Characterisation of Four New Genes in the Ovine KAP19 Family
Abstract
1. Introduction
2. Results
2.1. Identification of New KRTAP19-n on the Sheep Genome
2.2. Sequence Variants and Polymorphisms in sKRTAP19-n
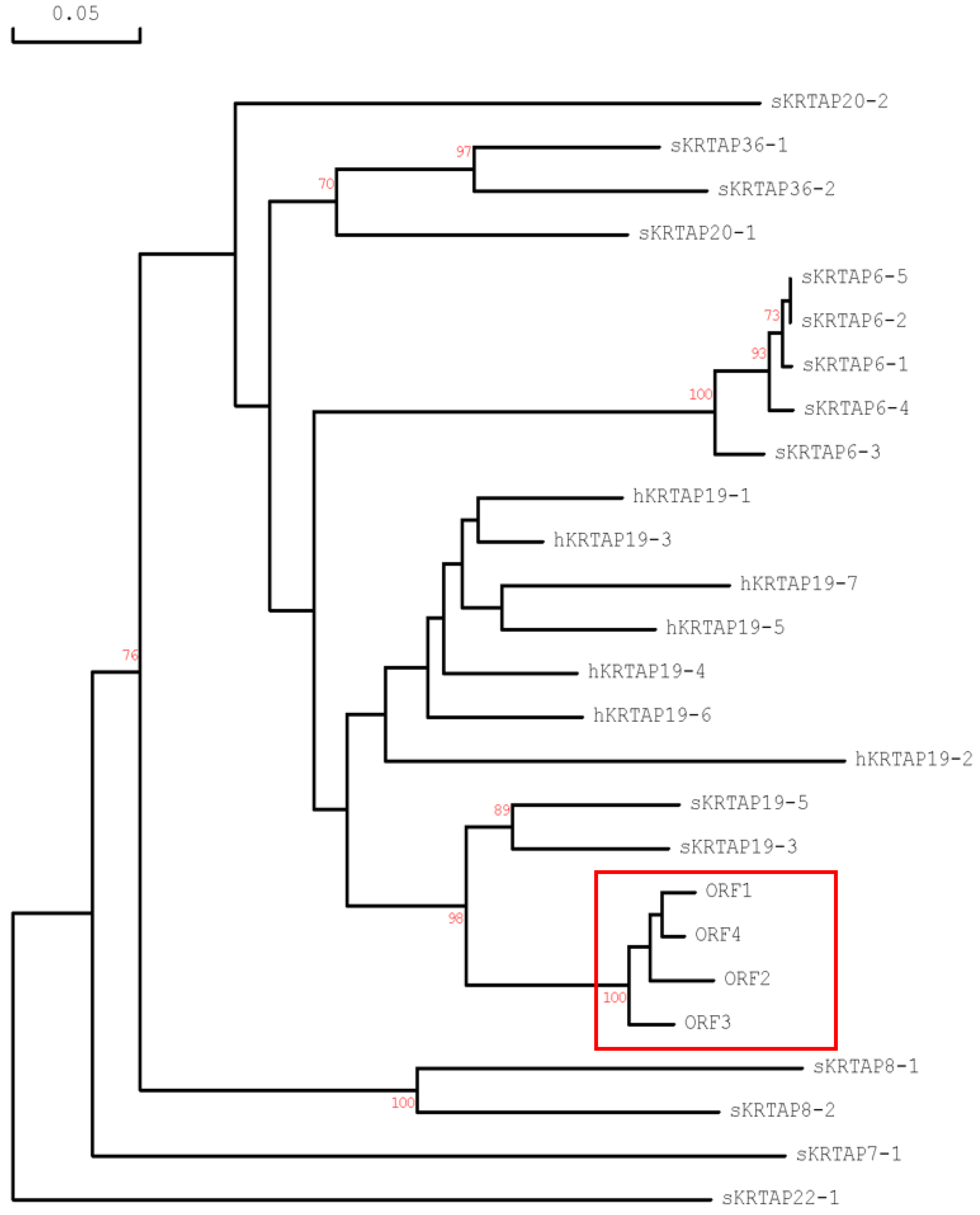
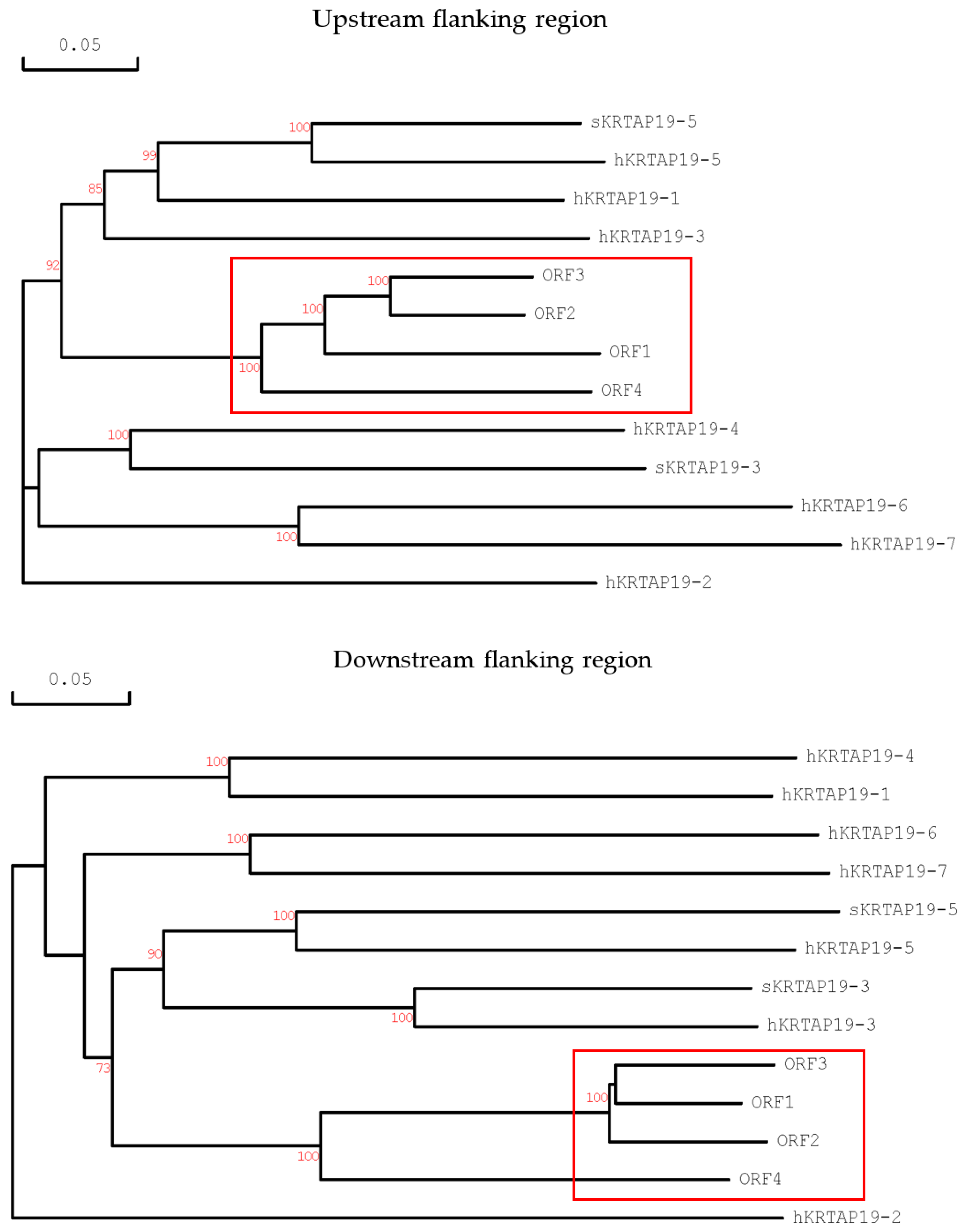
2.3. Characterisation of Distinctive sKRTAP19-n Variants and Their Sequence Comparison
3. Discussion
4. Materials and Methods
4.1. Selection of Sheep and DNA Purification
4.2. BLAST Search of Sheep Genome Assembly
4.3. PCR Amplification and SSCP Analysis
4.4. DNA Sequencing and Sequence Analysis
4.5. Analysis of Genetic Diversity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Popescu, C.; Stanescu, M.D. Eco-friendly processing of wool and sustainable valorization of this natural bioresource. Sustainability 2024, 16, 4661. [Google Scholar] [CrossRef]
- Ozek, H.Z. Sustainability, biodegradability and life cycle analysis of wool. In The Wool Handbook; Jose, S., Thomas, S., Basu, G., Eds.; Woodhead Publishing: Cambridge, UK, 2024; pp. 401–440. [Google Scholar]
- Scobie, D.; Grosvenor, A.; Bray, A.; Tandon, S.; Meade, W.; Cooper, A. A review of wool fibre variation across the body of sheep and the effects on wool processing. Small Rumin. Res. 2015, 133, 43–53. [Google Scholar] [CrossRef]
- Powell, B.A.; Rogers, G. The role of keratin proteins and their genes in the growth, structure and properties of hair. EXS 1997, 78, 59–148. [Google Scholar] [PubMed]
- Yu, Z.; Wildermoth, J.E.; Wallace, O.A.; Gordon, S.W.; Maqbool, N.J.; Maclean, P.H.; Nixon, A.J.; Pearson, A.J. Annotation of sheep keratin intermediate filament genes and their patterns of expression. Exp. Dermatol. 2011, 20, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Bai, L.; Li, S.; Li, W.; Wang, J.; Tao, J.; Hickford, J.G. Genetics of wool and cashmere fibre: Progress, challenges, and future research. Animals 2024, 14, 3228. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Gong, H.; Wang, J.; Luo, Y.; Li, S.; Tao, J.; Hickford, J.G. The complexity of the ovine and caprine keratin-associated protein genes. Int. J. Mol. Sci. 2021, 22, 12838. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.M. The proteins of hair and other hard α-keratins. In Cellular and Molecular Biology of Intermediate Filaments; Goldman, R.D.S., Peter, M., Eds.; Plenum: New York, NY, USA, 1990; pp. 95–128. [Google Scholar]
- Li, S.W.; Ouyang, H.S.; Rogers, G.E.; Bawden, C.S. Characterization of the structural and molecular defects in fibres and follicles of the merino felting lustre mutant. Exp. Dermatol. 2009, 18, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.E. Biology of the wool follicle: An excursion into a unique tissue interaction system waiting to be re-discovered. Exp. Dermatol. 2006, 15, 931–949. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Schweizer, J. Human KAP genes, only the half of it? Extensive size polymorphisms in hair keratin-associated protein genes. J. Investig. Dermatol. 2005, 124, vii–ix. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Langbein, L.; Winter, H.; Ehmann, C.; Praetzel, S.; Schweizer, J. Characterization of a first domain of human high glycine-tyrosine and high sulfur keratin-associated protein (KAP) genes on chromosome 21q22.1. J. Biol. Chem. 2002, 277, 48993–49002. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhou, H.; Tao, J.; Hickford, J.G. Characterisation of ovine KRTAP19-3 and its impact on wool traits in Chinese Tan sheep. Animals 2024, 14, 2772. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhou, H.; Li, W.; Tao, J.; Hickford, J.G. Exploring variation in ovine KRTAP19-5 and its effect on fine wool fibre curvature in Chinese Tan sheep. Animals 2024, 14, 2155. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Visnovska, T.; Gong, H.; Schmeier, S.; Hickford, J.; Ganley, A.R. Contrasting patterns of coding and flanking region evolution in mammalian keratin associated protein-1 genes. Mol. Phylogenet. Evol. 2019, 133, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Gong, H.; Wang, J.; Dyer, J.M.; Luo, Y.; Hickford, J.G. Identification of four new gene members of the KAP6 gene family in sheep. Sci. Rep. 2016, 6, 24074. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhou, H.; He, J.; Tao, J.; Hickford, J.G. Characterisation of three ovine KRTAP13 family genes and their association with wool traits in Chinese Tan sheep. Animals 2024, 14, 2862. [Google Scholar] [CrossRef] [PubMed]
- Reudelhuber, T. Molecular biology: Upstream and downstream control of eukaryotic genes. Nature 1984, 312, 700–701. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Wainwright, M.S.; Comeron, J.M.; Saitou, N.; Sanders, A.R.; Gelernter, J.; Gejman, P.V. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum. Mol. Genet. 2003, 12, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Kimchi-Sarfaty, C.; Oh, J.M.; Kim, I.W.; Sauna, Z.E.; Calcagno, A.M.; Ambudkar, S.V.; Gottesman, M.M. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 2007, 315, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Wang, J.; Zhou, H.; Gong, H.; Tao, J.; Hickford, J.G. Identification of ovine KRTAP28-1 and its association with wool fibre diameter. Animals 2019, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, Z.; Xu, H.; Qu, L.; Zhao, H.; Li, T.; Zhang, Z. Variation and expression of KAP9.2 gene affecting cashmere trait in goats. Mol. Biol. Rep. 2012, 39, 10525–10529. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.; Visser, C.; van Marle-Köster, E. Identification of novel variants for KAP 1.1, KAP 8.1 and KAP 13.3 in South African goats. Small Rumin. Res. 2017, 149, 176–180. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, W.; Zhang, F.; Shao, Y.; Yu, S. The polymorphism of a novel mutation of KAP13.1 gene and its associations with cashmere traits on Xinjiang local goat breed in China. Asian J. Anim. Vet. Adv. 2010, 5, 34–42. [Google Scholar] [CrossRef]
- Li, M.; Liu, X.; Wang, J.; Li, S.; Luo, Y. Molecular characterization of caprine KRTAP13-3 in Liaoning cashmere goat in China. J. Appl. Anim. Res. 2014, 42, 140–144. [Google Scholar] [CrossRef]
- Broda, J.; Paczkowska, E. Surface morphology and physical properties of sheep wool of selected Polish breeds. Pol. J. Mater. Environ. Eng. 2021, 1, 46–53. [Google Scholar] [CrossRef]
- Dmitriev, N.G.; Ernst, L.K. (Eds.) Animal Genetic Resources of the USSR; FAO Animal Production and Health Paper No. 65; Food and Agricultural Organization of the Unite Nations: Rome, Italy, 1989. [Google Scholar]
- Cao, C.; Kang, Y.; Zhou, Q.; Nanaei, H.A.; Bo, D.; Liu, P.; Bai, Y.; Li, R.; Jiang, Y.; Lan, X. Whole-genome resequencing reveals the genomic diversity and signatures of selection in Romanov sheep. J. Anim. Sci. 2023, 101, skad291. [Google Scholar] [CrossRef] [PubMed]
- Deniskova, T.; Dotsev, A.; Selionova, M.; Wimmers, K.; Reyer, H.; Kharzinova, V.; Brem, G.; Zinovieva, N. Whole-genome single nucleotide polymorphism study of Romanov sheep. J. Anim. Sci. 2017, 95, 339–340. [Google Scholar] [CrossRef][Green Version]
- Deniskova, T.E.; Dotsev, A.V.; Selionova, M.I.; Kunz, E.; Medugorac, I.; Reyer, H.; Wimmers, K.; Barbato, M.; Traspov, A.A.; Brem, G. Population structure and genetic diversity of 25 Russian sheep breeds based on whole-genome genotyping. Genet. Sel. Evol. 2018, 50, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hickford, J.G.H.; Fang, Q. A two-step procedure for extracting genomic DNA from dried blood spots on filter paper for polymerase chain reaction amplification. Anal. Biochem. 2006, 354, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.O.; Fang, Q.; Zhou, H.; Hickford, J.G.H. An effective method for silver-staining DNA in large numbers of polyacrylamide gels. Anal. Biochem. 2009, 385, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhou, H.; Plowman, J.E.; Dyer, J.M.; Hickford, J.G. Analysis of variation in the ovine ultra-high sulphur keratin-associated protein KAP5-4 gene using PCR-SSCP technique. Electrophoresis 2010, 31, 3545–3547. [Google Scholar] [CrossRef] [PubMed]
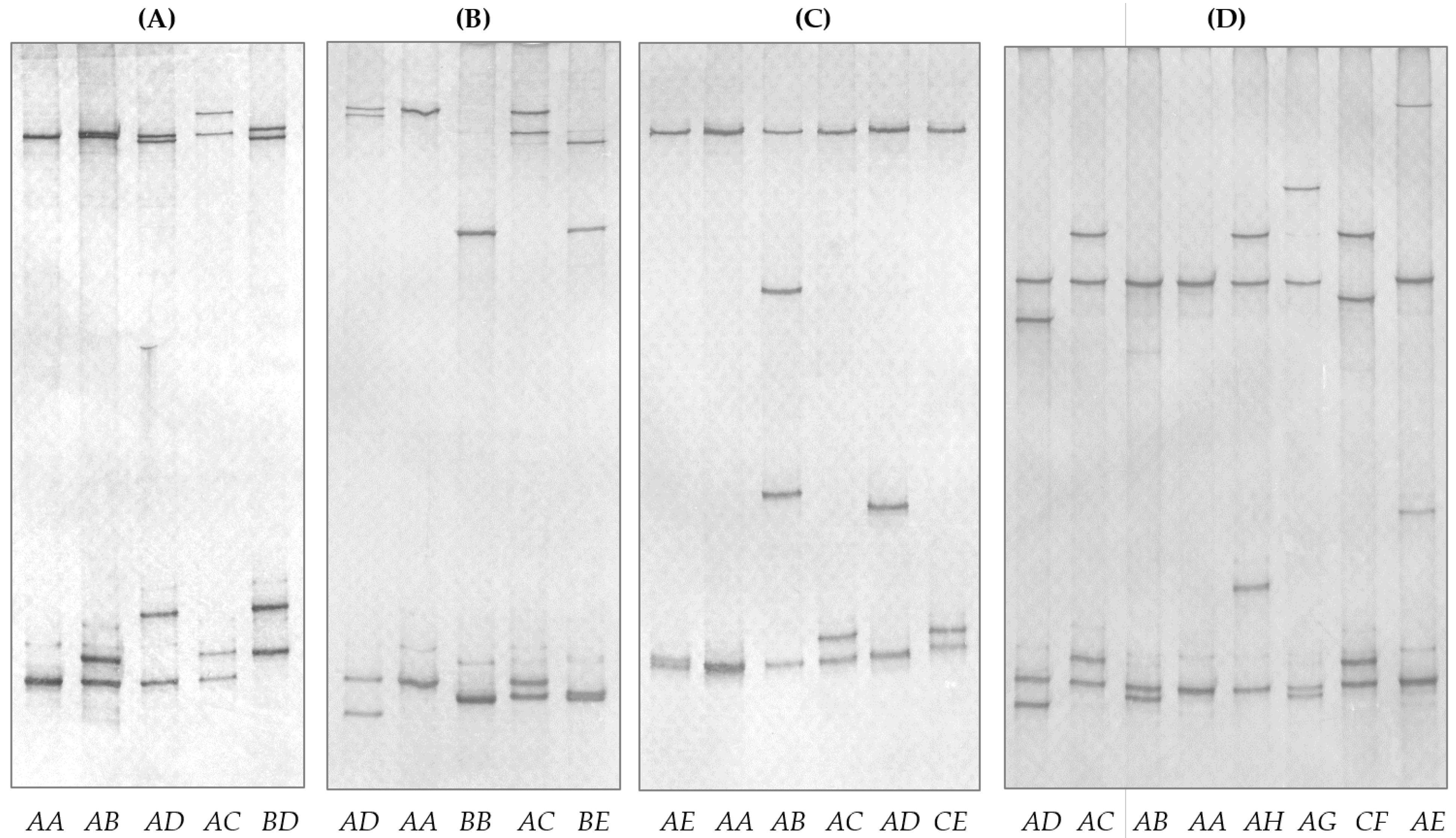


| Gene 1 | Variant Frequency (%) | Het 2 (%) | PIC 3 (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | |||
| 19-1 | 76.1 | 15.2 | 8.4 | 0.3 | 39.1 | 35.6 | ||||
| 19-2 | 50.5 | 26.1 | 12.8 | 6.7 | 3.9 | 65.5 | 60.1 | |||
| 19-4 | 94.7 | 3.0 | 1.0 | 1.0 | 0.3 | 10.2 | 10.1 | |||
| 19-6 | 62.4 | 22.3 | 7.7 | 3.9 | 3.0 | 0.3 | 0.2 | 0.2 | 55.3 | 50.6 |
| Gene 1 | Primer Sequence (5′-3′) | Expected Size | Annealing Temperature | SSCP Condition |
|---|---|---|---|---|
| 19-1 | CTCCCATAAATGCACACTTTG | 484 bp | 58 °C | 22 °C, 360 V |
| TCTTAAGGTTTACATGTATACAG | ||||
| 19-2 | TTCCCACGTGTTCTGACAAG | 366 bp | 60 °C | 22 °C, 320 V |
| CATTCTTGGTAGCAGATGTTG | ||||
| 19-4 | AAGGAATGACCACATGTTTTC | 416 bp | 60 °C | 17.5 °C, 360 V |
| CAGTTCTTCAACCGAATAATG | ||||
| 19-6 | GCCTCCCGCAAATGTACAG | 462 bp | 58 °C | 22 °C, 320 V |
| AGAGTAATCTTAATTCATTGATTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, L.; Zhou, H.; He, J.; Tao, J.; Yang, G.; Hickford, J.G.H. Characterisation of Four New Genes in the Ovine KAP19 Family. Int. J. Mol. Sci. 2025, 26, 6863. https://doi.org/10.3390/ijms26146863
Bai L, Zhou H, He J, Tao J, Yang G, Hickford JGH. Characterisation of Four New Genes in the Ovine KAP19 Family. International Journal of Molecular Sciences. 2025; 26(14):6863. https://doi.org/10.3390/ijms26146863
Chicago/Turabian StyleBai, Lingrong, Huitong Zhou, Jianning He, Jinzhong Tao, Guo Yang, and Jon G. H. Hickford. 2025. "Characterisation of Four New Genes in the Ovine KAP19 Family" International Journal of Molecular Sciences 26, no. 14: 6863. https://doi.org/10.3390/ijms26146863
APA StyleBai, L., Zhou, H., He, J., Tao, J., Yang, G., & Hickford, J. G. H. (2025). Characterisation of Four New Genes in the Ovine KAP19 Family. International Journal of Molecular Sciences, 26(14), 6863. https://doi.org/10.3390/ijms26146863








