The Role of TRP Channels in Colitis and Inflammatory Bowel Disease: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Framework and Objectives
2.2. Literature Search Strategy
2.3. Study Selection Process
3. Results
3.1. TRP Channels and IBD in Humans
3.1.1. Effects of TRPA1 in Humans
3.1.2. Effects of TRPV1 in Humans
3.1.3. Effects of TRPV2 in Humans
3.1.4. Effects of TRPV3 in Humans
3.1.5. Effects of TRPV4 in Humans
3.1.6. Effects of TRPV5 in Humans
3.1.7. Effects of TRPV6 in Humans
3.1.8. Effects of TRPM2 in Humans
3.1.9. Effects of TRPC1 in Humans
3.2. TRP Channels and Colitis in Animal Models
3.2.1. Effects of TRPA1 in Animal Models of Colitis
3.2.2. Effects of TRPV1 in Animal Models of Colitis
3.2.3. Effects of TRPV2 in Animal Models of Colitis
3.2.4. Effects of TRPV4 in Animal Models of Colitis
3.2.5. Effects of TRPM2 in Animal Models of Colitis
3.2.6. Effects of TRPM3 in Animal Models of Colitis
3.2.7. Effects of TRPM8 in Animal Models of Colitis
3.2.8. Effects of TRPC6 in Animal Models of Colitis
4. Discussion
4.1. TRPA1
4.2. TRPV1-TRPV6
4.3. TRPM2, TRPM3, and TRPM8
4.4. TRPC1 and TRPC6
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TRP | Transient receptor potential channel |
| IBD | Inflammatory Bowel Disease |
| CD | Crohn’s disease |
| UC | Ulcerative colitis |
| GI | Gastrointestinal tract |
| IBS | Irritable bowel syndrome-like |
| VHS | Visceral hypersensitivity |
| PICO | Population, Intervention, Comparison, Outcome |
| PBMCs | Peripheral blood mononuclear cells |
| SP | Substance P |
| NKA | Neurokinin A |
| NKB | Neurokinin B |
| TNF-α | Tumor necrosis factor alpha |
| TRPV1 | Transient receptor potential vanilloid 1 |
| TRPV2 | Transient receptor potential vanilloid 2 |
| TRPV3 | Transient receptor potential vanilloid 3 |
| TRPV4 | Transient receptor potential vanilloid 4 |
| TRPV5 | Transient receptor potential vanilloid 5 |
| TRPV6 | Transient receptor potential vanilloid 6 |
| TRPA1 | Transient receptor potential ankyrin 1 |
| TRPM2 | Transient receptor potential melastatin 2 |
| TRPM3 | Transient receptor potential melastatin 3 |
| TRPM8 | Transient receptor potential melastatin 8 |
| TRPC1 | Transient receptor potential canonical 1 |
| TRPC6 | Transient receptor potential canonical 6 |
| IL | Interleukin |
| DAG | Diacylglycerol |
| CRP | C-reactive protein |
| 4αPDD | 4α-phorbol-12,13-didecanoate |
| DSS | Dextran Sulfate Sodium |
| DNBS | Dinitrobenzene sulfonic acid |
| TNBS | 2,4,6-Trinitrobenzene sulfonic acid |
| OM | Oil of mustard |
| CGRP | Calcitonin Gene-Related Peptide |
| AITC | Allyl isothiocyanate |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| TLR4 | Toll-like receptor 4 |
| SPF | Specific pathogen free status |
| ROS | Reactive oxygen species |
| 5-ASA | 5-aminosalicylic acid |
| GPRs | G protein-coupled receptors |
References
- Ungaro, R.; Colombel, J.-F.; Lissoos, T.; Peyrin-Biroulet, L. A Treat-to-Target Update in Ulcerative Colitis: A Systematic Review. Off. J. Am. Coll. Gastroenterol. ACG 2019, 114, 874. [Google Scholar] [CrossRef]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet 2017, 390, 2769–2778, Erratum in Lancet 2020, 396, e56. [Google Scholar] [CrossRef]
- Buie, M.J.; Quan, J.; Windsor, J.W.; Coward, S.; Hansen, T.M.; King, J.A.; Kotze, P.G.; Gearry, R.B.; Ng, S.C.; Mak, J.W.Y.; et al. Global Hospitalization Trends for Crohn’s Disease and Ulcerative Colitis in the 21st Century: A Systematic Review with Temporal Analyses. Clin. Gastroenterol. Hepatol. 2023, 21, 2211–2221. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Li, Y.-Y. Inflammatory Bowel Disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Mitsuyama, K.; Yamasaki, H.; Mori, A.; Yoshimura, T.; Araki, T.; Morita, M.; Tsuruta, K.; Yamasaki, S.; Kuwaki, K.; et al. Gene Expression of Transient Receptor Potential Channels in Peripheral Blood Mononuclear Cells of Inflammatory Bowel Disease Patients. J. Clin. Med. 2020, 9, 2643. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, J.; Zhu, M.; Mukherjee, A.; Zhang, H. Transient Receptor Potential Channels and Inflammatory Bowel Disease. Front. Immunol. 2020, 11, 180. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Armache, J.-P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the TRPA1 Ion Channel Suggests Regulatory Mechanisms. Nature 2015, 520, 511–517. [Google Scholar] [CrossRef]
- Iftinca, M.; Defaye, M.; Altier, C. TRPV1-Targeted Drugs in Development for Human Pain Conditions. Drugs 2021, 81, 7–27. [Google Scholar] [CrossRef]
- Wijst, J.; van der Goor, M.K.; van Schreuder, M.F.; Hoenderop, J.G. TRPV5 in Renal Tubular Calcium Handling and Its Potential Relevance for Nephrolithiasis. Kidney Int. 2019, 96, 1283–1291. [Google Scholar] [CrossRef]
- Fecher-Trost, C.; Wissenbach, U.; Weissgerber, P. TRPV6: From Identification to Function. Cell Calcium 2017, 67, 116–122. [Google Scholar] [CrossRef]
- Khattar, V.; Wang, L.; Peng, J.-B. Calcium Selective Channel TRPV6: Structure, Function, and Implications in Health and Disease. Gene 2022, 817, 146192. [Google Scholar] [CrossRef]
- Thiel, G.; Rubil, S.; Lesch, A.; Guethlein, L.A.; Rössler, O.G. Transient Receptor Potential TRPM3 Channels: Pharmacology, Signaling, and Biological Functions. Pharmacol. Res. 2017, 124, 92–99. [Google Scholar] [CrossRef]
- Held, K.; Voets, T.; Vriens, J. TRPM3 in Temperature Sensing and Beyond. Temp. Multidiscip. Biomed. J. 2015, 2, 201–213. [Google Scholar] [CrossRef]
- Thiel, G.; Rössler, O.G. Stimulus-Transcription Coupling of TRPM3 Channels: A Signaling Pathway from the Plasma Membrane to the Nucleus. Biomolecules 2025, 15, 521. [Google Scholar] [CrossRef]
- Fouad, A.; Matsumoto, K.; Amagase, K.; Yasuda, H.; Tominaga, M.; Kato, S. Protective Effect of TRPM8 against Indomethacin-Induced Small Intestinal Injury via the Release of Calcitonin Gene-Related Peptide in Mice. Biol. Pharm. Bull. 2021, 44, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Sun, Y.; Sukumaran, P.; Quenum Zangbede, F.O.; Jondle, C.N.; Sharma, A.; Evans, D.L.; Chauhan, P.; Szlabick, R.E.; Aaland, M.O.; et al. M1 Macrophage Polarization Is Dependent on TRPC1-Mediated Calcium Entry. iScience 2018, 8, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Medic, N.; Desai, A.; Olivera, A.; Abramowitz, J.; Birnbaumer, L.; Beaven, M.A.; Gilfillan, A.M.; Metcalfe, D.D. Knockout of the Trpc1 Gene Reveals That TRPC1 Can Promote Recovery from Anaphylaxis by Negatively Regulating Mast Cell TNF-α Production. Cell Calcium 2013, 53, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. Transient Receptor Potential (TRP) Channels as Drug Targets for Diseases of the Digestive System. Pharmacol. Ther. 2011, 131, 142–170. [Google Scholar] [CrossRef]
- Khalil, M.; Alliger, K.; Weidinger, C.; Yerinde, C.; Wirtz, S.; Becker, C.; Engel, M.A. Functional Role of Transient Receptor Potential Channels in Immune Cells and Epithelia. Front. Immunol. 2018, 9, 174. [Google Scholar] [CrossRef]
- Zheng, J. Molecular Mechanism of TRP Channels. Compr. Physiol. 2013, 3, 221–242. [Google Scholar] [CrossRef]
- Engel, M.A.; Becker, C.; Reeh, P.W.; Neurath, M.F. Role of Sensory Neurons in Colitis: Increasing Evidence for a Neuroimmune Link in the Gut. Inflamm. Bowel Dis. 2011, 17, 1030–1033. [Google Scholar] [CrossRef]
- Kun, J.; Szitter, I.; Kemény, A.; Perkecz, A.; Kereskai, L.; Pohóczky, K.; Vincze, A.; Gódi, S.; Szabó, I.; Szolcsányi, J.; et al. Upregulation of the Transient Receptor Potential Ankyrin 1 Ion Channel in the Inflamed Human and Mouse Colon and Its Protective Roles. PLoS ONE 2014, 9, e108164. [Google Scholar] [CrossRef] [PubMed]
- Gombert, S.; Rhein, M.; Winterpacht, A.; Münster, T.; Hillemacher, T.; Leffler, A.; Frieling, H. Transient Receptor Potential Ankyrin 1 Promoter Methylation and Peripheral Pain Sensitivity in Crohn’s Disease. Clin. Epigenet. 2019, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Utsumi, D.; Matsumoto, K.; Tsukahara, T.; Amagase, K.; Tominaga, M.; Kato, S. Transient Receptor Potential Vanilloid 1 and Transient Receptor Potential Ankyrin 1 Contribute to the Progression of Colonic Inflammation in Dextran Sulfate Sodium-Induced Colitis in Mice: Links to Calcitonin Gene-Related Peptide and Substance P. J. Pharmacol. Sci. 2018, 136, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, W.; De Man, J.G.; De Schepper, H.U.; Bult, H.; Moreels, T.G.; Pelckmans, P.A.; De Winter, B.Y. Role of TRPV1 and TRPA1 in Visceral Hypersensitivity to Colorectal Distension during Experimental Colitis in Rats. Eur. J. Pharmacol. 2013, 698, 404–412. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, V.; Devi, K.; Kumar, A.; Khan, R.; Singh, R.P.; Rajarammohan, S.; Kondepudi, K.K.; Chopra, K.; Bishnoi, M. Intrarectal Capsazepine Administration Modulates Colonic Mucosal Health in Mice. Int. J. Mol. Sci. 2022, 23, 9577. [Google Scholar] [CrossRef]
- Kistner, K.; Siklosi, N.; Babes, A.; Khalil, M.; Selescu, T.; Zimmermann, K.; Wirtz, S.; Becker, C.; Neurath, M.F.; Reeh, P.W.; et al. Systemic Desensitization through TRPA1 Channels by Capsazepine and Mustard Oil—A Novel Strategy against Inflammation and Pain. Sci. Rep. 2016, 6, 28621. [Google Scholar] [CrossRef]
- Mitrovic, M.; Shahbazian, A.; Bock, E.; Pabst, M.A.; Holzer, P. Chemo-Nociceptive Signalling from the Colon Is Enhanced by Mild Colitis and Blocked by Inhibition of Transient Receptor Potential Ankyrin 1 Channels. Br. J. Pharmacol. 2010, 160, 1430–1442. [Google Scholar] [CrossRef]
- Jain, P.; Materazzi, S.; De Logu, F.; Rossi Degl’Innocenti, D.; Fusi, C.; Li Puma, S.; Marone, I.M.; Coppi, E.; Holzer, P.; Geppetti, P.; et al. Transient Receptor Potential Ankyrin 1 Contributes to Somatic Pain Hypersensitivity in Experimental Colitis. Sci. Rep. 2020, 10, 8632. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Kobayashi, K.; Hao, Y.; Kanda, H.; Kondo, T.; Kogure, Y.; Yamanaka, H.; Yamamoto, S.; Li, J.; et al. TRPA1-Expressing Lamina Propria Mesenchymal Cells Regulate Colonic Motility. JCI Insight 2019, 4, e122402. [Google Scholar] [CrossRef]
- Chen, J.; Winston, J.H.; Sarna, S.K. Neurological and Cellular Regulation of Visceral Hypersensitivity Induced by Chronic Stress and Colonic Inflammation in Rats. Neuroscience 2013, 248, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Kurahara, L.H.; Hiraishi, K.; Hu, Y.; Koga, K.; Onitsuka, M.; Doi, M.; Aoyagi, K.; Takedatsu, H.; Kojima, D.; Fujihara, Y.; et al. Activation of Myofibroblast TRPA1 by Steroids and Pirfenidone Ameliorates Fibrosis in Experimental Crohn’s Disease. Cell Mol. Gastroenterol. Hepatol. 2018, 5, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guo, C.-H.; Chowdhury, M.A.; Dai, T.-L.; Han, W. TRPA1 in the Spinal Dorsal Horn Is Involved in Post-Inflammatory Visceral Hypersensitivity: In Vivo Study Using TNBS-Treated Rat Model. J. Pain. Res. 2016, 9, 1153–1160. [Google Scholar] [CrossRef]
- Brierley, S.M.; Hughes, P.A.; Page, A.J.; Kwan, K.Y.; Martin, C.M.; O’Donnell, T.A.; Cooper, N.J.; Harrington, A.M.; Adam, B.; Liebregts, T.; et al. The Ion Channel TRPA1 Is Required for Normal Mechanosensation and Is Modulated by Algesic Stimuli. Gastroenterology 2009, 137, 2084–2095.e3. [Google Scholar] [CrossRef]
- Cattaruzza, F.; Spreadbury, I.; Miranda-Morales, M.; Grady, E.F.; Vanner, S.; Bunnett, N.W. Transient Receptor Potential Ankyrin-1 Has a Major Role in Mediating Visceral Pain in Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G81–G91. [Google Scholar] [CrossRef]
- Kimball, E.S.; Prouty, S.P.; Pavlick, K.P.; Wallace, N.H.; Schneider, C.R.; Hornby, P.J. Stimulation of Neuronal Receptors, Neuropeptides and Cytokines during Experimental Oil of Mustard Colitis. Neurogastroenterol. Motil. 2007, 19, 390–400. [Google Scholar] [CrossRef]
- Duo, L.; Wu, T.; Ke, Z.; Hu, L.; Wang, C.; Teng, G.; Zhang, W.; Wang, W.; Ge, Q.; Yang, Y.; et al. Gain of Function of Ion Channel TRPV1 Exacerbates Experimental Colitis by Promoting Dendritic Cell Activation. Mol. Ther. Nucleic Acids 2020, 22, 924–936. [Google Scholar] [CrossRef]
- Fichna, J.; Mokrowiecka, A.; Cygankiewicz, A.I.; Zakrzewski, P.K.; Małecka-Panas, E.; Janecka, A.; Krajewska, W.M.; Storr, M.A. Transient Receptor Potential Vanilloid 4 Blockade Protects against Experimental Colitis in Mice: A New Strategy for Inflammatory Bowel Diseases Treatment? Neurogastroenterol. Motil. 2012, 24, e557–e560. [Google Scholar] [CrossRef]
- D’Aldebert, E.; Cenac, N.; Rousset, P.; Martin, L.; Rolland, C.; Chapman, K.; Selves, J.; Alric, L.; Vinel, J.-P.; Vergnolle, N. Transient Receptor Potential Vanilloid 4 Activated Inflammatory Signals by Intestinal Epithelial Cells and Colitis in Mice. Gastroenterology 2011, 140, 275–285.e3. [Google Scholar] [CrossRef]
- Rizopoulos, T.; Papadaki-Petrou, H.; Assimakopoulou, M. Expression Profiling of the Transient Receptor Potential Vanilloid (TRPV) Channels 1, 2, 3 and 4 in Mucosal Epithelium of Human Ulcerative Colitis. Cells 2018, 7, 61. [Google Scholar] [CrossRef]
- Toledo Mauriño, J.J.; Fonseca-Camarillo, G.; Furuzawa-Carballeda, J.; Barreto-Zuñiga, R.; Martínez Benítez, B.; Granados, J.; Yamamoto-Furusho, J.K. TRPV Subfamily (TRPV2, TRPV3, TRPV4, TRPV5, and TRPV6) Gene and Protein Expression in Patients with Ulcerative Colitis. J. Immunol. Res. 2020, 2020, 2906845. [Google Scholar] [CrossRef]
- Toledo-Mauriño, J.J.; Furuzawa-Carballeda, J.; Villeda-Ramírez, M.A.; Fonseca-Camarillo, G.; Meza-Guillen, D.; Barreto-Zúñiga, R.; Yamamoto-Furusho, J.K. The Transient Receptor Potential Vanilloid 1 Is Associated with Active Inflammation in Ulcerative Colitis. Mediat. Inflamm. 2018, 2018, 6570371. [Google Scholar] [CrossRef]
- Akbar, A.; Yiangou, Y.; Facer, P.; Brydon, W.G.; Walters, J.R.F.; Anand, P.; Ghosh, S. Expression of the TRPV1 Receptor Differs in Quiescent Inflammatory Bowel Disease with or without Abdominal Pain. Gut 2010, 59, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Wang, Z.; Mu, J.; Zhu, M.; Zhen, Y.; Zhang, H. Upregulation of the Transient Receptor Potential Vanilloid 1 in Colonic Epithelium of Patients with Active Inflammatory Bowel Disease. Int. J. Clin. Exp. Pathol. 2017, 10, 11335–11344. [Google Scholar] [PubMed]
- Matsumoto, K.; Sugimoto, F.; Mizuno, T.; Hayashi, T.; Okamura, R.; Nishioka, T.; Yasuda, H.; Horie, S.; Kido, M.A.; Kato, S. Immunohistochemical Characterization of Transient Receptor Potential Vanilloid Types 2 and 1 in a Trinitrobenzene Sulfonic Acid-Induced Rat Colitis Model with Visceral Hypersensitivity. Cell Tissue Res. 2023, 391, 287–303. [Google Scholar] [CrossRef]
- Lapointe, T.K.; Basso, L.; Iftinca, M.C.; Flynn, R.; Chapman, K.; Dietrich, G.; Vergnolle, N.; Altier, C. TRPV1 Sensitization Mediates Postinflammatory Visceral Pain Following Acute Colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G87–G99. [Google Scholar] [CrossRef] [PubMed]
- Engel, M.A.; Khalil, M.; Mueller-Tribbensee, S.M.; Becker, C.; Neuhuber, W.L.; Neurath, M.F.; Reeh, P.W. The Proximodistal Aggravation of Colitis Depends on Substance P Released from TRPV1-Expressing Sensory Neurons. J. Gastroenterol. 2012, 47, 256–265. [Google Scholar] [CrossRef]
- Matsumoto, K.; Lo, M.W.; Hosoya, T.; Tashima, K.; Takayama, H.; Murayama, T.; Horie, S. Experimental Colitis Alters Expression of 5-HT Receptors and Transient Receptor Potential Vanilloid 1 Leading to Visceral Hypersensitivity in Mice. Lab. Investig. 2012, 92, 769–782. [Google Scholar] [CrossRef]
- Massa, F.; Sibaev, A.; Marsicano, G.; Blaudzun, H.; Storr, M.; Lutz, B. Vanilloid Receptor (TRPV1)-Deficient Mice Show Increased Susceptibility to Dinitrobenzene Sulfonic Acid Induced Colitis. J. Mol. Med. 2006, 84, 142–146. [Google Scholar] [CrossRef]
- Mazor, Y.; Engelmayer, N.; Nashashibi, H.; Rottenfußer, L.; Lev, S.; Binshtok, A.M. Attenuation of Colitis-Induced Visceral Hypersensitivity and Pain by Selective Silencing of TRPV1-Expressing Fibers in Rat Colon. Inflamm. Bowel Dis. 2024, 30, 1843–1851. [Google Scholar] [CrossRef]
- De Schepper, H.U.; De Man, J.G.; Ruyssers, N.E.; Deiteren, A.; Van Nassauw, L.; Timmermans, J.-P.; Martinet, W.; Herman, A.G.; Pelckmans, P.A.; De Winter, B.Y. TRPV1 Receptor Signaling Mediates Afferent Nerve Sensitization during Colitis-Induced Motility Disorders in Rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G245–G253. [Google Scholar] [CrossRef]
- De Schepper, H.U.; De Winter, B.Y.; Van Nassauw, L.; Timmermans, J.-P.; Herman, A.G.; Pelckmans, P.A.; De Man, J.G. TRPV1 Receptors on Unmyelinated C-Fibres Mediate Colitis-Induced Sensitization of Pelvic Afferent Nerve Fibres in Rats. J. Physiol. 2008, 586, 5247–5258. [Google Scholar] [CrossRef]
- Miranda, A.; Nordstrom, E.; Mannem, A.; Smith, C.; Banerjee, B.; Sengupta, J.N. The Role of Transient Receptor Potential Vanilloid 1 in Mechanical and Chemical Visceral Hyperalgesia Following Experimental Colitis. Neuroscience 2007, 148, 1021–1032. [Google Scholar] [CrossRef]
- Shen, S.; Al-Thumairy, H.W.; Hashmi, F.; Qiao, L.-Y. Regulation of Transient Receptor Potential Cation Channel Subfamily V1 Protein Synthesis by the Phosphoinositide 3-Kinase/Akt Pathway in Colonic Hypersensitivity. Exp. Neurol. 2017, 295, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Wang, J.; Fan, Q.; Zhu, J.; Yang, L.; Rong, W. TLR4 Mediates Upregulation and Sensitization of TRPV1 in Primary Afferent Neurons in 2,4,6-Trinitrobenzene Sulfate-Induced Colitis. Mol. Pain. 2019, 15, 1744806919830018. [Google Scholar] [CrossRef]
- Lee, J.; Yamamoto, T.; Kuramoto, H.; Kadowaki, M. TRPV1 Expressing Extrinsic Primary Sensory Neurons Play a Protective Role in Mouse Oxazolone-Induced Colitis. Auton. Neurosci. 2012, 166, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Issa, C.M.; Hambly, B.D.; Wang, Y.; Maleki, S.; Wang, W.; Fei, J.; Bao, S. TRPV2 in the Development of Experimental Colitis. Scand. J. Immunol. 2014, 80, 307–312. [Google Scholar] [CrossRef]
- Matsumoto, K.; Yamaba, R.; Inoue, K.; Utsumi, D.; Tsukahara, T.; Amagase, K.; Tominaga, M.; Kato, S. Transient Receptor Potential Vanilloid 4 Channel Regulates Vascular Endothelial Permeability during Colonic Inflammation in Dextran Sulphate Sodium-Induced Murine Colitis. Br. J. Pharmacol. 2018, 175, 84–99. [Google Scholar] [CrossRef]
- Matsumoto, K.; Takagi, K.; Kato, A.; Ishibashi, T.; Mori, Y.; Tashima, K.; Mitsumoto, A.; Kato, S.; Horie, S. Role of Transient Receptor Potential Melastatin 2 (TRPM2) Channels in Visceral Nociception and Hypersensitivity. Exp. Neurol. 2016, 285, 41–50. [Google Scholar] [CrossRef]
- Yamamoto, S.; Shimizu, S.; Kiyonaka, S.; Takahashi, N.; Wajima, T.; Hara, Y.; Negoro, T.; Hiroi, T.; Kiuchi, Y.; Okada, T.; et al. TRPM2-Mediated Ca2+ Influx Induces Chemokine Production in Monocytes That Aggravates Inflammatory Neutrophil Infiltration. Nat. Med. 2008, 14, 738–747. [Google Scholar] [CrossRef]
- Nakamoto, T.; Matsumoto, K.; Yasuda, H.; Mori, Y.; Kato, S. Transient Receptor Potential Melastatin 2 Is Involved in Trinitrobenzene Sulfonic Acid-Induced Acute and Chronic Colitis-Associated Fibrosis Progression in Mice. J. Pharmacol. Sci. 2024, 154, 18–29. [Google Scholar] [CrossRef] [PubMed]
- King, J.W.; Bennett, A.S.W.; Wood, H.M.; Baker, C.C.; Alsaadi, H.; Topley, M.; Vanner, S.A.; Reed, D.E.; Lomax, A.E. Expression and Function of Transient Receptor Potential Melastatin 3 in the Spinal Afferent Innervation of the Mouse Colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2024, 326, G176–G186. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, T.; Matsumoto, K.; Tashima, K.; Nakamura, H.; Fujino, H.; Murayama, T.; Horie, S. TRPM8 Has a Key Role in Experimental Colitis-Induced Visceral Hyperalgesia in Mice. Neurogastroenterol. Motil. 2014, 26, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Hyun, E.; Zhao, L.; Lapointe, T.K.; Chapman, K.; Hirota, C.L.; Ghosh, S.; McKemy, D.D.; Vergnolle, N.; Beck, P.L.; et al. TRPM8 Activation Attenuates Inflammatory Responses in Mouse Models of Colitis. Proc. Natl. Acad. Sci. USA 2013, 110, 7476–7481. [Google Scholar] [CrossRef]
- de Jong, P.R.; Takahashi, N.; Peiris, M.; Bertin, S.; Lee, J.; Gareau, M.G.; Paniagua, A.; Harris, A.R.; Herdman, D.S.; Corr, M.; et al. TRPM8 on Mucosal Sensory Nerves Regulates Colitogenic Responses by Innate Immune Cells via CGRP. Mucosal Immunol. 2015, 8, 491–504. [Google Scholar] [CrossRef]
- Khalil, M.; Babes, A.; Lakra, R.; Försch, S.; Reeh, P.W.; Wirtz, S.; Becker, C.; Neurath, M.F.; Engel, M.A. Transient Receptor Potential Melastatin 8 Ion Channel in Macrophages Modulates Colitis through a Balance-Shift in TNF-Alpha and Interleukin-10 Production. Mucosal Immunol. 2016, 9, 1500–1513. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, X.; Kang, L.; Leng, Z.; Ji, Y.; Yang, S.; Du, X.; Fang, K.; Wang, Z.; Li, Z.; et al. TRPM8 Inhibits Substance P Release from Primary Sensory Neurons via PKA/GSK-3beta to Protect Colonic Epithelium in Colitis. Cell Death Dis. 2024, 15, 91. [Google Scholar] [CrossRef]
- Nishiyama, K.; Kato, Y.; Nishimura, A.; Mi, X.; Nagata, R.; Mori, Y.; Azuma, Y.-T.; Nishida, M. Pharmacological Activation of TRPC6 Channel Prevents Colitis Progression. Int. J. Mol. Sci. 2024, 25, 2401. [Google Scholar] [CrossRef]
- Talavera, K.; Startek, J.B.; Alvarez-Collazo, J.; Boonen, B.; Alpizar, Y.A.; Sanchez, A.; Naert, R.; Nilius, B. Mammalian Transient Receptor Potential TRPA1 Channels: From Structure to Disease. Physiol. Rev. 2020, 100, 725–803. [Google Scholar] [CrossRef]
- Stewart, J.M. TRPV6 as A Target for Cancer Therapy. J. Cancer 2020, 11, 374–387. [Google Scholar] [CrossRef]
- Rohacs, T.; Fluck, E.C.; Jesús-Pérez, J.J.D.; Moiseenkova-Bell, V.Y. What Structures Did, and Did Not, Reveal about the Function of the Epithelial Ca2+ Channels TRPV5 and TRPV6. Cell Calcium 2022, 106, 102620. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.A. TRPM2 in Cancer. Cell Calcium 2019, 80, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, Z.; Zhao, H.; Qiu, M.; Wen, Y.; Yao, X.; Tang, W.H. Identification of TRPM2 as a Marker Associated with Prognosis and Immune Infiltration in Kidney Renal Clear Cell Carcinoma. Front. Mol. Biosci. 2022, 8, 774905. [Google Scholar] [CrossRef]
- Zhao, C.; MacKinnon, R. Structural and Functional Analyses of a GPCR-Inhibited Ion Channel TRPM3. Neuron 2023, 111, 81–91.e7. [Google Scholar] [CrossRef]
- Wu, Z.; Peng, S.; Huang, W.; Zhang, Y.; Liu, Y.; Yu, X.; Shen, L. The Role and Function of TRPM8 in the Digestive System. Biomolecules 2024, 14, 877. [Google Scholar] [CrossRef]
- Li, H. TRP Channel Classification. Adv. Exp. Med. Biol. 2017, 976, 1–8. [Google Scholar] [CrossRef]
- Hori, A.; Fukazawa, A.; Katanosaka, K.; Mizuno, M.; Hotta, N. Mechanosensitive Channels in the Mechanical Component of the Exercise Pressor Reflex. Auton. Neurosci. 2023, 250, 103128. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, X.; Tian, J.; Xiao, Y.; Tian, T.; Xu, F.; Hong, X.; Zhu, M.X. TRPC Channels: Structure, Function, Regulation and Recent Advances in Small Molecular Probes. Pharmacol. Ther. 2020, 209, 107497. [Google Scholar] [CrossRef]
- Liu, Y.; Lyu, Y.; Wang, H. TRP Channels as Molecular Targets to Relieve Endocrine-Related Diseases. Front. Mol. Biosci. 2022, 9, 895814. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated Guidance for Trusted Systematic Reviews: A New Edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 2019, ED000142. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; deBeer, H.; et al. GRADE Guidelines: 1. Introduction—GRADE Evidence Profiles and Summary of Findings Tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s Risk of Bias Tool for Animal Studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Mohan, G.H.; Shwetha Reddy, R.V.; Yogesh, C. Management of Specific Pathogen-Free (SPF) Mice and Rats. In Essentials of Laboratory Animal Science: Principles and Practices; Nagarajan, P., Gudde, R., Srinivasan, R., Eds.; Springer: Singapore, 2021; pp. 633–653. ISBN 9789811609879. [Google Scholar]
- Bondareva, E.D.; Barcheva, A.V.; Bolotova, V.T.; Vasyutina, M.L.; Kildibekov, K.Y.; Kovaleva, M.A.; Konovalova, G.V.; Lobova, P.S.; Lovat, M.L.; Loginova, M.V.; et al. Development of a quality identification system for laboratory animals. Identification of the level of barriers (Post 1). Lab. Anim. Sci. 2025, 1, 4–20. [Google Scholar] [CrossRef]
- Csekő, K.; Beckers, B.; Keszthelyi, D.; Helyes, Z. Role of TRPV1 and TRPA1 Ion Channels in Inflammatory Bowel Diseases: Potential Therapeutic Targets? Pharmaceuticals 2019, 12, 48. [Google Scholar] [CrossRef]
- Bertin, S.; Aoki-Nonaka, Y.; Lee, J.; de Jong, P.R.; Kim, P.; Han, T.; Yu, T.; To, K.; Takahashi, N.; Boland, B.S.; et al. The TRPA1 Ion Channel Is Expressed in CD4+ T Cells and Restrains T-Cell-Mediated Colitis through Inhibition of TRPV1. Gut 2017, 66, 1584–1596. [Google Scholar] [CrossRef]
- Landini, L.; de Araujo, D.S.M.; Titiz, M.; Geppetti, P.; Nassini, R.; De Logu, F. TRPA1 Role in Inflammatory Disorders: What Is Known So Far? Int. J. Mol. Sci. 2022, 23, 4529. [Google Scholar] [CrossRef]
- Rahman, M.M.; Jo, Y.-Y.; Kim, Y.H.; Park, C.-K. Current Insights and Therapeutic Strategies for Targeting TRPV1 in Neuropathic Pain Management. Life Sci. 2024, 355, 122954. [Google Scholar] [CrossRef]
- Do, N.; Zuo, D.; Kim, M.; Kim, M.; Ha, H.-J.; Blumberg, P.M.; Ann, J.; Hwang, S.W.; Lee, J. Discovery of Dual TRPA1 and TRPV1 Antagonists as Novel Therapeutic Agents for Pain. Pharmaceuticals 2024, 17, 1209. [Google Scholar] [CrossRef]
- Fricke, T.C.; Leffler, A. TRPV2: A Universal Regulator in Cellular Physiology with a yet Poorly Defined Thermosensitivity. J. Physiol. Sci. 2024, 74, 42. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Nizamuddin, P.B.; Uddin, S.; Al-Thani, M.; Frenneaux, M.P.; Janahi, I.A.; Steinhoff, M.; Azizi, F. TRPV2: A Cancer Biomarker and Potential Therapeutic Target. Dis. Markers 2020, 2020, 8892312. [Google Scholar] [CrossRef] [PubMed]
- Py, B.F.; Jin, M.; Desai, B.N.; Penumaka, A.; Zhu, H.; Kober, M.; Dietrich, A.; Lipinski, M.M.; Henry, T.; Clapham, D.E.; et al. Caspase-11 Controls Interleukin-1β Release through Degradation of TRPC1. Cell Rep. 2014, 6, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Zergane, M.; Kuebler, W.M.; Michalick, L. Heteromeric TRP Channels in Lung Inflammation. Cells 2021, 10, 1654. [Google Scholar] [CrossRef]
- Da Conceicao, V.N.; Sun, Y.; Ramachandran, K.; Chauhan, A.; Raveendran, A.; Venkatesan, M.; DeKumar, B.; Maity, S.; Vishnu, N.; Kotsakis, G.A.; et al. Resolving Macrophage Polarization through Distinct Ca2+ Entry Channel That Maintains Intracellular Signaling and Mitochondrial Bioenergetics. iScience 2021, 24, 103339. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, S.; Chu, Y.; Zhang, Y.; Liu, Y.; Yu, F.; Feng, L.; Zhu, Y. Loss of Endothelial TRPC1 Aggravates Metabolic Dysfunction in Obesity via Disrupting Adipose Tissue Homeostasis. Front. Mol. Biosci. 2025, 12, 1619559. [Google Scholar] [CrossRef]
- Shin, S.; Gombedza, F.C.; Awuah Boadi, E.; Yiu, A.J.; Roy, S.K.; Bandyopadhyay, B.C. Reduction of TRPC1/TRPC3 Mediated Ca2+-Signaling Protects Oxidative Stress-Induced COPD. Cell. Signal. 2023, 107, 110681. [Google Scholar] [CrossRef]
- Tang, N.; Tian, W.; Ma, G.-Y.; Xiao, X.; Zhou, L.; Li, Z.-Z.; Liu, X.-X.; Li, C.-Y.; Wu, K.-H.; Liu, W.; et al. TRPC Channels Blockade Abolishes Endotoxemic Cardiac Dysfunction by Hampering Intracellular Inflammation and Ca2+ Leakage. Nat. Commun. 2022, 13, 7455. [Google Scholar] [CrossRef]
- Chen, X.; Sooch, G.; Demaree, I.S.; White, F.A.; Obukhov, A.G. Transient Receptor Potential Canonical (TRPC) Channels: Then and Now. Cells 2020, 9, 1983. [Google Scholar] [CrossRef]
- Eide, D.J. The Oxidative Stress of Zinc Deficiency. Metallomics 2011, 3, 1124–1129. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and Its Role in Immunity and Inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef]

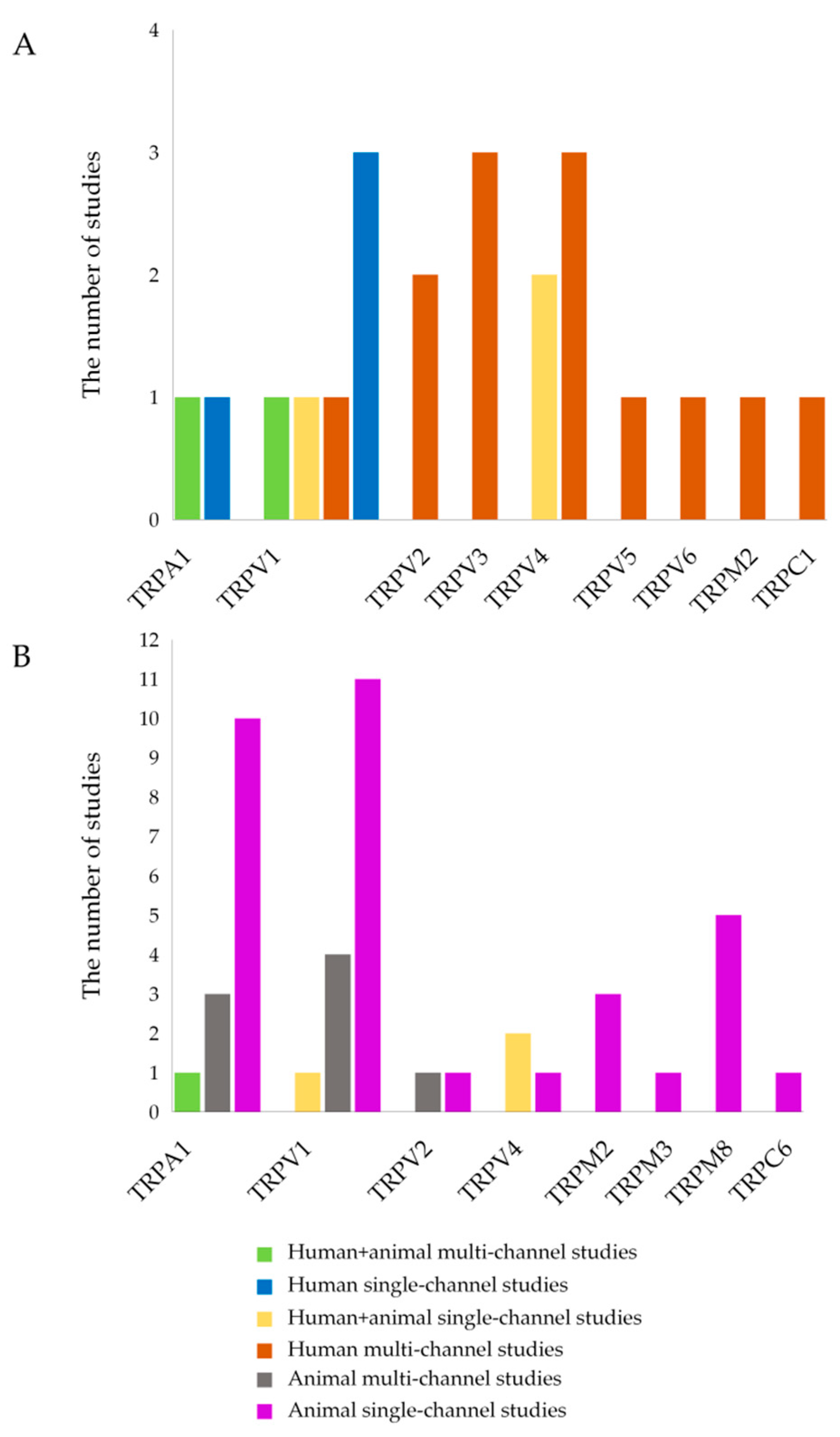
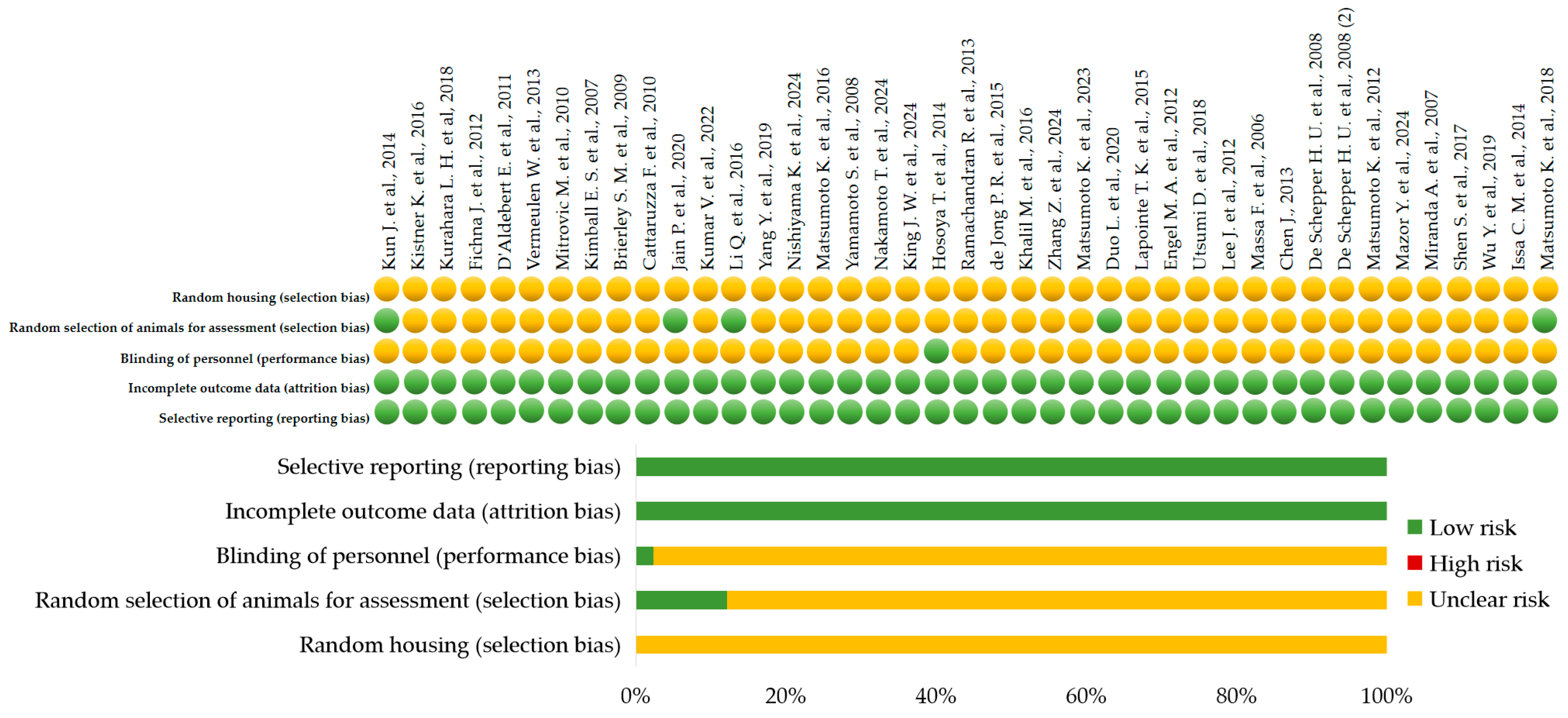
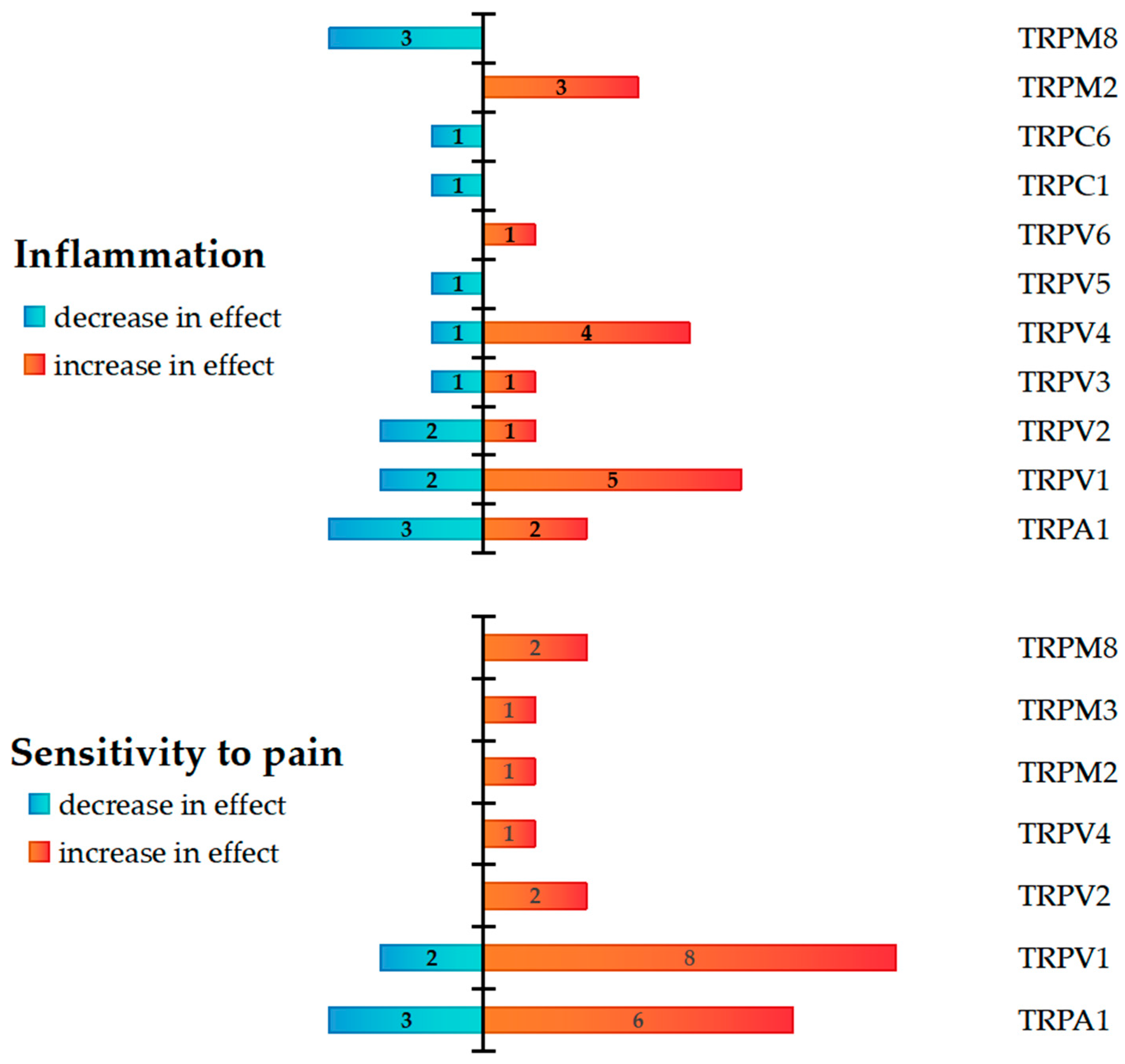

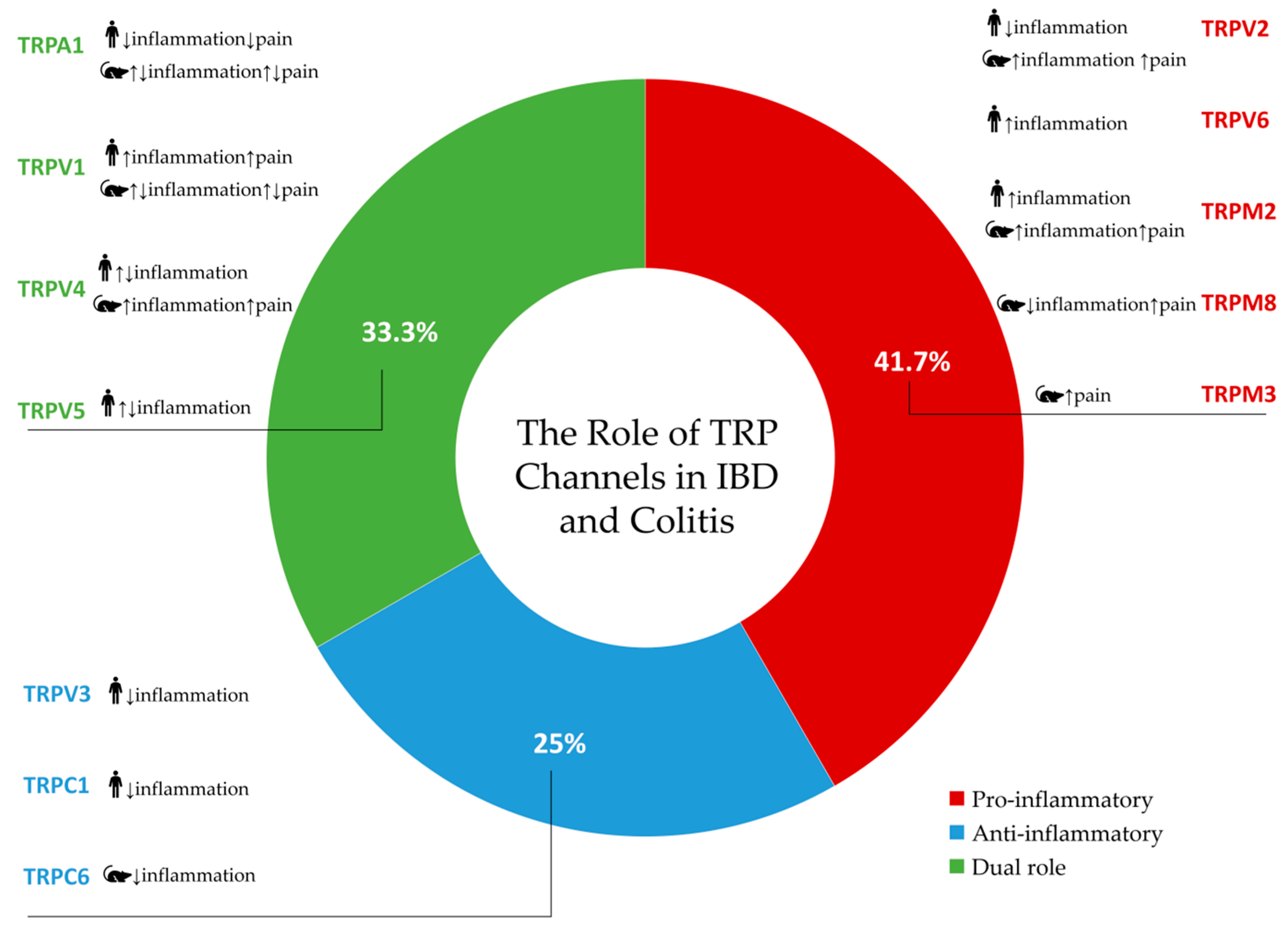
| TRP Channel | Category of Studies | Author, Year | Confirmed and/or Expected Effects | Conclusion |
|---|---|---|---|---|
| TRPA1 | Human + animal multi-channel studies | Kun J. et al., 2014 [22] | TRPA1 expression is significantly increased in patients with active IBD, but not inactive IBD, compared with non-inflamed samples | Anti-inflammatory role in active IBD and reduction in pain sensitivity |
| Human single-channel studies | Gombert S. et al., 2019 [23] | Increased TRPA1 promoter methylation correlates with dysregulated TRPA1 expression and enhanced peripheral pain sensitivity in CD patients | ||
| TRPV1 | Human + animal multi-channel studies | Kun J. et al., 2014 [22] | TRPV1 mRNA is significantly decreased in patients with active IBD compared to non-inflamed group | Mainly pro-inflammatory role and increased pain sensitivity |
| Human single-channel studies | Luo C. et al., 2017 [44] | TRPV1 immunoreactivity was highly expressed on epithelial cells and infiltrating inflammatory cells in colon biopsies from patients with active IBD | ||
| Human single-channel studies | Akbar A. et al., 2010 [43] | Abdominal pain and visceral hypersensitivity | ||
| Human single-channel studies | Toledo-Mauriño et al., 2018 [42] | Increased TRPV1 gene expression in remission UC patients compared to active UC patients; higher TRPV1 protein expression observed in all intestinal layers of active UC patients compared to non-IBD controls | ||
| Human multi-channel studies | Rizopoulos T., 2018 [40] | Statistically decreased TRPV1 expression levels were demonstrated for patients with active UC compared to the control group | ||
| Human + animal single-channel studies | Duo L. et al., 2020 [37] | TRPV1 is highly expressed in patients with IBD | ||
| TRPV2 | Human multi-channel studies | Morita T. et al., 2020 [5] | TRPV2 mRNA expression was negatively correlated with leukocyte count in UC; decreased TRPV2 mRNA expression levels in PBMCs of both UC and CD patients which negatively correlated with disease activity in both groups, suggesting a potential role in modulating inflammation | Anti-inflammatory role |
| Human multi-channel studies | Toledo Mauriño et al., 2020 [41] | The inner layers of the intestine had increased expression of TRPV2; TRPV2 gene expression was lower in samples of active and remission UC patients compared to control group; TRPV2 protein expression was upregulated in the mucosa and submucosa but lower in the muscular layer and serosa cells of controls compared to UC patients | ||
| TRPV3 | Human multi-channel studies | Morita T. et al., 2020 [5] | TRPV3 expression was reduced in patients with CD | Anti-inflammatory role |
| Human multi-channel studies | Rizopoulos T., 2018 [40] | No significant difference for TRPV3 expression levels between UC and control samples | ||
| Human multi-channel studies | Toledo Mauriño et al., 2020 [41] | TRPV3 gene and protein expression was higher in controls than in active UC patients, suggesting downregulated TRPV3 expression to be associated with disease activity | ||
| TRPV4 | Human multi-channel studies | Morita T. et al., 2020 [5] | Heightened TRPV4 mRNA expression levels in PBMCs of CD patients compared to healthy controls, positive correlation of its mRNA expression with the serum albumin level in the UC group and with the CRP level in the CD group | Pro- and anti-inflammatory roles |
| Human + animal single-channel studies | Fichna J. et al., 2012 [38] | TRPV4 mRNA expression was significantly elevated in patients with CD and UC compared with healthy subjects (2.9 and 4.5-fold, respectively) | ||
| Human multi-channel studies | Rizopoulos T., 2018 [40] | TRPV4 expression levels were significantly increased in the colonic epithelium of UC patients compared to non-IBD controls | ||
| Human multi-channel studies | Toledo Mauriño et al., 2020 [41] | TRPV4 expression in UC patients in remission and control groups was increased compared to active UC; high TRPV4 expression may be associated with a healthy colon | ||
| Human + animal single-channel studies | D’Aldebert E. et al., 2011 [39] | TRPV4 activation in Caco-2 and human colon epithelial cells increased Ca2+ and chemokine release, supporting a pro-inflammatory epithelial phenotype | ||
| TRPV5 | Human multi-channel studies | Toledo Mauriño et al., 2020 [41] | The absence of TRPV5 appears to correlate with UC induction | Anti-inflammatory role if this channel is activated, and pro-inflammatory role if this channel is inhibited |
| TRPV6 | Human multi-channel studies | Toledo Mauriño et al., 2020 [41] | TRPV6 is highly expressed in all layers of the intestine in patients with UC, and this appears to be clearly associated with disease activity | Pro-inflammatory role |
| TRPM2 | Human multi-channel studies | Morita T. et al., 2020 [5] | Increased expression levels in PBMCs from UC and CD patients | Pro-inflammatory role |
| TRPC1 | Human multi-channel studies | Morita T. et al., 2020 [5] | Reduced expression in PBMCs from UC and CD patients may enhance disease progression | Anti-inflammatory role |
| TRP Channel | Category of Studies | Author, Year | Model | Confirmed and/or Expected Effects | Conclusion |
|---|---|---|---|---|---|
| TRPA1 | Human + animal multi-channel studies | Kun J. et al., 2014 [22] | DSS-induced colitis in mice | Protective role in colitis | Pro- and anti-inflammatory roles with pain sensitivity increase |
| Animal single-channel studies | Kistner K. et al., 2016 [27] | DSS-induced colitis in mice | Desensitization of TRPA1 attenuates neurogenic inflammation | ||
| Animal single-channel studies | Mitrovic M. et al., 2010 [28] | DSS-induced colitis in mice | Visceral hypersensitivity mediated by TRPA1 agonist AITC | ||
| Animal single-channel studies | Jain P. et al., 2020 [29] | DSS-induced colitis in mice | TRPA1 contributes to colitis-associated mechanical hypersensitivity via increased expression and activity in DRG neurons, with its blockade reducing hypersensitivity without affecting colitis severity | ||
| Animal single-channel studies | Yang Y. et al., 2019 [30] | DSS-induced colitis in mice and rats | In DSS-induced colitis, TRPA1 expression is upregulated, and TRPA1 activation exacerbates abnormal colonic motility; pharmacological or genetic inhibition of TRPA1 alleviates these motility disturbances | ||
| Animal multi-channel studies | Utsumi D. et al., 2018 [24] | DSS-induced colitis in mice | TRPV1 and TRPA1 expression in sensory neurons plays a critical role in the progression of colonic inflammation in DSS-induced colitis in mice | ||
| Animal single-channel studies | Chen J., 2013 [31] | DNBS-induced colitis in rats | Increased visceral hypersensitivity; TRPA1 antagonism mitigates these effect | ||
| Animal single-channel studies | Kurahara L. H. et al., 2018 [32] | TNBS-induced colitis in mice | In TRPA1 knockout mice, the extent of inflammation and fibrosis is more pronounced compared to wild-type mice | ||
| Animal multi-channel studies | Vermeulen W. et al., 2013 [25] | TNBS-induced colitis in rats | Visceral hypersensitivity | ||
| Animal single-channel studies | Li Q. et al., 2016 [33] | TNBS-induced colitis in rats | Visceral hypersensitivity | ||
| Animal single-channel studies | Brierley S. M. et al., 2009 [34] | TNBS-induced colitis in mice | Visceral hypersensitivity | ||
| Animal single-channel studies | Cattaruzza F. et al., 2010 [35] | TNBS-induced colitis in mice | Visceral hypersensitivity | ||
| Animal multi-channel studies | Kumar V. et al., 2022 [26] | C57BL/6 mice treated with intrarectal capsazepine | Damaged mucosa, increased intestinal permeability | ||
| Animal single-channel studies | Kimball E. S. et al., 2007 [36] | OM colitis in mice | Increased mRNA levels of various neuropeptides and mediators associated with pain and inflammation | ||
| TRPV1 | Human + animal single-channel studies | Duo L. et al., 2020 [37] | DSS-induced colitis in mice | Enhanced Th17 cell differentiation and dendritic cell-mediated inflammation | Pro-inflammatory role with pain sensitivity increase, but only antagonism reduces inflammation and pain |
| Animal single-channel studies | Lapointe T. K. et al., 2015 [46] | DSS-induced colitis in mice | Increased inflammation, increased release of CGRP and SP, visceral hypersensitivity and pain-related behavior | ||
| Animal single-channel studies | Engel M. A. et al., 2012 [47] | DSS-induced colitis in mice | Increased inflammation, increased release of CGRP and SP | ||
| Animal multi-channel studies | Utsumi D. et al., 2018 [24] | DSS-induced colitis in mice | TRPV1 and TRPA1 expression in sensory neurons plays a critical role in the progression of colonic inflammation in DSS-induced colitis in mice | ||
| Animal single-channel studies | Matsumoto K. et al., 2012 [48] | DSS-induced colitis in rats | DSS-induced colitis leads to increased TRPV1 and 5-HT3 receptor expression and decreased 5-HT4 receptor expression in colonic mucosa, contributing to visceral hypersensitivity | ||
| Animal single-channel studies | Massa F. et al., 2006 [49] | DNBS-induced colitis in mice | Modulating of sensory pathways involved in colonic inflammation, possible protective effect | ||
| Animal single-channel studies | Mazor Y. et al., 2024 [50] | DNBS-induced colitis in rats | TRPV1 antagonism reduces pain and inflammation | ||
| Animal multi-channel studies | Vermeulen W. et al., 2013 [25] | TNBS-induced colitis in rats | Visceral hypersensitivity | ||
| Animal multi-channel studies | Matsumoto K. et al., 2023 [45] | TNBS-induced colitis in rats | Visceral hypersensitivity | ||
| Animal single-channel studies | De Schepper H. U. et al., 2008 [51] | TNBS-induced colitis in rats | TRPV1 receptor activation mediates afferent nerve sensitization during colitis-induced motility disorders in rats. Inhibition of TRPV1 signaling reduces colitis-induced motility disorders and afferent nerve sensitization | ||
| Animal single-channel studies | De Schepper H. U. et al., 2008 (2) [52] | TNBS-induced colitis in rats | TRPV1 receptors on unmyelinated C-fibers mediate colitis-induced sensitization of pelvic afferent nerve fibers in rats. Inhibition of TRPV1 signaling reduces colitis-induced sensitization and associated pain | ||
| Animal single-channel studies | Miranda A. et al., 2007 [53] | TNBS-induced colitis in rats | Increased visceral sensitivity; TRPV1 antagonism reduces both inflammation and hypersensitivity | ||
| Animal single-channel studies | Shen S. et al., 2017 [54] | TNBS-induced colitis in rats | Visceral hypersensitivity | ||
| Animal single-channel studies | Wu Y. et al., 2019 [55] | TNBS-induced colitis in mice | TLR4 signaling contributes to TRPV1 upregulation and peripheral sensitization in inflammatory conditions | ||
| Animal single-channel studies | Lee J. et al., 2012 [56] | oxazolone-induced colitis in mice | Excessive neutrophil accumulation; a protective role of TRPV1 expressing extrinsic sensory neurons in oxazolone induced colitis | ||
| Animal multi-channel studies | Kumar V. et al., 2022 [26] | C57BL/6 mice treated with intrarectal capsazepine | Damaged mucosa, increased intestinal permeability | ||
| TRPV2 | Animal multi-channel studies | Matsumoto K. et al., 2023 [45] | TNBS-induced colitis in rats | Visceral hypersensitivity | Pro-inflammatory role and increased pain sensitivity |
| Animal single-channel studies | Issa C. M. et al., 2014 [57] | DSS-induced colitis in mice | Increased inflammation | ||
| TRPV4 | Human + animal single-channel studies | Fichna J. et al., 2012 [38] | TNBS-induced colitis in mice | Intestinal inflammation and colitis-associated pain | Pro-inflammatory role and increased pain sensitivity |
| Human + animal single-channel studies | D’Aldebert E. et al., 2011 [39] | DSS-induced colitis in mice | TRPV4 mRNA expression was up-regulated when compared with control naïve tissues | ||
| Animal single-channel studies | Matsumoto K. et al., 2018 [58] | DSS-induced colitis in mice | Pro-inflammatory effects | ||
| TRPM2 | Animal single-channel studies | Matsumoto K. et al., 2016 [59] | TNBS-induced colitis in mice and rats | Increased visceromotor reflexes caused by balloon pressure, visceral hypersensitivity | Pro-inflammatory role and increased pain sensitivity |
| Animal single-channel studies | Yamamoto S. et al., 2008 [60] | DSS-induced colitis in mice | Progression of colitis through its possible implication in oxidative stress signaling | ||
| Animal single-channel studies | Nakamoto T. et al., 2024 [61] | TNBS-induced colitis in mice | TRPM2 contributes to inflammation via Th1/Th17 pathways; TRPM2 mediates ROS-induced cytokine release and MAPK activation | ||
| TRPM3 | Animal single-channel studies | King J. W. et al., 2024 [62] | DSS-induced colitis in mice | Perception of noxious stimuli in colitis, colonic hypersensitivity | Increased pain sensitivity |
| TRPM8 | Animal single-channel studies | Hosoya T. et al., 2014 [63] | TNBS-induced colitis in mice DSS-induced colitis in mice | Visceral hyperalgesia | Anti-inflammatory role, but with increased pain sensitivity |
| Animal single-channel studies | Ramachandran R. et al., 2013 [64] | DSS-induced colitis in mice | Anti-inflammatory role of TRPM8 activation, partly mediated by inhibition of neuropeptide release | ||
| Animal single-channel studies | de Jong P. R. et al., 2015 [65] | TNBS-induced colitis in mice DSS-induced colitis in mice | TRPM8 deficiency leads to increased susceptibility to colitis, while CGRP administration ameliorates inflammation | ||
| Animal single-channel studies | Khalil M. et al., 2016 [66] | DSS-induced colitis in mice | TRPM8 deficiency leads to increased colitis severity, while activation of TRPM8 with menthol enemas provides protection | ||
| Animal single-channel studies | Zhang Z. et al., 2024 [67] | DSS-induced colitis in mice | The activation of TRPM8 attenuated DSS-induced colitis in mice | ||
| TRPC6 | Animal single-channel studies | Nishiyama K. et al., 2024 [68] | DSS-induced colitis in mice | TRPC6 expression increased in DSS-induced colitis; treatment with TRPC6 activator PPZ2 prevented DSS-induced colitis progression | Anti-inflammatory role |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvornikova, K.A.; Platonova, O.N.; Bystrova, E.Y. The Role of TRP Channels in Colitis and Inflammatory Bowel Disease: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 9390. https://doi.org/10.3390/ijms26199390
Dvornikova KA, Platonova ON, Bystrova EY. The Role of TRP Channels in Colitis and Inflammatory Bowel Disease: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(19):9390. https://doi.org/10.3390/ijms26199390
Chicago/Turabian StyleDvornikova, Kristina A., Olga N. Platonova, and Elena Y. Bystrova. 2025. "The Role of TRP Channels in Colitis and Inflammatory Bowel Disease: A Systematic Review" International Journal of Molecular Sciences 26, no. 19: 9390. https://doi.org/10.3390/ijms26199390
APA StyleDvornikova, K. A., Platonova, O. N., & Bystrova, E. Y. (2025). The Role of TRP Channels in Colitis and Inflammatory Bowel Disease: A Systematic Review. International Journal of Molecular Sciences, 26(19), 9390. https://doi.org/10.3390/ijms26199390





