Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviated the Inflammatory Response in Mice Infected with the Influenza Virus A (H1N1)
Abstract
1. Introduction
2. Results
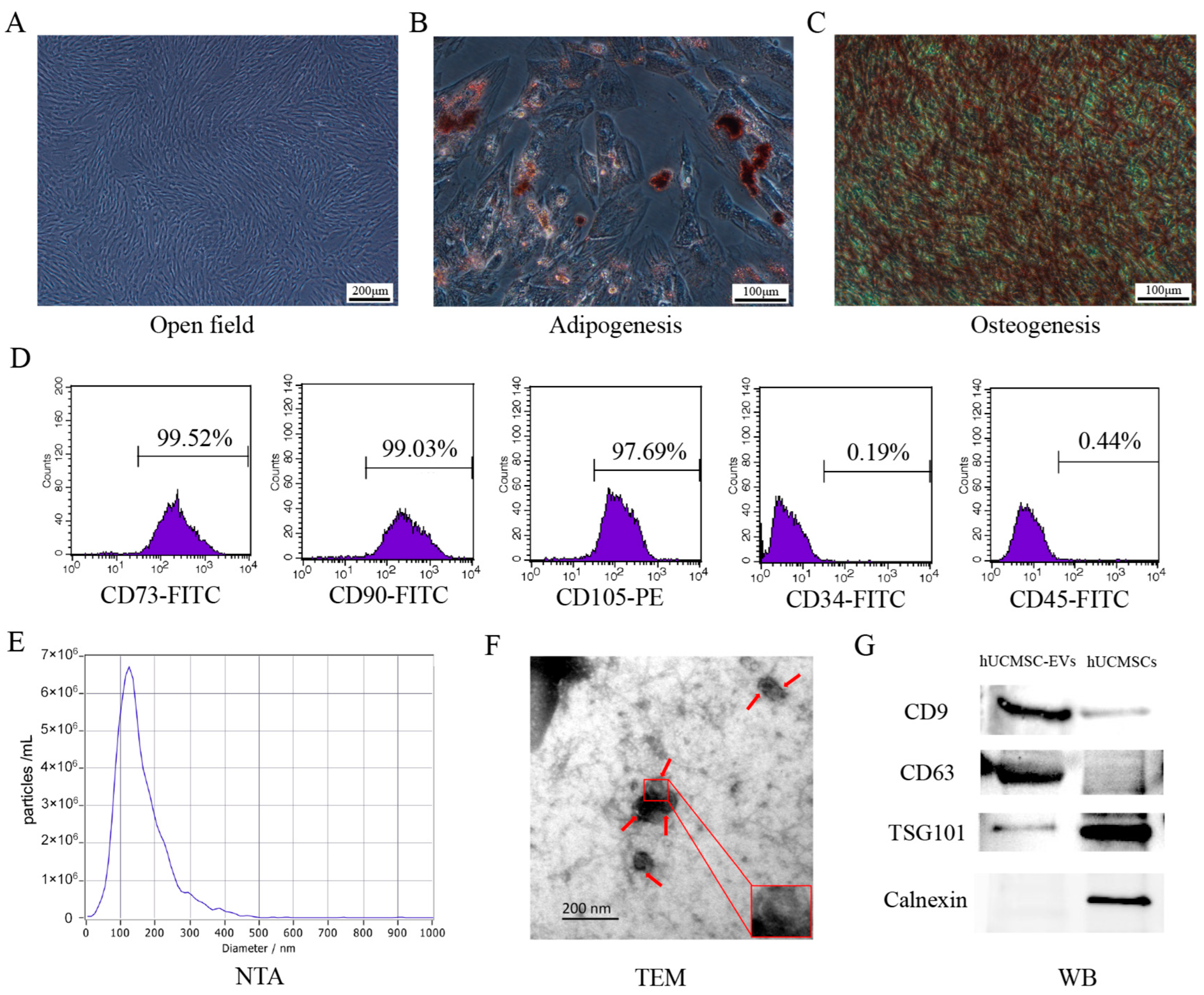
2.1. Characterization of hUCMSCs and hUCMSC-EVs
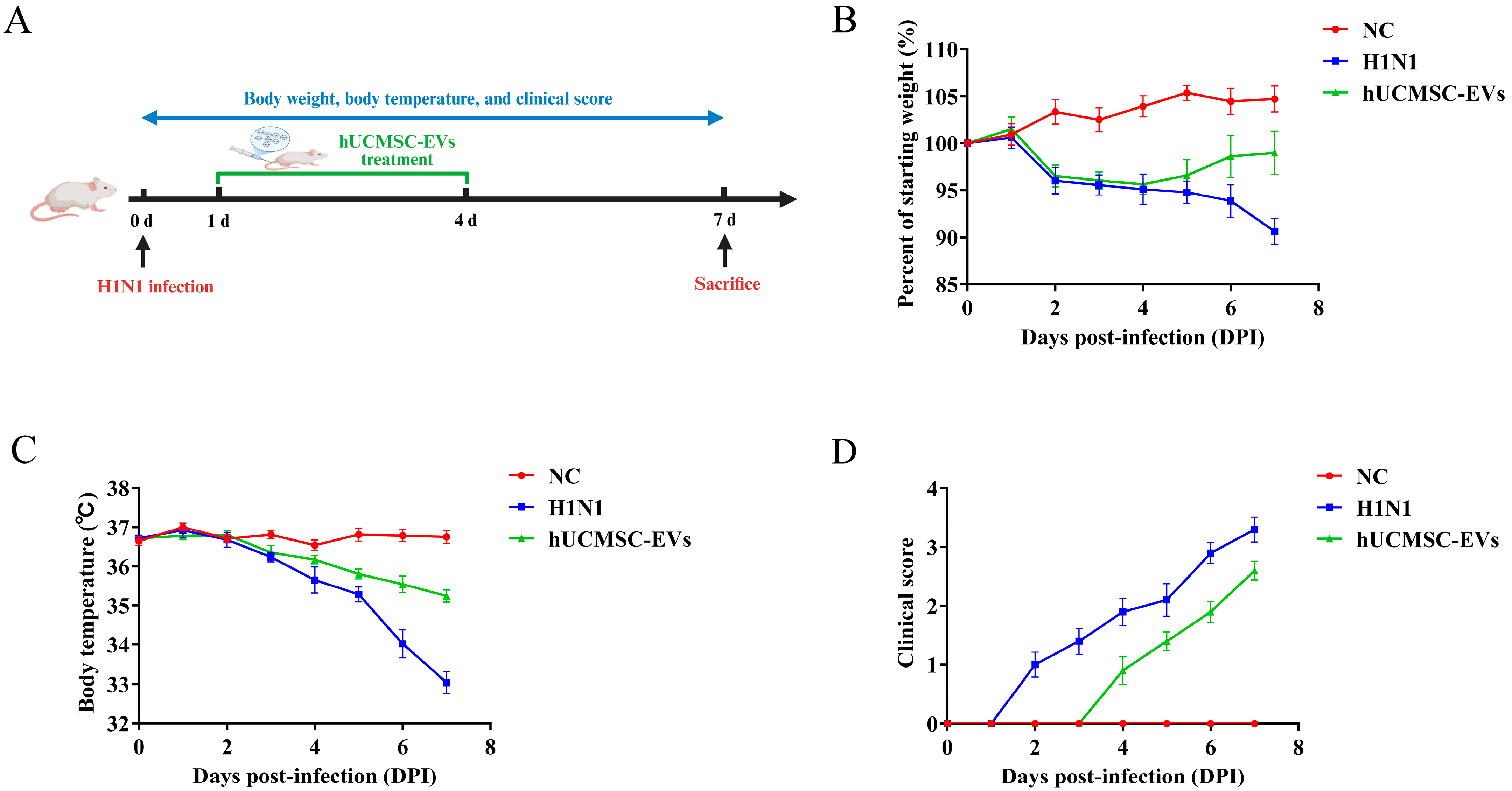
2.2. hUCMSC-EVs Attenuated Clinical Symptoms in Influenza A Virus (H1N1)-Infected Mice
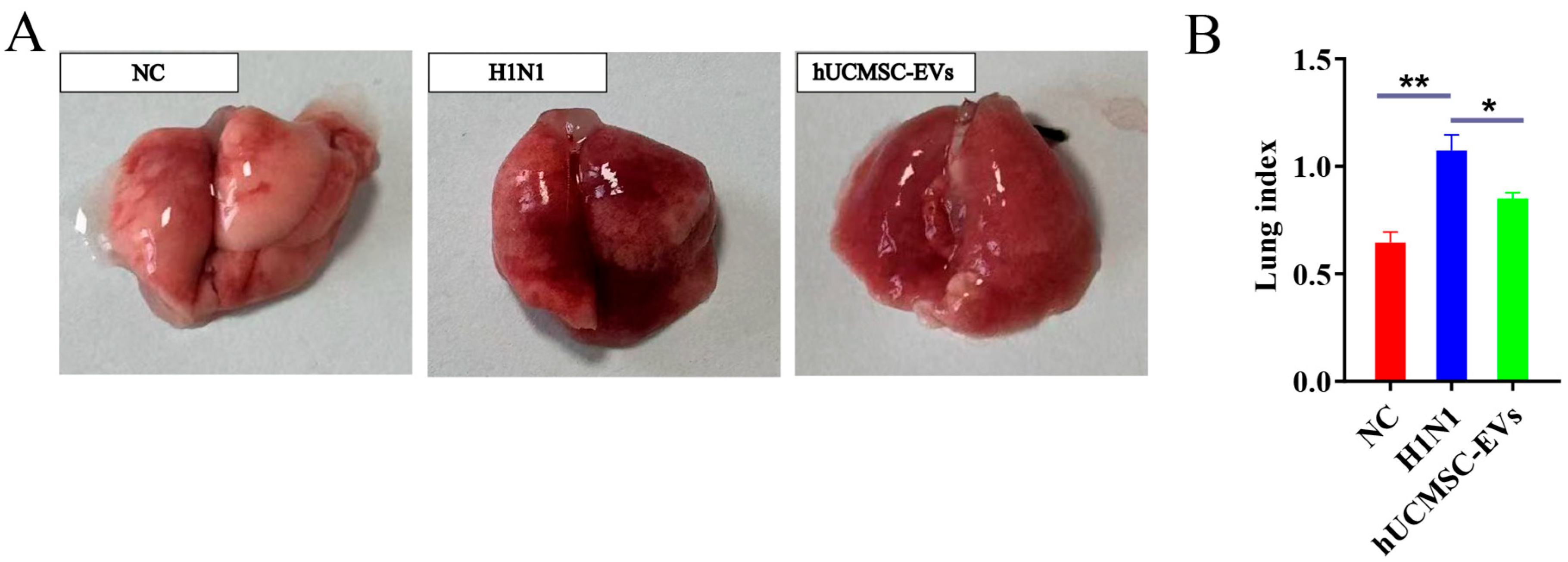
2.3. hUCMSC-EVs Reduced Lung Pathology
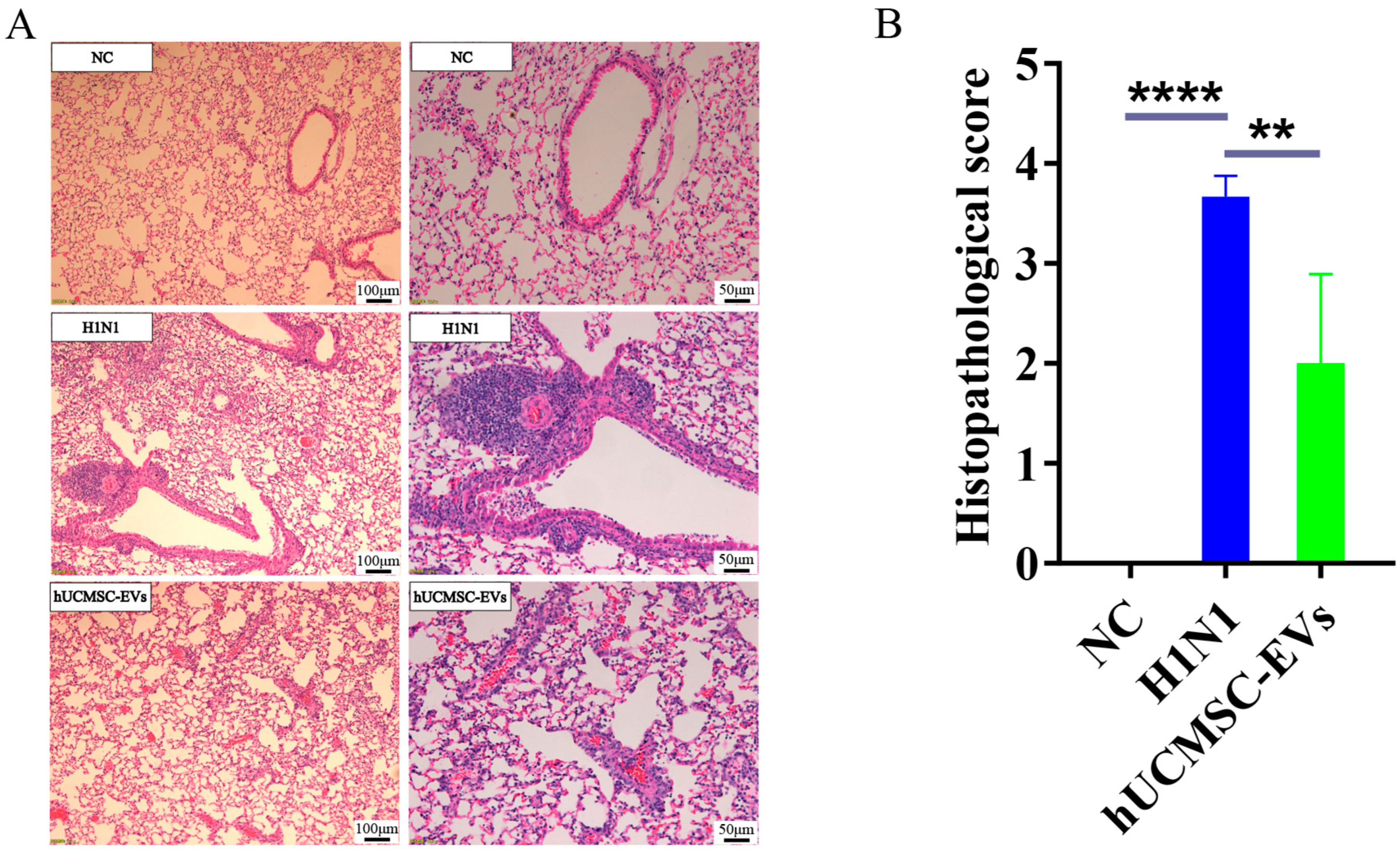
2.4. hUCMSC-EVs Improved Lung Histopathology
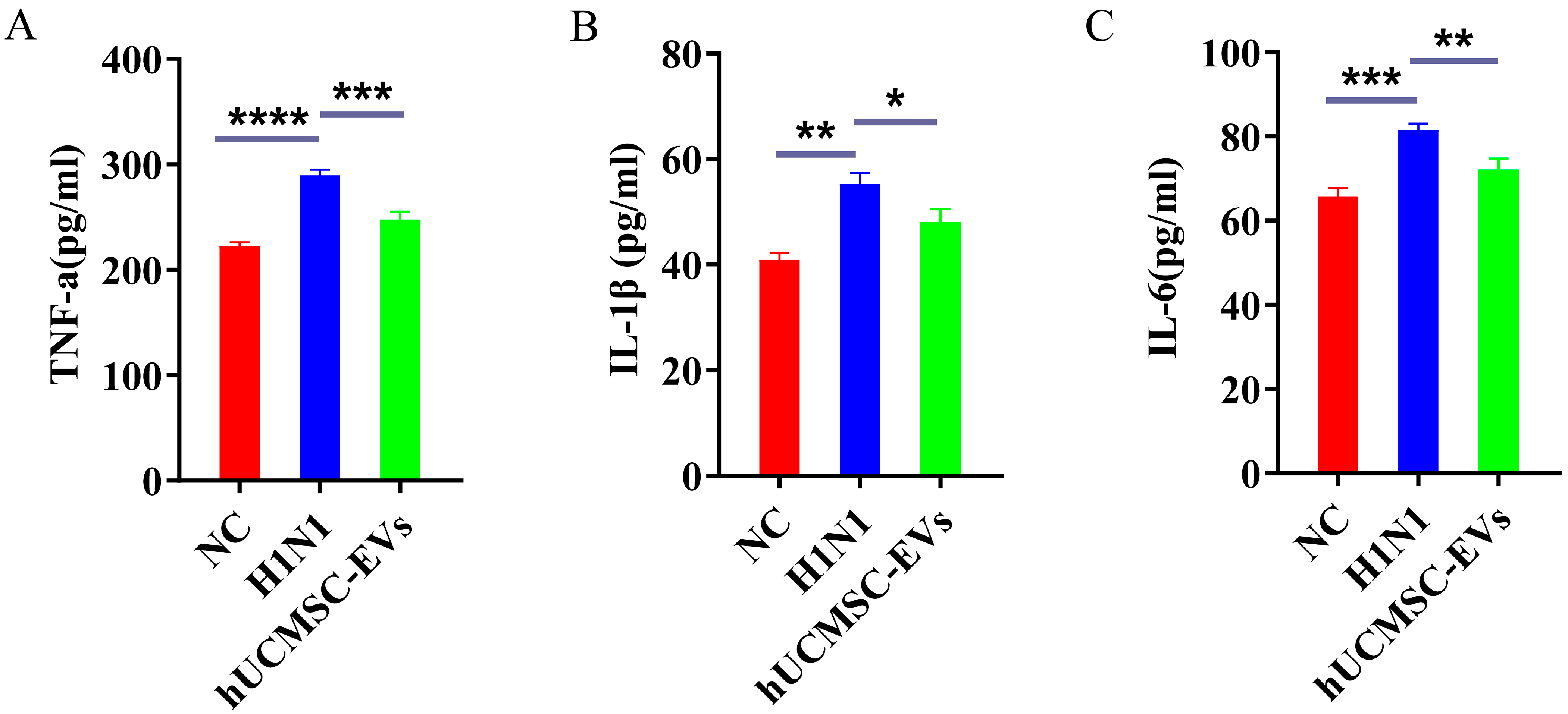
2.5. hUCMSC-EVs Suppressed Systemic Inflammation
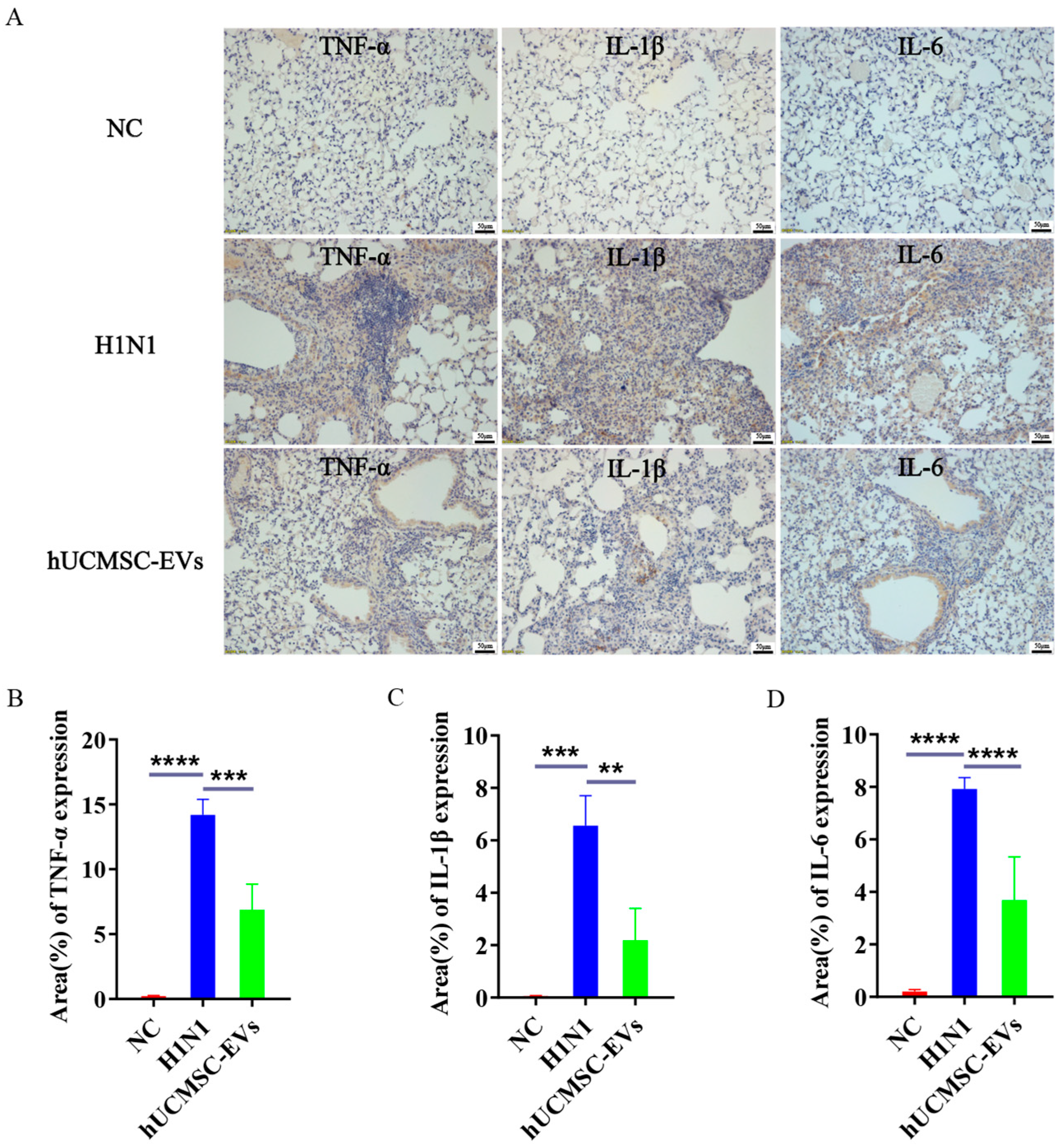
2.6. hUCMSC-EVs Mitigated Lung Inflammation
3. Discussion
4. Materials and Methods
4.1. Experimental Reagents
4.2. Experimental Animals
4.3. Virus Strain
4.4. Culture of hUCMSCs
4.5. Charaterization of hUCMSCs
4.6. Multidirectional Differentiation of hUCMSCs
4.7. Isolation and Characterization of hUCMSC-EVs
4.8. TEM Observation of hUCMSC-EVs Morphology
4.9. Western Blotting
4.10. Influenza Virus A (H1N1) Infection Model and Treatment
4.11. H&E Staining and Lung Pathological Scoring
4.12. Enzyme-Linked Immunosorbent Assay (ELISA)
4.13. Immunohistochemical Staining
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almutairi, F.; Sarr, D.; Tucker, S.L.; Fantone, K.; Lee, J.K.; Rada, B. RGS10 Reduces Lethal Influenza Infection and Associated Lung Inflammation in Mice. Front. Immunol. 2021, 12, 772288. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, F.; Xu, J.; Zhu, X.; Zhao, Y.; Wen, R.; Anirudhan, V.; Rong, L.; Tian, J.; Cui, Q. Qingjin Huatan decoction protects mice against influenza a virus pneumonia via the chemokine signaling pathways. J. Ethnopharmacol. 2023, 317, 116745. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.; García-Sastre, A.; Schotsaert, M. Host immune response-inspired development of the influenza vaccine. Ann. Allergy Asthma Immunol. 2020, 125, 28–35. [Google Scholar] [CrossRef]
- Tanner, A.R.; Dorey, R.B.; Brendish, N.J.; Clark, T.W. Influenza vaccination: Protecting the most vulnerable. Eur. Respir. Rev. 2021, 30, 200258. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.B.; Davey, R.T.; Steigbigel, R.T.; Fang, F. Targeting pandemic influenza: A primer on influenza antivirals and drug resistance. J. Antimicrob. Chemother. 2010, 65, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Kayani, M.D. Oseltamivir-resistant h1n1 influenza virus: Case report. J. Pak. Med. Assoc. 2022, 72, 2112–2113. [Google Scholar] [CrossRef]
- Ding, W.; Li, R.; Song, T.; Yang, Z.; Xu, D.; Huang, C.; Shen, S.; Zhong, N.; Lai, K.; Deng, Z. AMG487 alleviates influenza A (H1N1) virus-induced pulmonary inflammation through decreasing IFN-γ-producing lymphocytes and IFN-γ concentrations. Br. J. Pharmacol. 2024, 181, 2053–2069. [Google Scholar] [CrossRef]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, A.G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef]
- Naji, A.; Eitoku, M.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Suganuma, N. Biological functions of mesenchymal stem cells and clinical implications. Cell. Mol. Life Sci. 2019, 76, 3323–3348. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef]
- Arabpour, M.; Saghazadeh, A.; Rezaei, N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int. Immunopharmacol. 2021, 97, 107823. [Google Scholar] [CrossRef]
- Wu, R.; Fan, X.; Wang, Y.; Shen, M.; Zheng, Y.; Zhao, S.; Yang, L. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Liver Immunity and Therapy. Front. Immunol. 2022, 13, 833878. [Google Scholar] [CrossRef]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J. Transl. Med. 2020, 18, 449. [Google Scholar] [CrossRef]
- Dong, L.; Wang, Y.; Zheng, T.; Pu, Y.; Ma, Y.; Qi, X.; Zhang, W.; Xue, F.; Shan, Z.; Liu, J.; et al. Hypoxic hUCMSC-derived extracellular vesicles attenuate allergic airway inflammation and airway remodeling in chronic asthma mice. Stem Cell Res. Ther. 2021, 12, 4. [Google Scholar] [CrossRef]
- Dong, B.; Wang, C.; Zhang, J.; Zhang, J.; Gu, Y.; Guo, X.; Zuo, X.; Pan, H.; Hsu, A.C.; Wang, G.; et al. Exosomes from human umbilical cord mesenchymal stem cells attenuate the inflammation of severe steroid-resistant asthma by reshaping macrophage polarization. Stem Cell Res. Ther. 2021, 12, 204. [Google Scholar] [CrossRef]
- Abbaszadeh, H.; Ghorbani, F.; Abbaspour-Aghdam, S.; Kamrani, A.; Valizadeh, H.; Nadiri, M.; Sadeghi, A.; Shamsasenjan, K.; Jadidi-Niaragh, F.; Roshangar, L.; et al. Chronic obstructive pulmonary disease and asthma: Mesenchymal stem cells and their extracellular vesicles as potential therapeutic tools. Stem Cell Res. Ther. 2022, 13, 262. [Google Scholar] [CrossRef] [PubMed]
- Maremanda, K.P.; Sundar, I.K.; Rahman, I. Protective role of mesenchymal stem cells and mesenchymal stem cell-derived exosomes in cigarette smoke-induced mitochondrial dysfunction in mice. Toxicol. Appl. Pharmacol. 2019, 385, 114788. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Jin, L.; Lai, X.; Li, Y.; Sheng, L.; Xie, G.; Fang, J. Adverse events associated with oseltamivir and baloxavir marboxil in against influenza virus therapy: A pharmacovigilance study using the FAERS database. PLoS ONE 2024, 19, e0308998. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, L. Curcumin alleviates macrophage activation and lung inflammation induced by influenza virus infection through inhibiting the NF-κB signaling pathway. Influenza Other Respir. Viruses 2017, 11, 457–463. [Google Scholar] [CrossRef]
- Li, R.; Han, Q.; Li, X.; Liu, X.; Jiao, W. Natural Product-Derived Phytochemicals for Influenza A Virus (H1N1) Prevention and Treatment. Molecules 2024, 29, 2371. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.Y.; Wang, Y.; Feng, Q.; Sun, L.J.; Feng, Y.M.; Dong, Y.H.; Xu, C.X. Umbilical Cord Mesenchymal Stem Cells Could Reduce Lung Damage Caused by H1N1 Influenza Virus Infection. J. Med. Virol. 2025, 97, e70214. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, Y.; Souza-Moreira, L.; Wang, C.; Murray, A.B.P.; Salkhordeh, M.; Florian, M.; McIntyre, L.; Stewart, D.J.; Mei, S.H.J. Mesenchymal stem cells induce dynamic immunomodulation of airway and systemic immune cells in vivo but do not improve survival for mice with H1N1 virus-induced acute lung injury. Front. Bioeng. Biotechnol. 2023, 11, 1203387. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Zhang, X.; Chen, H.; Zhang, Q.; Zeng, Y.; Zheng, S.; Zou, J.; Zhao, Y.; Zheng, X.; et al. BMSC-Exosomes attenuate ALP dysfunction by restoring lysosomal function via the mTOR/TFEB Axis to reduce cerebral ischemia-reperfusion injury. Exp. Neurol. 2024, 376, 114726. [Google Scholar] [CrossRef]
- Sang, H.; Zhao, R.; Lai, G.; Deng, Z.; Zhuang, W.; Wu, M.; Wu, J. Bone marrow mesenchymal stem cell-derived exosomes attenuate the maturation of dendritic cells and reduce the rejection of allogeneic transplantation. Adv. Clin. Exp. Med. 2023, 32, 551–561. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Liu, M.; Zhao, J.; Xu, X.; Wei, D.; Chen, J. Mechanism of exosomes from adipose-derived mesenchymal stem cells on sepsis-induced acute lung injury by promoting TGF-β secretion in macrophages. Surgery 2023, 174, 1208–1219. [Google Scholar] [CrossRef]
- Chu, M.; Wang, H.; Bian, L.; Huang, J.; Wu, D.; Zhang, R.; Fei, F.; Chen, Y.; Xia, J. Nebulization Therapy with Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes for COVID-19 Pneumonia. Stem Cell Rev. Rep. 2022, 18, 2152–2163. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.; Geng, Z.; Bao, L.; Gao, S.; Sun, J.; Liu, X.; Yang, X.; Zhao, R.; Li, S.; et al. Geniposide attenuates influenza virus-induced pneumonia by regulating inflammatory cytokines production. Evidences to elucidate the followed pathway. Phytomedicine 2024, 135, 156018. [Google Scholar] [CrossRef]
- Gao, T.; Liu, J.; Huang, N.; Zhou, Y.; Li, C.; Chen, Y.; Hong, Z.; Deng, X.; Liang, X. Sangju Cold Granule exerts anti-viral and anti-inflammatory activities against influenza A virus in vitro and in vivo. J. Ethnopharmacol. 2024, 334, 118521. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.J.; Lu, Y.; Zhang, Y.Y.; Zhu, H.Y.; Tu, P.; Li, H.; Chen, D.F. Flavonoids from Houttuynia cordata attenuate H1N1-induced acute lung injury in mice via inhibition of influenza virus and Toll-like receptor signalling. Phytomedicine 2020, 67, 153150. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Qu, S.; Qin, M.; Huang, L.; Huang, Y.; Du, Y.; Yu, Z.; Fan, F.; Sun, J.; Li, Q.; et al. Immunomodulatory and antiviral effects of Lycium barbarum glycopeptide on influenza a virus infection. Microb. Pathog. 2023, 176, 106030. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeon, H.; Lötvall, J.; Cho, B.S. Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles in SARS-CoV-2 and H1N1 influenza-induced acute lung injury. J. Extracell. Vesicles 2024, 13, e12495. [Google Scholar] [CrossRef]
- Liu, P.; Yang, S.; Shao, X.; Li, C.; Wang, Z.; Dai, H.; Wang, C. Mesenchymal Stem Cells-Derived Exosomes Alleviate Acute Lung Injury by Inhibiting Alveolar Macrophage Pyroptosis. Stem Cells Transl. Med. 2024, 13, 371–386. [Google Scholar] [CrossRef]
- Mizuta, Y.; Akahoshi, T.; Guo, J.; Zhang, S.; Narahara, S.; Kawano, T.; Murata, M.; Tokuda, K.; Eto, M.; Hashizume, M.; et al. Exosomes from adipose tissue-derived mesenchymal stem cells ameliorate histone-induced acute lung injury by activating the PI3K/Akt pathway in endothelial cells. Stem Cell Res. Ther. 2020, 11, 508. [Google Scholar] [CrossRef]
- Deng, H.; Zhu, L.; Zhang, Y.; Zheng, L.; Hu, S.; Zhou, W.; Zhang, T.; Xu, W.; Chen, Y.; Zhou, H.; et al. Differential Lung Protective Capacity of Exosomes Derived from Human Adipose Tissue, Bone Marrow, and Umbilical Cord Mesenchymal Stem Cells in Sepsis-Induced Acute Lung Injury. Oxid. Med. Cell. Longev. 2022, 2022, 7837837. [Google Scholar] [CrossRef]
- Nagamura-Inoue, T.; He, H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J. Stem Cells 2014, 6, 195–202. [Google Scholar] [CrossRef]
- Meng, M.; Zhang, W.W.; Chen, S.F.; Wang, D.R.; Zhou, C.H. Therapeutic utility of human umbilical cord-derived mesenchymal stem cells-based approaches in pulmonary diseases: Recent advancements and prospects. World J. Stem Cells 2024, 16, 70–88. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.G.; Zhang, Q.J.; Zhou, J.R. Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev. Rep. 2011, 7, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, S.; Zou, J.; Wang, Y.; Ge, H.; Hui, Y.; Huang, S.; Li, W.; Na, W.; Huang, X.; et al. Comparison of efficacy of exosomes derived from human umbilical cord blood mesenchymal stem cells in treating mouse acute lung injury via different routes. Front. Pediatr. 2025, 13, 1560915. [Google Scholar] [CrossRef]
- Khatri, M.; Richardson, L.A.; Meulia, T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res. Ther. 2018, 9, 17. [Google Scholar] [CrossRef]
- Buchweitz, J.P.; Harkema, J.R.; Kaminski, N.E. Time-dependent airway epithelial and inflammatory cell responses induced by influenza virus A/PR/8/34 in C57BL/6 mice. Toxicol. Pathol. 2007, 35, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Wang, L.; Yang, S.; Liang, Y.; Zhang, Y.; Pan, X.; Li, J. Rosmarinic acid treatment protects against lethal H1N1 virus-mediated inflammation and lung injury by promoting activation of the h-PGDS-PGD(2)-HO-1 signal axis. Chin. Med. 2023, 18, 139. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Qiu, Z.; Huang, L.; Yang, S.; Li, J.; Li, K.; Liang, Y.; Liu, X.; Chen, Z.; et al. Insights into the mechanism of action of pterostilbene against influenza A virus-induced acute lung injury. Phytomedicine 2024, 129, 155534. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.P.; Lorenzo, M.E.; Jian, W.; Klein, S.L. Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog. 2011, 7, e1002149. [Google Scholar] [CrossRef]
- Anggraeni, N.; Vuong, C.K.; Silvia, P.; Fukushige, M.; Yamashita, T.; Obata-Yasuoka, M.; Hamada, H.; Ohneda, O. Mesenchymal stem cell-derived extracellular vesicles reduce inflammatory responses to SARS-CoV-2 and Influenza viral proteins via miR-146a/NF-κB pathway. Sci. Rep. 2024, 14, 26649. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Zou, Y.; Wang, B.; Li, Y.; Zhu, J.; Luo, Y.; Li, J. Ginsenoside Rg1 improves lipopolysaccharide-induced acute lung injury by inhibiting inflammatory responses and modulating infiltration of M2 macrophages. Int. Immunopharmacol. 2015, 28, 429–434. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, H.; Yu, X.; Dong, Y.; Bao, S.; Meng, X.; Zhao, J.; Dong, Z. Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviated the Inflammatory Response in Mice Infected with the Influenza Virus A (H1N1). Int. J. Mol. Sci. 2025, 26, 8839. https://doi.org/10.3390/ijms26188839
Xiao H, Yu X, Dong Y, Bao S, Meng X, Zhao J, Dong Z. Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviated the Inflammatory Response in Mice Infected with the Influenza Virus A (H1N1). International Journal of Molecular Sciences. 2025; 26(18):8839. https://doi.org/10.3390/ijms26188839
Chicago/Turabian StyleXiao, Hui, Xiao Yu, Yiding Dong, Shilong Bao, Xiaoting Meng, Jia Zhao, and Zhiyong Dong. 2025. "Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviated the Inflammatory Response in Mice Infected with the Influenza Virus A (H1N1)" International Journal of Molecular Sciences 26, no. 18: 8839. https://doi.org/10.3390/ijms26188839
APA StyleXiao, H., Yu, X., Dong, Y., Bao, S., Meng, X., Zhao, J., & Dong, Z. (2025). Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Alleviated the Inflammatory Response in Mice Infected with the Influenza Virus A (H1N1). International Journal of Molecular Sciences, 26(18), 8839. https://doi.org/10.3390/ijms26188839



