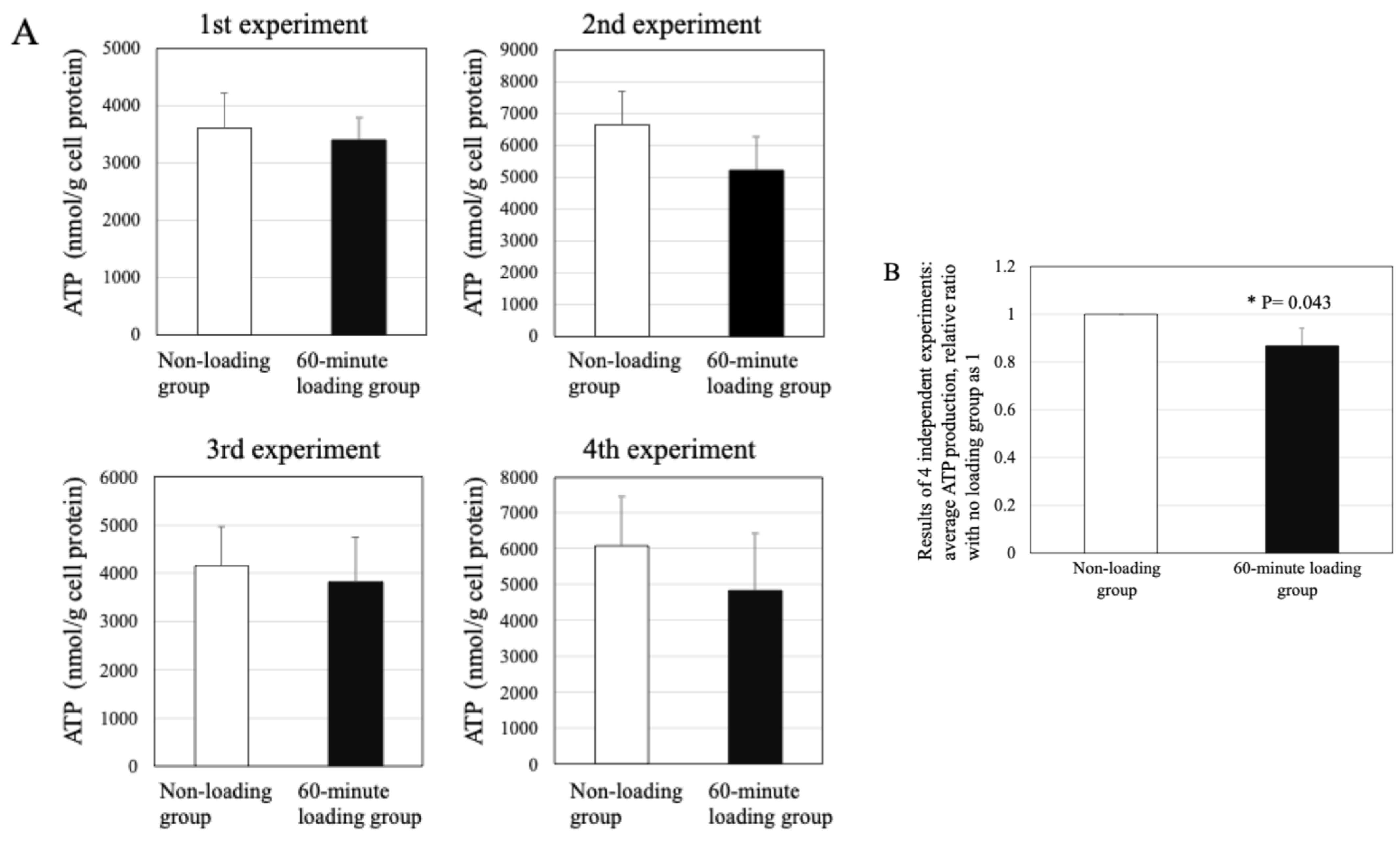
2.9. Effect of Repetitive Compressive Loading on ATP Production by PBMCs
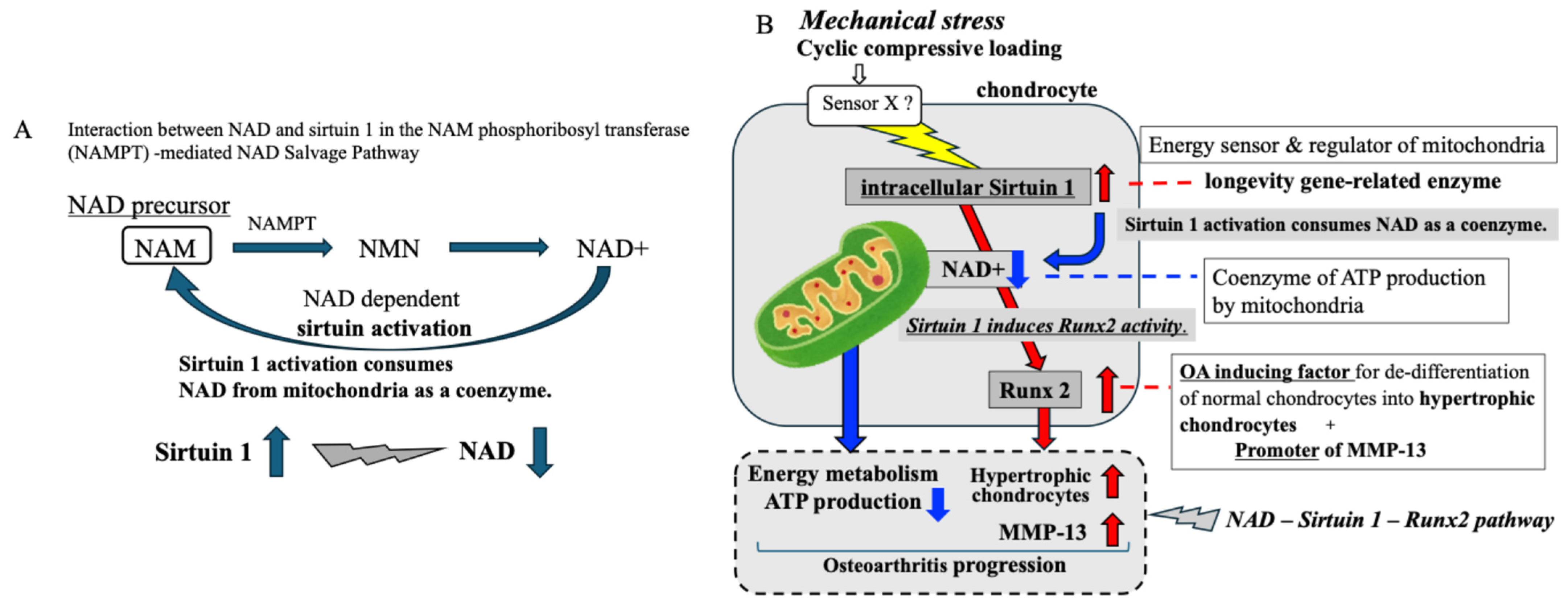
3. Discussion
4. Materials and Methods
4.1. Monolayer Human Chondrocyte Culture
4.2. Generation of a 3D Cell−Collagen Scaffold Construct for Cultured Human Cells
4.3. Repetitive Compressive Loading of the 3D Cell Culture Construct
4.4. Cellular Protein Content in the 3D Cell-Culture Construct
4.5. Effects of Repetitive Compressive Loading on Chondrocyte Viability
4.6. Scanning Electron Microscopy
4.7. Effects of Repetitive Compressive Loading on Chondrocyte Activity
4.8. Western Blot Assay
4.9. Effect of Repetitive Compressive Loading on the Level of NAD in Chondrocytes
4.10. ATP Production by Chondrocytes
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Liu-Bryan, R. Inflammation and intracellular metabolism: New targets in OA. Osteoarthr. Cartil. 2015, 23, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Macri, E.M.; Selles, R.W.; Stefanik, J.J.; Reijman, M. OARSI year in review 2023: Rehabilitation and outcomes. Osteoarthr. Cartil. 2023, 31, 1534–1547. [Google Scholar] [CrossRef]
- Yoshimura, N. Epidemiology of osteoarthritis in Japan: The ROAD study. Clin. Calcium 2011, 21, 821–825. [Google Scholar]
- Yoshimura, N.; Muraki, S.; Iidaka, T.; Oka, H.; Horii, C.; Kawaguchi, H.; Akune, T.; Nakamura, K.; Tanaka, S. Prevalence and co-existence of locomotive syndrome, sarcopenia, and frailty: The third survey of Research on Osteoarthritis/Osteoporosis Against Disability (ROAD) study. J. Bone Miner. Metab. 2019, 37, 1058–1066. [Google Scholar] [CrossRef]
- Yoshimura, N.; Iidaka, T.; Horii, C.; Mure, K.; Muraki, S.; Oka, H.; Kawaguchi, H.; Akune, T.; Ishibashi, H.; Ohe, T.; et al. Epidemiology of locomotive syndrome using updated clinical decision limits: 6-year follow-ups of the ROAD study. J. Bone Miner. Metab. 2022, 40, 623–635. [Google Scholar] [CrossRef]
- Hiligsmann, M.; Cooper, C.; Guillemin, F.; Hochberg, M.C.; Tugwell, P.; Arden, N.; Berenbaum, F.; Boers, M.; Boonen, A.; Branco, J.C.; et al. A reference case for economic evaluations in osteoarthritis: An expert consensus article from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Semin. Arthritis Rheum. 2014, 44, 271–282. [Google Scholar] [CrossRef]
- Bruyère, O.; Honvo, G.; Veronese, N.; Arden, N.K.; Branco, J.; Curtis, E.; Al-Daghri, N.M.; Herrero-Beaumont, G.; Martel-Pelletier, J.; Pelletier, J.-P.; et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin. Arthritis Rheum. 2019, 49, 337–350. [Google Scholar] [CrossRef]
- Allen, K.; Huffman, K.; Cleveland, R.; van der Esch, M.; Abbott, J.; Abbott, A.; Bennell, K.; Bowden, J.; Eyles, J.; Healey, E.; et al. Evaluating Osteoarthritis Management Programs: Outcome domain recommendations from the OARSI Joint Effort Initiative. Osteoarthr. Cartil. 2023, 31, 954–965. [Google Scholar] [CrossRef]
- Conley, B.; Bunzli, S.; Bullen, J.; O’Brien, P.; Persaud, J.; Gunatillake, T.; Dowsey, M.M.; Choong, P.F.M.; Lin, I. Core Recommendations for Osteoarthritis Care: A Systematic Review of Clinical Practice Guidelines. Arthritis Care Res. 2023, 75, 1897–1907. [Google Scholar] [CrossRef] [PubMed]
- Sampath, S.J.P.; Vijayalakshmi, V.; Sudip, G.; Nagasuryaprasad, K. Obesity, Metabolic Syndrome, and Osteoarthritis-An Updated Review. Curr. Obes. Rep. 2023, 12, 308–331. [Google Scholar] [CrossRef] [PubMed]
- Poulami, D.; Yue, Z.; Alexa, P.; Anirudh, S.; Evgeny, R.; Helal, E.; Brian, W.; Izabela, K.; Nizar, N.M.; Rajiv, G.; et al. High-fat diet-induced acceleration of osteoarthritis is associated with a distinct and sustained plasma metabolite signature. Sci. Rep. 2017, 7, 8205. [Google Scholar]
- Masson, A.O.; Krawetz, R.J. Understanding cartilage protection in OA and injury: A spectrum of possibilities. BMC Musculoskelet. Disord. 2020, 21, 432. [Google Scholar] [CrossRef]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic signaling pathways and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Namane, M.; Cicuttini, F. Osteoarthritis. Lancet 2025, 405, 71–85. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Kong, Y.; Zhang, X.; Zhang, H.; Gang, Y.; Bai, L. Mechanical stress protects against osteoarthritis via regulation of the AMPK/NF-kappaB signaling pathway. J. Cell. Physiol. 2018, 234, 9156–9167. [Google Scholar] [CrossRef]
- Zhen, G.; Guo, Q.; Li, Y.; Wu, C.; Zhu, S.; Wang, R.; Guo, X.E.; Kim, B.C.; Huang, J.; Hu, Y.; et al. Mechanical stress determines the configuration of TGFβ activation in articular cartilage. Nat. Commun. 2021, 12, 1706. [Google Scholar] [CrossRef]
- Lou, Y.; Song, F.; Kang, Y.; Xu, Y. Periodic Mechanical Stress Inhibits the Development of Osteoarthritis via Regulating ATF3-Akt Axis. J. Inflamm. Res. 2023, 16, 5613–5628. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, P.; Hu, B.; Xiao, Y.; Su, T.; Luo, X.; Tu, M.; Cai, G. Excessive mechanical loading promotes osteoarthritis development by upregulating Rcn2. Biochim. Biophys. Acta. Mol. Basis. Dis. 2024, 1870, 167251. [Google Scholar] [CrossRef]
- Kurz, B.; Lemke, A.; Kehn, M.; Domm, C.; Patwari, P.; Frank, E.H.; Grodzinsky, A.J.; Schünke, M. Influence of tissue maturation and antioxidants on the apoptotic response of articular cartilage after injurious compression. Arthritis Rheum. 2004, 50, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Kurz, B.; Lemke, A.K.; Fay, J.; Pufe, T.; Grodzinsky, A.J.; Schunke, M. Pathomechanisms of cartilage destruction by mechanical injury. Ann. Anat. 2005, 2187, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Green, D.M.; Noble, P.C.; Ahuero, J.S.; Birdsall, H.H. Cellular events leading to chondrocyte death after cartilage impact injury. Arthritis Rheum. 2006, 54, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Mori, D.; Kobayashi, H.; Mori, Y.; Nakamoto, H.; Okada, K.; Taniguchi, Y.; Sugita, S.; Yano, F.; Chung, U.-I.; et al. Excessive mechanical loading promotes osteoarthritis through the gremlin-1–NF-κB pathway. Nat. Commun. 2019, 10, 1442. [Google Scholar] [CrossRef]
- He, Y.; Makarczyk, M.J.; Lin, H. Role of mitochondria in mediating chondrocyte response to mechanical stimuli. Life Sci. 2020, 263, 118602. [Google Scholar] [CrossRef]
- Takeda, Y.; Niki, Y.; Fukuhara, Y.; Fukuda, Y.; Udagawa, K.; Shimoda, M.; Kikuchi, T.; Kobayashi, S.; Harato, K.; Miyamoto, T.; et al. Compressive mechanical stress enhances susceptibility to interleukin-1 by increasing interleukin-1 receptor expression in 3D-cultured ATDC5 cells. BMC Musculoskelet. Disord. 2021, 22, 238. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, H.; Guan, H.; Wu, C.; Qi, W.; Yang, L.; Yin, J.; Zhang, H.; Liu, L.; Lu, Y.; et al. PDZK1 protects against mechanical overload-induced chondrocyte senescence and osteoarthritis by targeting mitochondrial function. Bone Res. 2024, 12, 41. [Google Scholar] [CrossRef]
- Kan, S.; Duan, M.; Liu, Y.; Wang, C.; Xie, J. Role of Mitochondria in Physiology of Chondrocytes and Diseases of Osteoarthritis and Rheumatoid Arthritis. CARTILAGE 2021, 13 (Suppl. S2), 1102S–1121S. [Google Scholar] [CrossRef]
- He, Y.; Wu, Z.; Xu, L.; Xu, K.; Chen, Z.; Ran, J.; Wu, L. The role of SIRT3-mediated mitochondrial homeostasis in osteoarthritis. Cell. Mol. Life Sci. 2020, 77, 3729–3743. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.-Y.; Si, H.-B.; Lu, Y.-R.; Shen, B. Mechanistic insights into AMPK-SIRT3 positive feedback loop-mediated chondrocyte mitochondrial quality control in osteoarthritis pathogenesis. Pharmacol. Res. 2021, 166, 105497. [Google Scholar] [CrossRef]
- Liu, D.; Cai, Z.-J.; Yang, Y.-T.; Lu, W.-H.; Pan, L.-Y.; Xiao, W.-F.; Li, Y.-S. Mitochondrial quality control in cartilage damage and osteoarthritis: New insights and potential therapeutic targets. Osteoarthr. Cartil. 2022, 30, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, W.; Cheng, Y.; Ren, Z.; Liu, X.; Yang, H.; Ding, G.; Huang, H. Intrauterine hyperglycemia induces SIRT3-mediated mitochondrial dysfunction: The fetal origin pathogenesis of precocious osteoarthritis. Osteoarthr. Cartil. 2024, 32, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Somemura, S.; Kumai, T.; Yatabe, K.; Sasaki, C.; Fujiya, H.; Niki, H.; Yudoh, K. Physiologic Mechanical Stress Directly Induces Bone Formation by Activating Glucose Transporter 1 (Glut 1) in Osteoblasts, Inducing Signaling via NAD+-Dependent Deacetylase (Sirtuin 1) and Runt-Related Transcription Factor 2 (Runx2). Int. J. Mol. Sci. 2021, 22, 9070. [Google Scholar] [CrossRef] [PubMed]
- Sofia, P.; Alessandro, A.; Elisa, B.; Assunta, P.; Chiara, G.F.; Pietro, R.; Valentina, S.; Carmelo, M.; Alice, B. Biomechanics of Chondrocytes and Chondrons in Healthy Conditions and Osteoarthritis: A Review of the Mechanical Characterisations at the Microscale. Biomedicines 2023, 11, 1942. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 2020, 22, 119–141. [Google Scholar] [CrossRef]
- Chini, C.C.; Zeidler, J.D.; Kashyap, S.; Warner, G.; Chini, E.N. Evolving concepts in NAD+ metabolism. Cell Metab. 2021, 33, 1076–1087. [Google Scholar] [CrossRef]
- Mouchiroud, L.; Houtkooper, R.H.; Moullan, N.; Katsyuba, E.; Ryu, D.; Canto, C.; Mottis, A.; Jo, Y.S.; Viswanathan, M.; Schoonjans, K.; et al. The NAD+/Sirtuin Pathway Modulates Longevity through Activation of Mitochondrial UPR and FOXO Signaling. Cell 2013, 154, 430–441. [Google Scholar] [CrossRef]
- Katsyuba, E.; Mottis, A.; Zietak, M.; De Franco, F.; Van Der Velpen, V.; Gariani, K.; Ryu, D.; Cialabrini, L.; Matilainen, O.; Liscio, P.; et al. De novo NAD+ synthesis enhances mitochondrial function and improves health. Nature 2018, 563, 354–359. [Google Scholar] [CrossRef]
- Wang, G.; Lin, N. NAD-Dependent Protein Deacetylase Sirtuin-1 Mediated Mitophagy Regulates Early Brain Injury After Subarachnoid Hemorrhage. J. Inflamm. Res. 2024, 17, 1971–1981. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, M.C.; Tomanek, L. Sirtuins as regulators of the cellular stress response and metabolism in marine ectotherms. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 236, 110528. [Google Scholar] [CrossRef]
- Ji, Z.; Liu, G.H.; Qu, J. Mitochondrial sirtuins, metabolism, and aging. J. Genet. Genom. 2021, 49, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Muroi, Y.; Kakudo, K.; Nakata, K. Effects of Compressive Loading on Human Synovium-derived Cells. J. Dent. Res. 2007, 86, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Akamine, Y.; Kakudo, K.; Kondo, M.; Ota, K.; Muroi, Y.; Yoshikawa, H.; Nakata, K. Prolonged matrix metalloproteinase-3 high expression after cyclic compressive load on human synovial cells in three-dimensional cultured tissue. Int. J. Oral Maxillofac. Surg. 2012, 41, 874–881. [Google Scholar] [CrossRef]
- Oyama, S.; Kanamoto, T.; Ebina, K.; Etani, Y.; Hirao, M.; Goshima, A.; Otani, S.; Hikida, M.; Yamakawa, S.; Ito, S.; et al. Cyclic compressive loading induces a mature meniscal cell phenotype in mesenchymal stem cells with an atelocollagen-based scaffold. Front. Bioeng. Biotechnol. 2024, 12, 1394093. [Google Scholar] [CrossRef]
- Goh, S.L.; Persson, M.S.M.; Stocks, J.; Hou, Y.; Welton, N.J.; Lin, J.; Hall, M.C.; Doherty, M.; Zhang, W. Relative Efficacy of Different Exercises for Pain, Function, Performance and Quality of Life in Knee and Hip Osteoarthritis: Systematic Review and Network Meta-Analysis. Sports Med. 2019, 49, 743–761. [Google Scholar] [CrossRef]
- Smith, T.O.; Hawker, G.A.; Hunter, D.J.; March, L.M.; Boers, M.; Shea, B.J.; Christensen, R.; Guillemin, F.; Terwee, C.B.; Williamson, P.R.; et al. The OMERACT-OARSI core domain set for measurement in clinical trials of hip and/or knee osteoarthritis. J. Rheumatol. 2019, 46, 981–989. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Batushansky, A.; Zhu, S.; Komaravolu, R.K.; South, S.; Mehta-D'souza, P.; Griffin, T.M. Fundamentals of OA. An initiative of Osteoarthritis and Cartilage. Obesity and metabolic factors in OA. Osteoarthr. Cartil. 2021, 30, 501–515. [Google Scholar] [CrossRef]
- Nedunchezhiyan, U.; Varughese, I.; Sun, A.R.; Wu, X.; Crawford, R.; Prasadam, I. Obesity, Inflammation, and Immune System in Osteoarthritis. Front Immunol. 2022, 13, 907750. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Yu, H.; Lu, K.; Ruan, C.; Ding, C.; Tong, L.; Zhao, X.; Chen, D. AMPK Signaling in Energy Control, Cartilage Biology, and Osteoarthritis. Front. Cell Dev. Biol. 2021, 9, 696602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hou, M.; Liu, Y.; Liu, T.; Chen, X.; Shi, Q.; Geng, D.; Yang, H.; He, F.; Zhu, X. Recharge of chondrocyte mitochondria by sustained release of melatonin protects cartilage matrix homeostasis in osteoarthritis. J. Pineal Res. 2022, 73, e12815. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, C.A.; Yamamoto, H.; Fujita, T.; Furuichi, T.; Ito, K.; Inoue, K.; Yamana, K.; Zanma, A.; Takada, K.; Ito, Y.; et al. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev. 2004, 18, 952–963. [Google Scholar] [CrossRef]
- Komori, T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem. Cell. Biol. 2018, 149, 313–323. [Google Scholar] [CrossRef]
- Buhrmann, C.; Busch, F.; Shayan, P.; Shakibaei, M. Sirtuin-1 (SIRT1) Is Required for Promoting Chondrogenic Differentiation of Mesenchymal Stem Cells. J. Biol. Chem. 2014, 289, 22048–22062. [Google Scholar] [CrossRef]
- Kim, H.J.; Braun, H.J.; Dragoo, J.L. The effect of resveratrol on normal and osteoarthritic chondrocyte metabolism. Bone Jt. Res. 2014, 3, 51–59. [Google Scholar] [CrossRef]
- Wang, X.; Manner, P.A.; Horner, A.; Shum, L.; Tuan, R.S.; Nuckolls, G.H. Regulation of MMP-13 expression by RUNX2 and FGF2 in osteoarthritic cartilage. Osteoarthr. Cartil. 2004, 12, 963–973. [Google Scholar] [CrossRef]
- Kawaguchi, H. Endochondral Ossification Signals in Cartilage Degradation During Osteoarthritis Progression in Experimental Mouse Models. Mol. Cells 2008, 25, 1–6. [Google Scholar] [CrossRef]
- Goldring, M.B.; Tsuchimochi, K.; Ijiri, K. The control of chondrogenesis. J. Cell. Biochem. 2005, 97, 33–44. [Google Scholar] [CrossRef]
- Terauchi, K.; Kobayashi, H.; Yatabe, K.; Yui, N.; Fujiya, H.; Niki, H.; Musha, H.; Yudoh, K. The NAD-Dependent Deacetylase Sirtuin-1 Regulates the Expression of Osteogenic Transcriptional Activator Runt-Related Transcription Factor 2 (Runx2) and Production of Matrix Metalloproteinase (MMP)-13 in Chondrocytes in Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 1019. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takemoto, M.; Sugishita, Y.; Takahashi-Suzuki, Y.; Fujiya, H.; Niki, H.; Yudoh, K. Repetitive Compressive Loading Downregulates Mitochondria Function and Upregulates the Cartilage Matrix Degrading Enzyme MMP-13 Through the Coactivation of NAD-Dependent Sirtuin 1 and Runx2 in Osteoarthritic Chondrocytes. Int. J. Mol. Sci. 2025, 26, 4967. https://doi.org/10.3390/ijms26114967
Takemoto M, Sugishita Y, Takahashi-Suzuki Y, Fujiya H, Niki H, Yudoh K. Repetitive Compressive Loading Downregulates Mitochondria Function and Upregulates the Cartilage Matrix Degrading Enzyme MMP-13 Through the Coactivation of NAD-Dependent Sirtuin 1 and Runx2 in Osteoarthritic Chondrocytes. International Journal of Molecular Sciences. 2025; 26(11):4967. https://doi.org/10.3390/ijms26114967
Chicago/Turabian StyleTakemoto, Masahiro, Yodo Sugishita, Yuki Takahashi-Suzuki, Hiroto Fujiya, Hisateru Niki, and Kazuo Yudoh. 2025. "Repetitive Compressive Loading Downregulates Mitochondria Function and Upregulates the Cartilage Matrix Degrading Enzyme MMP-13 Through the Coactivation of NAD-Dependent Sirtuin 1 and Runx2 in Osteoarthritic Chondrocytes" International Journal of Molecular Sciences 26, no. 11: 4967. https://doi.org/10.3390/ijms26114967
APA StyleTakemoto, M., Sugishita, Y., Takahashi-Suzuki, Y., Fujiya, H., Niki, H., & Yudoh, K. (2025). Repetitive Compressive Loading Downregulates Mitochondria Function and Upregulates the Cartilage Matrix Degrading Enzyme MMP-13 Through the Coactivation of NAD-Dependent Sirtuin 1 and Runx2 in Osteoarthritic Chondrocytes. International Journal of Molecular Sciences, 26(11), 4967. https://doi.org/10.3390/ijms26114967





