Abstract
The increasing reliance on light-based antimicrobial technologies, such as antimicrobial blue light (aBL) and antimicrobial photodynamic inactivation (aPDI), underscores the urgent need to comprehend bacterial survival strategies beyond conventional resistance. Two key phenotypes—tolerance and resilience—have emerged as critical but often conflated mechanisms by which bacteria withstand oxidative and photodynamic stress. While tolerance refers to delayed bacterial killing without changes in MIC, resilience encompasses the active restoration of cellular function after transient stress exposure. Both phenomena may impair treatment outcomes and contribute to long-term persistence, even in the absence of genetic resistance. This review dissects the molecular mechanisms underlying tolerance and resilience, with a focus on their relevance to bacterial responses to reactive oxygen species generated by light-based or chemical stressors. The regulatory and effector overlap between these phenotypes is examined, including antioxidant defense systems, DNA repair pathways, and metabolic rewiring. Furthermore, the role of phenotypic heterogeneity and cross-stress protection in blurring the boundary between survival and recovery is discussed, highlighting challenges in experimental interpretation. Finally, the implications of these adaptive strategies are evaluated in the context of antimicrobial efficacy and safety, with an emphasis on kinetic assays and multidimensional profiling as tools to capture complex treatment outcomes. Clarifying the distinction between tolerance and resilience may help guide the development of robust and evolutionarily stable antimicrobial phototherapies.
1. Introduction
The rising failure of antibiotic therapies has intensified interest in non-conventional antimicrobials. Among them, light-based strategies such as antimicrobial photodynamic inactivation (aPDI) and antibacterial blue light (aBL) are particularly attractive because they generate reactive oxygen species (ROS) that inflict multi-target oxidative damage [1,2,3,4,5,6,7,8,9,10,11,12,13]. By acting through photochemical rather than specific molecular targets, these modalities may circumvent classical genetic resistance [14].
Yet bacterial survival under oxidative and photodynamic stress is more nuanced than simple killing. Sublethal or intermittent exposure to aBL or aPDI can trigger phenotypic adaptations that, although not involving resistance in the classical sense, still promote treatment failure and long-term survival [15,16,17,18]. Two such non-genetic strategies are tolerance and resilience.
Tolerance refers to the ability of bacteria to survive lethal conditions without an increase in minimal inhibitory concentration (MIC)—typically through slowed metabolism, biofilm protection, or persister formation [19,20,21,22]. Resilience, in contrast, reflects the capacity to regain physiological function and resume growth after stress removal, emphasizing recovery rather than endurance [23,24]. Although often conflated, these phenotypes embody distinct survival logics: tolerance delays killing, while resilience accelerates regrowth.
In addition, persistence represents yet another distinct phenomenon: a reversible state of dormancy adopted by a small subpopulation of cells that remain non-growing while the stressor is present. Unlike tolerant populations, which survive through slowed metabolic activity, or resilient populations, which recover rapidly after stress removal, persisters simply “wait out” the treatment in a quiescent state [20,21]. Once the antimicrobial challenge is lifted, they can resume growth without having acquired resistance mutations. This distinction is critical, as persistence explains transient treatment failures, while resilience explains how populations rebound after damage [20,23,24].
Together with resistance, persistence, and cross-stress adaptation, these traits form a spectrum of bacterial survival strategies (Figure 1). However, the specific distinction between tolerance and resilience has been largely overlooked—posing both conceptual and experimental challenges that this review aims to address.

Figure 1.
Conceptual distinctions between bacterial survival phenotypes under stress. Resistance refers to the heritable ability to grow in the presence of an antimicrobial agent, whereas tolerance describes a slowed killing rate without changes in MIC. Persistence is a subpopulation-level phenomenon characterized by dormancy in the presence of an agent. Resilience denotes the capacity of bacteria to recover and resume growth after transient stress exposure. Cross-stress adaptation represents cross-protection triggered by exposure to a different stressor.
Understanding the molecular underpinnings and phenotypic manifestations of both processes is essential for designing effective antimicrobial strategies [25]. In the context of light-based therapies, it remains unresolved whether photodynamic treatments predominantly elicit tolerance, resilience, or both. The overlap between these phenotypes complicates experimental discrimination [23,24]. Moreover, cross-stress protection—where exposure to one stressor enhances survival under another—further obscures interpretation, particularly in multifactorial environments such as infected tissues or food-processing surfaces [26,27,28,29,30,31].
Given the expanding interest in photodynamic approaches and the growing recognition of bacterial plasticity under oxidative pressure, this review aims to:
- i
- clearly define and differentiate tolerance and resilience in the context of ROS-generating treatments;
- ii.
- outline their molecular and regulatory mechanisms;
- iii.
- highlight areas of mechanistic overlap and cross-protection;
- iv.
- discuss the implications of these traits for the efficacy and design of next-generation photonic antimicrobial interventions.
2. Photodynamic and Oxidative Stress in Bacteria
Bacteria are constantly exposed to ROS, both from endogenous metabolic processes and external environmental sources. In natural and clinical settings, ROS may arise from the host immune system (e.g., neutrophilic oxidative bursts), environmental oxidants, disinfectants, or antibiotic therapies [25,32]. Additionally, light-based antimicrobial strategies such as aPDI and aBL act by inducing the formation of singlet oxygen (1O2) and other ROS through the excitation of endogenous or exogenous photosensitizers. In addition to ROS, several stressors and immune defenses generate reactive nitrogen species (RNS), such as nitric oxide (NO) and peroxynitrite (ONOO−), which further exacerbate damage to bacterial macromolecules and contribute to nitrosative stress [33]. For instance, activated macrophages and neutrophils produce NO via inducible nitric oxide synthase (iNOS), while acidified nitrite in phagosomes and nitrosylated compounds released at infection sites also serve as potent RNS sources [33,34,35] (Figure 2).
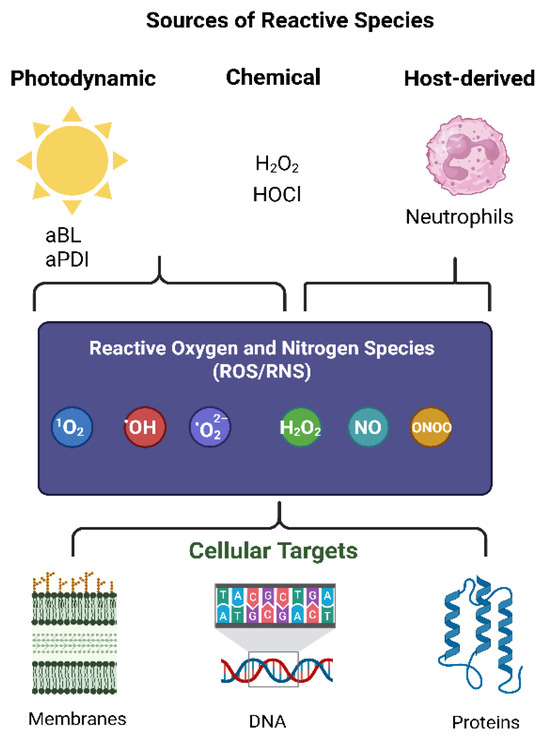
Figure 2.
Sources of reactive species and their cellular targets. Photodynamic inactivation (aBL, aPDI), chemical oxidants (e.g., H2O2, HOCl), and host-derived immune responses (e.g., neutrophils) generate reactive oxygen and nitrogen species (ROS/RNS) such as singlet oxygen (1O2), hydroxyl radical (•OH), superoxide (•O2−), hydrogen peroxide (H2O2), nitric oxide (NO), and peroxynitrite (ONOO−). These species attack cellular membranes, DNA, and proteins, leading to oxidative damage and stress responses [12,13,34,35].
The photodynamic stress elicited by aBL or aPDI typically involves the excitation of porphyrin-like chromophores, leading to electron transfer or energy transfer reactions that generate a spectrum of ROS, including superoxide anion (O2−), hydrogen peroxide (H2O2), hydroxyl radicals (•OH), and singlet oxygen (1O2). These species cause non-specific, multi-target damage to membranes, proteins, nucleic acids, and essential enzymes [12,13]. Oxidative stress may also be triggered by treatment with H2O2, hypochlorous acid (HOCl), peroxynitrite (ONOO−), or reactive halogen species (RHS) produced by host immune defenses [34,35]. Notably, many antibiotics (e.g., fluoroquinolones, aminoglycosides) indirectly cause oxidative stress by disturbing bacterial redox homeostasis and respiratory metabolism [36].
Despite shared effectors (ROS), photodynamic and oxidative stresses differ in their dynamics, localization, and intensity. For example:
Photodynamically generated ROS, especially 1O2, have very short half-lives and act locally near chromophores, often targeting membranes and periplasmic proteins [37,38].
In contrast, chemically induced ROS, such as H2O2, can penetrate into the cytoplasm and cause DNA damage or activate stress-responsive regulons, such as OxyR [39,40].
Understanding the sources, chemical nature, and subcellular localization of oxidative stress is critical when investigating bacterial survival strategies. These factors influence the type of cellular damage incurred and the subsequent adaptive responses bacteria deploy [41]. Such responses may tip the balance between tolerance, resilience, and irreversible cell death [19,20,21,22,23].
3. Mechanisms of Tolerance
Bacterial tolerance refers to the transient ability to survive lethal stress without a corresponding increase in MIC. Unlike resistance, which is heritable and affects growth in the presence of antimicrobials, tolerance is a phenotypic adaptation that manifests as a delay in bacterial killing. This kinetic delay is best captured by time-dependent metrics such as the minimum duration for killing (MDK), which quantifies the time required to reduce a bacterial population by a defined fraction (e.g., MDK99). Tolerant populations often display elevated MDK values while maintaining unchanged MICs, reflecting survival without resistance [20,42,43,44,45].
A hallmark of tolerance is the reduction in metabolic activity, which diminishes the efficacy of treatments that rely on active cellular processes for killing. Within tolerant populations, a subpopulation of slow-growing or dormant cells, known as persisters, often underlies the phenomenon of biphasic killing curves, where a fraction of cells survives treatment much longer than expected under bactericidal conditions. Persisters are reversible phenotypic variants that can resume growth after stress removal and are commonly associated with antibiotic therapy failure and infection recurrence, particularly in biofilm-associated environments [46,47,48,49,50,51].
Mechanisms contributing to tolerance include:
Energy metabolism modulation: Under stress, bacteria may downregulate ATP-consuming biosynthetic pathways while redirecting resources toward protective functions such as efflux activity. Efflux systems transiently lower intracellular concentrations of antibiotics or photosensitizers, thereby reducing treatment efficacy. Because efflux is energetically demanding, these shifts represent a metabolic strategy that promotes transient tolerance [52,53,54,55]. Consistently, pharmacological inhibition of efflux pumps sensitizes Staphylococcus aureus biofilms to photodynamic inactivation, underscoring their role in short-term protection [56].
Induction of antioxidant enzymes: Bacteria deploy enzymatic scavengers such as catalases (KatG), superoxide dismutases (SodA), and peroxidases (AhpC) to detoxify ROS and mitigate lethal damage. These protective systems delay killing and protect biomolecules during stress exposure [40,41,57,58]. In contrast, light-based therapies exploit photogenerated ROS—including singlet oxygen and other highly reactive species—against which enzymatic defense is often insufficient, especially in high-flux conditions [37].
Biofilm formation: The extracellular polymeric matrix of biofilms limits the diffusion of antimicrobials and light, creating nutrient-limited zones with low metabolic rates. These conditions favor the survival of tolerant and persister cells [49,59,60,61,62].
Persister cells and stress-regulated dormancy: A subpopulation of tolerant cells, termed persisters, survives lethal treatment by entering a dormant or metabolically inactive state. This phenotype is regulated by global stress-response networks, including the general stress sigma factor RpoS, the alarmone (p)ppGpp, and multiple toxin–antitoxin (TA) modules, which are activated under oxidative or antibiotic pressure. These systems reduce metabolic activity, downregulate ROS-generating pathways, and promote cellular quiescence, thereby limiting the efficacy of ROS-based killing mechanisms. Persisters are not genetically resistant but can endure treatment by effectively “shutting down” susceptible processes until stress subsides. Their formation is enhanced in structured communities such as biofilms but can also arise in planktonic cultures under nutrient limitation or oxidative stress [20,21,42,45,46,47,48,49,50,51,52,57,58,59,60,61,62,63,64,65,66,67,68,69,70].
DNA repair systems (SOS response): Activation of RecA–LexA-dependent repair pathways stabilizes the genome under genotoxic stress. By halting replication and initiating DNA repair (e.g., via UmuD, RecBCD), cells gain time to survive otherwise lethal damage. This system is particularly relevant under ROS-generating treatments such as aBL or aPDI [16,71,72,73,74,75,76,77].
Chaperones and proteases: Under oxidative or proteotoxic stress, bacteria upregulate chaperones like GroEL, DnaK, and disaggregases such as ClpB. These proteins prevent misfolding and aggregation, maintaining proteostasis during exposure. ATP-dependent proteases like ClpXP degrade irreversibly damaged proteins, limiting cellular injury. In this context, chaperone and protease systems primarily function to stabilize protein structures and buffer damage, allowing cells to persist through stress without active recovery [78,79,80,81,82,83].
Envelope stabilization: Tolerant cells may reinforce or modify their outer membrane to limit stressor penetration. Envelope stress response (ESR) systems detect and repair ROS-induced membrane damage, preserving bacterial homeostasis [84,85]. Outer membrane (OM) proteins such as LptD, BamA, and the Mla pathway maintain OM integrity and asymmetry under oxidative challenge, indirectly reducing aBL-induced damage [86,87,88,89].
Metabolic rerouting: Under oxidative or photodynamic stress, bacteria often downregulate ATP-consuming biosynthetic pathways while redirecting carbon flux toward the pentose phosphate pathway (PPP), enhancing NADPH generation to fuel antioxidant defenses. In parallel, glycolysis and the TCA cycle may be suppressed to lower NADH levels and thereby reduce ROS formation from respiratory activity. Such redox reprogramming supports survival during exposure by limiting endogenous ROS and providing reducing power for detoxification and repair. Recent work also shows that pathogens can reconfigure glycolytic flux to maintain proton motive force and redox homeostasis under immune-derived oxidative stress [53,54,90,91].
Mechanisms underlying bacterial tolerance to antimicrobial stress are illustrated in Figure 3.
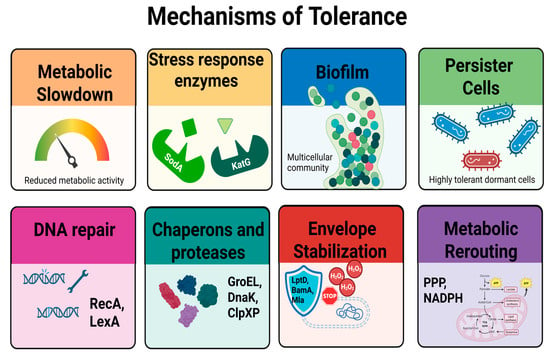
Figure 3.
Mechanisms underlying bacterial tolerance to antimicrobial stress. Tolerance is a transient, non-heritable phenotype characterized by delayed killing in the presence of lethal agents. Key mechanisms include metabolic slowdown, which limits the activity of treatments targeting active processes; induction of antioxidant enzymes such as catalase (KatG) and superoxide dismutase (SodA); formation of protective biofilms; and the presence of persister cells, which are metabolically quiescent and highly tolerant. Additional protective strategies involve activation of DNA repair systems (RecA/LexA), chaperones and proteases (GroEL, DnaK, ClpXP), stabilization of the cell envelope (LptD, BamA, Mla), and metabolic rerouting toward NADPH-producing pathways such as the pentose phosphate pathway (PPP). These adaptations collectively contribute to prolonged MDK99 values without changes in MIC, reflecting survival without resistance.
Bacterial tolerance represents a multifactorial and reversible survival strategy that enables a subpopulation to endure antimicrobial treatment without exhibiting resistance [20,42,43]. Key contributors include metabolic inactivation and slowdown [46,48], dormancy and toxin–antitoxin systems [59,60,61,62,63], oxidative stress regulation [53,57], and the SOS response [71,72,73,74,75]. Tolerant cells often downregulate growth-related processes while activating protective mechanisms such as chaperones and proteases [78,79,80,81,82], DNA repair systems [71,72,73,74,75,76,77], antioxidant enzymes [40,53,54], stabilization of the cell envelope [84,85,86,87,88,89], and metabolic rerouting toward NADPH-generating pathways such as the pentose phosphate pathway (PPP) [54,90,91]. This phenotype can emerge in both planktonic and biofilm-associated communities [29,49,50] in response to various stressors including antibiotics and photodynamic treatment [6,16,17]. While overlapping with resilience and persistence, tolerance is defined by its transient, non-heritable nature and lack of MIC increase [20,42]. Further insights into the interplay between these adaptive responses remain essential for optimizing light-based and conventional antimicrobial strategies.
4. Mechanisms of Resilience
While tolerance allows bacteria to endure lethal stress by slowing growth or entering dormancy, resilience describes their ability to actively recover once the stressor is removed. This dynamic phenotype involves repair of cellular damage, metabolic reactivation, and phenotypic plasticity. Unlike passive survival, resilience entails reprogramming of core systems to restore homeostasis and resume replication, particularly under fluctuating or sublethal exposures, such as intermittent oxidative or photodynamic stress [22,92].
Key mechanisms involved in resilience include:
Genomic repair and replication restart: As discussed in Section 3, DNA integrity is essential for survival under oxidative or photodynamic stress. In resilience, repair systems such as RecA, RecBCD, and UmuDC [93] contribute not only to lesion clearance but also to replication restart once stress is lifted. This coordination, often linked to the SOS response, enables resumption of cell division and population regrowth. In S. aureus, recA knockout strains showed markedly impaired recovery after sublethal aPDI, underscoring its role in post-stress regrowth [77]. Similarly, in Bacillus subtilis, sublethal photodynamic exposure was reported to upregulate nucleotide excision and transcription-coupled repair genes (uvrA, mfd), consistent with a role in damage clearance and replication restart [94].
Proteostasis-driven recovery: Chaperone systems such as GroEL, DnaK, and the disaggregase ClpB, together with ATP-dependent proteases (e.g., ClpXP), are induced by oxidative and photodynamic stress. While tolerance relies on their buffering role to stabilize proteins, resilience specifically engages their recovery function: refolding damaged proteins and degrading irreversibly oxidized peptides. This active proteome repair restores cellular functionality and supports rapid post-stress regrowth [81,95].
Membrane repair and PMF restoration: Resilient cells mobilize membrane-associated systems including LptD, BamA, OmpF, and the Mla pathway to restore outer membrane integrity and lipid asymmetry after oxidative damage [96]. RpoS-dependent remodeling contributes to envelope reinforcement during recovery [97]. Crucially, restoration of the proton motive force (PMF)—particularly its ΔpH component—supplies the energy required for periplasmic protein folding, solute transport, and reactivation of metabolism [98]. Envelope stress response systems such as the Cpx two-component system and the σ^E regulon orchestrate these repair programs, highlighting resilience as an active recovery strategy distinct from passive survival [99].
Redox rebalancing and metabolic reboot: Unlike tolerance, which dampens metabolic activity to reduce ROS generation, resilience requires rapid reactivation of metabolic fluxes. During post-stress recovery, Escherichia coli reroutes carbon through the oxidative branch of the pentose phosphate pathway (PPP), increasing NADPH production to support antioxidant defenses and anabolic repair [98,100]. This shift is accompanied by suppression of NADH-generating pathways to limit further oxidative damage. In contrast to tolerance strategies based on metabolic dormancy and lag time extension [101], resilience reflects a dynamic return to redox homeostasis and biosynthetic activity.
Regulatory plasticity during recovery: Global regulators such as RpoS and SoxRS in Gram-negative bacteria, σ^B in Gram-positive species, and OxyR in both lineages are essential in managing the transition from damage control to recovery. While these regulators initiate stress survival programs and growth arrest during the stress exposure (see Section 3), they also promote the reactivation of repair, metabolism, and cell division pathways after stress resolution. This dual-phase functionality supports the broader concept of stress-induced resilience [102,103,104].
Bacterial resilience represents an active and reversible recovery program, distinct from both tolerance and persistence. Instead of passively withstanding stress, resilient populations actively engage in redox rebalancing and metabolic rebooting, macromolecular repair, and regulatory reprogramming to restore physiological functions once the stressor is removed (Figure 4). These coordinated processes enable rapid resumption of growth after sublethal damage, whether triggered by antibiotics, oxidative agents, or photodynamic inactivation. Importantly, resilience does not involve stable genetic alterations or increased MIC values but rather reflects phenotypic plasticity and repair capacity [90,91,97,100,101,102]. Understanding the interplay between tolerance, persistence, and resilience is crucial for the optimization of antimicrobial strategies, particularly as light-based treatments may uniquely modulate recovery trajectories compared with conventional antibiotics.
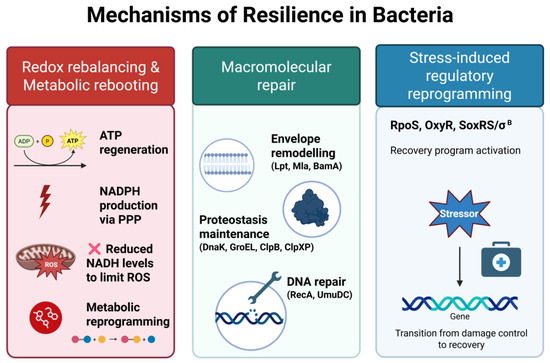
Figure 4.
Mechanisms of bacterial resilience. Resilience reflects the ability of bacteria to recover from transient, non-lethal stress. This process involves (1) redox rebalancing and metabolic re-booting, including ATP regeneration, enhanced NADPH production via the pentose phosphate pathway (PPP), reduced NADH levels to limit ROSs generation, and global metabolic reprogramming; (2) macromolecular repair, encompassing envelope remodeling (e.g., Lpt, Mla, BamA), proteostasis maintenance (e.g., DnaK, GroEL, ClpB, ClpXP), and DNA repair (e.g., RecA, UmuDC); (3) stress-induced regulatory reprogramming, coordinated by global regulators such as RpoS, OxyR, SoxRS, and σ^B, which enable a transcriptional shift from damage control to recovery. Together, these mechanisms promote the restoration of physiological functions once stress is relieved.
5. Cross-Talk and Mechanistic Overlap
Although bacterial tolerance and resilience are defined as distinct survival strategies—one enabling persistence during stress, the other promoting recovery post-stress—they often rely on overlapping molecular mechanisms, including global regulators (e.g., RpoS, OxyR), chaperone systems (e.g., DnaK, GroEL, ClpB), and DNA repair pathways (e.g., RecA, UmuDC) [77,81,95,97,102]. These phenotypes may coexist or even transition into one another depending on the timing, intensity, and nature of the stressor [42,77,103]. In clonal populations exposed to sublethal photodynamic stress, such overlap may manifest as phenotypic heterogeneity, where dormant-like cells exhibiting delayed killing (tolerance) coexist alongside metabolically active cells capable of rapid recovery (resilience). This supports the view that tolerance and resilience are not discrete, mutually exclusive categories, but rather points along a mechanistic continuum shaped by environmental context and regulatory architecture.
Mechanistic overlap between tolerance and resilience therefore suggests emergent, context-dependent behaviors within bacterial populations. Cross-stress protection and phenotypic heterogeneity have been documented as emergent phenomena arising from shared regulatory and repair mechanisms [104]. Additionally, antibiotic persistence has been shown to emerge as a spatial and metabolic property in structured microbial communities [105]. This conceptual continuum and its mechanistic overlap are illustrated in Figure 5.
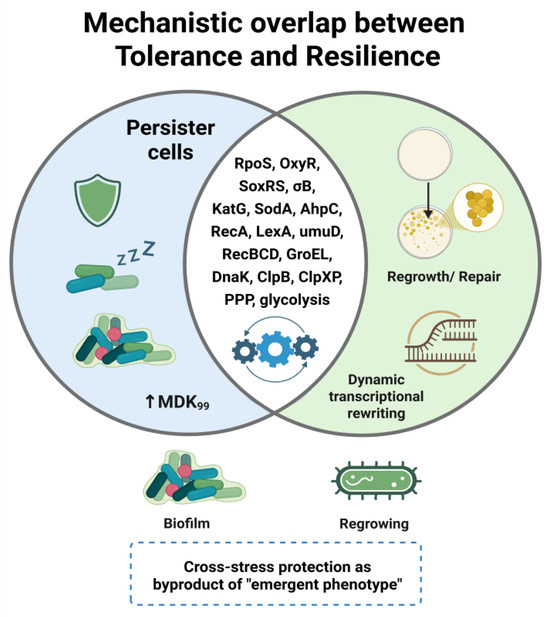
Figure 5.
Mechanistic overlap between bacterial tolerance and resilience. Tolerance is characterized by persister formation, metabolic slowdown, and prolonged MDK99, whereas resilience reflects active recovery programs, including redox rebalancing, macromolecular repair, and transcriptional reprogramming. Shared regulators and stress-response pathways (e.g., RpoS, OxyR, SoxRS, σ^B; antioxidant enzymes; DNA repair; chaperones/proteases; PPP/glycolysis) highlight a mechanistic continuum rather than strict dichotomy. Cross-stress protection may emerge as a byproduct of this continuum, underscoring resilience as a clinically and environmentally relevant hidden threat.
As summarized in Table 1, shared mechanisms and their differential roles in tolerance and resilience are outlined below.

Table 1.
Shared mechanisms between bacterial tolerance and resilience.
6. Reinterpreting Phototreatment Outcomes: Is Resilience the Missing Piece?
Resilience appears particularly relevant in light-based antimicrobial approaches such as aBL and aPDI, where multiple cellular targets are simultaneously affected. Under these conditions, bacteria may not evolve resistance or tolerance in a classical sense but instead transiently adjust their physiology to survive and regrow once stress ends.
While most studies on aBL and aPDI focus on resistance or tolerance, some findings suggest alternative interpretations. For example, Leanse et al. described cycle-dependent drops in aBL efficacy against Acinetobacter baumannii during sublethal exposure (notably cycles 9, 16, and 17), which later reverted spontaneously [108]. Though interpreted as a lack of stable tolerance, these fluctuations may reflect resilience-linked recovery. Similarly, Zhang et al. observed a transient decline in Candida albicans susceptibility to aBL around cycles 4–5, followed by partial recovery and stabilization. While no heritable change was detected, this trend could reflect a short-lived physiological adjustment rather than true tolerance [109]. Amin et al. reported that Pseudomonas aeruginosa subjected to ten repeated aBL cycles showed overall stable killing, but with a short transient dip in efficacy around cycles 3−4 that was later restored, again consistent with resilience-like recovery [110]. By contrast, the Tomb study showed that Staphylococcus aureus exposed to repeated aBL cycles maintained stable susceptibility throughout, with no evidence of transient fluctuations or resilience-like behavior [111].
In contrast, other phototreatment studies revealed the emergence of stable tolerance. Our previous work demonstrated that E. coli exposed to repeated aBL developed a persistent shift in killing kinetics without enhanced regrowth, consistent with tolerance rather than resilience [17]. Our group also showed that S. aureus developed stable tolerance after ~20 cycles of RB-mediated aPDI [16]. Similarly, Pieranski et al. demonstrated that Streptococcus agalactiae subjected to repeated RB-mediated aPDI cycles acquired a stable tolerant phenotype supported by oxidative stress gene upregulation and altered physiology [112]. Snell et al. reported a comparable outcome for MB-mediated aPDI, including cross-tolerance with TBO [18].
Although definitive proof remains elusive, as shown in Table 2, these cases highlight the importance of distinguishing biological variability from transient, non-heritable recovery. Standard tolerance assays (e.g., MIC shifts, survival curves) may overlook resilience, underscoring the need for refined experimental frameworks to disentangle survival during stress from recovery post-stress.

Table 2.
Re-evaluation of selected aBL studies in the context of bacterial resilience.
7. Resilience, Tolerance, and Resistance in the Context of Oxidative Phototreatments
The increasing use of oxidative, light-based antimicrobial therapies demands a refined view of bacterial survival dynamics. While resistance is typically tracked via stable increases in MIC, tolerance and persistence often escape standard susceptibility readouts. Tolerance denotes delayed killing (↑MDK) without MIC change; persistence reflects survival of small, dormant subpopulations; resilience captures rapid functional recovery once stress ceases [20,42,43,90,97,100,101,102,103]. These phenotypes may coexist within a single population, complicating eradication strategies [44,75,77].
Moreover, phototreatment-induced oxidative stress can engage cross-stress responses that modulate survival under other conditions (e.g., heat, desiccation, disinfectants), with species- and context-dependent outcomes [26,27,28,29,30]. Notably, recent work in E. coli suggests that aBL does not promote cross-stress resistance under the tested conditions [31].
To mitigate these challenges, phototreatment protocols should minimize sublethal exposures and integrate kinetic readouts. Useful approaches include combining light with adjuvants (e.g., redox disruptors or antibiotics), quantifying MDK and growth-delay kinetics, and explicitly monitoring post-exposure recovery [20,43,110].
Distinguishing resistance, tolerance, and resilience is not merely conceptual: it directly informs the design of robust and evolutionarily stable regimens, particularly for light-based strategies whose primary mode of action is oxidative damage [20,42]. Determining whether survivors exhibit transient reactivation (resilience), stable shifts in killing dynamics without MIC change (tolerance), or heritable increases in MIC (resistance) is essential for durable control in clinical and industrial settings [20,42,90,97,100,101,102,103]. Notably, antibiotic studies have demonstrated that tolerance can act as an evolutionary stepping-stone toward resistance development [23], underscoring the importance of distinguishing stress-specific trajectories when evaluating the long-term efficacy of antimicrobial interventions.
As a working model, survival strategies can be organized by their characteristic effects on MIC, MDK, and recovery, as illustrated in Figure 6:
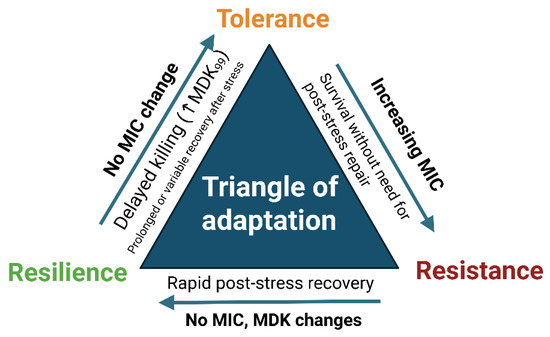
Figure 6.
Triangle of adaptation. Conceptual framework distinguishing three bacterial survival strategies under oxidative or photonic stress: resistance (increased MIC with no requirement for post-stress repair), tolerance (delayed killing; increased MDK99 with unchanged MIC), and resilience (rapid functional recovery after treatment without lasting MIC/MDK changes). Arrows indicate the typical experimental readouts associated with each phenotype [20,42,43].
Resistance—stable/elevated MIC with no recovery phase required (growth continues despite the presence of the stressor;
Tolerance—prolonged MDK without MIC increase;
Resilience—rapid post-stress regrowth without persistent MIC or MDK shifts [20,42,43,103].
Complementary experimental metrics to identify these traits are summarized in Table 3.

Table 3.
Diagnostic parameters proposed to distinguish resistance, tolerance, and resilience based on MIC shifts, MDK dynamics, and post-treatment regrowth behavior. These measurements complement standard susceptibility assays and reveal phenotypes often undetectable by MIC alone.
8. Materials and Methods
Literature Search Strategy
Relevant literature was identified through systematic searches in Google Scholar, PubMed, and Scopus using combinations of keywords including “bacterial tolerance”, “resilience”, “oxidative stress”, “antimicrobial blue light”, “antimicrobial photodynamic inactivation”, and “reactive oxygen species”. To complement this process, the AI-supported tool Elicit (elicit.org) was occasionally tested to explore query formulation and prioritization. However, it was not used as a primary search engine, and all references included in this review were verified manually by the author to ensure accuracy and relevance. Additional references were obtained via citation chaining and expert knowledge of the field.
9. Conclusions and Future Perspectives
Phenotypic strategies such as tolerance and resilience are increasingly recognized as critical contributors to bacterial survival under oxidative and photodynamic stress. While often conflated, this review underscores their distinct biological logic: tolerance prolongs survival by delaying bacterial killing, whereas resilience enables rapid recovery following exposure. Although both lead to treatment failure in practice, their mechanistic divergence has important conceptual and therapeutic implications.
Despite their differences, these phenotypes often share overlapping regulatory pathways, such as RpoS and oxidative stress regulons, making it challenging to discriminate between them experimentally. The decisive factor may lie in stress dynamics and population heterogeneity, which determine whether slowed killing (tolerance) or accelerated regrowth (resilience) predominates. This highlights the need for assays capable of disentangling survival during exposure from recovery after stress removal, particularly under sublethal or intermittent treatment conditions. The possibility of cross-stress priming, whereby exposure to ROS enhances survival under unrelated stressors (e.g., heat, desiccation, biocides) further complicates the picture and warrants careful consideration in both clinical and industrial contexts.
In the context of light-based antimicrobials, such as aBL and aPDI, assessing immediate killing efficacy is insufficient. Preventing survival and recovery of stress-adapted subpopulations is essential to avoid regrowth, recurrence, and the potential emergence of resistance. For tolerance, promising therapeutic directions include strategies targeting persister cells, biofilm matrix disruption, or metabolic reprogramming (e.g., PMF disruptors, ROS-boosting adjuvants). For resilience, potential interventions may aim at blocking repair and recovery pathways, such as RecA inhibitors, chaperone modulators, or envelope stress-response inhibitors. Rational combinatorial strategies—pairing light-based treatments with such adjuvants—should be prioritized and validated under physiologically relevant and fluctuating conditions (e.g., biofilms, food contact surfaces, or infected tissues).
While multi-omics and single-cell approaches remain indispensable to map the regulatory circuitry of stress responses, the highest priority should be the establishment of standardized kinetic benchmarks that clearly distinguish tolerance (prolonged MDK) from resilience (shortened recovery time). Without such harmonized assays, cross-study comparison will remain elusive, and therapeutic implications will remain blurred. Integrating resilience-aware metrics into susceptibility testing would represent a paradigm shift: beyond MIC and MDK, recovery kinetics should become a routine readout when evaluating novel antimicrobials, particularly light-based interventions.
In conclusion, tolerance and resilience are not redundant concepts but complementary pieces of the bacterial survival puzzle. Their mechanistic overlap highlights shared stress-defense nodes, while their divergence emphasizes temporal and population-level dynamics. Addressing both strategies will be essential for the development of next-generation antimicrobial regimens that are not only bactericidal but also resilience-proof.
Funding
This work was supported by National Science Centre grant no. 2022/47/D/NZ7/01795 (A.R.-Z.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was created.
Acknowledgments
The author sincerely thanks. Agata Woźniak-Pawlikowska for her creative input and assistance in preparing visual materials, and Mariusz Grinholc for his continuous support and valuable scientific guidance throughout the development of this manuscript. Figures were created in part using BioRender.com (https://biorender.com, accessed on 15 June 2025). The author also acknowledges the use of ChatGPT (OpenAI (https://openai.com/, accessed on 2 July 2025).) to support editing and structural refinement of the manuscript. All outputs were reviewed and edited by the author, who takes full responsibility for the final content of this publication.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| aBL | antimicrobial blue light |
| aPDI | antimicrobial photodynamic inactivation |
| CFU | colony-forming unit |
| MB | methylene blue |
| MDK | minimal duration of killing |
| MIC | minimal inhibitory concentration |
| NADH | nicotinamide adenine dinucleotide (reduced form) |
| NADPH | nicotinamide adenine dinucleotide phosphate (reduced form); |
| OD | optical density |
| PMF | proton motive force |
| PPP | pentose phosphate pathway |
| (p)ppGpp | guanosine tetraphosphate/pentaphosphate |
| qPCR | quantitative polymerase chain reaction |
| RB | rose bengal |
| RNS | reactive nitrogen species |
| ROS | reactive oxygen species |
| RNA-seq | RNA sequencing |
| TBO | toluidine blue O |
References
- Dai, T.; Huang, Y.Y.; Hamblin, M.R. Photodynamic therapy for localized infections—State of the art. Photodiagnosis Photodyn. Ther. 2009, 6, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Durantini, E.N. New insights into the antimicrobial blue light inactivation of Candida albicans. Virulence 2016, 7, 493–494. [Google Scholar] [CrossRef]
- Hamblin, M.R. Antimicrobial photodynamic inactivation: A bright new technique to kill resistant microbes. Curr. Opin. Microbiol. 2016, 33, 67–73. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, Y.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; Dai, T. Antimicrobial blue light inactivation of pathogenic microbes: State of the art. Drug Resist. Updates 2017, 33–35, 1–22. [Google Scholar] [CrossRef]
- Kashef, N.; Huang, Y.Y.; Hamblin, M.R. Advances in antimicrobial photodynamic inactivation at the nanoscale. Nanophotonics 2017, 6, 853–879. [Google Scholar] [CrossRef]
- Wozniak, A.; Grinholc, M. Combined antimicrobial activity of photodynamic inactivation and antimicrobials–state of the art. Front. Microbiol. 2018, 9, 930. [Google Scholar] [CrossRef]
- Hadi, J.; Wu, S.; Brightwell, G. Antimicrobial blue light versus pathogenic bacteria: Mechanism, application in the food industry, hurdle technologies and potential resistance. Foods 2020, 9, 1895. [Google Scholar] [CrossRef]
- Ghate, V.S.; Zhou, W.; Yuk, H.G. Perspectives and trends in the application of photodynamic inactivation for microbiological food safety. Compr. Rev. Food Sci. Food Saf. 2019, 18, 402–424. [Google Scholar] [CrossRef]
- Leanse, L.G.; Dos Anjos, C.; Mushtaq, S.; Dai, T. Antimicrobial blue light: A ‘Magic Bullet’for the 21st century and beyond? Adv. Drug Deliv. Rev. 2022, 180, 114057. [Google Scholar] [CrossRef]
- Sheng, L.; Li, X.; Wang, L. Photodynamic inactivation in food systems: A review of its application, mechanisms, and future perspective. Trends Food Sci. Technol. 2022, 124, 167–181. [Google Scholar] [CrossRef]
- Haridas, D.; Atreya, C.D. The microbicidal potential of visible blue light in clinical medicine and public health. Front. Med. 2022, 9, 905606. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos, C.; Leanse, L.G.; Ribeiro, M.S.; Sellera, F.P.; Dropa, M.; Arana-Chavez, V.E.; Lincopan, N.; Baptista, M.S.; Pogliani, F.C.; Dai, T.; et al. New insights into the bacterial targets of antimicrobial blue light. Microbiol. Spectr. 2023, 11, e02833-22. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, G.D.; Dos Anjos, C.; Ozdemir, M.A.; Leanse, L.G.; Dai, T. Lights out for Superbugs: Is antimicrobial blue light a potential approach for future infection Control? Adv. Drug Deliv. Rev. 2025, 224, 115654. [Google Scholar] [CrossRef]
- Maisch, T. Resistance in antimicrobial photodynamic inactivation of bacteria. Photochem. Photobiol. Sci. 2015, 14, 1518–1526. [Google Scholar] [CrossRef]
- Guffey, J.S.; Payne, W.; Jones, T.; Martin, K. Evidence of resistance development by Staphylococcus aureus to an in vitro, multiple stage application of 405 nm light from a supraluminous diode array. Photomed. Laser Surg. 2013, 31, 179–182. [Google Scholar] [CrossRef]
- Rapacka-Zdonczyk, A.; Wozniak, A.; Pieranski, M.; Woziwodzka, A.; Bielawski, K.P.; Grinholc, M. Development of Staphylococcus aureus tolerance to antimicrobial photodynamic inactivation and antimicrobial blue light upon sub-lethal treatment. Sci. Rep. 2019, 9, 9423. [Google Scholar] [CrossRef]
- Rapacka-Zdonczyk, A.; Wozniak, A.; Kruszewska, B.; Waleron, K.; Grinholc, M. Can gram-negative bacteria develop resistance to antimicrobial blue light treatment? Int. J. Mol. Sci. 2021, 22, 11579. [Google Scholar] [CrossRef]
- Snell, S.B.; Gill, A.L.; Haidaris, C.G.; Foster, T.H.; Baran, T.M.; Gill, S.R. Staphylococcus aureus tolerance and genomic response to photodynamic inactivation. Msphere 2021, 6, 10–1128. [Google Scholar] [CrossRef]
- Girvan, M.S.; Campbell, C.D.; Killham, K.; Prosser, J.I.; Glover, L.A. Bacterial diversity promotes community stability and functional resilience after perturbation. Environ. Microbiol. 2005, 7, 301–313. [Google Scholar] [CrossRef]
- Brauner, A.; Fridman, O.; Gefen, O.; Balaban, N.Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 2016, 14, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Trastoy, R.; Manso, T.; Fernández-García, L.; Blasco, L.; Ambroa, A.; Pérez Del Molino, M.L.; Bou, G.; García-Contreras, R.; Wood, T.K.; Tomás, M. Mechanisms of bacterial tolerance and persistence in the gastrointestinal and respiratory environments. Clin. Microbiol. Rev. 2018, 31, e00023-18. [Google Scholar] [CrossRef]
- Levin-Reisman, I.; Ronin, I.; Gefen, O.; Braniss, I.; Shoresh, N.; Balaban, N.Q. Antibiotic tolerance facilitates the evolution of resistance. Science 2017, 355, 826–830. [Google Scholar] [CrossRef]
- Carvalho, G.; Forestier, C.; Mathias, J.D. Antibiotic resilience: A necessary concept to complement antibiotic resistance? Proc. R. Soc. B 2019, 286, 20192408. [Google Scholar] [CrossRef] [PubMed]
- Guldimann, C.; Boor, K.J.; Wiedmann, M.; Guariglia-Oropeza, V. Resilience in the face of uncertainty: Sigma factor B fine-tunes gene expression to support homeostasis in Gram-positive bacteria. Appl. Environ. Microbiol. 2016, 82, 4456–4469. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.J.; Collins, J.J.; Walker, G.C. Unraveling the physiological complexities of antibiotic lethality. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 313–332. [Google Scholar] [CrossRef]
- Yang, H.; He, M.; Wu, C. Cross protection of lactic acid bacteria during environmental stresses: Stress responses and underlying mechanisms. Lwt 2021, 144, 111203. [Google Scholar] [CrossRef]
- Chapman, J.S. Disinfectant resistance mechanisms, cross-resistance, and co-resistance. Int. Biodeterior. Biodegrad. 2003, 51, 271–276. [Google Scholar] [CrossRef]
- Gnanadhas, D.P.; Marathe, S.A.; Chakravortty, D. Biocides–resistance, cross-resistance mechanisms and assessment. Expert Opin. Investig. Drugs 2013, 22, 191–206. [Google Scholar] [CrossRef]
- Salisbury, A.M.; Woo, K.; Sarkar, S.; Schultz, G.; Malone, M.; Mayer, D.O.; Percival, S.L. Tolerance of Biofilms to Antimicrobials and Significance to Antibiotic Resistance in Wounds. Surg. Technol. Int. 2018, 33, 59–66. [Google Scholar]
- de Souza Cândido, E.; de Barros, E.; Cardoso, M.H.; Franco, O.L. Bacterial cross-resistance to anti-infective compounds. Is it a real problem? Curr. Opin. Pharmacol. 2019, 48, 76–81. [Google Scholar] [CrossRef]
- Kruszewska-Naczk, B.; Pikulik-Arif, P.; Grinholc, M.; Rapacka-Zdonczyk, A. Antibacterial blue light is a promising tool for inactivating Escherichia coli in the food sector due to its low risk of cross-stress tolerance. Chem. Biol. Technol. Agric. 2024, 11, 126. [Google Scholar] [CrossRef]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454. [Google Scholar] [CrossRef]
- Fang, F.C. Antimicrobial reactive oxygen and nitrogen species: Concepts and controversies. Nat. Rev. Microbiol. 2004, 2, 820–832. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. The role of reactive species on innate immunity. Vaccines 2022, 10, 1735. [Google Scholar] [CrossRef]
- Martinvalet, D.; Walch, M. The role of reactive oxygen species in protective immunity. Front. Immunol. 2022, 12, 832946. [Google Scholar] [CrossRef]
- Dwyer, D.J.; Belenky, P.A.; Yang, J.H.; MacDonald, I.C.; Martell, J.D.; Takahashi, N.; Chan, C.T.Y.; Lobritz, M.A.; Braff, D.; Schwarz, E.G.; et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc. Natl. Acad. Sci. USA 2014, 111, E2100–E2109. [Google Scholar] [CrossRef]
- Przygoda, M.; Bartusik-Aebisher, D.; Dynarowicz, K.; Cieślar, G.; Kawczyk-Krupka, A.; Aebisher, D. Cellular mechanisms of singlet oxygen in photodynamic therapy. Int. J. Mol. Sci. 2023, 24, 16890. [Google Scholar] [CrossRef]
- Szewczyk, G.; Mokrzyński, K.; Sarna, T. Generation of singlet oxygen inside living cells: Correlation between phosphorescence decay lifetime, localization and outcome of photodynamic action. Photochem. Photobiol. Sci. 2024, 23, 1673–1685. [Google Scholar] [CrossRef]
- Asad, N.R.; Asad, L.M.B.O.; Almeida, C.E.B.D.; Felzenszwalb, I.; Cabral-Neto, J.B.; Leitão, A.C. Several pathways of hydrogen peroxide action that damage the E. coli genome. Genet. Mol. Biol. 2004, 27, 291–303. [Google Scholar] [CrossRef][Green Version]
- Imlay, J.A. Transcription factors that defend bacteria against reactive oxygen species. Annu. Rev. Microbiol. 2015, 69, 93–108. [Google Scholar] [CrossRef]
- Vatansever, F.; de Melo, W.C.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species–bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Helaine, S.; Lewis, K.; Ackermann, M.; Aldridge, B.; Andersson, D.I.; Brynildsen, M.P.; Bumann, D.; Camilli, A.; Collins, J.J.; et al. Definitions and guidelines for research on antibiotic persistence. Nat. Rev. Microbiol. 2019, 17, 441–448, Erratum in Nat. Rev. Microbiol. 2019, 17, 460.. [Google Scholar] [CrossRef]
- Brauner, A.; Shoresh, N.; Fridman, O.; Balaban, N.Q. An experimental framework for quantifying bacterial tolerance. Biophys. J. 2017, 112, 2664–2671. [Google Scholar] [CrossRef]
- Ahmad, Z.; Klinkenberg, L.G.; Pinn, M.L.; Fraig, M.M.; Peloquin, C.A.; Bishai, W.R.; Nuermberger, E.L.; Grosset, J.H.; Karakousis, P.C. Biphasic kill curve of isoniazid reveals the presence of drug-tolerant, not drug-resistant, Mycobacterium tuberculosis in the guinea pig. J. Infect. Dis. 2009, 200, 1136–1143. [Google Scholar] [CrossRef]
- Kim, J.S.; Wood, T.K. Tolerant, growing cells from nutrient shifts are not persister cells. Mbio 2017, 8, e00354-17. [Google Scholar] [CrossRef]
- Prax, M.; Bertram, R. Metabolic aspects of bacterial persisters. Front. Cell. Infect. Microbiol. 2014, 4, 148. [Google Scholar] [CrossRef]
- Zhou, Y.; Liao, H.; Pei, L.; Pu, Y. Combatting persister cells: The daunting task in post-antibiotics era. Cell Insight 2023, 2, 100104. [Google Scholar] [CrossRef]
- Cabral, D.J.; Wurster, J.I.; Belenky, P. Antibiotic persistence as a metabolic adaptation: Stress, metabolism, the host, and new directions. Pharmaceuticals 2018, 11, 14. [Google Scholar] [CrossRef]
- Yan, J.; Bassler, B.L. Surviving as a community: Antibiotic tolerance and persistence in bacterial biofilms. Cell Host Microbe 2019, 26, 15–21. [Google Scholar] [CrossRef]
- Niu, H.; Gu, J.; Zhang, Y. Bacterial persisters: Molecular mechanisms and therapeutic development. Signal Transduct. Target. Ther. 2024, 9, 174. [Google Scholar] [CrossRef]
- Rahman, K.T.; Amaratunga, R.; Butzin, X.Y.; Singh, A.; Hossain, T.; Butzin, N.C. Rethinking dormancy: Antibiotic persisters are metabolically active, non-growing cells. Int. J. Antimicrob. Agents 2025, 65, 107386. [Google Scholar] [CrossRef]
- Shan, Y.; Brown Gandt, A.; Rowe, S.E.; Deisinger, J.P.; Conlon, B.P.; Lewis, K. ATP-dependent persister formation in Escherichia coli. Mbio 2017, 8, e02267-16. [Google Scholar] [CrossRef]
- Lemire, J.; Alhasawi, A.; Appanna, V.P.; Tharmalingam, S.; Appanna, V.D. Metabolic defence against oxidative stress: The road less travelled so far. J. Appl. Microbiol. 2017, 123, 798–809. [Google Scholar] [CrossRef]
- Singh, R.; Mailloux, R.J.; Puiseux-Dao, S.; Appanna, V.D. Oxidative stress evokes a metabolic adaptation that favors increased NADPH synthesis and decreased NADH production in Pseudomonas fluorescens. J. Bacteriol. 2007, 189, 6665–6675. [Google Scholar] [CrossRef]
- Li, X.Z.; Plésiat, P.; Nikaido, H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol. Rev. 2015, 28, 337–418. [Google Scholar] [CrossRef]
- Grinholc, M.; Zawacka-Pankau, J.; Gwizdek-Wiśniewska, A.; Bielawski, K.P. Evaluation of the Role of the Pharmacological Inhibition of Staphylococcus aureus Multidrug Resistance Pumps and the Variable Levels of the Uptake of the Sensitizer in the Strain-Dependent Response of Staphylococcus aureus to PPArg2-Based Photodynamic Inactivation. Photochem. Photobiol. 2010, 86, 1118–1126. [Google Scholar]
- Zhang, R.; Hartline, C.; Zhang, F. The ability in managing reactive oxygen species affects Escherichia coli persistence to ampicillin after nutrient shifts. mSystems 2024, 9, e01295-24. [Google Scholar] [CrossRef]
- Zhao, X.; Drlica, K. Reactive oxygen species and the bacterial response to lethal stress. Curr. Opin. Microbiol. 2014, 21, 1–6. [Google Scholar] [CrossRef]
- Wang, X.; Wood, T.K. Toxin-antitoxin systems influence biofilm and persister cell formation and the general stress response. Appl. Environ. Microbiol. 2011, 77, 5577–5583. [Google Scholar] [CrossRef]
- Paul, P.; Sahu, B.R.; Suar, M. Plausible role of bacterial toxin–antitoxin system in persister cell formation and elimination. Mol. Oral Microbiol. 2019, 34, 97–107. [Google Scholar] [CrossRef]
- Fraikin, N.; Rousseau, C.J.; Goeders, N.; Van Melderen, L. Reassessing the role of the type II MqsRA toxin-antitoxin system in stress response and biofilm formation: mqsA is transcriptionally uncoupled from mqsR. Mbio 2019, 10, e02678-19. [Google Scholar] [CrossRef]
- Kędzierska, B.; Hayes, F. Emerging roles of toxin-antitoxin modules in bacterial pathogenesis. Molecules 2016, 21, 790. [Google Scholar] [CrossRef]
- Maisonneuve, E.; Gerdes, K. Molecular mechanisms underlying bacterial persisters. Cell 2014, 157, 539–548. [Google Scholar] [CrossRef]
- Zou, J.; Peng, B.; Qu, J.; Zheng, J. Are bacterial persisters dormant cells only? Front. Microbiol. 2022, 12, 708580. [Google Scholar] [CrossRef]
- Edelmann, D.; Berghoff, B.A. Type I toxin-dependent generation of superoxide affects the persister life cycle of Escherichia coli. Sci. Rep. 2019, 9, 14256. [Google Scholar] [CrossRef]
- Yuan, S.; Shen, Y.; Quan, Y.; Gao, S.; Zuo, J.; Jin, W.; Li, R.; Li, Y.; Wang, Y.; Wang, Y. Molecular mechanism and application of emerging technologies in study of bacterial persisters. BMC Microbiol. 2024, 24, 480. [Google Scholar] [CrossRef]
- Wu, N.; He, L.; Cui, P.; Wang, W.; Yuan, Y.; Liu, S.; Xu, T.; Zhang, S.; Wu, J.; Zhang, W.; et al. Ranking of persister genes in the same Escherichia coli genetic background demonstrates varying importance of individual persister genes in tolerance to different antibiotics. Front. Microbiol. 2015, 6, 1003. [Google Scholar] [CrossRef]
- Shi, X.; Zarkan, A. Bacterial survivors: Evaluating the mechanisms of antibiotic persistence. Microbiology 2022, 168, 001266. [Google Scholar] [CrossRef]
- Svenningsen, M.S.; Veress, A.; Harms, A.; Mitarai, N.; Semsey, S. Birth and resuscitation of (p) ppGpp induced antibiotic tolerant persister cells. Sci. Rep. 2019, 9, 6056. [Google Scholar] [CrossRef]
- Van den Bergh, B.; Fauvart, M.; Michiels, J. Formation, physiology, ecology, evolution and clinical importance of bacterial persisters. FEMS Microbiol. Rev. 2017, 41, 219–251. [Google Scholar] [CrossRef]
- Janion, C. Inducible SOS response system of DNA repair and mutagenesis in Escherichia coli. Int. J. Biol. Sci. 2008, 4, 338. [Google Scholar] [CrossRef]
- Maslowska, K.H.; Makiela-Dzbenska, K.; Fijalkowska, I.J. The SOS system: A complex and tightly regulated response to DNA damage. Environ. Mol. Mutagen. 2019, 60, 368–384. [Google Scholar] [CrossRef]
- Misra, H.S.; Rajpurohit, Y.S. DNA damage response and cell cycle regulation in bacteria: A twist around the paradigm. Front. Microbiol. 2024, 15, 1389074. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Skiebe, E.; Wilharm, G. Contributions of RecA and RecBCD DNA repair pathways to the oxidative stress response and sensitivity of Acinetobacter baumannii to antibiotics. Int. J. Antimicrob. Agents 2018, 52, 629–636. [Google Scholar] [CrossRef]
- Lima-Noronha, M.A.; Fonseca, D.L.; Oliveira, R.S.; Freitas, R.R.; Park, J.H.; Galhardo, R.S. Sending out an SOS-the bacterial DNA damage response. Genet. Mol. Biol. 2022, 45, e20220107. [Google Scholar] [CrossRef]
- Henry, C.; Loiseau, L.; Vergnes, A.; Vertommen, D.; Merida-Floriano, A.; Chitteni-Pattu, S.; Wood, E.A.; Casadesús, J.; Cox, M.M.; Barras, F.; et al. Redox controls RecA protein activity via reversible oxidation of its methionine residues. Elife 2021, 10, e63747. [Google Scholar] [CrossRef]
- Grinholc, M.; Rodziewicz, A.; Forys, K.; Rapacka-Zdonczyk, A.; Kawiak, A.; Domachowska, A.; Golunski, G.; Wolz, C.; Mesak, L.; Becker, K.; et al. Fine-tuning recA expression in Staphylococcus aureus for antimicrobial photoinactivation: Importance of photo-induced DNA damage in the photoinactivation mechanism. Appl. Microbiol. Biotechnol. 2015, 99, 9161–9176. [Google Scholar] [CrossRef]
- Susin, M.F.; Baldini, R.L.; Gueiros-Filho, F.; Gomes, S.L. GroES/GroEL and DnaK/DnaJ have distinct roles in stress responses and during cell cycle progression in Caulobacter crescentus. J. Bacteriol. 2006, 188, 8044–8053. [Google Scholar] [CrossRef]
- Cardoso, K.; Gandra, R.F.; Wisniewski, E.S.; Osaku, C.A.; Kadowaki, M.K.; Felipach-Neto, V.; Haus, L.F.A.; Simão, R.D.C.G. DnaK and GroEL are induced in response to antibiotic and heat shock in Acinetobacter baumannii. J. Med. Microbiol. 2010, 59, 1061–1068. [Google Scholar] [CrossRef]
- Figaj, D. The role of heat shock protein (Hsp) chaperones in environmental stress adaptation and virulence of plant pathogenic bacteria. Int. J. Mol. Sci. 2025, 26, 528. [Google Scholar] [CrossRef]
- Fourie, K.R.; Wilson, H.L. Understanding GroEL and DnaK stress response proteins as antigens for bacterial diseases. Vaccines 2020, 8, 773. [Google Scholar] [CrossRef]
- St. Denis, T.G.; Huang, L.; Dai, T.; Hamblin, M.R. Analysis of the bacterial heat shock response to photodynamic therapy-mediated oxidative stress. Photochem. Photobiol. 2011, 87, 707–713. [Google Scholar] [CrossRef]
- Alam, A.; Bröms, J.E.; Kumar, R.; Sjöstedt, A. The role of ClpB in bacterial stress responses and virulence. Front. Mol. Biosci. 2021, 8, 668910. [Google Scholar] [CrossRef] [PubMed]
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J.F. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396. [Google Scholar] [CrossRef]
- Chautrand, T.; Souak, D.; Chevalier, S.; Duclairoir-Poc, C. Gram-negative bacterial envelope homeostasis under oxidative and nitrosative stress. Microorganisms 2022, 10, 924. [Google Scholar] [CrossRef] [PubMed]
- Lundstedt, E.; Kahne, D.; Ruiz, N. Assembly and maintenance of lipids at the bacterial outer membrane. Chem. Rev. 2020, 121, 5098–5123. [Google Scholar] [CrossRef]
- Nikaido, H. Multidrug resistance in bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef]
- De Carvalho, C.C.; Caramujo, M.J. The various roles of fatty acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Liu, H.; Chen, X.; Liu, L. Engineering microbial membranes to increase stress tolerance of industrial strains. Metab. Eng. 2019, 53, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Liu, L.; Fitzsimmons, L.; Porwollik, S.; Kim, J.S.; Desai, P.; McClelland, M.; Vazquez-Torres, A. Glycolytic reprograming in Salmonella counters NOX2-mediated dissipation of ΔpH. Nat. Commun. 2020, 11, 1783. [Google Scholar] [CrossRef]
- Bhat, S.A.; Iqbal, I.K.; Kumar, A. Imaging the NADH: NAD+ homeostasis for understanding the metabolic response of Mycobacterium to physiologically relevant stresses. Front. Cell. Infect. Microbiol. 2016, 6, 145. [Google Scholar] [CrossRef]
- Meirelles, L.A.; Perry, E.K.; Bergkessel, M.; Newman, D.K. Bacterial defenses against a natural antibiotic promote collateral resilience to clinical antibiotics. PLoS Biol. 2021, 19, e3001093. [Google Scholar] [CrossRef]
- Reuven, N.B.; Tomer, G.; Livneh, Z. The mutagenesis proteins UmuD′ and UmuC prevent lethal frameshifts while increasing base substitution mutations. Mol. Cell 1998, 2, 191–199. [Google Scholar] [CrossRef]
- Pohland, S.; Vourvoutsiotou, J.; Brandtner, L.; Geißler, D.; Wiesmeth, S.; Scudlo, V.; Richter, P.; Burkovski, A.; Lebert, M. Chlorophyllin-Mediated Photodynamic Inactivation: Dosage and Time Dependency in the Inhibition of Bacillus subtilis. Microorganisms 2025, 13, 1189. [Google Scholar] [CrossRef] [PubMed]
- Pranjic, M.; Spät, P.; Curkovic, M.S.; Macek, B.; Gruic-Sovulj, I.; Mocibob, M. Resilience and proteome response of Escherichia coli to high levels of isoleucine mistranslation. Int. J. Biol. Macromol. 2024, 262, 130068. [Google Scholar] [CrossRef]
- Konovalova, A.; Kahne, D.E.; Silhavy, T.J. Outer membrane biogenesis. Annu. Rev. Microbiol. 2017, 71, 539–556. [Google Scholar] [CrossRef]
- Charoenwong, D.; Andrews, S.; Mackey, B. Role of rpoS in the development of cell envelope resilience and pressure resistance in stationary-phase Escherichia coli. Appl. Environ. Microbiol. 2011, 77, 5220–5229. [Google Scholar] [CrossRef]
- Christodoulou, D.; Link, H.; Fuhrer, T.; Kochanowski, K.; Gerosa, L.; Sauer, U. Reserve flux capacity in the pentose phosphate pathway enables Escherichia coli’s rapid response to oxidative stress. Cell Syst. 2018, 6, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Macritchie, D.M.; Raivio, T.L. Envelope stress responses. EcoSal Plus 2009, 3, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Meredith, H.R.; Andreani, V.; Ma, H.R.; Lopatkin, A.J.; Lee, A.J.; Anderson, D.J.; Batt, G.; You, L. Applying ecological resistance and resilience to dissect bacterial antibiotic responses. Sci. Adv. 2018, 4, eaau1873. [Google Scholar] [CrossRef]
- Fridman, O.; Goldberg, A.; Ronin, I.; Shoresh, N.; Balaban, N.Q. Optimization of lag time underlies antibiotic tolerance in evolved bacterial populations. Nature 2014, 513, 418–421. [Google Scholar] [CrossRef]
- Bouillet, S.; Bauer, T.S.; Gottesman, S. RpoS and the bacterial general stress response. Microbiol. Mol. Biol. Rev. 2024, 88, e00151-22. [Google Scholar] [CrossRef] [PubMed]
- Hecker, M.; Pané-Farré, J.; Uwe, V. SigB-dependent general stress response in Bacillus subtilis and related gram-positive bacteria. Annu. Rev. Microbiol. 2007, 61, 215–236. [Google Scholar] [CrossRef] [PubMed]
- Dragosits, M.; Mozhayskiy, V.; Quinones-Soto, S.; Park, J.; Tagkopoulos, I. Evolutionary potential, cross-stress behavior and the genetic basis of acquired stress resistance in Escherichia coli. Mol. Syst. Biol. 2013, 9, 643. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Othmer, H.G.; Harcombe, W.R. Emergent antibiotic persistence in a spatially structured synthetic microbial mutualism. ISME J. 2024, 18, wrae075. [Google Scholar] [CrossRef]
- Almatroudi, A. Biofilm resilience: Molecular mechanisms driving antibiotic resistance in clinical contexts. Biology 2025, 14, 165. [Google Scholar] [CrossRef]
- Kruszewska-Naczk, B.; Grinholc, M.; Rapacka-Zdonczyk, A. Mimicking the Effects of Antimicrobial Blue Light: Exploring Single Stressors and Their Impact on Microbial Growth. Antioxidants 2024, 13, 1583. [Google Scholar] [CrossRef]
- Leanse, L.G.; Harrington, O.D.; Fang, Y.; Ahmed, I.; Goh, X.S.; Dai, T. Evaluating the potential for resistance development to antimicrobial blue light (at 405 nm) in Gram-negative bacteria: In vitro and in vivo studies. Front. Microbiol. 2018, 9, 2403. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.; Chen, J.; Wang, Y.; Sherwood, M.E.; Murray, C.K.; Vrahas, M.S.; Hooper, D.C.; Hamblin, M.R.; Dai, T. Antimicrobial blue light inactivation of Candida albicans: In vitro and in vivo studies. Virulence 2016, 7, 536–545. [Google Scholar] [CrossRef]
- Amin, R.M.; Bhayana, B.; Hamblin, M.R.; Dai, T. Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: In vitro and in vivo studies. Lasers Surg. Med. 2016, 48, 562–568. [Google Scholar] [CrossRef]
- Tomb, R.M.; Maclean, M.; Coia, J.E.; MacGregor, S.J.; Anderson, J.G. Assessment of the potential for resistance to antimicrobial violet-blue light in Staphylococcus aureus. Antimicrob. Resist. Infect. Control. 2017, 6, 100. [Google Scholar] [CrossRef] [PubMed]
- Pieranski, M.; Sitkiewicz, I.; Grinholc, M. Increased photoinactivation stress tolerance of Streptococcus agalactiae upon consecutive sublethal phototreatments. Free Radic. Biol. Med. 2020, 160, 657–669. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).