Neuronutrition and Nrf2 Brain Resilience Signaling: Epigenomics and Metabolomics for Personalized Medicine in Nervous System Disorders from Bench to Clinic
Abstract
1. Introduction
2. Neuronutrition Targeting Nrf2 Brain Resilience Signaling in Nervous System Disorders: Personalized Preventive and Therapeutic Innovations
2.1. Ellagic Acid
2.1.1. Brain Resilience Potential of Ellagic Acid in AD
2.1.2. Brain Resilience Potential of Ellagic Acid in PD
2.1.3. Brain Resilience Potential of Ellagic Acid in Autism
2.2. Anthocyanins
2.2.1. Brain Resilience Potential of Anthocyanins in AD
2.2.2. Brain Resilience Potential of Anthocyanins in PD
2.2.3. Brain Resilience Potential of Anthocyanins in Autism
2.3. Centella asiatica and Caffeoylquinic Acid Metabolites
2.3.1. Brain Resilience Potential of Caffeoylquinic Acids in AD
2.3.2. Brain Resilience Potential of Caffeoylquinic Acids in PD
2.3.3. Brain Resilience Potential of Caffeoylquinic Acids in Autism
2.4. Genistein
2.4.1. Brain Resilience Potential of Genistein in AD
2.4.2. Brain Resilience Potential of Genistein in PD
2.4.3. Brain Resilience Potential of Genistein in Autism
2.5. Fisetin
2.5.1. Brain Resilience Potential of Fisetin in AD
2.5.2. Brain Resilience Potential of Fisetin in PD
2.5.3. Brain Resilience Potential of Fisetin in Autism
| Neuronutrients | Molecular Pathways | AD | PD | Autism | Ref. |
|---|---|---|---|---|---|
| Ellagic acid | ↓ NF-κB ↓ JAK-STAT ↑ Nrf2 | ↓ NF-κB, IL-1β, TLR4 ↑ Nrf2/Keap1 ↑ IRS/PI3K/Akt/GS3Kβ | ↓ Bax/Bcl-2, caspasi-3 ↓ 6-OHDA, Cox-2 ↑ Nrf2/HO-1 | - - - | [30,33,36] [30,32,42,46] [31,35,39] |
| Anthocyanins | ↓ NF-κB | ↓ IL-Iβ, IL-6, TNF-α ↑ PI3K/AKT/Nrf2 | ↓ 6-OHDA | ↓ COX-2 ↓ IL-1β, IL-6 ↓ TNF-α | [50,51,58] [51] [61] |
| Caffeoylquinic acids | ↑ Nrf2 | ↓ JNK, c-JUN ↑ ERK1/2 ↓ AKT, GSK-3β | ↓ 6-OHDA | - | [65,70,72] [66] |
| Genistein | ↑ Nrf2/HO-1/PI3K | ↑ PI3K/Akt/Nrf2 ↑ ERK/CREB/BDNF ↑ GSK-3β/ERK/JNK ↓ NF-kB, TNF-α, IL-1β | ↓ 6-OHDA | ↓ TNF-α, IL-1β ↓ Bax, Bcl2 ↓ Caspase-3 | [80,81,95] [83,97] [84,98] [88] |
| Fisetin | ↑ Nrf2 | ↓ p-JNK/NF-kB, IL-6 ↑ GST ↑ Nrf2/HO-1 | ↓ TNF-α, IL-6 ↑ GSH, SOD, CAT | ↑ GSH | [99,100,110] [104,108] [105] |
3. Neuroinflammation: Role of Neuronutrients
3.1. Neuronutrients Inhibit Neuroinflammatory Cascade and Promote Brain Resilience
3.1.1. Potential Anti-Neuroinflammatory Effects of Genistein
3.1.2. Potential Anti-Neuroinflammatory Effects of Fisetin
3.1.3. Potential Anti-Neuroinflammatory Effects of Coffee and Chlorogenic Acids Metabolites
3.1.4. Potential Anti-Neuroinflammatory Effects of Anthocyanins
4. Metabolomics for Studying Nervous System Disorders: Personalized Neuronutritional Medicine
4.1. Tryptophan, Kynurenine, and Serotonin Metabolic Pathways
4.1.1. Tryptophan Metabolites in AD
4.1.2. Tryptophan Metabolites in PD
4.1.3. Tryptophan Metabolites in Autism
4.2. Pharmacological Inhibitors and Neuronutrients Modulate Tryptophan Metabolites
4.2.1. Preclinical Studies
4.2.2. Clinical Studies
4.3. SCFAs Metabolism Along the Gut–Brain Axis: Focus on Nutrients
4.3.1. Neuronutrients Regulate SCFA Metabolites in Nervous System Disorders: SCFA Metabolites in AD
Preclinical Studies
Clinical Studies
4.4. SCFA Metabolites in PD
4.4.1. Preclinical Studies
4.4.2. Clinical Studies
4.5. SCFA Metabolites in Autism
4.5.1. Preclinical Studies
4.5.2. Clinical Studies
4.6. Sulfur-Containing Nutrient Metabolites
4.6.1. Dihydroasparagusic Acid
4.6.2. S-Allyl Cysteine
4.6.3. 6-(Methylsulfinyl)hexyl Isothiocyanate
4.6.4. Hydrogen Sulfide
4.7. Tyrosine Metabolism and Neuronutrition: Tyrosine Metabolites in AD
4.7.1. Preclinical Studies
4.7.2. Clinical Studies
4.8. Tyrosine Metabolites in PD
| Metabolites | Upregulation | Downregulation | Outcomes: AD | Outcomes: PD | Outcomes: Autism | Ref. |
|---|---|---|---|---|---|---|
| Kynurenic acid | Erk/JNK/MAPK | DR3/IKK/NF-κB | Neuroprotective effects | Protection neuronal | Neuroprotection | [185] |
| SCFAs | Nrf2 PI3K/Akt/mTOR | IL-6, IL-8, Il-12, IL-17, IL-1β, TNF-α | Strengthen BBB integrity | Neuroprotective role | Reduction in autism severity | [197,226] |
| Dihydroasparagusic acid | - | TNF-α, PGE2 Cyclooxygenase-2 Lipoxygenase | Inhibits the processes neuroinflammatory | Inhibits oxidative processes | - | [239] |
| S-Allyl cysteine | Nrf2/TLR4 | - | Neuroprotective and anti-amyloidogenic effects | Neuroprotective effects | - | [241] |
| 6-(Methylsulfinyl) hexyl isothiocyanate | Nrf2 | GSK-3β/NF-κB | Protection from oxidative stress and inflammation | Preserved nigral dopaminergic neurons | - | [251] |
| Hydrogen sulfide | Akt/glycogen synthase kinase-3β/β-catenin | - | Increases neurogenesis improved cognitive deficits | Neuroprotective properties | - | [256] |
| Tyrosine | PI3K/AKT/Nrf2 | MAPK/NF-κB | Neuroprotective effects | Protective action on dopaminergic neurons | Alleviates behavioral disorders, social communication deficits and reduced repetitive behavior | [262,278] |
4.9. Tyrosine Metabolites in Autism
4.9.1. Preclinical Studies
4.9.2. Clinical Studies
5. Neuro-Epigenetic Interactions: Role of Nrf2 in Nervous System Disorders
5.1. Nrf2 Epigenetic Regulation in AD and PD
5.2. Nutritional Modulators Regulate Epigenetic Modifications Targeting the Nrf2 Pathway
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scuto, M.; Majzúnová, M.; Torcitto, G.; Antonuzzo, S.; Rampulla, F.; Di Fatta, E.; Trovato Salinaro, A. Functional Food Nutrients, Redox Resilience Signaling and Neurosteroids for Brain Health. Int. J. Mol. Sci. 2024, 25, 12155. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef]
- Scuto, M.; Rampulla, F.; Reali, G.M.; Spanò, S.M.; Trovato Salinaro, A.; Calabrese, V. Hormetic Nutrition and Redox Regulation in Gut-Brain Axis Disorders. Antioxidants 2024, 13, 484. [Google Scholar] [CrossRef]
- Scuto, M.; Lombardo, C.M.G.; Lo Sasso, B.; Di Fatta, E.; Ferri, R.; Trovato Salinaro, A. Microplastics as Emerging Contaminants and Human Health: Exploring Functional Nutrition in Gastric–Colon–Brain Axis Cancer. Toxics 2025, 13, 438. [Google Scholar] [CrossRef]
- Su, K.P.; Tseng, P.T.; Lin, P.Y.; Okubo, R.; Chen, T.Y.; Chen, Y.W.; Matsuoka, Y.J. Association of Use of Omega-3 Polyunsaturated Fatty Acids with Changes in Severity of Anxiety Symptoms: A Systematic Review and Meta-analysis. JAMA Netw. Open 2018, 1, e182327. [Google Scholar] [CrossRef]
- Markun, S.; Gravestock, I.; Jäger, L.; Rosemann, T.; Pichierri, G.; Burgstaller, J.M. Effects of Vitamin B12 Supplementation on Cognitive Function, Depressive Symptoms, and Fatigue: A Systematic Review, Meta-Analysis, and Meta-Regression. Nutrients 2021, 13, 923. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, M.; Graham, A.M.; Feczko, E.; Miranda-Dominguez, O.; Rasmussen, J.M.; Nardos, R.; Entringer, S.; Wadhwa, P.D.; Buss, C.; Fair, D.A. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat. Neurosci. 2018, 21, 765–772. [Google Scholar] [CrossRef]
- Nogueira-De-Almeida, C.A.; Zotarelli-Filho, I.J.; Nogueira-De-Almeida, M.E.; Souza, C.G.; Kemp, V.L.; Ramos, W.S. Neuronutrients and Central Nervous System: A Systematic Review. Cent. Nerv. Syst. Agents Med. Chem. 2022, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Topcuoglu, M.A.; Arsava, E.M. Neuronutrition: An Emerging Concept. In Nutrition in Neurologic Disorders; Springer: Berlin/Heidelberg, Germany, 2017; pp. 155–206. [Google Scholar]
- Badaeva, A.V.; Danilov, A.B.; Clayton, P.; Moskalev, A.A.; Karasev, A.V.; Tarasevich, A.F.; Vorobyeva, Y.D.; Novikov, V.N. Perspectives on Neuronutrition in Prevention and Treatment of Neurological Disorders. Nutrients 2023, 28, 2505. [Google Scholar] [CrossRef]
- Yasuda, Y.; Tokumatsu, T.; Ueda, C.; Sakai, M.; Sasaki, Y.; Norikura, T.; Matsui-Yuasa, I.; Kojima-Yuasa, A. Ecklonia cava Polyphenols Have a Preventive Effect on Parkinson’s Disease through the Activation of the Nrf2-ARE Pathway. Nutrients 2024, 28, 2076. [Google Scholar] [CrossRef] [PubMed]
- Duarte, R.M.F.; Ribeiro-Barbosa, E.R.; Ferreira, F.R.; Espindola, F.S.; Spini, V.B.M.G. Resveratrol prevents offspring’s behavioral impairment associated with immunogenic stress during pregnancy. Prog. Neuropsychopharmacol. Biol. Psychiatry 2025, 10, 136, 111188. [Google Scholar] [CrossRef]
- Chen, Y.; Demnitz, N.; Yamamoto, S.; Yaffe, K.; Lawlor, B.; Leroi, I. Defining brain health: A concept analysis. Int. J. Geriatr. Psychiatry 2021, 37, 1–13. [Google Scholar] [CrossRef]
- Devi, A.; Narayanan, R. A Review on Neuronutrition. Asian J. Dairy Food Res. 2019, 38, 128–133. [Google Scholar] [CrossRef]
- Umeno, A.; Biju, V.; Yoshida, Y. In vivo ROS production and use of oxidative stress-derived biomarkers to detect the onset of diseases such as Alzheimer’s disease, Parkinson’s disease, and diabetes. Free Radic. Res. 2017, 51, 413–427. [Google Scholar] [CrossRef]
- Khan, M.S.; Ali, T.; Kim, M.W.; Jo, M.H.; Jo, M.G.; Badshah, H.; Kim, M.O. Anthocyanins protect against LPS-induced oxidative stress-mediated neuroinflammation and neurodegeneration in the adult mouse cortex. Neurochem. Int. 2016, 100, 1–10. [Google Scholar] [CrossRef]
- Arora, M.K.; Kisku, A.; Jangra, A. Mangiferin ameliorates intracerebroventricular-quinolinic acid-induced cognitive deficits, oxidative stress, and neuroinflammation in Wistar rats. Indian J. Pharmacol. 2020, 52, 296–305. [Google Scholar]
- Cahoon, D.S.; Fisher, D.R.; Lamon-Fava, S.; Wu, D.; Zheng, T.; Shukitt-Hale, B. Blueberry treatment administered before and/or after lipopolysaccharide stimulation attenuates inflammation and oxidative stress in rat microglial cells. Nutr. Neurosci. 2023, 26, 127–137. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, Z.; Zhan, G.; Li, X.; Li, S.; Wang, X.; Li, S.; Luo, A. Caffeic acid phenethyl ester suppresses oxidative stress and regulates M1/M2 microglia polarization via Sirt6/Nrf2 pathway to mitigate cognitive impairment in aged mice following anesthesia and surgery. Antioxidants 2023, 12, 714. [Google Scholar] [CrossRef] [PubMed]
- Corpas, R.; Griñán-Ferré, C.; Rodríguez-Farré, E.; Pallàs, M.; Sanfeliu, C. Resveratrol Induces Brain Resilience Against Alzheimer Neurodegeneration Through Proteostasis Enhancement. Mol. Neurobiol. 2019, 56, 1502–1516. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Hofford, R.S.; Meckel, K.R.; Dave, Y.A.; Zeldin, S.M.; Shipman, A.L.; Lucerne, K.E.; Trageser, K.J.; Oguchi, T.; Kiraly, D.D. Dietary polyphenols drive dose-dependent behavioral and molecular alterations to repeated morphine. Sci. Rep. 2023, 13, 12223. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.; Kuhad, A. Resveratrol suppresses neuroinflammation in the experimental paradigm of autism spectrum disorders. Neurochem. Int. 2017, 103, 8–23. [Google Scholar] [CrossRef]
- Scuto, M.; Trovato Salinaro, A.; Caligiuri, I.; Ontario, M.L.; Greco, V.; Sciuto, N.; Crea, R.; Calabrese, E.J.; Rizzolio, F.; Canzonieri, V.; et al. Redox modulation of vitagenes via plant polyphenols and vitamin D: Novel insights for chemoprevention and therapeutic interventions based on organoid technology. Mech. Ageing Dev. 2021, 199, 111551. [Google Scholar] [CrossRef]
- Scuto, M.; Ontario, M.L.; Salinaro, A.T.; Caligiuri, I.; Rampulla, F.; Zimbone, V.; Modafferi, S.; Rizzolio, F.; Canzonieri, V.; Calabrese, E.J.; et al. Redox modulation by plant polyphenols targeting vitagenes for chemoprevention and therapy: Relevance to novel anti-cancer interventions and mini-brain organoid technology. Free Radic. Biol. Med. 2022, 179, 59–75. [Google Scholar] [CrossRef]
- Scuto, M.; Modafferi, S.; Rampulla, F.; Zimbone, V.; Tomasello, M.; Spano, S.; Ontario, M.L.; Palmeri, A.; Trovato Salinaro, A.; Siracusa, R.; et al. Redox modulation of stress resilience by Crocus sativus L. for potential neuroprotective and anti-neuroinflammatory applications in brain disorders: From molecular basis to therapy. Mech. Ageing Dev. 2022, 205, 111686. [Google Scholar] [CrossRef]
- Zamroziewicz, M.K.; Barbey, A.K. Nutritional Cognitive Neuroscience: Innovations for Healthy Brain Aging. Front. Neurosci. 2016, 10, 240. [Google Scholar] [CrossRef]
- Calabrese, V.; Trovato, A.; Scuto, M.; Ontario, M.L.; Tomasello, M.; Perrotta, R.; Calabrese, E. Resilience signaling and hormesis in brain health and disease. In Human Aging: From Cellular Mechanisms to Therapeutic Strategies; Academic Press: Cambridge, MA, USA, 2021; pp. 155–172. [Google Scholar]
- Kris-Etherton, P.M.; Petersen, K.S.; Hibbeln, J.R.; Hurley, D.; Kolick, V.; Peoples, S.; Rodriguez, N.; Woodward-Lopez, G. Nutrition and behavioral health disorders: Depression and anxiety. Nutr. Rev. 2021, 79, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Singh, A.K.; Kumar, R.; Jamieson, S.; Pandey, A.K.; Bishayee, A. Neuroprotective Potential of Ellagic Acid: A Critical Review. Adv. Nutr. 2021, 12, 1211–1238. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liang, X.; Liang, M.; Qin, R.; Qin, F.; Wang, X. Ellagic Acid Ameliorates Renal Ischemic-Reperfusion Injury Through NOX4/JAK/STAT Signaling Pathway. Inflammation 2020, 43, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Aslan, A.; Gok, O.; Beyaz, S.; Arslan, E.; Erman, O.; Ağca, C.A. The preventive effect of ellagic acid on brain damage in rats via regulating of Nrf-2, NF-kB and apoptotic pathway. J. Food Biochem. 2020, 44, e13217. [Google Scholar] [CrossRef]
- Yang, X.; Chu, F.; Jiao, Z.; Yu, H.; Yang, W.; Li, Y.; Lu, C.; Ma, H.; Wang, S.; Liu, Z.; et al. Ellagic acid ameliorates arsenic-induced neuronal ferroptosis and cognitive impairment via Nrf2/GPX4 signaling pathway. Ecotoxicol. Environ. Saf. 2024, 283, 116833. [Google Scholar] [CrossRef]
- Zhu, W.; Tang, H.; Li, J.; Guedes, R.M.; Cao, L.; Guo, C. Ellagic acid attenuates interleukin-1β-induced oxidative stress and exerts protective effects on chondrocytes through the Kelch-like ECH-associated protein 1 (Keap1)/Nuclear factor erythroid 2-related factor 2 (Nrf2) pathway. Bioengineered 2022, 13, 9233–9247. [Google Scholar] [CrossRef]
- Kuatov, R.; Takano, J.; Arie, H.; Kominami, M.; Tateishi, N.; Wakabayashi, K.I.; Takemoto, D.; Izumo, T.; Nakao, Y.; Nakamura, W.; et al. Urolithin A Modulates PER2 Degradation via SIRT1 and Enhances the Amplitude of Circadian Clocks in Human Senescent Cells. Nutrients 2024, 17, 20. [Google Scholar] [CrossRef]
- Alfaris, N.A.; Alshammari, G.M.; Altamimi, J.Z.; Aljabryn, D.H.; Alagal, R.I.; Aldera, H.; Alkhateeb, M.A.; Yahya, M.A. Ellagic acid prevents streptozotocin-induced hippocampal damage and memory loss in rats by stimulating Nrf2 and nuclear factor-κB, and activating insulin receptor substrate/PI3K/Akt axis. J. Physiol. Pharmacol. 2021, 72, 503–515. [Google Scholar]
- Wang, W.; Yang, L.; Liu, T.; Wang, J.; Wen, A.; Ding, Y. Ellagic acid protects mice against sleep deprivation-induced memory impairment and anxiety by inhibiting TLR4 and activating Nrf2. Aging 2020, 12, 10457–10472. [Google Scholar] [CrossRef]
- Chen, F.; Lu, K.; Bai, N.; Hao, Y.; Wang, H.; Zhao, X.; Yue, F. Oral administration of ellagic acid mitigates perioperative neurocognitive disorders, hippocampal oxidative stress and neuroinflammation in aged mice by restoring IGF-1 signaling. Sci. Rep. 2024, 14, 2509. [Google Scholar] [CrossRef]
- Belcaro, G.; Saggino, A.; Cornelli, U.; Luzzi, R.; Dugall, M.; Hosoi, M.; Feragalli, B.; Cesarone, M.R. Improvement in mood, oxidative stress, fatigue, and insomnia following supplementary management with Robuvit®. J. Neurosurg. Sci. 2018, 62, 423–427. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, C.; Hao, Z.; Shen, J.; Tang, S.; Dai, C. Ellagic Acid Reduces Cadmium Exposure-Induced Apoptosis in HT22 Cells via Inhibiting Oxidative Stress and Mitochondrial Dysfunction and Activating Nrf2/HO-1 Pathway. Antioxidants 2024, 13, 1296. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Qiu, J.; Wang, L.; Zhuo, J.; Wang, B.; Sun, D.; Yu, S.; Lou, H. Urolithin A protects dopaminergic neurons in experimental models of Parkinson’s disease by promoting mitochondrial biogenesis through the SIRT1/PGC-1α signaling pathway. Food Funct. 2022, 13, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Ardah, M.T.; Eid, N.; Kitada, T.; Haque, M.E. Ellagic Acid Prevents α-Synuclein Aggregation and Protects SH-SY5Y Cells from Aggregated α-Synuclein-Induced Toxicity via Suppression of Apoptosis and Activation of Autophagy. Int. J. Mol. Sci. 2021, 22, 13398. [Google Scholar] [CrossRef] [PubMed]
- Ardah, M.T.; Bharathan, G.; Kitada, T.; Haque, M.E. Ellagic Acid Prevents Dopamine Neuron Degeneration from Oxidative Stress and Neuroinflammation in MPTP Model of Parkinson’s Disease. Biomolecules 2020, 10, 1519. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.Z.; Zhu, G.F.; Zheng, C.Q.; Li, J.J.; Sheng, S.; Li, D.D.; Wang, G.Q.; Zhang, F. Ellagic acid protects dopamine neurons from rotenone-induced neurotoxicity via activation of Nrf2 signalling. J. Cell. Mol. Med. 2020, 24, 9446–9456. [Google Scholar] [CrossRef] [PubMed]
- Radwan, N.; Khan, E.; Ardah, M.T.; Kitada, T.; Haque, M.E. Ellagic Acid Prevents α-Synuclein Spread and Mitigates Toxicity by Enhancing Autophagic Flux in an Animal Model of Parkinson’s Disease. Nutrients 2023, 16, 85. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Rabiee, N.; Zabihnejad, S.; Roghani, M. Ellagic acid exerts protective effect in intrastriatal 6-hydroxydopamine rat model of Parkinson’s disease: Possible involvement of ERβ/Nrf2/HO-1 signaling. Brain Res. 2017, 1662, 23–30. [Google Scholar] [CrossRef]
- He, X.M.; Zhou, Y.Z.; Sheng, S.; Li, J.J.; Wang, G.Q.; Zhang, F. Ellagic Acid Protects Dopamine Neurons via Inhibition of NLRP3 Inflammasome Activation in Microglia. Oxid. Med. Cell. Longev. 2020, 20, 2963540. [Google Scholar] [CrossRef] [PubMed]
- Jamali, Z.; Salimi, A.; Khezri, S.; Norozi, P.; Garmabi, B.; Khaksari, M. Protective Role of Ellagic Acid Against Ethanol-Induced Neurodevelopmental Disorders in Newborn Male Rats: Insights into Maintenance of Mitochondrial Function and Inhibition of Oxidative Stress. J. Stud. Alcohol Drugs 2025, 86, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, W.J.C.; Price, J.R.; Robinson, G.M.; Robinson, R. The distribution of anthocyanins in flowers, fruits and leaves. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1939, 230, 149–178. [Google Scholar]
- Salama, A.; Elgohary, R.; Amin, M.M.; Elwahab, S.A. Immunomodulatory effect of protocatechuic acid on cyclophosphamide induced brain injury in rat: Modulation of inflammosomes NLRP3 and SIRT1. Eur. J. Pharmacol. 2022, 932, 175217. [Google Scholar] [CrossRef]
- Sanjay; Shin, J.H.; Park, M.; Lee, H.J. Cyanidin-3-O-Glucoside Regulates the M1/M2 Polarization of Microglia via PPARγ and Aβ42 Phagocytosis Through TREM2 in an Alzheimer’s Disease Model. Mol. Neurobiol. 2022, 59, 5135–5148, Correction in Mol. Neurobiol. 2024, 61, 1223. [Google Scholar] [CrossRef]
- Ali, T.; Kim, T.; Rehman, S.U.; Khan, M.S.; Amin, F.U.; Khan, M.; Ikram, M.; Kim, M.O. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 6076–6093. [Google Scholar] [CrossRef]
- Borda, M.G.; Barreto, G.E.; Baldera, J.P.; de Lucia, C.; Khalifa, K.; Bergland, A.K.; Pola, I.; Botero-Rodríguez, F.; Siow, R.C.; Kivipelto, M.; et al. A randomized, placebo-controlled trial of purified anthocyanins on cognitive function in individuals at elevated risk for dementia: Analysis of inflammatory biomarkers toward personalized interventions. Exp. Gerontol. 2024, 196, 112569. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.G.; Hamilton, D.A.; Joseph, J.A.; Shukitt-Hale, B. Dietary blueberry improves cognition among older adults in a randomized, double-blind, placebo-controlled trial. Eur. J. Nutr. 2018, 57, 1169–1180. [Google Scholar] [CrossRef]
- McNamara, R.K.; Kalt, W.; Shidler, M.D.; McDonald, J.; Summer, S.S.; Stein, A.L.; Stover, A.N.; Krikorian, R. Cognitive response to fish oil, blueberry, and combined supplementation in older adults with subjective cognitive impairment. Neurobiol. Aging 2018, 64, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Skelton, M.R.; Summer, S.S.; Shidler, M.D.; Sullivan, P.G. Blueberry Supplementation in Midlife for Dementia Risk Reduction. Nutrients 2022, 14, 1619. [Google Scholar] [CrossRef]
- Tambe, M.A.; de Rus Jacquet, A.; Strathearn, K.E.; Hensel, J.A.; Colón, B.D.; Chandran, A.; Yousef, G.G.; Grace, M.H.; Ferruzzi, M.G.; Wu, Q.; et al. Protective Effects of Polyphenol-Rich Extracts against Neurotoxicity Elicited by Paraquat or Rotenone in Cellular Models of Parkinson’s Disease. Antioxidants 2023, 12, 1463. [Google Scholar] [CrossRef]
- Castro, S.L.; Tapias, V.; Gathagan, R.; Emes, A.; Brandon, T.E.; Smith, A.D. Blueberry juice augments exercise-induced neuroprotection in a Parkinson’s disease model through modulation of GDNF levels. IBRO Neurosci. Rep. 2022, 12, 217–227. [Google Scholar] [CrossRef]
- Maulik, M.; Mitra, S.; Hunter, S.; Hunstiger, M.; Oliver, S.R.; Bult-Ito, A.; Taylor, B.E. Sir-2.1 mediated attenuation of α-synuclein expression by Alaskan bog blueberry polyphenols in a transgenic model of Caenorhabditis elegans. Sci. Rep. 2018, 8, 10216. [Google Scholar] [CrossRef]
- Fan, D.; Alamri, Y.; Liu, K.; MacAskill, M.; Harris, P.; Brimble, M.; Dalrymple-Alford, J.; Prickett, T.; Menzies, O.; Laurenson, A.; et al. Supplementation of Blackcurrant Anthocyanins Increased Cyclic Glycine-Proline in the Cerebrospinal Fluid of Parkinson Patients: Potential Treatment to Improve Insulin-Like Growth Factor-1 Function. Nutrients 2018, 10, 714. [Google Scholar] [CrossRef]
- Serra, D.; Henriques, J.F.; Sousa, F.J.; Laranjo, M.; Resende, R.; Ferreira-Marques, M.; de Freitas, V.; Silva, G.; Peça, J.; Dinis, T.C.P.; et al. Attenuation of Autism-like Behaviors by an Anthocyanin-Rich Extract from Portuguese Blueberries via Microbiota-Gut-Brain Axis Modulation in a Valproic Acid Mouse Model. Int. J. Mol. Sci. 2022, 23, 9259. [Google Scholar] [CrossRef] [PubMed]
- Hamed, Y.S.; Abdin, M.; Chen, G.; Akhtar, H.M.S.; Zeng, X. Effects of impregnate temperature on extraction of caffeoylquinic acid derivatives from Moringa oleifera leaves and evaluation of inhibitory activity on digestive enzyme, antioxidant, anti-proliferative and antibacterial activities of the extract. Int. J. Food Sci. Technol. 2020, 55, 3082–3090. [Google Scholar] [CrossRef]
- Matthews, D.G.; Caruso, M.; Murchison, C.F.; Zhu, J.Y.; Wright, K.M.; Harris, C.J.; Gray, N.E.; Quinn, J.F.; Soumyanath, A. Centella asiatica improves memory and promotes antioxidative signaling in 5XFAD mice. Antioxidants 2019, 8, 630. [Google Scholar] [CrossRef]
- Sasaki, K.; Davies, J.; Doldan, N.G.; Arao, S.; Ferdousi, F.; Szele, F.G.; Isoda, H. 3,4,5-Tricaffeoylquinic acid induces adult neurogenesis and improves deficit of learning and memory in aging model senescence accelerated prone 8 mice. Aging 2019, 11, 401–422. [Google Scholar] [CrossRef]
- Ge, L.; Jiang, Y.; Li, Y.; Xie, Q.; Miao, Y.; Wu, Z.; Zeng, X. Caffeoylquinic acids isolated from Lonicera japonica Thunb. as TAK1 inhibitors protects against LPS plus IFN-γ-stimulated inflammation by interacting with KEAP1-regulated NRF2 activation. Biomed. Pharmacother. 2023, 165, 115038. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Gao, L.; An, L.; Jiang, X.; Bai, J.; Huang, J.; Meng, W.; Zhao, Q. Pretreatment of MQA, a caffeoylquinic acid derivative compound, protects against H2O2-induced oxidative stress in SH-SY5Y cells. Neurol. Res. 2016, 38, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.M.; Bollen, M.; David, J.; Speers, A.B.; Brandes, M.S.; Gray, N.E.; Alcázar Magaña, A.; McClure, C.; Stevens, J.F.; Maier, C.S.; et al. Pharmacokinetics and Pharmacodynamics of Key Components of a Standardized Centella asiatica Product in Cognitively Impaired Older Adults: A Phase 1, Double-Blind, Randomized Clinical Trial. Antioxidants 2022, 11, 215. [Google Scholar] [CrossRef]
- Alcázar Magaña, A.; Vaswani, A.; Brown, K.S.; Jiang, Y.; Alam, M.N.; Caruso, M.; Lak, P.; Cheong, P.; Gray, N.E.; Quinn, J.F.; et al. Integrating High-Resolution Mass Spectral Data, Bioassays and Computational Models to Annotate Bioactives in Botanical Extracts: Case Study Analysis of C. asiatica Extract Associates Dicaffeoylquinic Acids with Protection against Amyloid-β Toxicity. Molecules 2024, 29, 838. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.G.; Caruso, M.; Alcazar Magana, A.; Wright, K.M.; Maier, C.S.; Stevens, J.F.; Gray, N.E.; Quinn, J.F.; Soumyanath, A. Caffeoylquinic Acids in Centella asiatica Reverse Cognitive Deficits in Male 5XFAD Alzheimer’s Disease Model Mice. Nutrients 2020, 12, 3488. [Google Scholar] [CrossRef]
- Morroni, F.; Sita, G.; Graziosi, A.; Turrini, E.; Fimognari, C.; Tarozzi, A.; Hrelia, P. Neuroprotective Effect of Caffeic Acid Phenethyl Ester in A Mouse Model of Alzheimer’s Disease Involves Nrf2/HO-1 Pathway. Aging Dis. 2018, 9, 605–622. [Google Scholar] [CrossRef]
- Wright, K.M.; Bollen, M.; David, J.; Mepham, B.; Alcázar Magaña, A.; McClure, C.; Maier, C.S.; Quinn, J.F.; Soumyanath, A. Bioanalytical method validation and application to a phase 1, double-blind, randomized pharmacokinetic trial of a standardized Centella asiatica (L.) Urban water extract product in healthy older adults. Front. Pharmacol. 2023, 14, 1228030. [Google Scholar] [CrossRef]
- Canoyra, A.; Martín-Cordero, C.; Muñoz-Mingarro, D.; León-González, A.J.; Parsons, R.B.; Acero, N. Corema album Berry Juice as a Protective Agent Against Neurodegeneration. Pharmaceuticals 2024, 17, 1535. [Google Scholar] [CrossRef]
- Hamdi, A.; Córdoba-Rojano, M.A.; Monje-Moreno, J.M.; Guillén-Izquierdo, E.; Rodríguez-Arcos, R.; Jiménez-Araujo, A.; Muñoz-Ruiz, M.J.; Guillén-Bejarano, R. Harnessing the Potential of Walnut Leaves from Nerpio: Unveiling Extraction Techniques and Bioactivity Through Caenorhabditis elegans Studies. Foods 2025, 14, 1048. [Google Scholar] [CrossRef] [PubMed]
- Kardani, A.; Soltani, A.; Sewell, R.D.E.; Shahrani, M.; Rafieian-Kopaei, M. Neurotransmitter, Antioxidant and Anti-neuroinflammatory Mechanistic Potentials of Herbal Medicines in Ameliorating Autism Spectrum Disorder. Curr. Pharm. Des. 2019, 25, 4421–4429. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, Z.; Kumar, D.; Singh, S.K.; Singh, T.D. Centella asiatica Alleviates AlCl3-induced Cognitive Impairment, Oxidative Stress, and Neurodegeneration by Modulating Cholinergic Activity and Oxidative Burden in Rat Brain. Biol. Trace Elem. Res. 2022, 200, 5115–5126. [Google Scholar] [CrossRef] [PubMed]
- Bonet-Costa, V.; Herranz-Pérez Viña, J. Clearing amyloid-beta through PPARgamma/ApoE activation by genistein is a treatment of experimental Alzheimer’s disease. J. Alzheimer’s Dis. 2016, 51, 701–711. [Google Scholar] [CrossRef]
- Kaur, S.; Sarma, S.J.; Marshall, B.L.; Liu, Y.; Kinkade, J.A.; Bellamy, M.M.; Mao, J.; Helferich, W.G.; Schenk, A.K.; Bivens, N.J.; et al. Developmental exposure of California mice to endocrine disrupting chemicals and potential effects on the microbiome-gut-brain axis at adulthood. Sci. Rep. 2020, 10, 10902, Correction in Sci. Rep. 2020, 10, 12524. [Google Scholar]
- Smit, S.; Szymańska, E.; Kunz, I.; Gomez Roldan, V.; van Tilborg, M.W.; Weber, P.; Prudence, K.; van der Kloet, F.M.; van Duynhoven, J.P.; Smilde, A.K.; et al. Nutrikinetic modeling reveals order of genistein phase II metabolites appearance in human plasma. Mol. Nutr. Food Res. 2014, 58, 2111–2121. [Google Scholar] [CrossRef]
- Petry, F.S.; Coelho, B.P.; Gaelzer, M.M.; Kreutz, F.; Guma, F.T.C.R.; Salbego, C.G.; Trindade, V.M.T. Genistein protects against amyloid-beta-induced toxicity in SH-SY5Y cells by regulation of Akt and Tau phosphorylation. Phytother. Res. 2020, 34, 796–807. [Google Scholar] [CrossRef]
- Yi, S.; Chen, S.; Xiang, J.; Tan, J.; Huang, K.; Zhang, H.; Wang, Y.; Wu, H. Genistein exerts a cell-protective effect via Nrf2/HO-1/ /PI3K signaling in Ab25-35-induced Alzheimer’s disease models in vitro. Folia Histochem. Cytobiol. 2021, 59, 49–56. [Google Scholar] [CrossRef]
- Guo, J.; Yang, G.; He, Y.; Xu, H.; Fan, H.; An, J.; Zhang, L.; Zhang, R.; Cao, G.; Hao, D.; et al. Involvement of α7nAChR in the Protective Effects of Genistein Against β-Amyloid-Induced Oxidative Stress in Neurons via a PI3K/Akt/Nrf2 Pathway-Related Mechanism. Cell. Mol. Neurobiol. 2021, 41, 377–393. [Google Scholar] [CrossRef]
- Rassu, G.; Porcu, E.P.; Fancello, S.; Obinu, A.; Senes, N.; Galleri, G.; Migheli, R.; Gavini, E.; Giunchedi, P. Intranasal Delivery of Genistein-Loaded Nanoparticles as a Potential Preventive System against Neurodegenerative Disorders. Pharmaceutics 2018, 11, 8. [Google Scholar] [CrossRef]
- Lu, C.; Wang, Y.; Xu, T.; Li, Q.; Wang, D.; Zhang, L.; Fan, B.; Wang, F.; Liu, X. Genistein ameliorates scopolamine-induced amnesia in mice through the regulation of the cholinergic neurotransmission, antioxidant system and the ERK/CREB/BDNF signaling. Front. Pharmacol. 2018, 9, 1153. [Google Scholar] [CrossRef] [PubMed]
- Petry, F.D.S.; Hoppe, J.B.; Klein, C.P.; Dos Santos, B.G.; Hözer, R.M.; Bifi, F.; Matté, C.; Salbego, C.G.; Trindade, V.M.T. Genistein attenuates amyloid-beta-induced cognitive impairment in rats by modulation of hippocampal synaptotoxicity and hyperphosphorylation of Tau. J. Nutr. Biochem. 2021, 87, 108525. [Google Scholar] [CrossRef]
- Xu, H.; Li, L.; Wang, Y.; Wang, H.; An, D.; Heng, B.; Liu, Y.Q. Genistein inhibits A β25-35-induced SH-SY5Y cell damage by modulating the expression of apoptosis-related proteins and Ca2+ influx through ionotropic glutamate receptors. Phytother. Res. 2019, 33, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Lei, X.; Ye, S.; Ye, T.; Hua, R.; Wang, G.; Song, H.; Zhou, P.; Wang, Y.; Cai, B. Genistein attenuates memory impairment in Alzheimer’s disease via ERS-mediated apoptotic pathway in vivo and in vitro. J. Nutr. Biochem. 2022, 109, 109118, Correction in J. Nutr. Biochem. 2024, 131, 109710. [Google Scholar] [CrossRef]
- Rita, L.; Neumann, N.R.; Laponogov, I.; Gonzalez, G.; Veselkov, D.; Pratico, D.; Aalizadeh, R.; Thomaidis, N.S.; Thompson, D.C.; Vasiliou, V.; et al. Alzheimer’s disease: Using gene/protein network machine learning for molecule discovery in olive oil. Hum. Genom. 2023, 17, 57. [Google Scholar] [CrossRef]
- Shi, B.; Chen, M.; Xia, Z.; Tang, W.; Li, Y.; Qin, C.; Ahmadi, A.; Huang, C.; Xu, H. Genistein attenuates neuroinflammation and oxidative stress and improves cognitive impairment in a rat model of sepsis-associated encephalopathy: Potential role of the Nrf2 signaling pathway. Metab. Brain Dis. 2023, 38, 339–347. [Google Scholar] [CrossRef]
- Viña, J.; Escudero, J.; Baquero, M.; Cebrián, M.; Carbonell-Asíns, J.A.; Muñoz, J.E.; Satorres, E.; Meléndez, J.C.; Ferrer-Rebolleda, J.; Cózar-Santiago, M.D.P.; et al. Genistein effect on cognition in prodromal Alzheimer’s disease patients. The GENIAL clinical trial. Alzheimer’s Res. Ther. 2022, 14, 164. [Google Scholar]
- Viña, J.; Borrás, C.; Mas-Bargues, C. Genistein, A Phytoestrogen, Delays the Transition to Dementia in Prodromal Alzheimer’s Disease Patients. J. Alzheimer’s Dis. 2024, 101, S275–S283. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hernandez, G.; Mack, W.J.; Schneider, L.S.; Yin, F.; Brinton, R.D. Retrospective analysis of phytoSERM for management of menopause-associated vasomotor symptoms and cognitive decline: A pilot study on pharmacogenomic effects of mitochondrial haplogroup and APOE genotype on therapeutic efficacy. Menopause 2020, 27, 57–65. [Google Scholar] [CrossRef]
- Liu, L.X.; Chen, W.F.; Xie, J.X.; Wong, M.S. Neuroprotective effects of genistein on dopaminergic neurons in the mice model of Parkinson’s disease. Neurosci. Res. 2008, 60, 156–161. [Google Scholar] [CrossRef]
- Wu, H.C.; Hu, Q.L.; Zhang, S.J.; Wang, Y.M.; Jin, Z.K.; Lv, L.F.; Zhang, S.; Liu, Z.L.; Wu, H.L.; Cheng, O.M. Neuroprotective effects of genistein on SH-SY5Y cells overexpressing A53T mutant α-synuclein. Neural Regen. Res. 2018, 13, 1375–1383. [Google Scholar]
- Du, Z.R.; Gu, Y.; Xie, X.M.; Zhang, M.; Jiang, G.Y.; Chen, W.F. GPER and IGF-1R mediate the anti-inflammatory effect of genistein against lipopolysaccharide (LPS)-induced nigrostriatal injury in rats. J. Steroid Biochem. Mol. Biol. 2021, 214, 105989. [Google Scholar] [CrossRef] [PubMed]
- Arbabi, E.; Hamidi, G.; Talaei, S.A.; Salami, M. Estrogen agonist genistein differentially influences the cognitive and motor disorders in an ovariectomized animal model of Parkinsonism. Iran. J. Basic Med. Sci. 2016, 19, 1285–1290. [Google Scholar]
- Khera, R.; Mehan, S.; Kumar, S.; Sethi, P.; Bhalla, S.; Prajapati, A. Role of JAK-STAT and PRPAR-gamma signalling modulators in the prevention of autism and neurological dysfunctions. Mol. Neurobiol. 2022, 59, 3888–3912. [Google Scholar] [CrossRef]
- Kumar, M.; Mehan, S.; Kumar, A.; Sharma, T.; Khan, Z.; Tiwari, A.; Das Gupta, G.; Narula, A.S. Therapeutic efficacy of Genistein in activation of neuronal AC/cAMP/CREB/PKA and mitochondrial ETC-Complex pathways in experimental model of autism: Evidence from CSF, blood plasma and brain analysis. Brain Res. 2025, 1846, 149251. [Google Scholar] [CrossRef]
- Marshall, B.L.; Liu, Y.; Farrington, M.J.; Mao, J.; Helferich, W.G.; Schenk, A.K.; Bivens, N.J.; Sarma, S.J.; Lei, Z.; Sumner, L.W.; et al. Early genistein exposure of California mice and effects on the gut microbiota-brain axis. J. Endocrinol. 2019, 242, 139–157. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, A.; Ali, W.; Jo, M.H.; Park, J.; Ikram, M.; Kim, M.O. Fisetin Rescues the Mice Brains Against D-Galactose-Induced Oxidative Stress, Neuroinflammation and Memory Impairment. Front. Pharmacol. 2021, 12, 612078. [Google Scholar]
- Khatoon, S.; Agarwal, N.B.; Samim, M.; Alam, O. Neuroprotective Effect of Fisetin Through Suppression of IL-1R/TLR Axis and Apoptosis in Pentylenetetrazole-Induced Kindling in Mice. Front. Neurol. 2021, 12, 689069. [Google Scholar] [CrossRef]
- Ciapała, K.; Rojewska, E.; Pawlik, K.; Ciechanowska, A.; Mika, J. Analgesic Effects of Fisetin, Peimine, Astaxanthin, Artemisinin, Bardoxolone Methyl and 740 Y-P and Their Influence on Opioid Analgesia in a Mouse Model of Neuropathic Pain. Int. J. Mol. Sci. 2023, 24, 9000. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, I.M.; Jaja-Chimedza, A.; Joseph, A.; Balakrishnan, A.; Maliakel, B.; Swick, A. Enhanced bioavailability and pharmacokinetics of a novel hybrid-hydrogel formulation of fisetin orally administered in healthy individuals: A randomised double-blinded comparative crossover study. J. Nutr. Sci. 2022, 11, e74. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, B.K.; Park, S.K. Effects of Fisetin, a Plant-Derived Flavonoid, on Response to Oxidative Stress, Aging, and Age-Related Diseases in Caenorhabditis elegans. Pharmaceuticals 2022, 15, 1528. [Google Scholar] [CrossRef]
- Anyanwu, G.E.; Nwachukwu, I.J.; Oria, S.R.; Obasi, K.K.; Ekwueme, E.P.; Nto, J.N.; Anyanwu, N.C. Fisetin attenuates AlCl3-induced neurodegeneration by modulating oxidative stress and inflammatory cytokine release in adult albino wistar rats. Toxicol. Rep. 2024, 13, 101812. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, D.; Goyal, R.; Yadav, V.; Gore, S.K.; Sen, S.; Ranjan, O.P.; Mishra, A. Nanoformulated fisetin ameliorates Alzheimer’s disease via reducing proinflammatory cytokines and activating the NRF2/HO-1 pathway. Nanomedicine 2024, 19, 2537–2553. [Google Scholar] [CrossRef]
- Alikatte, K.; Palle, S.; Rajendra Kumar, J.; Pathakala, N. Fisetin Improved Rotenone-Induced Behavioral Deficits, Oxidative Changes, and Mitochondrial Dysfunctions in Rat Model of Parkinson’s Disease. J. Diet. Suppl. 2021, 18, 57–71. [Google Scholar]
- Chen, T.J.; Feng, Y.; Liu, T.; Wu, T.T.; Chen, Y.J.; Li, X.; Li, Q.; Wu, Y.C. Fisetin Regulates Gut Microbiota and Exerts Neuroprotective Effect on Mouse Model of Parkinson’s Disease. Front. Neurosci. 2020, 14, 549037. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kumar, R.; Khurana, N.; Singh, S.K.; Khurana, S.; Verma, S.; Sharma, N.; Kapoor, B.; Vyas, M.; Khursheed, R.; et al. Enhanced oral bioavailability and neuroprotective effect of fisetin through its SNEDDS against rotenone-induced Parkinson’s disease rat model. Food Chem. Toxicol. 2020, 144, 111590. [Google Scholar] [CrossRef]
- Mehra, S.; Ahsan, A.U.; Sharma, M.; Budhwar, M.; Chopra, M. Neuroprotective Efficacy of Fisetin Against VPA-Induced Autistic Neurobehavioral Alterations by Targeting Dysregulated Redox Homeostasis. J. Mol. Neurosci. 2023, 73, 403–422. [Google Scholar] [CrossRef]
- Mehra, S.; Ahsan, A.U.; Sharma, M.; Budhwar, M.; Chopra, M. Gestational Fisetin Exerts Neuroprotection by Regulating Mitochondria-Directed Canonical Wnt Signaling, BBB Integrity, and Apoptosis in Prenatal VPA-Induced Rodent Model of Autism. Mol. Neurobiol. 2024, 61, 4001–4020. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Melamed, I.R.; Heffron, M.; Testori, A.; Lipe, K. A pilot study of high-dose intravenous immunoglobulin 5% for autism: Impact on autism spectrum and markers of neuroinflammation. Autism Res. 2018, 11, 421–433. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, H.S.; Wang, X.; Dumont, A.S.; Liu, Q. Cellular senescence, DNA damage, and neuroinflammation in the aging brain. Trends Neurosci. 2024, 47, 461–474. [Google Scholar] [CrossRef]
- Ozben, T.; Ozben, S. Neuro-inflammation and antiinflammatory treatment options for Alzheimer’s disease. Clin. Biochem. 2019, 72, 87–89. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS generation in Microglia: Understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef]
- Asensi, M.T.; Napoletano, A.; Sofi, F.; Dinu, M. Low-Grade Inflammation and Ultra-Processed Foods Consumption: A Review. Nutrients 2023, 15, 1546. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, S.; Yang, H.; Zhang, Y.; Zhang, S.; Ma, Y.; Hou, Y.; Zhang, X.; Niu, K.; Borné, Y.; et al. Association of Ultraprocessed Food Consumption with Risk of Dementia: A Prospective Cohort Study. Neurology 2022, 99, e1056–e1066. [Google Scholar] [CrossRef]
- Adjibade, M.; Julia, C.; Allès, B.; Touvier, M.; Lemogne, C.; Srour, B.; Hercberg, S.; Galan, P.; Assmann, K.E.; Kesse-Guyot, E. Prospective association between ultra-processed food consumption and incident depressive symptoms in the French NutriNet-Santé cohort. BMC Med. 2019, 17, 78. [Google Scholar] [CrossRef] [PubMed]
- Gahtani, R.M.; Shoaib, S.; Hani, U.; Jayachithra, R.; Alomary, M.N.; Chauhan, W.; Jahan, R.; Tufail, S.; Ansari, M.A. Combating Parkinson’s disease with plant-derived polyphenols: Targeting oxidative stress and neuroinflammation. Neurochem. Int. 2024, 178, 105798. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Guo, Z.; Lei, J.; Wang, Y. Pomegranate polyphenol punicalin ameliorates lipopolysaccharide-induced memory impairment, behavioral disorders, oxidative stress, and neuroinflammation via inhibition of TLR4-NF-κB pathway. Phytother. Res. 2024, 38, 3489–3508. [Google Scholar]
- Rehman, S.U.; Shah, S.A.; Ali, T.; Chung, J.I.; Kim, M.O. Anthocyanins Reversed D-Galactose-Induced Oxidative Stress and Neuroinflammation Mediated Cognitive Impairment in Adult Rats. Mol. Neurobiol. 2017, 54, 255–271. [Google Scholar] [CrossRef]
- Ali, M.A.; Menze, E.T.; Tadros, M.G.; Tolba, M.F. Caffeic acid phenethyl ester counteracts doxorubicin-induced chemobrain in Sprague-Dawley rats: Emphasis on the modulation of oxidative stress and neuroinflammation. Neuropharmacology 2020, 181, 108334. [Google Scholar] [CrossRef] [PubMed]
- Rumman, M.; Pandey, S.; Singh, B.; Gupta, M.; Ubaid, S.; Mahdi, A.A. Genistein Prevents Hypoxia-Induced Cognitive Dysfunctions by Ameliorating Oxidative Stress and Inflammation in the Hippocampus. Neurotox. Res. 2021, 39, 1123–1133. [Google Scholar] [CrossRef]
- Mossine, V.V.; Waters, J.K.; Gu, Z.; Sun, G.Y.; Mawhinney, T.P. Bidirectional Responses of Eight Neuroinflammation-Related Transcriptional Factors to 64 Flavonoids in Astrocytes with Transposable Insulated Signaling Pathway Reporters. ACS Chem. Neurosci. 2022, 13, 613–623. [Google Scholar] [CrossRef]
- Huang, R.; Zhu, Z.; Wu, Q.; Bekhit, A.E.-D.A.; Wu, S.; Chen, M.; Wang, J.; Ding, Y. Whole-plant foods and their macromolecules: Untapped approaches to modulate neuroinflammation in Alzheimer’s disease. Crit. Rev. Food Sci. Nutr. 2021, 63, 2388–2406. [Google Scholar]
- Ronchetti, S.; Labombarda, F.; Del Core, J.; Roig, P.; De Nicola, A.F.; Pietranera, L. The phytoestrogen genistein improves hippocampal neurogenesis and cognitive impairment and decreases neuroinflammation in an animal model of metabolic syndrome. J. Neuroendocrinol. 2025, 37, e13480. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Xu, S.; Shen, Y.; Xu, Y.; Li, Y. Genistein Attenuates Isoflurane-Induced Neuroinflammation by Inhibiting TLR4-Mediated Microglial-Polarization in vivo and in vitro. J. Inflamm. Res. 2021, 14, 2587–2600. [Google Scholar]
- Li, Y.; Zhang, J.J.; Chen, R.J.; Chen, L.; Chen, S.; Yang, X.F.; Min, J.W. Genistein mitigates oxidative stress and inflammation by regulating Nrf2/HO-1 and NF-κB signaling pathways in hypoxic-ischemic brain damage in neonatal mice. Ann. Transl. Med. 2022, 10, 32. [Google Scholar] [CrossRef]
- Liu, Y.; Chu, W.; Ma, H.; Peng, W.; Li, Q.; Han, L.; Wang, H.; Wang, L.; Zhang, B.; Yang, J.; et al. Fisetin orchestrates neuroinflammation resolution and facilitates spinal cord injury recovery through enhanced autophagy in pro-inflammatory glial cells. Int. Immunopharmacol. 2024, 130, 111738. [Google Scholar] [CrossRef]
- Ding, H.; Li, Y.; Chen, S.; Wen, Y.; Zhang, S.; Luo, E.; Li, X.; Zhong, W.; Zeng, H. Fisetin ameliorates cognitive impairment by activating mitophagy and suppressing neuroinflammation in rats with sepsis-associated encephalopathy. CNS Neurosci. Ther. 2022, 28, 247–258. [Google Scholar] [CrossRef]
- Yang, W.; Tian, Z.K.; Yang, H.X.; Feng, Z.J.; Sun, J.M.; Jiang, H.; Cheng, C.; Ming, Q.L.; Liu, C.M. Fisetin improves lead-induced neuroinflammation, apoptosis and synaptic dysfunction in mice associated with the AMPK/SIRT1 and autophagy pathway. Food Chem. Toxicol. 2019, 134, 110824. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Liao, P.; Lu, J.; Liang, G.; Wei, Q.; Song, W.; Lan, Y.; Zeng, J.; Zou, C.; Pan, M.; et al. Single-nucleus RNA sequencing and network pharmacology reveal the mediation of fisetin on neuroinflammation in Alzheimer’s disease. Phytomedicine 2025, 141, 156724. [Google Scholar] [CrossRef]
- Geertsema, J.; Kratochvil, M.; González-Domínguez, R.; Lefèvre-Arbogast, S.; Low, D.Y.; Du Preez, A.; Lee, H.; Urpi-Sarda, M.; Sánchez-Pla, A.; Aigner, L.; et al. Coffee polyphenols ameliorate early-life stress-induced cognitive deficits in male mice. Neurobiol. Stress 2024, 31, 100641. [Google Scholar] [CrossRef] [PubMed]
- Boccella, S.; Iannotta, M.; Cristiano, C.; Iannotti, F.A.; Bello, F.D.; Guida, F.; Belardo, C.; Infantino, R.; Ricciardi, F.; Giannella, M.; et al. Treatment with 2-Pentadecyl-2-Oxazoline Restores Mild Traumatic Brain Injury-Induced Sensorial and Neuropsychiatric Dysfunctions. Front. Pharmacol. 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Jing, P. Red Cabbage Anthocyanins Attenuate Cognitive Impairment by Attenuating Neuroinflammation and Regulating Gut Microbiota in Aging Mice. J. Agric. Food Chem. 2023, 71, 15064–15072. [Google Scholar] [CrossRef]
- Muhammad, I.; Cremonini, E.; Mathieu, P.; Adamo, A.M.; Oteiza, P.I. Dietary Anthocyanins Mitigate High-Fat Diet-Induced Hippocampal Inflammation in Mice. J. Nutr. 2024, 154, 2752–2762. [Google Scholar]
- Baek, H.; Sanjay; Park, M.; Lee, H.J. Cyanidin-3-O-glucoside protects the brain and improves cognitive function in APPswe/PS1ΔE9 transgenic mice model. J. Neuroinflamm. 2023, 20, 268. [Google Scholar] [CrossRef]
- Jang, B.K.; Shin, S.J.; Park, H.H.; Kumar, V.; Park, Y.H.; Kim, J.Y.; Kang, H.Y.; Park, S.; Kwon, Y.; Shin, S.E.; et al. Investigation of Novel Aronia Bioactive Fraction-Alginic Acid Nanocomplex on the Enhanced Modulation of Neuroinflammation and Inhibition of Aβ Aggregation. Pharmaceutics 2024, 17, 13. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, M.; Chen, X.; Zhang, R.; Le, A.; Hong, M.; Zhang, Y.; Jia, L.; Zang, W.; Jiang, C.; et al. Tryptophan Metabolism in Central Nervous System Diseases: Pathophysiology and Potential Therapeutic Strategies. Aging Dis. 2023, 14, 858–878. [Google Scholar] [CrossRef]
- Xue, C.; Li, G.; Zheng, Q.; Gu, X.; Shi, Q.; Su, Y.; Chu, Q.; Yuan, X.; Bao, Z.; Lu, J.; et al. Tryptophan metabolism in health and disease. Cell Metab. 2023, 35, 1304–1326. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Xia, X.; Zhou, X.; Du, Y.; Yin, Z.; Wang, J. Integration of Urine Proteomic and Metabolomic Profiling Reveals Novel Insights into Neuroinflammation in Autism Spectrum Disorder. Front. Psychiatry 2022, 13, 780747. [Google Scholar] [CrossRef]
- Song, Z.; Wu, Z.; Luo, R.; He, C.; Li, Z.; Yang, M.; Yu, W.; He, J.; Deng, S.; Cheng, S. Identification of tryptophan metabolism-related genes in immunity and immunotherapy in Alzheimer’s disease. Aging 2023, 15, 13077–13099. [Google Scholar]
- Zhai, L.; Ladomersky, E.; Lenzen, A.; Nguyen, B.; Patel, R.; Lauing, K.L.; Wu, M.; Wainwright, D.A. IDO1 in cancer: A Gemini of immune checkpoints. Cell. Mol. Immunol. 2018, 15, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, J.; Zielińska, M. Exchange-mode glutamine transport across CNS cell membranes. Neuropharmacology 2019, 161, 107560. [Google Scholar] [CrossRef] [PubMed]
- Vécsei, L.; Szalárdy, L.; Fülöp, F.; Toldi, J. Kynurenines in the CNS: Recent advances and new questions. Nat. Rev. Drug Discov. 2013, 12, 64–82. [Google Scholar] [PubMed]
- Plitman, E.; Iwata, Y.; Caravaggio, F.; Nakajima, S.; Chung, J.K.; Gerretsen, P.; Kim, J.; Takeuchi, H.; Chakravarty, M.M.; Remington, G.; et al. Kynurenic acid in schizophrenia: A systematic review and meta-analysis. Schizophr. Bull. 2017, 43, 764–777. [Google Scholar] [CrossRef]
- Barone, P. The ‘Yin’ and the ‘Yang’ of the kynurenine pathway: Excitotoxicity and neuroprotection imbalance in stress-induced disorders. Behav. Pharmacol. 2019, 30, 163–186. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, X.; Zhou, Y.; Du, J.; Lu, C.; Zhang, L.; Sun, S.; Wang, S.; Li, Y. Kynurenic acid inhibits macrophage pyroptosis by suppressing ROS production via activation of the NRF2 pathway. Mol. Med. Rep. 2023, 28, 211. [Google Scholar] [CrossRef]
- Ferreira, F.S.; Biasibetti-Brendler, H.; Pierozan, P.; Schmitz, F.; Bertó, C.G.; Prezzi, C.A.; Manfredini, V.; Wyse, A.T.S. Kynurenic Acid Restores Nrf2 Levels and Prevents Quinolinic Acid-Induced Toxicity in Rat Striatal Slices. Mol. Neurobiol. 2018, 55, 8538–8549. [Google Scholar] [CrossRef]
- Turner, E.H.; Loftis, J.M.; Blackwell, A.D. Serotonin a la carte: Supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacol. Ther. 2006, 109, 325–338. [Google Scholar] [CrossRef]
- Gao, K.; Mu, C.-L.; Farzi, A.; Zhu, W.-Y. Tryptophan metabolism: A link between the gut microbiota and brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, S.; Ma, N.; Johnston, L.J.; Wu, C.; Ma, X. Metabolites of microbiota response to tryptophan and intestinal mucosal immunity: A therapeutic target to control intestinal inflammation. Med. Res. Rev. 2021, 41, 1061–1088. [Google Scholar] [CrossRef]
- Wojciech, L.; Png, C.W.; Koh, E.Y.; Kioh, D.Y.Q.; Deng, L.; Wang, Z.; Wu, L.Z.; Hamidinia, M.; Tung, D.W.; Zhang, W.; et al. A tryptophan metabolite made by a gut microbiome eukaryote induces pro-inflammatory T cells. EMBO J. 2023, 42, e112963. [Google Scholar] [CrossRef] [PubMed]
- Oxenkrug, G.; van der Hart, M.; Roeser, J.; Summergrad, P. Peripheral Tryptophan—Kynurenine Metabolism Associated with Metabolic Syndrome is Different in Parkinson’s and Alzheimer’s Diseases. Endocrinol. Diabetes Metab. J. 2017, 1, 4. [Google Scholar]
- Fongsaran, C.; Dineley, K.T.; Paessler, S.; Cisneros, I.E. VEEV TC-83 Triggers Dysregulation of the Tryptophan-Kynurenine Pathway in the Central Nervous System That Correlates with Cognitive Impairment in Tg2576 Mice. Pathogens 2024, 13, 397. [Google Scholar] [CrossRef]
- Mu, J.L.; Liu, X.D.; Dong, Y.H.; Fang, Y.Y.; Qiu, S.D.; Zhang, F.; Liu, K.X. Peripheral interleukin-6-associated microglial QUIN elevation in basolateral amygdala contributed to cognitive dysfunction in a mouse model of postoperative delirium. Front. Med. 2022, 9, 998397. [Google Scholar] [CrossRef] [PubMed]
- Bonda, D.J.; Mailankot, M.; Stone, J.G.; Garrett, M.R.; Staniszewska, M.; Castellani, R.J.; Siedlak, S.L.; Zhu, X.; Lee, H.G.; Perry, G.; et al. Indoleamine 2,3-dioxygenase and 3-hydroxykynurenine modifications are found in the neuropathology of Alzheimer’s disease. Redox Rep. 2010, 15, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, G.J.; Smythe, G.; Takikawa, O.; Brew, B.J. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia 2005, 49, 15–23. [Google Scholar] [CrossRef]
- Wu, W.; Nicolazzo, J.A.; Wen, L.; Chung, R.; Stankovic, R.; Bao, S.S.; Lim, C.K.; Brew, B.J.; Cullen, K.M.; Guillemin, G.J. Expression of tryptophan 2,3-dioxygenase and production of kynurenine pathway metabolites in triple transgenic mice and human Alzheimer’s disease brain. PLoS ONE 2013, 8, e59749. [Google Scholar] [CrossRef]
- Minhas, P.S.; Jones, J.R.; Latif-Hernandez, A.; Sugiura, Y.; Durairaj, A.S.; Wang, Q.; Mhatre, S.D.; Uenaka, T.; Crapser, J.; Conley, T.; et al. Restoring hippocampal glucose metabolism rescues cognition across Alzheimer’s disease pathologies. Science 2024, 385, eabm6131. [Google Scholar] [CrossRef]
- Choe, K.; Ali, M.; Lardenoije, R.; Riemens, R.J.M.; Pishva, E.; Bickel, H.; Weyerer, S.; Hoffmann, P.; Pentzek, M.; Riedel-Heller, S.; et al. Alzheimer’s disease-specific transcriptomic and epigenomic changes in the tryptophan catabolic pathway. Alzheimer’s Res. Ther. 2024, 16, 259. [Google Scholar] [CrossRef]
- Ogawa, T.; Matson, W.R.; Beal, M.F.; Myers, R.H.; Bird, E.D.; Milbury, P.; Saso, S. Kynurenine pathway abnormalities in Parkinson’s disease. Neurology 1992, 42, 1702–1706. [Google Scholar] [CrossRef] [PubMed]
- Heilman, P.L.; Wang, E.W.; Lewis, M.M.; Krzyzanowski, S.; Capan, C.D.; Burmeister, A.R.; Du, G.; Escobar Galvis, M.L.; Brundin, P.; Huang, X.; et al. Tryptophan Metabolites Are Associated with Symptoms and Nigral Pathology in Parkinson’s Disease. Mov. Disord. 2020, 35, 2028–2037. [Google Scholar] [CrossRef]
- Hajihassani, A.; Nourazarian, A.; Nikanfar, M.; Laghousi, D.; Khaki-Khatibi, F. Kynurenine and Beta-Alanine Serum Levels are Associated with the Expression of Wnt Pathway Genes in Patients with Parkinson’s Disease. Clin. Lab. 2023, 69, 2289–2298. [Google Scholar] [CrossRef]
- O’Farrell, K.; Fagan, E.; Connor, T.J.; Harkin, A. Inhibition of the kynurenine pathway protects against reactive microglial-associated reductions in the complexity of primary cortical neurons. Eur. J. Pharmacol. 2017, 810, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Jellen, L.C.; Escobar Galvis, M.L.; Sha, Q.; Isaguirre, C.; Johnson, A.; Madaj, Z.; Lewis, M.M.; Sheldon, R.D.; Kong, L.; Huang, X.; et al. Sex differences in peripheral and central dysregulation of the kynurenine pathway in Parkinson’s disease. npj Park. Dis. 2025, 11, 116. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, W.; Wu, W.; Wang, X.; Lin, Y.; Wu, L.; Wang, J.; Huan, F.; Ding, H.; Gao, R. Serum neurotransmitter analysis of motor and non-motor symptoms in Parkinson’s patients. Front. Aging Neurosci. 2024, 16, 1423120. [Google Scholar] [CrossRef]
- Chung, S.H.; Yoo, D.; Ahn, T.B.; Lee, W.; Hong, J. Profiling Analysis of Tryptophan Metabolites in the Urine of Patients with Parkinson’s Disease Using LC-MS/MS. Pharmaceuticals 2023, 16, 1495. [Google Scholar] [CrossRef]
- Babu, H.W.S.; Elangovan, A.; Iyer, M.; Kirola, L.; Muthusamy, S.; Jeeth, P.; Muthukumar, S.; Vanlalpeka, H.; Gopalakrishnan, A.V.; Kadhirvel, S.; et al. Association Study Between Kynurenine 3-Monooxygenase (KMO) Gene and Parkinson’s Disease Patients. Mol. Neurobiol. 2024, 61, 3867–3881. [Google Scholar] [CrossRef] [PubMed]
- Fargher, E.; Keatinge, M.; Pearce, O.; Piepponen, P.; Panula, P.; van Eeden, F.J.M.; MacDonald, R.B.; Bandmann, O. A zebrafish model of acmsd deficiency does not support a prominent role for ACMSD in Parkinson’s disease. npj Park. Dis. 2025, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- García-Aguilar, R.; Ortega, A.; López-Bayghen, E.; Ramírez-Martínez, L.; Rodriguez-Campuzano, A.; Murillo-González, F.; Elizondo, G.; Veja, L. Kynurenine attenuates mitochondrial depolarization and neuronal cell death induced by rotenone exposure independently of AhR-mediated parkin induction in SH-SY5Y differentiated cells. Neurotoxicology 2023, 99, 282–291. [Google Scholar] [CrossRef]
- Dhiman, N.R.; Singh, S.; Singh, R.; Kumar, A.; Singh, V.K.; Pathak, A.; Chaurasia, R.N.; Mishra, V.N.; Srivastava, N.K.; Sahu, S.; et al. Urinary based biomarkers identification and genetic profiling in Parkinson’s disease: A systematic review of metabolomic studies. Front. Bioinform. 2025, 5, 1513790. [Google Scholar] [CrossRef]
- Galley, J.D.; Chen, H.J.; Antonson, A.M.; Gur, T.L. Prenatal stress-induced disruptions in microbial and host tryptophan metabolism and transport. Behav. Brain Res. 2021, 414, 113471. [Google Scholar] [CrossRef]
- Aziz-Zadeh, L.; Ringold, S.M.; Jayashankar, A.; Kilroy, E.; Butera, C.; Jacobs, J.P.; Tanartkit, S.; Mahurkar-Joshi, S.; Bhatt, R.R.; Dapretto, M.; et al. Relationships between brain activity, tryptophan-related gut metabolites, and autism symptomatology. Nat. Commun. 2025, 16, 3465. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef]
- Jašarević, E.; Bale, T.L. Prenatal and postnatal contributions of the maternal microbiome on offspring programming. Front. Neuroendocrinol. 2019, 55, 100797. [Google Scholar] [CrossRef]
- Sun, Z.; Lee-Sarwar, K.; Kelly, R.S.; Lasky-Su, J.A.; Litonjua, A.A.; Weiss, S.T.; Liu, Y.Y. Revealing the importance of prenatal gut microbiome in offspring neurodevelopment in humans. eBioMedicine 2023, 90, 104491. [Google Scholar] [CrossRef] [PubMed]
- Moura, C.A.; Cagni, F.C.; Costa, L.R.F.; Tiago, P.R.F.; Croyal, M.; Aguesse, A.; Reyes-Castro, L.A.; Zambrano, E.; Bolaños-Jiménez, F.; Gavioli, E.C. Maternal Stress during Pregnancy in Mice Induces Sex-Dependent Behavioral Alterations in Offspring along with Impaired Serotonin and Kynurenine Pathways of Tryptophan Metabolism. Dev. Neurosci. 2022, 44, 603–614. [Google Scholar] [CrossRef]
- Liu, D.; Bu, D.; Li, H.; Wang, Q.; Ding, X.; Fang, X. Intestinal metabolites and the risk of autistic spectrum disorder: A two-sample Mendelian randomization study. Front. Psychiatry 2023, 13, 1034214. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Yamamoto, Y.; Wulaer, B.; Kunisawa, K.; Fujigaki, H.; Ando, T.; Kimura, H.; Kushima, I.; Arioka, Y.; Torii, Y.; et al. Indoleamine 2,3-dioxygenase 2 deficiency associates with autism-like behavior via dopaminergic neuronal dysfunction. FEBS J. 2024, 291, 945–964. [Google Scholar] [CrossRef]
- Bilgiç, A.; Abuşoğlu, S.; Sadıç Çelikkol, Ç.; Oflaz, M.B.; Akça, Ö.F.; Sivrikaya, A.; Baysal, T.; Ünlü, A. Altered kynurenine pathway metabolite levels in toddlers and preschool children with autism spectrum disorder. Int. J. Neurosci. 2022, 132, 826–834. [Google Scholar] [CrossRef]
- Launay, J.M.; Delorme, R.; Pagan, C.; Callebert, J.; Leboyer, M.; Vodovar, N. Impact of IDO activation and alterations in the kynurenine pathway on hyperserotonemia, NAD+ production, and AhR activation in autism spectrum disorder. Transl. Psychiatry 2023, 13, 380. [Google Scholar] [CrossRef]
- Yildirim, V.; Simsek, S.; Cetin, I.; Dokuyucu, R. Kynurenine, Kynurenic Acid, Quinolinic Acid and Interleukin-6 Levels in the Serum of Patients with Autism Spectrum Disorder. Medicina 2023, 59, 1906. [Google Scholar] [CrossRef]
- Li, S.; Cai, Y.; Guan, T.; Zhang, Y.; Huang, K.; Zhang, Z.; Cao, W.; Guan, X. Quinic acid alleviates high-fat diet-induced neuroinflammation by inhibiting DR3/IKK/NF-κB signaling via gut microbial tryptophan metabolites. Gut Microbes 2024, 16, 2374608. [Google Scholar] [CrossRef] [PubMed]
- Koshiguchi, M.; Komazaki, H.; Hirai, S.; Egashira, Y. Ferulic acid suppresses expression of tryptophan metabolic key enzyme indoleamine 2, 3-dioxygenase via NFκB and p38 MAPK in lipopolysaccharide-stimulated microglial cells. Biosci. Biotechnol. Biochem. 2017, 81, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Kurniati, D.; Hirai, S.; Egashira, Y. Effect of apigenin on tryptophan metabolic key enzymes expression in lipopolysaccharide-induced microglial cells and its mechanism. Heliyon 2022, 9, e12743. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, S.S.; Bae, M.A.; Kim, S.H. Neuroprotective Activities of Sertraline, Tiagabine, and Bicifadine with Autophagy-Inducing Potentials in a 6-Hydroxidopamine-Treated Parkinson’s Disease Cell Model. Neurochem. Res. 2025, 50, 154. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, M.; Shi, Q.; Huang, Z.; Zhu, J.; Sun, P.; Zhang, H.; Yang, L.; Chen, X.; Zhang, Y. Proof of concept study for developing 1-thienyl-β-carboline derivatives as IDO1 and TDO dual inhibitors to treat Parkinson’s disease complicating depression. Eur. J. Med. Chem. 2025, 291, 117597. [Google Scholar] [CrossRef]
- Kong, Q.; Chen, Q.; Mao, X.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium longum CCFM1077 Ameliorated Neurotransmitter Disorder and Neuroinflammation Closely Linked to Regulation in the Kynurenine Pathway of Autistic-like Rats. Nutrients 2022, 14, 1615. [Google Scholar] [CrossRef]
- Bytowska, Z.K.; Korewo-Labelle, D.; Kowalski, K.; Libionka, W.; Przewłócka, K.; Kloc, W.; Kaczor, J.J. Impact of 12 Weeks of Vitamin D3 Administration in Parkinson’s Patients with Deep Brain Stimulation on Kynurenine Pathway and Inflammatory Status. Nutrients 2023, 15, 3839. [Google Scholar] [CrossRef]
- Wilson, E.N.; Umans, J.; Swarovski, M.S.; Minhas, P.S.; Mendiola, J.H.; Ulvik, A.; Shahid-Besanti, M.; Linortner, P.; Mhatre, S.D.; Wang, Q. Parkinson’s disease is characterized by vitamin B6-dependent inflammatory kynurenine pathway dysfunction. npj Park. Dis. 2025, 11, 96. [Google Scholar] [CrossRef]
- Peron, G.; Meroño, T.; Gargari, G.; Hidalgo-Liberona, N.; Miñarro, A.; Lozano, E.V.; Castellano-Escuder, P.; González-Domínguez, R.; del Bo’, C.; Bernardi, S.; et al. A Polyphenol-Rich Diet Increases the Gut Microbiota Metabolite Indole 3-Propionic Acid in Older Adults with Preserved Kidney Function. Mol. Nutr. Food Res. 2022, 66, e2100349. [Google Scholar] [CrossRef]
- Wang, H.; Chen, D.; Li, H.; Fu, C.; Fang, L.; Wang, R.; Xu, J. Bifidobacterium regulates premature infant gut metabolites, reducing serum inflammatory factors: A randomised controlled trial. Pediatr. Res. 2025, 97, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Fernando, D.; Dimelow, R.; Gorey, C.; Zhu, X.; Muya, C.; Parker, C.; Wright, W.; Soleman, S.; Walsh, S.; Crause, M. Assessment of the safety, pharmacokinetics and pharmacodynamics of GSK3335065, an inhibitor of kynurenine monooxygenase, in a randomised placebo-controlled first-in-human study in healthy volunteers. Br. J. Clin. Pharmacol. 2022, 88, 865–870. [Google Scholar] [CrossRef]
- Lai, S.; Yan, Y.; Pu, Y.; Lin, S.; Qiu, J.G.; Jiang, B.H.; Keller, M.I.; Wang, M.; Bork, P.; Chen, W.H.; et al. Enterotypes of the human gut mycobiome. Microbiome 2023, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Li, J.; Gao, X.; Yang, H.; Su, J.; Weng, R.; Gao, Y.; Ni, W.; Gu, Y. Involvement of the gut-brain axis in vascular depression via tryptophan metabolism: A benefit of short chain fatty acids. Exp. Neurol. 2022, 358, 114225. [Google Scholar] [CrossRef]
- Vijay, N.; Morris, M.E. Role of monocarboxylate transporters in drug delivery to the brain. Curr. Pharm. Des. 2014, 20, 1487–1498. [Google Scholar] [CrossRef]
- Zheng, S.; Zhang, H.; Liu, R.; Huang, C.-L.; Li, H.; Deng, Z.Y.; Tsao, R. Do short chain fatty acids and phenolic metabolites of the gut have synergistic anti-inflammatory effects?–New insights from a TNF-α-induced Caco-2 cell model. Food Res. Int. 2021, 139, 109833. [Google Scholar] [CrossRef]
- Church, J.S.; Bannish, J.A.M.; Adrian, L.A.; Rojas Martinez, K.; Henshaw, A.; Schwartzer, J.J. Serum short chain fatty acids mediate hippocampal BDNF and correlate with decreasing neuroinflammation following high pectin fiber diet in mice. Front. Neurosci. 2023, 17, 1134080. [Google Scholar] [CrossRef]
- Fock, E.; Parnova, R. Mechanisms of Blood-Brain Barrier Protection by Microbiota-Derived Short-Chain Fatty Acids. Cells 2023, 12, 657. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, D.C.; Lukic, I.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158, Erratum in Sci. Transl. Med. 2014, 6, 266er7. [Google Scholar] [CrossRef]
- Hoyles, L.; Snelling, T.; Umlai, U.K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome-host systems interactions: Protective effects of propionate upon the blood-brain barrier. Microbiome 2018, 6, 55. [Google Scholar] [CrossRef]
- Li, H.; Sun, J.; Du, J.; Wang, F.; Fang, R.; Yu, C.; Xiong, J.; Chen, W.; Lu, Z.; Liu, J. Clostridium butyricum exerts a neuroprotective effect in a mouse model of traumatic brain injury via the gut-brain axis. Neurogastroenterol. Motil. 2018, 30, e13260. [Google Scholar] [CrossRef]
- Herath, M.; Hosie, S.; Bornstein, J.C.; Franks, A.E.; Hill-Yardin, E.L. The Role of the Gastrointestinal Mucus System in Intestinal Homeostasis: Implications for Neurological Disorders. Front. Cell. Infect. Microbiol. 2020, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Belzer, C.; Goossens, M.; Kleerebezem, M.; De Vos, W.M.; Thas, O.; De Weirdt, R.; Kerckhof, F.M.; Van de Wiele, T. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013, 7, 949–961. [Google Scholar] [CrossRef]
- Chen, T.; Yang, C.S. Biological fates of tea polyphenols and their interactions with microbiota in the gastrointestinal tract: Implications on health effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 2691–2709. [Google Scholar] [PubMed]
- Zhang, Y.; Chang, H.; Shao, S.; Zhao, L.; Zhang, R.; Zhang, S. Anthocyanins from Opuntia ficus-indica Modulate Gut Microbiota Composition and Improve Short-Chain Fatty Acid Production. Biology 2022, 11, 1505. [Google Scholar] [CrossRef] [PubMed]
- Marizzoni, M.; Cattaneo, A.; Mirabelli, P.; Festari, C.; Lopizzo, N.; Nicolosi, V.; Mombelli, E.; Mazzelli, M.; Luongo, D.; Naviglio, D.; et al. Short-Chain Fatty Acids and Lipopolysaccharide as Mediators Between Gut Dysbiosis and Amyloid Pathology in Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 78, 683–697. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, L.; Schröder, J.; Schuster, I.S.; Nakai, M.; Sun, G.; Sun, Y.B.Y.; Mariño, E.; Degli-Esposti, M.A.; Marques, F.Z.; et al. Dietary Fiber and Microbiota Metabolite Receptors Enhance Cognition and Alleviate Disease in the 5xFAD Mouse Model of Alzheimer’s Disease. J. Neurosci. 2023, 43, 6460–6475. [Google Scholar] [CrossRef]
- Baranowski, B.J.; Oliveira, B.F.; Falkenhain, K.; Little, J.P.; Mohammad, A.; Beaudette, S.M.; Finch, M.S.; Caldwell, H.G.; Neudorf, H.; MacPherson, R.E.K.; et al. Effect of exogenous β-hydroxybutyrate on BDNF signaling, cognition, and amyloid precursor protein processing in humans with T2D and insulin-resistant rodents. Am. J. Physiol.-Cell Physiol. 2025, 328, C541–C556. [Google Scholar] [CrossRef]
- Tran, T.T.T.; Corsini, S.; Kellingray, L.; Hegarty, C.; Le Gall, G.; Narbad, A.; Müller, M.; Tejera, N.; O’Toole, P.W.; Minihane, A.M.; et al. APOE genotype influences the gut microbiome structure and function in humans and mice: Relevance for Alzheimer’s disease pathophysiology. FASEB J. 2019, 33, 8221–8231. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Yuan, B.; Liu, L.; Zhang, H.; Zhu, M.; Chai, H.; Peng, J.; Huang, Y.; Zhou, S.; et al. Akkermansia muciniphila and its metabolite propionic acid maintains neuronal mitochondrial division and autophagy homeostasis during Alzheimer’s disease pathologic process via GPR41 and GPR43. Microbiome 2025, 13, 16. [Google Scholar] [CrossRef]
- Mateo, D.; Marquès, M.; Domingo, J.L.; Torrente, M. Influence of gut microbiota on the development of most prevalent neurodegenerative dementias and the potential effect of probiotics in elderly: A scoping review. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2024, 195, e32959. [Google Scholar] [CrossRef]
- Fu, J.; Li, J.; Sun, Y.; Liu, S.; Song, F.; Liu, Z. In-depth investigation of the mechanisms of Schisandra chinensis polysaccharide mitigating Alzheimer’s disease rat via gut microbiota and feces metabolomics. Int. J. Biol. Macromol. 2023, 232, 123488. [Google Scholar]
- Xiao-Hang, Q.; Si-Yue, C.; Hui-Dong, T. Multi-strain probiotics ameliorate Alzheimer’s-like cognitive impairment and pathological changes through the AKT/GSK-3β pathway in senescence-accelerated mouse prone 8 mice. Brain Behav. Immun. 2024, 119, 14–27. [Google Scholar]
- Xie, M.; Gu, S.; Hong, Y.; Liu, Y.; Rong, X.; Lu, W.; Liu, H.; Algradi, A.M.; Naseem, A.; Shu, Z.; et al. Study on the mechanism of Coptis chinensis Franch. And its main active components in treating Alzheimer’s disease based on SCFAs using Orbitrap Fusion Lumos Tribrid MS. J. Ethnopharmacol. 2023, 311, 116392. [Google Scholar]
- Bayazid, A.B.; Lim, B.O. Therapeutic Effects of Plant Anthocyanin against Alzheimer’s Disease and Modulate Gut Health, Short-Chain Fatty Acids. Nutrients 2024, 16, 1554. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, J.; Liu, C.; Zhang, Z.; Zhang, Y.; Meng, D. Xanthoceraside exerts anti-Alzheimer’s disease effect by remodeling gut microbiota and modulating microbial-derived metabolites level in rats. Phytomedicine 2022, 98, 153937. [Google Scholar] [PubMed]
- Han, L.; Chen, W.; Li, J.; Zhao, Y.; Zong, Y.; He, Z.; Du, R. Palmatine improves cognitive dysfunction in Alzheimer’s disease model rats through autophagy pathway and regulation of gut microbiota. Brain Res. 2024, 1835, 148932. [Google Scholar] [PubMed]
- Hu, F.; Gao, Q.; Zheng, C.; Zhang, W.; Yang, Z.; Wang, S.; Zhang, Y.; Lu, T. Encapsulated lactiplantibacillus plantarum improves Alzheimer’s symptoms in APP/PS1 mice. J. Nanobiotechnol. 2024, 22, 582. [Google Scholar]
- Parilli-Moser, I.; Domínguez-López, I.; Trius-Soler, M.; Castellví, M.; Bosch, B.; Castro-Barquero, S.; Estruch, R.; Hurtado-Barroso, S.; Lamuela-Raventós, R.M. Consumption of peanut products improves memory and stress response in healthy adults from the ARISTOTLE study: A 6-month randomized controlled trial. Clin. Nutr. 2021, 40, 5556–5567. [Google Scholar] [CrossRef]
- Mi, N.; Ma, L.; Li, X.; Fu, J.; Bu, X.; Liu, F.; Yang, F.; Zhang, Y.; Yao, L. Metabolomic analysis of serum short-chain fatty acid concentrations in a mouse of MPTP-induced Parkinson’s disease after dietary supplementation with branched-chain amino acids. Open Med. 2023, 18, 20230849. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; An, K.; Wang, D.; Yu, H.; Li, J.; Min, Z.; Xiong, Y.; Xue, Z.; Mao, Z. Short-Chain Fatty Acid Aggregates Alpha-Synuclein Accumulation and Neuroinflammation via GPR43-NLRP3 Signaling Pathway in a Model Parkinson’s Disease. Mol. Neurobiol. 2025, 62, 6612–6625. [Google Scholar]
- Qiao, C.M.; Sun, M.F.; Jia, X.B.; Shi, Y.; Zhang, B.P.; Zhou, Z.L.; Zhao, L.P.; Cui, C.; Shen, Y.Q. Sodium butyrate causes α-synuclein degradation by an Atg5-dependent and PI3K/Akt/mTOR-related autophagy pathway. Exp. Cell Res. 2020, 387, 111772. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Shi, H.; Xu, Y.; Ji, L. The gut microbiota metabolite propionate ameliorates intestinal epithelial barrier dysfunction-mediated Parkinson’s disease via the AKT signaling pathway. Neuroreport 2021, 32, 244–251. [Google Scholar] [CrossRef]
- Vascellari, S.; Palmas, V.; Melis, M.; Pisanu, S.; Cusano, R.; Uva, P.; Perra, D.; Madau, V.; Sarchioto, M.; Oppo, V.; et al. Gut Microbiota and Metabolome Alterations Associated with Parkinson’s Disease. mSystems 2020, 5, e00561-20. [Google Scholar] [CrossRef]
- Hall, D.A.; Voigt, R.M.; Cantu-Jungles, T.M.; Hamaker, B.; Engen, P.A.; Shaikh, M.; Raeisi, S.; Green, S.J.; Naqib, A.; Forsyth, C.B.; et al. An open label, non-randomized study assessing a prebiotic fiber intervention in a small cohort of Parkinson’s disease participants. Nat. Commun. 2023, 14, 926. [Google Scholar] [CrossRef]
- Bedarf, J.R.; Romano, S.; Heinzmann, S.S.; Duncan, A.; Traka, M.H.; Ng, D.; Segovia-Lizano, D.; Simon, M.C.; Narbad, A.; Wüllner, U.; et al. A prebiotic dietary pilot intervention restores faecal metabolites and may be neuroprotective in Parkinson’s Disease. npj Park. Dis. 2025, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; Chang, J.; Rudi, K.; Paulin, L.; Hertzberg, V.; Auvinen, P.; Tansey, M.G.; Scheperjans, F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 2021, 16, 6. [Google Scholar] [PubMed]
- Zahedi, E.; Sadr, S.S.; Sanaeierad, A.; Roghani, M. Chronic acetyl-L-carnitine treatment alleviates behavioral deficits and neuroinflammation through enhancing microbiota derived-SCFA in valproate model of autism. Biomed. Pharmacother. 2023, 163, 114848. [Google Scholar] [CrossRef]
- Zhong, J.G.; Lan, W.T.; Feng, Y.Q.; Li, Y.H.; Shen, Y.Y.; Gong, J.H.; Zou, Z.; Hou, X. Associations between dysbiosis gut microbiota and changes of neurotransmitters and short-chain fatty acids in valproic acid model rats. Front. Physiol. 2023, 14, 1077821. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Mervosh, N.L.; Strat, A.N.; Euston, T.J.; Zipursky, G.; Pollak, R.M.; Meckel, K.R.; Tyler, S.R.; Chan, K.L.; Buxbaum Grice, A.; et al. Acetate supplementation rescues social deficits and alters transcriptional regulation in prefrontal cortex of Shank3 deficient mice. Brain Behav. Immun. 2023, 114, 311–324. [Google Scholar]
- He, J.; Gong, X.; Hu, B.; Lin, L.; Lin, X.; Gong, W.; Zhang, B.; Cao, M.; Xu, Y.; Xia, R.; et al. Altered Gut Microbiota and Short-chain Fatty Acids in Chinese Children with Constipated Autism Spectrum Disorder. Sci. Rep. 2023, 13, 19103. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Yang, J.J.; Zhao, D.M.; Chen, B.; Zhang, G.Q.; Chen, S.; Cao, R.F.; Yu, H.; Zhao, C.Y.; et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacol. Res. 2020, 157, 104784. [Google Scholar] [CrossRef]
- Berding, K.; Donovan, S.M. Dietary Patterns Impact Temporal Dynamics of Fecal Microbiota Composition in Children with Autism Spectrum Disorder. Front. Nutr. 2020, 6, 193. [Google Scholar] [CrossRef]
- Venditti, A. Sulfur-containing Secondary Metabolites as Neuroprotective Agents. Curr. Med. Chem. 2020, 27, 4421–4436. [Google Scholar] [CrossRef]
- Salemme, A.; Togna, A.R.; Mastrofrancesco, A.; Cammisotto, V.; Ottaviani, M.; Bianco, A.; Venditti, A. Anti-inflammatory effects and antioxidant activity of dihydroasparagusic acid in lipopolysaccharide-activated microglial cells. Brain Res. Bull. 2016, 120, 151–158. [Google Scholar]
- Javed, H.; Khan, M.M.; Khan, A.; Vaibhav, K.; Ahmad, A.; Khuwaja, G.; Ahmed, E.; Raza, S.S.; Ashafaq, M.; Tabassum, R.; et al. S-allyl cysteine attenuates oxidative stress associated cognitive impairment and neurodegeneration in mouse model of streptozotocin-induced experimental dementia of Alzheimer’s type. Brain Res. 2011, 1389, 133–142. [Google Scholar]
- Shi, H.; Jing, X.; Wei, X.; Perez, R.G.; Ren, M.; Zhang, X.; Lou, H. S-allyl cysteine activates the Nrf2-dependent antioxidant response and protects neurons against ischemic injury in vitro and in vivo. J. Neurochem. 2015, 133, 298–308. [Google Scholar] [PubMed]
- García, E.; Santana-Martínez, R.; Silva-Islas, C.A.; Colín-González, A.L.; Galván-Arzate, S.; Heras, Y.; Maldonado, P.D.; Sotelo, J.; Santamaría, A. S-allyl cysteine protects against MPTP-induced striatal and nigral oxidative neurotoxicity in mice: Participation of Nrf2. Free Radic. Res. 2013, 48, 159–167. [Google Scholar] [PubMed]
- Ito, Y.; Kosuge, Y.; Sakikubo, T.; Horie, K.; Ishikawa, N.; Obokata, N.; Yokoyama, E.; Yamashina, K.; Yamamoto, M.; Saito, H.; et al. Protective effect of S-allyl-l-cysteine, a garlic compound, on amyloid β-protein-induced cell death in nerve growth factor-differentiated PC12 cells. Neurosci. Res. 2003, 46, 119–125. [Google Scholar] [PubMed]
- Kosuge, Y.; Koen, Y.; Ishige, K.; Minami, K.; Urasawa, H.; Saito, H.; Ito, Y. S-allyl-l-cysteine selectively protects cultured rat hippocampal neurons from amyloid β-protein- and tunicamycin-induced neuronal death. Neuroscience 2003, 122, 885–895. [Google Scholar]
- Tsai, S.-J.; Chiu, C.P.; Yang, H.-T.; Yin, M.-C. S-Allyl cysteine, S-ethyl cysteine, and S-propyl cysteine alleviate β-amyloid, glycative, and oxidative injury in brain of mice treated by d-galactose. J. Agric. Food Chem. 2011, 59, 6319–6326. [Google Scholar] [PubMed]
- Baluchnejadmojarad, T.; Kiasalari, Z.; Afshin-Majd, S.; Ghasemi, Z.; Roghani, M. S-allyl cysteine ameliorates cognitive deficits in streptozotocin-diabetic rats via suppression of oxidative stress, inflammation, and acetylcholinesterase. Eur. J. Pharmacol. 2017, 794, 69–76. [Google Scholar]
- Reyes-Soto, C.Y.; Ramírez-Carreto, R.J.; Ortíz-Alegría, L.B.; Silva-Palacios, A.; Zazueta, C.; Galván-Arzate, S.; Karasu, Ç.; Túnez, I.; Tinkov, A.A.; Aschner, M.; et al. S-allyl-cysteine triggers cytotoxic events in rat glioblastoma RG2 and C6 cells and improves the effect of temozolomide through the regulation of oxidative responses. Discov. Oncol. 2024, 15, 272. [Google Scholar]
- Morroni, F.; Sita, G.; Tarozzi, A.; Cantelli-Forti, G.; Hrelia, P. Neuroprotection by 6-(methylsulfinyl)hexyl isothiocyanate in a 6-hydroxydopamine mouse model of Parkinson׳s disease. Brain Res. 2014, 1589, 93–104. [Google Scholar] [PubMed]
- Trio, P.Z.; Fujisaki, S.; Tanigawa, S.; Hisanaga, A.; Sakao, K.; Hou, D.X. DNA Microarray Highlights Nrf2-Mediated Neuron Protection Targeted by Wasabi-Derived Isothiocyanates in IMR-32 Cells. Gene Regul. Syst. Biol. 2016, 10, 73–83. [Google Scholar]
- Morroni, F.; Sita, G.; Graziosi, A.; Turrini, E.; Fimognari, C.; Tarozzi, A.; Hrelia, P. Protective Effects of 6-(Methylsulfinyl) hexyl isothiocyanate on aβ1–42-induced cognitive deficit, oxidative stress, inflammation, and apoptosis in mice. Int. J. Mol. Sci. 2018, 19, 2083. [Google Scholar]
- Lohning, A.; Kidachi, Y.; Kamiie, K.; Sasaki, K.; Ryoyama, K.; Yamaguchi, H. 6-(methylsulfinyl)hexyl isothiocyanate (6-MITC) from Wasabia japonica alleviates inflammatory bowel disease (IBD) by potential inhibition of glycogen synthase kinase 3 beta (GSK-3β). Eur. J. Med. Chem. 2021, 216, 113250. [Google Scholar] [CrossRef]
- Uruno, A.; Matsumaru, D.; Ryoke, R.; Saito, R.; Kadoguchi, S.; Saigusa, D.; Saito, T.; Saido, T.C.; Kawashima, R.; Yamamoto, M. Nrf2 Suppresses Oxidative Stress and Inflammation in App Knock-In Alzheimer’s Disease Model Mice. Mol. Cell. Biol. 2020, 40, e00467-19. [Google Scholar] [CrossRef]
- Nouchi, R.; Kawata, N.Y.S.; Saito, T.; Nouchi, H.; Kawashima, R. Benefits of Wasabi Supplements with 6-MSITC (6-Methylsulfinyl Hexyl Isothiocyanate) on Memory Functioning in Healthy Adults Aged 60 Years and Older: Evidence from a Double-Blinded Randomized Controlled Trial. Nutrients 2023, 15, 4608. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Scuto, M.; Salinaro, A.T.; Dionisio, G.; Modafferi, S.; Ontario, M.L.; Greco, V.; Sciuto, S.; Schmitt, C.P.; Calabrese, E.J.; et al. Hydrogen Sulfide and Carnosine: Modulation of Oxidative Stress and Inflammation in Kidney and Brain Axis. Antioxidants 2020, 9, 1303. [Google Scholar] [CrossRef]
- Reekes, T.H.; Ledbetter, C.R.; Alexander, J.S.; Stokes, K.Y.; Pardue, S.; Bhuiyan, M.A.N.; Patterson, J.C.; Lofton, K.T.; Kevil, C.G.; Disbrow, E.A. Elevated plasma sulfides are associated with cognitive dysfunction and brain atrophy in human Alzheimer’s disease and related dementias. Redox Biol. 2023, 62, 102633. [Google Scholar] [CrossRef]
- Giovinazzo, D.; Bursac, B.; Sbodio, J.I.; Nalluru, S.; Vignane, T.; Snowman, A.M.; Albacarys, L.M.; Sedlak, T.W.; Torregrossa, R.; Whiteman, M.; et al. Hydrogen sulfide is neuroprotective in Alzheimer’s disease by sulfhydrating GSK3β and inhibiting Tau hyperphosphorylation. Proc. Natl. Acad. Sci. USA 2021, 118, e2017225118. [Google Scholar] [CrossRef]
- Wang, M.; Tang, J.J.; Wang, L.X.; Yu, J.; Zhang, L.; Qiao, C. Hydrogen sulfide enhances adult neurogenesis in a mouse model of Parkinson’s disease. Neural Regen. Res. 2021, 16, 1353–1358. [Google Scholar] [CrossRef]
- Tian, Q.; Tang, H.L.; Tang, Y.Y.; Zhang, P.; Kang, X.; Zou, W.; Tang, X.Q. Hydrogen Sulfide Attenuates the Cognitive Dysfunction in Parkinson’s Disease Rats via Promoting Hippocampal Microglia M2 Polarization by Enhancement of Hippocampal Warburg Effect. Oxid. Med. Cell. Longev. 2022, 2022, 2792348. [Google Scholar] [CrossRef]
- Sun, Y.; Li, D.; Su, Y.; Zhao, H.; Pang, W.; Zhao, W.; Wu, S. Protective effect of hydrogen sulfide is mediated by negative regulation of epigenetic histone acetylation in Parkinson’s disease. Arch. Med. Sci. 2020, 19, 1124–1135. [Google Scholar] [CrossRef]
- Olson, K.R.; Gao, Y.; Briggs, A.; Devireddy, M.; Iovino, N.A.; Licursi, M.; Skora, N.C.; Whelan, J.; Villa, B.P.; Straub, K.D. ‘Antioxidant’ berries, anthocyanins, resveratrol and rosmarinic acid oxidize hydrogen sulfide to polysulfides and thiosulfate: A novel mechanism underlying their biological actions. Free Radic. Biol. Med. 2021, 165, 67–78. [Google Scholar] [CrossRef]
- Wang, A.; Pi, Z.; Liu, S.; Zheng, Z.; Liu, Z.; Song, F. Mass Spectrometry-Based Urinary Metabolomics for Exploring the Treatment Effects of Radix Ginseng-Schisandra Chinensis Herb Pair on Alzheimer’s Disease in Rats. J. Sep. Sci. 2021, 44, 3158–3166. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, L.; Ding, W.; Wu, J.; Liu, F.; Liu, J.; Zhang, J.; Wang, J. The Improvement Effects of Sika Deer Antler Protein in an Alzheimer’s Disease Mouse Model via the Microbe-Gut-Brain Axis. Food Sci. Nutr. 2024, 13, e4656. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, M.; Zheng, Q.; Song, Y.; Yan, Z.; Wang, H.; Xiong, Y.; Chen, Y.; Cai, Z.; Yuan, J. Exploring the Mechanism of Kai-Xin-San to Improve Cognitive Deficits in AD Rats Induced by D-Gal and Aβ25-35 Based on Multi-Omics and Network Analysis. Biomed. Chromatogr. 2025, 39, e70047. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Zeng, M.N.; Hao, F.X.; Hao, Z.Y.; Zhang, Z.K.; Liang, X.W.; Wu, Y.Y.; Zhang, Y.H.; Feng, W.S.; Zheng, X.K. P-coumaric acid ameliorates Aβ25-35-induced brain damage in mice by modulating gut microbiota and serum metabolites. Biomed. Pharmacother. 2023, 168, 115825. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Y.; Zhang, J.; Liu, Q.; Zhu, L.; Mao, B.; Ma, Y.; Liu, Y. Serum metabolomics combined with gut microbiota reveals the effects of Polygala tenuifolia polysaccharide on the metabolic and microbial profiles in SAMP8 mouse. J. Pharm. Biomed. Anal. 2024, 251, 116442. [Google Scholar] [CrossRef]
- Xu, X.; Ding, Y.; Liu, M.; Zhang, X.; Wang, D.; Pan, Y.; Ren, S.; Liu, X. Neuroprotective mechanisms of defatted walnut powder against scopolamine-induced Alzheimer’s disease in mice revealed through metabolomics and proteomics analyses. J. Ethnopharmacol. 2024, 319, 117107. [Google Scholar] [CrossRef]
- Tu, H.J.; Su, C.J.; Peng, C.S.; Lin, T.E.; HuangFu, W.C.; Hsu, K.C.; Hwang, T.L.; Pan, S.L. Urolithin A exhibits a neuroprotective effect against Alzheimer’s disease by inhibiting DYRK1A activity. J. Food Drug Anal. 2023, 31, 358–370. [Google Scholar] [CrossRef]
- Tan, T.H.; Li, S.W.; Chang, C.W.; Chen, Y.C.; Liu, Y.H.; Ma, J.T.; Chang, C.P.; Liao, P.C. Rat Hair Metabolomics Analysis Reveals Perturbations of Unsaturated Fatty Acid Biosynthesis, Phenylalanine, and Arachidonic Acid Metabolism Pathways Are Associated with Amyloid-β-Induced Cognitive Deficits. Mol. Neurobiol. 2023, 60, 4373–4395. [Google Scholar] [CrossRef]
- François, M.; Pascovici, D.; Wang, Y.; Vu, T.; Liu, J.W.; Beale, D.; Hor, M.; Hecker, J.; Faunt, J.; Maddison, J.; et al. Saliva Proteome, Metabolome and Microbiome Signatures for Detection of Alzheimer’s Disease. Metabolites 2024, 14, 714. [Google Scholar] [CrossRef]
- Xu, J.; Green, R.; Kim, M.; Lord, J.; Ebshiana, A.; Westwood, S.; Baird, A.L.; Nevado-Holgado, A.J.; Shi, L.; Hye, A.; et al. Sex-Specific Metabolic Pathways Were Associated with Alzheimer’s Disease (AD) Endophenotypes in the European Medical Information Framework for AD Multimodal Biomarker Discovery Cohort. Biomedicines 2021, 9, 1610. [Google Scholar] [CrossRef]
- Breitel, D.; Brett, P.; Alseekh, S.; Fernie, A.R.; Butelli, E.; Martin, C. Diverting tyrosine: Data from untargeted metabolic analysis of tomato fruit accumulating L-DOPA. Data Brief. 2020, 34, 106678. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, Phenylalanine, and Catecholamine Synthesis and Function in the Brain. J. Nutr. 2007, 137, 1539S–1547S. [Google Scholar] [CrossRef]
- Stefani, A.; Pierantozzi, M.; Olivola, E.; Galati, S.; Cerroni, R.; D’Angelo, V.; Hainsworth, A.H.; Saviozzi, V.; Fedele, E.; Liguori, C. Homovanillic acid in CSF of mild stage Parkinson’s disease patients correlates with motor impairment. Neurochem. Int. 2017, 105, 58–63. [Google Scholar] [CrossRef]
- Breitel, D.; Brett, P.; Alseekh, S.; Fernie, A.R.; Butelli, E.; Martin, C. Metabolic engineering of tomato fruit enriched in L-DOPA. Metab. Eng. 2021, 65, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R.P.; Koledova, V.V.; Subramaniam, A.; Schneider, K.; Artamonova, A.; Sambanthamurthi, R.; Hayes, K.C.; Sinskey, A.J.; Rha, C. Palm Fruit Bioactives augment expression of Tyrosine Hydroxylase in the Nile Grass Rat basal ganglia and alter the colonic microbiome. Sci. Rep. 2019, 9, 18625, Correction in Sci. Rep. 2020, 10, 2878. [Google Scholar] [CrossRef]
- Patel, D.; Jana, A.; Roy, A.; Pahan, K. Cinnamon and its Metabolite Protect the Nigrostriatum in a Mouse Model of Parkinson’s Disease Via Astrocytic GDNF. J. Neuroimmune Pharmacol. 2019, 14, 503–518. [Google Scholar] [CrossRef]
- Zhao, T.T.; Shin, K.S.; Kim, K.S.; Park, H.J.; Kim, H.J.; Lee, K.E.; Lee, M.K. Effects of (-)-sesamin on motor and memory deficits in an MPTP-lesioned mouse model of Parkinson’s disease treated with l-DOPA. Neuroscience 2016, 339, 644–654. [Google Scholar] [PubMed]
- Fang, J.; Guo, J.; Lao, Y.; Kang, S.G.; Huang, K.; Tong, T. L-tyrosine alleviates autism-like behavior in mice by remodeling the gut microbiota. Brain Behav. Immun. 2025, 127, 358–374. [Google Scholar] [PubMed]
- Yang, X.; Wei, H.; Li, J.; Li, G.; Zhang, Y.; Li, H. Efficacy of sialic acid supplementation in early life in autism model rats. Sci. Rep. 2025, 15, 8576. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Vargason, T.; Santiago, M.; Hahn, J.; Krajmalnik-Brown, R. Distinct Fecal and Plasma Metabolites in Children with Autism Spectrum Disorders and Their Modulation after Microbiota Transfer Therapy. mSphere 2020, 5, e00314-20. [Google Scholar] [CrossRef]
- Flynn, C.K.; Adams, J.B.; Krajmalnik-Brown, R.; Khoruts, A.; Sadowsky, M.J.; Nirmalkar, K.; Takyi, E.; Whiteley, P. Review of Elevated Para-Cresol in Autism and Possible Impact on Symptoms. Int. J. Mol. Sci. 2025, 26, 1513. [Google Scholar] [CrossRef]
- Shaw, W. Increased urinary excretion of a 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylalanine metabolite of Clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophrenia. Nutr. Neurosci. 2010, 13, 135–143. [Google Scholar]
- Timperio, A.M.; Gevi, F.; Cucinotta, F.; Ricciardello, A.; Turriziani, L.; Scattoni, M.L.; Persico, A.M. Urinary Untargeted Metabolic Profile Differentiates Children with Autism from Their Unaffected Siblings. Metabolites 2022, 12, 797. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M.; Gao, B.; Hybertson, B.M. The Complex Genetic and Epigenetic Regulation of the Nrf2 Pathways: A Review. Antioxidants 2023, 12, 366. [Google Scholar] [CrossRef] [PubMed]
- Heurtaux, T.; Bouvier, D.S.; Benani, A.; Helgueta Romero, S.; Frauenknecht, K.B.M.; Mittelbronn, M.; Sinkkonen, L. Normal and Pathological NRF2 Signalling in the Central Nervous System. Antioxidants 2022, 11, 1426. [Google Scholar] [CrossRef]
- Liu, S.; Park, T.; Krüger, D.M.; Pena-Centeno, T.; Burkhardt, S.; Schutz, A.L.; Huang, Y.N.; Rosewood, T.; Chaudhuri, S.; Cho, M.; et al. Plasma miRNAs across the Alzheimer’s disease continuum: Relationship to central biomarkers. Alzheimer’s Dement. 2024, 20, 7698–7714. [Google Scholar] [CrossRef]
- Masoud, A.M.; Bihaqi, S.W.; Alansi, B.; Dash, M.; Subaiea, G.M.; Renehan, W.E.; Zawia, N.H. Altered microRNA, mRNA, and Protein Expression of Neurodegeneration-Related Biomarkers and Their Transcriptional and Epigenetic Modifiers in a Human Tau Transgenic Mouse Model in Response to Developmental Lead Exposure. J. Alzheimer’s Dis. 2018, 63, 273–282. [Google Scholar] [CrossRef]
- Sato, T.; Greco, C.M. Expanding the link between circadian rhythms and redox metabolism of epigenetic control. Free Radic. Biol. Med. 2021, 170, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Luan, R.; Ding, D.; Yang, J. The protective effect of natural medicines against excessive inflammation and oxidative stress in acute lung injury by regulating the Nrf2 signaling pathway. Front. Pharmacol. 2022, 13, 1039022. [Google Scholar] [CrossRef]
- Atalay Ekiner, S.; Gegotek, A.; Skrzydlewska, E. The molecular activity of cannabidiol in the regulation of Nrf2 system interacting with NF-κB pathway under oxidative stress. Redox Biol. 2022, 57, 102489. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- McMahon, M.; Thomas, N.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Redox-regulated turnover of Nrf2 is determined by at least two separate protein domains, the redox-sensitive Neh2 degron and the redox-insensitive Neh6 degron. J. Biol. Chem. 2004, 279, 31556–31567. [Google Scholar] [CrossRef]
- Silva-Llanes, I.; Shin, C.H.; Jiménez-Villegas, J.; Gorospe, M.; Lastres-Becker, I. The Transcription Factor NRF2 Has Epigenetic Regulatory Functions Modulating HDACs, DNMTs, and miRNA Biogenesis. Antioxidants 2023, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Khor, T.O.; Cheung, K.L.; Li, W.; Wu, T.Y.; Huang, Y. Nrf2 expression is regulated by epigenetic mechanisms in prostate cancer of TRAMP mice. PLoS ONE 2010, 5, e8579. [Google Scholar] [CrossRef]
- Taheri, Z.; Asadzadeh Aghdaei, H.; Irani, S.; Modarressi, M.H.; Zahra, N. Evaluation of the Epigenetic Demethylation of NRF2, a Master Transcription Factor for Antioxidant Enzymes, in Colorectal Cancer. Rep. Biochem. Mol. Biol. 2020, 9, 33–39. [Google Scholar] [CrossRef]
- Quiles, J.M.; Pepin, M.E.; Sunny, S.; Shelar, S.B.; Challa, A.K.; Dalley, B.; Hoidal, J.R.; Pogwizd, S.M.; Wende, A.R. Identification of Nrf2-responsive microRNA networks as putative mediators of myocardial reductive stress. Sci. Rep. 2021, 11, 11977. [Google Scholar] [CrossRef]
- Kaundal, R.K.; Datusalia, A.K.; Sharma, S.S. Posttranscriptional regulation of Nrf2 through miRNAs and their role in Alzheimer’s disease. Pharmacol. Res. 2022, 175, 106018. [Google Scholar] [CrossRef]
- Cheng, X.; Ku, C.H.; Siow, R.C. Regulation of the Nrf2 antioxidant pathway by microRNAs: New players in micromanaging redox homeostasis. Free Radic. Biol. Med. 2013, 64, 4–11. [Google Scholar] [CrossRef]
- Joo, M.S.; Lee, C.G.; Koo, H.; Kim, S.G. miR-125b transcriptionally increased by Nrf2 inhibits AhR repressor, which protects kidney from cisplatin-induced injury. Cell Death Dis. 2013, 4, e899. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, S.; Jain, A.K.; Bloom, D.A.; Jaiswal, A.K. Bach1 Competes with Nrf2 Leading to Negative Regulation of the Antioxidant Response Element (ARE)-mediated NAD(P)H:Quinone Oxidoreductase 1 Gene Expression and Induction in Response to Antioxidants. J. Biol. Chem. 2005, 280, 16891–16900. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Hu, H.; Zhong, Y.; Chen, Y.; Tang, K.; Pan, Z.; Huang, J.; Yang, X.; Wang, Q.; Gao, Y. Microglia Sirt6 modulates the transcriptional activity of NRF2 to ameliorate high-fat diet-induced obesity. Mol. Med. 2023, 29, 108, Correction in Mol. Med. 2023, 29, 128. [Google Scholar] [CrossRef]
- Bell, K.F.S.; Al-Mubarak, B.; Martel, M.-A.; McKay, S.; Wheelan, N.; Hasel, P.; Márkus, N.M.; Baxter, P.; Deighton, R.F.; Serio, A.; et al. Neuronal development is promoted by weakened intrinsic antioxidant defences due to epigenetic repression of Nrf2. Nat. Commun. 2015, 6, 7066. [Google Scholar] [CrossRef] [PubMed]
- Boas, S.M.; Joyce, K.L.; Cowell, R.M. The NRF2-Dependent Transcriptional Regulation of Antioxidant Defense Pathways: Relevance for Cell Type-Specific Vulnerability to Neurodegeneration and Therapeutic Intervention. Antioxidants 2021, 11, 8. [Google Scholar] [CrossRef]
- Rojo, A.I.; Pajares, M.; García-Yagüe, A.J.; Buendia, I.; Van Leuven, F.; Yamamoto, M.; López, M.G.; Cuadrado, A. Deficiency in the transcription factor NRF2 worsens inflammatory parameters in a mouse model with combined tauopathy and amyloidopathy. Redox Biol. 2018, 18, 173–180. [Google Scholar] [CrossRef]
- Jing, X.; Shi, H.; Zhang, C.; Ren, M.; Han, M.; Wei, X.; Zhang, X.; Lou, H. Dimethyl fumarate attenuates 6-OHDA-induced neurotoxicity in SH-SY5Y cells and in animal model of Parkinson’s disease by enhancing Nrf2 activity. Neuroscience 2015, 286, 131–140. [Google Scholar] [CrossRef]
- Ahuja, M.; Ammal Kaidery, N.; Attucks, O.C.; McDade, E.; Hushpulian, D.M.; Gaisina, A.; Gaisina, I.; Ahn, Y.H.; Nikulin, S.; Poloznikov, A.; et al. Bach1 derepression is neuroprotective in a mouse model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2021, 118, e2111643118, Correction in Proc. Natl. Acad. Sci. USA 2024, 121, e2411303121. [Google Scholar] [CrossRef]
- Coppieters, N.; Dieriks, B.V.; Lill, C.; Faull, R.L.; Curtis, M.A.; Dragunow, M. Global changes in DNA methylation and hydroxymethylation in Alzheimer’s disease human brain. Neurobiol. Aging 2014, 35, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Griñán-Ferré, C.; Marsal-García, L.; Bellver-Sanchis, A.; Kondengaden, S.M.; Turga, R.C.; Vázquez, S.; Pallàs, M. Pharmacological inhibition of G9a/GLP restores cognition and reduces oxidative stress, neuroinflammation and β-Amyloid plaques in an early-onset Alzheimer’s disease mouse model. Aging 2019, 11, 11591–11608. [Google Scholar] [CrossRef]
- Liu, H.X.; Yang, M.K.; Li, Y.C.; Liu, C.X.; Li, G.P.; Meng, X.L.; Pei, K.; Wen, S.Y. Shenqi granules enhance recovery from cerebral ischemia-reperfusion injury by modulating tryptophan and tyrosine metabolism and activating NFE2L2/NRF2. Phytomedicine 2025, 140, 156623. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Deng, F.; Wu, W.; Jiang, L.; Yamashiro, T.; Yano, S.; Hou, D.X. Baicalein modulates Nrf2/Keap1 system in both Keap1-dependent and Keap1-independent mechanisms. Arch. Biochem. Biophys. 2014, 559, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Y.; Pyo, M.C.; Nam, M.-H.; Lee, K.-W. ERK/Nrf2 pathway activation by caffeic acid in HepG2 cells alleviates its hepatocellular damage caused by t-butylhydroperoxide-induced oxidative stress. BMC Complement. Altern. Med. 2019, 19, 139. [Google Scholar] [CrossRef]
- Hu, Y.-R.; Ma, H.; Zou, Z.-Y.; He, K.; Xiao, Y.-B.; Wang, Y.; Feng, M.; Ye, X.L.; Li, X.G. Activation of Akt and JNK/Nrf2/NQO1pathway contributes to the protective effect of coptisine against AAPH-induced oxidative stress. Biomed. Pharmacother. 2017, 85, 313–322. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Venkidasamy, B.; Subramanian, U.; Samynathan, R.; Ali Shariati, M.; Rebezov, M.; Girish, S.; Thangavel, S.; Dhanapal, A.R.; Fedoseeva, N.; et al. Bioactive Compounds in Oxidative Stress-Mediated Diseases: Targeting the NRF2/ARE Signaling Pathway and Epigenetic Regulation. Antioxidants 2021, 10, 1859. [Google Scholar] [CrossRef]
- Culbreth, M.; Aschner, M. GSK-3β, a double-edged sword in Nrf2 regulation: Implications for neurological dysfunction and disease. F1000Research 2018, 7, 1043. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Zhu, J.; Lei, S.; Dong, Y.; Li, L.; Jiang, B.; Tan, L.; Wu, J.; Yu, S.; et al. GSK-3β downregulates Nrf2 in cultured cortical neurons and in a rat model of cerebral ischemia-reperfusion. Sci. Rep. 2016, 6, 20196. [Google Scholar] [CrossRef]
- Baxter, P.S.; Márkus, N.M.; Dando, O.; He, X.; Al-Mubarak, B.R.; Qiu, J.; Hardingham, G.E. Targeted de-repression of neuronal Nrf2 inhibits α-synuclein accumulation. Cell Death Dis. 2021, 12, 218. [Google Scholar]
- Kraft, A.D.; Johnson, D.A.; Johnson, J.A. Nuclear Factor E2-Related Factor 2-Dependent Antioxidant Response Element Activation by tert-Butylhydroquinone and Sulforaphane Occurring Preferentially in Astrocytes Conditions Neurons against Oxidative Insult. J. Neurosci. 2004, 24, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, V.; Palomera-Ávalos, V.; López-Ruiz, S.; Canudas, A.M.; Pallàs, M.; Griñán-Ferré, C. Maternal Resveratrol Supplementation Prevents Cognitive Decline in Senescent Mice Offspring. Int. J. Mol. Sci. 2019, 20, 1134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhang, J.; Chang, N. Epigenetic modification of Nrf2 by sulforaphane increases the antioxidative and anti-inflammatory capacity in a cellular model of Alzheimer’s disease. Eur. J. Pharmacol. 2018, 824, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.M.; Catanzaro, M.; Fagiani, F.; Simoni, E.; Caporaso, R.; Dacrema, M.; Romanoni, I.; Govoni, S.; Racchi, M.; Daglia, M.; et al. Modulation of Keap1/Nrf2/ARE Signaling Pathway by Curcuma- and Garlic-Derived Hybrids. Front. Pharmacol. 2020, 10, 1597. [Google Scholar] [CrossRef]
- Harithpriya, K.; Ganesan, K.; Ramkumar, K.M. Pterostilbene Reverses Epigenetic Silencing of Nrf2 and Enhances Antioxidant Response in Endothelial Cells in Hyperglycemic Microenvironment. Nutrients 2024, 16, 2045. [Google Scholar] [CrossRef]
- Guo, C.; Zhu, J.; Wang, J.; Duan, J.; Ma, S.; Yin, Y.; Quan, W.; Zhang, W.; Guan, Y.; Ding, Y.; et al. Neuroprotective effects of protocatechuic aldehyde through PLK2/p-GSK3β/Nrf2 signaling pathway in both in vivo and in vitro models of Parkinson’s disease. Aging 2019, 11, 9424–9441. [Google Scholar] [CrossRef]
- Bhatt, T.; Dey, R.; Hegde, A.; Ketkar, A.A.; Pulianmackal, A.J.; Pulianmackal, A.J.; Deb, A.P.; Rampalli, S.; Jamora, C. Initiation of wound healing is regulated by the convergence of mechanical and epigenetic cues. PLoS Biol. 2022, 20, e3001777. [Google Scholar]
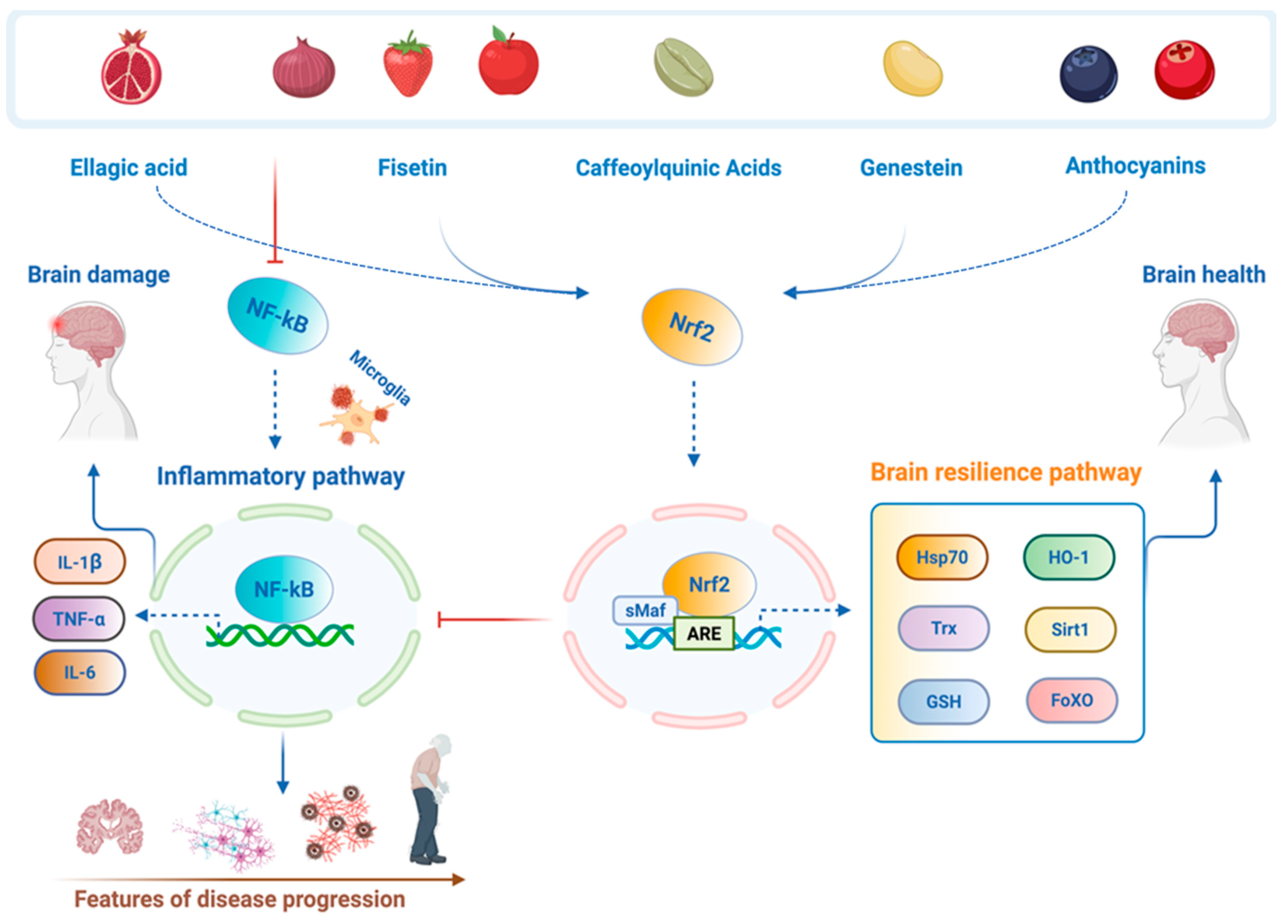
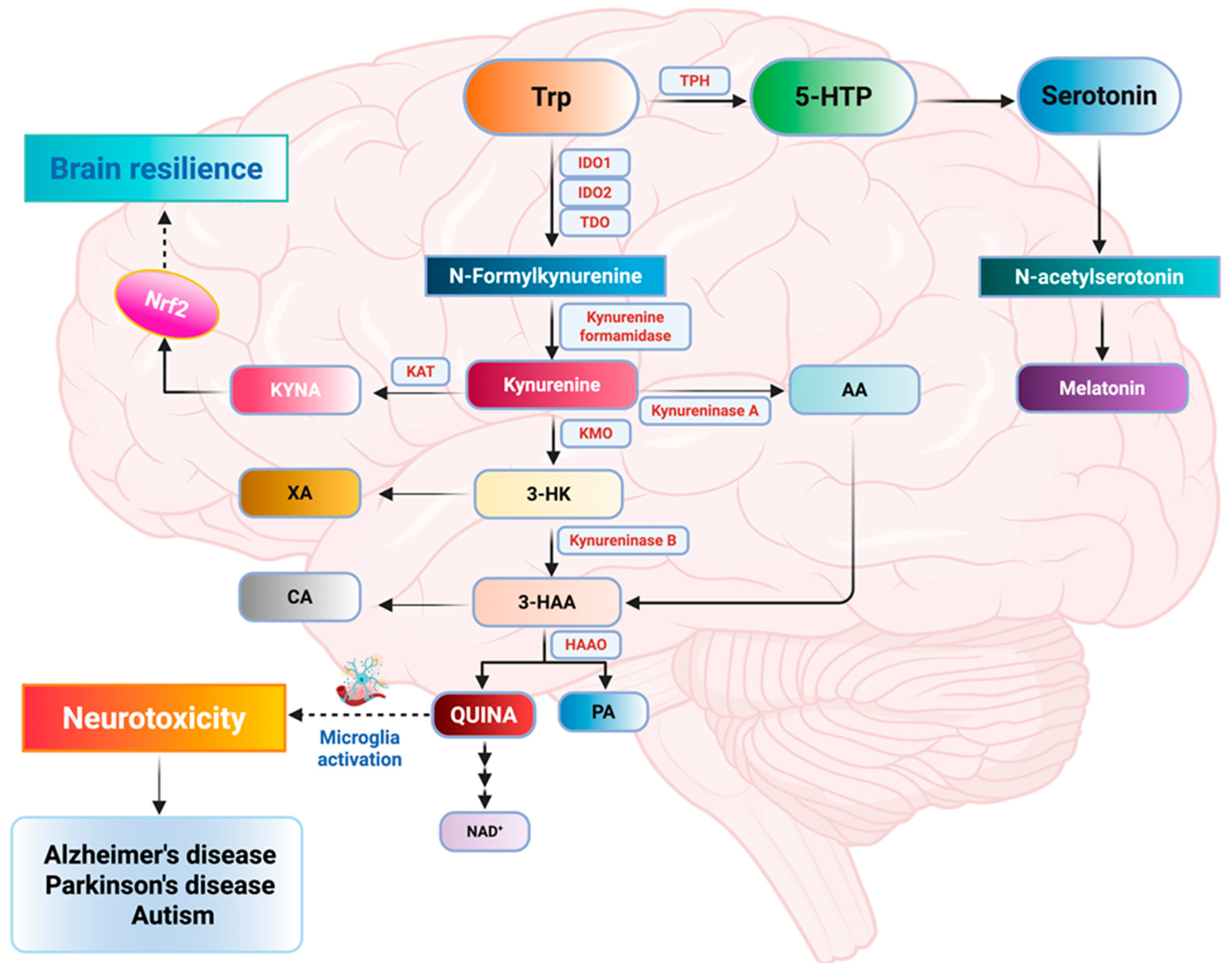
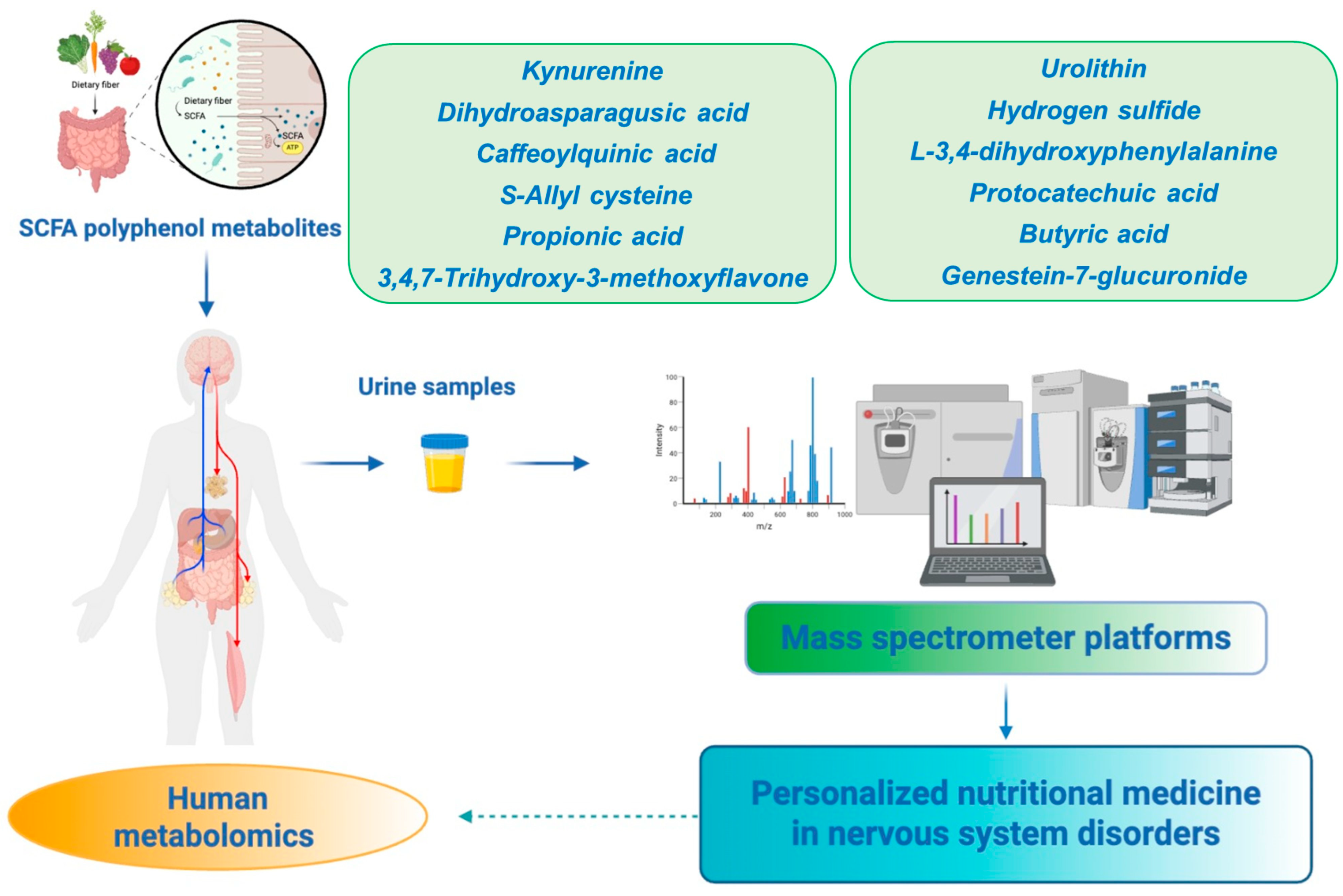
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scuto, M.C.; Anfuso, C.D.; Lombardo, C.; Di Fatta, E.; Ferri, R.; Musso, N.; Zerbo, G.; Terrana, M.; Majzúnová, M.; Lupo, G.; et al. Neuronutrition and Nrf2 Brain Resilience Signaling: Epigenomics and Metabolomics for Personalized Medicine in Nervous System Disorders from Bench to Clinic. Int. J. Mol. Sci. 2025, 26, 9391. https://doi.org/10.3390/ijms26199391
Scuto MC, Anfuso CD, Lombardo C, Di Fatta E, Ferri R, Musso N, Zerbo G, Terrana M, Majzúnová M, Lupo G, et al. Neuronutrition and Nrf2 Brain Resilience Signaling: Epigenomics and Metabolomics for Personalized Medicine in Nervous System Disorders from Bench to Clinic. International Journal of Molecular Sciences. 2025; 26(19):9391. https://doi.org/10.3390/ijms26199391
Chicago/Turabian StyleScuto, Maria Concetta, Carmelina Daniela Anfuso, Cinzia Lombardo, Eleonora Di Fatta, Raffaele Ferri, Nicolò Musso, Giulia Zerbo, Morena Terrana, Miroslava Majzúnová, Gabriella Lupo, and et al. 2025. "Neuronutrition and Nrf2 Brain Resilience Signaling: Epigenomics and Metabolomics for Personalized Medicine in Nervous System Disorders from Bench to Clinic" International Journal of Molecular Sciences 26, no. 19: 9391. https://doi.org/10.3390/ijms26199391
APA StyleScuto, M. C., Anfuso, C. D., Lombardo, C., Di Fatta, E., Ferri, R., Musso, N., Zerbo, G., Terrana, M., Majzúnová, M., Lupo, G., & Trovato Salinaro, A. (2025). Neuronutrition and Nrf2 Brain Resilience Signaling: Epigenomics and Metabolomics for Personalized Medicine in Nervous System Disorders from Bench to Clinic. International Journal of Molecular Sciences, 26(19), 9391. https://doi.org/10.3390/ijms26199391











