Ultrafast Photochemical Dynamics of Dinitrosyl Iron Complexes Investigated by Femtosecond Time-Resolved Infrared Spectroscopy
Abstract
1. Introduction
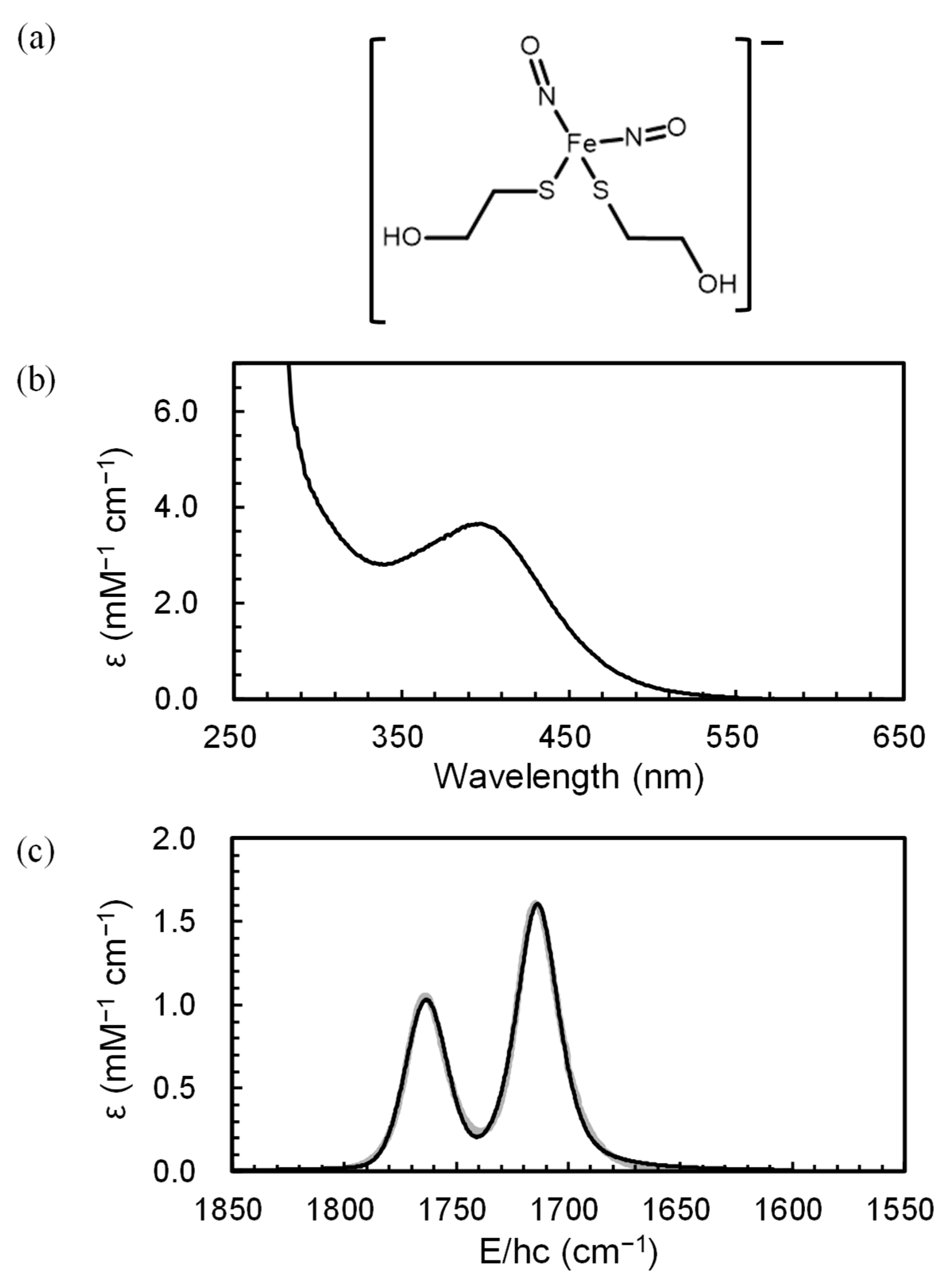
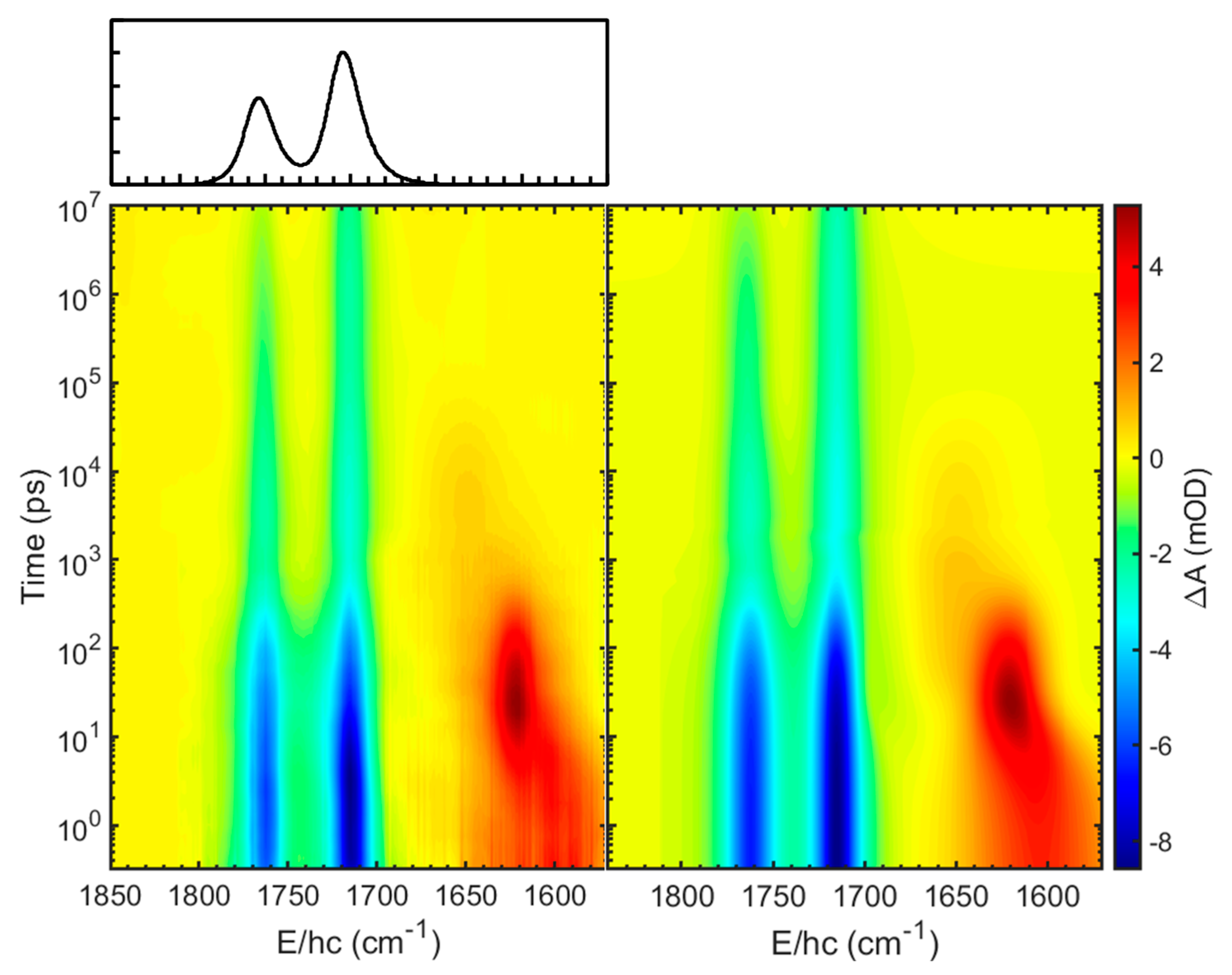
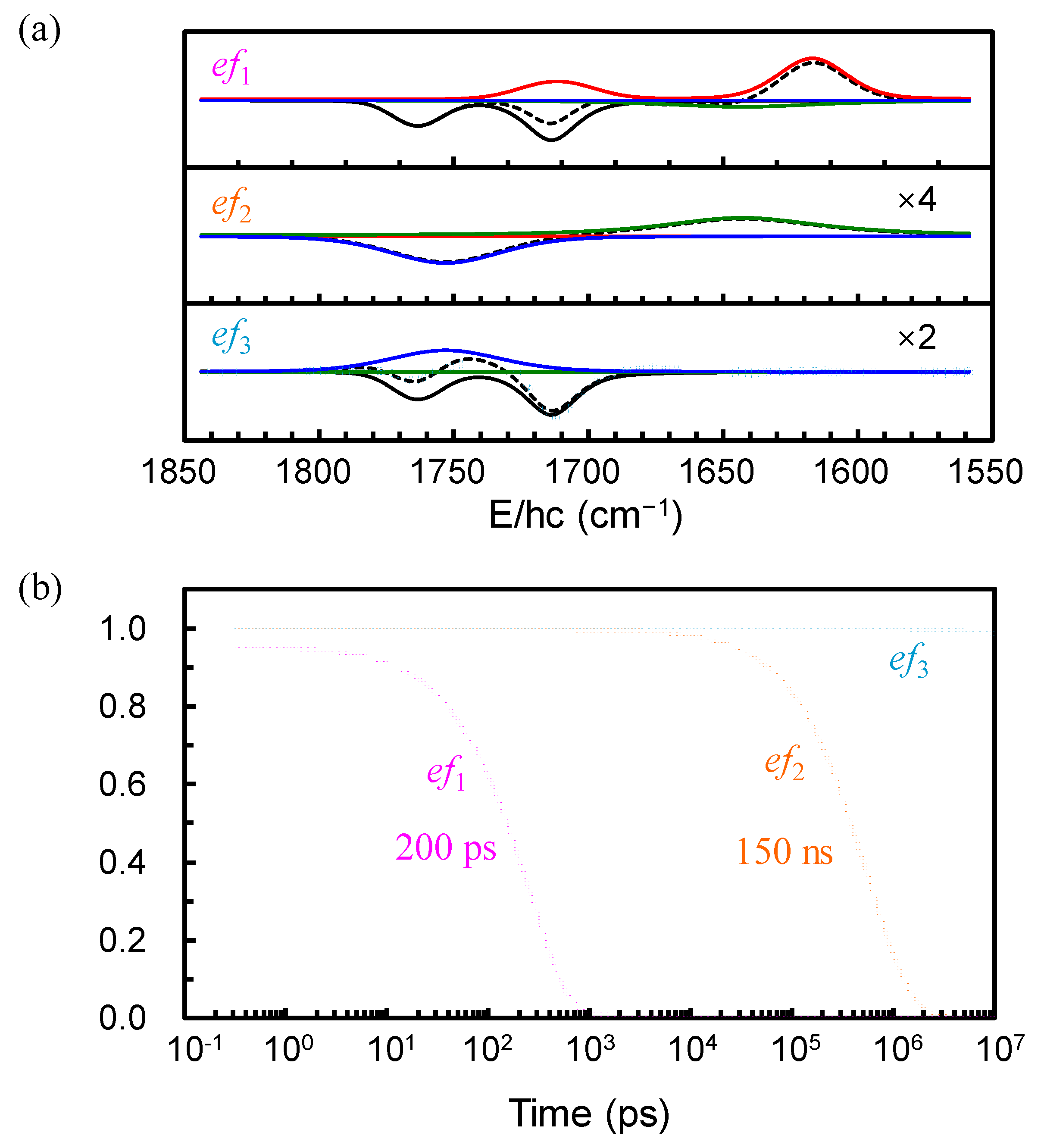
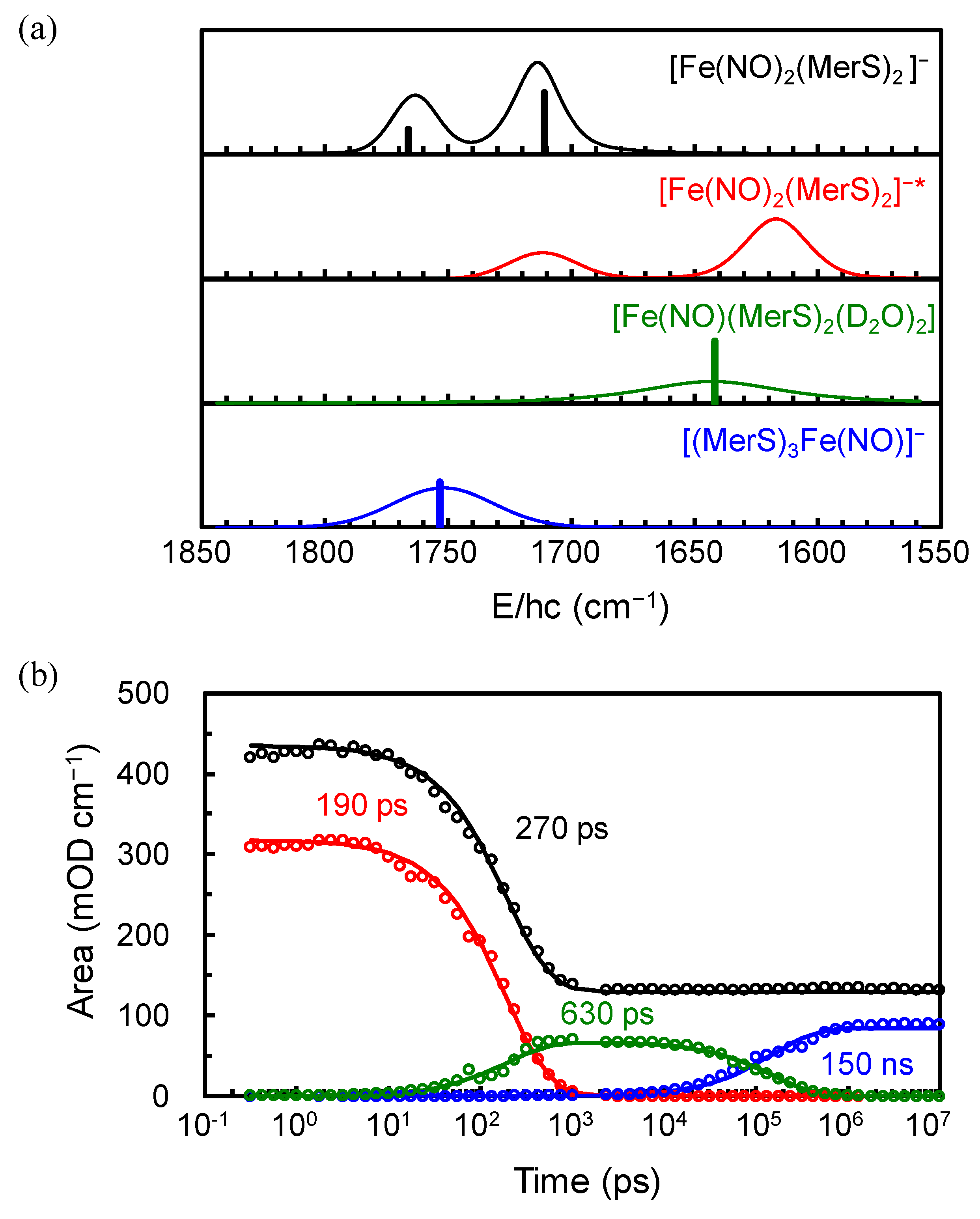
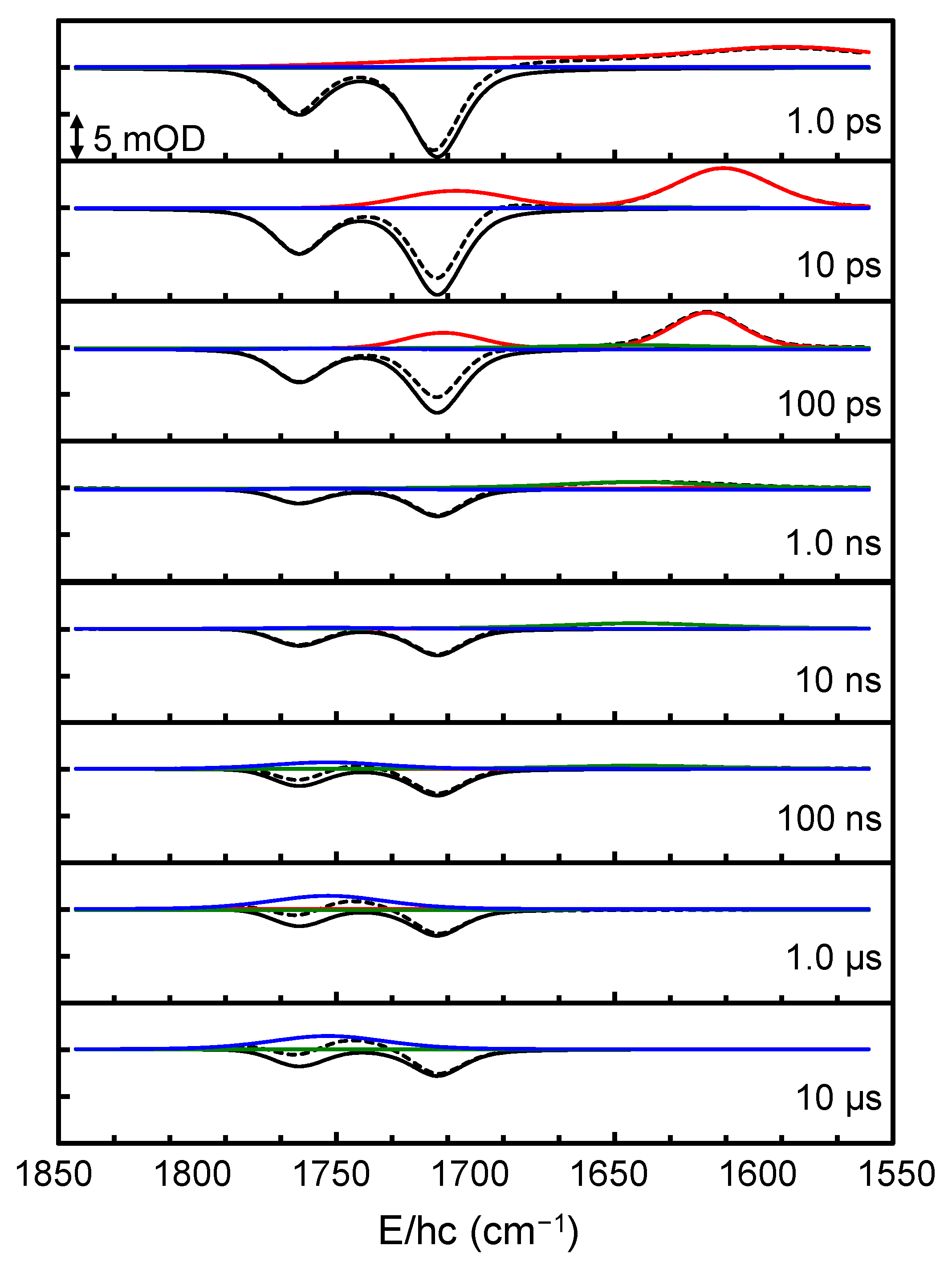
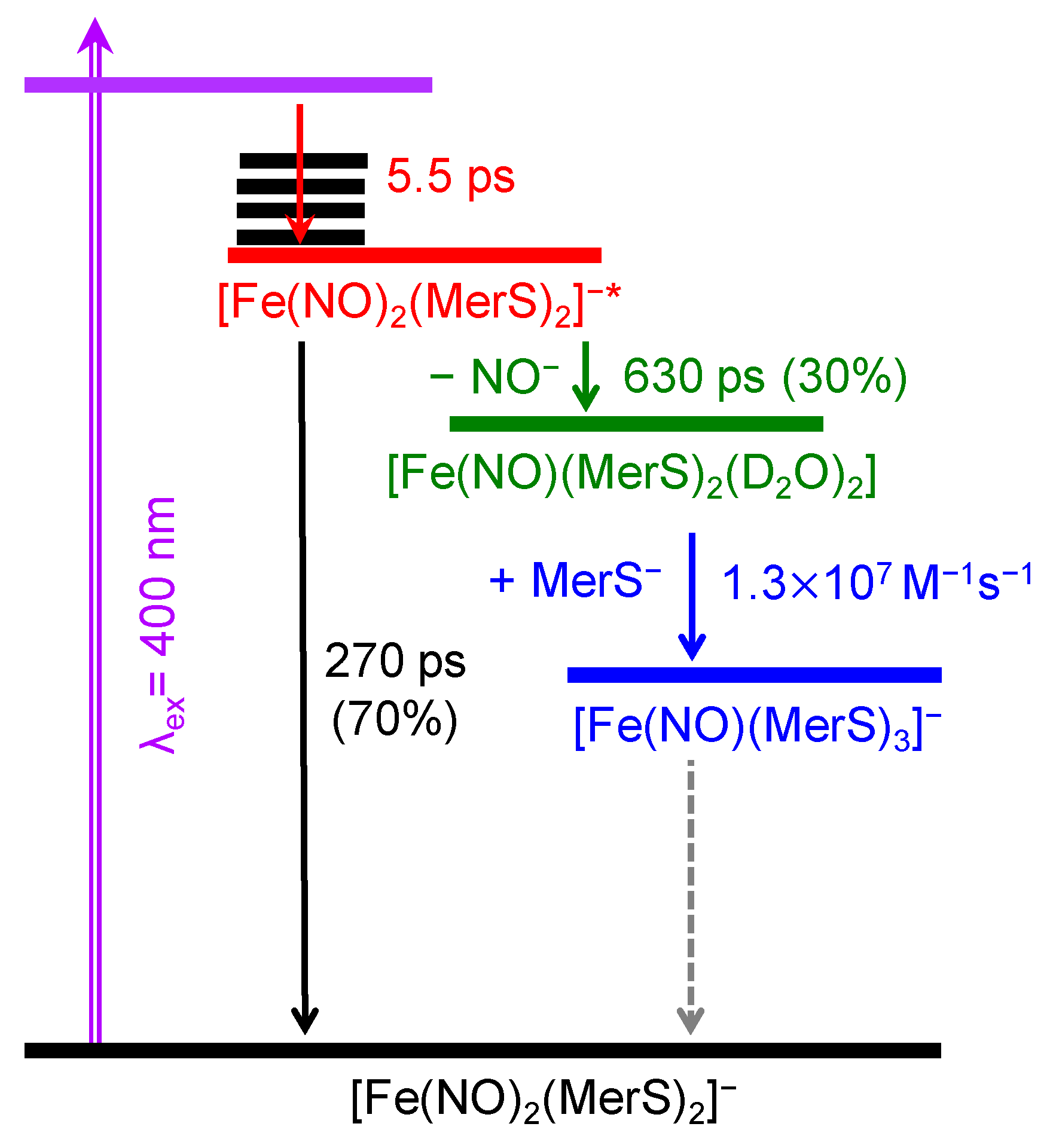
2. Results and Discussion
3. Materials and Methods
3.1. Sample Preparation
3.2. Time-Resolved Infrared (IR) Spectroscopy
3.3. Computational Details
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DNIC | Dinitrosyl iron complex |
| NO | Nitric oxide |
| MNIC | Mononitrosyl iron complex |
| RRE | Roussin’s red ester |
| TRIR | Time-resolved infrared |
| EPR | Electron paramagnetic resonance |
| DFT | Density functional theory |
| MLCT | Metal-to-ligand charge transfer |
| SVD | Singular value decomposition |
| IR | Infrared |
| OPA | Optical parametric amplifier |
| PCM | Polarized continuum model |
References
- Chiang, C.-Y.; Darensbourg, M.Y. Iron nitrosyl complexes as models for biological nitric oxide transfer reagents. J. Biol. Inorg. Chem. 2006, 11, 359–370. [Google Scholar] [CrossRef]
- Shumaev, K.B.; Kosmachevskaya, O.V.; Chumikina, L.V.; Topunov, A.F. Dinitrosyl iron complexes and other physiological metabolites of nitric oxide: Multifarious role in plants. Nat. Prod. Commun. 2016, 11, 1189–1192. [Google Scholar] [CrossRef]
- Truzzi, D.R.; Medeiros, N.M.; Augusto, O.; Ford, P.C. Dinitrosyl iron complexes (DNICs). From spontaneous assembly to biological roles. Inorg. Chem. 2021, 60, 15835–15845. [Google Scholar] [CrossRef]
- Wu, S.-C.; Lu, C.-Y.; Chen, Y.-L.; Lo, F.-C.; Wang, T.-Y.; Chen, Y.-J.; Yuan, S.-S.; Liaw, W.-F.; Wang, Y.-M. Water-soluble dinitrosyl iron complex (DNIC): A nitric oxide vehicle triggering cancer cell death via apoptosis. Inorg. Chem. 2016, 55, 9383–9392. [Google Scholar] [CrossRef]
- Foster, M.W.; Cowan, J. Chemistry of nitric oxide with protein-bound iron sulfur centers. Insights on physiological reactivity. J. Am. Chem. Soc. 1999, 121, 4093–4100. [Google Scholar] [CrossRef]
- Rogers, P.A.; Ding, H. L-cysteine-mediated destabilization of dinitrosyl iron complexes in proteins. J. Biol. Chem. 2001, 276, 30980–30986. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Rogers, P.A.; Ding, H. Repair of nitric oxide-modified ferredoxin [2Fe-2S] cluster by cysteine desulfurase (IscS). J. Biol. Chem. 2002, 277, 12868–12873. [Google Scholar] [CrossRef]
- Ding, H.; Demple, B. Direct nitric oxide signal transduction via nitrosylation of iron-sulfur centers in the SoxR transcription activator. Proc. Natl. Acad. Sci. USA 2000, 97, 5146–5150. [Google Scholar] [CrossRef] [PubMed]
- Boese, M.; Mordvintcev, P.I.; Vanin, A.F.; Busse, R.; Mülsch, A. S-nitrosation of serum albumin by dinitrosyl-iron complex. J. Biol. Chem. 1995, 270, 29244–29249. [Google Scholar] [CrossRef]
- Medeiros, N.M.; Garcia, F.A.; Truzzi, D.R. Insight into the relevance of dinitrosyl iron complex (DNIC) formation in the absence of thiols in aqueous media. Dalton Trans. 2024, 53, 1951–1955. [Google Scholar] [CrossRef] [PubMed]
- Hickok, J.R.; Sahni, S.; Shen, H.; Arvind, A.; Antoniou, C.; Fung, L.W.; Thomas, D.D. Dinitrosyliron complexes are the most abundant nitric oxide-derived cellular adduct: Biological parameters of assembly and disappearance. Free Radic. Biol. Med. 2011, 51, 1558–1566. [Google Scholar] [CrossRef]
- Lehnert, N.; Kim, E.; Dong, H.T.; Harland, J.B.; Hunt, A.P.; Manickas, E.C.; Oakley, K.M.; Pham, J.; Reed, G.C.; Alfaro, V.S. The biologically relevant coordination chemistry of iron and nitric oxide: Electronic structure and reactivity. Chem. Rev. 2021, 121, 14682–14905. [Google Scholar] [CrossRef] [PubMed]
- Fugami, K.M.; Black, G.S.; Kowalczyk, T.; Seda, T.; Gilbertson, J.D. Intermolecular N–N Coupling of a Dinitrosyl Iron Complex Induced by Hydrogen Bond Donors in the Secondary Coordination Sphere. J. Am. Chem. Soc. 2025, 147, 7274–7281. [Google Scholar] [CrossRef]
- Chiu, H.; Chau Fang, A.; Chen, Y.H.; Koi, R.X.; Yu, K.C.; Hsieh, L.H.; Lu, T.T. Mechanistic and kinetic insights into cellular uptake of biomimetic Dinitrosyl iron complexes and intracellular delivery of NO for activation of cytoprotective HO-1. J. Am. Chem. Soc. Au 2024, 4, 1550–1569. [Google Scholar] [CrossRef]
- Luzhkov, V.B.; Krapivin, V.B.; Sanina, N.A.; Aldoshin, S.M. Thio ligand binding in dinitrosyl iron-sulfur complexes: Quantitative structure–activity relationships. Polyhedron 2024, 257, 117013. [Google Scholar] [CrossRef]
- Pereira, J.C.M.; Iretskii, A.V.; Han, R.-M.; Ford, P.C. Dinitrosyl iron complexes with cysteine. Kinetics studies of the formation and reactions of DNICs in aqueous solution. J. Am. Chem. Soc. 2015, 137, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Ku, W.-C.; Feng, L.-T.; Tsai, M.-L.; Hsieh, C.-H.; Hsu, W.-H.; Liaw, W.-F.; Hung, C.-H.; Chen, Y.-J. Nitric oxide physiological responses and delivery mechanisms probed by water-soluble Roussin’s red ester and {Fe(NO)2}10 DNIC. J. Am. Chem. Soc. 2008, 130, 10929–10938. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Tsai, F.-T.; Lu, T.-T.; Tsai, M.-L.; Wei, Y.-C.; Hsu, I.-J.; Lee, J.-F.; Liaw, W.-F. Relative binding affinity of thiolate, imidazolate, phenoxide, and nitrite toward the {Fe(NO)2} motif of dinitrosyl iron complexes (DNICs): The characteristic pre-edge energy of {Fe(NO) 2}9 DNICs. Inorg. Chem. 2009, 48, 9579–9591. [Google Scholar] [CrossRef]
- Lu, T.-T.; Lai, S.-H.; Li, Y.-W.; Hsu, I.-J.; Jang, L.-Y.; Lee, J.-F.; Chen, I.-C.; Liaw, W.-F. Discrimination of mononuclear and dinuclear dinitrosyl iron complexes (DNICs) by S K-edge X-ray absorption spectroscopy: Insight into the electronic structure and reactivity of DNICs. Inorg. Chem. 2011, 50, 5396–5406. [Google Scholar] [CrossRef]
- Dai, R.J.; Ke, S.C. Detection and determination of the {Fe (NO)2} core vibrational features in dinitrosyl−iron complexes from experiment, normal coordinate analysis, and density functional theory: An avenue for probing the nitric oxide oxidation state. J. Phys. Chem. B 2007, 111, 2335–2346. [Google Scholar] [CrossRef]
- Ye, S.; Neese, F. The unusual electronic structure of dinitrosyl iron complexes. J. Am. Chem. Soc. 2010, 132, 3646–3647. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-Y.; Miller, M.L.; Reibenspies, J.H.; Darensbourg, M.Y. Bismercaptoethanediazacyclooctane as a N2S2 chelating agent and Cys−X−Cys mimic for Fe(NO) and Fe (NO)2. J. Am. Chem. Soc. 2004, 126, 10867–10874. [Google Scholar] [CrossRef] [PubMed]
- Tonzetich, Z.J.; Héroguel, F.; Do, L.H.; Lippard, S.J. Chemistry of nitrosyliron complexes supported by a β-diketiminate ligand. Inorg. Chem. 2011, 50, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Park, S.; Lim, M. Photorelease dynamics of nitric oxide from cysteine-bound Roussin’s red ester. J. Phys. Chem. Lett. 2020, 11, 3198–3202. [Google Scholar] [CrossRef]
- Yoon, H.; Park, S.; Lim, M. Photodissociation dynamics of nitric oxide from N-acetylcysteine-or N-acetylpenicillamine-bound Roussin’s red ester. ACS Omega 2021, 6, 27158–27169. [Google Scholar] [CrossRef]
- Tonzetich, Z.J.; Do, L.H.; Lippard, S.J. Dinitrosyl iron complexes relevant to Rieske cluster nitrosylation. J. Am. Chem. Soc. 2009, 131, 7964–7965. [Google Scholar] [CrossRef]
- Tsai, F.-T.; Lee, Y.-C.; Chiang, M.-H.; Liaw, W.-F. Nitrate-to-nitrite-to-nitric oxide conversion modulated by nitrate-containing {Fe (NO)2}9 dinitrosyl iron complex (DNIC). Inorg. Chem. 2013, 52, 464–473. [Google Scholar] [CrossRef]
- Yeh, S.-W.; Lin, C.-W.; Li, Y.-W.; Hsu, I.-J.; Chen, C.-H.; Jang, L.-Y.; Lee, J.-F.; Liaw, W.-F. Insight into the dinuclear {Fe(NO)2}10 {Fe(NO)2}10 and mononuclear {Fe(NO)2}10 dinitrosyliron complexes. Inorg. Chem. 2012, 51, 4076–4087. [Google Scholar] [CrossRef]
- Tsou, C.-C.; Tsai, F.-T.; Chen, H.-Y.; Hsu, I.-J.; Liaw, W.-F. Insight into one-electron oxidation of the {Fe(NO)2}9 dinitrosyl iron complex (DNIC): Aminyl radical stabilized by [Fe(NO)2] motif. Inorg. Chem. 2013, 52, 1631–1639. [Google Scholar] [CrossRef]
- Lin, Z.-S.; Lo, F.-C.; Li, C.-H.; Chen, C.-H.; Huang, W.-N.; Hsu, I.-J.; Lee, J.-F.; Horng, J.-C.; Liaw, W.-F. Peptide-bound dinitrosyliron complexes (DNICs) and neutral/reduced-form Roussin’s red esters (RREs/rRREs): Understanding nitrosylation of [Fe–S] clusters leading to the formation of DNICs and RREs using a de novo design strategy. Inorg. Chem. 2011, 50, 10417–10431. [Google Scholar] [CrossRef]
- Wittkamp, F.; Nagel, C.; Lauterjung, P.; Mallick, B.; Schatzschneider, U.; Apfel, U.-P. Phosphine-ligated dinitrosyl iron complexes for redox-controlled NO release. Dalton Trans. 2016, 45, 10271–10279. [Google Scholar] [CrossRef]
- Yoon, H.; Park, S.; Lim, M. Dynamics of irreversible NO release from photoexcited Molsidomine. J. Phys. Chem. Lett. 2023, 14, 516–523. [Google Scholar] [CrossRef] [PubMed]
- van Wilderen, L.J.; Lincoln, C.N.; van Thor, J.J. Modelling multi-pulse population dynamics from ultrafast spectroscopy. PLoS ONE 2011, 6, e17373. [Google Scholar] [CrossRef]
- Truzzi, D.R.; Augusto, O.; Iretskii, A.V.; Ford, P.C. Dynamics of dinitrosyl iron complex (DNIC) formation with low molecular weight thiols. Inorg. Chem. 2019, 58, 13446–13456. [Google Scholar] [CrossRef]
- Truzzi, D.R.; Augusto, O.; Ford, P.C. Thiyl radicals are co-products of dinitrosyl iron complex (DNIC) formation. Chem. Commun. 2019, 55, 9156–9159. [Google Scholar] [CrossRef]
- Park, S.; Shin, J.; Yoon, H.; Lim, M. Rotational isomerization of carbon–carbon single bonds in ethyl radical derivatives in a room-temperature solution. J. Phys. Chem. Lett. 2022, 13, 11551–11557. [Google Scholar] [CrossRef]
- Yoon, H.; Park, S.; Jeon, S.; Lim, M. Photoexcitation dynamics of V-PYRRO/NO investigated using femtosecond time-resolved infrared spectroscopy. J. Phys. Chem. Lett. 2024, 15, 11975–11981. [Google Scholar] [CrossRef]
- Yoon, H.; Park, S.; Kim, S.Y.; Hong, D.; Park, J.W.; Lim, M. Dynamics of NO release and linkage isomer formation from S-nitroso-mercaptoethanol in aqueous solutions: Insights from femtosecond infrared spectroscopy. J. Phys. Chem. Lett. 2024, 15, 8829–8837. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jin, G.; Lim, M. Dynamics of geminate recombination of NO with myoglobin in aqueous solution probed by femtosecond mid-IR spectroscopy. J. Phys. Chem. B 2004, 108, 20366–20375. [Google Scholar] [CrossRef]
- Park, S.; Shin, J.; Yoon, H.; Pak, Y.; Lim, M. Complete photodissociation dynamics of CF2I2 in solution. Phys. Chem. Chem. Phys. 2019, 21, 6859–6867. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Perdew, J.P.; Staroverov, V.N.; Scuseria, G.E. Climbing the density functional ladder: Nonempirical meta–generalized gradient approximation designed for molecules and solids. Phys. Rev. Lett. 2003, 91, 146401. [Google Scholar] [CrossRef]
- Staroverov, V.N.; Scuseria, G.E.; Tao, J.; Perdew, J.P. Comparative assessment of a new nonempirical density functional: Molecules and hydrogen-bonded complexes. J. Chem. Phys. 2003, 119, 12129–12137. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.P. Bioinorganic chemistry modeled with the TPSSh density functional. Inorg. Chem. 2008, 47, 10357–10365. [Google Scholar] [CrossRef] [PubMed]
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef] [PubMed]
| Compound | Spin | ν1 (cm−1) | ν2 (cm−1) | ΔG (kcal/mol) | G (Hartree) |
|---|---|---|---|---|---|
| [Fe(NO)2(MerS)2]− | doublet | 1839.75 | 1788.88 | 0 | −2399.38 |
| [Fe(NO)(MerS)2] | doublet | 1899.48 | 71.9 | −2269.37 | |
| [Fe(NO)(MerS)2(D2O)] | doublet | 1870.24 | 66.8 | −2345.74 | |
| [Fe(NO)(MerS)2(D2O)2] | doublet | 1806.56 | 64.2 | −2422.11 | |
| [Fe(NO)(MerS)2(D2O)3] | doublet | 1788.96 | 84.4 | −2498.44 | |
| [Fe(NO)(MerS)2]− | triplet | 1772.29 | 30.1 | −2269.53 | |
| [Fe(NO)(MerS)2(D2O)]− | triplet | 1753.69 | 31.1 | −2345.89 | |
| [Fe(NO)(MerS)2(D2O)2]− | triplet | 1732.24 | 28.3 | −2422.26 | |
| [Fe(NO)(MerS)2(D2O)3]− | triplet | Not converged | |||
| [Fe(NO)(MerS)3]− | doublet | 1812.75 | 37.8 | −2707.49 | |
| [Fe(NO)(MerS)3(D2O)]− | doublet | 1737.64 | 41.9 | −2783.84 | |
| [Fe(NO)(MerS)3(D2O)2]− | doublet | 1731.08 | 59.2 | −2860.18 | |
| [Fe(NO)(MerS)3]2− | triplet | 1669.39 | 19.8 | −2707.56 | |
| [Fe(NO)(MerS)3(D2O)]2− | triplet | 1661.53 | 19.1 | −2783.92 | |
| [Fe(NO)(MerS)3(D2O)2]2− | triplet | 1671.19 | 32.6 | −2860.27 | |
| [Fe(NO)(MerS)] | triplet | 1821.47 | 57.5 | −1831.42 | |
| [Fe(NO)(MerS)(D2O)] | triplet | 1814.10 | 52.5 | −1907.80 | |
| [Fe(NO)(MerS)(D2O)2] | triplet | 1775.38 | 52.6 | −1984.16 | |
| NO− | triplet | −129.90 | |||
| NO− | singlet | −129.84 | |||
| NO | doublet | −129.81 | |||
| MerS | doublet | −437.92 | |||
| MerS− | singlet | −438.06 | |||
| D2O | singlet | −76.36 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, H.; Shin, J.; Park, S.; Lim, M. Ultrafast Photochemical Dynamics of Dinitrosyl Iron Complexes Investigated by Femtosecond Time-Resolved Infrared Spectroscopy. Int. J. Mol. Sci. 2025, 26, 8835. https://doi.org/10.3390/ijms26188835
Yoon H, Shin J, Park S, Lim M. Ultrafast Photochemical Dynamics of Dinitrosyl Iron Complexes Investigated by Femtosecond Time-Resolved Infrared Spectroscopy. International Journal of Molecular Sciences. 2025; 26(18):8835. https://doi.org/10.3390/ijms26188835
Chicago/Turabian StyleYoon, Hojeong, Juhyang Shin, Seongchul Park, and Manho Lim. 2025. "Ultrafast Photochemical Dynamics of Dinitrosyl Iron Complexes Investigated by Femtosecond Time-Resolved Infrared Spectroscopy" International Journal of Molecular Sciences 26, no. 18: 8835. https://doi.org/10.3390/ijms26188835
APA StyleYoon, H., Shin, J., Park, S., & Lim, M. (2025). Ultrafast Photochemical Dynamics of Dinitrosyl Iron Complexes Investigated by Femtosecond Time-Resolved Infrared Spectroscopy. International Journal of Molecular Sciences, 26(18), 8835. https://doi.org/10.3390/ijms26188835







