Real-Time FTIR-ATR Spectroscopy for Monitoring Ethanolysis: Spectral Evaluation, Regression Modelling, and Molecular Insight
Abstract
1. Introduction
2. Results and Discussion
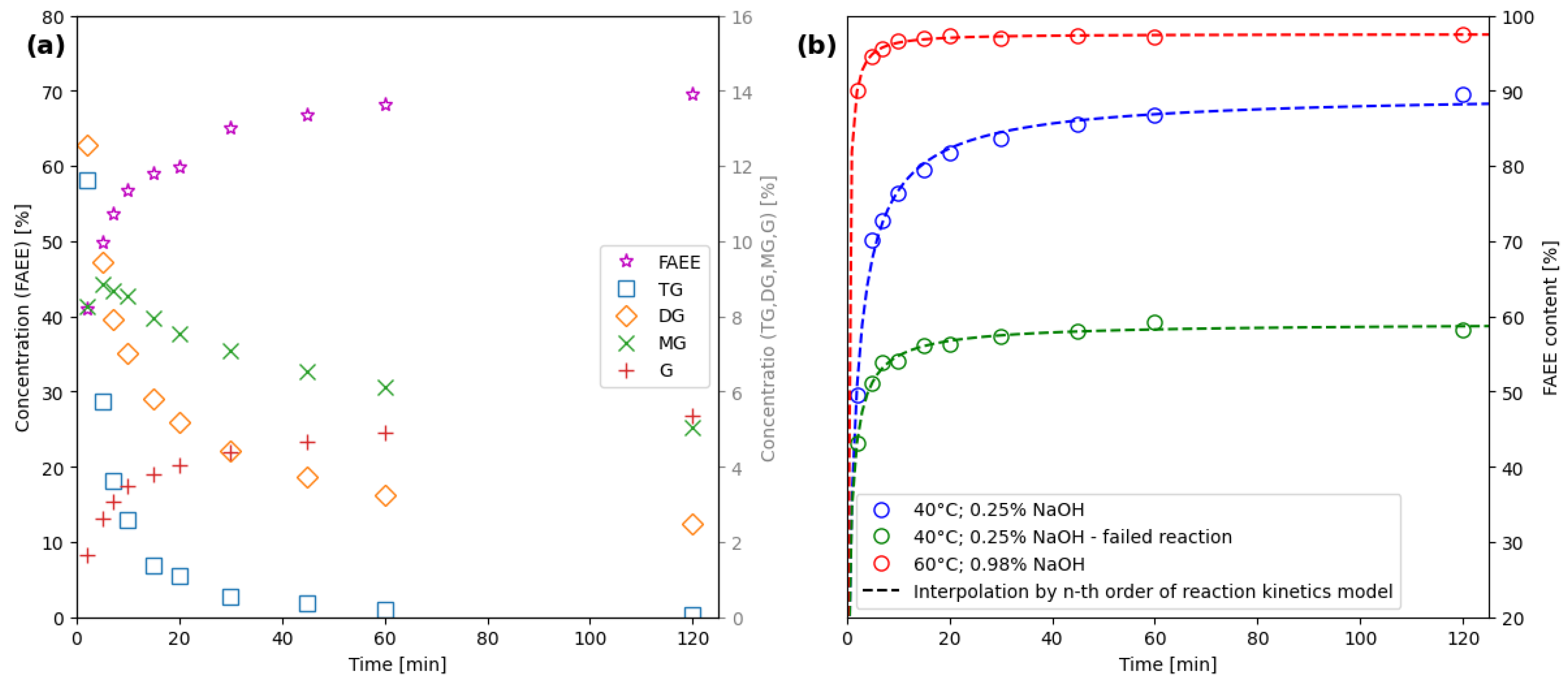
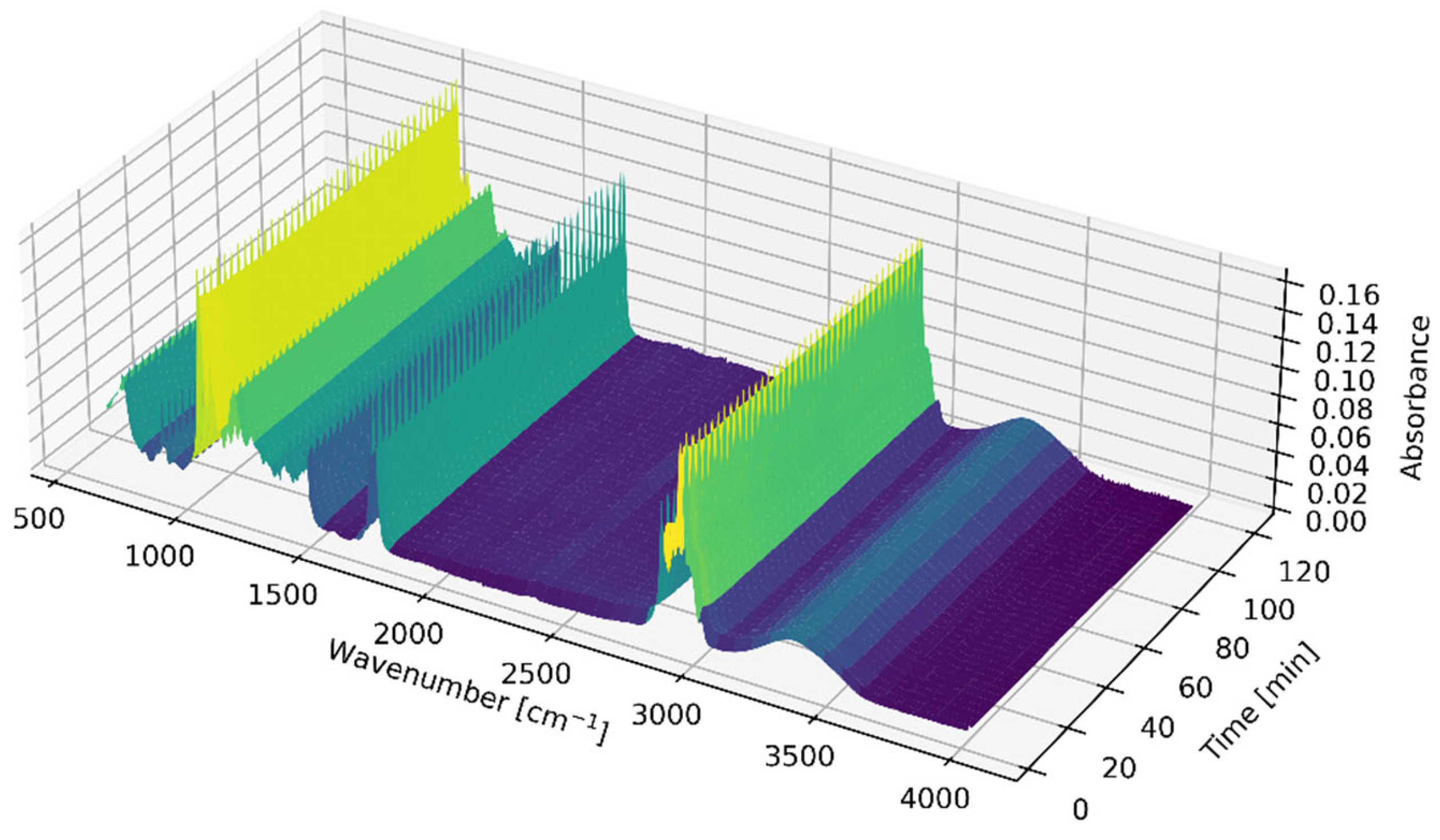
2.1. Calibration Data Acquisition
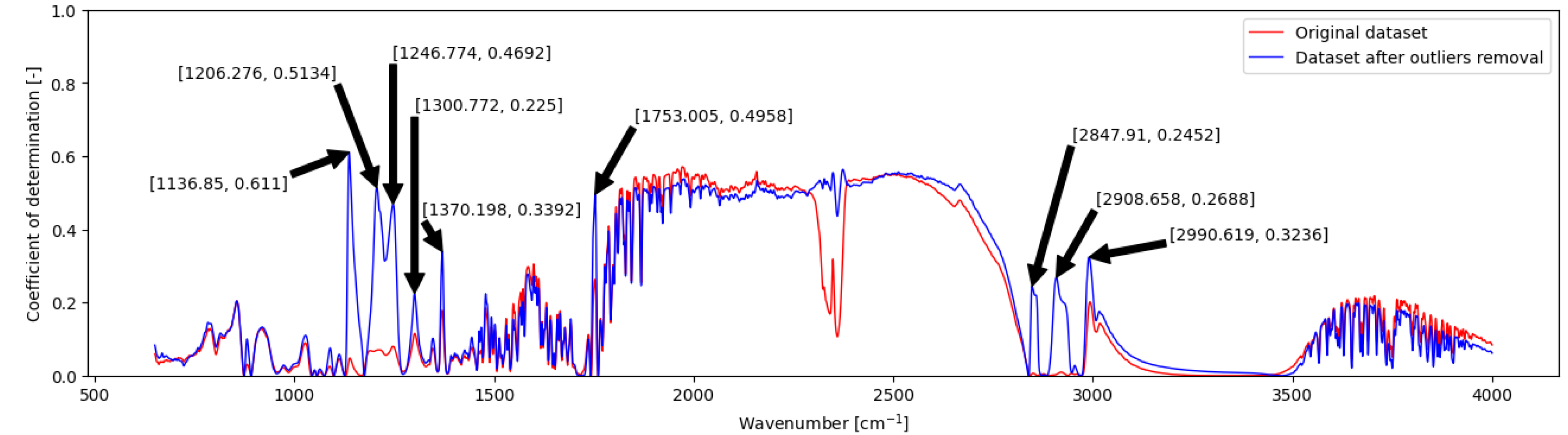
2.2. Correlation Analysis of the Dataset
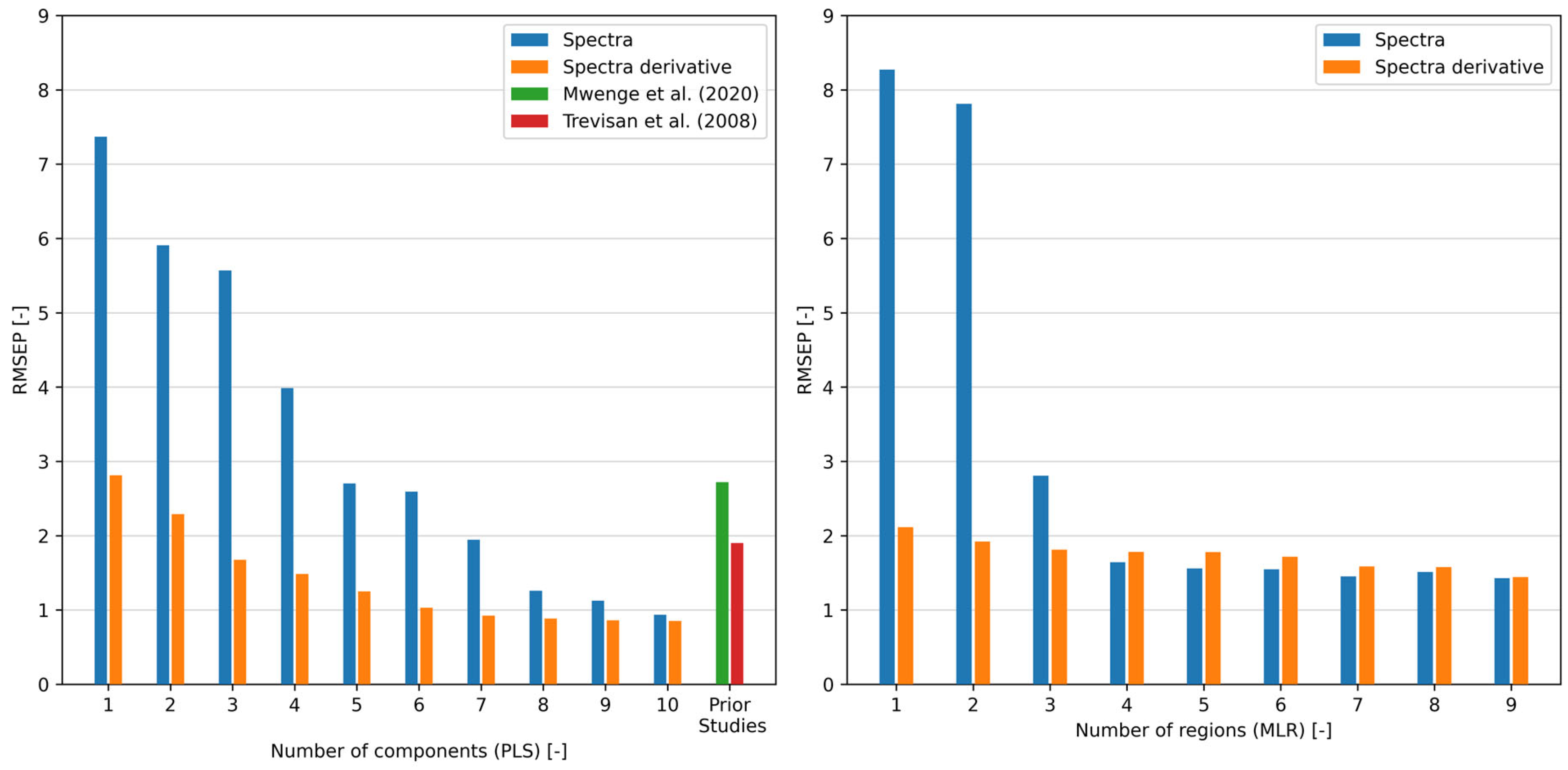
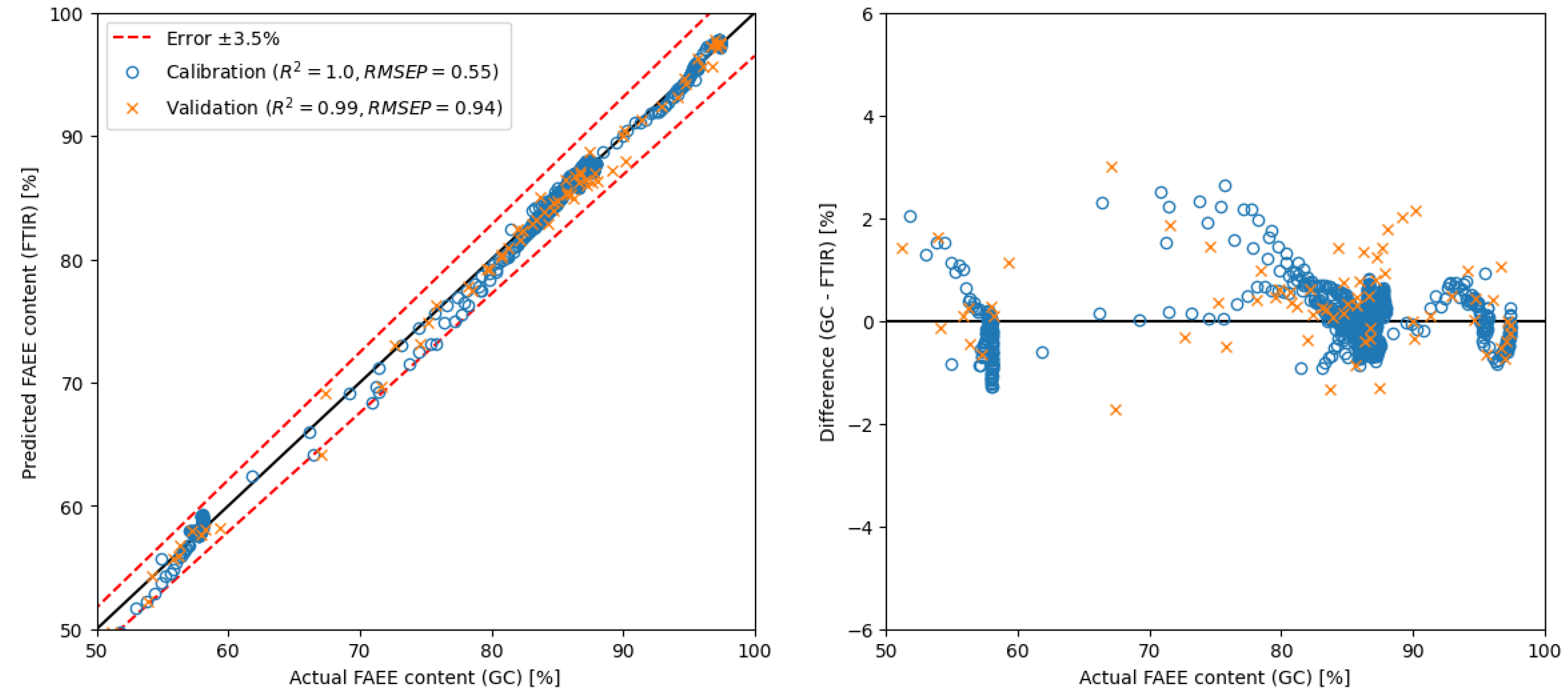
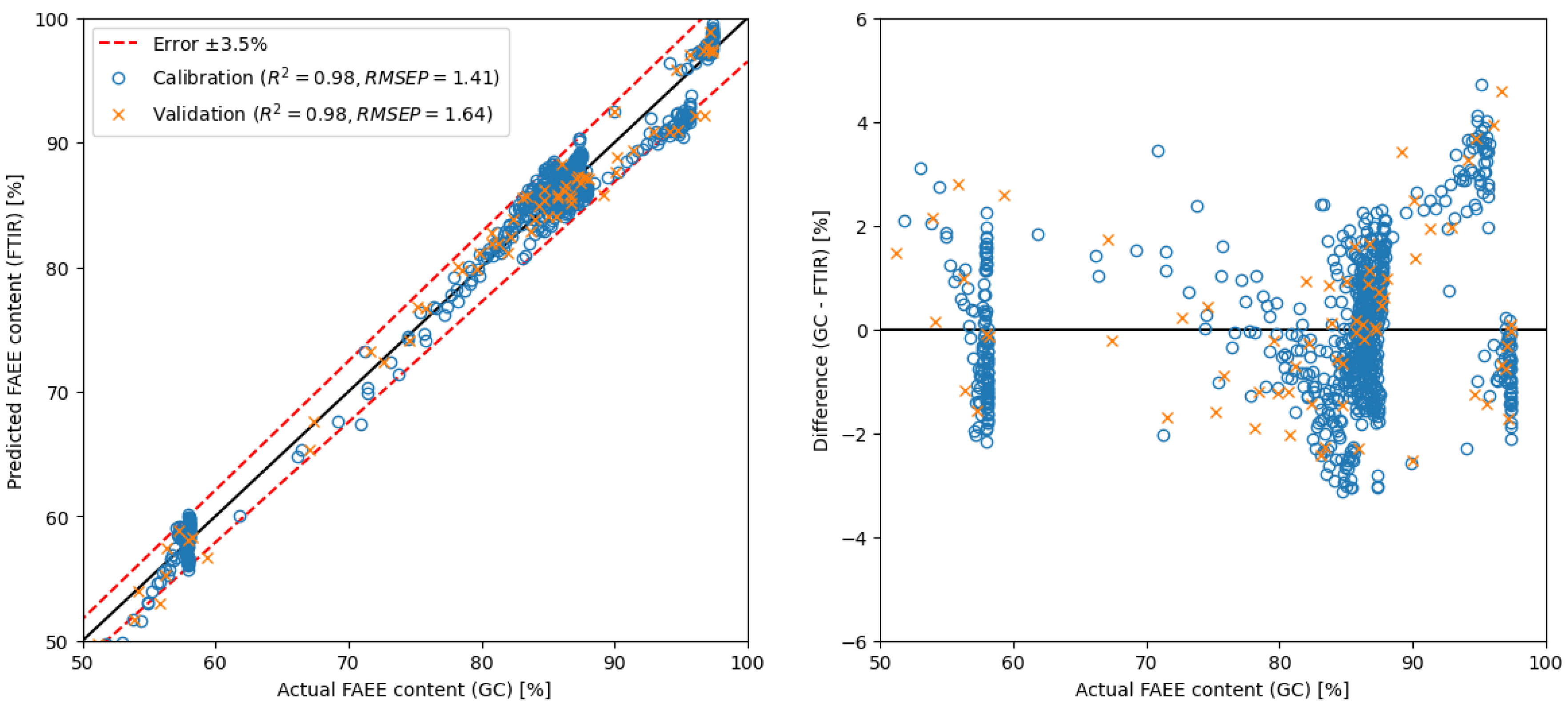
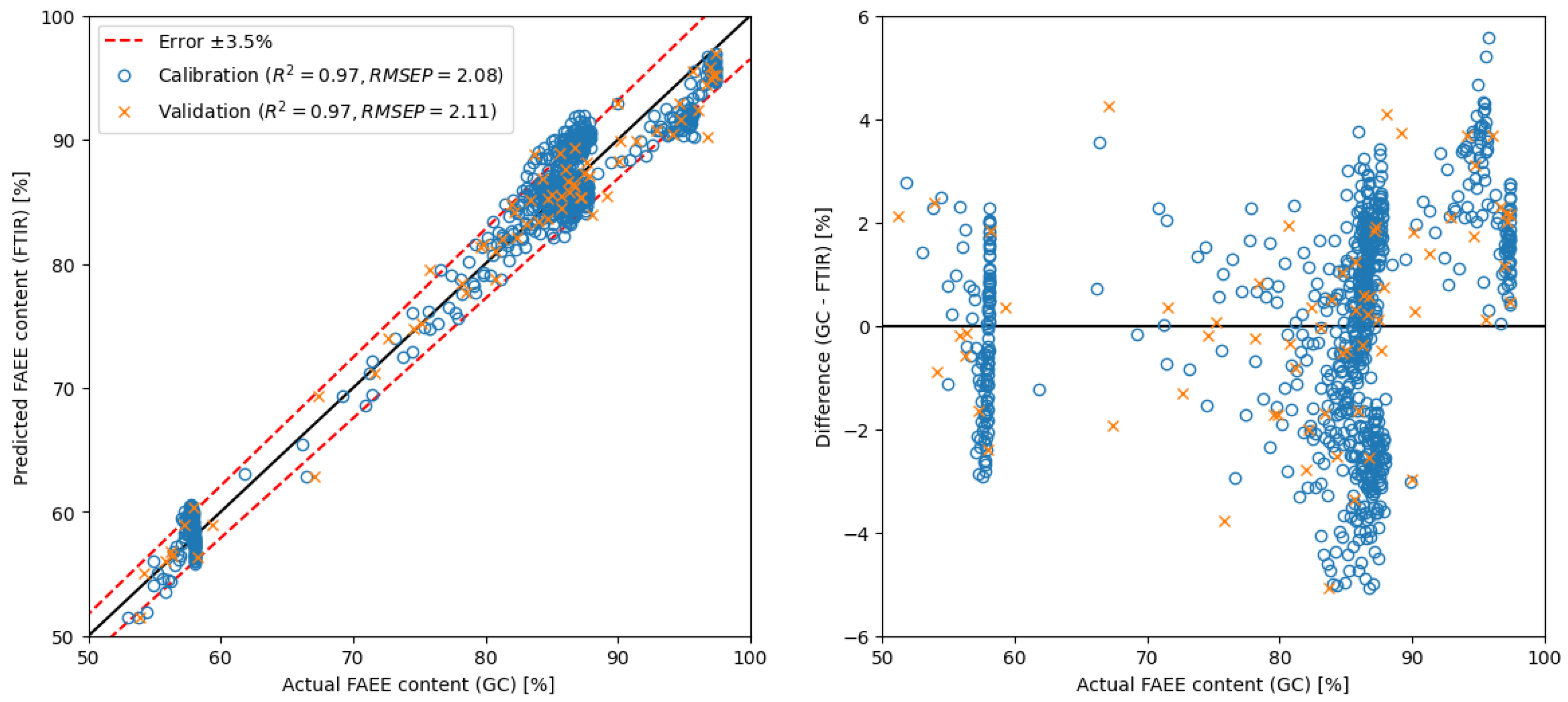
2.3. Development and Comparison of Regression Models
| Regression Method | Data Used | Spectral Range [cm−1] | Number of Components/Regions [-] | Reference Method | RMSEP | Source |
|---|---|---|---|---|---|---|
| PLS | preprocessed spectra | 650–2500 | 11 | GC | 2.72 | * Mwenge et al. [40] |
| PLS | preprocessed spectra | 814–3707 | 6 | NMR | 1.90 | Trevisan et al. [44] |
| PLS | raw spectra | 650–4000 | 7 | GC | 1.95 | ** |
| PLS | raw spectra | 650–4000 | 10 | GC | 0.94 | ** see Figure 6 |
| PLS | spectra derivative | 650–4000 | 3 | GC | 1.68 | ** |
| PLS | spectra derivative | 650–4000 | 7 | GC | 0.92 | ** |
| MLR | raw spectra | 650–4000 | 4 | GC | 1.64 | ** see Figure 7 |
| MLR | raw spectra | 650–4000 | 7 | GC | 1.45 | ** see Figure S2 |
| SLR | spectra derivative | 650–4000 | 1 | GC | 2.11 | ** see Figure 8 |
| MLR | spectra derivative | 650–4000 | 9 | GC | 1.44 | ** see Figure S3 |
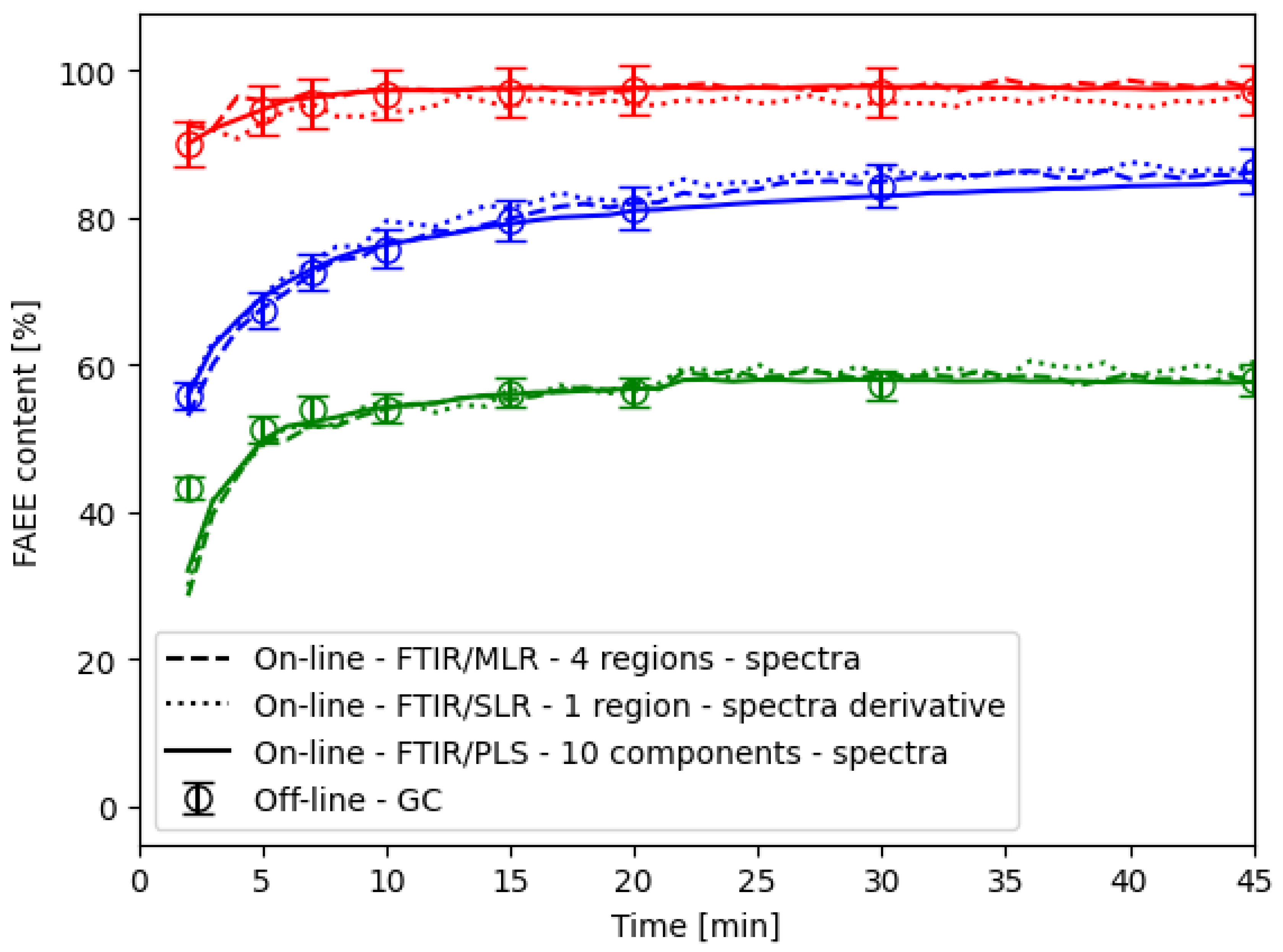
2.4. Method Application
2.5. Economic and Industrial Implementation Considerations
3. Materials and Methods
3.1. Materials
3.2. Small-Scale Transesterification System
3.3. Process Monitoring Methods
3.3.1. FTIR
3.3.2. GC—Reference Method
3.4. Experimental Data Evaluation
3.5. Regression Models and Their Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GC | Gas Chromatography |
| HPLC | High-Performance Liquid Chromatography |
| NMR | Nuclear Magnetic Resonance |
| NIR | Near-infrared spectroscopy |
| FTIR | Fourier Transform Infrared Spectroscopy |
| ATR | Attenuated Total Reflection |
| FAEE | Fatty Acid Ethyl Esters |
| PCA | Principal Component Analysis |
| PLS | Partial Least Squares |
| G | Glycerol |
| TG | Triglycerides |
| DG | Diglycerides |
| MG | Monoglycerides |
| SLR | Simple Linear Regression |
| MLR | Multiple Linear Regression |
| RMSEP | Root Mean Squared Error on Prediction |
References
- Biofuels. Available online: https://energy.ec.europa.eu/topics/renewable-energy/bioenergy/biofuels_en (accessed on 15 August 2025).
- Five Reasons Europe Needs to Do Better on Biofuels—POLITICO. Available online: https://www.politico.eu/sponsored-content/five-reasons-europe-needs-to-do-better-on-biofuels/ (accessed on 15 August 2025).
- Shay, E.G. Diesel Fuel from Vegetable Oils: Status and Opportunities. Biomass Bioenergy 1993, 4, 227–242. [Google Scholar] [CrossRef]
- Bookstaff, R.C.; PaiBir, S.; Bharaj, S.S.; Kelm, G.R.; Kulick, R.M.; Balm, T.K.; Murray, J.V. The Safety of the Use of Ethyl Oleate in Food Is Supported by Metabolism Data in Rats and Clinical Safety Data in Humans. Regul. Toxicol. Pharmacol. 2003, 37, 133–148. [Google Scholar] [CrossRef]
- Saravacos, G.D.; Marousis, S.N.; Raouzeos, G.S. Effect of Ethyl Oleate on the Rate of Air-Drying of Foods. J. Food Eng. 1988, 7, 263–270. [Google Scholar] [CrossRef]
- Apparent Canola Oil Wetter 840—Apparent, Ag. Available online: https://apparentag.com.au/product/apparent-canola-oil-wetter/ (accessed on 21 October 2022).
- Varnadoe, G.R.; Spence, S.K. Coating Compositions Containing Methyl/Ethyl Esters and Methods of Using Same. U.S. Patent 6,514,332, 4 February 2003. [Google Scholar]
- Freedman, B.; Butterfield, R.O.; Pryde, E.H. Transesterification Kinetics of Soybean Oil 1. J. Am. Oil Chem. Soc. 1986, 63, 1375–1380. [Google Scholar] [CrossRef]
- dos Santos, R.C.M.; Gurgel, P.C.; Pereira, N.S.; Breves, R.A.; de Matos, P.R.R.; Silva, L.P.; Sales, M.J.A.; Lopes, R. de V.V. Ethyl Esters Obtained from Pequi and Macaúba Oils by Transesterification with Homogeneous Acid Catalysis. Fuel 2020, 259, 116206. [Google Scholar] [CrossRef]
- Chen, J.; Bian, X.; Rapp, G.; Lang, J.; Montoya, A.; Trethowan, R.; Bouyssiere, B.; Portha, J.-F.; Jaubert, J.-N.; Pratt, P.; et al. From Ethyl Biodiesel to Biolubricants: Options for an Indian Mustard Integrated Biorefinery toward a Green and Circular Economy. Ind. Crops Prod. 2019, 137, 597–614. [Google Scholar] [CrossRef]
- Changmai, B.; Vanlalveni, C.; Ingle, A.P.; Bhagat, R.; Rokhum, L. Widely Used Catalysts in Biodiesel Production: A Review. RSC Adv. 2020, 10, 41625–41679. [Google Scholar] [CrossRef]
- Weinfeld, J.A.; Owens, S.A.; Eldridge, R.B. Reactive Dividing Wall Columns: A Comprehensive Review. Chem. Eng. Process.—Process Intensif. 2018, 123, 20–33. [Google Scholar] [CrossRef]
- Benavides, P.T.; Salazar, J.; Diwekar, U. Economic Comparison of Continuous and Batch Production of Biodiesel Using Soybean Oil. Environ. Prog. Sustain. Energy 2013, 32, 11–24. [Google Scholar] [CrossRef]
- Brásio, A.S.R.; Romanenko, A.; Fernandes, N.C.P.; Santos, L.O. First Principle Modeling and Predictive Control of a Continuous Biodiesel Plant. J. Process Control 2016, 47, 11–21. [Google Scholar] [CrossRef]
- EN 14103; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Ester and Linolenic Acid Methyl Ester Contents. European Committee for Standardization CEN: Brussels, Belgium, 2004.
- EN 14105; Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Free and Total Glycerol and Mono-, Di-, Triglyceride Contents. European Committee for Standardization CEN: Brussels, Belgium, 2011.
- EN 14214; Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods. European Committee for Standardization CEN: Brussels, Belgium, 2012.
- Knothe, G. Analytical Methods Used in the Production and Fuel Quality Assessment of Biodiesel. Trans. Am. Soc. Agric. Eng. 2001, 44, 193–200. [Google Scholar] [CrossRef]
- Mittelbach, M. Biodiesel. In Renewable Energy Systems; Springer: New York, NY, USA, 2013; pp. 27–44. [Google Scholar] [CrossRef]
- Šánek, L.; Pecha, J.; Kolomazník, K. Simultaneous Determination of Main Reaction Components in the Reaction Mixture during Biodiesel Production. J. Sep. Sci. 2013, 36, 1029–1036. [Google Scholar] [CrossRef]
- Syed, M.B. Analysis of Biodiesel by High Performance Liquid Chromatography Using Refractive Index Detector. MethodsX 2017, 4, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Galvan, D.; de Aguiar, L.M.; Rohwedder, J.J.R.; Borsato, D.; Killner, M.H.M. Online Monitoring of Transesterification Reaction by Medium-Resolution Benchtop 1H NMR and NIR Spectroscopy. Fuel Process. Technol. 2020, 208, 106511. [Google Scholar] [CrossRef]
- Dimmig, T.; Radig, W.; Knoll, C.; Dittmar, T. 13C-NMR-Spectroscopy for Determination of Conversion and Reaction Kinetics of Transesterification of Triglyceride to Methylester. Chem. Tech. 1999, 51, 326–328. [Google Scholar]
- Killner, M.H.M.; Rohwedder, J.J.R.; Pasquini, C. A PLS Regression Model Using NIR Spectroscopy for On-Line Monitoring of the Biodiesel Production Reaction. Fuel 2011, 90, 3268–3273. [Google Scholar] [CrossRef]
- Knothe, G. Monitoring a Progressing Transesterification Reaction by Fiber-Optic near Infrared Spectroscopy with Correlation to 1H Nuclear Magnetic Resonance Spectroscopy. JAOCS J. Am. Oil Chem. Soc. 2000, 77, 489–493. [Google Scholar] [CrossRef]
- De Lima, S.M.; Silva, B.F.A.; Pontes, D.V.; Pereira, C.F.; Stragevitch, L.; Pimentel, M.F. In-Line Monitoring of the Transesterification Reactions for Biodiesel Production Using NIR Spectroscopy. Fuel 2014, 115, 46–53. [Google Scholar] [CrossRef]
- Izida, T.; Bussler, L.; Silva, J.R.; Andrade, L.H.C.; Simionatto, E.; Simionatto, E.L.; Scharf, D.R.; Lima, S.M. On-Line in Situ Monitoring of the Soybean Oil and Ethanol Transesterification Reaction by Fluorescence Spectroscopy. Fuel 2015, 145, 109–115. [Google Scholar] [CrossRef]
- Richard, R.; Dubreuil, B.; Thiebaud-Roux, S.; Prat, L. On-Line Monitoring of the Transesterification Reaction Carried out in Microreactors Using near Infrared Spectroscopy. Fuel 2013, 104, 318–325. [Google Scholar] [CrossRef]
- De Filippis, P.; Giavarini, C.; Scarsella, M.; Sorrentino, M. Transesterification Processes for Vegetable Oils: A Simple Control Method of Methyl Ester Content. J. Am. Oil Chem. Soc. 1995, 72, 1399–1404. [Google Scholar] [CrossRef]
- Kanda, L.R.S.; Yamamoto, C.I.; Lopes, A.R.; Voll, F.A.P.; Corazza, M.L.; Wypych, F. Density, Refractive Index and Viscosity as Content Monitoring Tool of Acylglycerols and Fatty Acid Methyl Esters in the Transesterification of Soybean Oil. Anal. Methods 2016, 8, 5619–5627. [Google Scholar] [CrossRef]
- Talavera-Prieto, N.M.C.; Ferreira, A.G.M.; Portugal, A.A.; Egas, P. Density of Cottonseed Oil and Biodiesel. J. Chem. Eng. Data 2018, 63, 3438–3448. [Google Scholar] [CrossRef]
- Husár, J.; Pecha, J.; Šánek, L. Development and Validation of a Simple and Reliable Alternative Method for Process Monitoring and Final Product Quality Control during Fatty Acid Ethyl Esters Production. Talanta 2021, 235, 122752. [Google Scholar] [CrossRef]
- Rosset, M.; Perez-Lopez, O.W. FTIR Spectroscopy Analysis for Monitoring Biodiesel Production by Heterogeneous Catalyst. Vib. Spectrosc. 2019, 105, 102990. [Google Scholar] [CrossRef]
- Mahamuni, N.N.; Adewuyi, Y.G. Fourier Transform Infrared Spectroscopy (FTIR) Method to Monitor Soy Biodiesel and Soybean Oil in Transesterification Reactions, Petrodiesel- Biodiesel Blends, and Blend Adulteration with Soy Oil. Energy Fuels 2009, 23, 3773–3782. [Google Scholar] [CrossRef]
- de Lima Furtado, W.; Corgozinho, C.N.C.; Tauler, R.; Sena, M.M. Monitoring Biodiesel and Its Intermediates in Transesterification Reactions with Multivariate Curve Resolution Alternating Least Squares Calibration Models. Fuel 2021, 283, 119275. [Google Scholar] [CrossRef]
- Rabelo, S.N.; Ferraz, V.P.; Oliveira, L.S.; Franca, A.S. FTIR Analysis for Quantification of Fatty Acid Methyl Esters in Biodiesel Produced by Microwave-Assisted Transesterification. Int. J. Environ. Sci. Dev. 2015, 6, 964–969. [Google Scholar] [CrossRef]
- Dubé, M.A.; Zheng, S.; McLean, D.D.; Kates, M. A Comparison of Attenuated Total Reflectance-FTIR Spectroscopy and GPC for Monitoring Biodiesel Production. JAOCS J. Am. Oil Chem. Soc. 2004, 81, 599–603. [Google Scholar] [CrossRef]
- Natalello, A.; Sasso, F.; Secundo, F. Enzymatic Transesterification Monitored by an Easy-to-Use Fourier Transform Infrared Spectroscopy Method. Biotechnol. J. 2013, 8, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Zagonel, G.F.; Peralta-Zamora, P.; Ramos, L.P. Multivariate Monitoring of Soybean Oil Ethanolysis by FTIR. Talanta 2004, 63, 1021–1025. [Google Scholar] [CrossRef]
- Mwenge, P.; Seodigeng, T. In Situ Real-Time Multivariate Analysis of Methanolysis Monitoring of Sunflower Oil Using FTIR. Int. J. Chem. Mol. Eng. 2020, 14, 136–148. [Google Scholar]
- Reyman, D.; Saiz Bermejo, A.; Ramirez Uceda, I.; Rodriguez Gamero, M. A New FTIR Method to Monitor Transesterification in Biodiesel Production by Ultrasonication. Environ. Chem. Lett. 2014, 12, 235–240. [Google Scholar] [CrossRef]
- Yuan, T.; Akochi-Koble, E.; Pinchuk, D.; de Voort, F. FTIR On-Line Monitoring of Biodiesel Transesterification. Int. J. Renew. Energy Biofuels 2014, 2014, 178474. [Google Scholar] [CrossRef]
- Siatis, N.G.; Kimbaris, A.C.; Pappas, C.S.; Tarantilis, P.A.; Polissiou, M.G. Improvement of Biodiesel Production Based on the Application of Ultrasound: Monitoring of the Procedure by FTIR Spectroscopy. J. Am. Oil Chem. Soc. 2006, 83, 53–57. [Google Scholar] [CrossRef]
- Trevisan, M.G.; Garcia, C.M.; Schuchardt, U.; Poppi, R.J. Evolving Factor Analysis-Based Method for Correcting Monitoring Delay in Different Batch Runs for Use with PLS: On-Line Monitoring of a Transesterification Reaction by ATR-FTIR. Talanta 2008, 74, 971–976. [Google Scholar] [CrossRef]
- Ortega, M.F.; Donoso, D.; Bousbaa, H.; Bolonio, D.; Ballesteros, R.; García-Martínez, M.J.; Lapuerta, M.; Canoira, L. Optimized Production of Fatty Acid Ethyl Esters (FAEE) from Waste Frying Oil by Response Surface Methodology. Waste Biomass Valorization 2021, 12, 2303–2310. [Google Scholar] [CrossRef]
- Torres, A.; Fuentes, B.; Rodríguez, K.E.; Brito, A.; Díaz, L. Analysis of the Content of Fatty Acid Methyl Esters in Biodiesel by Fourier-Transform Infrared Spectroscopy: Method and Comparison with Gas Chromatography. J. Am. Oil Chem. Soc. 2020, 97, 651–661. [Google Scholar] [CrossRef]
- Chen, W.; Wang, C.W.; Liu, S.W.; Wu, Y.X.; Tang, Z.J. Reaction Processes and Mechanism of Supercritical Transesterification in Situ ATR Infrared Spectrum. J. Fuel Chem. Technol. 2011, 39, 817–822. [Google Scholar] [CrossRef]
- Lu, C.; Zeng, J.; Dong, Y.; Xu, X. Streaming Variational Probabilistic Principal Component Analysis for Monitoring of Nonstationary Process. J. Process Control 2024, 133, 103134. [Google Scholar] [CrossRef]
- Huo, K.; Huang, D.; Shang, C. A Novel White Component Analysis for Dynamic Process Monitoring. J. Process Control 2023, 127, 102998. [Google Scholar] [CrossRef]
- Back to Basics: Spectral Pre-Treatments, Derivatives|Spectroscopy Europe/World. Available online: https://www.spectroscopyeurope.com/td-column/back-basics-spectral-pre-treatments-derivatives (accessed on 10 April 2024).
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. Encycl. Anal. Chem. 2000, 12, 10815–10837. [Google Scholar] [CrossRef]
- Smith, B. The C=O Bond, Part VI: Esters and the Rule of Three. Available online: https://www.spectroscopyonline.com/view/co-bond-part-vi-esters-and-rule-three (accessed on 4 May 2025).
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; Wiley: New York, NY, USA, 1998; p. 704. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husar, J.; Sanek, L.; Pecha, J. Real-Time FTIR-ATR Spectroscopy for Monitoring Ethanolysis: Spectral Evaluation, Regression Modelling, and Molecular Insight. Int. J. Mol. Sci. 2025, 26, 9381. https://doi.org/10.3390/ijms26199381
Husar J, Sanek L, Pecha J. Real-Time FTIR-ATR Spectroscopy for Monitoring Ethanolysis: Spectral Evaluation, Regression Modelling, and Molecular Insight. International Journal of Molecular Sciences. 2025; 26(19):9381. https://doi.org/10.3390/ijms26199381
Chicago/Turabian StyleHusar, Jakub, Lubomir Sanek, and Jiri Pecha. 2025. "Real-Time FTIR-ATR Spectroscopy for Monitoring Ethanolysis: Spectral Evaluation, Regression Modelling, and Molecular Insight" International Journal of Molecular Sciences 26, no. 19: 9381. https://doi.org/10.3390/ijms26199381
APA StyleHusar, J., Sanek, L., & Pecha, J. (2025). Real-Time FTIR-ATR Spectroscopy for Monitoring Ethanolysis: Spectral Evaluation, Regression Modelling, and Molecular Insight. International Journal of Molecular Sciences, 26(19), 9381. https://doi.org/10.3390/ijms26199381







