Unravelling High Nuclear Genomic Similarity and Mitochondria Linked Epigenetic Divergence in SCNT Derived Buffalo Clones via Long-Read Nanopore Genome Sequencing
Abstract
1. Introduction
2. Results
2.1. DNA Methylation Mapping and Patterns
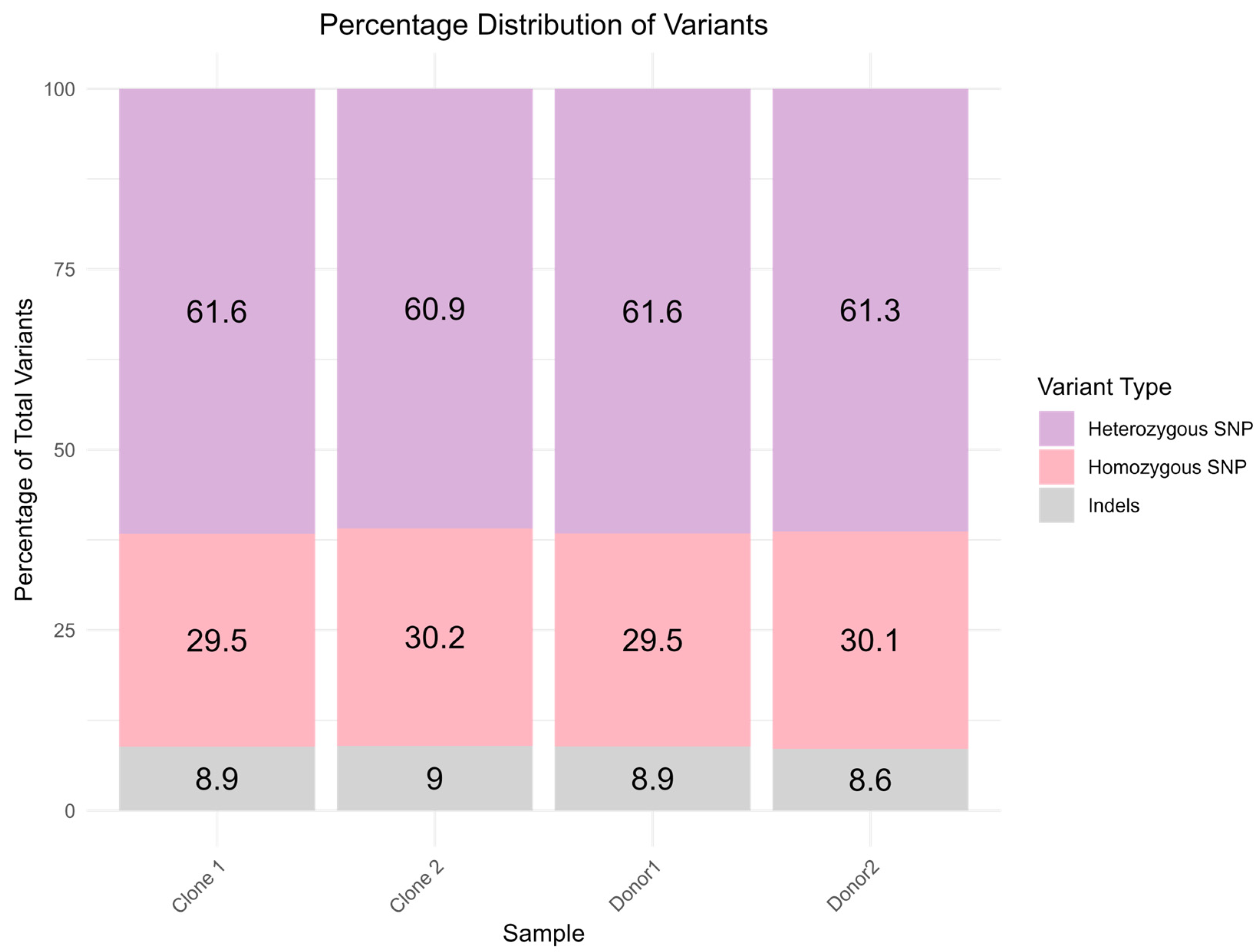
2.2. Variant Analysis
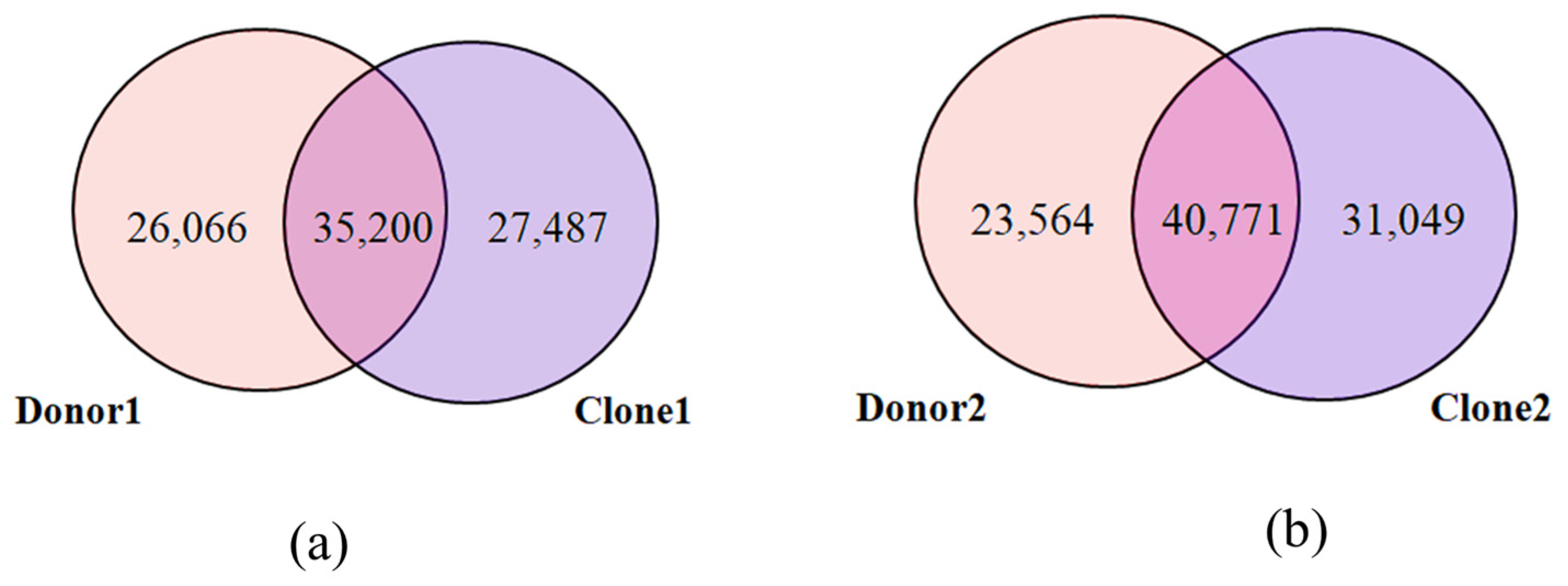
2.3. Structural Variant (SV) Analysis
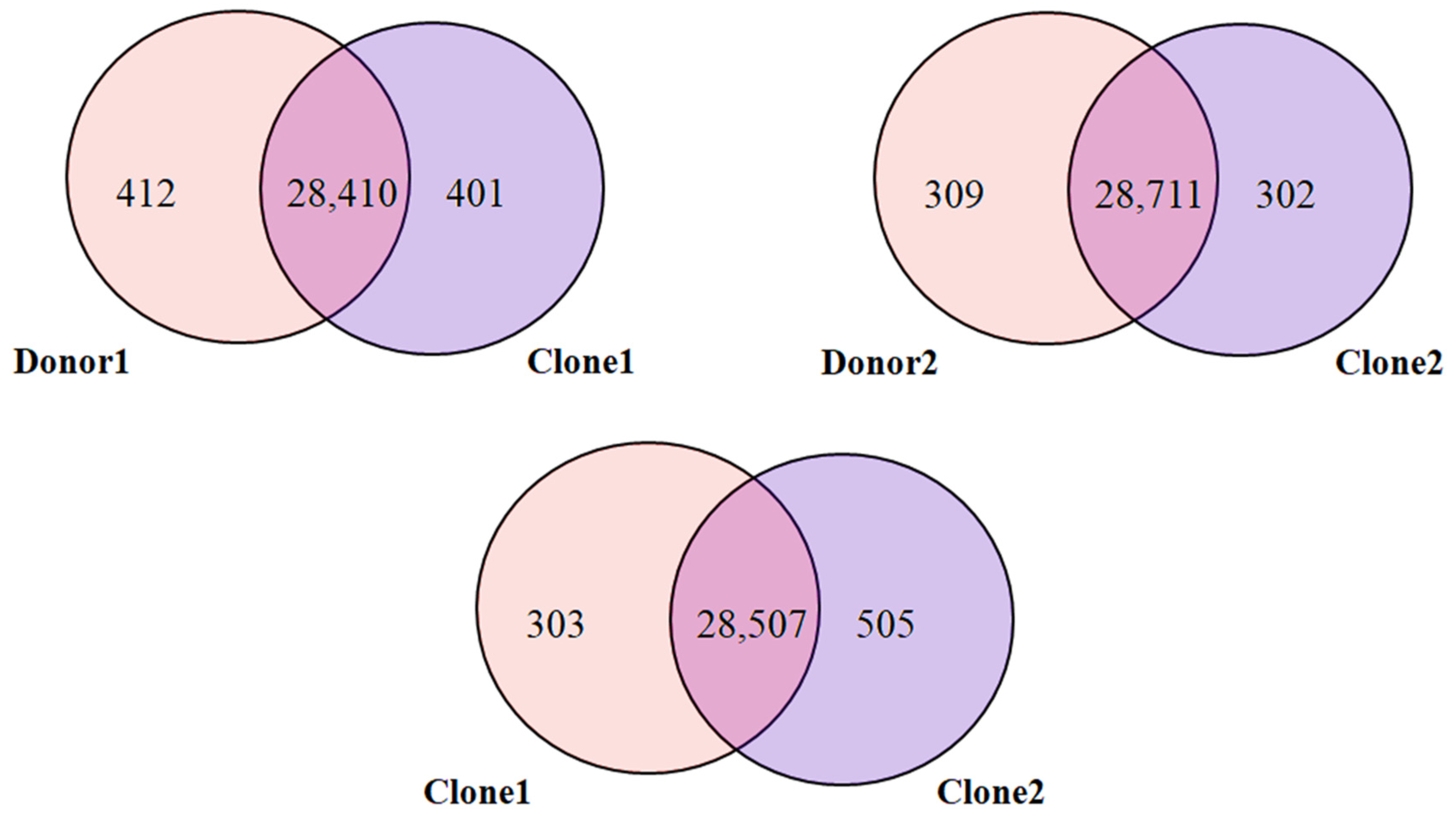
2.4. SV Overlap Between Donors and Clones
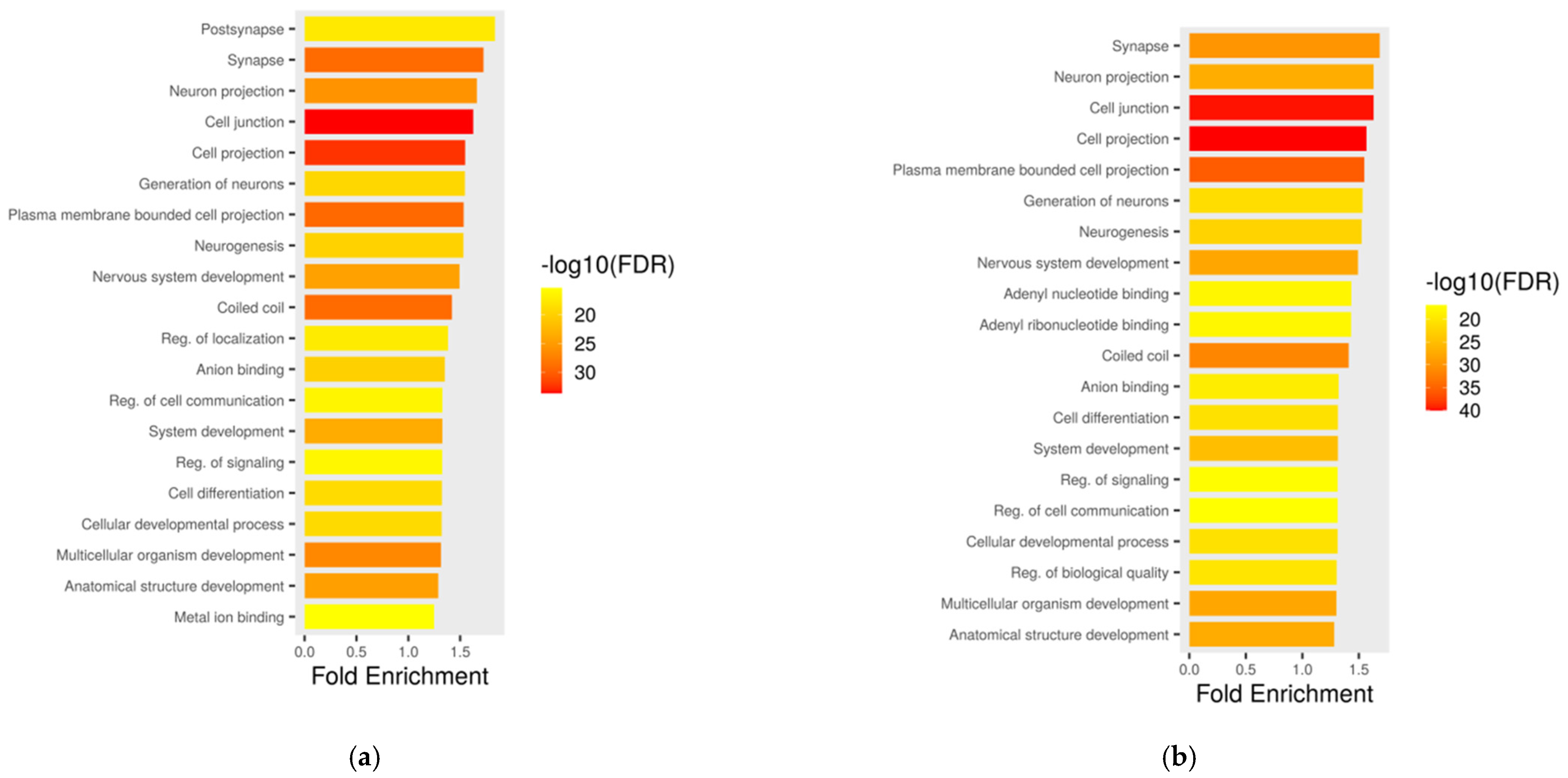
2.5. Functional Annotation of Affected Genomic Regions
2.6. Gene- and Protein-Level Similarity Between Donor–Clone Pairs
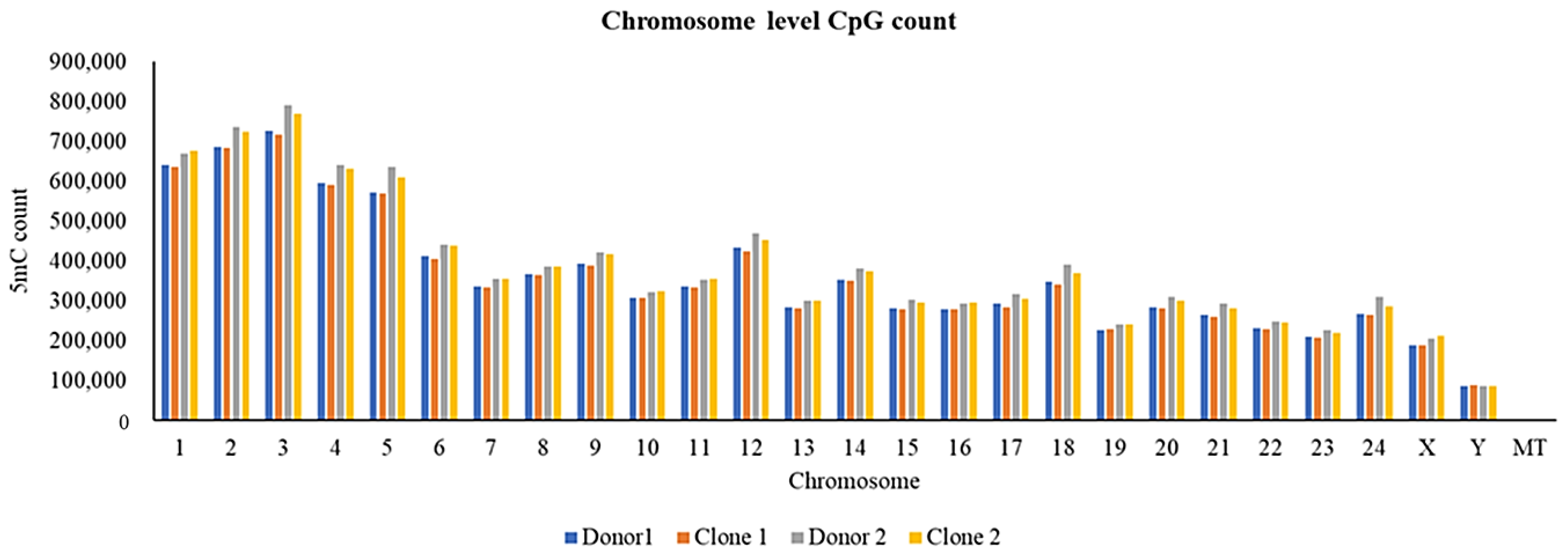
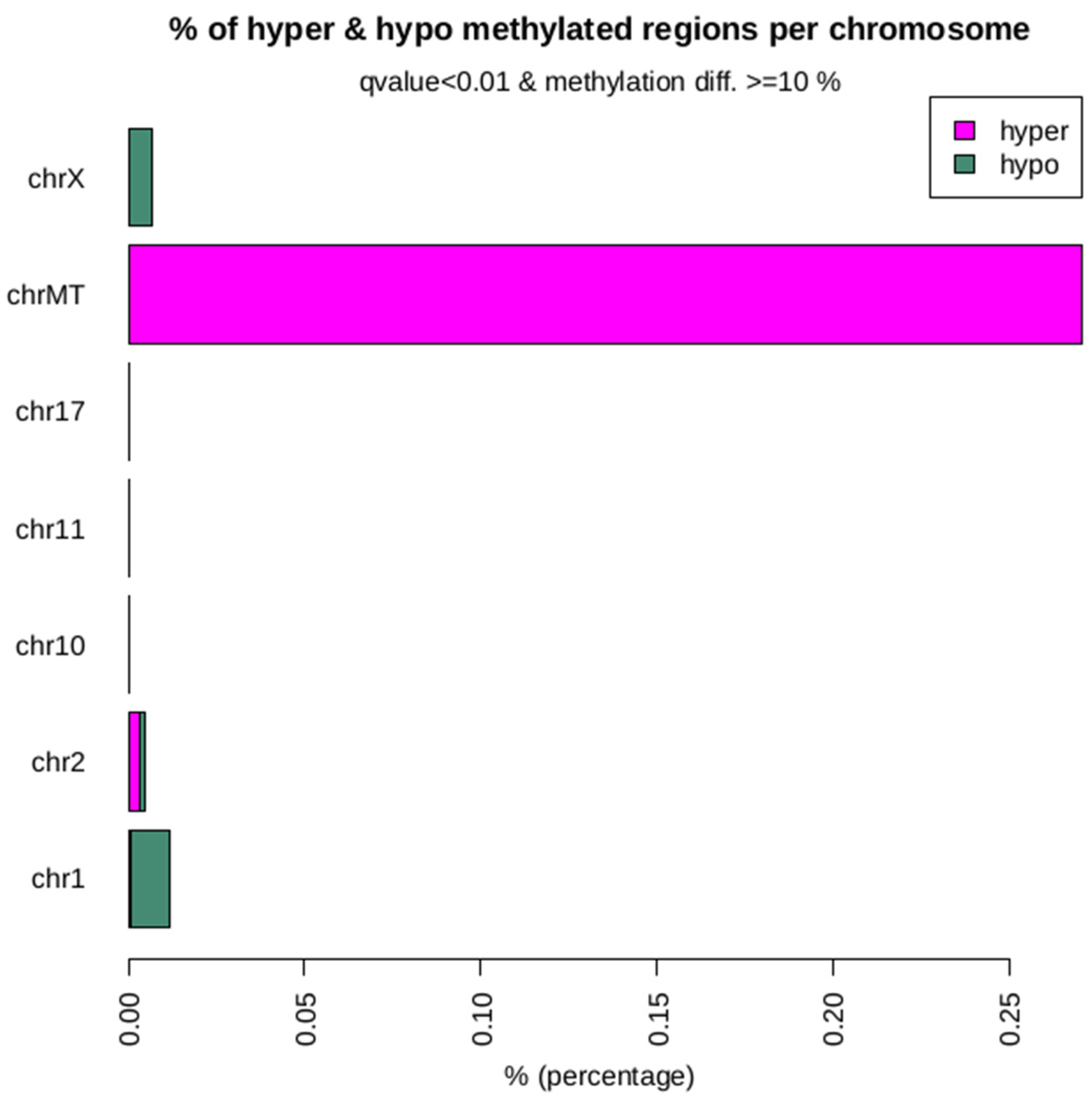
2.7. Chromosomal Distribution of SVs
2.8. Characterization of Methylated Regions
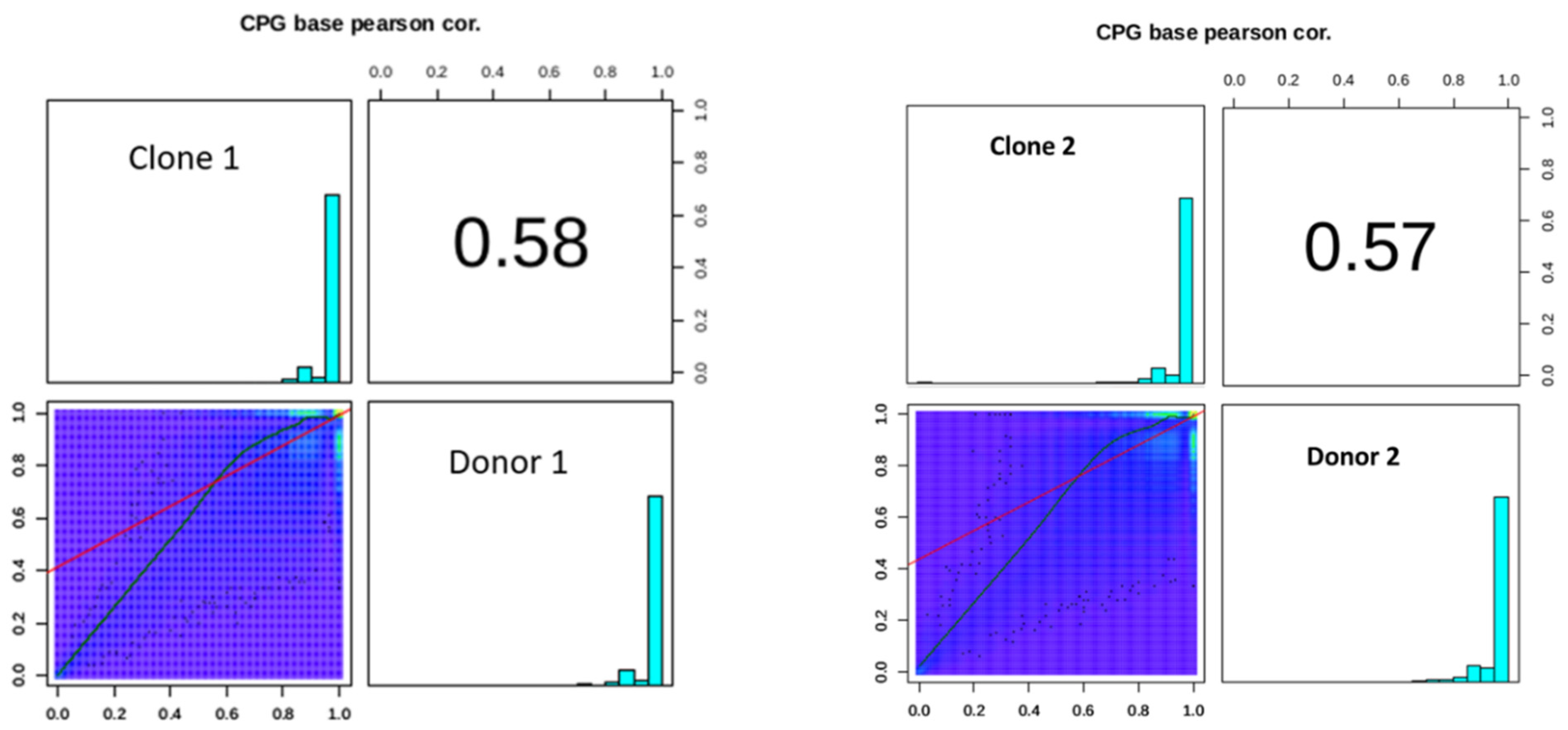
2.9. DNA Methylation Conservation Between the Donor and Clone Bulls
2.10. Differential Methylation of the Hypermethylated and Hypomethylated Island Annotation Between the Clone and Donor
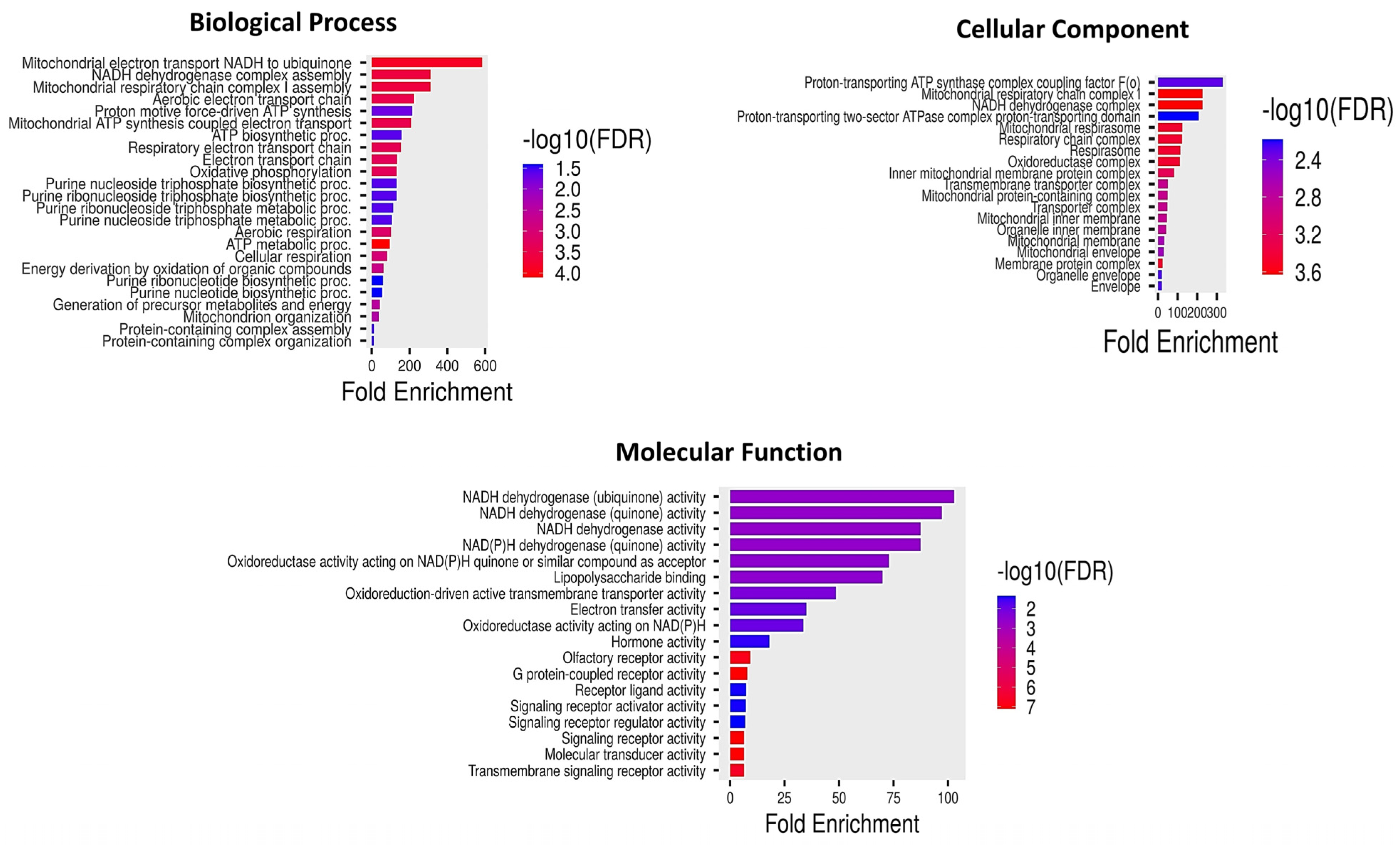
2.11. GO Enrichment and Pathway Analyses of Differentially Methylated Genes
3. Discussion
4. Materials and Methods
4.1. Animals and Sampling
4.2. DNA Extraction and Quality Control
4.3. Library Preparation
4.4. Nanopore Sequencing
4.5. Bioinformatics and Data Analysis
4.5.1. Base Calling and Quality Assessment
4.5.2. Read Alignment and Reference Mapping
4.5.3. DNA Methylation Analysis
4.5.4. Variant Calling and Annotation
4.5.5. Structural Variant Detection
4.5.6. Comparative Analysis of Donor and Clone Samples
4.5.7. Gene Ontology and Pathway Enrichment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCNT | Somatic cell nuclear transfer |
| ONT | Oxford Nanopore Technologies |
| WGS | Whole-genome sequencing |
| SMRT | PacBio’s single molecule technologies |
| SNV | Single nucleotide variants |
| SV | Structural variants |
| HMC | Handmade cloning |
| SNP | Single nucleotide polymorphism |
| Indels | Insertion and deletion |
| DMR | Differentially methylated regions |
| DEL | Deletions |
| INS | Insertion |
| BND | Breakends |
| INV | Inversion |
| NPR2 | Natriuretic peptide receptor 2 |
| chrMT | Chromosome of mitochondria |
| EDN1 | Endothelin 1 |
| NPPC | Natriuretic peptide C |
| EDNRA | Endothelin receptor type A |
| PGRMC1 | Progesterone receptor membrane component 1 |
| SPEM1 | Spermatid maturation 1 |
| APOE | Apolipoprotein E |
| XKR6 | XK related 6 |
| ATP8B1 | ATPase phospholipid transporting 8B1 |
| GO | Gene ontology |
| ND1 | NADH dehydrogenase subunit 1 |
| ND2 | NADH dehydrogenase subunit 2 |
| ATP6 | ATP synthase F0 subunit 6 |
| OR6Z9 | Olfactory receptor family 6 subfamily Z member 9 |
| OR9K15 | Olfactory receptor family 9 subfamily K member 15 |
| OR4C1F | Olfactory receptor family 4 subfamily C member 1F |
| mtDNA | Mitochondrial DNA |
References
- Yadav, P.S.; Kumar, D.; Saini, M.; Sharma, R.K.; Dua, S.; Selokar, N.L.; Bansal, S.; Punetha, M.; Gupta, A.; Kumar, R.; et al. Evaluation of postnatal growth, hematology, telomere length and semen attributes of multiple clones and re-clone of superior buffalo breeding bulls. Theriogenology 2024, 213, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Selokar, N.L.; Sharma, P.; Saini, M.; Sheoran, S.; Rajendran, R.; Kumar, D.; Sharma, R.K.; Motiani, R.K.; Kumar, P.; Jerome, A.; et al. Successful cloning of a superior buffalo bull. Sci. Rep. 2019, 9, 11366. [Google Scholar] [CrossRef] [PubMed]
- Latham, K.E. Early and delayed aspects of nuclear reprogramming during cloning. Biol. Cell 2005, 97, 119–132. [Google Scholar] [CrossRef]
- Ogura, A.; Inoue, K.; Wakayama, T. Recent advancements in cloning by somatic cell nuclear transfer. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002, 357, 1207–1212. [Google Scholar] [CrossRef]
- Rideout, W.M.; Eggan, K.; Jaenisch, R. Nuclear cloning and epigenetic reprogramming of the genome. Science 2001, 293, 1093–1098. [Google Scholar] [CrossRef]
- Shimozawa, N.; Sotomaru, Y.; Eguchi, N.; Suzuki, S.; Hioki, K.; Usui, T.; Kono, T.; Ito, M. Phenotypic abnormalities observed in aged cloned mice from embryonic stem cells after long-term maintenance. Reproduction 2006, 132, 435–441. [Google Scholar] [CrossRef][Green Version]
- Amiridze, N.S.; Darwish, R.; Griffith, G.M.; Zoarskia, G.H. Treatment of arteriovenous malformations with hydrocoils in a Swine model. Interv. Neuroradiol. 2008, 14, 165–171. [Google Scholar] [CrossRef]
- Swindle, M.M. Swine as surgical models in biomedical research. In Proceedings of the 2009 ACVP/ASVCP Annual Meetings, Monterey, CA, USA, 5–9 December 2009. [Google Scholar]
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J., Jr.; Frazier, K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H.; Tian, X.C.; King, W.A.; Lee, R.S. Epigenetic reprogramming in embryonic and foetal development upon somatic cell nuclear transfer cloning. Reproduction 2008, 135, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H. Epigenetic reprogramming in mammalian species after SCNT-based cloning. Theriogenology 2016, 86, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, X.; Wang, J.; Meydan, C.; Glass, J.L.; Shih, A.H.; Delwel, R.; Levine, R.L.; Mason, C.E.; Melnick, A.M. Somatic mutations drive specific, but reversible, epigenetic heterogeneity states in AML. Cancer Discov. 2020, 10, 1934–1949. [Google Scholar] [CrossRef]
- Laszlo, A.H.; Derrington, I.M.; Brinkerhoff, H.; Langford, K.W.; Nova, I.C.; Samson, J.M.; Bartlett, J.J.; Pavlenok, M.; Gundlach, J.H. Detection and mapping of 5-methylcytosine and 5-hydroxymethylcytosine with nanoporeMspA. Proc. Natl. Acad. Sci. USA 2013, 110, 18904–18909. [Google Scholar] [CrossRef]
- Schreiber, J.; Wescoe, Z.L.; Abu-Shumays, R.; Vivian, J.T.; Baatar, B.; Karplus, K.; Akeson, M. Error rates for nanopore discrimination among cytosine, methylcytosine, and hydroxymethylcytosine along individual DNA strands. Proc. Natl. Acad. Sci. USA 2013, 110, 18910–18915. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, S.; McPherson, J.D.; McCombie, W.R. Coming of age: Ten years of next-generation sequencing technologies. Nat. Rev. Genet. 2016, 17, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Olsen, H.E.; Paten, B.; Akeson, M. The Oxford Nanopore MinION: Delivery of nanopore sequencing to the genomics community. Genome Biol. 2016, 17, 239. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, S.; Momozawa, Y.; Liu, X.; Terao, C.; Kubo, M.; Kamatani, Y. Comprehensive evaluation of structural variation detection algorithms for whole genome sequencing. Genome Biol. 2019, 20, 117. [Google Scholar] [CrossRef]
- Alkan, C.; Coe, B.P.; Eichler, E.E. Genome structural variation discovery and genotyping. Nat. Rev. Genet. 2011, 12, 363–376. [Google Scholar] [CrossRef]
- Sudmant, P.H.; Rausch, T.; Gardner, E.J.; Handsaker, R.E.; Abyzov, A.; Huddleston, J.; Zhang, Y.; Ye, K.; Jun, G.; Fritz, M.H.-Y.; et al. An integrated map of structural variation in 2,504 human genomes. Nature 2015, 526, 75–81. [Google Scholar] [CrossRef]
- Kwon, S.G.; Bae, G.H.; Hong, J.H.; Choi, J.-W.; Choi, J.H.; Lim, N.S.; Jeon, C.; Mali, N.M.; Jun, M.S.; Shin, J.; et al. Comprehensive analysis of somatic mutations and structural variations in domestic pig. Mamm. Genome 2024, 35, 645–656. [Google Scholar] [CrossRef]
- Juyal, R.C.; Figuera, L.E.; Hauge, X.; Elsea, S.H.; Lupski, J.R.; Greenberg, F.; Baldini, A.; Patel, P.I. Molecular analyses of 17p11. 2 deletions in 62 Smith-Magenis syndrome patients. Am. J. Hum. Genet. 1996, 58, 998–1007. [Google Scholar] [PubMed]
- Ji, Y.; Eichler, E.E.; Schwartz, S.; Nicholls, R.D. Structure of chromosomal duplicons and their role in mediating human genomic disorders. Genome Res. 2000, 10, 597–610. [Google Scholar] [CrossRef]
- Lupski, J.R.; Stankiewicz, P. Genomic disorders: Molecular mechanisms for rearrangements and conveyed phenotypes. PLoS Genet. 2005, 1, e49. [Google Scholar] [CrossRef]
- Weischenfeldt, J.; Symmons, O.; Spitz, F.; Korbel, J.O. Phenotypic impact of genomic structural variation: Insights from and for human disease. Nat. Rev. Genet. 2013, 14, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Østern, R.; Fagerheim, T.; Hjellnes, H.; Nygård, B.; Mellgren, S.I.; Nilssen, Ø. Diagnostic laboratory testing for Charcot Marie Tooth disease (CMT): The spectrum of gene defects in Norwegian patients with CMT and its implications for future genetic test strategies. BMC Med. Genet. 2013, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.B.; Lupski, J.R. Mechanisms underlying structural variant formation in genomic disorders. Nat. Rev. Genet. 2016, 17, 224–238. [Google Scholar] [CrossRef]
- Hiendleder, S.; Prelle, K.; Bruggerhoff, K.; Reichenbach, H.D.; Wenigerkind, H.; Bebbere, D.; Stojkovic, M.; Mular, S.; Brem, G.; Zakhartchenko, V.; et al. Nuclear-cytoplasmic interactions affect in utero developmental capacity, phenotype, and cellular metabolism of bovine nuclear transfer fetuses. Biol. Reprod. 2004, 70, 1196–1205. [Google Scholar] [CrossRef]
- John, J.C.; Moffatt, O.; D’Souza, N. Aberrant heteroplasmic transmission of mtDNA in cloned pigs arising from double nuclear transfer. Mol. Reprod. Dev. 2005, 72, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.K.H.; Bellis, E.S.; Calkins, J.; Adrion, J.R.; Latta, L.C., Iv.; Schaack, S. Engines of change: Transposable element mutation rates are high and variable within Daphnia magna. PLoS Genet. 2021, 17, e1009827. [Google Scholar] [CrossRef] [PubMed]
- Mérot, C.; Oomen, R.A.; Tigano, A.; Wellenreuther, M. A roadmap for understanding the evolutionary significance of structural genomic variation. Trends Ecol. Evol. 2020, 35, 561–572. [Google Scholar] [CrossRef]
- Catanach, A.; Crowhurst, R.; Deng, C.; David, C.; Bernatchez, L.; Wellenreuther, M. The genomic pool of standing structural variation outnumbers single nucleotide polymorphism by threefold in the marine teleost Chrysophrys auratus. Mol. Ecol. 2019, 28, 1210–1223. [Google Scholar] [CrossRef]
- Feulner, P.G.D.; Chain, F.J.J.; Panchal, M.; Eizaguirre, C.; Kalbe, M.; Lenz, T.L.; Mundry, M.; Samonte, I.E.; Stoll, M.; Milinski, M.; et al. Genome-wide patterns of standing genetic variation in a marine population of three-spined sticklebacks. Mol. Ecol. 2013, 22, 635–649. [Google Scholar] [CrossRef]
- Feulner, P.; De-Kayne, R. Genome evolution, structural rearrangements and speciation. J. Evol. Biol. 2017, 30, 1488–1490. [Google Scholar] [CrossRef]
- Kirkpatrick, M.; Barton, N. Chromosome inversions, local adaptation and speciation. Genetics 2006, 173, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Wellenreuther, M.; Bernatchez, L. Eco-evolutionary genomics of chromosomal inversions. Trends Ecol. Evol. 2018, 33, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Dean, W.; Santos, F.; Stojkovic, M.; Zakhartchenko, V.; Walter, J.; Reik, W. Conservation of methylation reprogramming in mammalian development: Aberrant reprogramming in cloned embryos. Proc. Natl. Acad. Sci. USA 2001, 98, 13734–13738. [Google Scholar] [CrossRef] [PubMed]
- Peat, J.; Reik, W. Incomplete methylation reprogramming in SCNT embryos. Nat. Genet. 2012, 44, 965–966. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Liu, W.; Li, J.; Li, C.; Kou, X.; Chen, J.; Zhao, Y.; Gao, H.; Wang, H.; et al. Distinct features of H3K4me3 and H3K27me3 chromatin domains in pre-implantation embryos. Nature 2016, 537, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Matoba, S.; Zhang, Y. Somatic cell nuclear transfer reprogramming: Mechanisms and applications. Cell Stem Cell 2018, 23, 471–485. [Google Scholar] [CrossRef]
- Wang, X.; Qu, J.; Li, J.; He, H.; Liu, Z.; Huan, Y. Epigenetic reprogramming during somatic cell nuclear transfer: Recent progress and future directions. Front. Genet. 2020, 11, 205. [Google Scholar] [CrossRef]
- Bi, Y.; Tu, Z.; Zhang, Y.; Yang, P.; Guo, M.; Zhu, X.; Zhao, C.; Zhou, J.; Wang, H.; Wang, Y.; et al. Identification of ALPPL2 as a naive pluripotent state-specific surface protein essential for human naive pluripotency regulation. Cell Rep. 2020, 30, 3917–3931. [Google Scholar] [CrossRef]
- Shen, C.J.; Lin, C.C.; Shen, P.C.; Cheng, W.T.; Chen, H.L.; Chang, T.C.; Liu, S.S.; Chen, C.M. Imprinted genes and satellite loci are differentially methylated in bovine somatic cell nuclear transfer clones. Cell Reprogram 2013, 15, 413–424. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Hannigan, B.; Crews, D. Temperature shift alters DNA methylation and histone modification patterns in gonadal aromatase (cyp19a1) gene in species with temperature-dependent sex determination. PLoS ONE 2016, 11, e0167362. [Google Scholar] [CrossRef]
- Niemann, H.; Carnwath, J.W.; Herrmann, D.; Wieczorek, G.; Lemme, E.; Lucas-Hahn, A.; Olek, S. DNA methylation pattern reflects epigenetic reprogramming in bovine embryos. Cell Reprogram 2010, 12, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.K.; Koo, D.B.; Park, J.S.; Choi, Y.H.; Kim, H.N.; Chang, W.K.; Lee, K.K.; Han, Y.M. Typical demethylation events in cloned pig embryos: Clues on species-specific differences in epigenetic reprogramming of a cloned donor genome. J. Biol. Chem. 2001, 276, 39980–39984. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.S.; Østrup, O.; Østrup, E.; Vejlsted, M.; Niemann, H.; Lucas-Hahn, A.; Petersen, B.; Li, J.; Callesen, H.; Hyttel, P. DNA methylation in porcine preimplantation embryos developed in vivo and produced by in vitro fertilization, parthenogenetic activation and somatic cell nuclear transfer. Epigenetics 2011, 6, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Whitworth, K.M.; Spate, L.D.; Walters, E.M.; Zhao, J.; Prather, R.S. Regulation of oocyte mitochondrial DNA copy number by follicular fluid, EGF, and neuregulin 1 during in vitro maturation affects embryo development in pigs. Theriogenology 2012, 78, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Lu, J.; Wang, L.; Gao, M. Pro-inflammatory role of cell-free mitochondrial DNA in cardiovascular diseases. IUBMB Life 2020, 72, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Ryu, H.; Jang, W.; Chae, H.; Kim, M.; Kim, Y.; Kim, J.; Lee, J.W.; Chung, N.G.; Cho, B.; et al. Novel 5.712 kb mitochondrial DNA deletion in a patient with Pearson syndrome: A case report. Mol. Med. Rep. 2015, 11, 3741–3745. [Google Scholar] [CrossRef][Green Version]
- Srivastava, S. The mitochondrial basis of aging and age-related disorders. Genes 2017, 8, 398. [Google Scholar] [CrossRef]

| Sample | Average Depth (X) | Total Reads (Number) | Mean Read Length (bp) | % Mapped Reads | Average Mapping Depth (X) |
|---|---|---|---|---|---|
| Donor 1 | 16.67 | 6,892,605 | 6288.9 | 99.90 | 16.05 |
| Clone 1 | 16.54 | 6,337,029 | 6786.7 | 99.90 | 15.89 |
| Donor 2 | 19.66 | 8,525,247 | 5995.8 | 99.84 | 18.52 |
| Clone 2 | 17.96 | 7,132,741 | 6547.7 | 99.91 | 17.32 |
| Genomic Region | Donor 1 | Clone 1 | Donor 2 | Clone 2 |
|---|---|---|---|---|
| DOWNSTREAM | 5.0 | 5.1 | 6.9 | 5.0 |
| INTERGENIC | 18.8 | 19.9 | 14.5 | 18.8 |
| INTRAGENIC | 0.0 | 0.0 | 0.0 | 0.0 |
| UPSTREAM | 5.0 | 4.8 | 7.1 | 4.7 |
| UTR_3_PRIME | 0.7 | 0.7 | 1.0 | 0.6 |
| EXON | 1.3 | 1.2 | 2.0 | 1.2 |
| INTRON | 69.0 | 68.0 | 68.1 | 69.4 |
| UTR_5_PRIME | 0.22 | 0.21 | 0.4 | 0.2 |
| Variant Types | Donor 1 | Clone 1 | Donor 2 | Clone 2 |
|---|---|---|---|---|
| BND | 1.44% | 1.45% | 1.63% | 1.32% |
| DEL | 60.50% | 59.61% | 60.31% | 58.78% |
| DUP | 0.32% | 0.31% | 0.30% | 0.26% |
| INS | 37.62% | 38.52% | 37.65% | 39.55% |
| INV | 0.09% | 0.09% | 0.09% | 0.08% |
| Gene Biotype | Donor 1 | Clone 1 | Donor 2 | Clone 2 |
|---|---|---|---|---|
| Protein coding | 86.248% | 85.764% | 86.759% | 85.979% |
| lncRNA | 11.580% | 11.937% | 11.167% | 11.847% |
| pseudogene | 2.030% | 2.062% | 1.866% | 1.904% |
| C region (or Variable region) | 0.041% | 0.117% | 0.117% | 0.149% |
| Misc RNA | 0.045% | 0.058% | 0.025% | 0.059% |
| V segment | 0.020% | 0.034% | 0.025% | 0.032% |
| rRNA | 0.010% | 0.010% | 0.006% | 0.005% |
| snRNA | 0.006% | 0.006% | 0.015% | 0.011% |
| snoRNA | 0.006% | 0.003% | 0.009% | 0.005% |
| tRNA | 0.006% | 0.003% | 0.006% | 0.002% |
| Animal | Total CPG | Top 100 Gene Methylation Count |
|---|---|---|
| Donor 1 | 9,408,361 | 402,837 |
| Clone 1 | 9,319,431 | 400,398 |
| Donor 2 | 10,131,221 | 455,049 |
| Clone 2 | 9,963,519 | 428,795 |
| Total DMR Count | HyperMethylated Count | HypoMethylated Count | Significant Methylation Count (q-Value: 0.01) | |
|---|---|---|---|---|
| Donor 1 vs. Clone 1 | 7,743,726 | 15 | 12 | 27 |
| Donor 2 vs. Clone 2 | 8,821,206 | 38 | 103 | 141 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punetha, M.; Kumar, D.; Kumar, S.; Maggo, B.; Dahiya, P.; Kumar, P.; Sharma, R.K.; Pal, Y.; Yadav, P.S. Unravelling High Nuclear Genomic Similarity and Mitochondria Linked Epigenetic Divergence in SCNT Derived Buffalo Clones via Long-Read Nanopore Genome Sequencing. Int. J. Mol. Sci. 2025, 26, 8836. https://doi.org/10.3390/ijms26188836
Punetha M, Kumar D, Kumar S, Maggo B, Dahiya P, Kumar P, Sharma RK, Pal Y, Yadav PS. Unravelling High Nuclear Genomic Similarity and Mitochondria Linked Epigenetic Divergence in SCNT Derived Buffalo Clones via Long-Read Nanopore Genome Sequencing. International Journal of Molecular Sciences. 2025; 26(18):8836. https://doi.org/10.3390/ijms26188836
Chicago/Turabian StylePunetha, Meeti, Dharmendra Kumar, Satish Kumar, Bhavya Maggo, Priya Dahiya, Pradeep Kumar, Rakesh K. Sharma, Yash Pal, and Prem S. Yadav. 2025. "Unravelling High Nuclear Genomic Similarity and Mitochondria Linked Epigenetic Divergence in SCNT Derived Buffalo Clones via Long-Read Nanopore Genome Sequencing" International Journal of Molecular Sciences 26, no. 18: 8836. https://doi.org/10.3390/ijms26188836
APA StylePunetha, M., Kumar, D., Kumar, S., Maggo, B., Dahiya, P., Kumar, P., Sharma, R. K., Pal, Y., & Yadav, P. S. (2025). Unravelling High Nuclear Genomic Similarity and Mitochondria Linked Epigenetic Divergence in SCNT Derived Buffalo Clones via Long-Read Nanopore Genome Sequencing. International Journal of Molecular Sciences, 26(18), 8836. https://doi.org/10.3390/ijms26188836






