Lipid Signature of Motile Human Sperm: Characterization of Sphingomyelin, Ceramide, and Phospholipids with a Focus on Very Long Chain Polyunsaturated Fatty Acids
Abstract
1. Introduction
2. Results
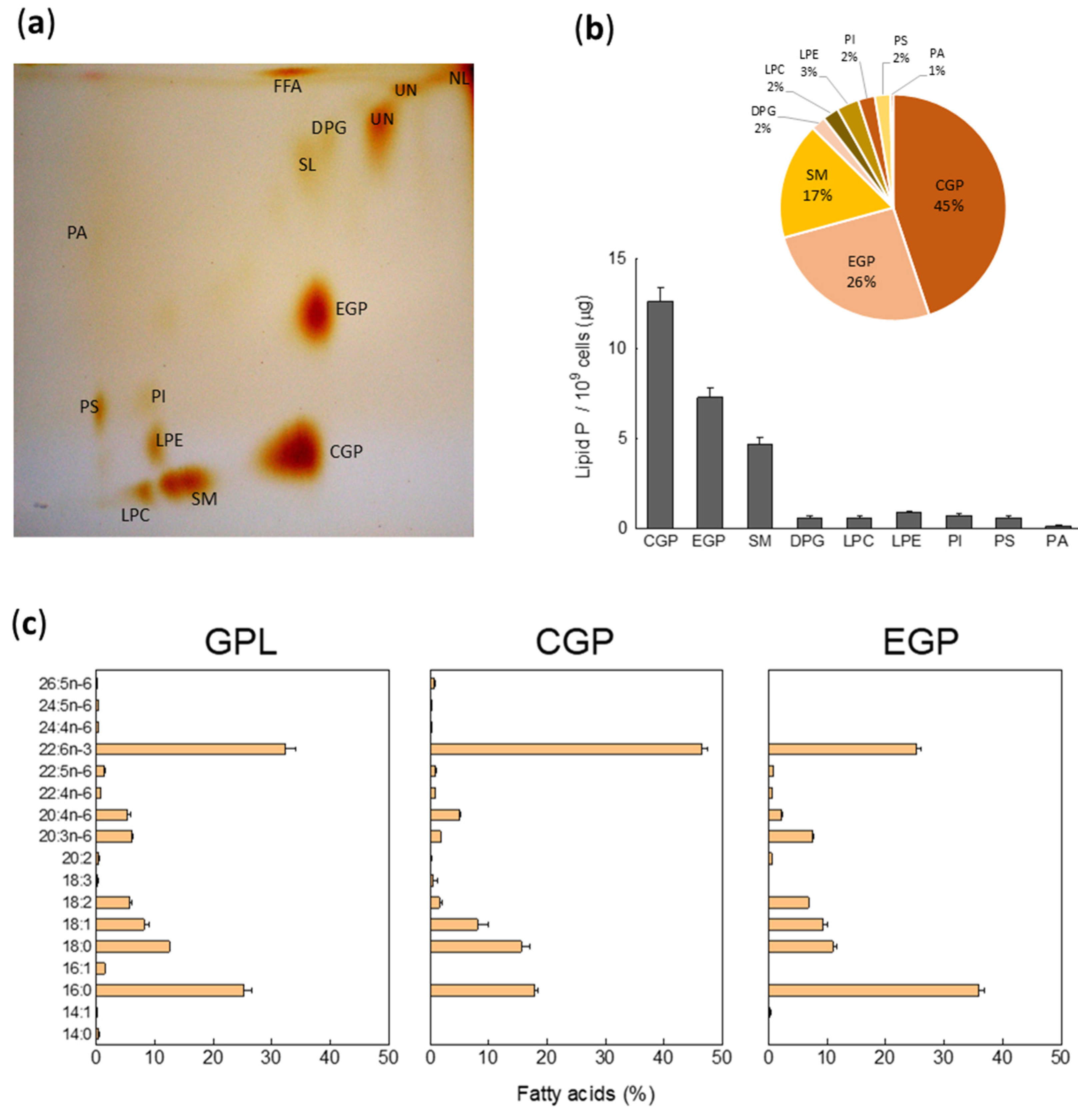
2.1. Phospholipid Composition and Fatty Acid of Total Glycerophospholipids (GPL) in Non-Capacitated Motile Human Sperm
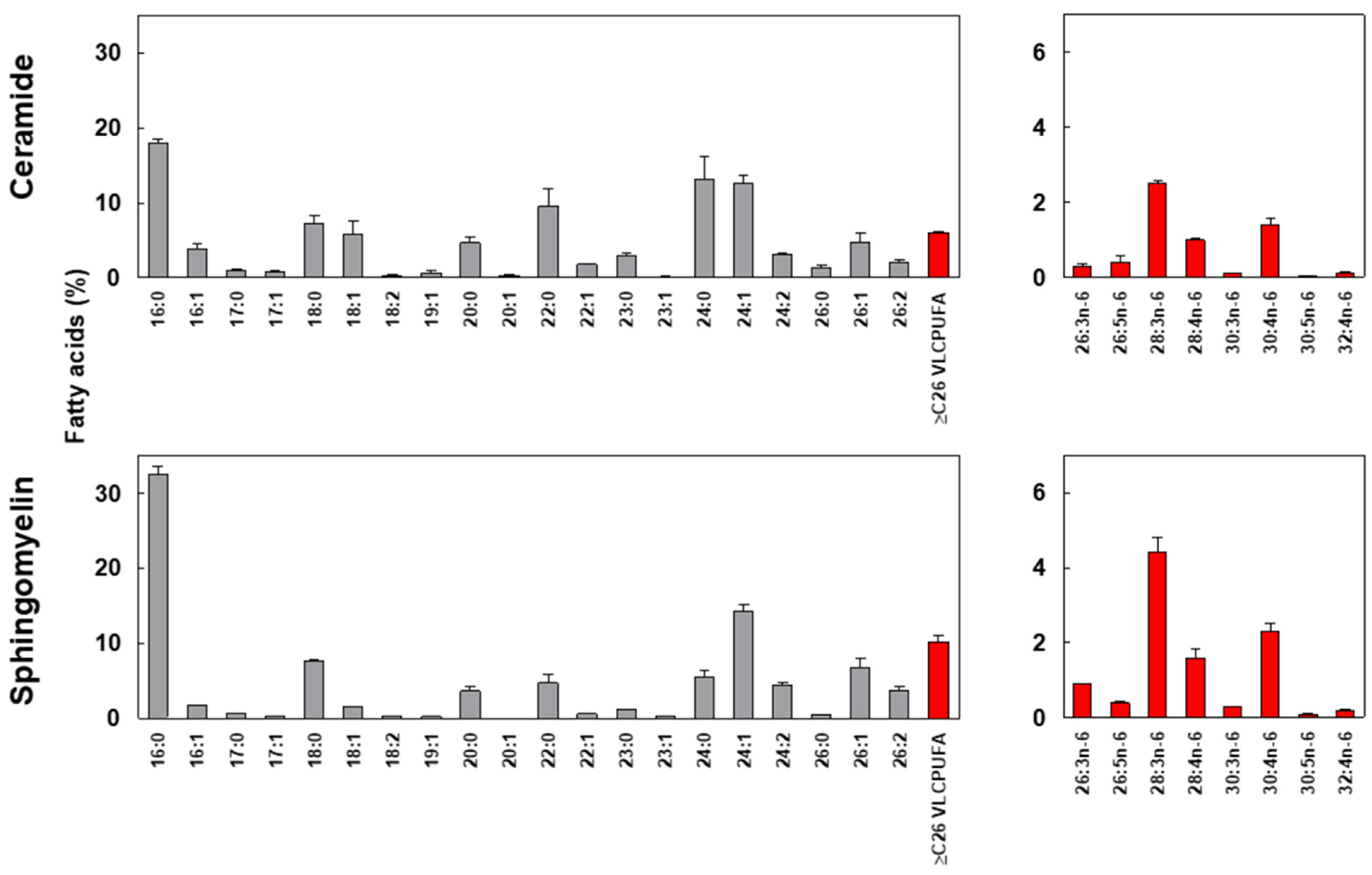
2.2. Fatty Acid Composition in Sphingomyelin (SM) and Ceramide (Cer) from Human Motile Spermatozoa
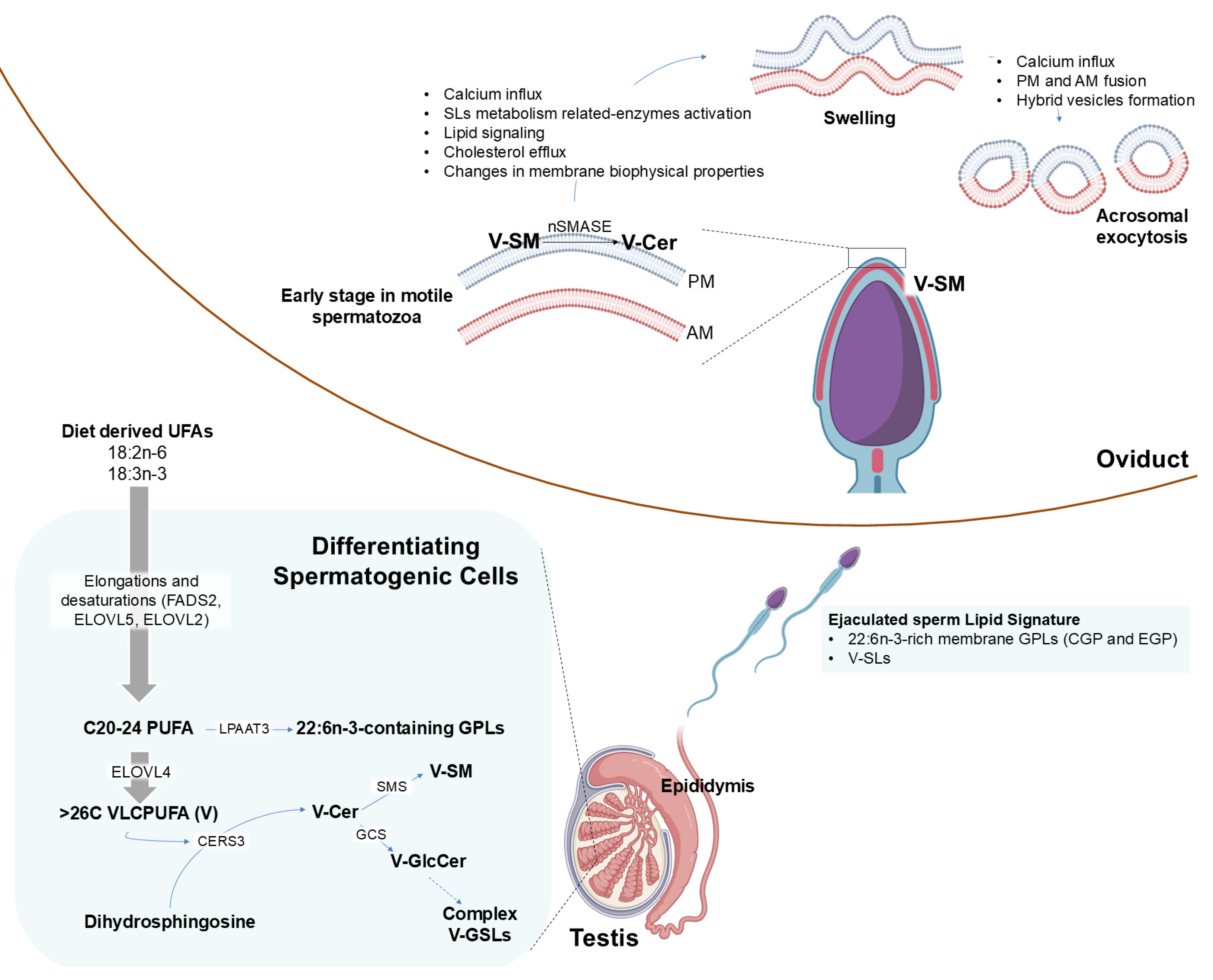
3. Discussion
4. Materials and Methods
4.1. Ethics Statement and Human Sperm Preparation
4.2. Lipid Separation and Analysis
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ART | Assisted reproductive technology |
| Cer | Ceramide |
| CERS3 | Ceramide synthase 3 |
| CGP | Choline glycerophospholipids |
| DHA | Docosahexaenoic acid |
| EGP | Ethanolamine glycerophospholipids |
| ELOVL | Elongases |
| FADS2 | Δ6 desaturase |
| GCS | Glucosylceramide synthase |
| GluCer | Glucosylceramide |
| GPL | Glycerophospholipids |
| GSLs | Glycosphingolipids |
| LPAAT3 | Lysophosphatidic acid acyltransferase |
| LPC | Lysophosphatidylcholine |
| LPE | Lysophosphoethanolamine |
| NL | Neutral lipids |
| nSMASE | Neutral sphingomyelinase |
| PA | Phosphatidic acid |
| PI | Phosphatidylinositol |
| PS | Phosphatidylserine |
| PUFA | Polyunsaturated fatty acid |
| SL | Seminolipid |
| SLs | Sphingolipids |
| SM | Sphingomyelin |
| SMS | Sphingomyelin synthase |
| UFAs | Unsaturated fatty acids |
| VLCPUFA | Very long-chain polyunsaturated fatty acid |
References
- Flesch, F.M.; Gadella, B.M. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim. Biophys. Acta 2000, 1469, 197–235. [Google Scholar] [CrossRef] [PubMed]
- Aveldano, M.I.; Rotstein, N.P.; Vermouth, N.T. Occurrence of long and very long polyenoic fatty acids of the n-9 series in rat spermatozoa. Lipids 1992, 27, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Carro, M.L.M.; Ramirez-Vasquez, R.R.A.; Penalva, D.A.; Buschiazzo, J.; Hozbor, F.A. Desmosterol Incorporation Into Ram Sperm Membrane Before Cryopreservation Improves in vitro and in vivo Fertility. Front. Cell Dev. Biol. 2021, 9, 660165. [Google Scholar] [CrossRef] [PubMed]
- Carro, M.; Luquez, J.M.; Penalva, D.A.; Buschiazzo, J.; Hozbor, F.A.; Furland, N.E. PUFA-rich phospholipid classes and subclasses of ram spermatozoa are unevenly affected by cryopreservation with a soybean lecithin-based extender. Theriogenology 2022, 186, 122–134. [Google Scholar] [CrossRef]
- Poulos, A.; Johnson, D.W.; Beckman, K.; White, I.G.; Easton, C. Occurrence of unusual molecular species of sphingomyelin containing 28-34-carbon polyenoic fatty acids in ram spermatozoa. Biochem. J. 1987, 248, 961–964. [Google Scholar] [CrossRef]
- Furland, N.E.; Oresti, G.M.; Antollini, S.S.; Venturino, A.; Maldonado, E.N.; Aveldano, M.I. Very long-chain polyunsaturated fatty acids are the major acyl groups of sphingomyelins and ceramides in the head of mammalian spermatozoa. J. Biol. Chem. 2007, 282, 18151–18161. [Google Scholar] [CrossRef]
- Oresti, G.M.; Luquez, J.M.; Furland, N.E.; Aveldano, M.I. Uneven distribution of ceramides, sphingomyelins and glycerophospholipids between heads and tails of rat spermatozoa. Lipids 2011, 46, 1081–1090. [Google Scholar] [CrossRef]
- Sandhoff, R. Very long chain sphingolipids: Tissue expression, function and synthesis. FEBS Lett. 2010, 584, 1907–1913. [Google Scholar] [CrossRef]
- Yeboah, G.K.; Lobanova, E.S.; Brush, R.S.; Agbaga, M.P. Very long chain fatty acid-containing lipids: A decade of novel insights from the study of ELOVL4. J. Lipid Res. 2021, 62, 100030. [Google Scholar] [CrossRef]
- Santiago Valtierra, F.X.; Penalva, D.A.; Luquez, J.M.; Furland, N.E.; Vasquez, C.; Reyes, J.G.; Aveldano, M.I.; Oresti, G.M. Elovl4 and Fa2h expression during rat spermatogenesis: A link to the very-long-chain PUFAs typical of germ cell sphingolipids. J. Lipid Res. 2018, 59, 1175–1189. [Google Scholar] [CrossRef]
- Rabionet, M.; van der Spoel, A.C.; Chuang, C.C.; von Tumpling-Radosta, B.; Litjens, M.; Bouwmeester, D.; Hellbusch, C.C.; Korner, C.; Wiegandt, H.; Gorgas, K.; et al. Male germ cells require polyenoic sphingolipids with complex glycosylation for completion of meiosis: A link to ceramide synthase-3. J. Biol. Chem. 2008, 283, 13357–13369. [Google Scholar] [CrossRef] [PubMed]
- Rabionet, M.; Bayerle, A.; Jennemann, R.; Heid, H.; Fuchser, J.; Marsching, C.; Porubsky, S.; Bolenz, C.; Guillou, F.; Grone, H.J.; et al. Male meiotic cytokinesis requires ceramide synthase 3-dependent sphingolipids with unique membrane anchors. Hum. Mol. Genet. 2015, 24, 4792–4808. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.B.; Brush, R.S.; Sullivan, M.T.; Zavy, M.T.; Agbaga, M.P.; Anderson, R.E. Decreased very long chain polyunsaturated fatty acids in sperm correlates with sperm quantity and quality. J. Assist. Reprod. Genet. 2019, 36, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Xie, H.; Xiong, Y.; Sun, P.; Xue, Y.; Li, K. Lipidomics profiles of human spermatozoa: Insights into capacitation and acrosome reaction using UPLC-MS-based approach. Front. Endocrinol. 2023, 14, 1273878. [Google Scholar] [CrossRef] [PubMed]
- Gavrizi, S.Z.; Hosseinzadeh, P.; Brush, R.S.; Tytanic, M.; Eckart, E.; Peck, J.D.; Craig, L.B.; Diamond, M.P.; Agbaga, M.P.; Hansen, K.R. Sperm very long-chain polyunsaturated fatty acids: Relation to semen parameters and live birth outcome in a multicenter trial. Fertil. Steril. 2023, 119, 753–760. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Futerman, A.H.; Hannun, Y.A. The complex life of simple sphingolipids. EMBO Rep. 2004, 5, 777–782. [Google Scholar] [CrossRef]
- Suhaiman, L.; De Blas, G.A.; Obeid, L.M.; Darszon, A.; Mayorga, L.S.; Belmonte, S.A. Sphingosine 1-phosphate and sphingosine kinase are involved in a novel signaling pathway leading to acrosomal exocytosis. J. Biol. Chem. 2010, 285, 16302–16314. [Google Scholar] [CrossRef]
- Vaquer, C.C.; Suhaiman, L.; Pavarotti, M.A.; Arias, R.J.; Pacheco Guinazu, A.B.; De Blas, G.A.; Belmonte, S.A. The pair ceramide 1-phosphate/ceramide kinase regulates intracellular calcium and progesterone-induced human sperm acrosomal exocytosis. Front. Cell Dev. Biol. 2023, 11, 1148831. [Google Scholar] [CrossRef]
- Belmonte, S.A.; Suhaiman, L. Optimized protocols to analyze sphingosine-1-phosphate signal transduction pathways during acrosomal exocytosis in human sperm. Methods Mol. Biol. 2012, 874, 99–128. [Google Scholar] [CrossRef]
- Vaquer, C.C.; Suhaiman, L.; Pavarotti, M.A.; De Blas, G.A.; Belmonte, S.A. Ceramide induces a multicomponent intracellular calcium increase triggering the acrosome secretion in human sperm. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118704. [Google Scholar] [CrossRef] [PubMed]
- Kolesnick, R.N.; Goni, F.M.; Alonso, A. Compartmentalization of ceramide signaling: Physical foundations and biological effects. J. Cell Physiol. 2000, 184, 285–300. [Google Scholar] [CrossRef] [PubMed]
- van Blitterswijk, W.J.; van der Luit, A.H.; Veldman, R.J.; Verheij, M.; Borst, J. Ceramide: Second messenger or modulator of membrane structure and dynamics? Biochem. J. 2003, 369, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Ahumada-Gutierrez, H.; Penalva, D.A.; Enriz, R.D.; Antollini, S.S.; Cascales, J.J.L. Mechanical properties of bilayers containing sperm sphingomyelins and ceramides with very long-chain polyunsaturated fatty acids. Chem. Phys. Lipids 2019, 218, 178–186. [Google Scholar] [CrossRef]
- Penalva, D.A.; Antollini, S.S.; Ambroggio, E.E.; Aveldano, M.I.; Fanani, M.L. Membrane Restructuring Events during the Enzymatic Generation of Ceramides with Very Long-Chain Polyunsaturated Fatty Acids. Langmuir 2018, 34, 4398–4407. [Google Scholar] [CrossRef]
- Zanetti, S.R.; Monclus, M.L.; Rensetti, D.E.; Fornes, M.W.; Aveldano, M.I. Differential involvement of rat sperm choline glycerophospholipids and sphingomyelin in capacitation and the acrosomal reaction. Biochimie 2010, 92, 1886–1894. [Google Scholar] [CrossRef]
- Zanetti, S.R.; de Los Angeles, M.M.; Rensetti, D.E.; Fornes, M.W.; Aveldano, M.I. Ceramides with 2-hydroxylated, very long-chain polyenoic fatty acids in rodents: From testis to fertilization-competent spermatozoa. Biochimie 2010, 92, 1778–1786. [Google Scholar] [CrossRef]
- Furse, S.; Kusinski, L.C.; Ray, A.; Glenn-Sansum, C.; Williams, H.E.L.; Koulman, A.; Meek, C.L. Relative Abundance of Lipid Metabolites in Spermatozoa across Three Compartments. Int. J. Mol. Sci. 2022, 23, 11655. [Google Scholar] [CrossRef]
- Suhaiman, L.; Belmonte, S.A. Lipid remodeling in acrosome exocytosis: Unraveling key players in the human sperm. Front. Cell Dev. Biol. 2024, 12, 1457638. [Google Scholar] [CrossRef]
- Penalva, D.A.; Oresti, G.M.; Dupuy, F.; Antollini, S.S.; Maggio, B.; Aveldano, M.I.; Fanani, M.L. Atypical surface behavior of ceramides with nonhydroxy and 2-hydroxy very long-chain (C28–C32) PUFAs. Biochim. Biophys. Acta 2014, 1838, 731–738. [Google Scholar] [CrossRef]
- Santiago Valtierra, F.X.; Mateos, M.V.; Aveldano, M.I.; Oresti, G.M. Sphingomyelins and ceramides with VLCPUFAs are excluded from low-density raft-like domains in differentiating spermatogenic cells. J. Lipid Res. 2017, 58, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Gafurova, C.R.; Tsentsevitsky, A.N.; Fedorov, N.S.; Khaziev, A.N.; Malomouzh, A.I.; Petrov, A.M. beta2-Adrenergic Regulation of the Neuromuscular Transmission and Its Lipid-Dependent Switch. Mol. Neurobiol. 2024, 61, 6805–6821. [Google Scholar] [CrossRef] [PubMed]
- Tsentsevitsky, A.N.; Gafurova, C.R.; Mukhutdinova, K.A.; Giniatullin, A.R.; Fedorov, N.S.; Malomouzh, A.I.; Petrov, A.M. Sphingomyelinase modulates synaptic vesicle mobilization at the mice neuromuscular junctions. Life Sci. 2023, 318, 121507. [Google Scholar] [CrossRef] [PubMed]
- Furland, N.E.; Luquez, J.M.; Oresti, G.M.; Aveldano, M.I. Mild testicular hyperthermia transiently increases lipid droplet accumulation and modifies sphingolipid and glycerophospholipid acyl chains in the rat testis. Lipids 2011, 46, 443–454. [Google Scholar] [CrossRef]
- Oresti, G.M.; Reyes, J.G.; Luquez, J.M.; Osses, N.; Furland, N.E.; Aveldano, M.I. Differentiation-related changes in lipid classes with long-chain and very long-chain polyenoic fatty acids in rat spermatogenic cells. J. Lipid Res. 2010, 51, 2909–2921. [Google Scholar] [CrossRef]
- Furland, N.E.; Zanetti, S.R.; Oresti, G.M.; Maldonado, E.N.; Aveldano, M.I. Ceramides and sphingomyelins with high proportions of very long-chain polyunsaturated fatty acids in mammalian germ cells. J. Biol. Chem. 2007, 282, 18141–18150. [Google Scholar] [CrossRef]
- Guillou, H.; Zadravec, D.; Martin, P.G.; Jacobsson, A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog. Lipid Res. 2010, 49, 186–199. [Google Scholar] [CrossRef]
- Gregory, M.K.; Gibson, R.A.; Cook-Johnson, R.J.; Cleland, L.G.; James, M.J. Elongase reactions as control points in long-chain polyunsaturated fatty acid synthesis. PLoS ONE 2011, 6, e29662. [Google Scholar] [CrossRef]
- Iizuka-Hishikawa, Y.; Hishikawa, D.; Sasaki, J.; Takubo, K.; Goto, M.; Nagata, K.; Nakanishi, H.; Shindou, H.; Okamura, T.; Ito, C.; et al. Lysophosphatidic acid acyltransferase 3 tunes the membrane status of germ cells by incorporating docosahexaenoic acid during spermatogenesis. J. Biol. Chem. 2017, 292, 12065–12076. [Google Scholar] [CrossRef]
- Pinot, M.; Vanni, S.; Pagnotta, S.; Lacas-Gervais, S.; Payet, L.A.; Ferreira, T.; Gautier, R.; Goud, B.; Antonny, B.; Barelli, H. Lipid cell biology. Polyunsaturated phospholipids facilitate membrane deformation and fission by endocytic proteins. Science 2014, 345, 693–697. [Google Scholar] [CrossRef]
- Aveldano, M.I.; Robinson, B.S.; Johnson, D.W.; Poulos, A. Long and very long chain polyunsaturated fatty acids of the n-6 series in rat seminiferous tubules. Active desaturation of 24:4n-6 to 24:5n-6 and concomitant formation of odd and even chain tetraenoic and pentaenoic fatty acids up to C32. J. Biol. Chem. 1993, 268, 11663–11669. [Google Scholar] [CrossRef] [PubMed]
- Zadravec, D.; Tvrdik, P.; Guillou, H.; Haslam, R.; Kobayashi, T.; Napier, J.A.; Capecchi, M.R.; Jacobsson, A. ELOVL2 controls the level of n-6 28:5 and 30:5 fatty acids in testis, a prerequisite for male fertility and sperm maturation in mice. J. Lipid Res. 2011, 52, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Oresti, G.M.; Penalva, D.A.; Luquez, J.M.; Antollini, S.S.; Aveldano, M.I. Lipid Biochemical and Biophysical Changes in Rat Spermatozoa During Isolation and Functional Activation In Vitro. Biol. Reprod. 2015, 93, 140. [Google Scholar] [CrossRef]
- Bollinger, C.R.; Teichgraber, V.; Gulbins, E. Ceramide-enriched membrane domains. Biochim. Biophys. Acta 2005, 1746, 284–294. [Google Scholar] [CrossRef]
- Pinto, S.N.; Laviad, E.L.; Stiban, J.; Kelly, S.L.; Merrill, A.H., Jr.; Prieto, M.; Futerman, A.H.; Silva, L.C. Changes in membrane biophysical properties induced by sphingomyelinase depend on the sphingolipid N-acyl chain. J. Lipid Res. 2014, 55, 53–61. [Google Scholar] [CrossRef]
- Di Nisio, A.; De Toni, L.; Sabovic, I.; Vignoli, A.; Tenori, L.; Dall’Acqua, S.; Sut, S.; La Vignera, S.; Condorelli, R.A.; Giacone, F.; et al. Lipidomic Profile of Human Sperm Membrane Identifies a Clustering of Lipids Associated with Semen Quality and Function. Int. J. Mol. Sci. 2023, 25, 297. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; Cambridge University: Cambridge, UK, 2021.
- Suhaiman, L.; Altamirano, K.N.; Morales, A.; Belmonte, S.A. Different Approaches to Record Human Sperm Exocytosis. Methods Mol. Biol. 2021, 2233, 139–168. [Google Scholar] [CrossRef]
- Lopez, C.I.; Pelletan, L.E.; Suhaiman, L.; De Blas, G.A.; Vitale, N.; Mayorga, L.S.; Belmonte, S.A. Diacylglycerol stimulates acrosomal exocytosis by feeding into a PKC- and PLD1-dependent positive loop that continuously supplies phosphatidylinositol 4,5-bisphosphate. Biochim. Biophys. Acta 2012, 1821, 1186–1199. [Google Scholar] [CrossRef]
- Pelletan, L.E.; Suhaiman, L.; Vaquer, C.C.; Bustos, M.A.; De Blas, G.A.; Vitale, N.; Mayorga, L.S.; Belmonte, S.A. ADP Ribosylation Factor 6 (ARF6) Promotes Acrosomal Exocytosis by Modulating Lipid Turnover and Rab3A Activation. J. Biol. Chem. 2015, 290, 9823–9841. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Holub, B.J.; Skeaff, C.M. Nutritional regulation of cellular phosphatidylinositol. Methods Enzymol. 1987, 141, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Rouser, G.; Fkeischer, S.; Yamamoto, A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 1970, 5, 494–496. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oresti, G.M.; Luquez, J.M.; Belmonte, S.A. Lipid Signature of Motile Human Sperm: Characterization of Sphingomyelin, Ceramide, and Phospholipids with a Focus on Very Long Chain Polyunsaturated Fatty Acids. Int. J. Mol. Sci. 2025, 26, 9301. https://doi.org/10.3390/ijms26199301
Oresti GM, Luquez JM, Belmonte SA. Lipid Signature of Motile Human Sperm: Characterization of Sphingomyelin, Ceramide, and Phospholipids with a Focus on Very Long Chain Polyunsaturated Fatty Acids. International Journal of Molecular Sciences. 2025; 26(19):9301. https://doi.org/10.3390/ijms26199301
Chicago/Turabian StyleOresti, Gerardo Martín, Jessica Mariela Luquez, and Silvia Alejandra Belmonte. 2025. "Lipid Signature of Motile Human Sperm: Characterization of Sphingomyelin, Ceramide, and Phospholipids with a Focus on Very Long Chain Polyunsaturated Fatty Acids" International Journal of Molecular Sciences 26, no. 19: 9301. https://doi.org/10.3390/ijms26199301
APA StyleOresti, G. M., Luquez, J. M., & Belmonte, S. A. (2025). Lipid Signature of Motile Human Sperm: Characterization of Sphingomyelin, Ceramide, and Phospholipids with a Focus on Very Long Chain Polyunsaturated Fatty Acids. International Journal of Molecular Sciences, 26(19), 9301. https://doi.org/10.3390/ijms26199301





