The Synergistic Effect of Polysaccharides and Silane Coupling Agents on the Properties of Calcium Phosphate-Based Bone Substitutes
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical and Phase Composition
2.2. Setting Times
2.3. Mechanical Strength
2.4. Porosity
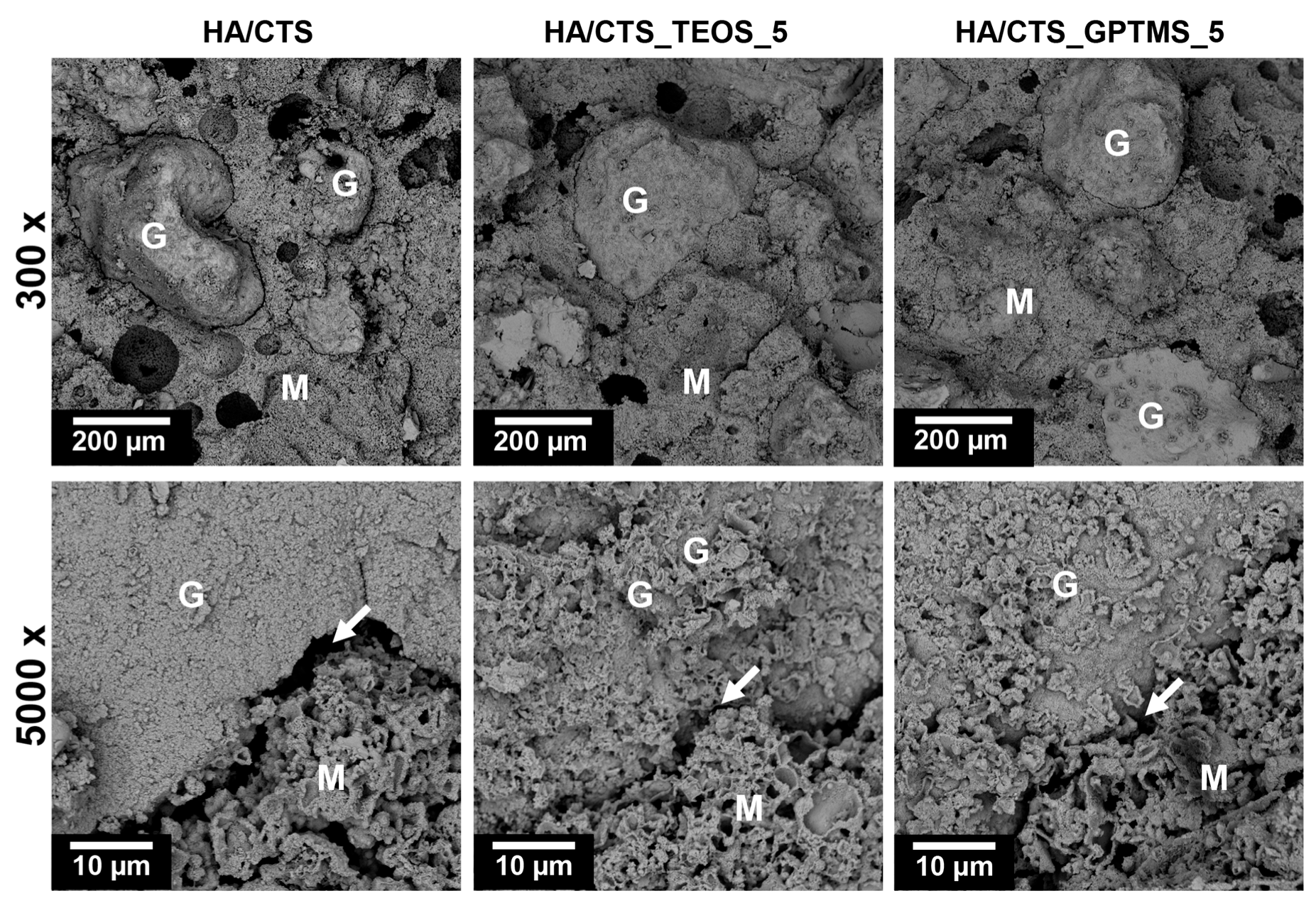
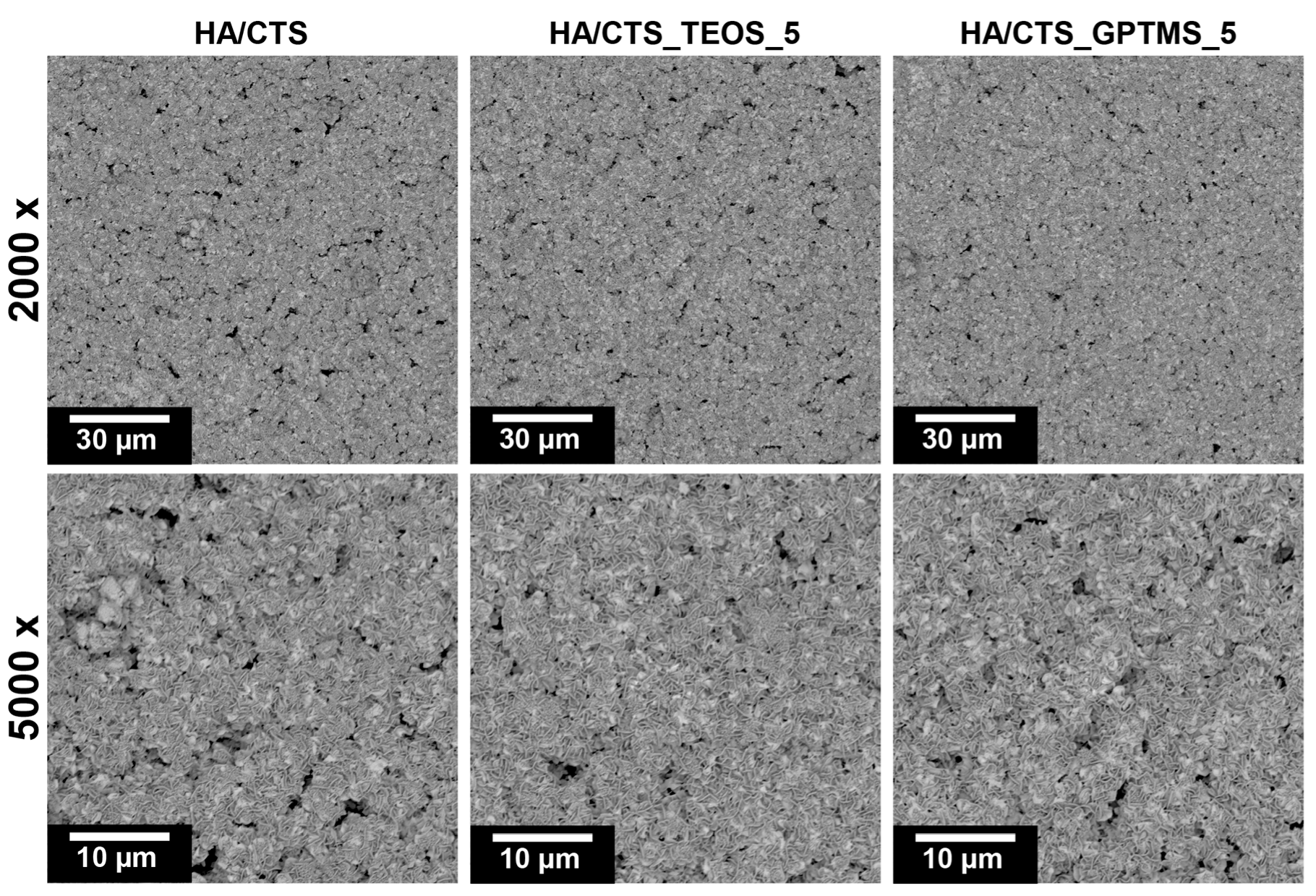
2.5. Microstructure
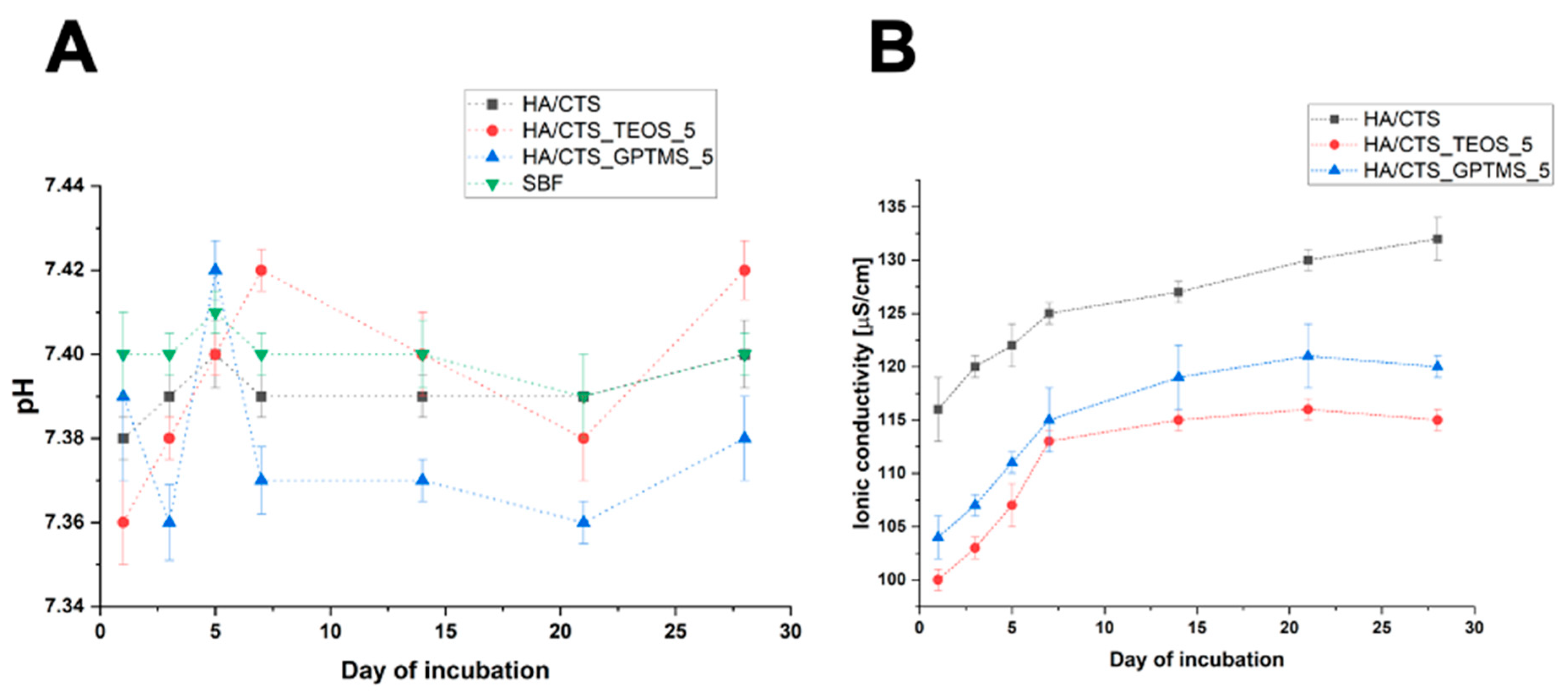
2.6. Chemical Stability and Bioactivity In Vitro
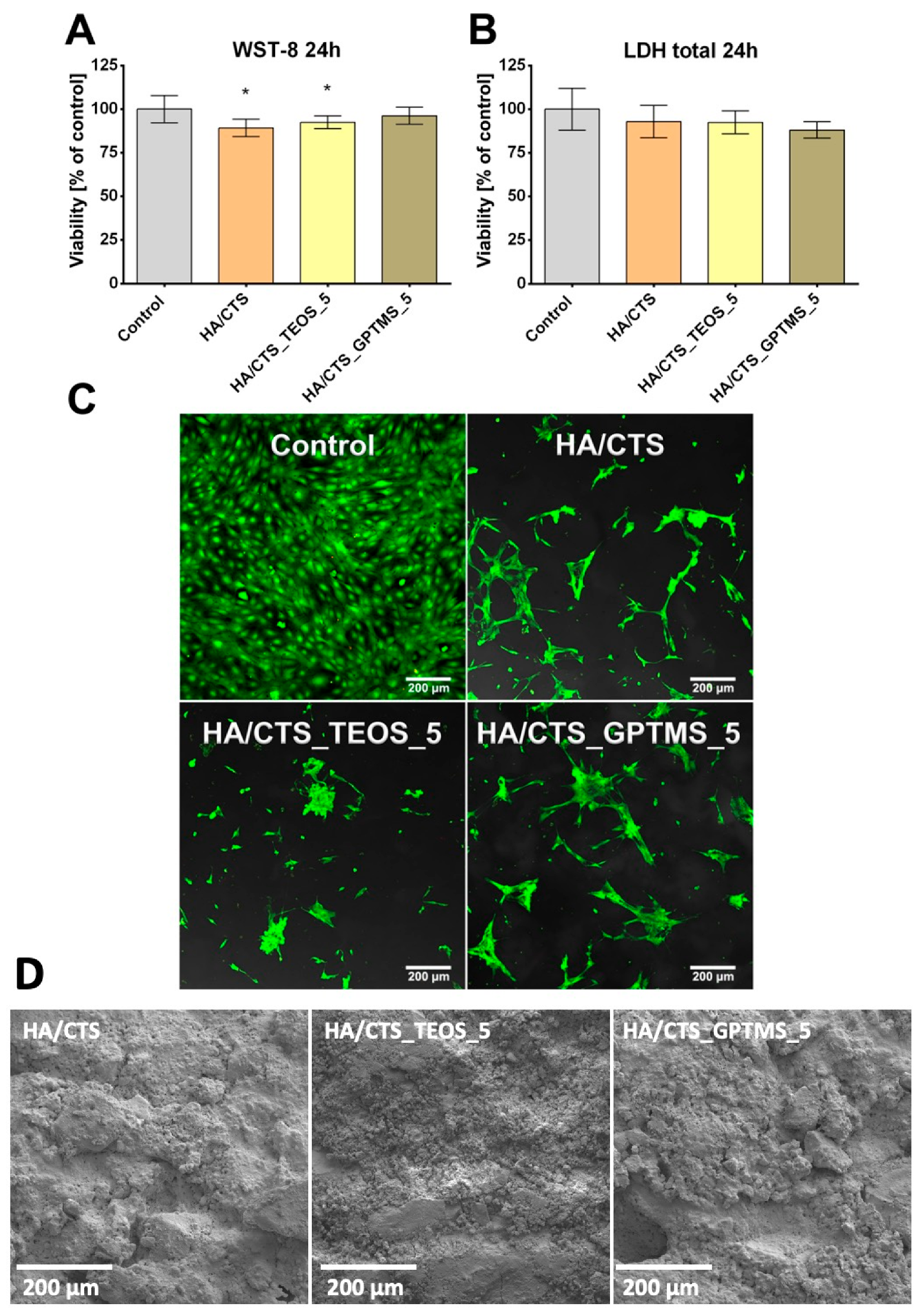
2.7. Cytotoxicity Tests
3. Materials and Methods
3.1. Materials
3.1.1. Synthesis of α-TCP and SCA-Modified α-TCP Powders
3.1.2. Synthesis of Hybrid Hydroxyapatite/Chitosan Granules
3.2. Preparation of Bone Cements
3.3. Methods
3.3.1. Chemical and Phase Composition
3.3.2. Setting Times
3.3.3. Mechanical Strength
3.3.4. Porosity
3.3.5. Microstructure
3.3.6. Chemical Stability and Bioactivity
3.3.7. Cell Studies
3.3.8. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tronco, M.C.; Cassel, J.B.; dos Santos, L.A. α-TCP-based calcium phosphate cements: A critical review. Acta Biomater. 2022, 151, 70–87. [Google Scholar] [CrossRef]
- Zhao, X. Introduction to bioactive materials in medicine. In Woodhead Publishing Series in Biomaterials, Bioactive Materials in Medicine; Woodhead Publishing: Sawston, UK, 2011; pp. 1–13. [Google Scholar] [CrossRef]
- Pylostomou, A.; Demir, Ö.; Loca, D. Calcium phosphate bone cements as local drug delivery systems for bone cancer treatment. Mater. Sci. Eng. C 2023, 148, 213367. [Google Scholar] [CrossRef] [PubMed]
- Schröter, L.; Kaiser, F.; Stein, S.; Gbureck, U.; Ignatius, A. Biological and mechanical performance and degradation characteristics of calcium phosphate cements in large animals and humans. Acta Biomater. 2020, 117, 1–20. [Google Scholar] [CrossRef]
- Steinacker, V.C.; Weichhold, J.; Renner, T.; Gubik, S.; Vollmer, A.; Breitenbücher, N.; Fuchs, A.; Straub, A.; Hartmann, S.; Kübler, A.C.; et al. Biological and mechanical performance of calcium phosphate cements modified with phytic acid. J. Mater. Sci. Mater. Med. 2024, 35, 36. [Google Scholar] [CrossRef]
- Graça, M.P.F.; Gavinho, S.R. Calcium Phosphate Cements in Tissue Engineering. In Contemporary Topics About Phosphorus in Biology and Materials; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Ginebra, M.; Rilliard, A.; Fernández, E.; Elvira, C.; Román, J.S.; Planell, J.A. Improvement of the Mechanical Properties of an α-TCP Cement by the Addition of a Polymeric Drug Containing Salicylic Acid. Key Eng. Mater. 2000, 192-195, 781–784. [Google Scholar] [CrossRef]
- Geffers, M.; Groll, J.; Gbureck, U. Reinforcement Strategies for Load-Bearing Calcium Phosphate Biocements. Materials 2015, 8, 2700–2717. [Google Scholar] [CrossRef]
- Dos Santos, L.; De Oliveira, L.; Rigo, E.; Carrodeguas, R.; Boschi, A.; De Arruda, A. Influence of polymeric additives on the mechanical properties of α-tricalcium phosphate cement. Bone 1999, 25, 99S–102S. [Google Scholar] [CrossRef] [PubMed]
- A Perez, R.; Kim, H.-W.; Ginebra, M.-P. Polymeric additives to enhance the functional properties of calcium phosphate cements. J. Tissue Eng. 2012, 3, 2041731412439555. [Google Scholar] [CrossRef]
- Czechowska, J.; Zima, A.; Siek, D.; Ślósarczyk, A. Influence of sodium alginate and methylcellulose on hydrolysis and physicochemical properties of α-TCP based materials. Ceram. Int. 2018, 44, 6533–6540. [Google Scholar] [CrossRef]
- Watanabe, M.; Tanaka, M.; Sakurai, M.; Maeda, M. Development of calcium phosphate cement. J. Eur. Ceram. Soc. 2006, 26, 549–552. [Google Scholar] [CrossRef]
- Dziadek, M.; Zima, A.; Cichoń, E.; Czechowska, J.; Ślósarczyk, A. Biomicroconcretes based on the hybrid HAp/CTS granules, α-TCP and pectins as a novel injectable bone substitutes. Mater. Lett. 2020, 265, 127457. [Google Scholar] [CrossRef]
- Guo, L.; Liang, Z.; Yang, L.; Du, W.; Yu, T.; Tang, H.; Li, C.; Qiu, H. The role of natural polymers in bone tissue engineering. J. Control. Release 2021, 338, 571–582. [Google Scholar] [CrossRef]
- Gu, T.; Shi, H.; Ye, J. Reinforcement of calcium phosphate cement by incorporating with high-strength β-tricalcium phosphate aggregates. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 100B, 350–359. [Google Scholar] [CrossRef]
- Xu, H.H.; Quinn, J.B. Calcium phosphate cement containing resorbable fibers for short-term reinforcement and macroporosity. Biomaterials 2002, 23, 193–202. [Google Scholar] [CrossRef]
- Boehm, A.V.; Meininger, S.; Gbureck, U.; Müller, F.A. Self-healing capacity of fiber-reinforced calcium phosphate cements. Sci. Rep. 2020, 10, 9430. [Google Scholar] [CrossRef]
- Czechowska, J.P.; Dorner-Reisel, A.; Zima, A. Hybrid Bone Substitute Containing Tricalcium Phosphate and Silver Modified Hydroxyapatite–Methylcellulose Granules. J. Funct. Biomater. 2024, 15, 196. [Google Scholar] [CrossRef]
- Pańtak, P.; Czechowska, J.P.; Zima, A. The influence of silane coupling agents on the properties of α-TCP-based ceramic bone substitutes for orthopaedic applications. RSC Adv. 2023, 13, 34020–34031. [Google Scholar] [CrossRef]
- Tang, Q.; Xiao, P.; Kou, C.; Lou, K.; Kang, A.; Wu, Z. Physical, chemical and interfacial properties of modified recycled concrete aggregates for asphalt mixtures: A review. Constr. Build. Mater. 2021, 312, 125357. [Google Scholar] [CrossRef]
- Kouhi, M.; Reddy, V.J.; Ramakrishna, S. GPTMS-Modified Bredigite/PHBV Nanofibrous Bone Scaffolds with Enhanced Mechanical and Biological Properties. Appl. Biochem. Biotechnol. 2018, 188, 357–368. [Google Scholar] [CrossRef]
- Bravaya, N.; Saratovskikh, S.; Panin, A.; Faingol’D, E.; Zharkov, I.; Babkina, O.; Vasil’EV, S.; Bubnova, M.; Volkov, V.; Lobanov, M. Influence of silane coupling agent on the synthesis and properties of nanocomposites obtained via in situ catalytic copolymerization of ethylene and propylene in the presence of modified Nafen™ Al2O3 nanofibers. Polymer 2019, 174, 114–122. [Google Scholar] [CrossRef]
- Varghese, J.T.; Cho, K.; Raju; Farrar, P.; Prentice, L.; Prusty, B.G. Influence of silane coupling agent on the mechanical performance of flowable fibre-reinforced dental composites. Dent. Mater. 2022, 38, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Götz, W.; Tobiasch, E.; Witzleben, S.; Schulze, M. Effects of Silicon Compounds on Biomineralization, Osteogenesis, and Hard Tissue Formation. Pharmaceutics 2019, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.; Konishi, T.; Wang, Z.; Feng, J.; Wang, L.; Han, J.; Yang, Z.; Thian, E. Enhancing osteoconductivity and biocompatibility of silver-substituted apatite in vivo through silicon co-substitution. Mater. Lett. 2018, 212, 90–93. [Google Scholar] [CrossRef]
- Reid, J.W.; Tuck, L.; Sayer, M.; Fargo, K.; Hendry, J.A. Synthesis and characterization of single-phase silicon-substituted α-tricalcium phosphate. Biomaterials 2006, 27, 2916–2925. [Google Scholar] [CrossRef]
- Mestres, G.; Le Van, C.; Ginebra, M.P. Silicon-stabilized α-tricalcium phosphate and its use in a calcium phosphate cement: Characterization and cell response. Acta Biomater. 2012, 8, 1169–1179. [Google Scholar] [CrossRef]
- Şahin, E. Calcium Phosphate Bone Cements. In Cement Based Materials; InTech Open: London, UK, 2018. [Google Scholar] [CrossRef]
- Yubao, L.; Xingdong, Z.; de Groot, K. Hydrolysis and phase transition of alpha-tricalcium phosphate. Biomaterials 1997, 18, 737–741. [Google Scholar] [CrossRef]
- Casagrande, C.A.; Jochem, L.F.; Repette, W.L. Analysis of the 3-Glycidoxypropyltrimethoxysilane (GPTMS) hydrolysis by infrared spectroscopy. Mater. De Jan. 2020, 25, e-12811. [Google Scholar] [CrossRef]
- Rubio, F.; Rubio, J.; Oteo, J.L. A FT-IR Study of the Hydrolysis of Tetraethylorthosilicate (TEOS). Spectrosc. Lett. 1998, 31, 199–219. [Google Scholar] [CrossRef]
- Gui-Long, X.; Changyun, D.; Yun, L.; Pi-Hui, P.; Jian, H.; Zhuoru, Y. Preparation and Characterization of Raspberry-like SiO2 Particles by the Sol-Gel Method. Nanomater. Nanotechnol. 2011, 1, 21. [Google Scholar] [CrossRef]
- He, Z.; Zhai, Q.; Hu, M.; Cao, C.; Wang, J.; Yang, H.; Li, B. Bone cements for percutaneous vertebroplasty and balloon kyphoplasty: Current status and future developments. J. Orthop. Transl. 2015, 3, 1–11. [Google Scholar] [CrossRef]
- Durucan, C.; Brown, P.W. Reactivity of α-tricalcium phosphate. J. Mater. Sci. 2002, 37, 963–969. [Google Scholar] [CrossRef]
- Pańtak, P.; Cichoń, E.; Czechowska, J.; Zima, A. Influence of Natural Polysaccharides on Properties of the Biomicroconcrete-Type Bioceramics. Materials 2021, 14, 7496. [Google Scholar] [CrossRef]
- Wei, X.; Ugurlu, O.; Ankit, A.; Acar, H.Y.; Akinc, M. Dissolution behavior of Si,Zn-codoped tricalcium phosphates. Mater. Sci. Eng. C 2009, 29, 126–135. [Google Scholar] [CrossRef]
- Ji, E.; Song, Y.H.; Seo, J.H.; Joo, K.I. Utilization of functionalized silane coatings for enhanced mechanical properties of hydroxyapatite filler. Korean J. Chem. Eng. 2023, 40, 1709–1714. [Google Scholar] [CrossRef]
- Vaz, C.; Reis, R.; Cunha, A. Use of coupling agents to enhance the interfacial interactions in starch–EVOH/hydroxylapatite composites. Biomaterials 2002, 23, 629–635. [Google Scholar] [CrossRef]
- Kotha, S.P.; Lieberman, M.; Vickers, A.; Schmid, S.R.; Mason, J.J. Adhesion enhancement of steel fibers to acrylic bone cement through a silane coupling agent. J. Biomed. Mater. Res. Part A 2005, 76A, 111–119. [Google Scholar] [CrossRef]
- Pańtak, P.; Czechowska, J.P.; Kashimbetova, A.; Čelko, L.; Montufar, E.B.; Wójcik, Ł.; Zima, A. Improving the processability and mechanical strength of self-hardening robocasted hydroxyapatite scaffolds with silane coupling agents. J. Mech. Behav. Biomed. Mater. 2024, 161, 106792. [Google Scholar] [CrossRef]
- Pape, P.G.; Promoters, A. Plastics Design Library, Applied Plastics Engineering Handbook; William Andrew Publishing: Norwich, NY, USA, 2011; pp. 503–517. [Google Scholar] [CrossRef]
- Wu, J.-K.; Zheng, K.-W.; Nie, X.-C.; Ge, H.-R.; Wang, Q.-Y.; Xu, J.-T. Promoters for Improved Adhesion Strength between Addition-Cured Liquid Silicone Rubber and Low-Melting-Point Thermoplastic Polyurethanes. Materials 2022, 15, 991. [Google Scholar] [CrossRef] [PubMed]
- Matinlinna, J.P.; Lung, C.Y.K.; Tsoi, J.K.H. Silane adhesion mechanism in dental applications and surface treatments: A review. Dent. Mater. 2018, 34, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yamaguchi, S.; Imazato, S. Quantitative evaluation of the degradation amount of the silane coupling layer of CAD/CAM resin composites by water absorption. J. Prosthodont. Res. 2023, 67, 55–61. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Kolmas, J.; Kaflak, A.; Zima, A.; Ślósarczyk, A. Alpha-tricalcium phosphate synthesized by two different routes: Structural and spectroscopic characterization. Ceram. Int. 2015, 41, 5727–5733. [Google Scholar] [CrossRef]
- Czechowska, J.; Zima, A.; Paszkiewicz, Z.; Lis, J.; Ślósarczyk, A. Physicochemical properties and biomimetic behaviour of α-TCP-chitosan based materials. Ceram. Int. 2014, 40, 5523–5532. [Google Scholar] [CrossRef]
- Zima, A. Hydroxyapatite-chitosan based bioactive hybrid biomaterials with improved mechanical strength. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2018, 193, 175–184. [Google Scholar] [CrossRef] [PubMed]
- ASTM C266-20; Standard Test Method for Time of Setting of Hydraulic-Cement Paste by Gillmore Needles. ASTM International: West Conshohocken, PA, USA, 2021. Available online: https://store.astm.org/c0266-20.html (accessed on 10 July 2025).
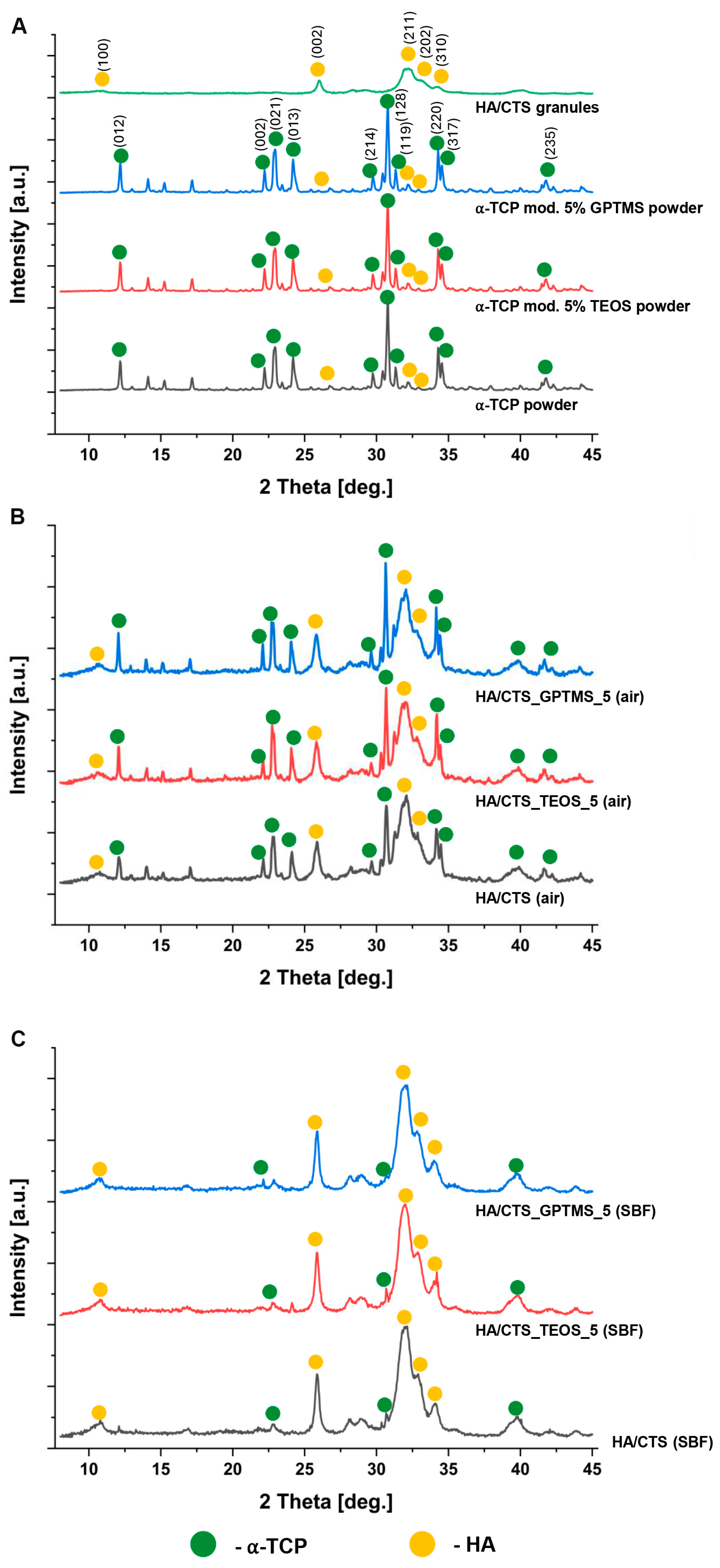
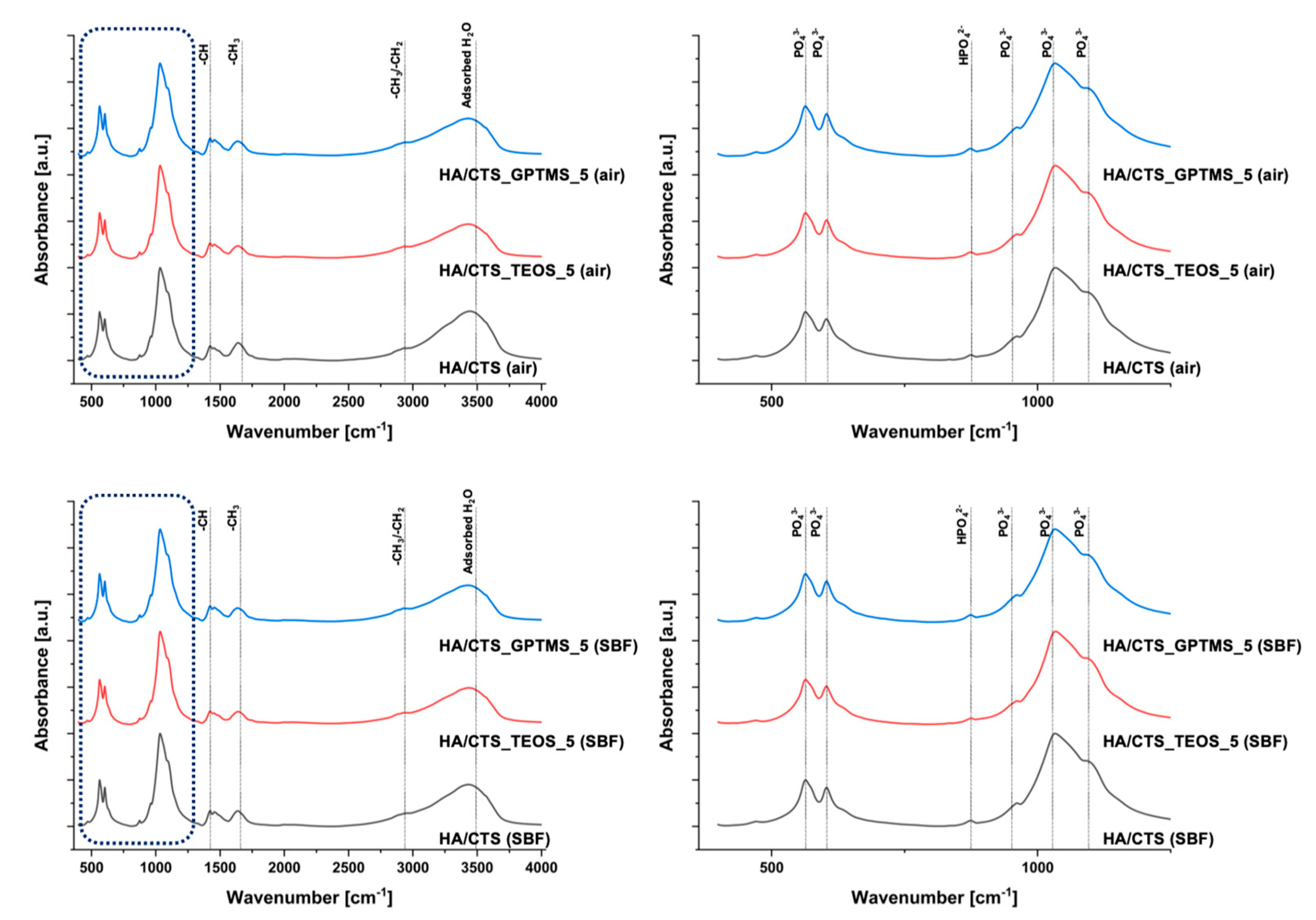
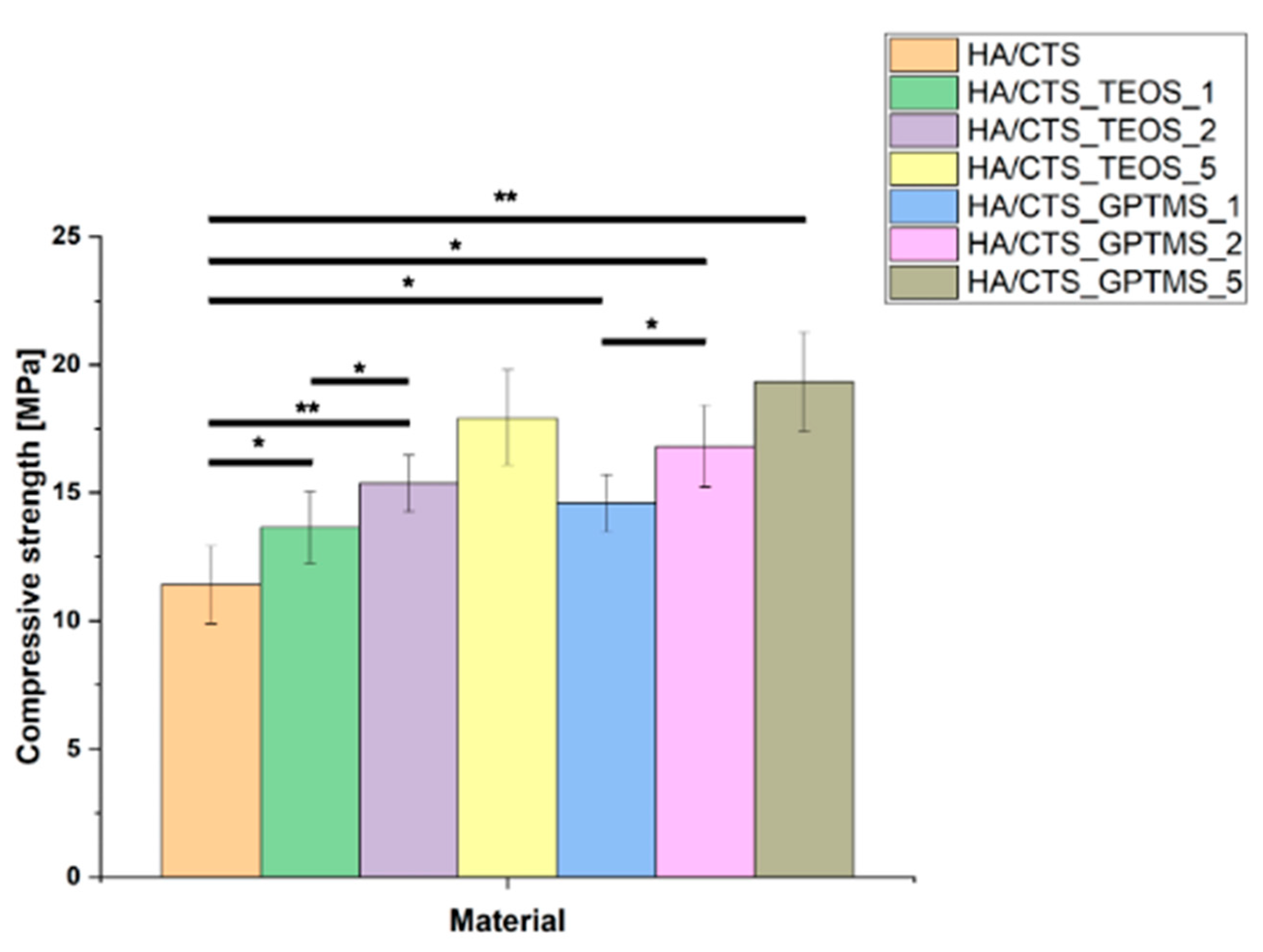
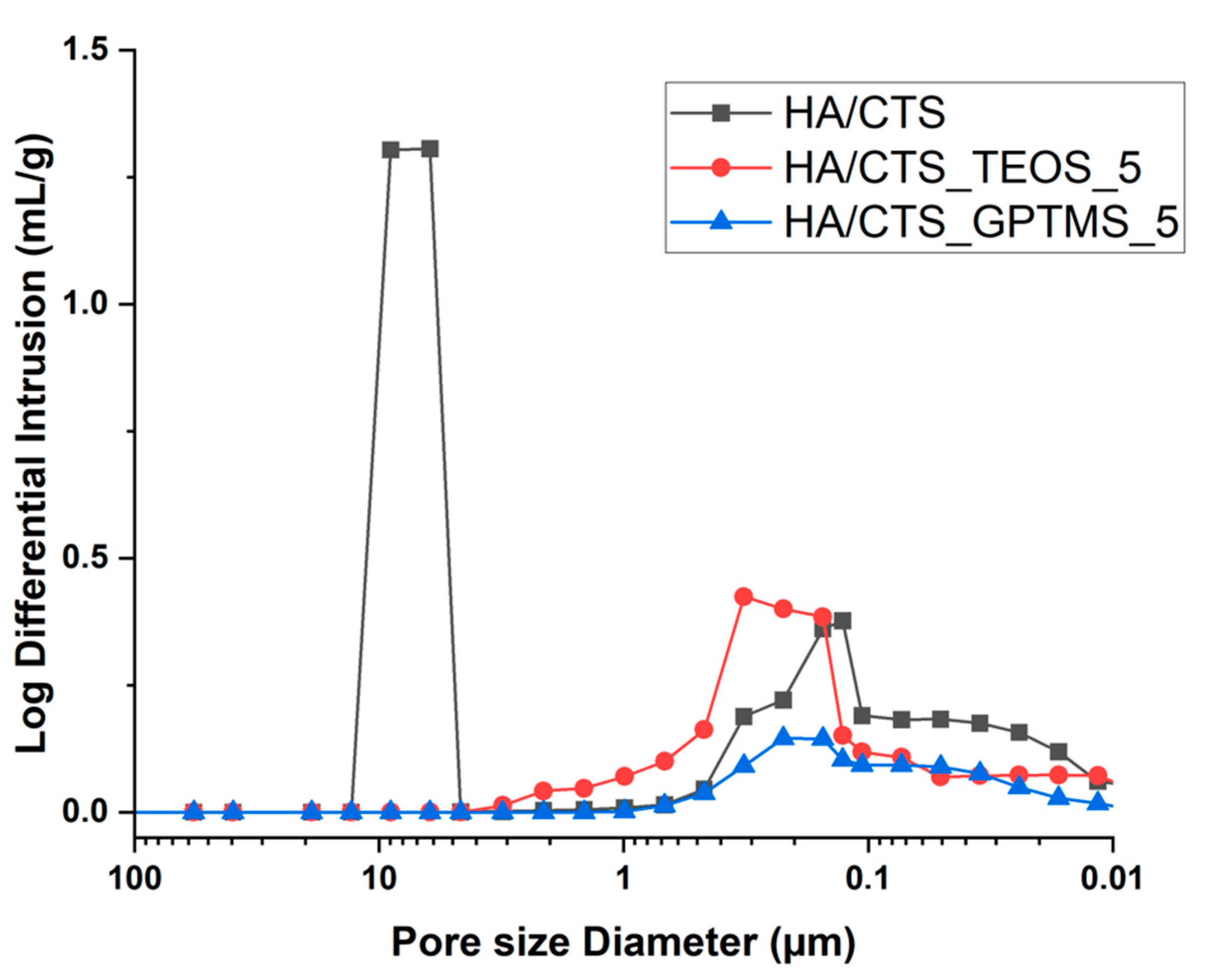
| Material | 7 Days in Air | 7 Days in SBF | ||
|---|---|---|---|---|
| α-TCP, wt.% | Hydroxyapatite, wt.% | α-TCP, wt.% | Hydroxyapatite, wt.% | |
| HA/CTS | 58 ± 2 | 42 ± 2 | 8 ± 2 | 92 ± 2 |
| HA/CTS_TEOS_5 | 52 ± 1 | 48 ± 1 | 7 ± 1 | 93 ± 1 |
| HA/CTS_GPTMS_5 | 51 ± 2 | 49 ± 2 | 6 ± 1 | 94 ± 2 |
| Material | Initial Setting Time (ti) [min] | Final Setting Time (tf) [min] |
|---|---|---|
| HA/CTS | 11.0 ± 1.0 | 19.5 ± 1.5 |
| HA/CTS_TEOS_1 | 8.5 ± 0.5 | 17.5 ± 0.5 |
| HA/CTS_TEOS_2 | 12.0 ± 1.5 | 15.0 ± 0.5 |
| HA/CTS_TEOS_5 | 6.5 ± 1.0 | 15.0 ± 1.0 |
| HA/CTS_GPTMS_1 | 10.0 ± 1.5 | 17.5 ± 1.5 |
| HA/CTS_GPTMS_2 | 7.5 ± 0.5 | 14.5 ± 1.0 |
| HA/CTS_GPTMS_5 | 5.5 ± 0.5 | 11.5 ± 1.0 |
| Material | Solid Phase (P) | Liquid Phase (L) | L/P [g/g] |
|---|---|---|---|
| HA/CTS | α-TCP: HA/CTS granules 3:2 by weight | 1.0 wt.% Na2HPO4 solution in 2.5 wt.% citrus pectin gel | 0.5 |
| HA/CTS_TEOS_1 | α-TCP (1 wt.% TEOS): HA/CTS granules 3:2 by weight | ||
| HA/CTS_TEOS_2 | α-TCP (2 wt.% TEOS): HA/CTS granules 3:2 by weight | ||
| HA/CTS_TEOS_5 | α-TCP (5 wt.% TEOS): HA/CTS granules 3:2 by weight | ||
| HA/CTS_GPTMS_1 | α-TCP (1 wt.% GPTMS): HA/CTS granules 3:2 by weight | ||
| HA/CTS_GPTMS_2 | α-TCP (2 wt.% GPTMS): HA/CTS granules 3:2 by weight | ||
| HA/CTS_GPTMS_5 | α-TCP (5 wt.% GPTMS): HA/CTS granules 3:2 by weight |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pańtak, P.; Czechowska, J.P.; Vivcharenko, V.; Dorner-Reisel, A.; Zima, A. The Synergistic Effect of Polysaccharides and Silane Coupling Agents on the Properties of Calcium Phosphate-Based Bone Substitutes. Int. J. Mol. Sci. 2025, 26, 8910. https://doi.org/10.3390/ijms26188910
Pańtak P, Czechowska JP, Vivcharenko V, Dorner-Reisel A, Zima A. The Synergistic Effect of Polysaccharides and Silane Coupling Agents on the Properties of Calcium Phosphate-Based Bone Substitutes. International Journal of Molecular Sciences. 2025; 26(18):8910. https://doi.org/10.3390/ijms26188910
Chicago/Turabian StylePańtak, Piotr, Joanna P. Czechowska, Vladyslav Vivcharenko, Annett Dorner-Reisel, and Aneta Zima. 2025. "The Synergistic Effect of Polysaccharides and Silane Coupling Agents on the Properties of Calcium Phosphate-Based Bone Substitutes" International Journal of Molecular Sciences 26, no. 18: 8910. https://doi.org/10.3390/ijms26188910
APA StylePańtak, P., Czechowska, J. P., Vivcharenko, V., Dorner-Reisel, A., & Zima, A. (2025). The Synergistic Effect of Polysaccharides and Silane Coupling Agents on the Properties of Calcium Phosphate-Based Bone Substitutes. International Journal of Molecular Sciences, 26(18), 8910. https://doi.org/10.3390/ijms26188910









