The Sirt1 Activator SRT1720 Mitigates Human Monocyte Activation and Improves Outcome During Gram-Negative Pneumosepsis in Mice
Abstract
1. Introduction
2. Results
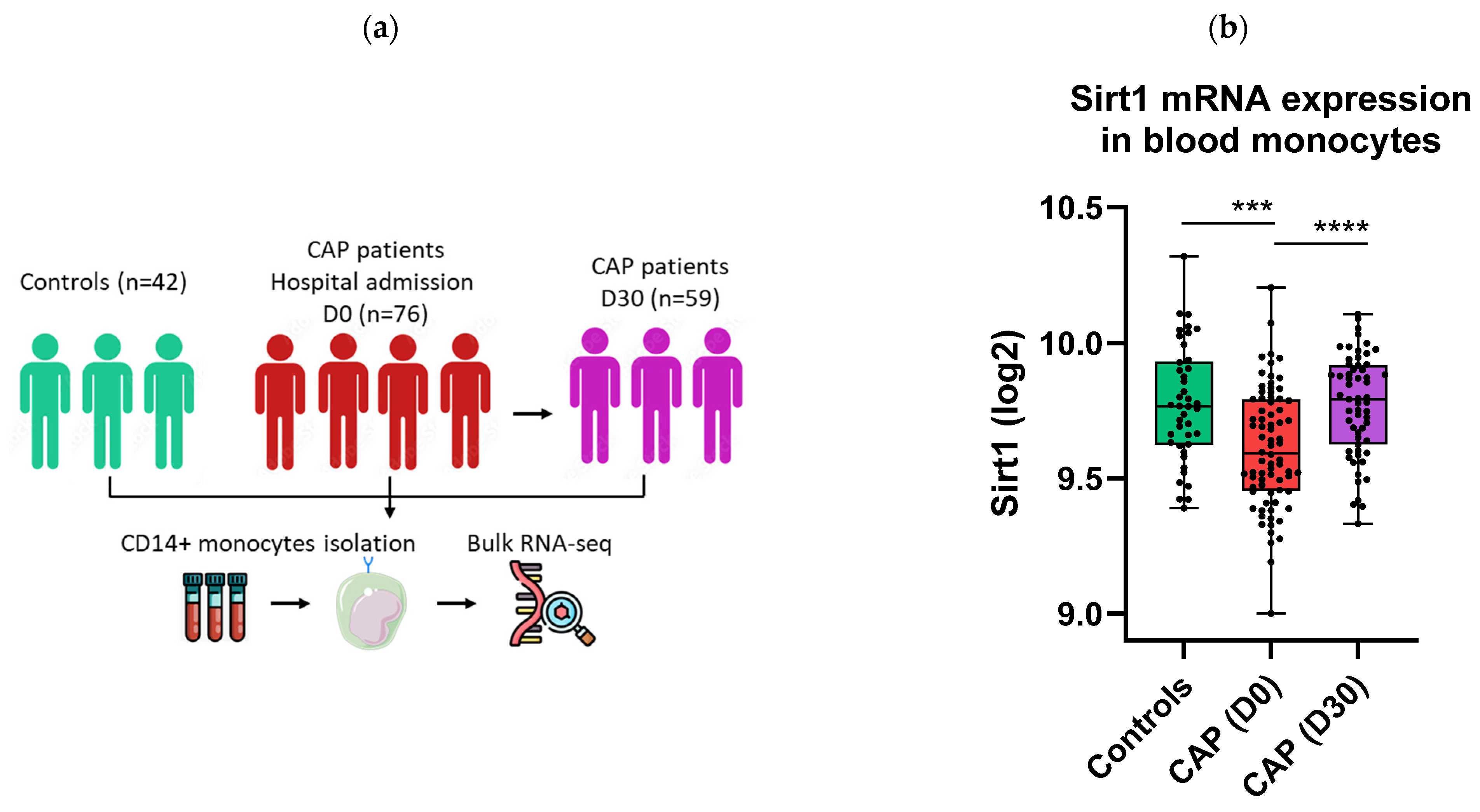
2.1. Sirt1 mRNA Expression Is Decreased in Monocytes of CAP Patients
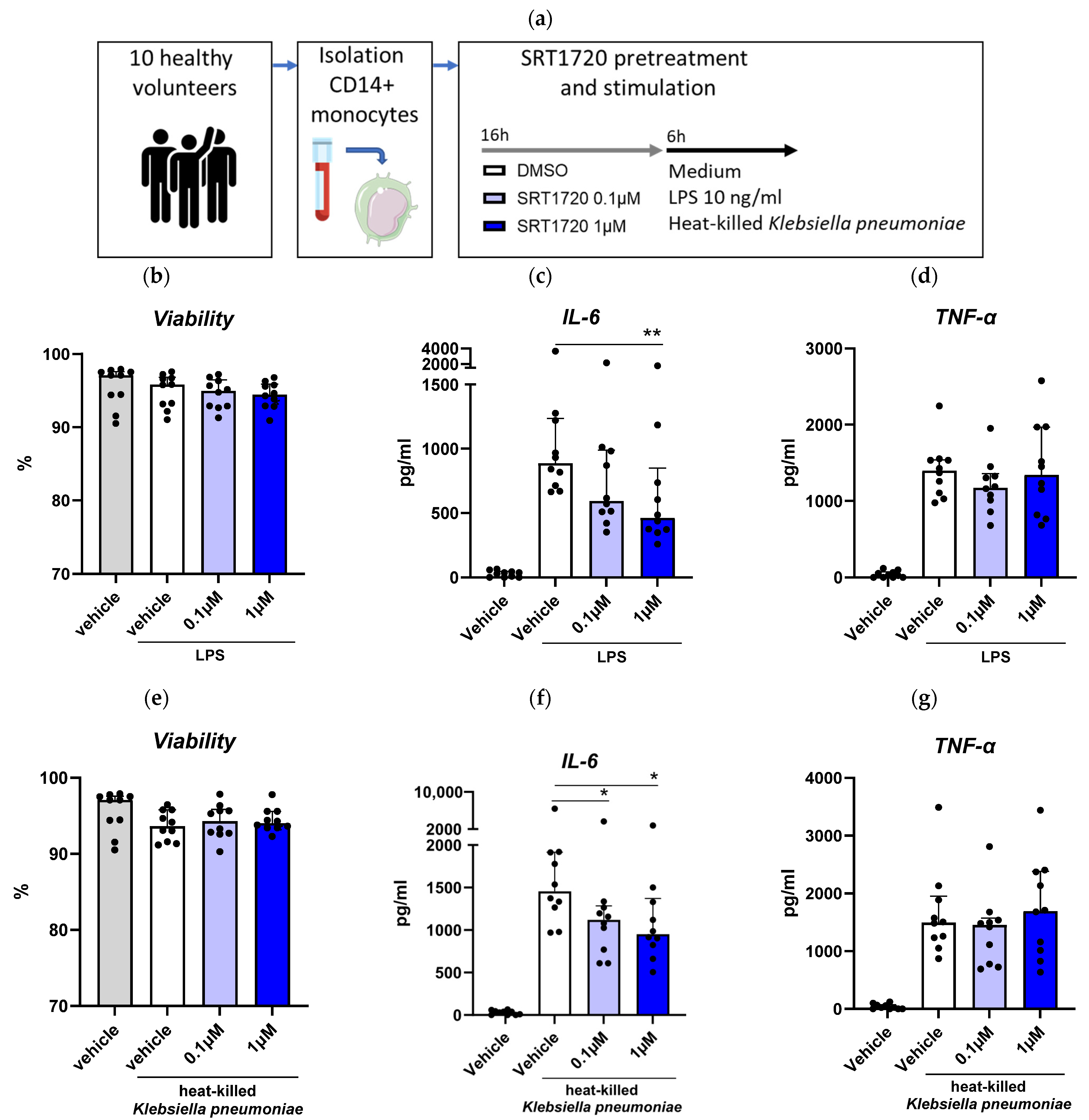
2.2. The Sirt1 Activator SRT1720 Inhibits LPS and K. Pneumoniae-Induced Monocyte Activation
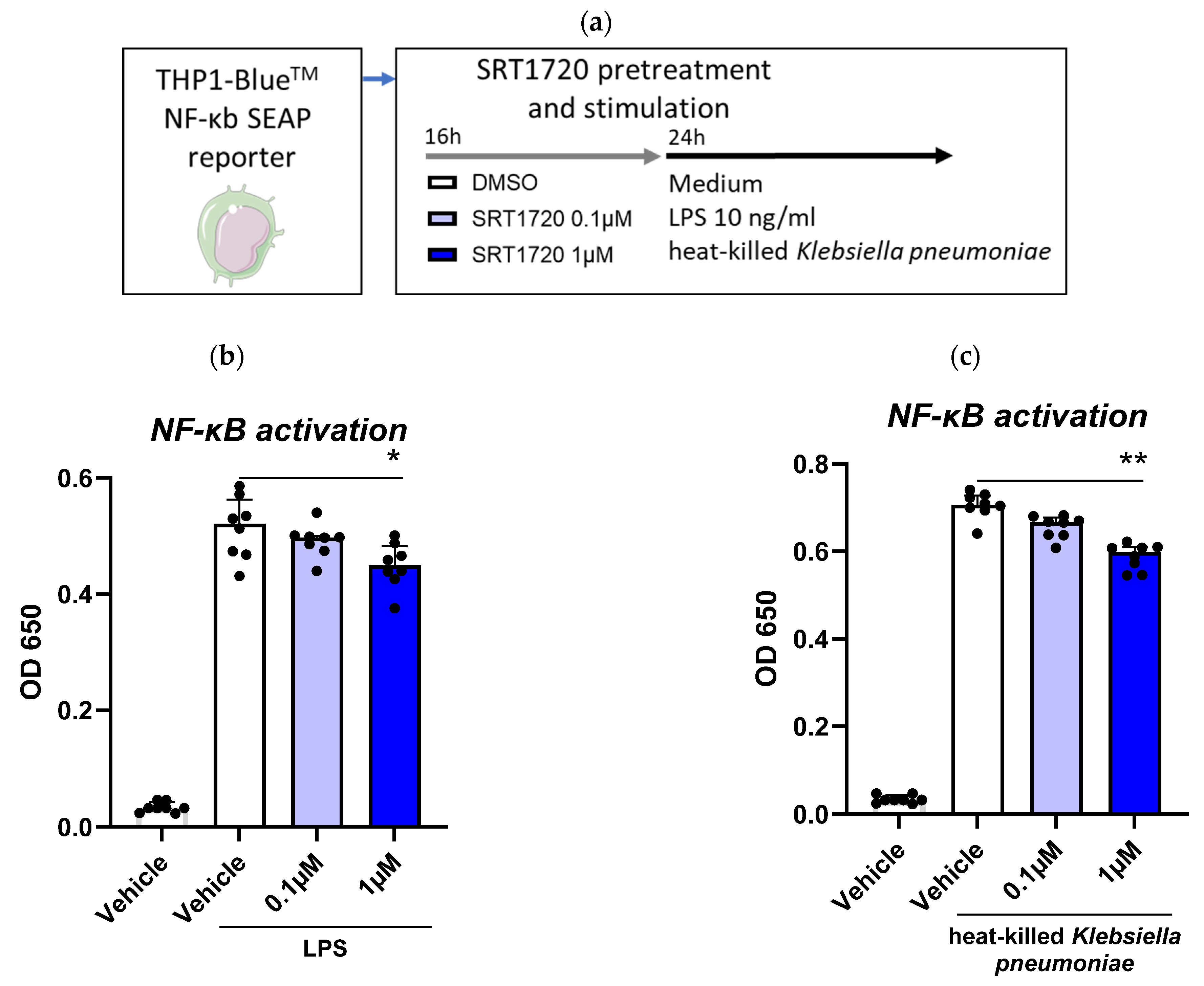
2.3. SRT1720 Reduces NF-κB Activation in THP1 Monocytes
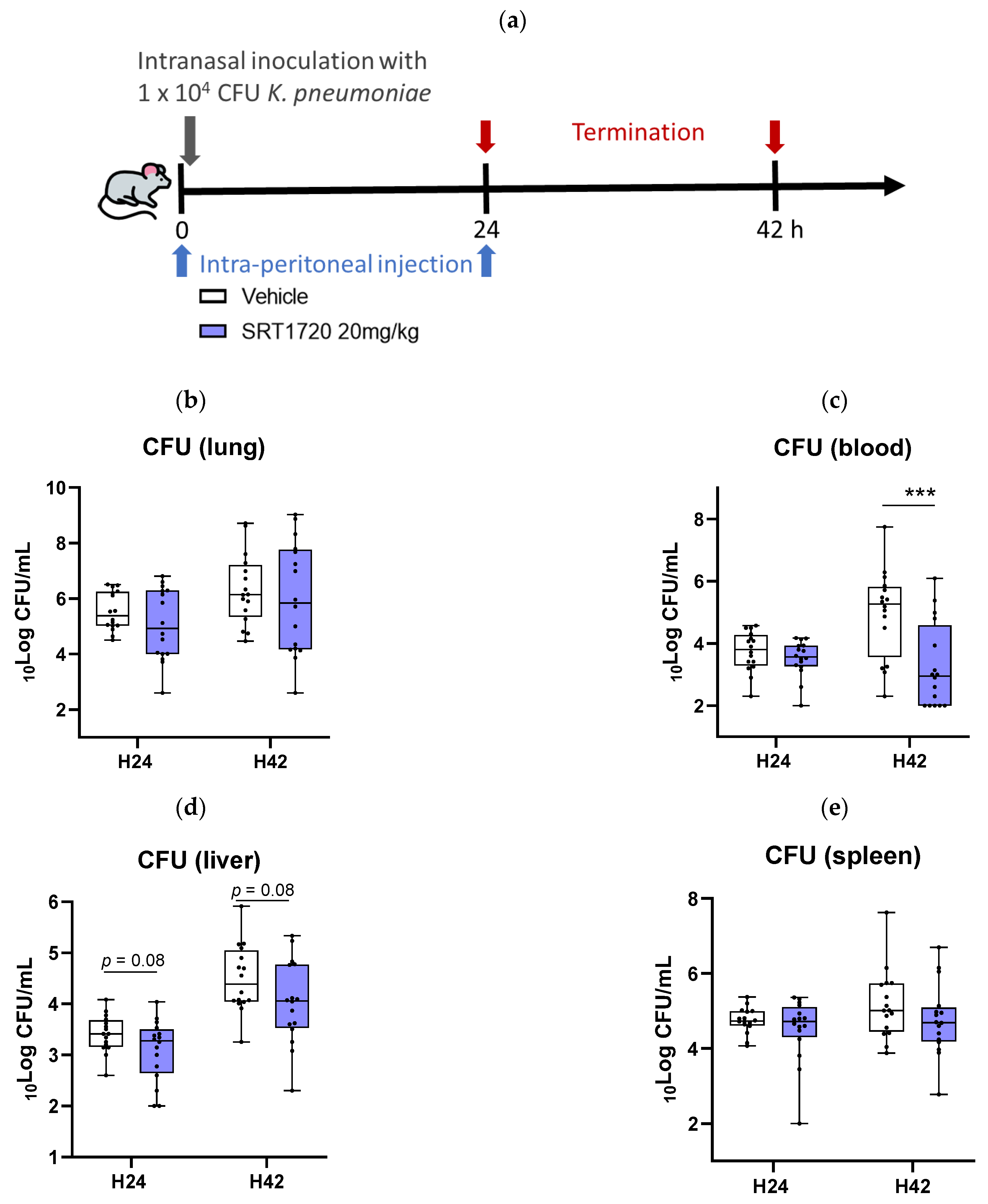
2.4. SRT1720 Reduces Bacterial Dissemination During in Vivo Klebsiella Pneumosepsis
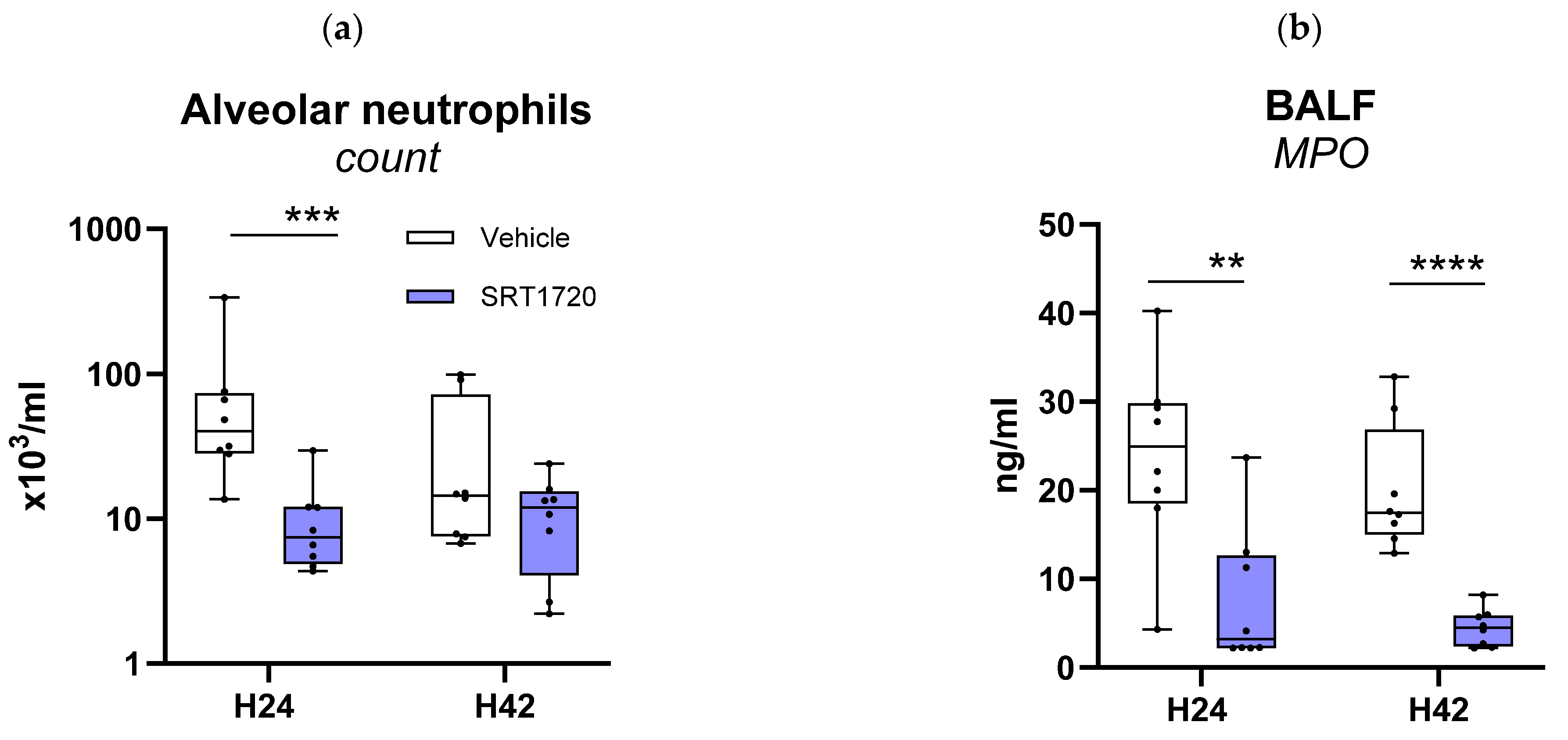
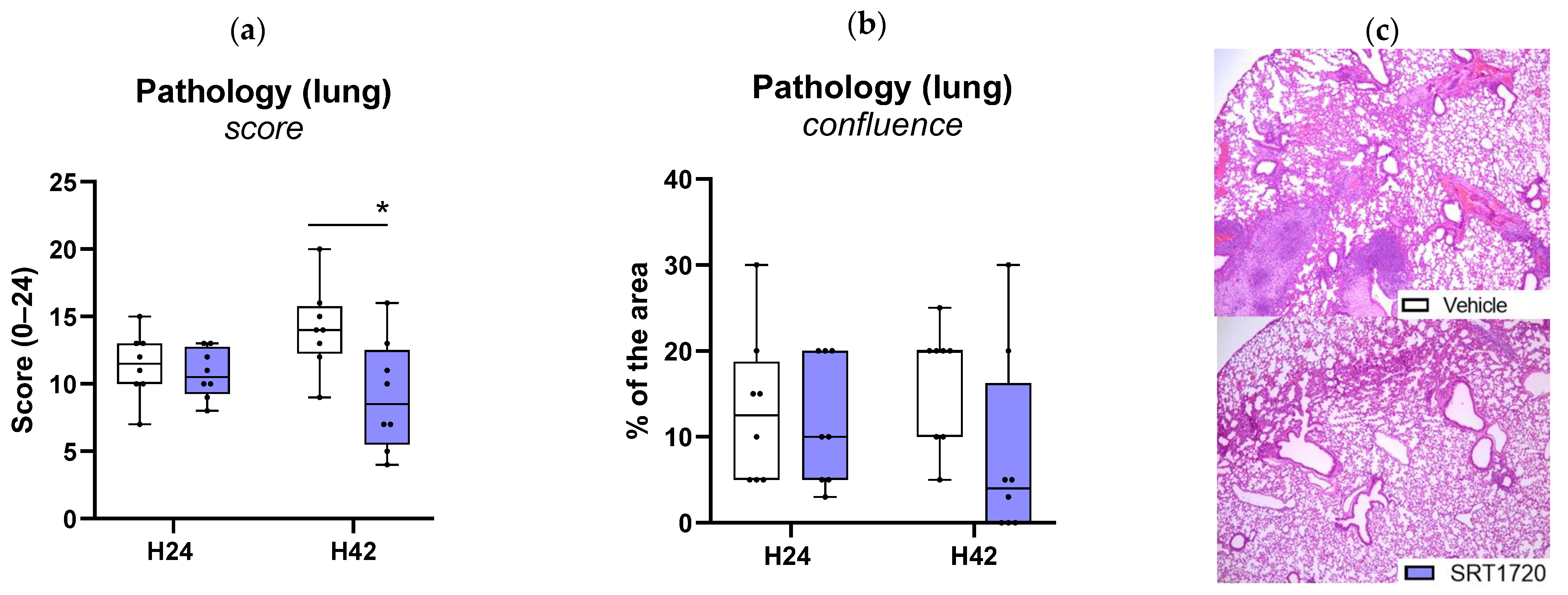
2.5. SRT1720 Mitigates Lung Inflammation and Pathology During Klebsiella Pneumosepsis
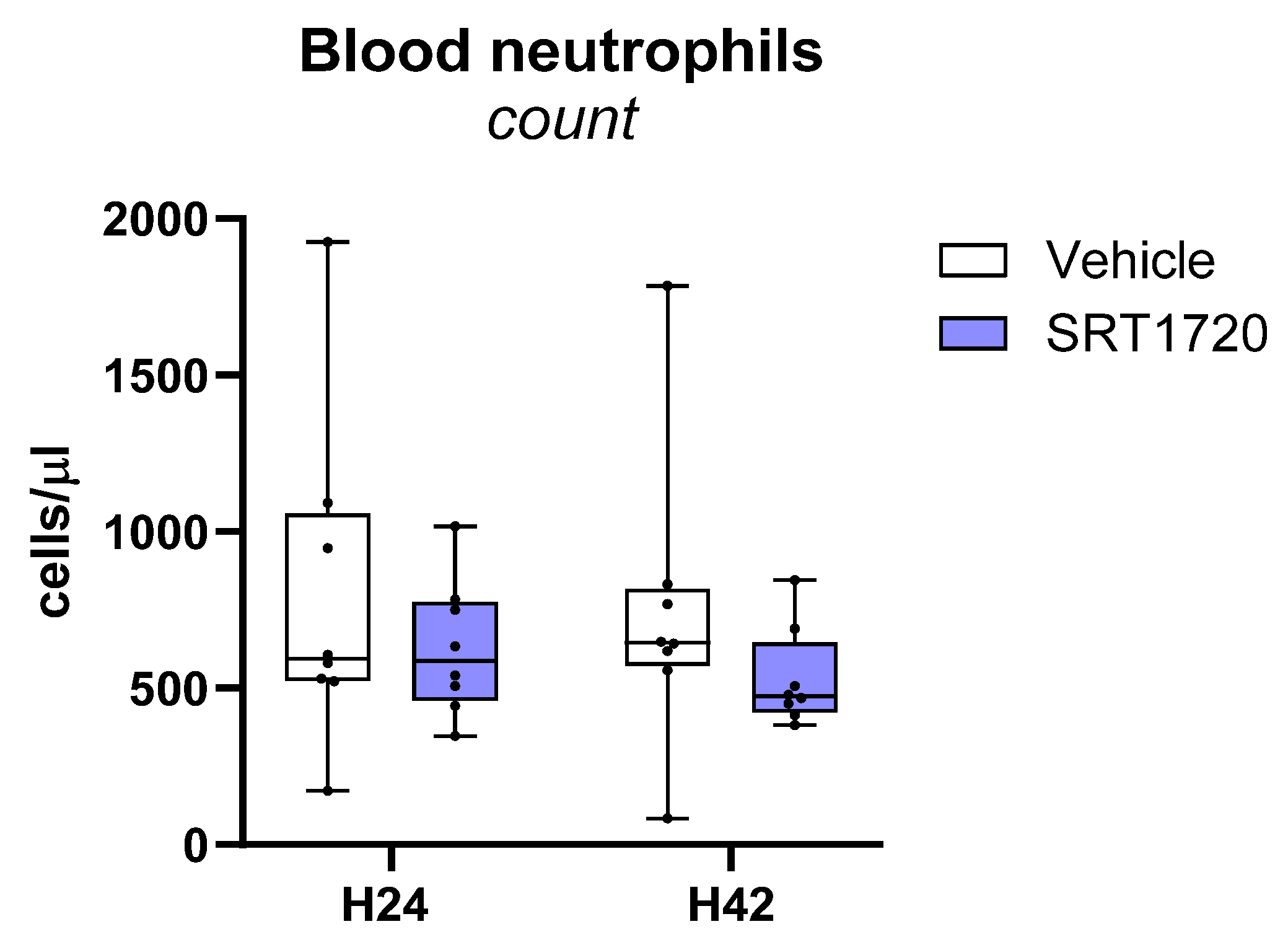
2.6. SRT1720 Mitigates Distant Organ Injury During Klebsiella Pneumosepsis
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Ex Vivo-Experiments Using Monocytes from Healthy Controls
4.3. Measurement of NF-κB Activity
4.4. Mice
4.5. Experimental Study Design
4.6. Bacterial Burden Determination
4.7. Histology and Immunohistochemistry
4.8. Protein Assays
4.9. Flow Cytometry
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data availability statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| ALT | alanine aminotransferase |
| AST | aspartate amino-transferase |
| BALF | bronchoalveolar lavage fluid |
| CAP | community-acquired pneumonia |
| CCL | CC chemokine ligand |
| CFU | colony-forming units |
| CXCL | C-X-C motif ligand |
| FDR | false discovery rate |
| H&E | hematoxylin and eosin |
| IFN | interferon |
| LDH | lactate dehydrogenase |
| NF-κB | nuclear factor-kappa B |
| PBMCs | peripheral blood mononuclear cells |
| RPMI | Roswell Park memorial institute |
| SEAP | secreted alkaline phosphatase |
| Sirt-1 | sirtuin-1 |
Appendix A
References
- Torres, A.; Cilloniz, C.; Niederman, M.S.; Menéndez, R.; Chalmers, J.D.; Wunderink, R.G.; van der Poll, T. Pneumonia. Nat. Rev. Dis. Primers 2021, 7, 25. [Google Scholar] [CrossRef]
- Stienstra, R.; Netea-Maier, R.T.; Riksen, N.P.; Joosten, L.A.B.; Netea, M.G. Specific and Complex Reprogramming of Cellular Metabolism in Myeloid Cells during Innate Immune Responses. Cell Metab. 2017, 26, 142–156. [Google Scholar] [CrossRef]
- Soares, M.P.; Teixeira, L.; Moita, L.F. Disease tolerance and immunity in host protection against infection. Nat. Rev. Immunol. 2017, 17, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.J.; Billiar, T.R.; Rosengart, M.R. Biology and Metabolism of Sepsis: Innate Immunity, Bioenergetics, and Autophagy. Surg. Infect. 2016, 17, 286–293. [Google Scholar] [CrossRef]
- Kosciuk, T.; Wang, M.; Hong, J.Y.; Lin, H. Updates on the epigenetic roles of sirtuins. Curr. Opin. Chem. Biol. 2019, 51, 18–29. [Google Scholar] [CrossRef]
- Wang, Y.; He, J.; Liao, M.; Hu, M.; Li, W.; Ouyang, H.; Wang, X.; Ye, T.; Zhang, Y.; Ouyang, L. An overview of Sirtuins as potential therapeutic target: Structure, function and modulators. Eur. J. Med. Chem. 2019, 161, 48–77. [Google Scholar] [CrossRef]
- Vachharajani, V.; McCall, C.E. Sirtuins: Potential therapeutic targets for regulating acute inflammatory response? Expert Opin. Ther. Targets 2020, 24, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Schug, T.T.; Xu, Q.; Gao, H.; Peres-da-Silva, A.; Draper, D.W.; Fessler, M.B.; Purushotham, A.; Li, X. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol. Cell. Biol. 2010, 30, 4712–4721. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Y.; Wang, Y.; Chao, Y.; Zhang, J.; Jia, Y.; Tie, J.; Hu, D. Regulation of SIRT1 and Its Roles in Inflammation. Front. Immunol. 2022, 13, 831168. [Google Scholar] [CrossRef]
- Imperatore, F.; Maurizio, J.; Vargas Aguilar, S.; Busch, C.J.; Favret, J.; Kowenz-Leutz, E.; Cathou, W.; Gentek, R.; Perrin, P.; Leutz, A.; et al. SIRT1 regulates macrophage self-renewal. EMBO J. 2017, 36, 2353–2372. [Google Scholar] [CrossRef]
- Wang, F.; Ma, J.; Wang, J.; Chen, M.; Xia, H.; Yao, S.; Zhang, D. SIRT1 ameliorated septic associated-lung injury and macrophages apoptosis via inhibiting endoplasmic reticulum stress. Cell. Signal. 2022, 97, 110398. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Duan, C.; Yang, X.; Liu, J.; Deng, Y.; Tiselius, H.-G.; Ye, Z.; Wang, T.; Xing, J.; Xu, H. Metformin suppresses calcium oxalate crystal-induced kidney injury by promoting Sirt1 and M2 macrophage-mediated anti-inflammatory activation. Signal Transduct. Target. Ther. 2023, 8, 38. [Google Scholar] [CrossRef]
- You, J.; Li, Y.; Chong, W. The role and therapeutic potential of SIRTs in sepsis. Front. Immunol. 2024, 15, 1394925. [Google Scholar] [CrossRef]
- Jia, Y.; Li, Z.; Cai, W.; Xiao, D.; Han, S.; Han, F.; Bai, X.; Wang, K.; Liu, Y.; Li, X.; et al. SIRT1 regulates inflammation response of macrophages in sepsis mediated by long noncoding RNA. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2018, 1864, 784–792. [Google Scholar] [CrossRef]
- Xu, W.; Lu, Y.; Yao, J.; Li, Z.; Chen, Z.; Wang, G.; Jing, H.; Zhang, X.; Li, M.; Peng, J.; et al. Novel role of resveratrol: Suppression of high-mobility group protein box 1 nucleocytoplasmic translocation by the upregulation of sirtuin 1 in sepsis-induced liver injury. Shock 2014, 42, 440–447. [Google Scholar] [CrossRef]
- Gao, R.; Chen, J.; Hu, Y.; Li, Z.; Wang, S.; Shetty, S.; Fu, J. Sirt1 deletion leads to enhanced inflammation and aggravates endotoxin-induced acute kidney injury. PLoS ONE 2014, 9, e98909. [Google Scholar] [CrossRef] [PubMed]
- Labiner, H.E.; Sas, K.M.; Baur, J.A.; Sims, C.A. Sirtuin 1 deletion increases inflammation and mortality in sepsis. J. Trauma Acute Care Surg. 2022, 93, 672–678. [Google Scholar] [CrossRef]
- van der Meer, A.J.; Scicluna, B.P.; Moerland, P.D.; Lin, J.; Jacobson, E.W.; Vlasuk, G.P.; van der Poll, T. The Selective Sirtuin 1 Activator SRT2104 Reduces Endotoxin-Induced Cytokine Release and Coagulation Activation in Humans. Crit. Care Med. 2015, 43, e199–e202. [Google Scholar] [CrossRef]
- Labiner, H.E.; Sas, K.M.; Hoying, J.; Sepeda, J.A.; Wolf, N.; Perez, E.C.; Sas, A.R.; Sims, C.A. SIRT1 downregulation in pneumonia is associated with an immature neutrophil response and increased disease severity. J. Trauma Acute Care Surg. 2024, 96, 557–565. [Google Scholar] [CrossRef]
- Brands, X.; Haak, B.W.; Klarenbeek, A.M.; Butler, J.; Uhel, F.; Qin, W.; Otto, N.A.; Jakobs, M.E.; Faber, D.R.; Lutter, R.; et al. An epigenetic and transcriptomic signature of immune tolerance in human monocytes through multi-omics integration. Genome Med. 2021, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- Milne, J.C.; Lambert, P.D.; Schenk, S.; Carney, D.P.; Smith, J.J.; Gagne, D.J.; Jin, L.; Boss, O.; Perni, R.B.; Vu, C.B.; et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature 2007, 450, 712–716. [Google Scholar] [CrossRef]
- Chauhan, D.; Bandi, M.; Singh, A.V.; Ray, A.; Raje, N.; Richardson, P.; Anderson, K.C. Preclinical evaluation of a novel SIRT1 modulator SRT1720 in multiple myeloma cells. Br. J. Haematol. 2011, 155, 588–598. [Google Scholar] [CrossRef]
- Claushuis, T.A.M.; de Vos, A.F.; Nieswandt, B.; Boon, L.; Roelofs, J.J.T.H.; de Boer, O.J.; van’t Veer, C.; van der Poll, T. Platelet glycoprotein VI aids in local immunity during pneumonia-derived sepsis caused by gram-negative bacteria. Blood 2018, 131, 864–876. [Google Scholar] [CrossRef]
- Achouiti, A.; Vogl, T.; Urban, C.F.; Röhm, M.; Hommes, T.J.; van Zoelen, M.A.D.; Florquin, S.; Roth, J.; van’t Veer, C.; de Vos, A.F.; et al. Myeloid-related protein-14 contributes to protective immunity in gram-negative pneumonia derived sepsis. PLoS Pathog 2012, 8, e1002987. [Google Scholar] [CrossRef]
- Qin, W.; Liu, Z.; van der Poll, T.; de Vos, A.F. Induction of Acute or Disseminating Bacterial Pneumonia in Mice and Sampling of Infected Organs for Studying the Host Response to Bacterial Pneumonia. Bio-Protocol 2022, 12, e4287. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Moral, I.; Blok, D.C.; Bernink, J.H.; Garcia-Laorden, M.I.; Florquin, S.; Boon, L.; Van’t Veer, C.; Mack, M.; Saluzzo, S.; Knapp, S.; et al. Interleukin-33 improves local immunity during Gram-negative pneumonia by a combined effect on neutrophils and inflammatory monocytes. J. Pathol. 2021, 253, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.L. Sirt1 and the Mitochondria. Mol. Cells 2016, 39, 87–95. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Hao, Q.; Jin, J.; Wang, Y. Metabolic mechanisms orchestrated by Sirtuin family to modulate inflammatory responses. Front. Immunol. 2024, 15, 1448535. [Google Scholar] [CrossRef]
- Sônego, F.; Castanheira, F.V.E.S.; Ferreira, R.G.; Kanashiro, A.; Leite, C.A.V.G.; Nascimento, D.C.; Colón, D.F.; Borges, V.d.F.; Alves-Filho, J.C.; Cunha, F.Q. Paradoxical Roles of the Neutrophil in Sepsis: Protective and Deleterious. Front. Immunol. 2016, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chai, H.; Du, X.; Cui, R.; Dong, Y. Overexpressing long non-coding RNA OIP5-AS1 ameliorates sepsis-induced lung injury in a rat model via regulating the miR-128-3p/Sirtuin-1 pathway. Bioengineered 2021, 12, 9723–9738. [Google Scholar] [CrossRef]
- Gu, C.; Li, Y.; Xu, W.-L.; Yan, J.-P.; Xia, Y.; Ma, Y.-Y.; Chen, C.; Wang, H.-J.; Tao, H. Sirtuin 1 Activator SRT1720 Protects Against Lung Injury via Reduction of Type II Alveolar Epithelial Cells Apoptosis in Emphysema. COPD 2015, 12, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Guo, X.; Zeng, Z.; Wu, J.; Liu, Y.; He, J.; Wang, R.; Huang, Q.; Chen, Z. Sirt1 Protects Endothelial Cells against LPS-Induced Barrier Dysfunction. Oxidative Med. Cell. Longev. 2017, 2017, 4082102. [Google Scholar] [CrossRef]
- Fu, C.; Hao, S.; Xu, X.; Zhou, J.; Liu, Z.; Lu, H.; Wang, L.; Jin, W.; Li, S. Activation of SIRT1 ameliorates LPS-induced lung injury in mice via decreasing endothelial tight junction permeability. Acta Pharmacol. Sin. 2019, 40, 630–641. [Google Scholar] [CrossRef]
- van der Poll, T.; Shankar-Hari, M.; Wiersinga, W.J. The immunology of sepsis. Immunity 2021, 54, 2450–2464. [Google Scholar] [CrossRef]
- Pacholec, M.; Bleasdale, J.E.; Chrunyk, B.; Cunningham, D.; Flynn, D.; Garofalo, R.S.; Griffith, D.; Griffor, M.; Loulakis, P.; Pabst, B.; et al. SRT1720, SRT2183, SRT1460, and Resveratrol Are Not Direct Activators of SIRT1. J. Biol. Chem. 2010, 285, 8340–8351. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, K.; Yi, Z.; Geng, S.; Li, L. Development of Exhausted Memory Monocytes and Underlying Mechanisms. Front. Immunol. 2021, 12, 778830. [Google Scholar] [CrossRef] [PubMed]


| 24 h | 42 h | |||
|---|---|---|---|---|
| Vehicle | SRT1720 | Vehicle | SRT1720 | |
| Alveolar IL-6 (pg/mL) | 133 (78-223) | 37 (31–75) ** | 49 (39–62) | 46 (33–59) |
| Alveolar TNF-α (pg/mL) | 225 (142–580) | 45 (71–96) ** | 153 (108–403) | 100 (60–132) * |
| Alveolar CXCL1 (pg/mL) | 287 (264–413) | 96 (78–172) *** | 278 (174–356) | 135 (102–262) |
| Alveolar CXCL2 (pg/mL) | 615 (536–751) | 443 (327–501) ** | 582 (523–705) | 511 (466–578) * |
| 24 h | 42 h | |||
|---|---|---|---|---|
| Vehicle | SRT1720 | Vehicle | SRT1720 | |
| Plasma IL-6 (pg/mL) | 694 (429–1052) | 527 (328–1022) | 602 (351–1554) | 207 (89–364) *** |
| Plasma TNF-α (pg/mL) | 165 (54–375) | 84 (49–482) | 98 (63–172) | 57 (24–103) * |
| Plasma IFN-γ (pg/mL) | 8.9 (2.5–20.6) | 2.5 (2.5–19.2) | 45.6 (15.8–94.2) | 47.6 (20.1–114.7) |
| Plasma IL-12 (pg/mL) | 111 (89–134) | 103 (82–157) | 233 (98–278) | 152 (123–217) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blot, M.; Léopold, V.; de Beer, R.; Florquin, S.; Butler, J.M.; van’t Veer, C.; de Vos, A.F.; van der Poll, T. The Sirt1 Activator SRT1720 Mitigates Human Monocyte Activation and Improves Outcome During Gram-Negative Pneumosepsis in Mice. Int. J. Mol. Sci. 2025, 26, 9309. https://doi.org/10.3390/ijms26199309
Blot M, Léopold V, de Beer R, Florquin S, Butler JM, van’t Veer C, de Vos AF, van der Poll T. The Sirt1 Activator SRT1720 Mitigates Human Monocyte Activation and Improves Outcome During Gram-Negative Pneumosepsis in Mice. International Journal of Molecular Sciences. 2025; 26(19):9309. https://doi.org/10.3390/ijms26199309
Chicago/Turabian StyleBlot, Mathieu, Valentine Léopold, Regina de Beer, Sandrine Florquin, Joe M. Butler, Cornelis van’t Veer, Alex F. de Vos, and Tom van der Poll. 2025. "The Sirt1 Activator SRT1720 Mitigates Human Monocyte Activation and Improves Outcome During Gram-Negative Pneumosepsis in Mice" International Journal of Molecular Sciences 26, no. 19: 9309. https://doi.org/10.3390/ijms26199309
APA StyleBlot, M., Léopold, V., de Beer, R., Florquin, S., Butler, J. M., van’t Veer, C., de Vos, A. F., & van der Poll, T. (2025). The Sirt1 Activator SRT1720 Mitigates Human Monocyte Activation and Improves Outcome During Gram-Negative Pneumosepsis in Mice. International Journal of Molecular Sciences, 26(19), 9309. https://doi.org/10.3390/ijms26199309







