Molecular Consequences of CCN6 Variants Encoding WISP3 in Progressive Pseudorheumatoid Dysplasia
Abstract
1. Introduction
2. Results
2.1. Stability Analyses of IGFBP N-Terminal Domain Variants of WISP3:
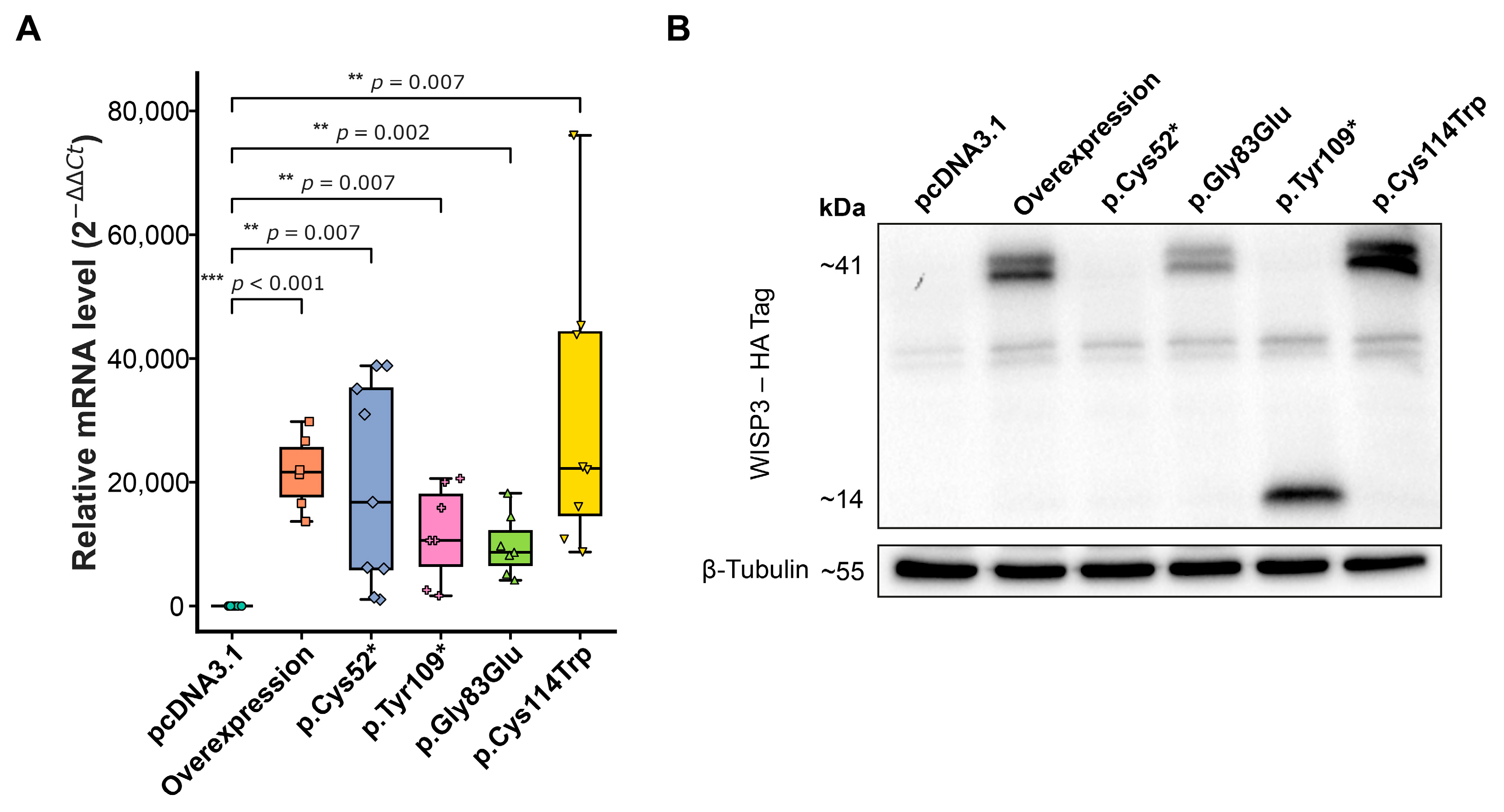
2.2. Verification of Plasmid Constructs
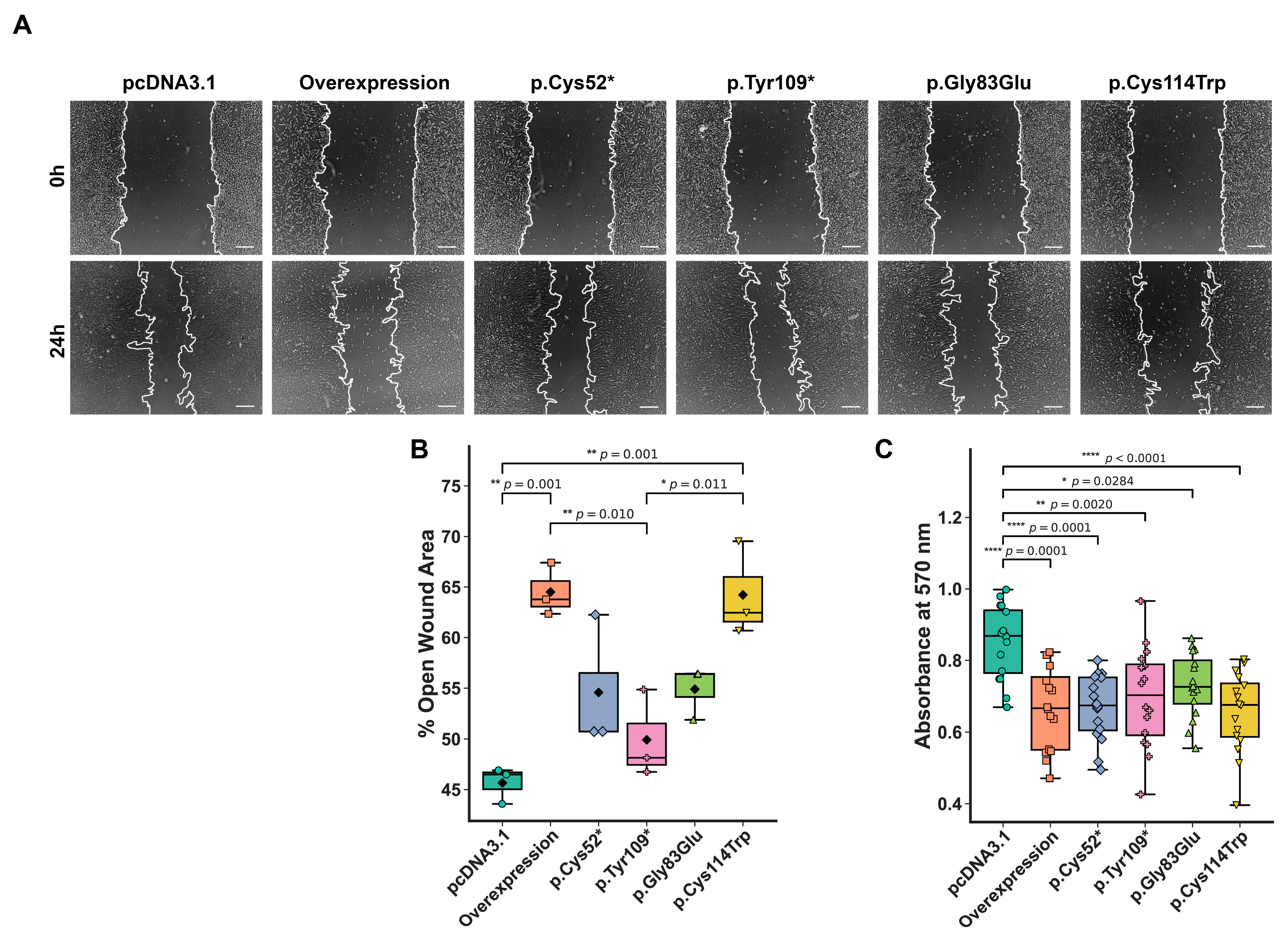
2.3. Migration and Viability of Chondrocytes Following WISP3 Variant Expression
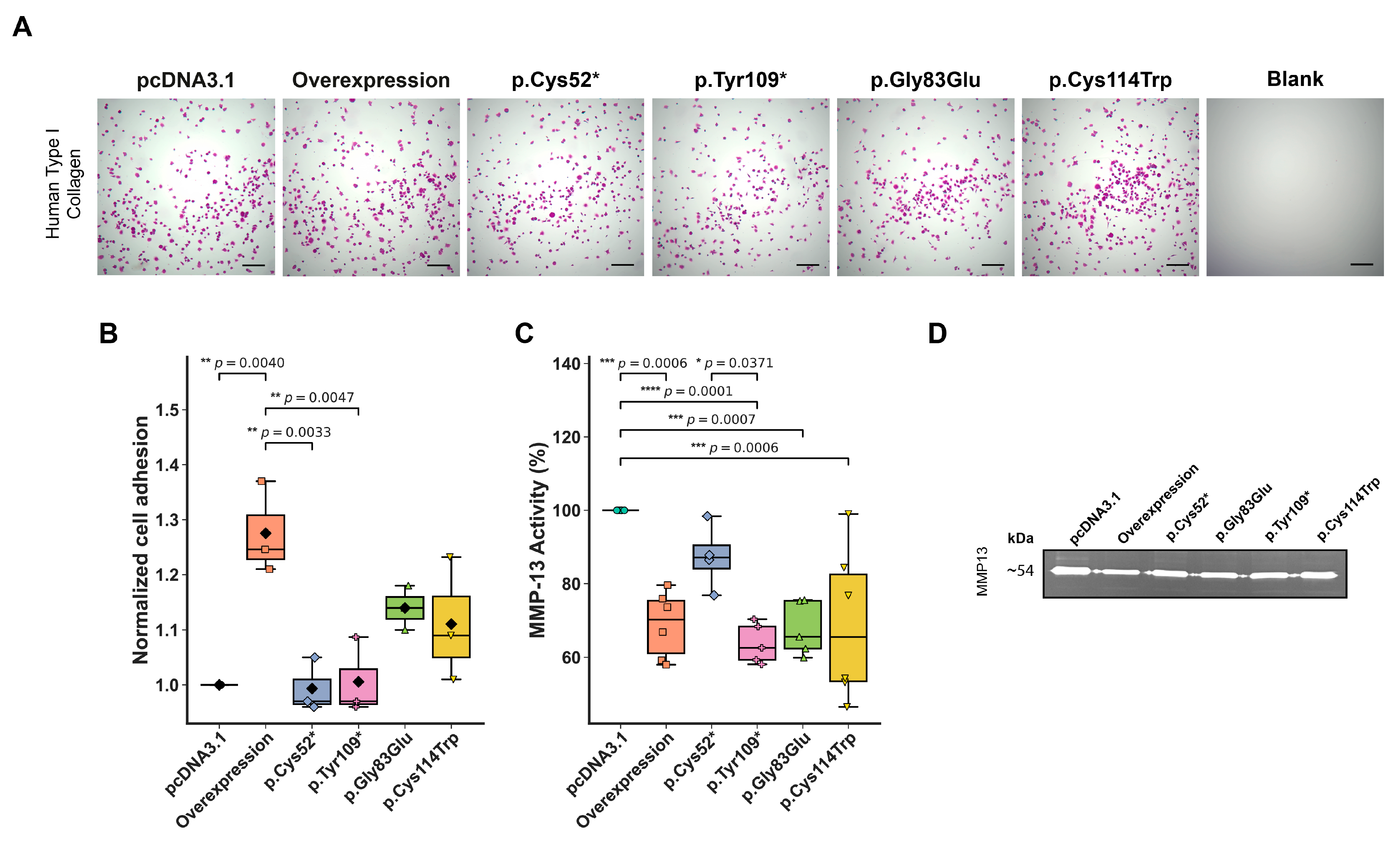
2.4. Adhesion and Matrix Remodeling Properties of Chondrocytes Expressing WISP3 Variants
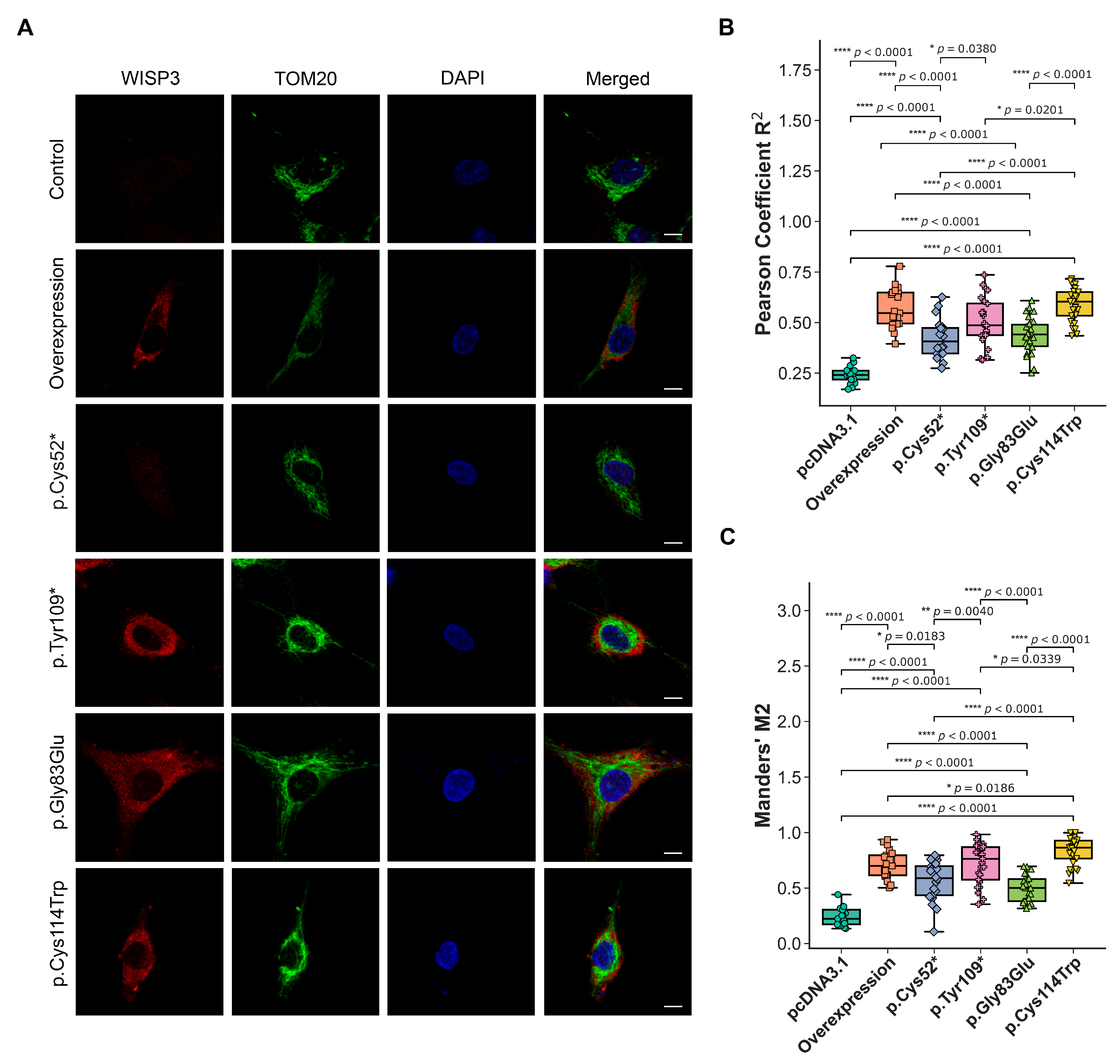
2.5. Mitochondrial Redox Imbalance, ER Stress Marker Expression, and Subcellular Localization of WISP3 Variants
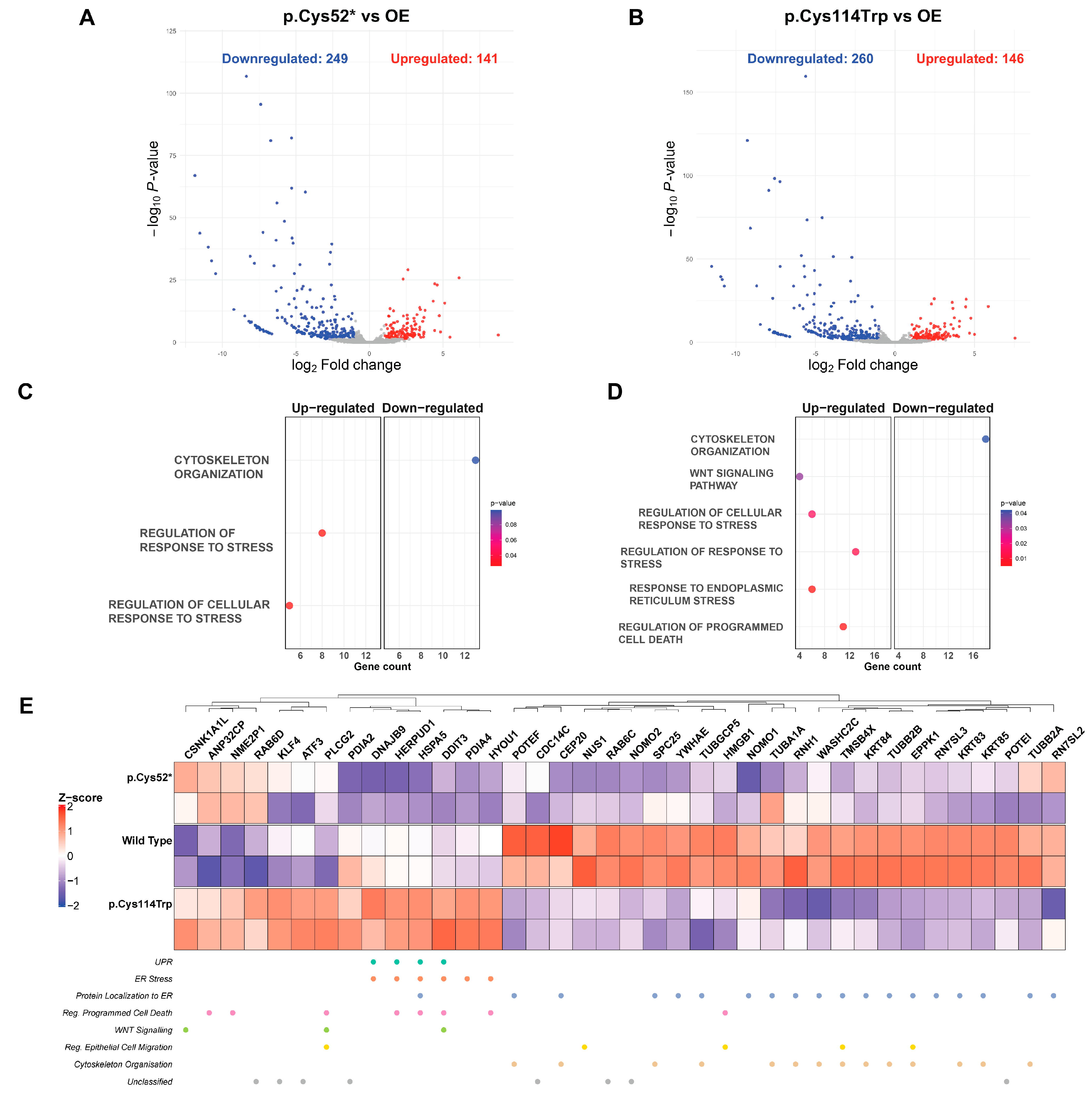
2.6. Whole Transcriptome Profiling Reveals Variant-Specific Enrichment of ER Stress, Apoptosis, and Cytoskeletal Pathways
2.7. Protein–Protein Interaction Profiling of Wild-Type and Disease-Associated WISP3
3. Discussion
3.1. Variant-Specific Destabilization of WISP3 in Chondrocytes via NMD or Structural Disruption
3.2. Truncating and Missense Variants Within the IGFBP Domain of WISP3 Impair Chondrocyte Function via Domain Loss or Disruption
3.3. WISP3 Variants Differentially Disrupt ER Stress Responses and Mitochondrial Redox Homeostasis in Chondrocytes
3.4. Variant-Specific Disruption of WISP3 Interactome Reveals Divergent Stress Adaptation and Cytoskeletal Dysregulation Mechanisms in PPD
4. Materials and Methods
4.1. Cell Culture Experiments
4.2. Protein Structure and Prediction of Protein Stability
4.3. Variant Construct Generation
4.4. MTT Assay
4.5. Adhesion Assay
4.6. Migration and Wound Healing Assay
4.7. RNA Isolation and Quantitative PCR
4.8. Gelatin Zymography
4.9. Flow Cytometry
4.10. Confocal Microscopy
4.11. Western Blot
4.12. RNA-Seq Quantification, Differential Gene Expression, and Enrichment Analysis
4.13. Co-Immunoprecipitation and LC–MS/MS Analysis
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PPD | Progressive pseudorheumatoid dysplasia |
| OMIM | Online Mendelian Inheritance in Man |
| CCN6 | Cellular Communication Network Factor 6 |
| WISP3 | Wnt1-inducible-signaling pathway protein 3 |
| JIA | Juvenile idiopathic arthritis |
| ESR | Erythrocyte Sedimentation Rate |
| CRP | C-Reactive Protein |
| ECM | Extracellular Matrix |
| IGFBP | Insulin-like Growth Factor Binding Protein |
| TSP1 | Thrombospondin Type 1 Repeat |
| CTCK | Cysteine Knot-Containing Domain |
| Wnt | Wingless/Integrated |
| TGF-β | Transforming Growth Factor Beta |
| CHOP | C/EBP Homologous Protein |
| sXBP1 | Spliced X-box Binding Protein 1 |
| uXBP1 | Unspliced X-box Binding Protein 1 |
| MMP | Matrix Metalloproteinase |
References
- Hurvitz, J.R.; Suwairi, W.M.; Van Hul, W.; El-Shanti, H.; Superti-Furga, A.; Roudier, J.; Holderbaum, D.; Pauli, R.M.; Herd, J.K.; Hul, E.V.; et al. Mutations in the CCN Gene Family Member WISP3 Cause Progressive Pseudorheumatoid Dysplasia. Nat. Genet. 1999, 23, 94–98. [Google Scholar] [CrossRef]
- Garcia Segarra, N.; Mittaz, L.; Campos-Xavier, A.B.; Bartels, C.F.; Tuysuz, B.; Alanay, Y.; Cimaz, R.; Cormier-Daire, V.; Di Rocco, M.; Duba, H.; et al. The Diagnostic Challenge of Progressive Pseudorheumatoid Dysplasia (PPRD): A Review of Clinical Features, Radiographic Features, and WISP3 Mutations in 63 Affected Individuals. Am. J. Med. Genet. C Semin. Med. Genet. 2012, 160C, 217–229. [Google Scholar] [CrossRef]
- Sen, M.; Cheng, Y.; Goldring, M.B.; Lotz, M.K.; Carson, D.A. WISP3-dependent Regulation of Type II Collagen and Aggrecan Production in Chondrocytes. Arthritis Rheum. 2004, 50, 488–497. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health Multidiscip. Approach 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, M.; Xing, X.; Li, F.; Song, Y.; Luo, Y.; Ma, H. Identification of a Mutation in the WISP3 Gene in Three Unrelated Families with Progressive Pseudorheumatoid Dysplasia. Mol. Med. Rep. 2015, 12, 419–425. [Google Scholar] [CrossRef][Green Version]
- Perbal, B. CCN Proteins: Multifunctional Signalling Regulators. Lancet 2004, 363, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.-I.; Lau, L.F. Taking Aim at the Extracellular Matrix: CCN Proteins as Emerging Therapeutic Targets. Nat. Rev. Drug Discov. 2011, 10, 945–963. [Google Scholar] [CrossRef]
- Holbourn, K.P.; Acharya, K.R.; Perbal, B. The CCN Family of Proteins: Structure–Function Relationships. Trends Biochem. Sci. 2008, 33, 461–473. [Google Scholar] [CrossRef]
- Patra, M.; Mahata, S.K.; Padhan, D.K.; Sen, M. CCN6 Regulates Mitochondrial Function. J. Cell Sci. 2016, 129, 2841–2851. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S.; Sen, M. Potential Role of WISP3 (CCN6) in Regulating the Accumulation of Reactive Oxygen Species. Biochem. Biophys. Res. Commun. 2007, 355, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.; Chen, Y.; Sen, M. WISP-3 Functions as a Ligand and Promotes Superoxide Dismutase Activity. Biochem. Biophys. Res. Commun. 2006, 342, 259–265. [Google Scholar] [CrossRef]
- Rao, R.S.; Patra, M.; Sen, M. WISP3-IGF1 Interaction Regulates Chondrocyte Hypertrophy. J. Cell Sci. 2013, 126, 1650–1658. [Google Scholar] [CrossRef]
- Kleer, C.G.; Zhang, Y.; Pan, Q.; Van Golen, K.L.; Wu, Z.-F.; Livant, D.; Merajver, S.D. WISP3 Is a Novel Tumor Suppressor Gene of Inflammatory Breast Cancer. Oncogene 2002, 21, 3172–3180. [Google Scholar] [CrossRef]
- Huang, W.; Martin, E.E.; Burman, B.; Gonzalez, M.E.; Kleer, C.G. The Matricellular Protein CCN6 (WISP3) Decreases Notch1 and Suppresses Breast Cancer Initiating Cells. Oncotarget 2016, 7, 25180–25193. [Google Scholar] [CrossRef]
- Nam, M.-W.; Lee, H.K.; Kim, C.-W.; Choi, Y.; Ahn, D.; Go, R.-E.; Choi, K.-C. Effects of CCN6 Overexpression on the Cell Motility and Activation of P38/Bone Morphogenetic Protein Signaling Pathways in Pancreatic Cancer Cells. Biomed. Pharmacother. 2023, 163, 114780. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.N.; Kleer, C.G. Matricellular CCN6 (WISP3) Protein: A Tumor Suppressor for Mammary Metaplastic Carcinomas. J. Cell Commun. Signal. 2018, 12, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Ehl, S.; Uhl, M.; Berner, R.; Bonafé, L.; Superti-Furga, A.; Kirchhoff, A. Clinical, Radiographic, and Genetic Diagnosis of Progressive Pseudorheumatoid Dysplasia in a Patient with Severe Polyarthropathy. Rheumatol. Int. 2004, 24, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Karalexi, M.A.; Papadopoulou, A.; Papakonstantinou, O.; Mitrogiorgou, M.; Skarantavos, G.; Papaevangelou, V.; Atsali, E. Late Diagnosis of Progressive Pseudorheumatoid Dysplasia in an Adolescent Patient: A Case Report and Review of the Literature. J. Pediatr. Res. 2021, 8, 209–213. [Google Scholar] [CrossRef]
- Uludağ Alkaya, D.; Kasapçopur, Ö.; Bursalı, A.; Adrovic, A.; Demir, B.; Aykut, A.; Tüysüz, B. Specific Early Signs and Long-Term Follow-up Findings of Progressive Pseudorheumatoid Dysplasia (PPRD) in the Turkish Cohort. Rheumatology 2022, 61, 3693–3703. [Google Scholar] [CrossRef]
- Pode-Shakked, B.; Vivante, A.; Barel, O.; Padeh, S.; Marek-Yagel, D.; Veber, A.; Abudi, S.; Eliyahu, A.; Tirosh, I.; Shpilman, S.; et al. Progressive Pseudorheumatoid Dysplasia Resolved by Whole Exome Sequencing: A Novel Mutation in WISP3 and Review of the Literature. BMC Med. Genet. 2019, 20, 53. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, K.; Yang, Y.; Wu, Z.; Jin, J.; Qiu, G.; Weng, X.; Zhao, X. CCN6 Mutation Detection in Chinese Patients with Progressive Pseudo-rheumatoid Dysplasia and Identification of Four Novel Mutations. Mol. Genet. Genom. Med. 2020, 8, e1261. [Google Scholar] [CrossRef]
- Delague, V.; Chouery, E.; Corbani, S.; Ghanem, I.; Aamar, S.; Fischer, J.; Levy-Lahad, E.; Urtizberea, J.A.; Mégarbané, A. Molecular Study of WISP3 in Nine Families Originating from the Middle-East and Presenting with Progressive Pseudorheumatoid Dysplasia: Identification of Two Novel Mutations, and Description of a Founder Effect. Am. J. Med. Genet. A 2005, 138A, 118–126. [Google Scholar] [CrossRef]
- Delgado, J.; Radusky, L.G.; Cianferoni, D.; Serrano, L. FoldX 5.0: Working with RNA, Small Molecules and a New Graphical Interface. Bioinformatics 2019, 35, 4168–4169. [Google Scholar] [CrossRef]
- Gao, H.; Yin, F.; Guan, D.; Feng, Y.; Zheng, Q.; Wang, X.; Zhu, M.; Zhang, X.; Cheng, S.; Chen, T.; et al. Liver Cancer: WISP3 Suppresses Hepatocellular Carcinoma Progression by Negative Regulation of Β-catenin/TCF/LEF Signalling. Cell Prolif. 2019, 52, e12583. [Google Scholar] [CrossRef]
- Zhou, F.; Sun, J.; Ye, L.; Jiang, T.; Li, W.; Su, C.; Ren, S.; Wu, F.; Zhou, C.; Gao, G. Fibronectin Promotes Tumor Angiogenesis and Progression of Non-Small-Cell Lung Cancer by Elevating WISP3 Expression via FAK/MAPK/ HIF-1α Axis and Activating Wnt Signaling Pathway. Exp. Hematol. Oncol. 2023, 12, 61. [Google Scholar] [CrossRef]
- Verma, R.P.; Hansch, C. Matrix Metalloproteinases (MMPs): Chemical–Biological Functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X. Targeting Mitochondrial Reactive Oxygen Species as Novel Therapy for Inflammatory Diseases and Cancers. J. Hematol. Oncol. 2013, 6, 19. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Valle, N.R.-D.; Huang, P. Redox Regulation of Cell Survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Helguera, P.; Seiglie, J.; Rodriguez, J.; Hanna, M.; Helguera, G.; Busciglio, J. Adaptive Downregulation of Mitochondrial Function in Down Syndrome. Cell Metab. 2013, 17, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Marzin, P.; Cormier-Daire, V. New Perspectives on the Treatment of Skeletal Dysplasia. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820904016. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Ichimura, S.; Sasaki, K.; Masuya, H.; Suzuki, T.; Wakana, S.; Ikegawa, S.; Furuichi, T. Endoplasmic Reticulum Stress-Mediated Apoptosis Contributes to a Skeletal Dysplasia Resembling Platyspondylic Lethal Skeletal Dysplasia, Torrance Type, in a Novel Col2a1 Mutant Mouse Line. Biochem. Biophys. Res. Commun. 2015, 468, 86–91. [Google Scholar] [CrossRef]
- Ritz, C.; Spiess, A.-N. qpcR: An R Package for Sigmoidal Model Selection in Quantitative Real-Time Polymerase Chain Reaction Analysis. Bioinformatics 2008, 24, 1549–1551. [Google Scholar] [CrossRef]
- Li, J.; Rouse, S.L.; Matthews, I.R.; Park, Y.; Eltawil, Y.; Sherr, E.H.; Chan, D.K. Modulating the Unfolded Protein Response with ISRIB Mitigates Cisplatin Ototoxicity. Sci. Rep. 2024, 14, 22382. [Google Scholar] [CrossRef] [PubMed]
- Mcdonald, J.H.; Dunn, K.W. Statistical Tests for Measures of Colocalization in Biological Microscopy. J. Microsc. 2013, 252, 295–302. [Google Scholar] [CrossRef]
- Baker, N.; Sharpe, P.; Culley, K.; Otero, M.; Bevan, D.; Newham, P.; Barker, W.; Clements, K.M.; Langham, C.J.; Goldring, M.B.; et al. Dual Regulation of Metalloproteinase Expression in Chondrocytes by Wnt-1–Inducible Signaling Pathway Protein 3/CCN6. Arthritis Rheum. 2012, 64, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Schweingruber, C.; Rufener, S.C.; Zünd, D.; Yamashita, A.; Mühlemann, O. Nonsense-Mediated mRNA Decay—Mechanisms of Substrate mRNA Recognition and Degradation in Mammalian Cells. Biochim. Biophys. Acta BBA—Gene Regul. Mech. 2013, 1829, 612–623. [Google Scholar] [CrossRef]
- Popp, M.W.-L.; Maquat, L.E. Organizing Principles of Mammalian Nonsense-Mediated mRNA Decay. Annu. Rev. Genet. 2013, 47, 139–165. [Google Scholar] [CrossRef]
- Carter, M.S.; Li, S.; Wilkinson, M.F. A Splicing-Dependent Regulatory Mechanism That Detects Translation Signals. EMBO J. 1996, 15, 5965–5975. [Google Scholar] [CrossRef]
- Buß, O.; Muller, D.; Jager, S.; Rudat, J.; Rabe, K.S. Improvement in the Thermostability of a β-Amino Acid Converting ω-Transaminase by Using FoldX. ChemBioChem 2018, 19, 379–387. [Google Scholar] [CrossRef]
- Buß, O.; Rudat, J.; Ochsenreither, K. FoldX as Protein Engineering Tool: Better Than Random Based Approaches? Comput. Struct. Biotechnol. J. 2018, 16, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Petrosino, M.; Novak, L.; Pasquo, A.; Chiaraluce, R.; Turina, P.; Capriotti, E.; Consalvi, V. Analysis and Interpretation of the Impact of Missense Variants in Cancer. Int. J. Mol. Sci. 2021, 22, 5416. [Google Scholar] [CrossRef] [PubMed]
- Tokuriki, N.; Stricher, F.; Serrano, L.; Tawfik, D.S. How Protein Stability and New Functions Trade Off. PLoS Comput. Biol. 2008, 4, e1000002. [Google Scholar] [CrossRef]
- Hernández, I.M.; Dehouck, Y.; Bastolla, U.; López-Blanco, J.R.; Chacón, P. Predicting Protein Stability Changes upon Mutation Using a Simple Orientational Potential. Bioinformatics 2023, 39, btad011. [Google Scholar] [CrossRef]
- Wang, M.; Man, X.-F.; Liu, Y.-Q.; Liao, E.-Y.; Shen, Z.-F.; Luo, X.-H.; Guo, L.-J.; Wu, X.-P.; Zhou, H.-D. Dysfunction of Collagen Synthesis and Secretion in Chondrocytes Induced by Wisp3 Mutation. Int. J. Endocrinol. 2013, 2013, 679763. [Google Scholar] [CrossRef] [PubMed]
- Padhan, D.K.; Sengupta, A.; Patra, M.; Ganguly, A.; Mahata, S.K.; Sen, M. CCN6 Regulates Mitochondrial Respiratory Complex Assembly and Activity. FASEB J. 2020, 34, 12163–12176. [Google Scholar] [CrossRef]
- Rehm, H.L.; Berg, J.S.; Brooks, L.D.; Bustamante, C.D.; Evans, J.P.; Landrum, M.J.; Ledbetter, D.H.; Maglott, D.R.; Martin, C.L.; Nussbaum, R.L.; et al. ClinGen—The Clinical Genome Resource. N. Engl. J. Med. 2015, 372, 2235–2242. [Google Scholar] [CrossRef]
- Nakamura, Y.; Weidinger, G.; Liang, J.O.; Aquilina-Beck, A.; Tamai, K.; Moon, R.T.; Warman, M.L. The CCN Family Member Wisp3, Mutant in Progressive Pseudorheumatoid Dysplasia, Modulates BMP and Wnt Signaling. J. Clin. Investig. 2007, 117, 3075–3086. [Google Scholar] [CrossRef]
- Zhou, H.-D.; Bu, Y.-H.; Peng, Y.-Q.; Xie, H.; Wang, M.; Yuan, L.-Q.; Jiang, Y.; Li, D.; Wei, Q.-Y.; He, Y.-L.; et al. Cellular and Molecular Responses in Progressive Pseudorheumatoid Dysplasia Articular Cartilage Associated with Compound Heterozygous WISP3 Gene Mutation. J. Mol. Med. 2007, 85, 985–996. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Alemany-Ribes, M.; Raftery, R.M.; Nwoko, U.; Warman, M.L.; Craft, A.M. Directed Differentiation of Human Pluripotent Stem Cells into Articular Cartilage Reveals Effects Caused by Absence of WISP3, the Gene Responsible for Progressive Pseudorheumatoid Arthropathy of Childhood. Ann. Rheum. Dis. 2023, 82, 1547–1557. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Sun, C.; Yu, H.-C.; Song, B.; Pan, Z.-X. Targeted Inhibition of β-Catenin by miR-320 and Decreased MMP-13 Expression in Suppressing Chondrocyte Collagen Degradation. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5828–5835. [Google Scholar] [CrossRef]
- Lechuga, C.G.; Hernández-Nazara, Z.H.; Rosales, J.-A.D.; Morris, E.R.; Rincón, A.R.; Rivas-Estilla, A.M.; Esteban-Gamboa, A.; Rojkind, M. TGF-Β1 Modulates Matrix Metalloproteinase-13 Expression in Hepatic Stellate Cells by Complex Mechanisms Involving p38MAPK, PI3-Kinase, AKT, and P70S6k. Am. J. Physiol.-Gastrointest. Liver Physiol. 2004, 287, G974–G987. [Google Scholar] [CrossRef]
- Yang, X.; Chen, L.; Xu, X.; Li, C.; Huang, C.; Deng, C.-X. TGF-β/Smad3 Signals Repress Chondrocyte Hypertrophic Differentiation and Are Required for Maintaining Articular Cartilage. J. Cell Biol. 2001, 153, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Leivonen, S.-K.; Chantry, A.; Häkkinen, L.; Han, J.; Kähäri, V.-M. Smad3 Mediates Transforming Growth Factor-β-Induced Collagenase-3 (Matrix Metalloproteinase-13) Expression in Human Gingival Fibroblasts. J. Biol. Chem. 2002, 277, 46338–46346. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; He, T.; Zhong, Y.; Chen, M.; Yao, Q.; Chen, D.; Shao, Z.; Xiao, G. Roles of Focal Adhesion Proteins in Skeleton and Diseases. Acta Pharm. Sin. B 2023, 13, 998–1013. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, Y.; Yin, Z.; Yang, X.; Jiang, Y.; Gui, J. Chondrocyte Migration Affects Tissue-Engineered Cartilage Integration by Activating the Signal Transduction Pathways Involving Src, PLCγ1, and ERK1/2. Tissue Eng. Part A 2013, 19, 2506–2516. [Google Scholar] [CrossRef]
- Gortazar, A.R.; Martin-Millan, M.; Bravo, B.; Plotkin, L.I.; Bellido, T. Crosstalk between Caveolin-1/Extracellular Signal-Regulated Kinase (ERK) and β-Catenin Survival Pathways in Osteocyte Mechanotransduction. J. Biol. Chem. 2013, 288, 8168–8175. [Google Scholar] [CrossRef]
- Wörthmüller, J.; Rüegg, C. The Crosstalk between FAK and Wnt Signaling Pathways in Cancer and Its Therapeutic Implication. Int. J. Mol. Sci. 2020, 21, 9107. [Google Scholar] [CrossRef]
- Cockburn, C.G.; Barnes, M.J. Characterization of Thrombospondin Binding to Collagen (Type I) Fibres: Role of Collagen Telopeptides. Matrix 1991, 11, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, H.; Zhang, Z.; Pan, J.; Yuan, H. Cartilage Targeting Therapy with Reactive Oxygen Species-Responsive Nanocarrier for Osteoarthritis. J. Nanobiotechnol. 2022, 20, 419. [Google Scholar] [CrossRef]
- Kan, S.; Duan, M.; Liu, Y.; Wang, C.; Xie, J. Role of Mitochondria in Physiology of Chondrocytes and Diseases of Osteoarthritis and Rheumatoid Arthritis. Cartilage 2021, 13, 1102S–1121S. [Google Scholar] [CrossRef]
- Pendergrass, W.; Wolf, N.; Poot, M. Efficacy of MitoTracker GreenTM and CMXrosamine to Measure Changes in Mitochondrial Membrane Potentials in Living Cells and Tissues. Cytometry A 2004, 61A, 162–169. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic Reticulum Stress: Molecular Mechanism and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Lamandé, S.R.; Bateman, J.F. Genetic Disorders of the Extracellular Matrix. Anat. Rec. 2020, 303, 1527–1542. [Google Scholar] [CrossRef]
- Tran, H.C.; Van Aken, O. Mitochondrial Unfolded Protein-Related Responses across Kingdoms: Similar Problems, Different Regulators. Mitochondrion 2020, 53, 166–177. [Google Scholar] [CrossRef]
- Falahatpisheh, H.; Nanez, A.; Montoya-Durango, D.; Qian, Y.; Tiffany-Castiglioni, E.; Ramos, K.S. Activation Profiles of HSPA5 during the Glomerular Mesangial Cell Stress Response to Chemical Injury. Cell Stress Chaperones 2007, 12, 209. [Google Scholar] [CrossRef]
- Huang, Y.; Arora, K.; Mun, K.S.; Yang, F.; Moon, C.; Yarlagadda, S.; Jegga, A.; Weaver, T.; Naren, A.P. Targeting DNAJB9, a Novel ER Luminal Co-Chaperone, to Rescue ΔF508-CFTR. Sci. Rep. 2019, 9, 9808. [Google Scholar] [CrossRef]
- Paredes, F.; Parra, V.; Torrealba, N.; Navarro-Marquez, M.; Gatica, D.; Bravo-Sagua, R.; Troncoso, R.; Pennanen, C.; Quiroga, C.; Chiong, M.; et al. HERPUD1 Protects against Oxidative Stress-Induced Apoptosis through Downregulation of the Inositol 1,4,5-Trisphosphate Receptor. Free Radic. Biol. Med. 2016, 90, 206–218. [Google Scholar] [CrossRef]
- Liu, S.; Li, Z.; Lan, S.; Hao, H.; Baz, A.A.; Yan, X.; Gao, P.; Chen, S.; Chu, Y. The Dual Roles of Activating Transcription Factor 3 (ATF3) in Inflammation, Apoptosis, Ferroptosis, and Pathogen Infection Responses. Int. J. Mol. Sci. 2024, 25, 824. [Google Scholar] [CrossRef] [PubMed]
- Turpin, J.; El-Safadi, D.; Lebeau, G.; Frumence, E.; Desprès, P.; Viranaïcken, W.; Krejbich-Trotot, P. CHOP Pro-Apoptotic Transcriptional Program in Response to ER Stress Is Hacked by Zika Virus. Int. J. Mol. Sci. 2021, 22, 3750. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.T.; Mulazzani, E.; Nutt, S.L.; Masters, S.L. The Role of PLCγ2 in Immunological Disorders, Cancer, and Neurodegeneration. J. Biol. Chem. 2021, 297, 100905. [Google Scholar] [CrossRef]
- Sojoudi, K.; Azizi, H.; Skutella, T. A Study of Dual Function and Expression Pattern of Klf4 between Two Different Processes of Differentiation and Maintenance of Pluripotency in Spermatogonial Stem Cells. Andrologia 2024, 2024, 8061627. [Google Scholar] [CrossRef]
- Gene: CSNK1A1L Casein Kinase 1 Alpha 1 like [Homo sapiens]. Available online: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=122011 (accessed on 13 July 2025).
- ANP32CP Acidic Nuclear Phosphoprotein 32 Family Member C, Pseudogene [Homo sapiens (Human)]. Available online: https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=23520 (accessed on 13 July 2025).
- NME2P1 NME2 Pseudogene 1 [Homo sapiens (Human)]. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=NME2P1 (accessed on 13 July 2025).
- Qazi, S.; Jit, B.P.; Das, A.; Karthikeyan, M.; Saxena, A.; Ray, M.D.; Singh, A.R.; Raza, K.; Jayaram, B.; Sharma, A. BESFA: Bioinformatics Based Evolutionary, Structural & Functional Analysis of Prostate, Placenta, Ovary, Testis, and Embryo (POTE) Paralogs. Heliyon 2022, 8, e10476. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Du, Y.; Zhao, D.; Chen, K. The Relationship between the Prognostic Marker LIMA1 in Head and Neck Squamous Cell Carcinoma and Immune Infiltration. J. Oncol. 2022, 2022, 1040116. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, C.; Song, H.; Yuan, J.; Zhang, X.; Yuan, Y.; Zhang, L.; He, J. Characterization of LIMA1 and Its Emerging Roles and Potential Therapeutic Prospects in Cancers. Front. Oncol. 2023, 13, 1115943. [Google Scholar] [CrossRef]
- Loughlin, J.; Sinsheimer, J.S.; Carr, A.; Chapman, K. The CALM1 Core Promoter Polymorphism Is Not Associated with Hip Osteoarthritis in a United Kingdom Caucasian Population. Osteoarthr. Cartil. 2006, 14, 295–298. [Google Scholar] [CrossRef]
- Mototani, H.; Mabuchi, A.; Saito, S.; Fujioka, M.; Iida, A.; Takatori, Y.; Kotani, A.; Kubo, T.; Nakamura, K.; Sekine, A.; et al. A Functional Single Nucleotide Polymorphism in the Core Promoter Region of CALM1 Is Associated with Hip Osteoarthritis in Japanese. Hum. Mol. Genet. 2005, 14, 1009–1017. [Google Scholar] [CrossRef]
- Koren, T.D.T.; Shrivastava, R.; Siddiqui, S.I.; Ghosh, S. Calmodulin Modulates the Gating Properties of Voltage-Dependent Anion Channel from Rat Brain Mitochondria. J. Phys. Chem. B 2022, 126, 4857–4871. [Google Scholar] [CrossRef] [PubMed]
- Despotidis, M.; Lyros, O.; Driva, T.S.; Sarantis, P.; Kapetanakis, E.I.; Mylonakis, A.; Mamilos, A.; Sakellariou, S.; Schizas, D. DKK1 and Its Receptors in Esophageal Adenocarcinoma: A Promising Molecular Target. Diagnostics 2025, 15, 85. [Google Scholar] [CrossRef]
- Li, C.M.; Kang, J.; Baek, J.; Kim, Y.; Park, H.; Jung, Y.-K. Cytosolic FKBPL and ER-Resident CKAP4 Co-Regulates ER-Phagy and Protein Secretion. Nat. Commun. 2024, 15, 7886. [Google Scholar] [CrossRef]
- Harada, T.; Sada, R.; Osugi, Y.; Matsumoto, S.; Matsuda, T.; Hayashi-Nishino, M.; Nagai, T.; Harada, A.; Kikuchi, A. Palmitoylated CKAP4 Regulates Mitochondrial Functions through an Interaction with VDAC2 at ER–Mitochondria Contact Sites. J. Cell Sci. 2020, 133, jcs249045. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, X.; Wang, S.; Zheng, Y.; Yang, S.; Hou, Y.; Zou, B.; Dong, L. Decreased Expression of DEAD-Box Helicase 5 Inhibits Esophageal Squamous Cell Carcinomas by Regulating Endoplasmic Reticulum Stress and Autophagy. Biochem. Biophys. Res. Commun. 2020, 533, 1449–1456. [Google Scholar] [CrossRef]
- Xu, K.; Sun, S.; Yan, M.; Cui, J.; Yang, Y.; Li, W.; Huang, X.; Dou, L.; Chen, B.; Tang, W.; et al. DDX5 and DDX17—Multifaceted Proteins in the Regulation of Tumorigenesis and Tumor Progression. Front. Oncol. 2022, 12, 943032. [Google Scholar] [CrossRef]
- Lambrecht, S.; Dhaenens, M.; Almqvist, F.; Verdonk, P.; Verbruggen, G.; Deforce, D.; Elewaut, D. Proteome Characterization of Human Articular Chondrocytes Leads to Novel Insights in the Function of Small Heat-Shock Proteins in Chondrocyte Homeostasis. Osteoarthr. Cartil. 2010, 18, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Bruey, J.-M.; Ducasse, C.; Bonniaud, P.; Ravagnan, L.; Susin, S.A.; Diaz-Latoud, C.; Gurbuxani, S.; Arrigo, A.-P.; Kroemer, G.; Solary, E.; et al. Hsp27 Negatively Regulates Cell Death by Interacting with Cytochrome c. Nat. Cell Biol. 2000, 2, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Li, Y.; Shi, C. HSPB1 as an RNA-Binding Protein Mediates the Pathological Process of Osteoarthritis. J. Orthop. Surg. 2024, 19, 156. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Lazarovici, P.; Marcinkiewicz, C.; Lelkes, P.I. Cell-Based Adhesion Assays for Isolation of Snake Venom’s Integrin Antagonists. In Methods in Molecular Biology; Springer US: New York, NY, USA, 2020; pp. 205–223. ISBN 978-1-4939-9844-9. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bormann, T.; Maus, R.; Stolper, J.; Tort Tarrés, M.; Brandenberger, C.; Wedekind, D.; Jonigk, D.; Welte, T.; Gauldie, J.; Kolb, M.; et al. Role of Matrix Metalloprotease-2 and MMP-9 in Experimental Lung Fibrosis in Mice. Respir. Res. 2022, 23, 180. [Google Scholar] [CrossRef] [PubMed]
- Bolte, S.; Cordelières, F.P. A Guided Tour into Subcellular Colocalization Analysis in Light Microscopy. J. Microsc. 2006, 224, 213–232. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-Based Genome Alignment and Genotyping with HISAT2 and HISAT-Genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Mudge, J.M.; Carbonell-Sala, S.; Diekhans, M.; Martinez, J.G.; Hunt, T.; Jungreis, I.; Loveland, J.E.; Arnan, C.; Barnes, I.; Bennett, R.; et al. GENCODE 2025: Reference Gene Annotation for Human and Mouse. Nucleic Acids Res. 2025, 53, D966–D975. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Lun, A.T.L.; Baldoni, P.L.; Smyth, G.K. edgeR v4: Powerful Differential Analysis of Sequencing Data with Expanded Functionality and Improved Support for Small Counts and Larger Datasets. Nucleic Acids Res. 2025, 53, gkaf018. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]
- Liberzon, A.; Subramanian, A.; Pinchback, R.; Thorvaldsdóttir, H.; Tamayo, P.; Mesirov, J.P. Molecular Signatures Database (MSigDB) 3.0. Bioinformatics 2011, 27, 1739–1740. [Google Scholar] [CrossRef]
- Kolde, R. Package ‘Pheatmap’; 2015; p. 790. version number 1.0.13. Available online: https://cran.r-project.org/web/packages/pheatmap/index.html (accessed on 13 July 2025).
- Wickham, H. Ggplot2. WIREs Comput. Stat. 2011, 3, 180–185. [Google Scholar] [CrossRef]
- Zhang, X.; Smits, A.H.; Van Tilburg, G.B.; Ovaa, H.; Huber, W.; Vermeulen, M. Proteome-Wide Identification of Ubiquitin Interactions Using UbIA-MS. Nat. Protoc. 2018, 13, 530–550. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.L. Tidygraph: A Tidy API for Graph Manipulation, R package version 1.3.1.9000; 2020. Available online: https://cran.r-project.org/web/packages/tidygraph/index.html (accessed on 19 July 2025).
- Pedersen, T.L. Ggforce: Accelerating ‘Ggplot2’, R package version 0.5.0.9000; 2019. Available online: https://cran.r-project.org/web/packages/ggforce/index.html (accessed on 19 July 2025).
- Fruchterman, T.M.J.; Reingold, E.M. Graph Drawing by Force-directed Placement. Softw. Pract. Exp. 1991, 21, 1129–1164. [Google Scholar] [CrossRef]
- Bressert, E. SciPy and NumPy: Examples to Jumpstart Your Scientific Python Programming, 1st ed., 3. release.; O’Reilly: Beijing, China; Köln, Germany, 2013; ISBN 978-1-4493-0546-8. [Google Scholar]
- Bisong, E. Building Machine Learning and Deep Learning Models on Google Cloud Platform: A Comprehensive Guide for Beginners; Apress: Berkeley, CA, USA, 2019; ISBN 978-1-4842-4469-2. [Google Scholar]
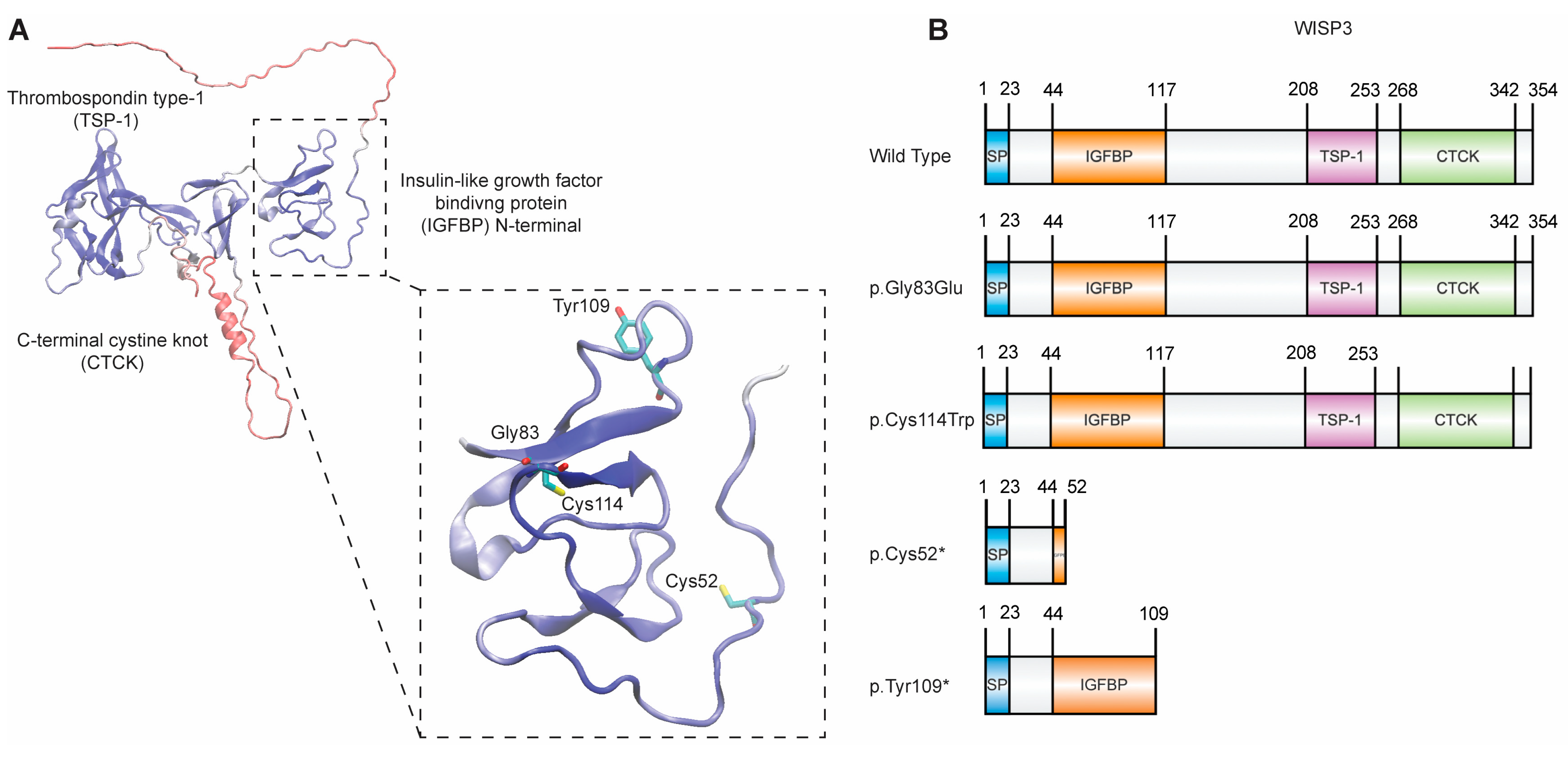


| Variant | ΔΔG Prediction for Protein Stability (kcal/mol) |
|---|---|
| p.Gly83Glu | +2.73 |
| p.Cys114Trp | +43.33 |
| Nucleotide Change | Protein Change | Forward and Reverse Primer (5′ → 3′) | GC Content (%) |
|---|---|---|---|
| c.156C>A | p.Cys52* | Fw: GTTTTGTCACTGGCCCTGAAAATGCCCTCAGCAGAAG | 51 |
| Rv: CTTCTGCTGAGGGCATTTTCAGGGCCAGTGACAAAAC | |||
| c.248G>A | p.Glu83Glu | Fw: CTGTGCCAAGCAACCAGAGGAAATCTGCAATGAAG | 49 |
| Rv: CTTCATTGCAGATTTCCTCTGGTTGCTTGGCACAG | |||
| c.327C>A | p.Tyr109* | Fw: GACAGGCCTAGGTAAGAGACTGGAGTGTG | 55 |
| Rv: CACACTCCAGTCTCTTACCTAGGCCTGTC | |||
| c.342T>G | p.Cys114Trp | Fw: GAGACTGGAGTGTGGGCATACCTTGTAGC | 55 |
| Rv: GCTACAAGGTATGCCCACACTCCAGTCTC |
| Gene | Forward and Reverse Primer (5′ → 3′) |
|---|---|
| CCN6 | Fw: ACTGTAGCCTGGAACCATTACT Rv: TGGTCACCCTGTTAGATATTCCC |
| CHOP | Fw: GGAAACAGAGTGGTCATTCCC Rv: CTGCTTGAGCCGTTCATTCTC |
| sXBP1 | Fw: GCTGAGTCCGCAGCAGGT Rw: CTGGGTCCAAGTTGTCCAGAAT |
| uXBP1 | Fw: CAGACTACGTGCACCTCTGC Rv: CTGGGTCCAAGTTGTCCAGAAT |
| ACTB | Fw: CACCATTGGCAATGAGCGGTTC Rv: AGGTCTTTGCGGATGTCCACGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guven Tasbicen, G.; Tufan, A.; Savsar, B.; Bulbul, A.; Tonbul, Z.; Guzel, E.; Ayhan, D.H.; Timucin, A.C.; Onat, U.I.; Bayram Akcapinar, G.; et al. Molecular Consequences of CCN6 Variants Encoding WISP3 in Progressive Pseudorheumatoid Dysplasia. Int. J. Mol. Sci. 2025, 26, 8838. https://doi.org/10.3390/ijms26188838
Guven Tasbicen G, Tufan A, Savsar B, Bulbul A, Tonbul Z, Guzel E, Ayhan DH, Timucin AC, Onat UI, Bayram Akcapinar G, et al. Molecular Consequences of CCN6 Variants Encoding WISP3 in Progressive Pseudorheumatoid Dysplasia. International Journal of Molecular Sciences. 2025; 26(18):8838. https://doi.org/10.3390/ijms26188838
Chicago/Turabian StyleGuven Tasbicen, Gulipek, Ali Tufan, Batuhan Savsar, Alper Bulbul, Zeynep Tonbul, Elif Guzel, Dilay Hazal Ayhan, Ahmet Can Timucin, Umut Inci Onat, Gunseli Bayram Akcapinar, and et al. 2025. "Molecular Consequences of CCN6 Variants Encoding WISP3 in Progressive Pseudorheumatoid Dysplasia" International Journal of Molecular Sciences 26, no. 18: 8838. https://doi.org/10.3390/ijms26188838
APA StyleGuven Tasbicen, G., Tufan, A., Savsar, B., Bulbul, A., Tonbul, Z., Guzel, E., Ayhan, D. H., Timucin, A. C., Onat, U. I., Bayram Akcapinar, G., Akgun Dogan, O., Alanay, Y., & Tahir Turanli, E. (2025). Molecular Consequences of CCN6 Variants Encoding WISP3 in Progressive Pseudorheumatoid Dysplasia. International Journal of Molecular Sciences, 26(18), 8838. https://doi.org/10.3390/ijms26188838








