Triacetin and a Mushroom Blend Restore Butyrate Production by IBS Microbiomes Ex Vivo, Thus Promoting Barrier Integrity
Abstract
1. Introduction
2. Results
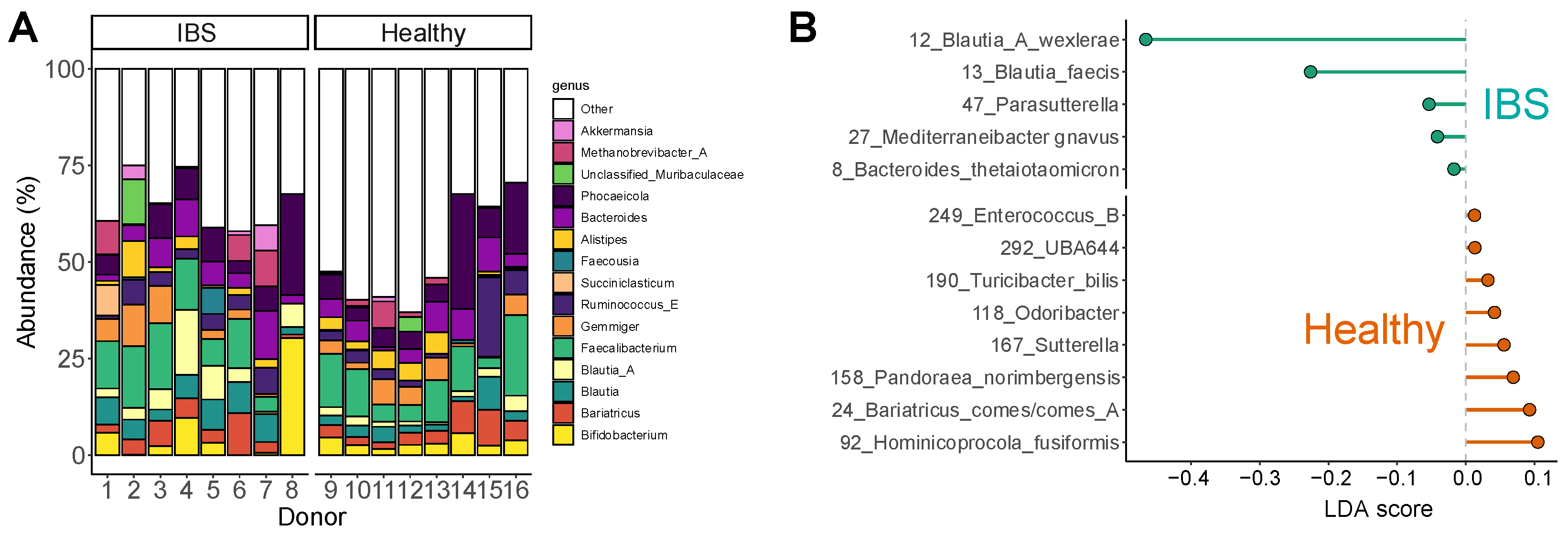
2.1. The IBS Microbiome Displayed Large Interpersonal Differences and an IBS-Associated Dysbiosis
2.2. Encapsulation Enhances Colonic Delivery of TA
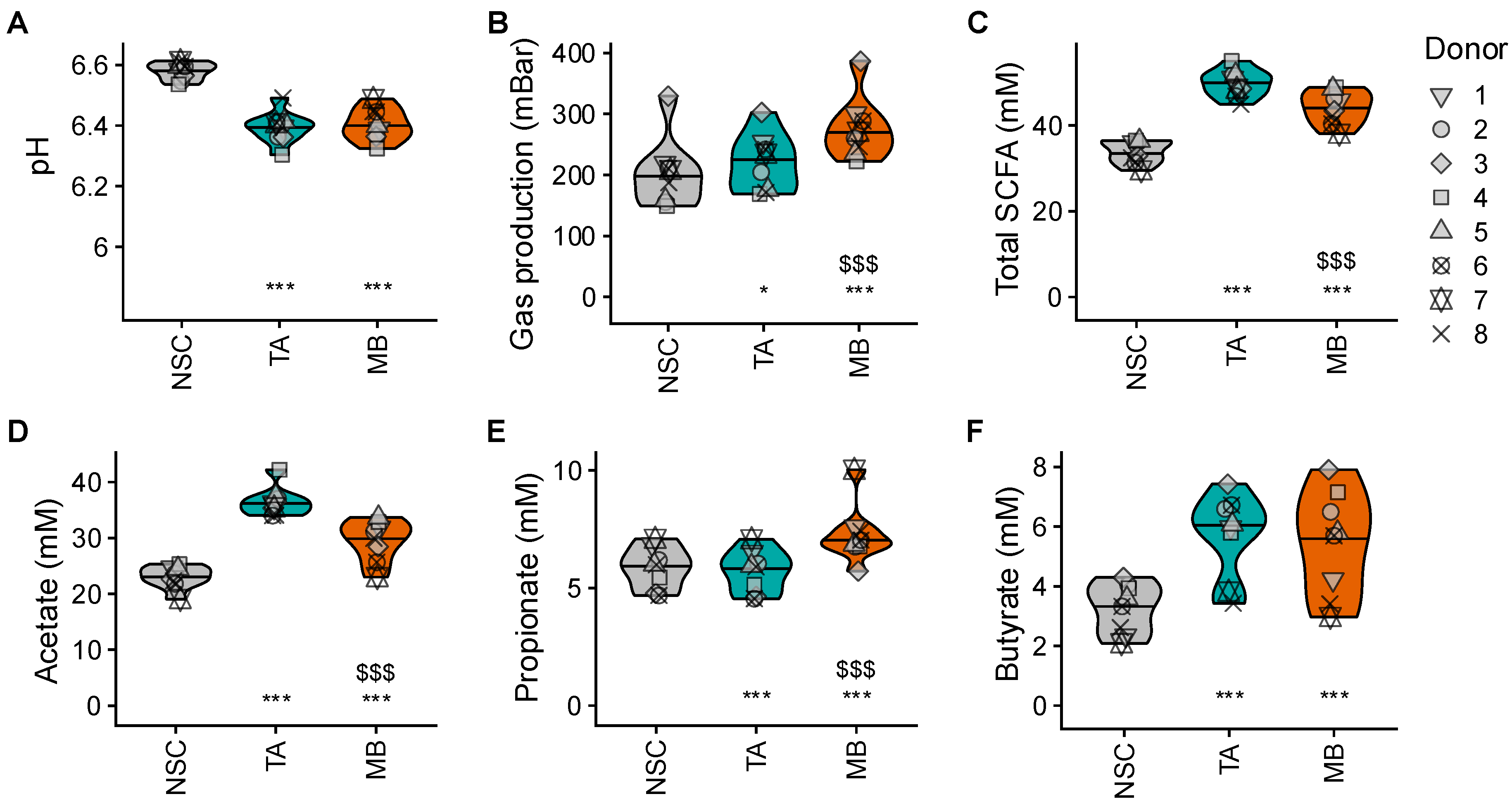
2.3. TA and MB Boosted SCFA, Which Was Accompagnied by Only Low Gas Production for TA
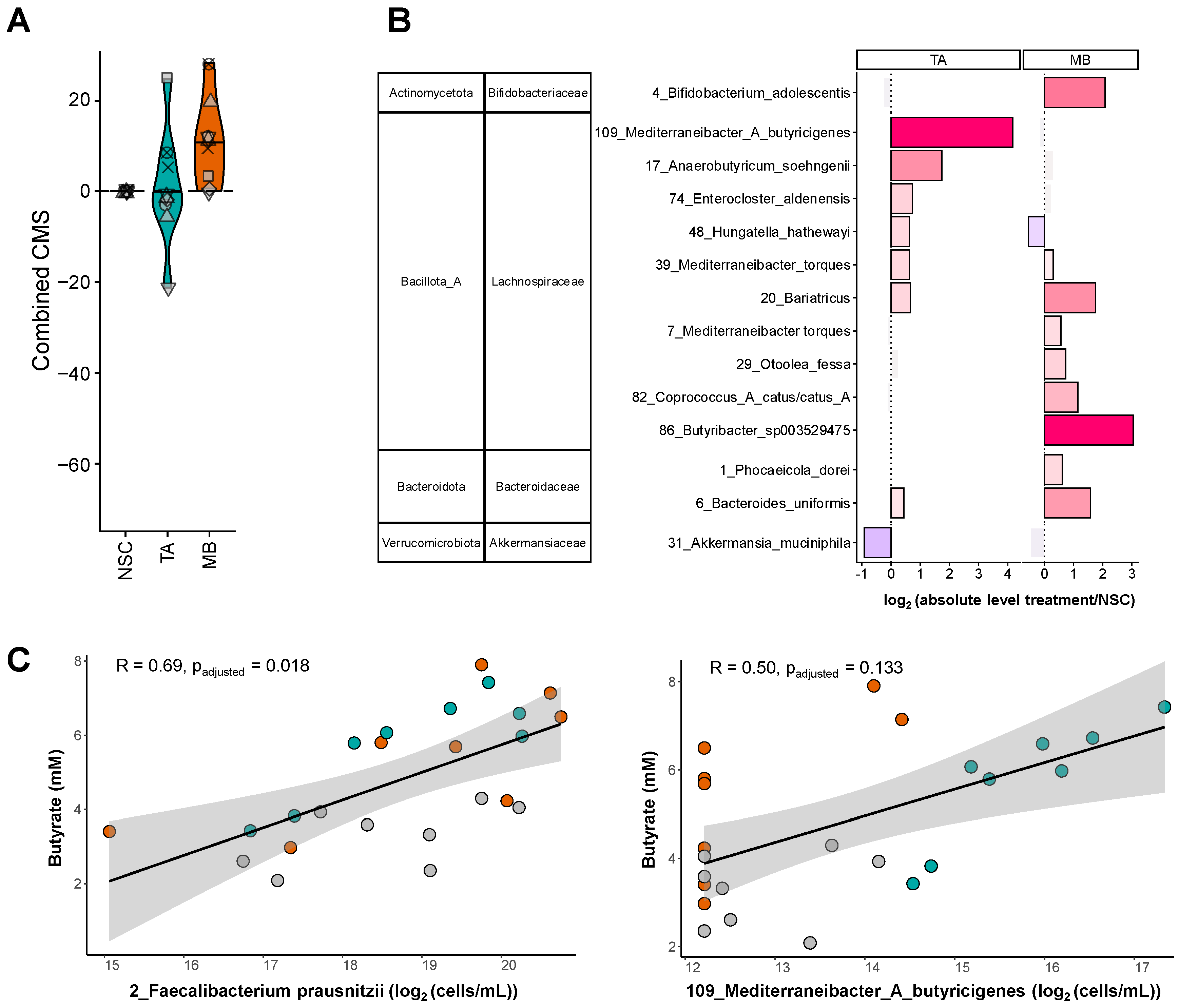
2.4. TA and MB Each Enhanced the Growth of Specific SCFA-Producing Gut Microbes
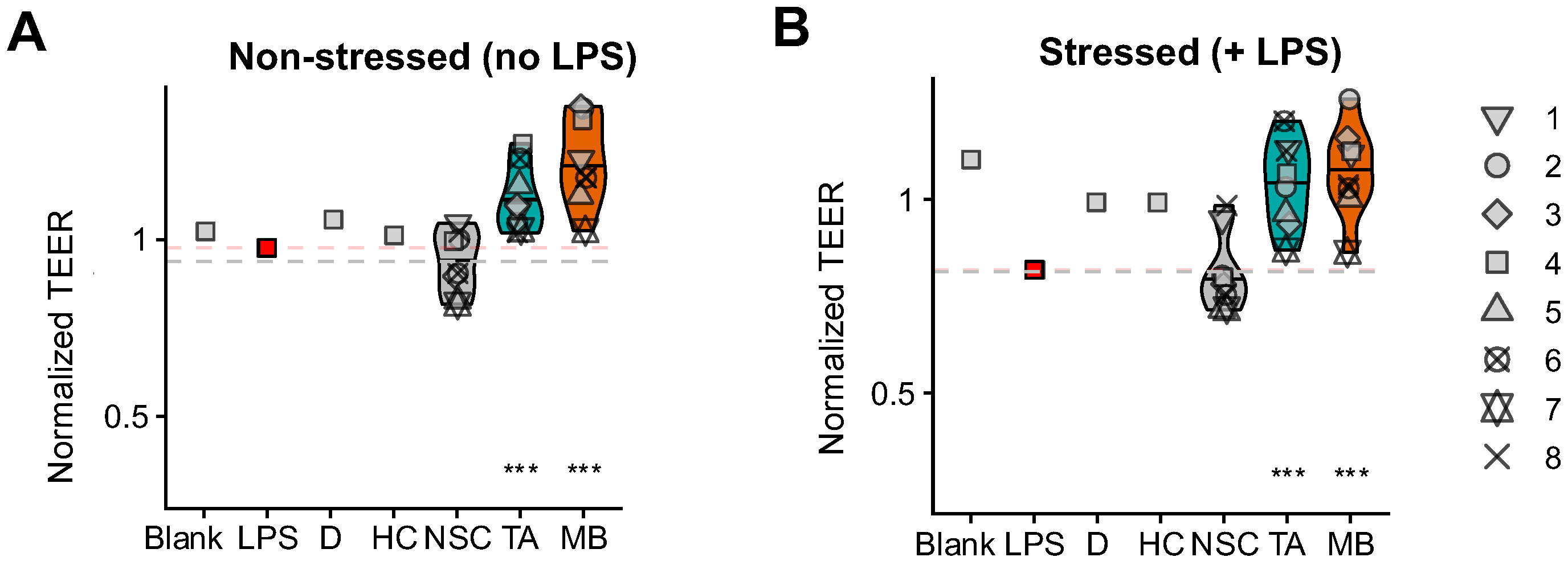
2.5. TA and MB Promoted Gut Barrier Integrity Under Non-Stressed and Stressed Conditions
3. Discussion
4. Materials and Methods
4.1. Selection Criteria Test Subjects
4.2. Upper Gastrointestinal Tract Simulation
4.3. Ex Vivo Colonic Fermentations (SIFR®): Experimental Configuration, Timeline and Analysis
4.4. Key Fermentative Parameters
4.5. Microbial Composition
4.6. Host/Microbiome Interaction Assay
4.7. Data Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| BSS | Bristol stool score |
| CMS | Community modulation score |
| IBS | Irritable Bowel Syndrome |
| MB | Mushroom blend |
| NSC | No-substrate control |
| OTU | Operational taxonomic unit |
| SCFA | Short-chain fatty acid |
| SIFR® | Systemic Intestinal Fermentation Research |
| TA | Triacetin |
| TEER | Transepithelial electrical resistance |
References
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M.; Tilg, H.; Hul, M.V.; Cani, P.D. Gut Microbiome and Health: Mechanistic Insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Koropatkin, N.M.; Cameron, E.A.; Martens, E.C. How Glycan Metabolism Shapes the Human Gut Microbiota. Nat. Rev. Microbiol. 2012, 10, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of Propionate and Butyrate by the Human Colonic Microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed]
- Pozuelo, M.; Panda, S.; Santiago, A.; Mendez, S.; Accarino, A.; Santos, J.; Guarner, F.; Azpiroz, F.; Manichanh, C. Reduction of Butyrate- and Methane-Producing Microorganisms in Patients with Irritable Bowel Syndrome. Sci. Rep. 2015, 5, 12693. [Google Scholar] [CrossRef]
- Quigley, E.M.M.; Abdel-Hamid, H.; Barbara, G.; Bhatia, S.J.; Boeckxstaens, G.; De Giorgio, R.; Delvaux, M.; Drossman, D.A.; Foxx-Orenstein, A.E.; Guarner, F.; et al. A Global Perspective on Irritable Bowel Syndrome: A Consensus Statement of the World Gastroenterology Organisation Summit Task Force on Irritable Bowel Syndrome. J. Clin. Gastroenterol. 2012, 46, 356–366. [Google Scholar] [CrossRef]
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef]
- Lacy, B.E.; Patel, N.K. Rome Criteria and a Diagnostic Approach to Irritable Bowel Syndrome. J. Clin. Med. 2017, 6, 99. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Bråthen Kristoffersen, A.; Hausken, T. Efficacy of Faecal Microbiota Transplantation for Patients with Irritable Bowel Syndrome in a Randomised, Double-Blind, Placebo-Controlled Study. Gut 2020, 69, 859–867. [Google Scholar] [CrossRef]
- Peng, L.; Li, Z.-R.; Green, R.S.; Holzmanr, I.R.; Lin, J. Butyrate Enhances the Intestinal Barrier by Facilitating Tight Junction Assembly via Activation of AMP-Activated Protein Kinase in Caco-2 Cell Monolayers. J. Nutr. 2009, 139, 1619–1625. [Google Scholar] [CrossRef]
- Dunlop, S.P.; Hebden, J.; Campbell, E.; Naesdal, J.; Olbe, L.; Perkins, A.C.; Spiller, R.C. Abnormal Intestinal Permeability in Subgroups of Diarrhea-Predominant Irritable Bowel Syndromes. Am. J. Gastroenterol. 2006, 101, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, B.; Verne, G.N. Intestinal Membrane Permeability and Hypersensitivity In the Irritable Bowel Syndrome. Pain 2009, 146, 41–46. [Google Scholar] [CrossRef]
- Hanning, N.; Edwinson, A.L.; Ceuleers, H.; Peters, S.A.; De Man, J.G.; Hassett, L.C.; De Winter, B.Y.; Grover, M. Intestinal Barrier Dysfunction in Irritable Bowel Syndrome: A Systematic Review. Therap. Adv. Gastroenterol. 2021, 14, 1756284821993586. [Google Scholar] [CrossRef] [PubMed]
- Vinelli, V.; Biscotti, P.; Martini, D.; Del Bo’, C.; Marino, M.; Meroño, T.; Nikoloudaki, O.; Calabrese, F.M.; Turroni, S.; Taverniti, V.; et al. Effects of Dietary Fibers on Short-Chain Fatty Acids and Gut Microbiota Composition in Healthy Adults: A Systematic Review. Nutrients 2022, 14, 2559. [Google Scholar] [CrossRef]
- Moens, F.; Verce, M.; De Vuyst, L. Lactate- and Acetate-Based Cross-Feeding Interactions between Selected Strains of Lactobacilli, Bifidobacteria and Colon Bacteria in the Presence of Inulin-Type Fructans. Int. J. Food Microbiol. 2017, 241, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Young, E.; Rojas Vasquez, L.S.; Lim, A.; Philpott, H. Low FODMAP diet for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2022, 8, CD014029. [Google Scholar] [CrossRef]
- Cantu-Jungles, T.M.; Hamaker, B.R. New View on Dietary Fiber Selection for Predictable Shifts in Gut Microbiota. mBio 2020, 11, e02179-19, Erratum in mBio 2020, 11, e00747-20. [Google Scholar] [CrossRef]
- Ruppin, H.; Bar-Meir, S.; Soergel, K.H.; Wood, C.M.; Schmitt, M.G. Absorption of Short-Chain Fatty Acids by the Colon. Gastroenterology 1980, 78, 1500–1507. [Google Scholar] [CrossRef]
- Procházková, N.; Falony, G.; Dragsted, L.O.; Licht, T.R.; Raes, J.; Roager, H.M. Advancing Human Gut Microbiota Research by Considering Gut Transit Time. Gut 2022, 72, 180–191. [Google Scholar] [CrossRef]
- Vujkovic-Cvijin, I.; Sklar, J.; Jiang, L.; Natarajan, L.; Knight, R.; Belkaid, Y. Host Variables Confound Gut Microbiota Studies of Human Disease. Nature 2020, 587, 448–454. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Deyaert, S.; Thabuis, C.; Perreau, C.; Bajic, D.; Wintergerst, E.; Joossens, M.; Firrman, J.; Walsh, D.; Baudot, A. Bridging Preclinical and Clinical Gut Microbiota Research Using the Ex Vivo SIFR® Technology. Front. Microbiol. 2023, 14, 1131662. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Kunkler, C.N.; Poppe, J.; Rose, A.; van Hengel, I.A.J.; Baudot, A.; Warner, C.D. Serum-Derived Bovine Immunoglobulin Promotes Barrier Integrity and Lowers Inflammation for 24 Human Adults Ex Vivo. Nutrients 2024, 16, 1585. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Dong, Q.; Chen, M.; Zhao, R.; Zha, L.; Zhao, Y.; Zhang, M.; Zhang, B.; Ma, A. The Effect of Mushroom Dietary Fiber on the Gut Microbiota and Related Health Benefits: A Review. J. Fungi 2023, 9, 1028. [Google Scholar] [CrossRef] [PubMed]
- Brunkwall, L.; Ericson, U.; Nilsson, P.M.; Orho-Melander, M.; Ohlsson, B. Self-reported Bowel Symptoms Are Associated with Differences in Overall Gut Microbiota Composition and Enrichment of Blautia in a Population-based Cohort. J. Gastro. Hepatol. 2021, 36, 174–180. [Google Scholar] [CrossRef]
- Kim, G.-H.; Lee, K.; Shim, J.O. Gut Bacterial Dysbiosis in Irritable Bowel Syndrome: A Case-Control Study and a Cross-Cohort Analysis Using Publicly Available Data Sets. Microbiol. Spectr. 2023, 11, e02125-22. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Wang, X.; Wang, Z.; Zhang, J.; Jiang, R.; Wang, X.; Wang, K.; Liu, Z.; Xia, Z.; et al. Similar Fecal Microbiota Signatures in Patients With Diarrhea-Predominant Irritable Bowel Syndrome and Patients With Depression. Clin. Gastroenterol. Hepatol. 2016, 14, 1602–1611.e5. [Google Scholar] [CrossRef]
- Jeffery, I.B.; Das, A.; O’Herlihy, E.; Coughlan, S.; Cisek, K.; Moore, M.; Bradley, F.; Carty, T.; Pradhan, M.; Dwibedi, C.; et al. Differences in Fecal Microbiomes and Metabolomes of People With vs Without Irritable Bowel Syndrome and Bile Acid Malabsorption. Gastroenterology 2020, 158, 1016–1028.e8. [Google Scholar] [CrossRef]
- Zhai, L.; Huang, C.; Ning, Z.; Zhang, Y.; Zhuang, M.; Yang, W.; Wang, X.; Wang, J.; Zhang, L.; Xiao, H.; et al. Ruminococcus gnavus Plays a Pathogenic Role in Diarrhea-Predominant Irritable Bowel Syndrome by Increasing Serotonin Biosynthesis. Cell Host Microbe 2023, 31, 33–44.e5. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Vermeire, S. The Intestinal Barrier: A Fundamental Role in Health and Disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Kim, J.-S.; Lee, K.C.; Suh, M.K.; Han, K.-I.; Eom, M.K.; Lee, J.H.; Park, S.-H.; Kang, S.W.; Park, J.-E.; Oh, B.S.; et al. Mediterraneibacter butyricigenes sp. nov., a Butyrate-Producing Bacterium Isolated from Human Faeces. J. Microbiol. 2019, 57, 38–44. [Google Scholar] [CrossRef]
- Shetty, S.A.; Zuffa, S.; Bui, T.P.N.; Aalvink, S.; Smidt, H.; De Vos, W.M. Reclassification of Eubacterium hallii as Anaerobutyricum hallii gen. nov., comb. nov., and Description of Anaerobutyricum soehngenii sp. nov., a Butyrate and Propionate-Producing Bacterium from Infant Faeces. Int. J. Syst. Evol. Microbiol. 2018, 68, 3741–3746. [Google Scholar] [CrossRef]
- Holdeman, L.V.; Moore, W.E.C. New Genus, Coprococcus, Twelve New Species, and Emended Descriptions of Four Previously Described Species of Bacteria from Human Feces. Int. J. Syst. Bacteriol. 1974, 24, 260–277. [Google Scholar] [CrossRef]
- Zou, Y.; Xue, W.; Lin, X.; Lv, M.; Luo, G.; Dai, Y.; Sun, H.; Liu, S.-W.; Sun, C.-H.; Hu, T.; et al. Butyribacter intestini gen. nov., sp. nov., a Butyric Acid-Producing Bacterium of the Family Lachnospiraceae Isolated from Human Faeces, and Reclassification of Acetivibrio ethanolgignens as Acetanaerobacter ethanolgignens gen. nov., comb. nov. Syst. Appl. Microbiol. 2021, 44, 126201. [Google Scholar] [CrossRef] [PubMed]
- Breyner, N.M.; Michon, C.; de Sousa, C.S.; Vilas Boas, P.B.; Chain, F.; Azevedo, V.A.; Langella, P.; Chatel, J.M. Microbial Anti-Inflammatory Molecule (MAM) from Faecalibacterium prausnitzii Shows a Protective Effect on DNBS and DSS-Induced Colitis Model in Mice through Inhibition of NF-κB Pathway. Front. Microbiol. 2017, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Wortelboer, K.; Koopen, A.M.; Herrema, H.; De Vos, W.M.; Nieuwdorp, M.; Kemper, E.M. From Fecal Microbiota Transplantation toward Next-Generation Beneficial Microbes: The Case of Anaerobutyricum soehngenii. Front. Med. 2022, 9, 1077275. [Google Scholar] [CrossRef]
- Cisbani, G.; Chouinard-Watkins, R.; Smith, M.E.; Malekanian, A.; Valenzuela, R.; Metherel, A.H.; Bazinet, R.P. Dietary Triacetin, but Not Medium Chain Triacylglycerides, Blunts Weight Gain in Diet-Induced Rat Model of Obesity. Lipids 2023, 58, 257–270. [Google Scholar] [CrossRef]
- Gunn, D.; Abbas, Z.; Harris, H.C.; Major, G.; Hoad, C.; Gowland, P.; Marciani, L.; Gill, S.K.; Warren, F.J.; Rossi, M.; et al. Psyllium Reduces Inulin-Induced Colonic Gas Production in IBS: MRI and in Vitro Fermentation Studies. Gut 2022, 71, 919–927. [Google Scholar] [CrossRef]
- Verbeke, K. Combining Dietary Fibres to Reduce Intestinal Gas Production in Patients with IBS. Gut 2022, 71, 848–849. [Google Scholar] [CrossRef]
- Hernandez, K.; Garcia-Verdugo, E.; Porcar, R.; Fernandez-Lafuente, R. Hydrolysis of Triacetin Catalyzed by Immobilized Lipases: Effect of the Immobilization Protocol and Experimental Conditions on Diacetin Yield. Enzym. Microb. Technol. 2011, 48, 510–517. [Google Scholar] [CrossRef]
- Walker, A.W.; Duncan, S.H.; McWilliam Leitch, E.C.; Child, M.W.; Flint, H.J. pH and Peptide Supply Can Radically Alter Bacterial Populations and Short-Chain Fatty Acid Ratios within Microbial Communities from the Human Colon. Appl. Environ. Microbiol. 2005, 71, 3692–3700. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Marzorati, M.; Derde, M.; De Weirdt, R.; Joan, V.; Possemiers, S.; Van de Wiele, T. Arabinoxylans, Inulin and Lactobacillus Reuteri 1063 Repress the Adherent-Invasive Escherichia Coli from Mucus in a Mucosa-Comprising Gut Model. NPJ Biofilms Microbiomes 2016, 2, 16016. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Collado, M.C.; Wopereis, H.; Salminen, S.; Knol, J.; Roeselers, G. The Bifidogenic Effect Revisited—Ecology and Health Perspectives of Bifidobacterial Colonization in Early Life. Microorganisms 2020, 8, 1855. [Google Scholar] [CrossRef] [PubMed]
- Laursen, M.F.; Sakanaka, M.; von Burg, N.; Mörbe, U.; Andersen, D.; Moll, J.M.; Pekmez, C.T.; Rivollier, A.; Michaelsen, K.F.; Mølgaard, C.; et al. Bifidobacterium Species Associated with Breastfeeding Produce Aromatic Lactic Acids in the Infant Gut. Nat. Microbiol. 2021, 6, 1367–1382. [Google Scholar] [CrossRef] [PubMed]
- Tintoré, M.; Cuñé, J.; Vu, L.D.; Poppe, J.; Van den Abbeele, P.; Baudot, A.; de Lecea, C. A Long-Chain Dextran Produced by Weissella Cibaria Boosts the Diversity of Health-Related Gut Microbes Ex Vivo. Biology 2024, 13, 51. [Google Scholar] [CrossRef]
- Esteban-Torres, M.; Mancheño, J.M.; de las Rivas, B.; Muñoz, R. Production and Characterization of a Tributyrin Esterase from Lactobacillus Plantarum Suitable for Cheese Lipolysis. J. Dairy Sci. 2014, 97, 6737–6744. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Matsui, T.; Kaneko, N.; Kobayashi, I. Digestion and Absorption of Triacetin, a Short-Chain Triacylglycerol. Lipids 2025, 60, 185–192. [Google Scholar] [CrossRef]
- Jensen, B.A.H.; Heyndrickx, M.; Jonkers, D.; Mackie, A.; Millet, S.; Naghibi, M.; Pærregaard, S.I.; Pot, B.; Saulnier, D.; Sina, C.; et al. Small Intestine vs. Colon Ecology and Physiology: Why It Matters in Probiotic Administration. Cell Rep. Med. 2023, 4, 101190. [Google Scholar] [CrossRef]
- Kim, M.-H.; Yun, K.E.; Kim, J.; Park, E.; Chang, Y.; Ryu, S.; Kim, H.-L.; Kim, H.-N. Gut Microbiota and Metabolic Health among Overweight and Obese Individuals. Sci. Rep. 2020, 10, 19417. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Detzel, C.; Rose, A.; Deyaert, S.; Baudot, A.; Warner, C. Serum-Derived Bovine Immunoglobulin Stimulates SCFA Production by Specific Microbes in the Ex Vivo SIFR® Technology. Microorganisms 2023, 11, 659. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Deyaert, S.; Albers, R.; Baudot, A.; Mercenier, A. Carrot RG-I Reduces Interindividual Differences between 24 Adults through Consistent Effects on Gut Microbiota Composition and Function Ex Vivo. Nutrients 2023, 15, 2090. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van den Abbeele, P.; Poppe, J.; Baudot, A.; Vu, L.D. Triacetin and a Mushroom Blend Restore Butyrate Production by IBS Microbiomes Ex Vivo, Thus Promoting Barrier Integrity. Int. J. Mol. Sci. 2025, 26, 9388. https://doi.org/10.3390/ijms26199388
Van den Abbeele P, Poppe J, Baudot A, Vu LD. Triacetin and a Mushroom Blend Restore Butyrate Production by IBS Microbiomes Ex Vivo, Thus Promoting Barrier Integrity. International Journal of Molecular Sciences. 2025; 26(19):9388. https://doi.org/10.3390/ijms26199388
Chicago/Turabian StyleVan den Abbeele, Pieter, Jonas Poppe, Aurélien Baudot, and Lam Dai Vu. 2025. "Triacetin and a Mushroom Blend Restore Butyrate Production by IBS Microbiomes Ex Vivo, Thus Promoting Barrier Integrity" International Journal of Molecular Sciences 26, no. 19: 9388. https://doi.org/10.3390/ijms26199388
APA StyleVan den Abbeele, P., Poppe, J., Baudot, A., & Vu, L. D. (2025). Triacetin and a Mushroom Blend Restore Butyrate Production by IBS Microbiomes Ex Vivo, Thus Promoting Barrier Integrity. International Journal of Molecular Sciences, 26(19), 9388. https://doi.org/10.3390/ijms26199388







