Reduced Expression of m6A Demethylases FTO and ALKBH5 in Monocytes from the Site of Inflammation in Patients with Juvenile Idiopathic Arthritis
Abstract
1. Introduction
2. Results
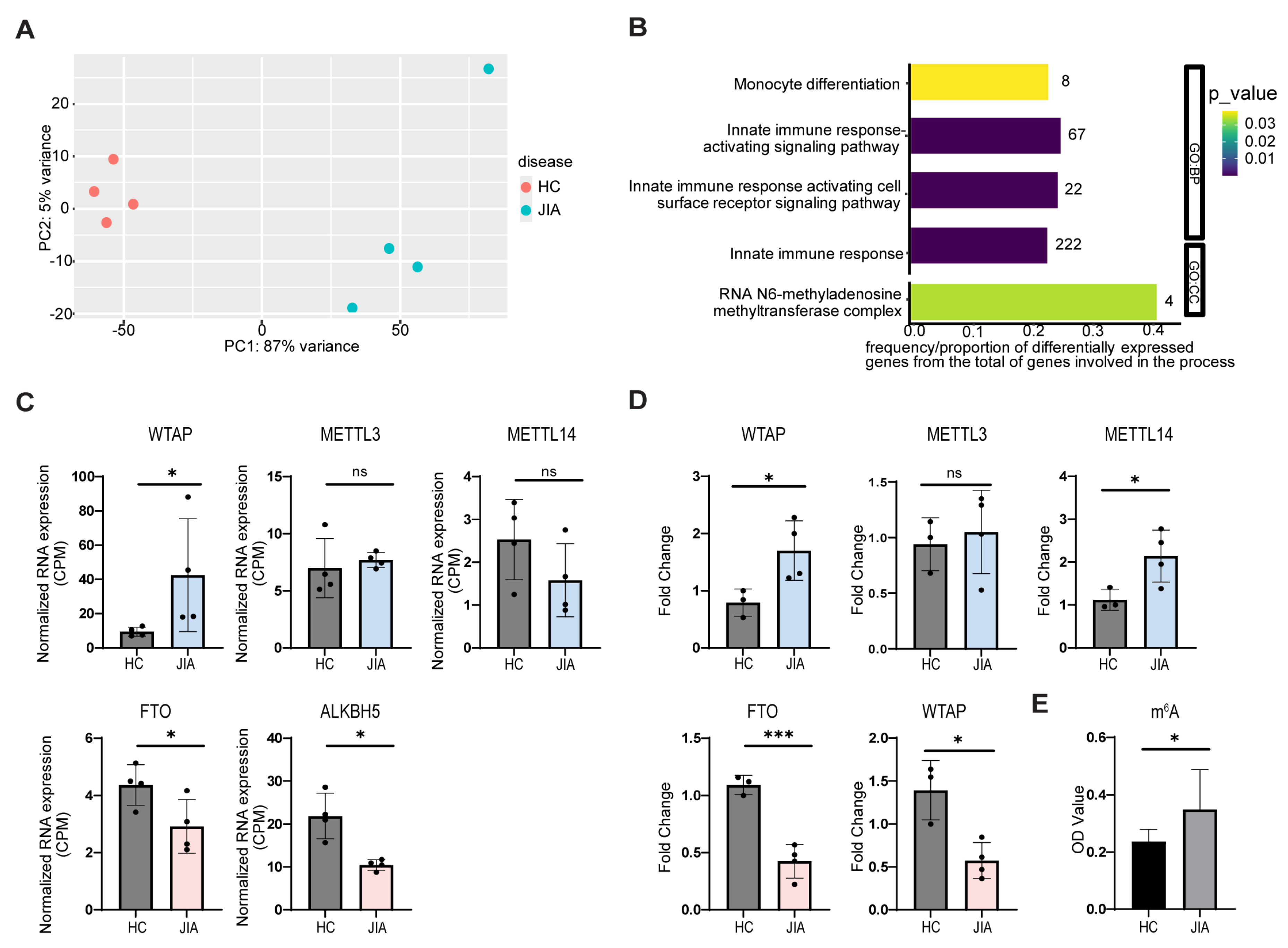
2.1. JIA Synovial Monocytes Exhibit Changes in m6A Compared to Controls
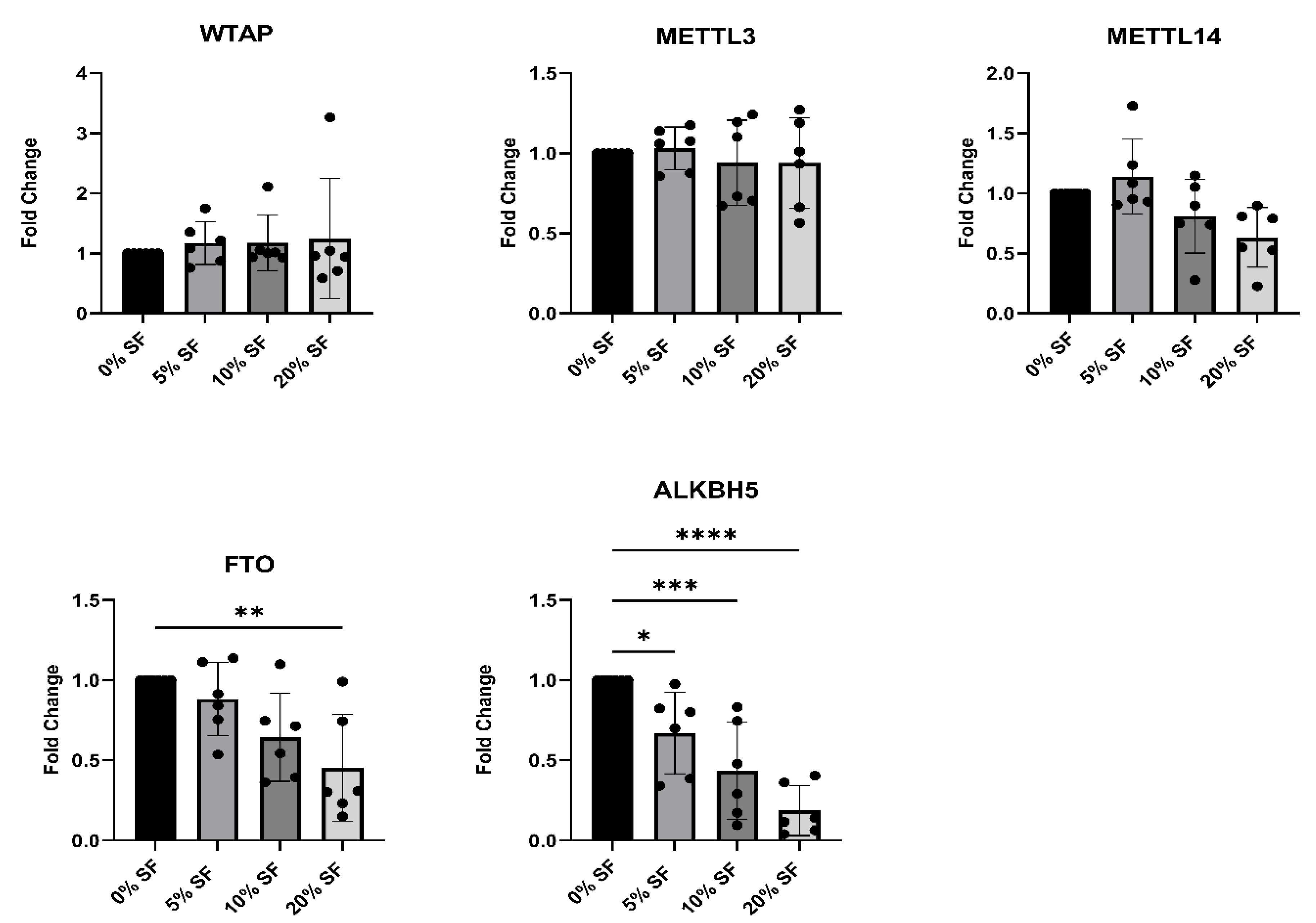
2.2. Synovial Fluid from JIA Patients Downregulates m6A Eraser Expression in Healthy Monocytes
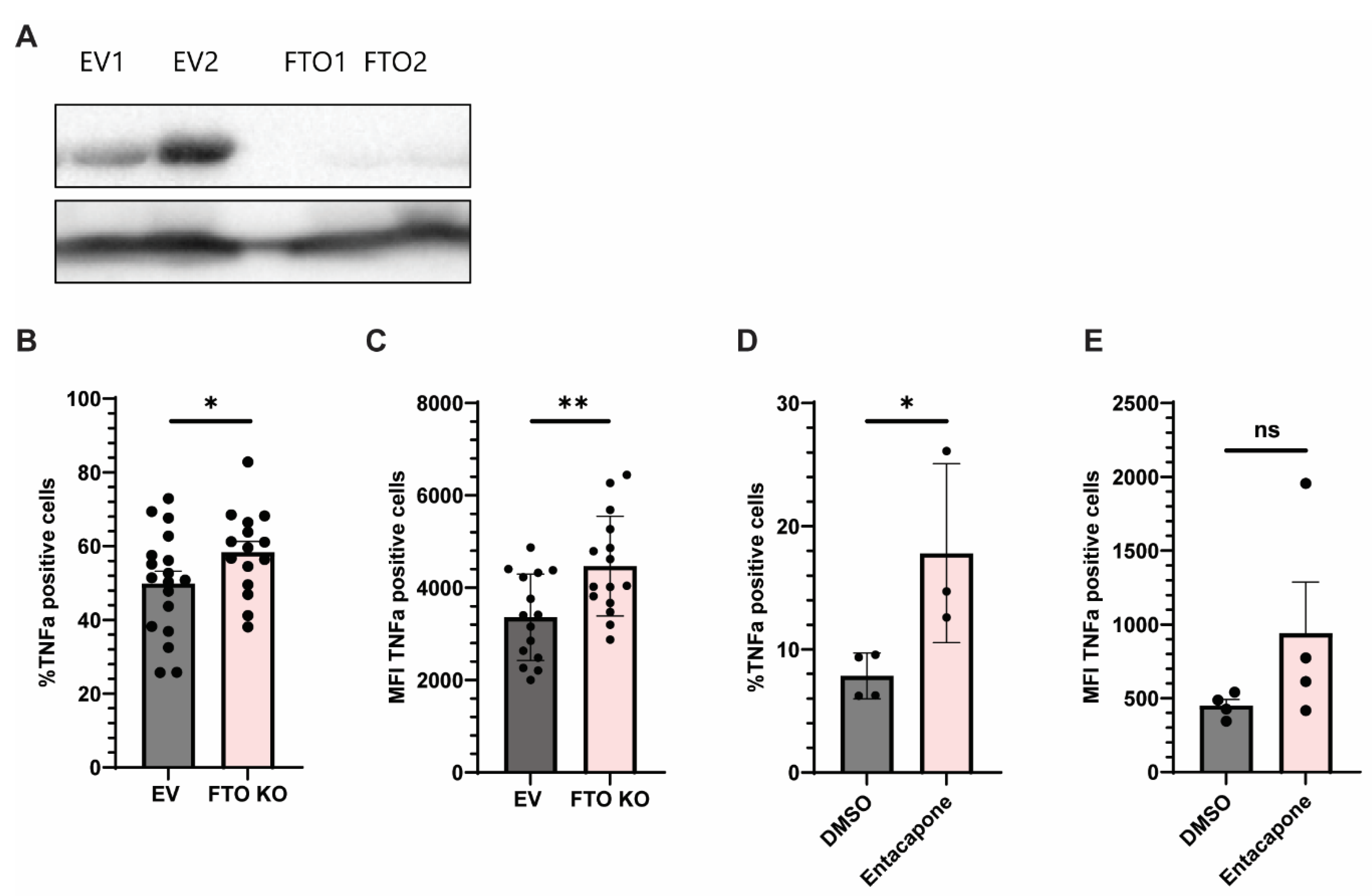
2.3. Genetic and Pharmacologic Inhibition of FTO Enhances TNFα Production in Monocytes
3. Discussion
4. Methods and Materials
4.1. Cell Culture
4.2. Culture with JIA Synovial Fluid
4.3. Next Generation RNA-Sequencing
4.4. Total m6A RNA Methylation Quantification
4.5. Quantitative PCR (RT-qPCR)
4.6. Cytokine Stimulation
4.7. Western-Blotting
4.8. CRISPR/Cas9 Knock-Out
4.9. FTO Inhibition and Flow Cytometry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALKBH5 | AlkB Homolog 5 |
| APC | Allophycocyanin |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| DESeq2 | Differential Expression Sequencing 2 |
| DMSO | Dimethyl Sulfoxide |
| FBS | Fetal Bovine Serum |
| FTO | Fat Mass and Obesity-Associated Protein |
| GO | Gene Ontology |
| JIA | Juvenile Idiopathic Arthritis |
| LPS | Lipopolysaccharide |
| MACS | Magnetic-Activated Cell Sorting |
| METTL3 | Methyltransferase-Like 3 |
| METTL14 | Methyltransferase-Like 14 |
| MFI | Mean Fluorescence Intensity |
| m6A | N6-methyladenosine |
| PBMC | Peripheral Blood Mononuclear Cell |
| PCA | Principal Component Analysis |
| PEI | Polyethylenimine |
| RT-qPCR | Reverse Transcription Quantitative Polymerase Chain Reaction |
| RA | Rheumatoid Arthritis |
| RPMI | Roswell Park Memorial Institute Medium |
| RT | Reverse Transcription |
| SF | Synovial Fluid |
| SFMC | Synovial Fluid Mononuclear Cell |
| TNFα | Tumor Necrosis Factor Alpha |
| WTAP | Wilms Tumor 1-Associating Protein |
References
- Kivity, S. Infection and autoimmunity in Sjogren’s syndrome: A clinical study and comprehensive review. J. Autoimmun. 2014, 51, 17–22. [Google Scholar] [PubMed]
- Colafrancesco, S.; Perricone, C.; Priori, R.; Valesini, G.; Shoenfeld, Y. Sjögren’s syndrome: Another facet of the autoimmune/inflammatory syndrome induced by adjuvants (ASIA). J. Autoimmun. 2014, 51, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Peng, J. Long term effect of gut microbiota transfer on diabetes development. J. Autoimmun. 2014, 53, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, F.S.; Gershwin, M.E. Human autoimmune diseases: A comprehensive update. J. Intern. Med. 2015, 278, 369–395. [Google Scholar] [CrossRef]
- Pisetsky, D.S. Pathogenesis of autoimmune disease. Nat. Rev. Nephrol. 2023, 19, 509–524. [Google Scholar] [CrossRef]
- Stansfield, B.K.; Ingram, D.A. Clinical significance of monocyte heterogeneity. Clin. Transl. Med. 2015, 4, 5. [Google Scholar] [CrossRef]
- Zhang, F. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 2019, 20, 928–942. [Google Scholar] [CrossRef]
- Sullivan, K.E. Inflammation in Juvenile Idiopathic Arthritis. Rheum. Dis. Clin. N. Am. 2007, 33, 365–388. [Google Scholar] [CrossRef]
- Eberhard, B.A.; Laxer, R.M.; Andersson, U.; Silverman, E.D. Local Synthesis of Both Macrophage and T Cell Cytokines by Synovial Fluid Cells from Children with Juvenile Rheumatoid Arthritis. Clin. Exp. Immunol. 1994, 96, 260–266. [Google Scholar] [CrossRef]
- Schmidt, T. Children with oligoarticular juvenile idiopathic arthritis have skewed synovial monocyte polarization pattern with functional impairment—A distinct inflammatory pattern for oligoarticular juvenile arthritis. Arthritis Res. Ther. 2020, 22, 186. [Google Scholar] [CrossRef]
- Mazzone, R. The emerging role of epigenetics in human autoimmune disorders. Clin. Epigenetics 2019, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K. Broadening our understanding of the genetics of Juvenile Idiopathic Arthritis (JIA): Interrogation of three dimensional chromatin structures and genetic regulatory elements within JIA-associated risk loci. PLoS ONE 2020, 15, e0235857. [Google Scholar] [CrossRef] [PubMed]
- Surace, A.E.A.; Hedrich, C.M. The role of epigenetics in autoimmune/inflammatory disease. Front. Immunol. 2019, 10, 1525. [Google Scholar] [CrossRef] [PubMed]
- Peeters, J.G.C. Epigenetic changes in autoimmune monocytes contribute to disease and can be targeted by JAK inhibition. Rheumatology 2023, 62, 2887–2897. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qian, C.; Cao, X. Post-Translational Modification Control of Innate Immunity. Immunity 2016, 45, 15–30. [Google Scholar] [CrossRef]
- Liu, C. Potential roles of N6-methyladenosine (m6A) in immune cells. J. Transl. Med. 2021, 19, 251. [Google Scholar] [CrossRef]
- Yu, R.; Li, Q.; Feng, Z.; Cai, L.; Xu, Q. m6A reader YTHDF2 regulates lps-induced inflammatory response. Int. J. Mol. Sci. 2019, 20, 1323. [Google Scholar] [CrossRef]
- Wang, J.; Yan, S.; Lu, H.; Wang, S.; Xu, D. METTL3 Attenuates LPS-Induced Inflammatory Response in Macrophages via NF-κB Signaling Pathway. Mediat. Inflamm. 2019, 2019, 3120391. [Google Scholar] [CrossRef]
- Zheng, Y. Mettl14 mediates the inflammatory response of macrophages in atherosclerosis through the NF-κB/IL-6 signaling pathway. Cell. Mol. Life Sci. 2022, 79, 311. [Google Scholar] [CrossRef]
- Liu, Y. The N6-methyladenosine (m6A)-forming enzyme METTL3 facilitates M1 macrophage polarization through the methylation of STAT1 mRNA. Am. J. Physiol. Cell Physiol. 2019, 317, 762–775. [Google Scholar] [CrossRef]
- Gu, X. N6-methyladenosine demethylase FTO promotes M1 and M2 macrophage activation. Cell Signal 2020, 69, 109553. [Google Scholar] [CrossRef]
- Luo, Q. Decreased ALKBH5, FTO, and YTHDF2 in Peripheral Blood Are as Risk Factors for Rheumatoid Arthritis. Biomed. Res. Int. 2020, 2020, 5735279. [Google Scholar] [CrossRef]
- Mo, X.B.; Zhang, Y.H.; Lei, S.F. Genome-wide identification of N6-methyladenosine (m6A) SNPs associated with rheumatoid arthritis. Front. Genet. 2018, 9, 299. [Google Scholar] [CrossRef] [PubMed]
- Lv, X. RNA Methylation in Systemic Lupus Erythematosus. Front. Cell Dev. Biol. 2021, 9, 696559. [Google Scholar] [CrossRef] [PubMed]
- Li, L.J.; Fan, Y.G.; Leng, R.X.; Pan, H.F.; Ye, D.Q. Potential link between m6A modification and systemic lupus erythematosus. Mol. Immunol. 2018, 93, 55–63. [Google Scholar] [PubMed]
- Luo, Q. Decreased Peripheral Blood ALKBH5 Correlates with Markers of Autoimmune Response in Systemic Lupus Erythematosus. Dis. Markers 2020, 2020, 8193895. [Google Scholar] [CrossRef]
- Luo, Q. The study of METTL14, ALKBH5, and YTHDF2 in peripheral blood mononuclear cells from systemic lupus erythematosus. Mol. Genet. Genom. Med. 2020, 8, e1298. [Google Scholar] [CrossRef]
- Wang, Y.N.; Jin, H.Z. Transcriptome-Wide m6A Methylation in Skin Lesions From Patients With Psoriasis Vulgaris. Front. Cell Dev. Biol. 2020, 8, 591629. [Google Scholar] [CrossRef]
- Ye, F. N6-Methyladenosine RNA modification in cerebrospinal fluid as a novel potential diagnostic biomarker for progressive multiple sclerosis. J. Transl. Med. 2021, 19, 316. [Google Scholar] [CrossRef]
- Zhang, S. Identification of molecular patterns and diagnostic biomarkers in juvenile idiopathic arthritis based on the gene expression of m6A regulators. Front. Pediatr. 2022, 10, 930119. [Google Scholar] [CrossRef]
- De Jager, W.; Hoppenreijs, E.P.A.H.; Wulffraat, N.M.; Wedderburn, L.R.; Kuis, W.; Prakken, B.J. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: A cross-sectional study. Ann. Rheum. Dis. 2007, 66, 589–598, Erratum in Ann. Rheum. Dis. 2008, 67, 280. [Google Scholar] [CrossRef]
- Hu, Z.; Li, Y.; Yuan, W.; Jin, L.; Leung, W.K.; Zhang, C.; Yang, Y. N6-methyladenosine of Socs1 modulates macrophage inflammatory response in different stiffness environments. Int. J. Biol. Sci. 2022, 18, 5753–5769. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Meng, W.; Chen, L.; Zhang, X.; Markazi, A.; Yuan, W.; Huang, Y.; Gao, S.J. N6-Methyladenosine and reader protein YTHDF2 enhance the innate immune response by mediating DUSP1 mRNA degradation and activating mitogen-activated protein kinases during bacterial and viral infections. mBio 2023, 14, e03349-22. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Yi, J.; Chen, J.; Yang, L. N6-Methyladenosine (m6A) Modification in Natural Immune Cell-Mediated Inflammatory Diseases. J. Innate Immun. 2023, 15, 804–821. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Fu, Y.; Zhao, X.; Dai, Q.; Zheng, G.; Yang, Y.; Yi, C.; Lindahl, T.; Pan, T.; Yang, Y.-G.; et al. N6-Methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011, 7, 885–887. [Google Scholar] [CrossRef]
- Zheng, G.; Dahl, J.A.; Niu, Y.; Fedorcsak, P.; Huang, C.M.; Li, C.J.; Vågbø, C.B.; Shi, Y.; Wang, W.L.; Song, S.H.; et al. ALKBH5 Is a Mammalian RNA Demethylase that Impacts RNA Metabolism and Mouse Fertility. Mol. Cell 2013, 49, 18–29. [Google Scholar] [CrossRef]
- Yazar, M.; Sarban, S.; Kocyigit, A.; Isikan, U.E. Synovial Fluid and Plasma Selenium, Copper, Zinc, and Iron Concentrations in Patients with Rheumatoid Arthritis and Osteoarthritis. Biol. Trace Elem. Res. 2005, 106, 123–132. [Google Scholar] [CrossRef]
- Konieczynski, P.; Szreder, G.; Tamowska, E.; Wesolowski, M. Essential elements in synovial fluid samples obtained from patients living in Northern Poland. J. Trace Elem. Med. Biol. 2018, 48, 20–24. [Google Scholar] [CrossRef]
- Konieczynski, P.; Szreder, G.; Wesolowski, M. Study of the Element Concentrations in Synovial Fluids of Patients with Arthritis and Rheumatic Diseases. Arch. Orthop. 2022, 3, 1–9. [Google Scholar] [CrossRef]
- Boltjes, A. Conventional dendritic cells type 1 are strongly enriched, quiescent and relatively tolerogenic in local inflammatory arthritis. Front. Immunol. 2023, 13, 1101999. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Zhu, A.; Ibrahim, J.G.; Love, M.I. Heavy-tailed prior distributions for sequence count data: Removing the noise and preserving large differences. Bioinformatics 2019, 35, 2084–2092. [Google Scholar] [CrossRef]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Vilo, J.; Peterson, H. gprofiler2—An R package for gene list functional enrichment analysis and namespace conversion toolset g:Profiler. F1000Research 2020, 9, 709. [Google Scholar] [CrossRef]
- Behera, A.K. Blocking intercellular adhesion molecule-1 on human epithelial cells decreases respiratory syncytial virus infection. Biochem. Biophys. Res. Commun. 2001, 280, 188–195. [Google Scholar] [CrossRef]
- Slobodin, B. Transcription Impacts the Efficiency of mRNA Translation via Co-transcriptional N6-adenosine Methylation. Cell 2017, 169, 326–337. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu-Tawil, H.I.; Picavet, L.W.; van Vroonhoven, E.C.N.; Bodelón, A.; Scholman, R.C.; ter Haar, N.; Boltjes, A.; Vastert, S.J.; van Loosdregt, J. Reduced Expression of m6A Demethylases FTO and ALKBH5 in Monocytes from the Site of Inflammation in Patients with Juvenile Idiopathic Arthritis. Int. J. Mol. Sci. 2025, 26, 9248. https://doi.org/10.3390/ijms26189248
Abu-Tawil HI, Picavet LW, van Vroonhoven ECN, Bodelón A, Scholman RC, ter Haar N, Boltjes A, Vastert SJ, van Loosdregt J. Reduced Expression of m6A Demethylases FTO and ALKBH5 in Monocytes from the Site of Inflammation in Patients with Juvenile Idiopathic Arthritis. International Journal of Molecular Sciences. 2025; 26(18):9248. https://doi.org/10.3390/ijms26189248
Chicago/Turabian StyleAbu-Tawil, Hisham I., Lucas W. Picavet, Ellen C. N. van Vroonhoven, Alejandra Bodelón, Rianne C. Scholman, Nienke ter Haar, Arjan Boltjes, Sebastiaan J. Vastert, and Jorg van Loosdregt. 2025. "Reduced Expression of m6A Demethylases FTO and ALKBH5 in Monocytes from the Site of Inflammation in Patients with Juvenile Idiopathic Arthritis" International Journal of Molecular Sciences 26, no. 18: 9248. https://doi.org/10.3390/ijms26189248
APA StyleAbu-Tawil, H. I., Picavet, L. W., van Vroonhoven, E. C. N., Bodelón, A., Scholman, R. C., ter Haar, N., Boltjes, A., Vastert, S. J., & van Loosdregt, J. (2025). Reduced Expression of m6A Demethylases FTO and ALKBH5 in Monocytes from the Site of Inflammation in Patients with Juvenile Idiopathic Arthritis. International Journal of Molecular Sciences, 26(18), 9248. https://doi.org/10.3390/ijms26189248





