Synergistic Crosstalk of PACAP and Notch Signaling Pathways in Bone Development
Abstract
1. Introduction
2. Results
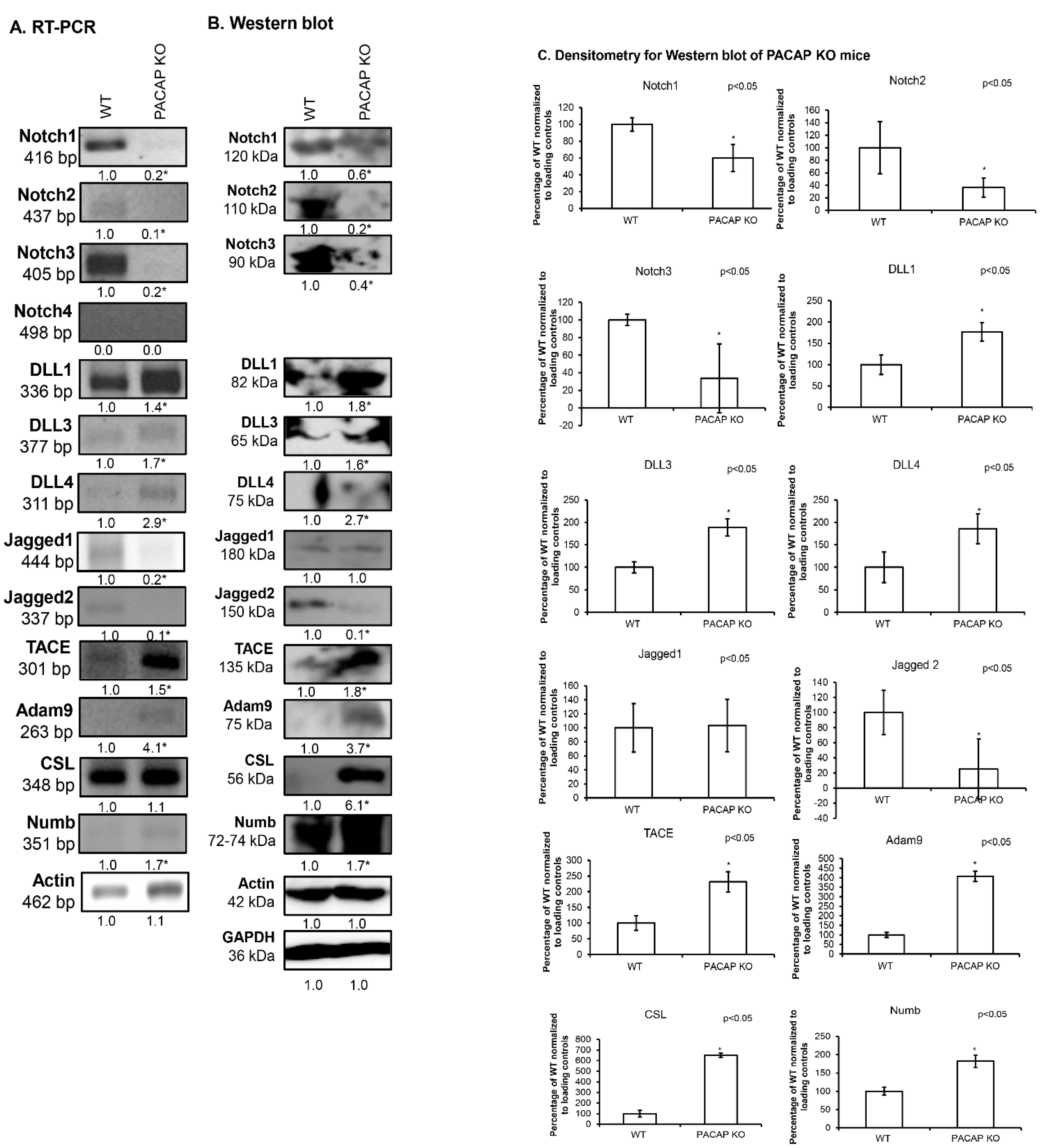
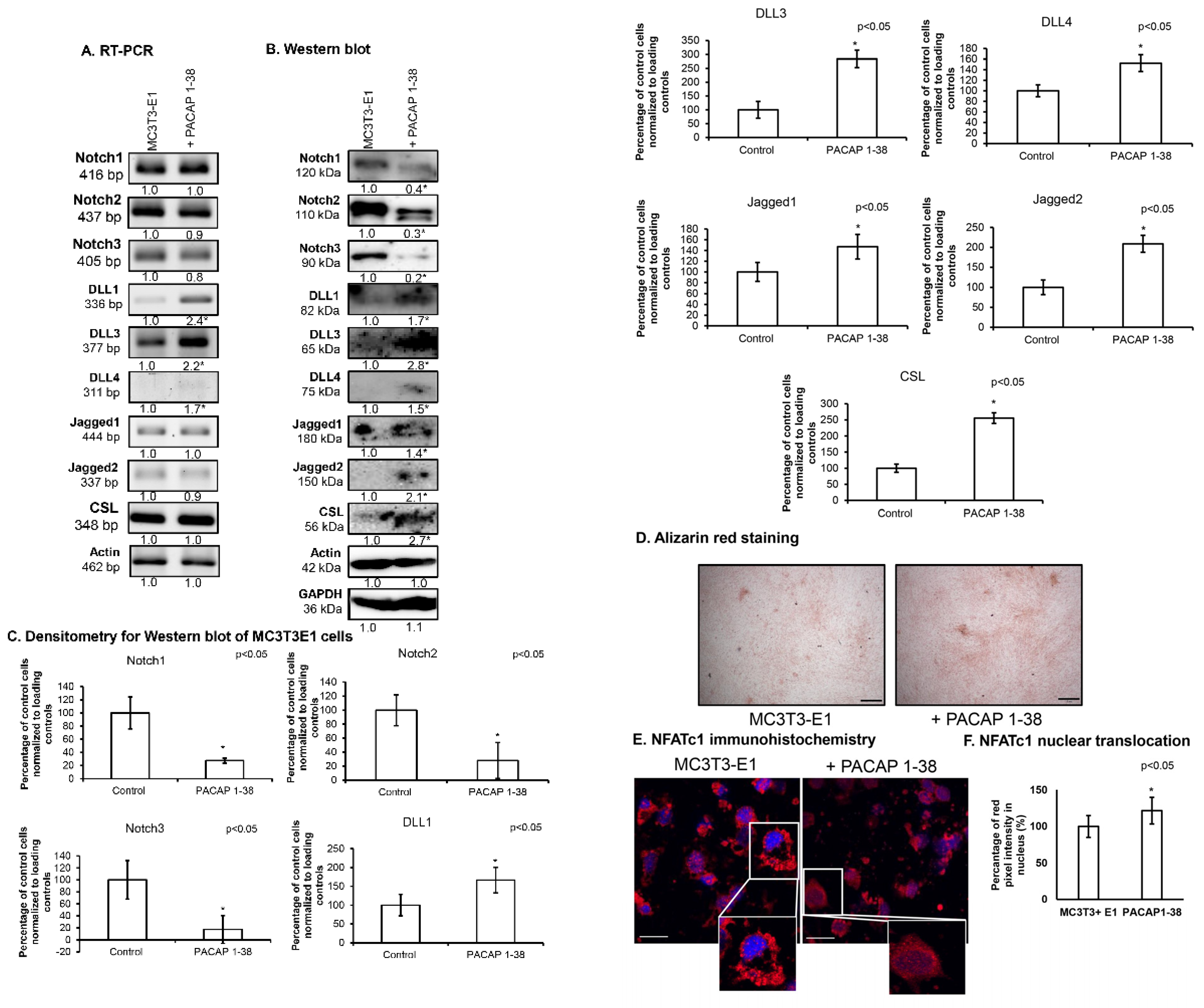
2.1. Notch Signalling Alterations in Femurs of PACAP-Gene-Deficient Mice
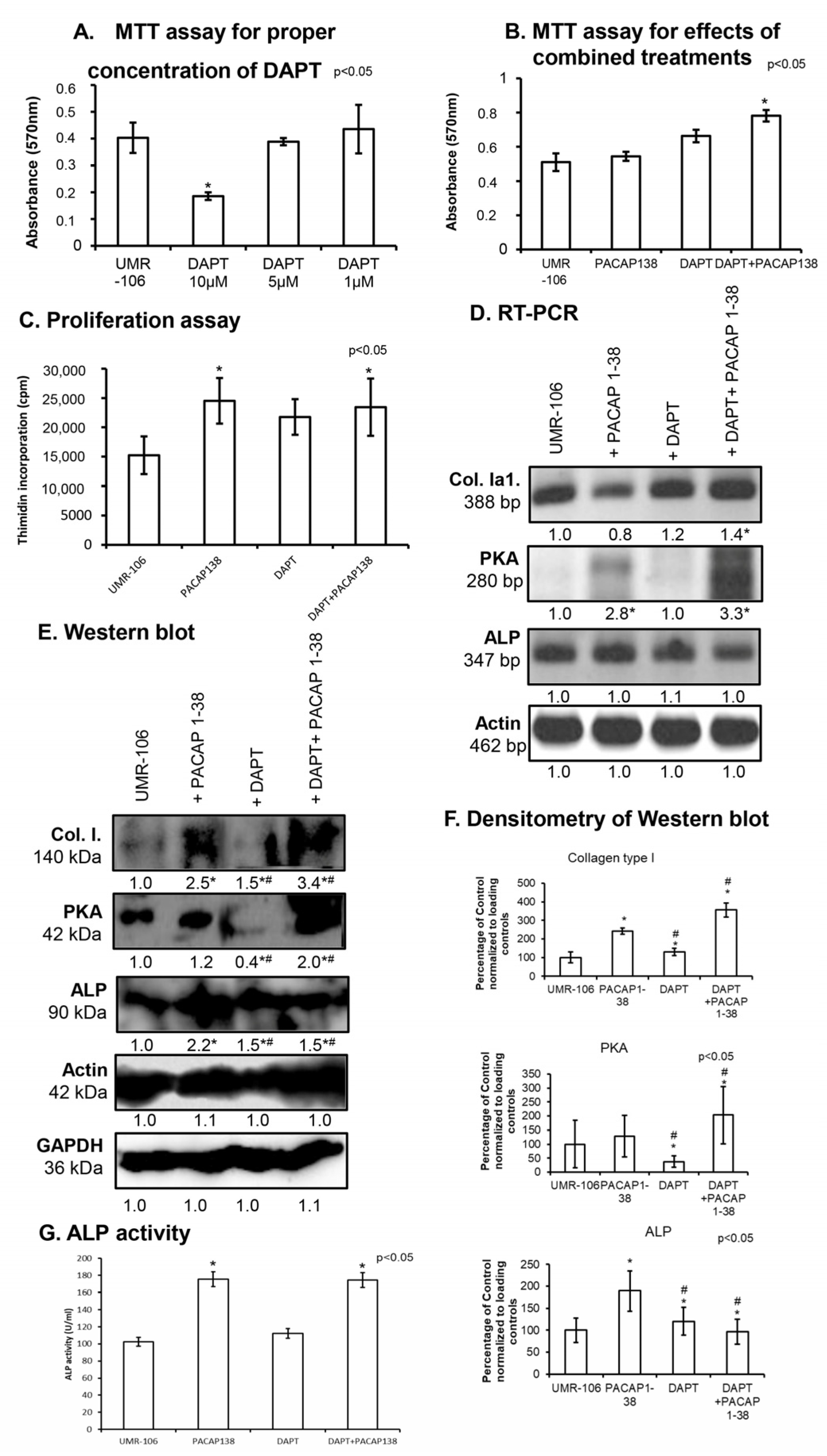
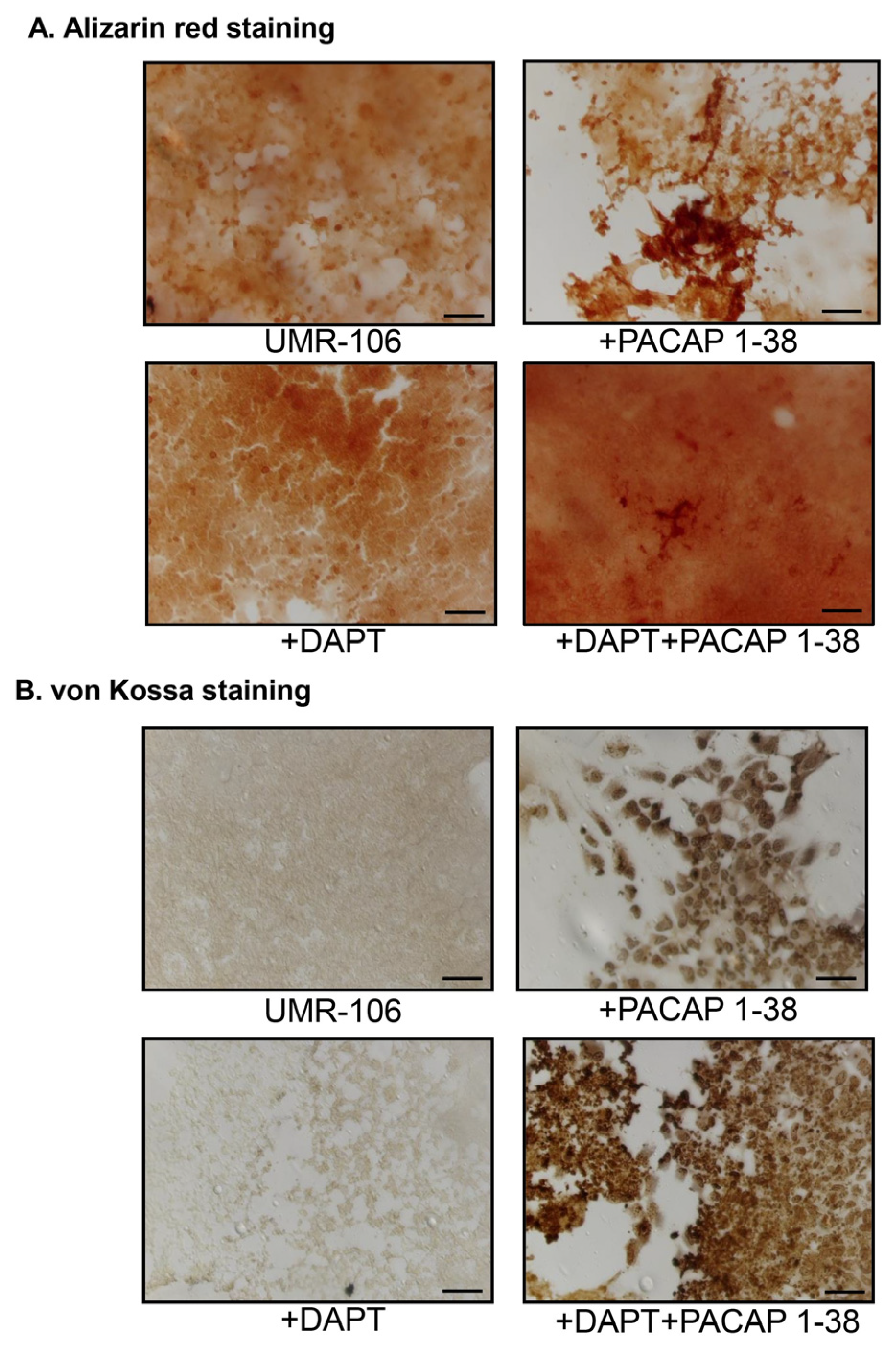
2.2. Inhibition of Notch Signalling Is Compensated by PACAP 1-38 in Osteoblastic Cell Line
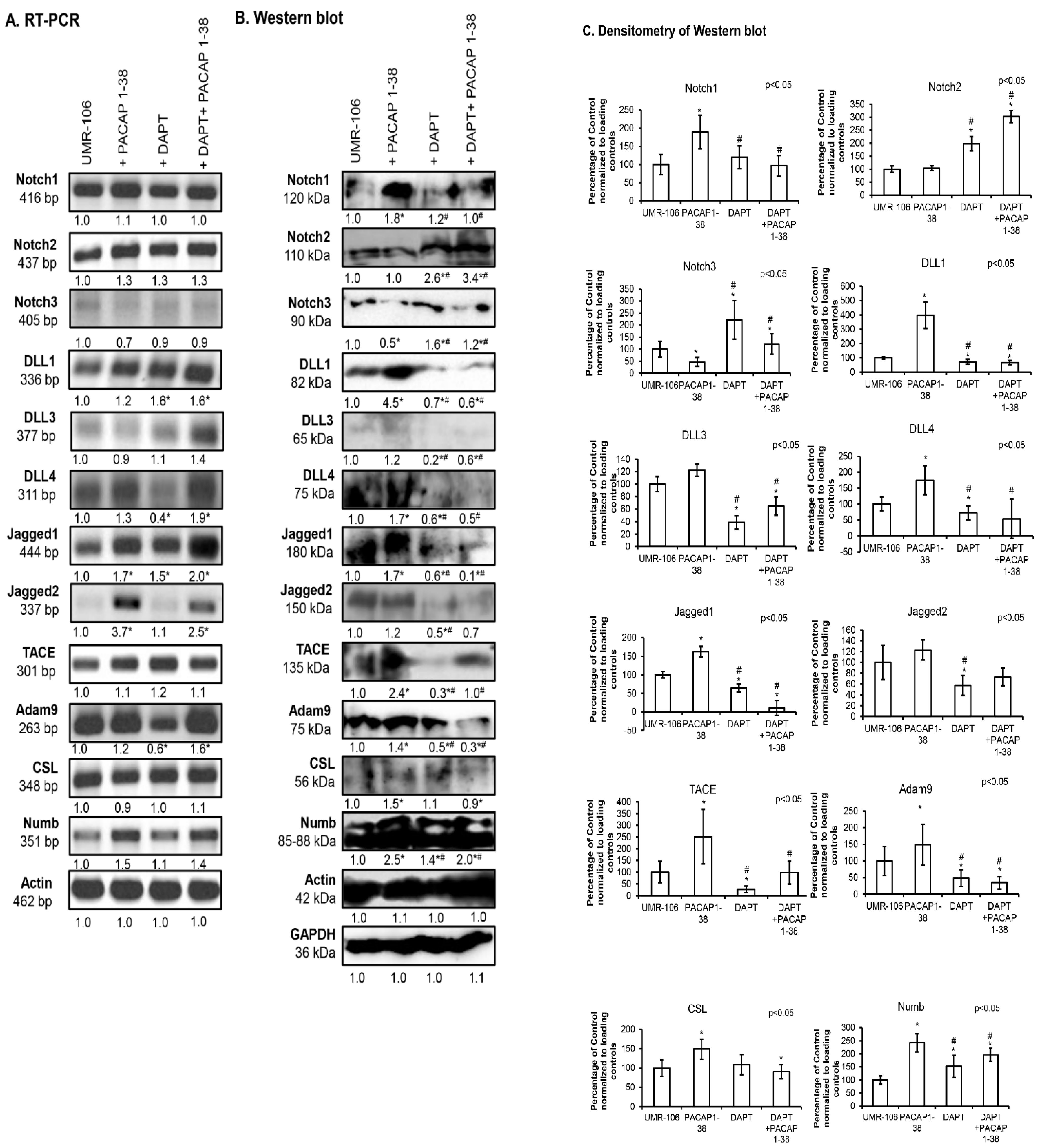
2.3. Notch Signalling Showed Altered Expression After the Addition of PACAP 1-38 in Osteoblast Cell Lines
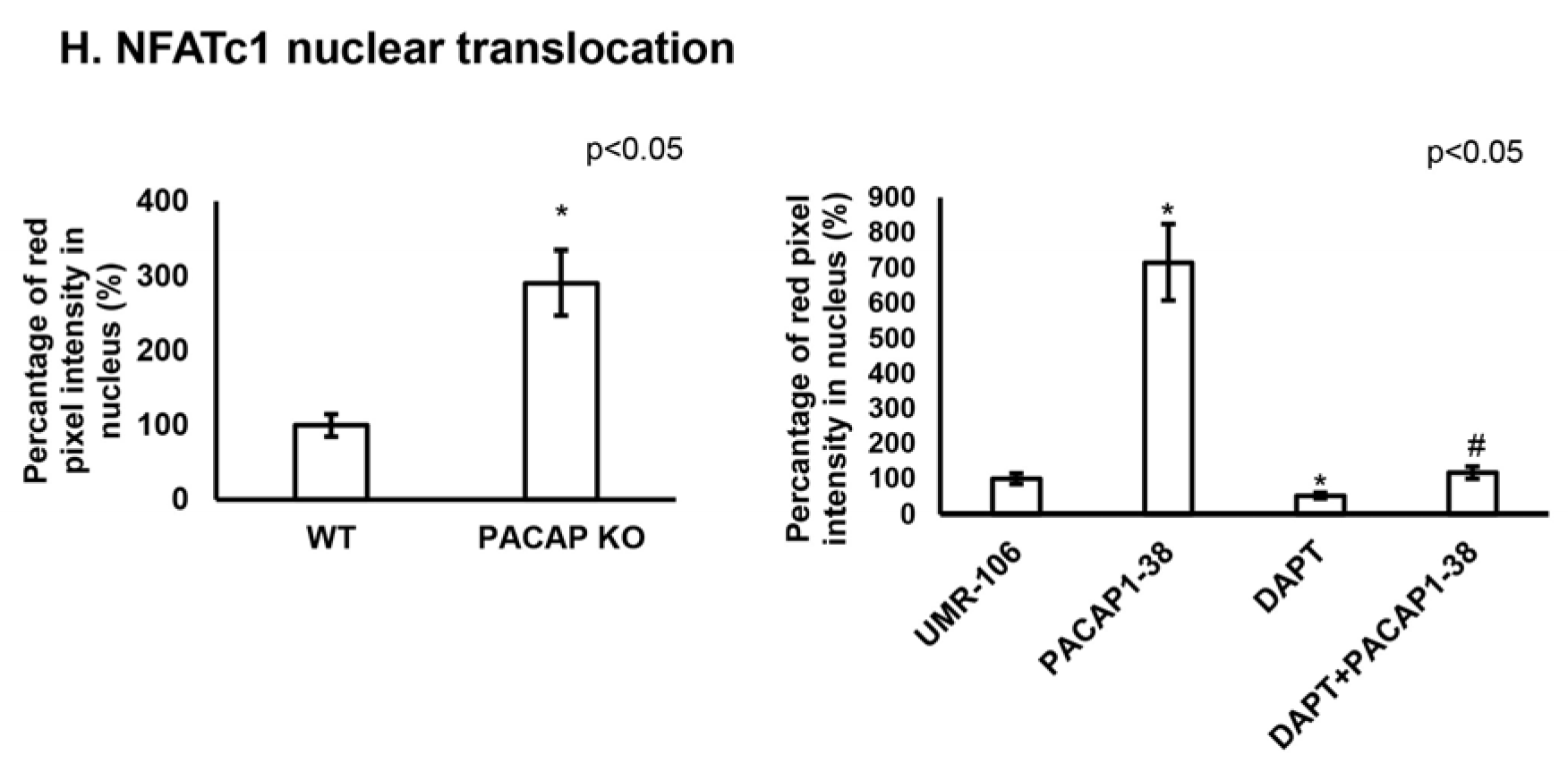
2.4. Altered NFATc1 Expression in the Presence of PACAP 1-38 and DAPT
2.5. PACAP 1-38 Alters Notch Signalisation in the Intramembranous Ossification Model
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Culture
4.3. PACAP 1-38 and DAPT Treatments
4.4. Determination of Non-Toxic Concentrations of DAPT and PACAP
4.5. Staining for Light Microscopy
4.6. Proliferation and Mitochondrial Activity Assessment
4.7. RT-PCR Analysis
4.8. Western Blot Analysis
4.9. Immunocytochemistry
4.10. ALP Activity Assay
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAM9 | a disintegrin and metalloproteinase 9 |
| ALP | alkaline phosphatase |
| BMP | bone morphogenetic protein |
| BSA | bovine serum albumin |
| cAMP | cyclic adenosine monophosphate |
| CSL | CBF1 humans/Su (H) Drosophila/LAG1Caenorhabditis elegans transcription factor |
| CREB | cAMP response element-binding protein |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DAPT | N-[N-(3,5-Difluorophenacetyl-L-alanyl)]-S-phenylglycine t-butyl ester |
| DLL | Delta-like ligands |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| dNTP | deoxynucleotide triphosphate |
| ECM | extracellular matrix |
| EDTA | ethylene diamine tetra-acetic acid |
| EGTA | ethylene glycol-bis(β-aminoethyl ether)-N,N,N’,N’-tetra acetic acid |
| FBS | fetal bovine serum |
| FGF | fibroblast growth factor |
| HH | hedgehog |
| KO | knock out |
| NA | numerical aperture |
| NICD | Notch intracellular domain |
| NFATc1 | nuclear factor of activated T-cells c1 |
| Notch | neurogenic locus notch homolog protein |
| NUMB | endocytic adaptor protein |
| PAC1 | pituitary adenylate cyclase-activating polypeptide type I receptor |
| PACAP | pituitary adenylate cyclase activating polypeptide |
| PBS | phosphate buffered saline |
| PBST | phosphate buffered saline supplemented with 1% Tween-20 |
| PP2B | protein phosphatase 2B |
| PKA | protein kinase A |
| PMSF | phenylmethylsulfonyl fluoride |
| RT-PCR | reverse transcription followed by polymerase chain reaction |
| Runx2 | Runt-related transcription factor 2 |
| Smad | small worm phenotype and mothers against decapentaplegic |
| SDS-PAGE | sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| TACE | tumor necrosis factor-alpha converting enzyme |
| WNT | wingless int1 |
| WT | wild type |
References
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. 3), S131–S139. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Mezour, M.A.; Badran, Z.; Tamimi, F. Extracellular Matrices for Bone Regeneration: A Literature Review. Tissue Eng. Part A 2017, 23, 1436–1451. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, H.P.; Binkley, D.M.; Luo, L.; Grandfield, K. A search for apatite crystals in the gap zone of collagen fibrils in bone using dark-field illumination. Bone 2020, 135, 115304. [Google Scholar] [CrossRef]
- Rolian, C. Endochondral ossification and the evolution of limb proportions. Wiley Interdiscip. Rev. Dev. Biol. 2020, 9, e373. [Google Scholar] [CrossRef] [PubMed]
- Khallaf, F.G.; Kehinde, E.O.; Mostafa, A. Growth factors and cytokines in patients with long bone fractures and associated spinal cord injury. J. Orthop. 2016, 13, 69–75. [Google Scholar] [CrossRef]
- Berendsen, A.D.; Olsen, B.R. Bone development. Bone 2015, 80, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Ponzetti, M.; Rucci, N. Osteoblast Differentiation and Signaling: Established Concepts and Emerging Topics. Int. J. Mol. Sci. 2021, 22, 6651. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C.; Li, Y.P. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef]
- Tan, Z.; Ding, N.; Lu, H.; Kessler, J.A.; Kan, L. Wnt signaling in physiological and pathological bone formation. Histol. Histopathol. 2019, 34, 303–312. [Google Scholar] [CrossRef]
- Su, N.; Du, X.; Chen, L. FGF signaling: Its role in bone development and human skeleton diseases. Front. Biosci. 2008, 13, 2842–2865. [Google Scholar] [CrossRef]
- Yang, J.; Andre, P.; Ye, L.; Yang, Y.Z. The Hedgehog signalling pathway in bone formation. Int. J. Oral Sci. 2015, 7, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Ohta, Y.; Nakao, Y.; Manaka, T.; Nakamura, H.; Takaoka, K. Epinephrine accelerates osteoblastic differentiation by enhancing bone morphogenetic protein signaling through a cAMP/protein kinase A signaling pathway. Bone 2010, 47, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, L.; Dong, Q.; Wang, Y.; Feng, Q.; Ou, X.; Zhou, P.; He, T.; Luo, J. Activation of PKA/CREB Signaling is Involved in BMP9-Induced Osteogenic Differentiation of Mesenchymal Stem Cells. Cell. Physiol. Biochem. 2015, 37, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Toth, D.; Szabo, E.; Tamas, A.; Juhasz, T.; Horvath, G.; Fabian, E.; Opper, B.; Szabo, D.; Maugeri, G.; D’Amico, A.G.; et al. Protective Effects of PACAP in Peripheral Organs. Front. Endocrinol. 2020, 11, 377. [Google Scholar] [CrossRef]
- Jozsa, G.; Szegeczki, V.; Palfi, A.; Kiss, T.; Helyes, Z.; Fulop, B.; Cserhati, C.; Daroczi, L.; Tamas, A.; Zakany, R.; et al. Signalling Alterations in Bones of Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) Gene Deficient Mice. Int. J. Mol. Sci. 2018, 19, 2538. [Google Scholar] [CrossRef]
- Juhasz, T.; Matta, C.; Katona, E.; Somogyi, C.; Takacs, R.; Hajdu, T.; Helgadottir, S.L.; Fodor, J.; Csernoch, L.; Toth, G.; et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) signalling enhances osteogenesis in UMR-106 cell line. J. Mol. Neurosci. 2014, 54, 555–573. [Google Scholar] [CrossRef]
- Juhasz, T.; Szentleleky, E.; Somogyi, C.S.; Takacs, R.; Dobrosi, N.; Engler, M.; Tamas, A.; Reglodi, D.; Zakany, R. Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) Pathway Is Induced by Mechanical Load and Reduces the Activity of Hedgehog Signaling in Chondrogenic Micromass Cell Cultures. Int. J. Mol. Sci. 2015, 16, 17344–17367. [Google Scholar] [CrossRef]
- Szentleleky, E.; Szegeczki, V.; Karanyicz, E.; Hajdu, T.; Tamas, A.; Toth, G.; Zakany, R.; Reglodi, D.; Juhasz, T. Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) Reduces Oxidative and Mechanical Stress-Evoked Matrix Degradation in Chondrifying Cell Cultures. Int. J. Mol. Sci. 2019, 20, 168. [Google Scholar] [CrossRef]
- Delgado, M.; Abad, C.; Martinez, C.; Juarranz, M.G.; Leceta, J.; Ganea, D.; Gomariz, R.P. PACAP in immunity and inflammation. Ann. N. Y. Acad. Sci. 2003, 992, 141–157. [Google Scholar] [CrossRef]
- Yu, R.; Cui, Z.; Li, M.; Yang, Y.; Zhong, J. Dimer-dependent intrinsic/basal activity of the class B G protein-coupled receptor PAC1 promotes cellular anti-apoptotic activity through Wnt/beta-catenin pathways that are associated with dimer endocytosis. PLoS ONE 2014, 9, e113913. [Google Scholar] [CrossRef]
- Jozsa, G.; Fulop, B.D.; Kovacs, L.; Czibere, B.; Szegeczki, V.; Kiss, T.; Hajdu, T.; Tamas, A.; Helyes, Z.; Zakany, R.; et al. Lack of Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Disturbs Callus Formation. J. Mol. Neurosci. 2021, 71, 1543–1555. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Fu, Z.; Guan, J.; Liu, X.; Zhang, Q. The role of Notch signaling pathway in metabolic bone diseases. Biochem. Pharmacol. 2023, 207, 115377. [Google Scholar] [CrossRef] [PubMed]
- Ugarte, F.; Ryser, M.; Thieme, S.; Fierro, F.A.; Navratiel, K.; Bornhauser, M.; Brenner, S. Notch signaling enhances osteogenic differentiation while inhibiting adipogenesis in primary human bone marrow stromal cells. Exp. Hematol. 2009, 37, 867–875.e1. [Google Scholar] [CrossRef]
- Zanotti, S.; Canalis, E. Notch regulation of bone development and remodeling and related skeletal disorders. Calcif. Tissue Int. 2012, 90, 69–75. [Google Scholar] [CrossRef]
- Takizawa, T.; Ochiai, W.; Nakashima, K.; Taga, T. Enhanced gene activation by Notch and BMP signaling cross-talk. Nucleic Acids Res. 2003, 31, 5723–5731. [Google Scholar] [CrossRef]
- Xu, Y.; Shu, B.; Tian, Y.; Chelly, M.; Morandi, M.M.; Barton, S.; Shang, X.; Dong, Y. Notch activation promotes osteoblast mineralization by inhibition of apoptosis. J. Cell. Physiol. 2018, 233, 6921–6928. [Google Scholar] [CrossRef]
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The roles of signaling pathways in bone repair and regeneration. J. Cell. Physiol. 2018, 233, 2937–2948. [Google Scholar] [CrossRef]
- Arya, P.N.; Saranya, I.; Selvamurugan, N. Crosstalk between Wnt and bone morphogenetic protein signaling during osteogenic differentiation. World J. Stem Cells 2024, 16, 102–113. [Google Scholar] [CrossRef]
- Ko, F.C.; Sumner, D.R. How faithfully does intramembranous bone regeneration recapitulate embryonic skeletal development? Dev. Dyn. 2021, 250, 377–392. [Google Scholar] [CrossRef]
- Yoshida, G.; Kawabata, T.; Takamatsu, H.; Saita, S.; Nakamura, S.; Nishikawa, K.; Fujiwara, M.; Enokidani, Y.; Yamamuro, T.; Tabata, K.; et al. Degradation of the NOTCH intracellular domain by elevated autophagy in osteoblasts promotes osteoblast differentiation and alleviates osteoporosis. Autophagy 2022, 18, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- Nowell, C.S.; Radtke, F. Notch as a tumour suppressor. Nat. Rev. Cancer 2017, 17, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Fulop, B.D.; Sandor, B.; Szentleleky, E.; Karanyicz, E.; Reglodi, D.; Gaszner, B.; Zakany, R.; Hashimoto, H.; Juhasz, T.; Tamas, A. Altered Notch Signaling in Developing Molar Teeth of Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP)-Deficient Mice. J. Mol. Neurosci. 2019, 68, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Kopan, R.; Ilagan, M.X. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell 2009, 137, 216–233. [Google Scholar] [CrossRef]
- Au, E.; Roskams, A.J. Olfactory ensheathing cells of the lamina propria in vivo and in vitro. Glia 2003, 41, 224–236. [Google Scholar] [CrossRef]
- Brifault, C.; Gras, M.; Liot, D.; May, V.; Vaudry, D.; Wurtz, O. Delayed pituitary adenylate cyclase-activating polypeptide delivery after brain stroke improves functional recovery by inducing M2 microglia/macrophage polarization. Stroke 2015, 46, 520–528. [Google Scholar] [CrossRef]
- Reglodi, D.; Vaczy, A.; Rubio-Beltran, E.; MaassenVanDenBrink, A. Protective effects of PACAP in ischemia. J. Headache Pain 2018, 19, 19. [Google Scholar] [CrossRef]
- Mustafa, T. Pituitary adenylate cyclase-activating polypeptide (PACAP): A master regulator in central and peripheral stress responses. Adv. Pharmacol. 2013, 68, 445–457. [Google Scholar] [CrossRef]
- Holland, P.R.; Barloese, M.; Fahrenkrug, J. PACAP in hypothalamic regulation of sleep and circadian rhythm: Importance for headache. J. Headache Pain 2018, 19, 20. [Google Scholar] [CrossRef]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef]
- Juhasz, T.; Helgadottir, S.L.; Tamas, A.; Reglodi, D.; Zakany, R. PACAP and VIP signaling in chondrogenesis and osteogenesis. Peptides 2015, 66, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, B.M.; Jafari, A.; Zaher, W.; Qiu, W.; Kassem, M. Skeletal (stromal) stem cells: An update on intracellular signaling pathways controlling osteoblast differentiation. Bone 2015, 70, 28–36. [Google Scholar] [CrossRef]
- Kovacs, C.S.; Chik, C.L.; Li, B.; Karpinski, E.; Ho, A.K. Pituitary adenylate cyclase-activating peptide stimulates cyclic AMP accumulation in UMR 106 osteoblast-like cells. J. Endocrinol. 1996, 149, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.J.; Li, X.G.; Wang, X.Q. Notch Signaling in Mammalian Intestinal Stem Cells: Determining Cell Fate and Maintaining Homeostasis. Curr. Stem Cell Res. Ther. 2019, 14, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Yang, J.; Zheng, Y.; Yao, L.; Peng, X.; Chen, Y.; Yang, C. The Role of the Notch Signaling Pathway in the Differentiation of Human Umbilical Cord-Derived Mesenchymal Stem Cells. Front. Biosci. (Landmark Ed.) 2024, 29, 74. [Google Scholar] [CrossRef]
- Siebel, C.; Lendahl, U. Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiol. Rev. 2017, 97, 1235–1294. [Google Scholar] [CrossRef]
- Han, L.; Wang, B.; Wang, R.; Gong, S.; Chen, G.; Xu, W. The shift in the balance between osteoblastogenesis and adipogenesis of mesenchymal stem cells mediated by glucocorticoid receptor. Stem Cell Res. Ther. 2019, 10, 377. [Google Scholar] [CrossRef]
- Luo, Z.; Shang, X.; Zhang, H.; Wang, G.; Massey, P.A.; Barton, S.R.; Kevil, C.G.; Dong, Y. Notch Signaling in Osteogenesis, Osteoclastogenesis, and Angiogenesis. Am. J. Pathol. 2019, 189, 1495–1500. [Google Scholar] [CrossRef]
- Jung, S.R.; Song, N.J.; Yang, D.K.; Cho, Y.J.; Kim, B.J.; Hong, J.W.; Yun, U.J.; Jo, D.G.; Lee, Y.M.; Choi, S.Y.; et al. Silk proteins stimulate osteoblast differentiation by suppressing the Notch signaling pathway in mesenchymal stem cells. Nutr. Res. 2013, 33, 162–170. [Google Scholar] [CrossRef]
- Xiao, D.; Bi, R.; Liu, X.; Mei, J.; Jiang, N.; Zhu, S. Notch Signaling Regulates MMP-13 Expression via Runx2 in Chondrocytes. Sci. Rep. 2019, 9, 15596. [Google Scholar] [CrossRef]
- Liu, P.; Ping, Y.; Ma, M.; Zhang, D.; Liu, C.; Zaidi, S.; Gao, S.; Ji, Y.; Lou, F.; Yu, F.; et al. Anabolic actions of Notch on mature bone. Proc. Natl. Acad. Sci. USA 2016, 113, E2152–E2161. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Ke, Y.; Gao, S. Intermittent activation of notch signaling promotes bone formation. Am. J. Transl. Res. 2017, 9, 2933–2944. [Google Scholar] [PubMed]
- Canalis, E. Notch in skeletal physiology and disease. Osteoporos. Int. 2018, 29, 2611–2621. [Google Scholar] [CrossRef]
- Lee, H.; Joung, J.G.; Shin, H.T.; Kim, D.H.; Kim, Y.; Kim, H.; Kwon, O.J.; Shim, Y.M.; Lee, H.Y.; Lee, K.S.; et al. Genomic alterations of ground-glass nodular lung adenocarcinoma. Sci. Rep. 2018, 8, 7691. [Google Scholar] [CrossRef]
- Wang, H.; Zang, C.; Liu, X.S.; Aster, J.C. The role of Notch receptors in transcriptional regulation. J. Cell. Physiol. 2015, 230, 982–988. [Google Scholar] [CrossRef]
- Bigas, A.; Guiu, J.; Gama-Norton, L. Notch and Wnt signaling in the emergence of hematopoietic stem cells. Blood Cells Mol. Dis. 2013, 51, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Marklund, U.; Hansson, E.M.; Sundstrom, E.; de Angelis, M.H.; Przemeck, G.K.; Lendahl, U.; Muhr, J.; Ericson, J. Domain-specific control of neurogenesis achieved through patterned regulation of Notch ligand expression. Development 2010, 137, 437–445. [Google Scholar] [CrossRef]
- Engler, A.; Zhang, R.; Taylor, V. Notch and Neurogenesis. Adv. Exp. Med. Biol. 2018, 1066, 223–234. [Google Scholar] [CrossRef]
- Mutyaba, P.L.; Belkin, N.S.; Lopas, L.; Gray, C.F.; Dopkin, D.; Hankenson, K.D.; Ahn, J. Notch signaling in mesenchymal stem cells harvested from geriatric mice. J. Orthop. Trauma 2014, 28 (Suppl. 1), S20–S23. [Google Scholar] [CrossRef]
- Ballhause, T.M.; Jiang, S.; Baranowsky, A.; Brandt, S.; Mertens, P.R.; Frosch, K.H.; Yorgan, T.; Keller, J. Relevance of Notch Signaling for Bone Metabolism and Regeneration. Int. J. Mol. Sci. 2021, 22, 1325. [Google Scholar] [CrossRef]
- Ho, D.M.; Artavanis-Tsakonas, S. The Notch-Mediated Proliferation Circuitry. Curr. Top. Dev. Biol. 2016, 116, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Sen, A.; Artavanis-Tsakonas, S. Notch signaling at a glance. J. Cell Sci. 2013, 126, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Hosseini-Alghaderi, S.; Woodcock, S.A.; Baron, M. Alternative mechanisms of Notch activation by partitioning into distinct endosomal domains. J. Cell Biol. 2024, 223, e202211041. [Google Scholar] [CrossRef] [PubMed]
- Zanotti, S.; Canalis, E. Notch suppresses nuclear factor of activated T cells (NFAT) transactivation and Nfatc1 expression in chondrocytes. Endocrinology 2013, 154, 762–772. [Google Scholar] [CrossRef]
- Zanotti, S.; Smerdel-Ramoya, A.; Canalis, E. Reciprocal regulation of Notch and nuclear factor of activated T-cells (NFAT) c1 transactivation in osteoblasts. J. Biol. Chem. 2011, 286, 4576–4588. [Google Scholar] [CrossRef]
- Hagen, B.M.; Bayguinov, O.; Sanders, K.M. VIP and PACAP regulate localized Ca2+ transients via cAMP-dependent mechanism. Am. J. Physiol. Cell Physiol. 2006, 291, C375–C385. [Google Scholar] [CrossRef]
- Delgado, M.; Ganea, D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit expression of Fas ligand in activated T lymphocytes by regulating c-Myc, NF-kappa B, NF-AT, and early growth factors 2/3. J. Immunol. 2001, 166, 1028–1040. [Google Scholar] [CrossRef]
- Stern, P.H. The calcineurin-NFAT pathway and bone: Intriguing new findings. Mol. Interv. 2006, 6, 193–196. [Google Scholar] [CrossRef][Green Version]
- Juhasz, T.; Matta, C.; Katona, E.; Somogyi, C.; Takacs, R.; Gergely, P.; Csernoch, L.; Panyi, G.; Toth, G.; Reglodi, D.; et al. Pituitary adenylate cyclase activating polypeptide (PACAP) signalling exerts chondrogenesis promoting and protecting effects: Implication of calcineurin as a downstream target. PLoS ONE 2014, 9, e91541. [Google Scholar] [CrossRef]
- Giaimo, B.D.; Friedrich, T.; Ferrante, F.; Bartkuhn, M.; Borggrefe, T. Comprehensive genomic features indicative for Notch responsiveness. Nucleic Acids Res. 2024, 52, 5179–5194. [Google Scholar] [CrossRef]
- Pearl, J.I.; Ma, T.; Irani, A.R.; Huang, Z.; Robinson, W.H.; Smith, R.L.; Goodman, S.B. Role of the Toll-like receptor pathway in the recognition of orthopedic implant wear-debris particles. Biomaterials 2011, 32, 5535–5542. [Google Scholar] [CrossRef]
- Rotolo, J.A.; Maj, J.G.; Feldman, R.; Ren, D.; Haimovitz-Friedman, A.; Cordon-Cardo, C.; Cheng, E.H.; Kolesnick, R.; Fuks, Z. Bax and Bak do not exhibit functional redundancy in mediating radiation-induced endothelial apoptosis in the intestinal mucosa. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 804–815. [Google Scholar] [CrossRef]
- Heyza, J.R.; Mikhova, M.; Schmidt, J.C. Live cell single-molecule imaging to study DNA repair in human cells. DNA Repair 2023, 129, 103540. [Google Scholar] [CrossRef]
- Hashimoto, H.; Hashimoto, R.; Shintani, N.; Tanaka, K.; Yamamoto, A.; Hatanaka, M.; Guo, X.; Morita, Y.; Tanida, M.; Nagai, K.; et al. Depression-like behavior in the forced swimming test in PACAP-deficient mice: Amelioration by the atypical antipsychotic risperidone. J. Neurochem. 2009, 110, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Shintani, N.; Tanaka, K.; Mori, W.; Hirose, M.; Matsuda, T.; Sakaue, M.; Miyazaki, J.; Niwa, H.; Tashiro, F.; et al. Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP). Proc. Natl. Acad. Sci. USA 2001, 98, 13355–13360. [Google Scholar] [CrossRef] [PubMed]
- Forrest, S.M.; Ng, K.W.; Findlay, D.M.; Michelangeli, V.P.; Livesey, S.A.; Partridge, N.C.; Zajac, J.D.; Martin, T.J. Characterization of an osteoblast-like clonal cell line which responds to both parathyroid hormone and calcitonin. Calcif. Tissue Int. 1985, 37, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Izumiya, M.; Haniu, M.; Ueda, K.; Ishida, H.; Ma, C.; Ideta, H.; Sobajima, A.; Ueshiba, K.; Uemura, T.; Saito, N.; et al. Evaluation of MC3T3-E1 Cell Osteogenesis in Different Cell Culture Media. Int. J. Mol. Sci. 2021, 22, 7752. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, W.; Zhang, Z.; Chen, X.; Jin, H.; Jiang, N.; Xu, H. The bone-protective benefits of kaempferol combined with metformin by regulation of osteogenesis-angiogenesis coupling in OVX rats. Biomed. Pharmacother. 2024, 173, 116364. [Google Scholar] [CrossRef]
- Zou, W.; Chen, Q.X.; Sun, X.W.; Chi, Q.B.; Kuang, H.Y.; Yu, X.P.; Dai, X.H. Acupuncture inhibits Notch1 and Hes1 protein expression in the basal ganglia of rats with cerebral hemorrhage. Neural Regen. Res. 2015, 10, 457–462. [Google Scholar] [CrossRef]
- Zakany, R.; Szijgyarto, Z.; Matta, C.; Juhasz, T.; Csortos, C.; Szucs, K.; Czifra, G.; Biro, T.; Modis, L.; Gergely, P. Hydrogen peroxide inhibits formation of cartilage in chicken micromass cultures and decreases the activity of calcineurin: Implication of ERK1/2 and Sox9 pathways. Exp. Cell Res. 2005, 305, 190–199. [Google Scholar] [CrossRef]

| Gene | Primer | Nucleotide Sequence (5′→3′) | GenBank ID | Annealing Temperature | Amplimer Size (bp) |
|---|---|---|---|---|---|
| Alkaline phosphatase (Alpl) | sense | GAA GTC CGT GGG CAT CGT (474–491) | NM_013059 | 59 °C | 347 |
| antisense | CAG TGC GGT TCC AGA CAT AG (801–820) | ||||
| DLL1 (Dll1) | sense | GAA ACA CCA GCC TCC ACC T (2305–2323) | NM_001379042.1 | 53 °C | 336 |
| antisense | GGA ATC TCC CCA CCC CTA (2623–2640) | ||||
| DLL3 (Dll3) | sense | CCT GGT TTC CAA GGC TCT AA (1060–1079) | NM_007866.2 | 57 °C | 377 |
| antisense | ACA GCG AAC TCG CAT CTC A (1418–1436) | ||||
| DLL4 (Dll4) | sense | TTG CCC TTC AAT TTC ACC (673–690) | NM_019454.4 | 54 °C | 311 |
| antisense | CAG GAC AGG CTG CCA TCT (966–983) | ||||
| Jagged2 (Jag2) | sense | CCT CGT CGT CAT TCC CTT TC (395–411) | NM_001409685.1 | 57 °C | 337 |
| antisense | GCA TTC TTT GCC CAT CCA G (713–731) | ||||
| Jagged1 (Jag1) | sense | TCA GGC ATG ATA AAC CCT AGC (735–755) | NM_013822.5 | 56 °C | 444 |
| antisense | GGG CTG ATG AGT CCC ACA (1161–1178) | ||||
| Collagen type I (Col1a1) | sense | GGG CGA GTG CTG TGC TTT (348–365) | NM_007742.3 | 60 °C | 388 |
| antisense | GGG ACC CAT TGG ACC TGA A (717–735) | ||||
| Notch1 (notch1) | sense | GGA TCA CAT GGA CCG ATT G (6504–6522) | NM_008714.3 | 56 °C | 416 |
| antisense | TGG ATG GAG ACT GCT GGA A (6901–6919) | ||||
| Notch2 (notch2) | sense | GTA TCT CCA AGC CGT GTA TG (2791–2810) | NM_010928.2 | 55 °C | 437 |
| antisense | GCA GAA GGG ACC AGT GAA (3210–3227) | ||||
| Notch3 (notch3) | sense | GCA CCA GTG ATG GAA TAG GC (2308–2327) | NM_008716.3 | 56 °C | 405 |
| antisense | AGC GAG GAC CAG CAA AGC (2695–2712) | ||||
| Notch4 (notch4) | sense | GCC ACT CTT TAG CCA ACG C (3207–3225) | NM_010929.2 | 57 °C | 498 |
| antisense | CAT CGC AGG TCC CAT CAC (3687–3704) | ||||
| NFATc1 (Nfatc1) | sense | CCT GAC CAC CGA TAG CAC (973–990) | NM_001164109.1 | 52 °C | 325 |
| antisense | CTC GTA TGG ACC AGA ATG T (1279–1297) | ||||
| Adam9 (Adam9) | sense | TGA TTC GCT TAG CAA ACT (857–874) | NM_001270996.1 | 49 °C | 263 |
| antisense | GTG GCT CCT TGA ACA TAC (1102–1119) | ||||
| PKA (Prkaca) | sense | GCA AAG GCT ACA ACA AGG C (847–865) | NM_008854 | 53 °C | 280 |
| antisense | ATG GCA ATC CAG TCA ATC G (1109–1126) | ||||
| CSL (Csl) | sense | TGG AGC TTC CTG GAC AAT (1054–1071) | NM_027945.4 | 51 °C | 348 |
| antisense | AGG CTG GTG GAG TAA ATG (1384–1401) | ||||
| Numb (Numb) | sense | ATT CCG TGT CAC AAC TGC (597–614) | NM_001136075.3 | 51 °C | 351 |
| antisense | AAA TCG GTC TTC CTC TGC (930–947) | ||||
| TACE (Adam17) | sense | AAG TCT GCC TGG CTC ATC (1192–1209) | NM_001277266.1 | 51 °C | 301 |
| antisense | CCT CCT TGG TCC TCA TTT (1475–1492) | ||||
| Actin (Actb) | sense | GCC AAC CGT GAA AAG ATG A (419–437) | NM_001014970 | 48 °C | 462 |
| antisense | CAA GAA GGA AGG CTG GAA AA (861–880) |
| Antibody | Host Animal | Dilution | Distributor |
|---|---|---|---|
| Anti-Notch1 | rabbit, polyclonal | 1:500 | Cell Signaling, Danvers, MA, USA |
| Anti-Notch2 | rabbit, polyclonal | 1:500 | Cell Signaling, Danvers, MA, USA |
| Anti-Notch3 | rabbit, polyclonal | 1:500 | Cell Signaling, Danvers, MA, USA |
| Anti-Coll. I. | mouse, monoclonal | 1:1000 | Sigma-Aldrich, St. Louis, MO, USA |
| Anti-DLL1 | rabbit, polyclonal | 1:500 | Cell Signaling, Danvers, MA, USA |
| Anti-DLL3 | rabbit, polyclonal | 1:500 | Cell Signaling, Danvers, MA, USA |
| Anti-DLL4 | rabbit, polyclonal | 1:500 | Cell Signaling, Danvers, MA, USA |
| Anti-Jagged1 | rabbit, polyclonal | 1:500 | Cell Signaling, Danvers, MA, USA |
| Anti-Jagged2 | rabbit, polyclonal | 1:500 | Abcam, Cambridge, UK |
| Anti-ALP | rabbit, polyclonal | 1:500 | Abcam, Cambridge, UK |
| Anti-CSL | rabbit, polyclonal | 1:500 | Cell Signaling, Danvers, MA, USA |
| Anti-TACE | rabbit, polyclonal | 1:500 | Cell Signaling, Danvers, MA, USA |
| Anti-PKA | rabbit, polyclonal | 1:800 | Cell Signaling, Danvers, MA, USA |
| Anti-Numb | rabbit, polyclonal | 1:500 | Cell Signaling, Danvers, MA, USA |
| Anti-Adam9 | rabbit, polyclonal | 1:600 | Cell Signaling, Danvers, MA, USA |
| Anti-NFATc1 | mouse, monoclonal | 1:500 | Abcam, Cambridge, UK |
| Anti-Actin | mouse, monoclonal | 1:10,000 | Sigma-Aldrich, St. Louis, MO, USA |
| Anti-GAPDH | rabbit, polyclonal | 1:800 | Abcam, Cambridge, UK |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szegeczki, V.; Pálfi, A.; Fillér, C.; Hinnah, B.; Tóth, A.; Kovács, L.S.; Jüngling, A.; Zákány, R.; Reglődi, D.; Juhász, T. Synergistic Crosstalk of PACAP and Notch Signaling Pathways in Bone Development. Int. J. Mol. Sci. 2025, 26, 5088. https://doi.org/10.3390/ijms26115088
Szegeczki V, Pálfi A, Fillér C, Hinnah B, Tóth A, Kovács LS, Jüngling A, Zákány R, Reglődi D, Juhász T. Synergistic Crosstalk of PACAP and Notch Signaling Pathways in Bone Development. International Journal of Molecular Sciences. 2025; 26(11):5088. https://doi.org/10.3390/ijms26115088
Chicago/Turabian StyleSzegeczki, Vince, Andrea Pálfi, Csaba Fillér, Barbara Hinnah, Anna Tóth, Lili Sarolta Kovács, Adél Jüngling, Róza Zákány, Dóra Reglődi, and Tamás Juhász. 2025. "Synergistic Crosstalk of PACAP and Notch Signaling Pathways in Bone Development" International Journal of Molecular Sciences 26, no. 11: 5088. https://doi.org/10.3390/ijms26115088
APA StyleSzegeczki, V., Pálfi, A., Fillér, C., Hinnah, B., Tóth, A., Kovács, L. S., Jüngling, A., Zákány, R., Reglődi, D., & Juhász, T. (2025). Synergistic Crosstalk of PACAP and Notch Signaling Pathways in Bone Development. International Journal of Molecular Sciences, 26(11), 5088. https://doi.org/10.3390/ijms26115088






