Growth Phase-Dependent Changes in the Carbohydrate Metabolism of Penicillium Strains from Diverse Temperature Classes in Response to Cold Stress
Abstract
1. Introduction
2. Results
2.1. Effect of Cold Stress on the Activity of Glycolytic Enzymes
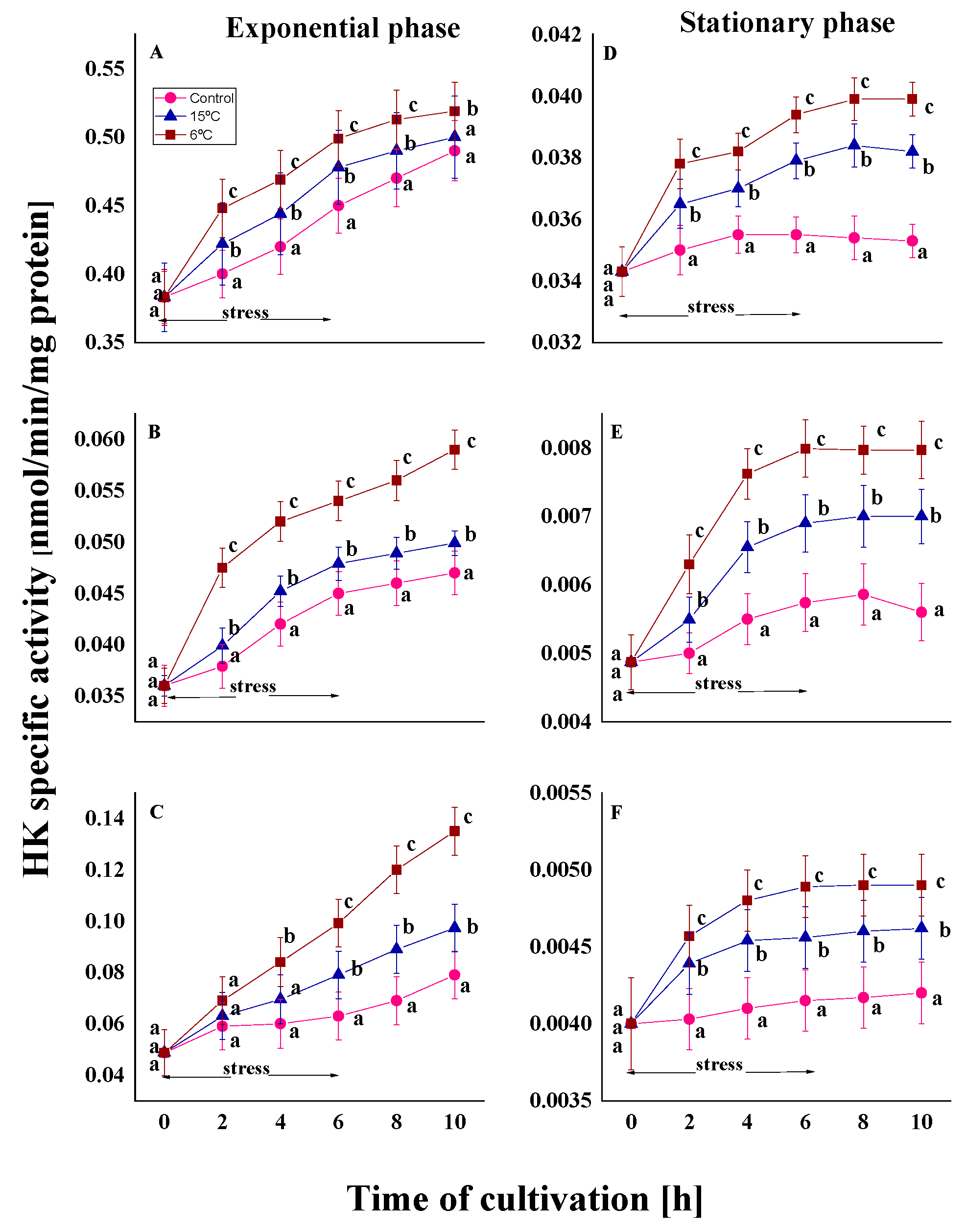
2.1.1. Changes in HK Activity
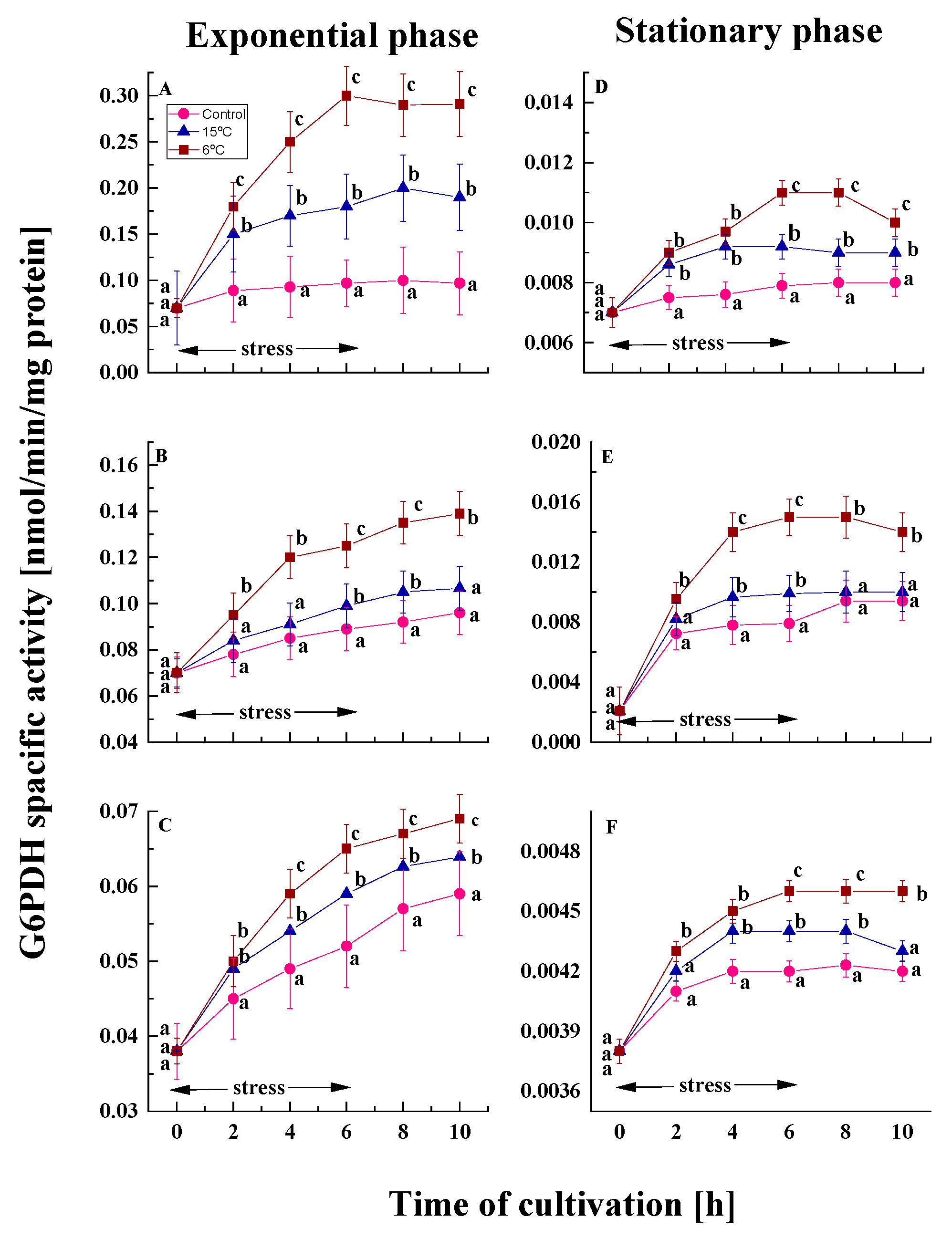
2.1.2. Changes in G6PDH Activity
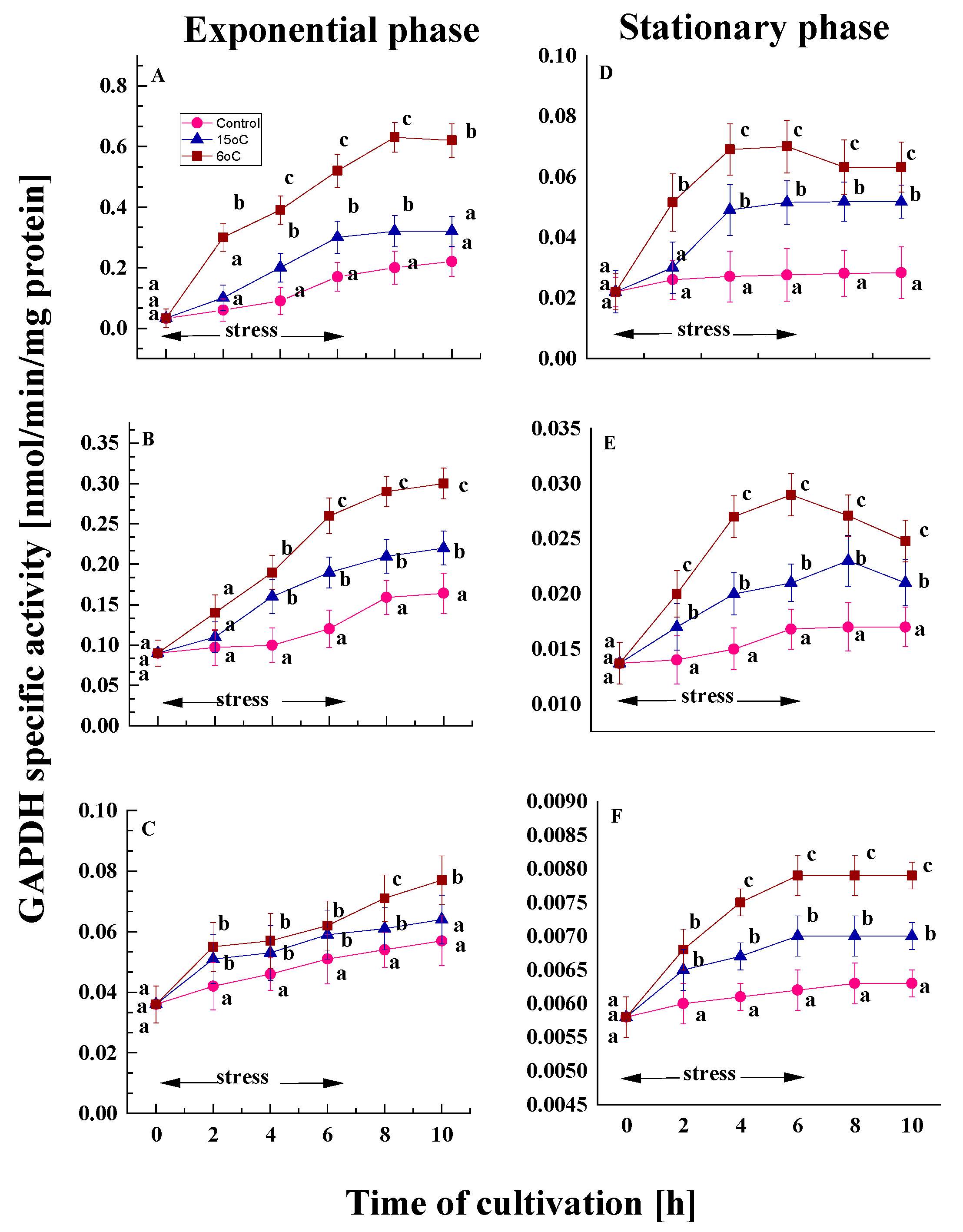
2.1.3. Changes in Glyceraldehyde Phosphate Dehydrogenase Activity
2.2. Effect of Cold Stress on the Enzymes Activity of TCA Cycle
2.2.1. Effect on the ICDH Activity
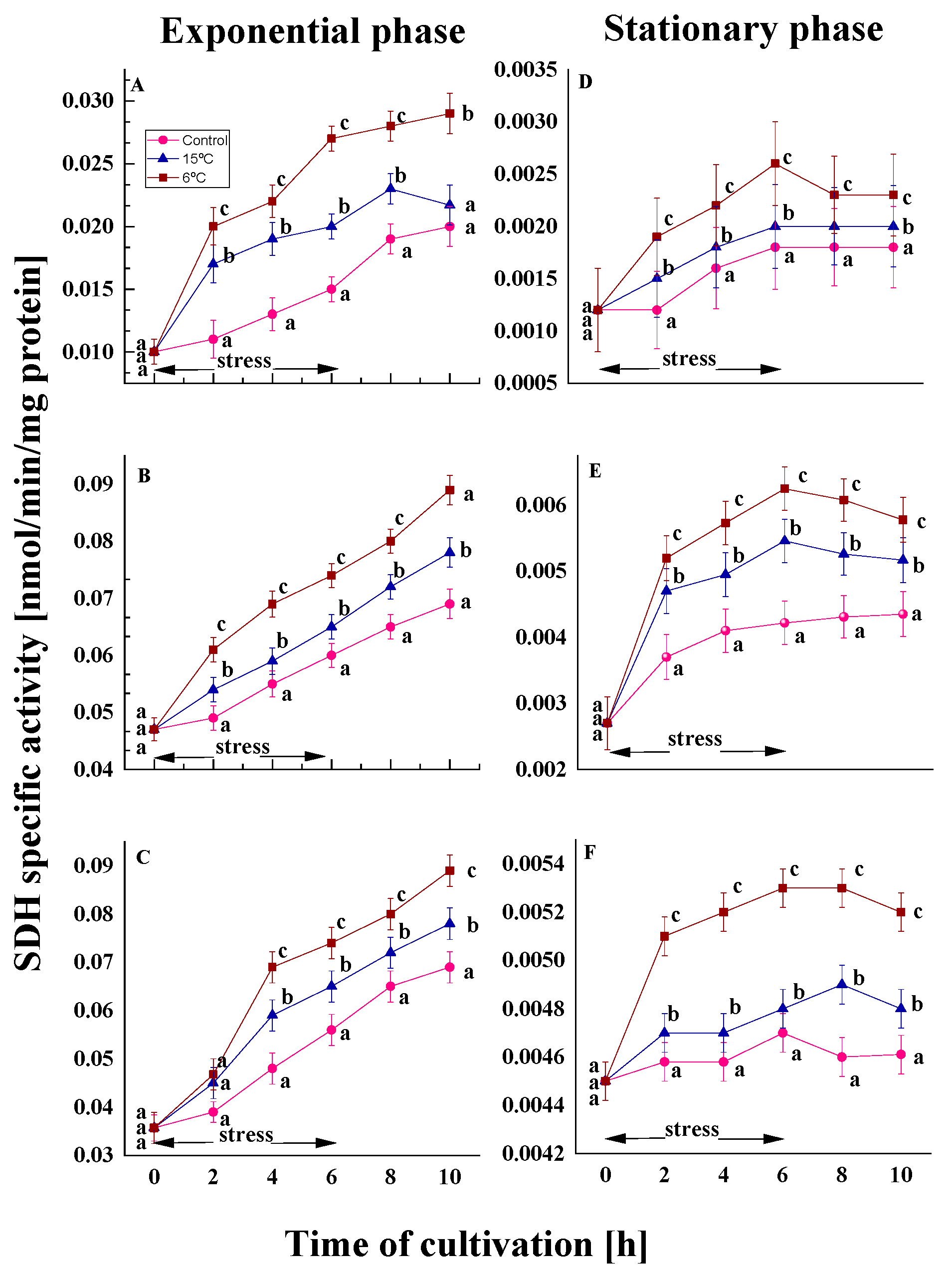
2.2.2. Effect on the SDH Activity
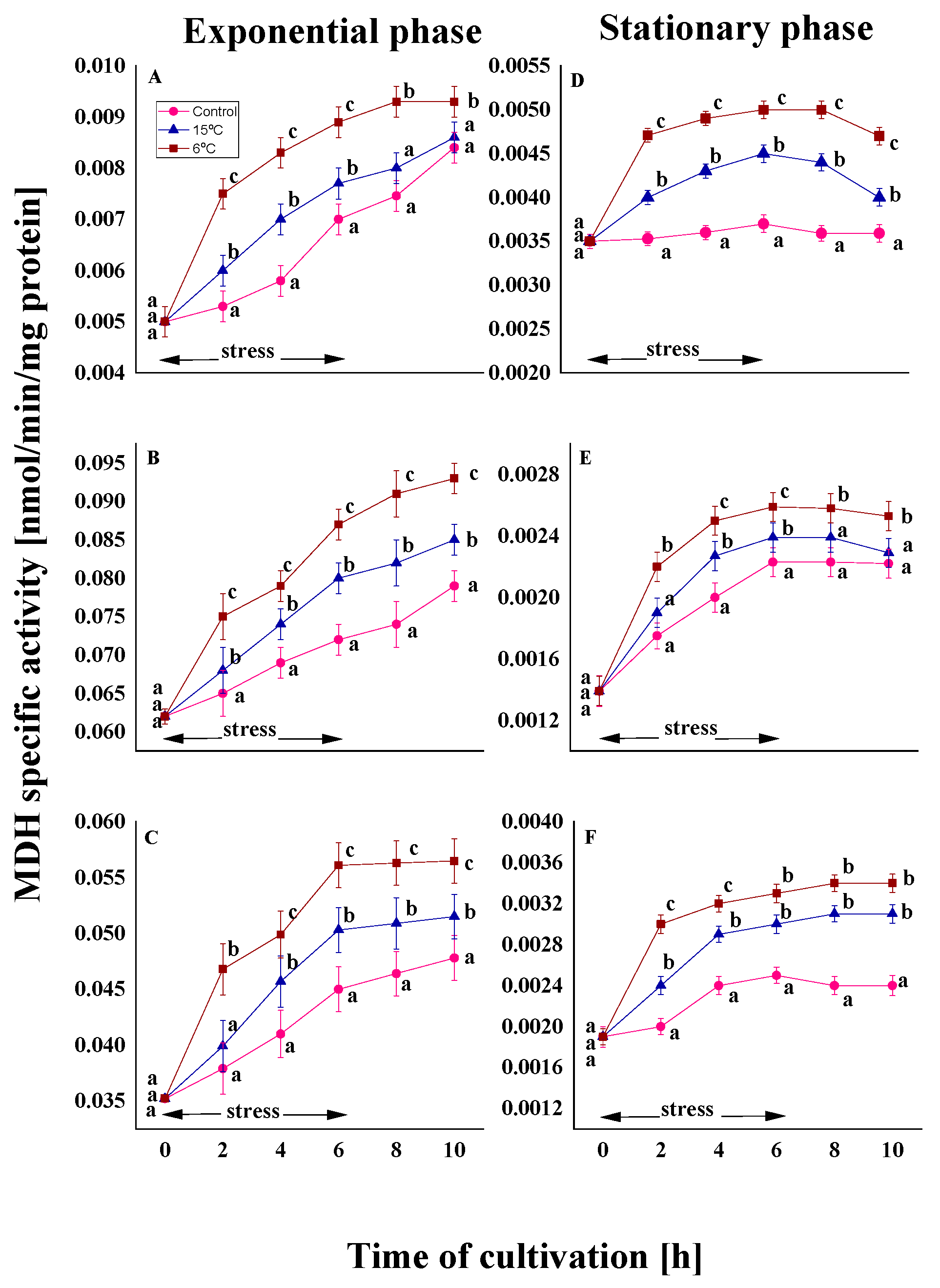
2.2.3. Effect on the Malat Dehydrogenase Activity
3. Discussion
3.1. Cold Stress Affected the Enzyme Activity of Carbohydrate Metabolism
3.1.1. Changes in the Enzyme Activity of Glycolysis
3.1.2. Changes in Enzyme Activity of the TCA Cycle
3.2. Cold Stress Response and Temperature Preference of the Tested Strains
3.3. Growth Phase-Dependent Cold Stress Response
3.3.1. Growth Phase-Dependent Changes Under Optimal Conditions
3.3.2. Growth Phase-Dependent Changes Under Cold Stress Conditions
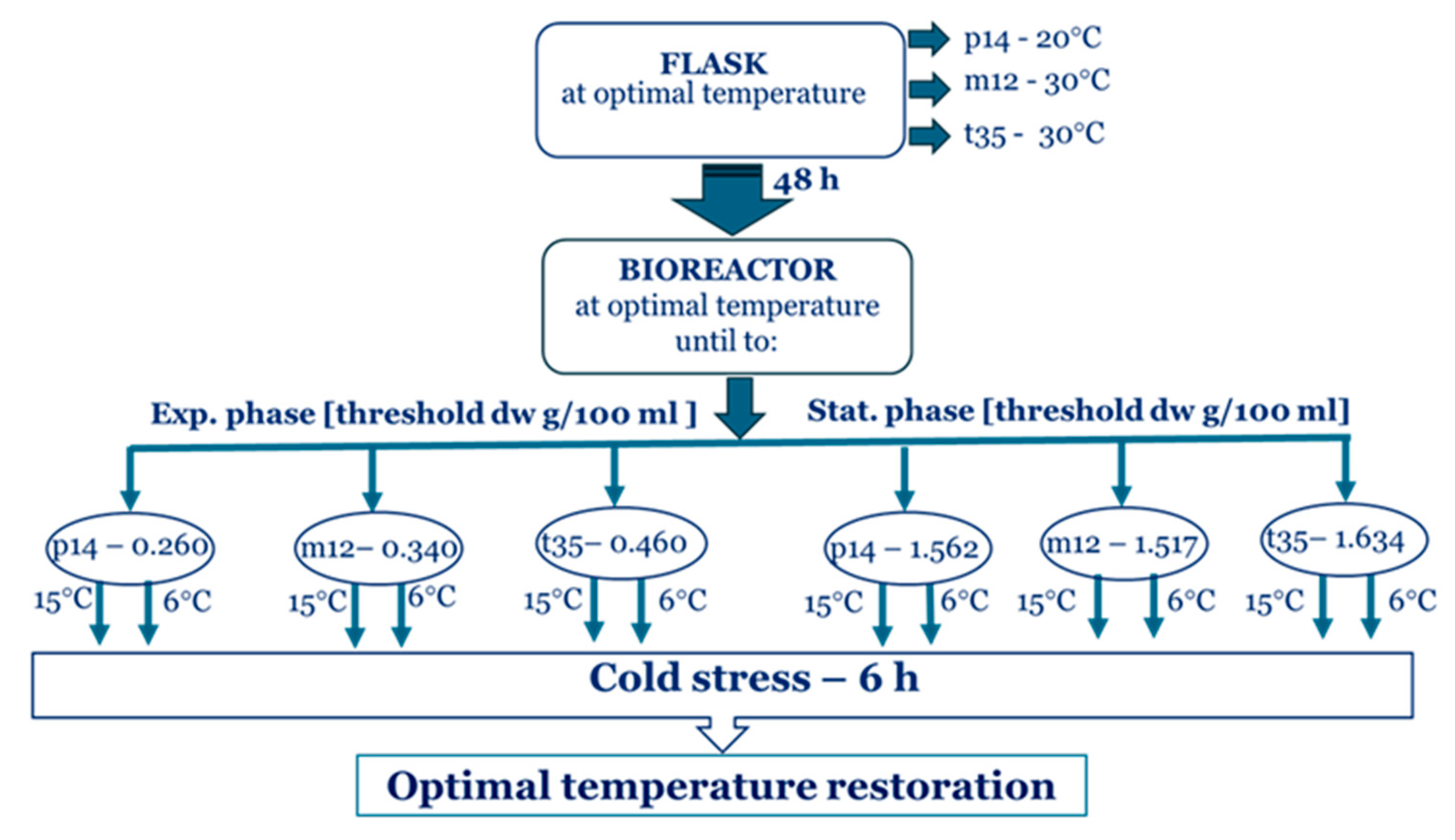
4. Materials and Methods
4.1. Fungal Strains and Cultivation
4.2. Preparation of Cell Fractions
4.3. Enzyme Activity Determination
4.3.1. Enzyme Activity of Glycolysis
4.3.2. Enzyme Activity of TCA Cycle
4.4. Statistical Evaluation of the Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Younger cells | cells from exponential-phase cultures |
| Older cells | cells from stationary-phase cultures |
| ATP | adenosine triphosphate |
| NADPH | nicotinamide adenine dinucleotide phosphate (reduced form) |
| ROS | reactive oxygen radicals |
| ●O2− | superoxide radicals |
| H2O2 | hydrogen peroxide |
| OH• | hydroxyl radicals |
| 1O2 | singlet oxygen |
| HK | hexokinase |
| G6PDH | glucose-6-phosphate-dehydrogenase |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| ICDH | isocitrate dehydrogenase |
| SDH | succinate dehydrogenase |
| MDH | malate dehydrogenase |
| TCA cycle | tricarboxylic acid cycle |
References
- Li, Y.; Tian, X.; Luo, J.; Bao, T.; Wang, S.; Wu, X. Molecular mechanisms of aging and anti-aging strategies. Cell Commun. Signal. 2024, 22, 285. [Google Scholar] [CrossRef]
- Ackermann, M.; Chao, L.; Bergstrom, C.T.; Doebeli, M. On the evolutionary origin of aging. Aging Cell 2007, 6, 235–244. [Google Scholar] [CrossRef]
- Osiewacz, H.D. Mitochondrial quality control in aging and lifespan control of the fungal aging model Podospora anserina. Biochem. Soc. Trans. 2011, 39, 1488–1492. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Yang, J.; Luo, J.; Tian, X.; Zhao, Y.; Li, Y.; Wu, X. Progress in understanding oxidative stress, aging, and aging-related diseases. Antioxidants 2024, 13, 394. [Google Scholar] [CrossRef]
- Maldonado, E.; Morales-Pison, S.; Urbina, F.; Solari, A. Aging hallmarks and the role of oxidative stress. Antioxidants 2023, 12, 651. [Google Scholar] [CrossRef] [PubMed]
- Van Breusegem, F.; Dat, J.F. Reactive oxygen species in plant cell death. Plant Physiol. 2006, 141, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.; Fernández-Ayala, D.J.M.; Stefanatos, R.K.A.; Jacobs, H.T. Mitochondrial ROS production correlates with, but does not directly regulate, lifespan in Drosophila. Aging 2010, 2, 200–223. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, W.; Biliński, T.; Bartosz, G. Oxidative stress during aging of stationary cultures of the yeast Saccharomyces cerevisiae. Free Radic. Biol. Med. 2000, 28, 659–664. [Google Scholar] [CrossRef]
- Osiewacz, H.D.; Borghouts, C. Mitochondrial oxidative stress and aging in the filamentous fungus Podospora anserina. Ann. N. Y. Acad. Sci. 2000, 908, 31–39. [Google Scholar] [CrossRef]
- Dukan, S.; Nyström, T. Bacterial senescence: Stasis results in increased and differential oxidation of cytoplasmic proteins leading to developmental induction of the heat shock regulon. Genes Dev. 1998, 12, 3431–3441. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidants in human health and disease. Annu. Rev. Nutr. 1996, 16, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Khalil, A.A.; Awadallah, S.; Khan, S.A.; Abu-Izneid, T.; Kamran, M.; Hemeg, H.A.; Mubarak, M.S.; Khalid, A.; Wilairatana, P. Reactive oxygen species in biological systems: Pathways, associated diseases, and potential inhibitors—A review. Food Sci. Nutr. 2024, 12, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Linnane, A.W.; Kios, M.; Vitetta, L. Healthy aging: Regulation of the metabolome by cellular redox modulation and prooxidant signaling systems: The essential roles of superoxide anion and hydrogen peroxide. Biogerontology 2007, 8, 445–467. [Google Scholar] [CrossRef]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Singh, S.D.; Choudhury, M.; Singh, D.; Singh, D.; Gogoi, J.B. Effect of free radicals on aging process in human health. Int. J. Pharm. Clin. Res. 2024, 16, 1612–1629. [Google Scholar]
- Pooja, G.; Shweta, S.; Patel, P. Oxidative stress and free radicals in disease pathogenesis: A review. Discov. Med. 2025, 2, 104. [Google Scholar] [CrossRef]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative stress and inflammation in the pathogenesis of neurological disorders: Mechanisms and implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef]
- Fang, M.; Lei, Z.; Ruilin, M.; Jing, W.; Leqiang, D. High temperature stress induced oxidative stress, gut inflammation and disordered metabolome and microbiome in tsinling lenok trout. Ecotoxicol. Environ. Saf. 2023, 266, 115607. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Thomas, T.; Curmi, P.M.G. Cold stress response in Archaea. Extremophiles 2000, 4, 321–331. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K. Low temperature and oxidative stress. Curr. Sci. 2002, 83, 109. [Google Scholar]
- Abrashev, R.; Miteva-Staleva, J.; Gocheva, Y.; Stoyancheva, G.; Dishliyska, V.; Spasova, B.; Krumova, E.; Angelova, M. Cell response to oxidative stress in Antarctic filamentous fungi. Appl. Sci. 2025, 15, 5149. [Google Scholar] [CrossRef]
- Qian, Z.; He, L.; Li, F. Understanding cold stress response mechanisms in plants: An overview. Front. Plant Sci. 2024, 15, 1443317. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.; Kong, X.; Khan, A.; Ullah, N.; Zhang, X. Plant coping with cold stress: Molecular and physiological adaptive mechanisms with future perspectives. Cells 2025, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kanamori, Y.; Moroishi, T. Cell attachment defines sensitivity to cold stress via the hippo pathway. Biochem. Biophys. Res. Commun. 2024, 730, 150373. [Google Scholar] [CrossRef]
- Lo Sterzo, M.; Iuso, D.; Palazzese, L.; Moncada, M.; Boffa, F.; Scudieri, A.; Gioia, L.; Czernik, M.; Loi, P. Exogenous LEA proteins expression enhances cold tolerance in mammalian cells by reducing oxidative stress. Sci. Rep. 2025, 15, 3351. [Google Scholar] [CrossRef]
- Cheng, J.H.; Lv, X.; Pan, Y.; Sun, D.W. Foodborne bacterial stress responses to exogenous reactive oxygen species (ROS) induced by cold plasma treatments. Trends Food Sci. Technol. 2020, 103, 239–247. [Google Scholar] [CrossRef]
- García-Ríos, E.; Ramos-Alonso, L.; Guillamón, J.M. Correlation between low temperature adaptation and oxidative stress in Saccharomyces cerevisiae. Front. Microbiol. 2016, 7, 1199. [Google Scholar] [CrossRef]
- Tian, F.; Lee, S.Y.; Woo, S.Y.; Choi, H.Y.; Heo, S.; Nah, G.; Chun, H.S. Transcriptomic responses of Aspergillus flavus to temperature and oxidative stresses during aflatoxin production. Sci. Rep. 2021, 11, 2803. [Google Scholar] [CrossRef]
- Wang, M.; Tian, J.; Xiang, M.; Liu, X. Living strategy of cold-adapted fungi with reference to several representative species. Mycology 2017, 8, 178–188, Correction in Mycology 2017, 8, iii. [Google Scholar] [CrossRef]
- Guo, R.; Liu, T.; Guo, C.; Chen, G.; Fan, J.; Zhang, Q. Carotenoid biosynthesis is associated with low-temperature adaptation in Rhodosporidium kratochvilovae. BMC Microbiol. 2022, 22, 319. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Shi, G.; Xiong, L.; Shi, J.; Kang, J.; Su, A.; Ding, X.; Li, Y.; Qiao, L.; et al. Quantitative proteomics insights into gel properties changes of myofibrillar protein from Procambarus clarkii under cold stress. Food Chem. 2022, 372, 130935. [Google Scholar] [CrossRef]
- Riccardi, C.; Calvanese, M.; Ghini, V.; Alonso-Vásquez, T.; Perrin, E.; Turano, P.; Giurato, G.; Weisz, A.; Parrilli, E.; Tutino, M.L.; et al. Metabolic robustness to growth temperature of a cold-adapted marine bacterium. Msystems 2023, 8, e01124-22. [Google Scholar] [CrossRef]
- Dumont, S.; Rivoal, J. Consequences of oxidative stress on plant glycolytic and respiratory metabolism. Front. Plant Sci. 2019, 10, 166. [Google Scholar] [CrossRef]
- Ortega-Martínez, P.; Roldán, M.; Díaz-Troya, S.; Florencio, F.J. Stress response requires an efficient connection between glycogen and central carbon metabolism by phosphoglucomutases in cyanobacteria. J. Exp. Bot. 2023, 74, 1532–1550. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Schwarzlander, M.; Obata, T.; Sirikantaramas, S.; Burow, M.; Olsen, C.E.; Tohge, T.; Fricker, M.D.; Moller, B.L.; Fernie, A.R.; et al. The metabolic response of Arabidopsis roots to oxidative stress is distinct from that of heterotrophic cells in culture and highlights a complex relationship between the levels of transcripts, metabolites, and flux. Mol. Plant 2009, 2, 390–406. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M. Cold-stress responses in the Antarctic basidiomycetous yeast Mrakia blollopis. R. Soc. Open Sci. 2016, 3, 160106. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Kudoh, S. Soil yeasts in the vicinity of Syowa Station, East Antarctica: Their diversity and extracellular enzymes, cold adaptation strategies, and secondary metabolites. Sustainability 2020, 12, 4518. [Google Scholar] [CrossRef]
- Gevi, F.; Leo, P.; Cassaro, A.; Pacelli, C.; de Vera, J.-P.P.; Rabbow, E.; Timperio, A.M.; Onofri, S. Metabolomic profile of the fungus Cryomyces antarcticus under simulated Martian and space conditions as support for life-detection missions on Mars. Front. Microbiol. 2022, 13, 749396. [Google Scholar] [CrossRef]
- Liu, J.Y.; Men, J.L.; Chang, M.C.; Feng, C.P.; Yuan, L.G. iTRAQ-based quantitative proteome revealed metabolic changes of Flammulina velutipes mycelia in response to cold stress. J. Proteom. 2017, 156, 75–84. [Google Scholar] [CrossRef]
- Gocheva, Y.G.; Tosi, S.; Krumova, E.T.; Slokoska, L.S.; Miteva, J.G.; Vassilev, S.V.; Angelova, M.B. Temperature downshift induces antioxidant response in fungi isolated from Antarctica. Extremophiles 2009, 13, 273–281. [Google Scholar] [CrossRef]
- Miteva-Staleva, J.G.; Krumova, E.T.; Vassilev, S.V.; Angelova, M.B. Cold-stress response during the stationary-growth phase of Antarctic and temperate-climate Penicillium strains. Microbiology 2017, 163, 1042–1051. [Google Scholar] [CrossRef]
- Miteva-Staleva, J.; Krumova, E.; Spasova, B.; Angelova, M. Age-related changes in protease activity as cold stress response by Penicillium strains from different temperature classes. Pol. Polar Res. 2024, 45, 67–79. [Google Scholar] [CrossRef]
- Branco, S.; Schauster, A.; Liao, H.L.; Ruytinx, J. Mechanisms of stress tolerance and their effects on the ecology and evolution of mycorrhizal fungi. New Phytol. 2022, 235, 2158–2175. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Lemus, E.; Usgame, K.; Fierro, A.; López-Alarcón, C. Enzymes of glycolysis and the pentose phosphate pathway as targets of oxidants: Role of redox reactions on the carbohydrate catabolism. Redox Biochem. Chem. 2025, 11, 100049. [Google Scholar] [CrossRef]
- McGuinness, O.P. The Citric Acid Cycle: A Pathway Central to Carbohydrate, Lipid, & Amino Acid Metabolism. In Harper’s Illustrated Biochemistry, 32nd ed.; Kennelly, P.J., Botham, K.M., McGuinness, O.P., Rodwell, V.W., Weil, P., Eds.; McGraw Hill Education: New York, NY, USA, 2023; Available online: https://www.accessscience.com/content/book/9781260469943/chapter/chapter16 (accessed on 20 August 2025).
- Wang, Q.; Li, M.; Zeng, N.; Zhou, Y.; Yan, J. Succinate dehydrogenase complex subunit C: Role in cellular physiology and disease. Exp. Biol. Med. 2023, 248, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Osiewacz, H.D.; Brust, D.; Hamann, A.; Kunstmann, B.; Luce, K.; Műller-Ohldach, M.; Scheckhuber, C.Q.; Servos, J.; Strobel, I. Mitochondrial pathways governing stress resistance, life, and death in the fungal aging model Podospora anserina. Ann. N. Y. Acad. Sci. 2010, 1197, 54–66. [Google Scholar] [CrossRef]
- Iakovou, E.; Kourti, M. A Comprehensive overview of the complex role of oxidative stress in aging, the contributing environmental stressors, and emerging antioxidant therapeutic interventions. Front. Aging Neurosci. 2022, 14, 827900. [Google Scholar] [CrossRef]
- Antibus, R.K. Temperature acclimation on growth, respiration and enzyme activities in an arctic and a temperate isolate of Cenococcum geophilum Fr. N. Am. Fungi. 2010, 5, 187–204. [Google Scholar] [CrossRef][Green Version]
- Wu, R.; Wyatt, E.; Chawla, K.; Tran, M.; Ghanefar, M.; Laakso, M.; Epting, C.L.; Ardehali, H. Hexokinase II knockdown results in exaggerated cardiac hypertrophy via increased ROS production. EMBO Mol. Med. 2012, 4, 633–646. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Dumouchel, T.; Aguer, C.; deKemp, R.; Beanlands, R.; Harper, M.E. Hexokinase II Acts through UCP3 to suppress mitochondrial reactive oxygen species production and maintain aerobic respiration. Biochem. J. 2011, 437, 301–311. [Google Scholar] [CrossRef][Green Version]
- Lin, X.W.; Xu, W.H. Hexokinase is a key regulator of energy metabolism and ROS activity in insect lifespan extension. Aging 2016, 8, 245–258. [Google Scholar] [CrossRef]
- Yan, Z.Y.; Zhao, M.R.; Huang, C.Y.; Zhang, L.J.; Zhang, J.X. Trehalose alleviates high-temperature stress in Pleurotus ostreatus by affecting central carbon metabolism. Microb. Cell Factories 2021, 20, 82. [Google Scholar] [CrossRef]
- Bagshaw, S.B.; Kitashova, A.; Özmen, B.; Yip, C.K.; Süling, B.E.; Schröder, L.; Kleine, T.; Nägele, T. Chloroplast positioning affects Hexokinase 1-mediated plant immunity under combined low temperature and high light. Plant Stress 2025, 15, 100791. [Google Scholar] [CrossRef]
- Wu, R.; Lin, X.; He, J.; Min, A.; Pang, L.; Wang, Y.; Lin, Y.; Zhang, Y.; He, W.; Li, M.; et al. Hexokinase1: A glucose sensor involved in drought stress response and sugar metabolism depending on its kinase activity in strawberry. Front. Plant Sci. 2023, 14, 1069830. [Google Scholar] [CrossRef] [PubMed]
- Landi, S.; Capasso, G.; Esposito, S. Different G6PDH isoforms show specific roles in acclimation to cold stress at various growth stages of Barley (Hordeum vulgare) and Arabidopsis thaliana. Plant Physiol. Biochem. 2021, 169, 190–202. [Google Scholar] [CrossRef]
- Kelavkar, U.P.; Chhatpar, H.S. Relations of enzymes in Aspergillus repens grown under sodium chloride stress. Curr. Microbiol. 1993, 27, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Angelova, M.; Pashova, S.; Spasova, B.; Vassilev, S.; Slokoska, L. Oxidative stress response of filamentous fungi induced by hydrogen peroxide and paraquat. Mycol. Res. 2005, 109, 150–158. [Google Scholar] [CrossRef]
- Thomas, P.A.; Geraldine, P.; Jayakumar, T. Pleurotus ostreatus, an edible mushroom, enhances glucose-6-phosphate dehydrogenase, ascorbate peroxidase and reduces xanthine dehydrogenase in major organs of aged rats. Pharm. Biol. 2014, 52, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, M.; Cheng, L.; Lin, Y.; Chen, Q.; Sun, B.; Gu, X.; Wang, Y.; Li, M.; Luo, Y.; et al. Identification of the cytosolic glucose-6-phosphate dehydrogenase gene from strawberry involved in cold stress response. Int. J. Mol. Sci. 2020, 21, 7322. [Google Scholar] [CrossRef]
- Nóbrega-Pereira, S.; Fernandez-Marcos, P.J.; Brioche, T.; Gomez-Cabrera, M.C.; Salvador-Pascual, A.; Flores, J.M.; Viña, J.; Serrano, M. G6PD Protects from oxidative damage and improves healthspan in mice. Nat. Commun. 2016, 7, 10894. [Google Scholar] [CrossRef]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.C.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.-M.; Krüger, A.; Alam, M.T.; et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. 2015, 90, 927–963. [Google Scholar] [CrossRef]
- Wang, Y.P.; Zhou, L.S.; Zhao, Y.Z.; Wang, S.W.; Chen, L.L.; Liu, L.X.; Ling, Z.Q.; Hu, F.J.; Sun, Y.P.; Zhang, J.Y.; et al. Regulation of G6PD acetylation by SIRT2 and KAT9 modulates NADPH homeostasis and cell survival during oxidative stress. EMBO J. 2014, 33, 1304–1320. [Google Scholar] [CrossRef]
- Cosentino, C.; Grieco, D.; Costanzo, V. ATM Activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 2011, 30, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Sun, H.; Zhang, S.; Shan, C. The Multiple Roles of glucose-6-phosphate dehydrogenase in tumorigenesis and cancer chemoresistance. Life 2022, 12, 271. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Guo, L.; Wang, X. Nuclear moonlighting of cytosolic glyceraldehyde-3-phosphate dehydrogenase regulates Arabidopsis response to heat stress. Nat. Commun. 2020, 11, 3439. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, J.; Xia, N.; Qu, Y.; Zhan, Y.; Teng, W.; Li, H.; Li, W.; Li, Y.; Zhao, X.; et al. Genome-wide identification and analysis of glyceraldehyde-3-phosphate dehydrogenase family reveals the role of GmGAPDH14 to improve salt tolerance in soybean (Glycine max, L.). Front. Plant Sci. 2023, 14, 1193044. [Google Scholar] [CrossRef]
- Thanonkeo, P.; Monkeang, R.; Saksirirat, W.; Thanonkeo, K.; Akiyama, T. Cloning and molecular characterization of glyceraldehyde-3-phosphate dehydrogenase gene from thermotolerant mushroom Lentinus polychrous. Afr. J. Biotechnol. 2010, 9, 3242–3251. [Google Scholar] [CrossRef]
- Kadooka, C.; Katsuki, N.; Masuo, S.; Kojima, S.; Amahisa, M.; Suzuki, K.; Doi, Y.; Takeshita, N.; Takaya, N. Fungal glyceraldehyde 3-phosphate dehydrogenase GpdC maintains glycolytic mechanism against reactive nitrogen stress-induced damage. Front. Microbiol. 2024, 15, 1475567. [Google Scholar] [CrossRef]
- Morigasaki, S.; Shimada, K.; Ikner, A.; Yanagida, M.; Shiozaki, K. Glycolytic enzyme, GAPDH promotes peroxide stress signaling through multistep phosphorelay to a MAPK Cascade. Mol. Cell 2008, 30, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Noster, J.; Persicke, M.; Chao, T.C.; Krone, L.; Heppner, B.; Hensel, M.; Hansmeier, N. Impact of ROS-induced damage of TCA cycle enzymes on metabolism and virulence of Salmonella enterica serovar typhimurium. Front. Microbiol. 2019, 10, 762. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Zhang, Z.; Yang, H.; Hu, D.; Wu, Y.; Xue, J.; Guo, D.; Xu, S. Characterization of the isocitrate dehydrogenase gene family and their response to drought stress in maize. Plants 2023, 12, 3466. [Google Scholar] [CrossRef] [PubMed]
- Bériault, R.; Hamel, R.; Chenier, D.; Mailloux, R.J.; Joly, H.; Appanna, V.D. The overexpression of NADPH-producing enzymes counters the oxidative stress evoked by gallium an iron mimetic. BioMetals 2007, 20, 165–176. [Google Scholar] [CrossRef]
- Su, Y.; Wang, T.; Wu, N.; Li, D.; Fan, X.; Xu, Z.; Mishra, S.K.; Yang, M. Alpha-ketoglutarate extends Drosophila lifespan by inhibiting mTOR and activating AMPK. Aging 2019, 11, 4183–4197. [Google Scholar] [CrossRef]
- Yoshihara, T.; Hamamoto, T.; Munakata, R.; Tajiri, R.; Ohsumi, M.; Yokota, S. Localization of cytosolic NADP-dependent isocitrate dehydrogenase in the peroxisomes of rat liver cells: Biochemical and immunocytochemical studies. J. Histochem. Cytochem. 2001, 49, 1123–1131. [Google Scholar] [CrossRef]
- Reitman, Z.J.; Hai, Y. Isocitrate dehydrogenase 1 and 2 mutations in cancer: Alterations at a crossroads of cellular metabolism. J. Natl. Cancer Inst. 2010, 102, 932–941. [Google Scholar] [CrossRef]
- Lin, Z.H.; Chang, S.Y.; Shen, W.C.; Lin, Y.H.; Shen, C.L.; Liao, S.B.; Liu, Y.C.; Chen, C.S.; Ching, T.T.; Wang, H.D. Isocitrate dehydrogenase alpha-1 modulates lifespan and oxidative stress tolerance in Caenorhabditis elegans. Int. J. Mol. Sci. 2022, 24, 612. [Google Scholar] [CrossRef]
- Ding, J.L.; Li, X.H.; Lei, J.H.; Feng, M.G.; Ying, S.H. Succinate dehydrogenase subunit c contributes to mycelial growth and development, stress response, and virulence in the insect parasitic fungus Beauveria bassiana. Microbiol. Spectr. 2022, 10, e0289122. [Google Scholar] [CrossRef]
- Fan, M.; Qi, H.; Shao, W.; Zhang, H.; Yin, Y.; Chen, Y.; Zhao, Y.; Ma, Z. Functional analysis of all succinate dehydrogenase subunits in Fusarium fujikuroi. Phytopathol. Res. 2024, 6, 35. [Google Scholar] [CrossRef]
- Shao, W.; Yang, Y.; Zhang, Y.; Lv, C.; Ren, W.; Chen, C. Involvement of BcStr2 in methionine biosynthesis, vegetative differentiation, multiple stress tolerance and virulence in Botrytis cinerea. Mol. Plant Pathol. 2016, 17, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yang, F.; Zhang, X.; Tang, M.; Wan, K.; Lai, C.; Lai, Z.; Lin, Y. Genome-wide identification and expression analysis unveil the involvement of the succinic semialdehyde dehydrogenase (SSADH) gene family in banana low-temperature stress. Int. J. Mol. Sci. 2025, 26, 3006. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, B.; Bansal, V.K.; Kav, N.N. Proteome-level investigation of Brassica carinata-derived resistance to Leptosphaeria maculans. J. Agric. Food Chem. 2005, 53, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Farahani, A.S.; Taghavi, S.M. Expression profiling of malate dehydrogenase, superoxide dismutase, and polygalacturonase-inhibiting protein in common bean in response to host and non-host pathogens. J. Plant Pathol. 2015, 97, 491–495. Available online: https://cabidigitallibrary.org (accessed on 15 June 2025).
- Zhao, Y.; Luo, L.; Xu, J.; Xin, P.; Guo, H.; Wu, J.; Bai, L.; Wang, G.; Chu, J.; Zuo, J.; et al. Malate transported from chloroplast to mitochondrion triggers production of ROS and PCD in Arabidopsis thaliana. Cell Res. 2018, 28, 448–461. [Google Scholar] [CrossRef]
- Wang, M.; Wang, H.; Wang, P.; Fu, H.H.; Li, C.Y.; Qin, Q.L.; Liang, Y.; Wang, M.; Chen, X.L.; Zhang, Y.Z.; et al. TCA cycle enhancement and uptake of monomeric substrates support growth of marine Roseobacter at low temperature. Commun. Biol. 2022, 5, 705. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Bériault, R.; Lemire, J.; Singh, R.; Chénier, D.R.; Hamel, R.D.; Appanna, V.D. The tricarboxylic acid cycle, an ancient metabolic network with a novel twist. PLoS ONE 2007, 2, e690. [Google Scholar] [CrossRef]
- Su, Y.; Jiang, X.; Wu, W.; Wang, M.; Hamid, M.I.; Xiang, M.; Liu, X. Genomic, transcriptomic, and proteomic analysis provide insights into the cold adaptation mechanism of the obligate psychrophilic fungus Mrakia psychrophila. G3 2016, 6, 3603–3613. [Google Scholar] [CrossRef]
- Abu Bakar, N.; Lau, B.Y.C.; González-Aravena, M.; Smykla, J.; Krzewicka, B.; Karsani, S.A.; Alias, S.A. Geographical diversity of proteomic responses to cold stress in the fungal genus Pseudogymnoascus. Microb. Ecol. 2023, 87, 11. [Google Scholar] [CrossRef]
- Larochelle, M.; Drouin, S.; Robert, F.; Turcotte, B. Oxidative stress-activated zinc cluster protein, Stb5, has dual activator/repressor functions required for pentose phosphate pathway regulation and NADPH production. Mol. Cell. Biol. 2006, 26, 6690–6701. [Google Scholar] [CrossRef]
- Touchette, D.; Altshuler, I.; Gostinčar, C.; Zalar, P.; Raymond-Bouchard, I.; Zajc, J.; McKay, C.P.; Gunde-Cimerman, N.; Whyte, L.G. Novel antarctic yeast adapts to cold by switching energy metabolism and increasing small RNA synthesis. ISME J. 2022, 16, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Corona, J.F.; González-Hernández, G.A.; Padilla-Guerrero, I.E.; Olmedo-Monfil, V.; Martínez-Rocha, A.L.; Patiño-Medina, J.A.; Meza-Carmen, V.; Torres-Guzmán, J.C. Fungal alcohol dehydrogenases: Physiological function, molecular properties, regulation of their production, and biotechnological potential. Cells 2023, 12, 2239. [Google Scholar] [CrossRef]
- Dufault-Thompson, K.; Chang, N.; Huahua, J.; Jian, F.; Wang, Y.; Zhang, F. Reconstruction and analysis of thermodynamically constrained models reveal metabolic responses of a deep-sea bacterium to temperature perturbations. Msystems 2022, 7, e00588-22. [Google Scholar] [CrossRef]
- García-Descalzo, L.; García-López, E.; Cid, C. Comparative proteomic analysis of psychrophilic vs. mesophilic bacterial species reveals different strategies to achieve temperature adaptation. Front. Microbiol. 2022, 13, 841359. [Google Scholar] [CrossRef]
- Grum-Grzhimaylo, O.A.; Bubnova, E.N.; Grum-Grzhimaylo, A.A.; Debets, A.J.M.; Aanen, D.K. Occurrence of Psychrophilomyces antarcticus in the Arctic. Fungal Ecol. 2025, 74, 101408. [Google Scholar] [CrossRef]
- Yusof, N.A.; Hashim, N.H.F.; Bharudin, I. Cold adaptation strategies and the potential of psychrophilic enzymes from the Antarctic yeast, Glaciozyma antarctica PI12. J. Fungi 2021, 7, 528. [Google Scholar] [CrossRef]
- Peixoto, D.N.; Panek, A.D. The involvement of hexokinases in trehalose synthesis. Biochem. Mol. Biol. Int. 1999, 47, 873–880. [Google Scholar] [CrossRef]
- Saavedra, E.; Ramos-Casillas, L.E.; Marín-Hernández, A.; Moreno-Sánchez, R.; Guerra-Sánchez, G. Glycolysis in Ustilago maydis. FEMS Yeast Res. 2008, 8, 1313–1323. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.E.; Braunberger, K.S.; Kelly, J.M. Role of HxkC, a mitochondrial hexokinase-like protein, in fungal programmed cell death. Fungal Genet. Biol. 2016, 97, 36–45. [Google Scholar] [CrossRef]
- den Ridder, M.J.; van den Brandeler, W.; Altiner, M.; Daran-Lapujade, P.A.S.; Pabst, M. Proteome dynamics during transition from exponential to stationary phase under aerobic and anaerobic conditions in yeast. Mol. Cell. Proteom. 2023, 22, 100552. [Google Scholar] [CrossRef] [PubMed]
- Fleck, C.B.; Brock, M. Aspergillus fumigatus catalytic glucokinase and hexokinase: Expression analysis and importance for germination, growth, and conidiation. Eukaryot. Cell 2010, 9, 1120–1135. [Google Scholar] [CrossRef]
- Samokhvalov, V.; Ignatov, M.; Kondrashova, M. Inhibition of Krebs cycle and activation of glyoxylate cycle in the course of chronological aging of Saccharomyces cerevisiae. Compensatory role of succinate oxidation. V. Biochim. 2004, 86, 39–46. [Google Scholar] [CrossRef]
- Kondoh, H.; Lleonart, M.E.; Bernard, D.; Gil, J. Protection from oxidative stress by enhanced glycolysis: A possible mechanism of cellular immortalization. Histol. Histopathol. 2007, 22, 85–90. [Google Scholar] [CrossRef]
- Kierans, S.J.; Taylor, C.T. Glycolysis: A multifaceted metabolic pathway and signaling hub. J. Biol. Chem. 2024, 300, 107906. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Zhang, L.; Gao, W.; Huang, C.; Huber, P.E.; Zhou, X.; Li, G.; Shen, B.; Zou, C. NAD+ metabolism: Pathophysiologic mechanisms and therapeutic potential. Sig. Transduct. Target Ther. 2020, 5, 227. [Google Scholar] [CrossRef] [PubMed]
- Borkum, J.M. The tricarboxylic acid cycle as a central regulator of the rate of aging: Implications for metabolic interventions. Adv. Biol. 2023, 7, 2300095. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.; Zhang, S.; Wang, Y.; Pei, X.; Chen, Y.; Zhang, J.; Zhang, Y.; Du, Y.; Hao, S.; et al. Mitochondrial dysfunction in the regulation of aging and aging-related diseases. Cell Commun. Signal. 2025, 23, 290. [Google Scholar] [CrossRef]
- Gocheva, Y.; Krumova, E.; Slokoska, L.; Miteva, J.; Angelova, M. Cell response of Antarctic and temperate strains of Penicillium spp. to different growth temperature. Mycol. Res. 2006, 110, 1347–1354. [Google Scholar] [CrossRef]
- Krumova, E.; Dolashki, A.; Pashova, S.; Dolashka-Angelova, P.; Stevanovic, S.; Hristova, R.; Stefanova, L.; Voelter, W.; Angelova, M. Unusual location and characterization of cu/zn-containing superoxide dismutase from filamentous fungus Humicola lutea. Arch. Microbiol. 2008, 189, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bergmeyer, H.U. Methods of Enzymatic Analysis, 2nd ed.; Academic Press: New York, NY, USA, 1974. [Google Scholar]
- Kornberg, A. Isocitric dehydrogenase. Meth. Enzymol. 1955, 1, 707–708. [Google Scholar]
- Veeger, C.; Dervastanian, V.; Zeylemacher, W. Succinate dehydrogenase. Methods Enzymol. 1969, 13, 81–82. [Google Scholar]
- Kornberg, A.; Horecker, R.L. Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA, 1955; Volume 1, p. 323. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miteva-Staleva, J.; Krumova, E.; Angelova, M. Growth Phase-Dependent Changes in the Carbohydrate Metabolism of Penicillium Strains from Diverse Temperature Classes in Response to Cold Stress. Int. J. Mol. Sci. 2025, 26, 9308. https://doi.org/10.3390/ijms26199308
Miteva-Staleva J, Krumova E, Angelova M. Growth Phase-Dependent Changes in the Carbohydrate Metabolism of Penicillium Strains from Diverse Temperature Classes in Response to Cold Stress. International Journal of Molecular Sciences. 2025; 26(19):9308. https://doi.org/10.3390/ijms26199308
Chicago/Turabian StyleMiteva-Staleva, Jeny, Ekaterina Krumova, and Maria Angelova. 2025. "Growth Phase-Dependent Changes in the Carbohydrate Metabolism of Penicillium Strains from Diverse Temperature Classes in Response to Cold Stress" International Journal of Molecular Sciences 26, no. 19: 9308. https://doi.org/10.3390/ijms26199308
APA StyleMiteva-Staleva, J., Krumova, E., & Angelova, M. (2025). Growth Phase-Dependent Changes in the Carbohydrate Metabolism of Penicillium Strains from Diverse Temperature Classes in Response to Cold Stress. International Journal of Molecular Sciences, 26(19), 9308. https://doi.org/10.3390/ijms26199308




