Antibacterial, Photoprotective, Anti-Inflammatory, and Selected Anticancer Properties of Honokiol Extracted from Plants of the Genus Magnolia and Used in the Treatment of Dermatological Problems—A Review
Abstract
1. Introduction
2. Systematics and Occurrence of Magnolia Genus Plants Serving as Honokiol Sources
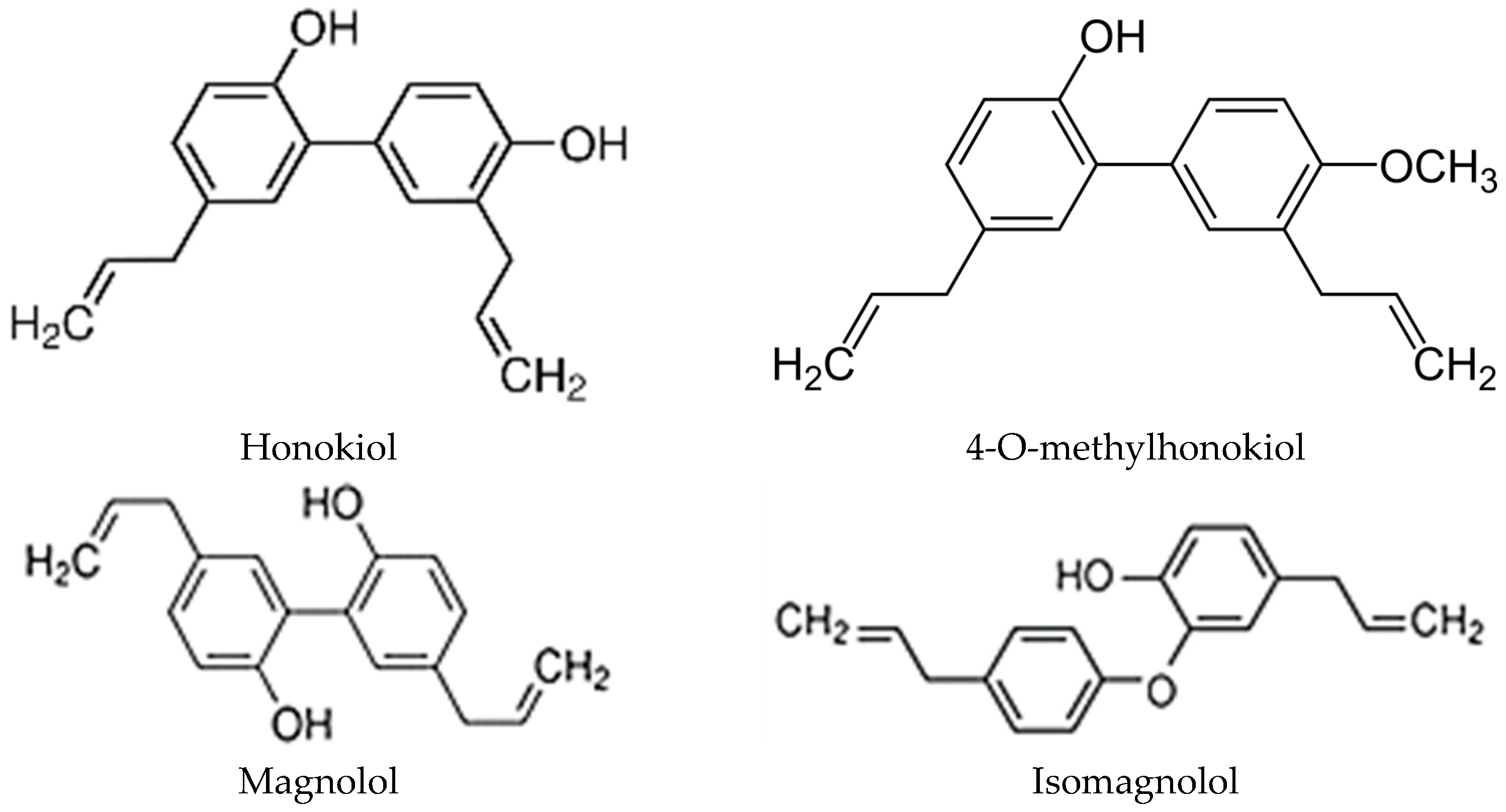
3. Honokiol, Its Derivatives, and Other Active Compounds in Magnolia Extracts
4. Methods of Honokiol Extraction
5. Honokiol in Experiments
6. Honokiol Content in Selected Organs of Several Magnolia Species
7. Structure and Physicochemical Properties of Honokiol
8. Therapeutic Properties of Honokiol in Certain Diseases
9. Selected Biological Properties of Honokiol
9.1. Antiviral and Virucidal Activity of Honokiol
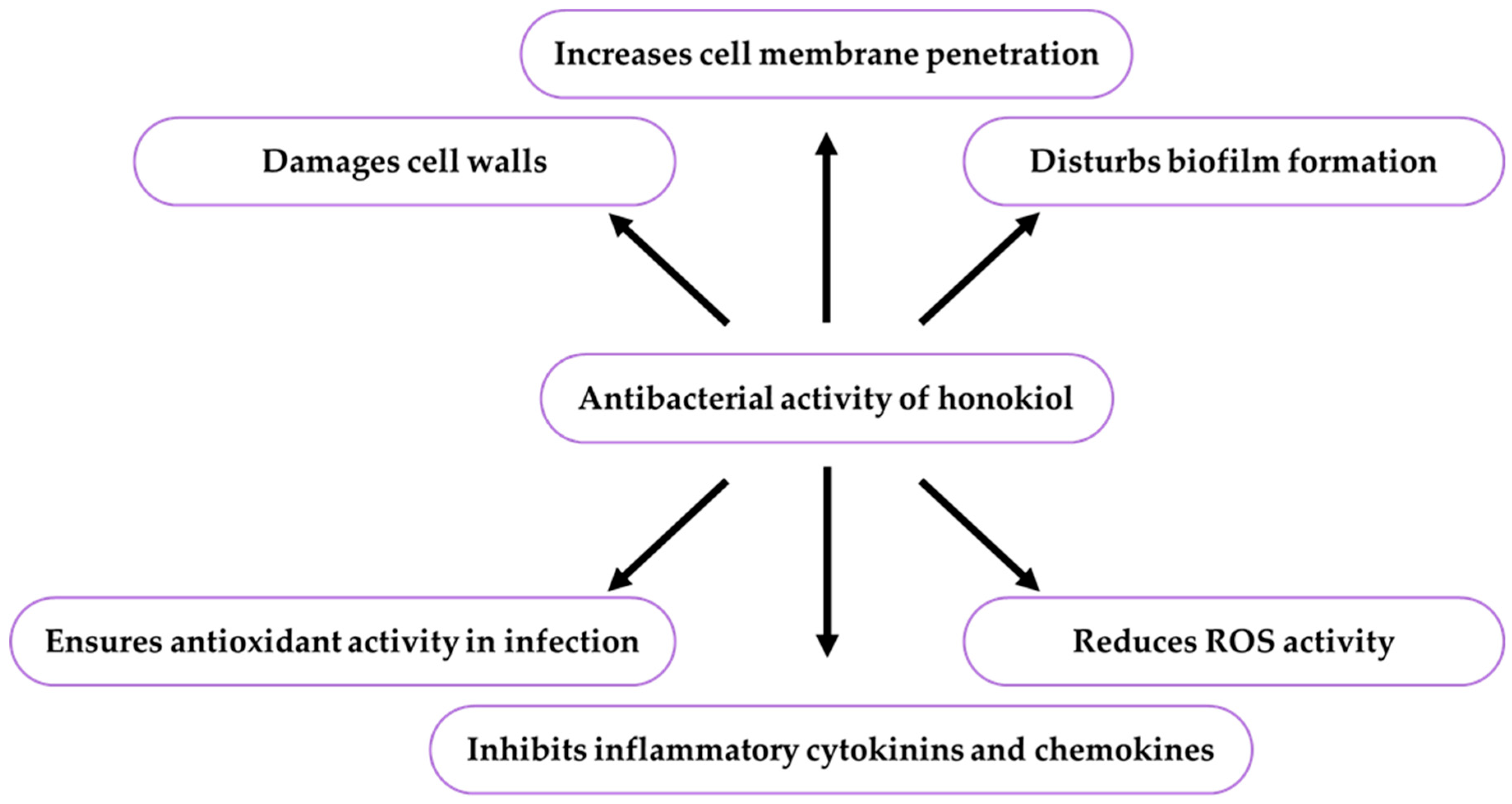
9.2. Antibacterial Activity of Honokiol
Effect of Honokiol on Biofilm Formation
9.3. Antifungal Activity of Honokiol
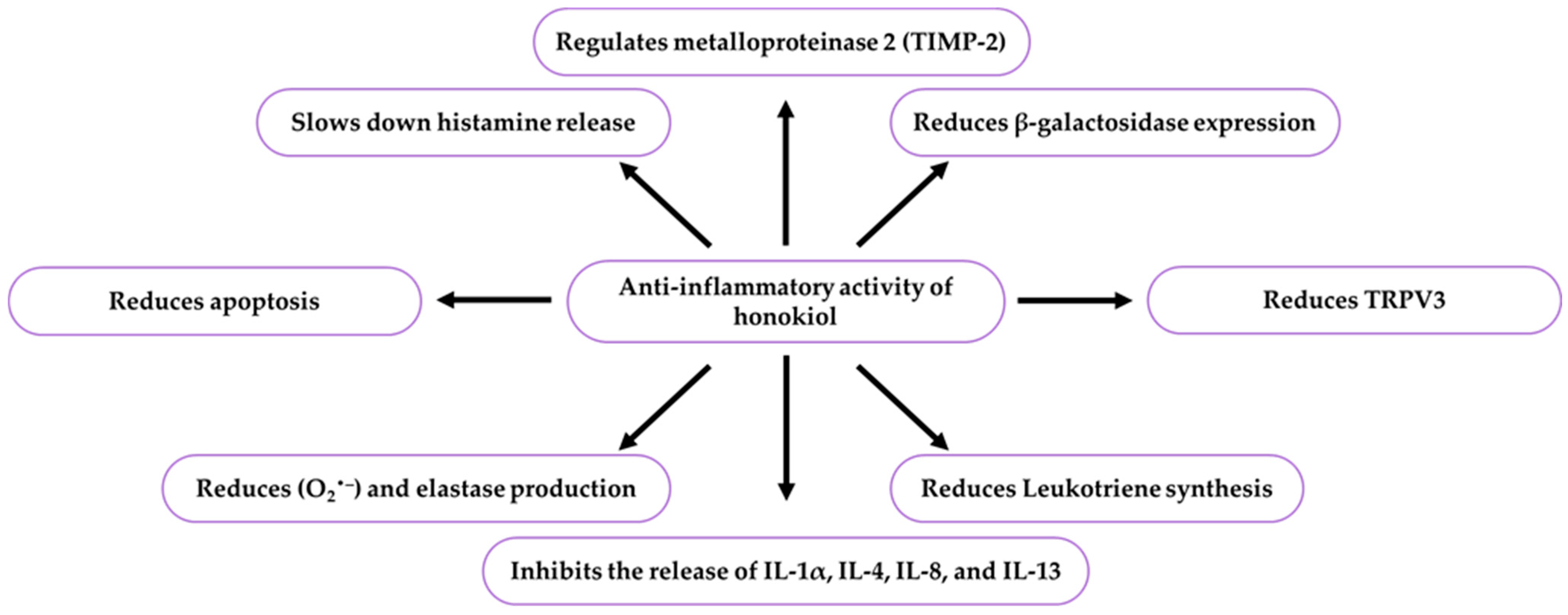
9.4. Anti-Inflammatory Activity of Honokiol
9.5. Photoprotective Activity of Honokiol
9.6. Anticancer Activity of Honokiol
9.7. Activity in Skin Cancer Treatment
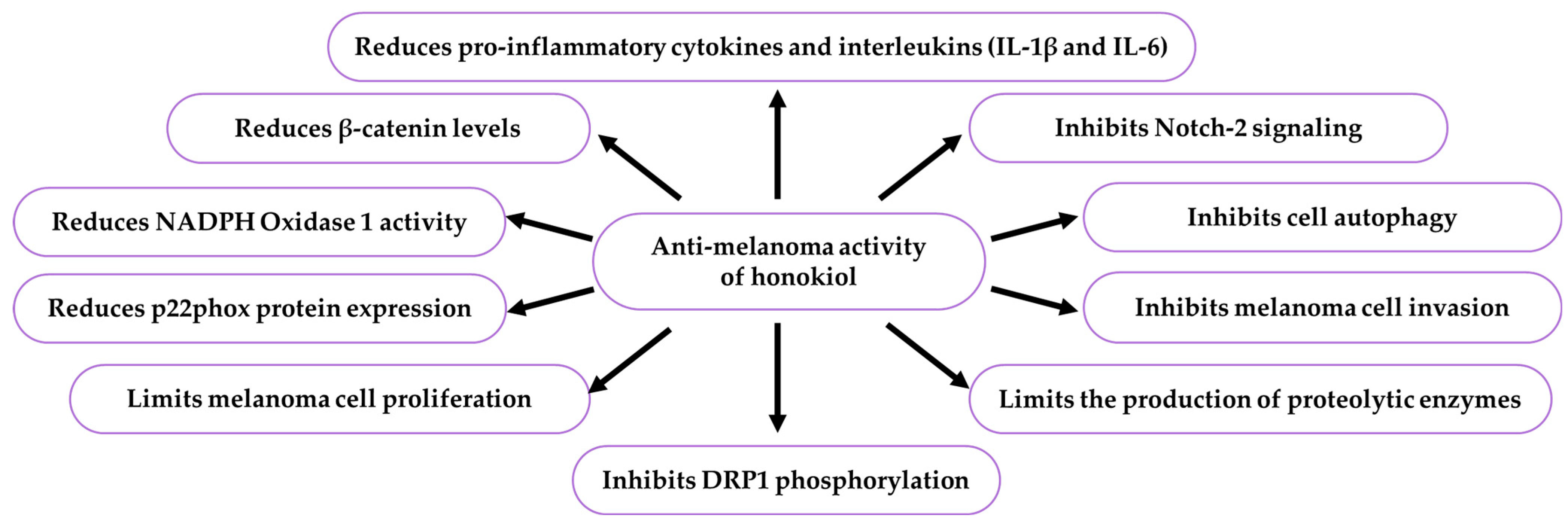
9.7.1. Activity Against Melanoma
9.7.2. Activity Against Non-Melanoma Skin Cancers
Honokiol in the Treatment of Basalioma
Honokiol in the Treatment of Squamous Cell Carcinoma
10. Future Directions
11. Materials and Methods
11.1. Review Design
11.2. Bibliographic Databases and Search Phrases
11.3. Number and Dates of Publication of Articles
11.4. Citations of Literature References
| Year of Publication | Numerical Position of Publications Cited in the Manuscript | Citations Number of the Individual Publications | Total Citations Number in Each Year |
|---|---|---|---|
| 2002–2005 | [54,55] *,[59] *, [78] E, [177] ***, [77] E | 17,16,13,133,332,9 | 520 |
| 2006, 2007, 2009,2010 | [76,80] E, [79] E, [60] *, [145] **, [166,197] ** | 71,22,136,1,6,86,73, | 392 |
| 2011–2013 | [56] *, [61] *, [73] E, [193] **, [62] * | 0,1,0,13,0 | 14 |
| 2015 | [108,114,123,128,148,156,158,180,207,220,222] *** | 125,6,48,48,79,31,47,740,53,84,0, | 1161 |
| 2016 | [1,71,75,102,113,118,126,160,162,177,184,186,190,197, 198,219,223] *** | 898,16,71,21,49,71,46,83,23,0,8,61,76, 32,29,36,36 | 1545 |
| 2017 | [9,10,72,100,124,136,176,192,193,195,201,221] *** | 29,45,95,187,0,37,0,59,44,2,63,24 | 585 |
| 2018 | [11,12,15,79,129,157,167,172,173,174,199,202,218] *** | 10,12,20,236,81,30,28,45,25,93,57,13,31 | 681 |
| 2019 | [4,6,13,29,30,76,88,90,95,101,103,104,109,121,131,133, 137,145,159,187,188,189,194,205] *** | 67,45,5,64,3,70,58,43,8,136,10,115,180, 44,7,49,29,10,36,77,48,3,24,0 | 1093 |
| 2020 | [31,47,53,68,73,80,82,93,94,116,122,143,164,168,185, 224,225,226] *** | 119,27,173,11,11,11,1,2,17,137,21,39, 39,19,6,5,21,1704 | 3023 |
| 2021 | [7,16,20,23,24,40,46,48,69,74,86,107,110,115,132,140, 144,146,150,154,155,161,163,166,170,182,191,200,203, 204,227,228,229] *** | 16,45,1,6,167,64,19,26,7,28,8,85,4,38,37,16,25, 20,16,100,47,50,6,21,12,39,28,0,30,20,316,12,18 | 2001 |
| 2022 | [2,3,14,35,45,63,64,87,97,105,106,125,147,149,151,183, 206,212,213,217,230] *** | 15,12,0,26,26,5,63,14,2,25,0,52,0,6,26, 3,23,4,0,10,0 | 312 |
| 2023 | [5,17,18,26,32,54,56,65,77,81,84,89,96,98,111,117,119, 130,138,141,152,165,169,171,209,215,231,232,233] *** | 4,35,16,19,5,5,20,9,15,9,8,34,4,3,6,15,32,23,7, 17,12,11,53,18,4,4,2,88,13 | 883 |
| 2024 | [8,21,22,25,27,28,33,39,52,66,78,83,85,91,92,99,120,134,135,142,208,210,211,216,234,235,236,237] *** | 9,4,7,0,3,3,5,0,8,18,7,2,0,7,3,0,5,3,19,9,13,12,19, 31,27,21,10,12,0 | 257 |
| 2025 | [19,34,49,50,51,57,67,70,112,181,196,214] *** | 1,10,0,2,8,2,2,2,0,0,0,5 | 33 |
| Total citation | 12,500 | ||
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef]
- Li, Y.; Liang, C.; Zhou, X. The application prospects of honokiol in dermatology. Dermatol. Ther. 2022, 35, e15658. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Im, D.S. Honokiol suppresses 2,6-dinitrochlorobenzene-induced atopic dermatitis in mice. J. Ethnopharmacol. 2022, 289, 115023. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Tong, C.; Fu, Q.; Xu, J.; Shi, S.; Xiao, Y. Identification of minor lignans, alkaloids, and phenylpropanoid glycosides in Magnolia officinalis by HPLC-DAD-QTOF-MS/MS. J. Pharm. Biomed. Anal. 2019, 170, 153–160. [Google Scholar] [CrossRef]
- Saha, P.; Saha, S.; Semwal, A.; Prinsa, P.; Parashar, T.; Jakhmola, V. Geographical distribution, chemical constituents, and activity profile of Magnolia. Trad. Med. J. 2023, 28, 122–131. [Google Scholar] [CrossRef]
- Oufensou, S.; Scherm, B.; Pani, G.; Balmas, V.; Fabbri, D.; Dettori, M.A.; Carta, P.; Malbrán, I.; Migheli, Q.; Delogu, G. Honokiol, magnolol and its monoacetyl derivative show strong anti-fungal effect on Fusarium isolates of clinical relevance. PLoS ONE 2019, 14, e0221249. [Google Scholar] [CrossRef]
- Trifan, A.; Bostănaru, A.C.; Luca, S.V.; Temml, V.; Akram, M.; Herdlinger, S.; Kulinowski, Ł.; Skalicka-Woźniak, K.; Granica, S.; Czerwińska, M.E.; et al. Honokiol and magnolol: Insights into their antidermatophytic effects. Plants 2021, 10, 2522. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Li, Q.; Xiong, S.; Yang, Y.; Yang, Y.; Huang, C.; Wang, Z.P. Delivery strategies, structural modification, and pharmacological mechanisms of honokiol: A comprehensive review. Chem. Biodivers. 2024, 21, e202302032. [Google Scholar] [CrossRef]
- Costa, A.; Facchini, G.; Pinheiro, A.L.T.A.; da Silva, M.S.; Bonner, M.Y.; Arbiser, J.; Eberlin, S. Honokiol protects skin cells against inflammation, collagenolysis, apoptosis, and senescence caused by cigarette smoke damage. Int. J. Dermatol. 2017, 56, 754–761. [Google Scholar] [CrossRef]
- Prasad, R.; Singh, T.; Katiyar, S.K. Honokiol inhibits ultraviolet radiation-induced immunosuppression through inhibition of ultraviolet-induced inflammation and DNA hypermethylation in mouse skin. Sci. Rep. 2017, 7, 1657. [Google Scholar] [CrossRef]
- Wu, W.; Tang, M.H.; Tang, H.; Chen, K.; Fu, J.; Wang, L.; Xue, L.L.; Peng, A.; Ye, H.; Chen, L.J. Identification, characterization and HPLC quantification of formulation-related impurities of honokiol, an antitumor natural drug candidate in clinical trials. J. Pharm. Biomed. Anal. 2018, 153, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Huang, T.H.; Hung, C.F.; Huang, Y.L.; Aljuffali, I.A.; Liao, W.C.; Lin, C.F. Derivatization of honokiol by integrated acetylation and methylation for improved cutaneous delivery and anti-inflammatory potency. Eur. J. Pharm. Sci. 2018, 114, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.P.; Lee, W.L.; Tang, Y.Q.; Yap, W.H. Honokiol: A review of its anticancer potential and mechanisms. Cancers 2019, 12, 48. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, L.; Zhou, X. Honokiol improves acne-like lesions in a rabbit ear model by alleviating hyperkeratosis and sebum secretion. Nat. Prod. Commun. 2022, 17, 1–7. [Google Scholar] [CrossRef]
- Zhou, C.; Guo, C.; Li, W.; Zhao, J.; Yang, Q.; Tan, T.; Wan, Z.; Dong, J.; Song, X.; Gong, T. A novel honokiol liposome: Formulation, pharmacokinetics, and antitumor studies. Drug Dev. Ind. Pharm. 2018, 44, 2005–2012. [Google Scholar] [CrossRef]
- Usach, I.; Alaimo, A.; Fernández, J.; Ambrosini, A.; Mocini, S.; Ochiuz, L.; Peris, J.E. Magnolol and honokiol: Two natural compounds with similar chemical structure but different physicochemical and stability properties. Pharmaceutics 2021, 13, 224. [Google Scholar] [CrossRef]
- Khatoon, F.; Ali, S.; Kumar, V.; Elasbali, A.M.; Alhassan, H.H.; Alharethi, S.H.; Islam, A.; Hassan, I. Pharmacological features, health benefits and clinical implications of honokiol. J. Biomol. Struct. Dyn. 2023, 41, 7511–7533. [Google Scholar] [CrossRef]
- Yang, J.; Shang, J.; Yang, L.; Wei, D.; Wang, X.; Deng, Q.; Zhong, Z.; Ye, Y.; Zhou, M. Nanotechnology-based drug delivery systems for honokiol: Enhancing therapeutic potential and overcoming limitations. Int. J. Nanomed. 2023, 18, 6639–6665. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.T.; Nguyen, N.H.; Do, H.T.M.; Vu, C.M.; Nguyen, P.T.M.; Chu, H.H. Honokiol-loaded PLGA-PEG nanoparticles with solubility in water for infusion treatment of solid cancer. J. Drug Deliv. Sci. Technol. 2025, 103, 106436. [Google Scholar] [CrossRef]
- Choi, Y.H. Honokiol attenuates oxidative stress-induced cytotoxicity in human keratinocytes via activating AMPK signaling. Asian Pac. J. Trop. Biomed. 2021, 11, 222–230. [Google Scholar] [CrossRef]
- Thi, H.D.; Kim, J.Y.; Kim, H.J.; Kim, W.K.; Kim, S.J.; Nam, J.H. Inhibition of Ca2+ permeable TRPV3 and inflammatory cytokine release by honokiol and magnolol in human epidermal keratinocytes. Biochem. Biophys. Res. Commun. 2024, 692, 149332. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, F.; Tao, L.; Jiang, Y.; Huang, T.; Li, Y.; Hu, Z.; Yang, J.; Hao, X.; Yuan, C. Three rare anti-inflammatory sesquiterpene lactones from Magnolia grandiflora. Chin. J. Nat. Med. 2024, 22, 265–272. [Google Scholar] [CrossRef]
- Chen, X.; Shi, B.L.; Qi, R.Z.; Chang, X.; Zheng, H.G. Ultra-performance liquid chromatography/mass spectrometry-based metabolomics for discovering potential biomarkers and metabolic pathways of colorectal cancer in mouse model (ApcMin/+) and revealing the effect of honokiol. Front. Oncol. 2021, 11, 671014. [Google Scholar] [CrossRef]
- Rauf, A.; Olatunde, A.; Imran, M.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Khan, S.A.; Uddin, M.S.; Mitra, S.; Emran, T.B.; Khayrullin, M.; et al. Honokiol: A review of its pharmacological potential and therapeutic insights. Phytomedicine 2021, 90, 153647, Erratum in: Phytomedicine 2021, 92, 153769. [Google Scholar] [CrossRef]
- Li, Z.; Jia, K.; Zhou, J. The improvement effect of Magnolia officinalis oil extract on non-alcoholic fatty liver disease and its mechanism of regulating inflammatory pathways and oxidative stress in HepG2 cells. Adv. Biomed. Res. 2024, 5, 65–74. [Google Scholar] [CrossRef]
- Faysal, M.; Khan, J.; Zehravi, M.; Nath, N.; Singh, L.P.; Kakkar, S.; Perusomula, R.; Khan, P.A.; Nainu, F.; Asiri, M.; et al. Neuropharmacological potential of honokiol and its derivatives from chinese herb Magnolia species: Understandings from therapeutic viewpoint. Chin. Med. 2023, 18, 154. [Google Scholar] [CrossRef]
- Xiao, L.; Zhao, A.; Qiu, J.; Liu, A.; Chen, S.; Jiang, J.; Zhang, J.; Guo, C.; Di, J.; Cheng, J.; et al. Comprehensive reutilization of herbal waste: Coproduction of magnolol, honokiol, and β-amyrin from Magnolia officinalis residue. Green Energy Environ. 2024, 9, 403–412. [Google Scholar] [CrossRef]
- Zang, Y.D.; Zang, C.X.; Tian, J.Y.; Xu, K.L.; Li, C.; Li, C.J.; Yang, Y.; Ye, F.; Zhang, D.; Zhang, D.M.; et al. Chiral separation and bioactivities of six pairs of enantiomeric dilignans from Magnolia officinalis var. biloba. Phytochemistry 2024, 219, 113964. [Google Scholar] [CrossRef]
- Li, Y.; Sylvester, S.P.; Li, M.; Zhang, C.; Li, X.; Duan, Y.; Wang, X. The complete plastid genome of Magnolia zenii and genetic comparison to Magnoliaceae species. Molecules 2019, 24, 261. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Park, S.; Sun, W.; Kim, S. Complete chloroplast genome sequence of Magnolia sinica (Y.W. Law) Noot. (magnoliaceae), a critically endangered species with extremely small populations in Magnoliaceae. Mitochondrial DNA B Resour. 2019, 4, 242–243. [Google Scholar] [CrossRef]
- Wang, Y.B.; Liu, B.B.; Nie, Z.L.; Chen, H.F.; Chen, F.J.; Figlar, R.B.; Wen, J. Major clades and a revised classification of Magnolia and Magnoliaceae based on whole plastid genome sequences via genome skimming. J. Syst. Evol. 2020, 58, 673–695. [Google Scholar] [CrossRef]
- Shen, Z.; Ding, X.; Cheng, J.; Wu, F.; Yin, H.; Wang, M. Phylogenetic studies of magnoliids: Advances and perspectives. Front. Plant Sci. 2023, 13, 1100302. [Google Scholar] [CrossRef]
- Doyle, J.A.; Endress, P.K. Integrating cretaceous fossils into the phylogeny of living angiosperms: Fossil Magnoliales and their evolutionary implications. Int. J. Plant Sci. 2024, 185, 42–70. [Google Scholar] [CrossRef]
- Helmstetter, A.J.; Ezedin, Z.; Lirio, E.J.; Oliveira, S.M.; Chatrou, L.W.; Erkens, R.H.J.; Larridon, I.; Leempoel, K.; Maurin, O.; Roy, S.; et al. Towards a phylogenomic classification of magnoliids. Am. J. Bot. 2025, 112, e16451. [Google Scholar] [CrossRef]
- Xie, H.; Tang, Y.; Fu, J.; Chi, X.; Du, W.; Dimitrov, D.; Liu, J.; Xi, Z.; Wu, J.; Xu, X. Diversity patterns and conservation gaps of Magnoliaceae species in China. Sci. Total Environ. 2022, 813, 152665. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Latif, Z.; Stewart, M.; Nahar, L. Phytochemistry of the genus Magnolia. In Magnolia. The Genus Magnolia, 1st ed.; Satyajit, D.S., Yuji, M., Eds.; Taylor & Francis: Hoboken, NJ, USA; CRC Press: London, UK, 2002; pp. 21–74. [Google Scholar]
- Watanabe, K.; Ikegami, F.; Horie, S. Introduction—The Genus Magnolia. In Magnolia. The Genus Magnolia, 1st ed.; Satyajit, D.S., Yuji, M., Eds.; Taylor & Francis: Hoboken, NJ, USA; CRC Press: London, UK, 2002; pp. 1–7. [Google Scholar]
- Turner, I.M. The taxa described by Paul Évariste Parmentier in histoire des magnoliacées. Adansonia 2011, 34, 237–249. [Google Scholar] [CrossRef]
- Barbosa, J.C.J.; Caruzo, M.B.R.; Simões, A.R.G.; Samain, M.S. Taxonomic revision of the native Magnolia (Magnoliaceae) species of Brazil. PhytoKeys 2024, 238, 33–64. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yin, Q.; Sang, Z.; Zhu, Z.; Jia, Z.; Ma, L. Prediction of potentially suitable areas for the introduction of Magnolia wufengensis under climate change. Ecol. Indic. 2021, 127, 107762. [Google Scholar] [CrossRef]
- Soika, G.; Łabanowski, G. Spider mites [Tetranychidae] recorded on ornamental trees and shrubs in nurseries. J. Plant Prot. Res. 2003, 43, 105–112. [Google Scholar]
- Marczewski, A. New woody cultivars from the botanical garden of the polish academy of sciences. Rocznik PTD 2009, 57, 39–43. [Google Scholar]
- Michalojć, Z.; Jarosz, Z. Decorative values and the nutritional status of some Magnolia L. species under the climatic conditions of Lublin (Poland). Part I. Decorative values of the plants. Acta Agrobot. 2012, 65, 125–131. [Google Scholar] [CrossRef][Green Version]
- Sobisz, Z.; Truchan, M.; Osadowski, Z. Monumental forme, manor parks of the Drawsko Lakeland. Rocz. Akad. Rol. W Poznaniu. Bot.-Steciana 2013, 17, 177–197. [Google Scholar][Green Version]
- Podwyszyńska, M.; Orlikowska, T.; Trojak-Goluch, A.; Wojtania, A. Application and improvement of in vitro culture systems for commercial production of ornamental, fruit, and industrial plants in Poland. Acta Soc. Bot. Pol. 2022, 91, 914. [Google Scholar] [CrossRef]
- Vázquez-García, J.A.; Muñiz-Castro, M.A.; Dahua-Machoa, A.; Osorio-Muñoz, E.A.; Hernández-Vera, G.; Ortega-Peña, A.S.; de Lourdes Romo-Campos, R.; Jacobo-Pereira, C.; de Román, Á.N.; Shalisko, V. How to save endangered magnolias? From population biology to conservation action: The case of allopatric radiation in western Mexico. In Endangered Plants; Kumar, S., Ed.; IntechOpen: London, UK, 2021; pp. 13–56. [Google Scholar] [CrossRef]
- Bui, D.; Li, L.; Yin, T.; Wang, X.; Gao, S.; You, M.; Singh, R.; Hu, M. Pharmacokinetic and metabolic profiling of key active components of dietary supplement Magnolia officinalis extract for prevention against oral carcinoma. J. Agric. Food Chem. 2020, 68, 6576–6587. [Google Scholar] [CrossRef]
- Szałabska-Rąpała, K.; Borymska, W.; Kaczmarczyk-Sedlak, I. Effectiveness of magnolol, a lignan from Magnolia bark, in diabetes, its complications and comorbidities—A review. Int. J. Mol. Sci. 2021, 22, 10050. [Google Scholar] [CrossRef]
- Li, S.; Jiao, G.; Ou, P.; Zhang, X.; Yu, Y.; Wang, Y.; Yao, Q.; Wang, W. Phytochemical characterization, antioxidant activity, and anti-melanoma mechanism of fower buds of Magnolia biondii Pamp. Plants 2025, 14, 1725. [Google Scholar] [CrossRef]
- Siudem, P.; Wasiak, A.; Zielińska, A.; Kowalska, V.; Paradowska, K. Using lignans from Magnolia officinalis bark in the assessment of the quality of dietary supplements—The application of 1H NMR and HPLC-DAD. Int. J. Mol. Sci. 2025, 26, 1659. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, J.; Cao, H.; Wang, C.; Shen, C.; Liu, J. Effect and mechanism of Magnolia officinalis in colorectal cancer: Multi-component-multi-target approach. J. Ethnopharmacol. 2025, 338, 119007. [Google Scholar] [CrossRef]
- Cristea, R.M.; Sava, C.; Căpățână, C.; Kanellou, A. Phytochemical analysis and specific activities of bark and flower extracts from four Magnolia plant species. Horticulturae 2024, 10, 141. [Google Scholar] [CrossRef]
- Luo, L.; Nong Wang, J.; Kong, L.D.; Jiang, Q.G.; Tan, R.X. Antidepressant effects of Banxia Houpu decoction, a traditional Chinese medicinal empirical formula. J. Ethnopharmacol. 2020, 73, 277–281. [Google Scholar] [CrossRef]
- Suzuki, T.; Kikuchi, A.; Kaneko, A.; Arita, R.; Nogami, T.; Saito, N.; Takayama, S. A review of frequently used Kampo prescriptions: Part 2—Hangekobokuto. Tradit. Kampo Med. 2023, 10, 103–119. [Google Scholar] [CrossRef]
- Zhang, D.; Yan, X.W.; Wang, G.; Wen, X.F.; Li, X.M.; Luo, D.; Cao, W.G. Optimization of extraction technology of magnolol and honokiol from the leaves of Magnolia officinalis Rehd. et Wils. Med. Plant 2012, 3, 45. [Google Scholar]
- Xu, J.; Xu, H. Magnolol: Chemistry and biology. Ind. Crops Prod. 2023, 205, 117493. [Google Scholar] [CrossRef]
- Sampieri-Morán, J.M.; Bravo-Alfaro, D.A.; Uribe-Lam, E.; Luna-Barcenas, G.; Montiel-Sanchez, M.; Velasco-Rodriguez, L.D.C.; Acosta-Osoria, A.; Ferrer, M.; Garcia, H.S. Delivery of Magnolia bark extract in nanoemulsions formed by high and low energy methods improves the bioavailability of honokiol and magnolol. Eur. J. Pharm. Biopharm. 2025, 208, 114627. [Google Scholar] [CrossRef]
- Amblard, F.; Delinsky, D.; Arbiser, J.L.; Schinazi, R.F. Facile purification of honokiol and its antiviral and cytotoxic properties. J. Med. Chem. 2006, 49, 3426–3427. [Google Scholar] [CrossRef]
- Sun, A.L.; Feng, L.; Liu, R.M. Preparative isolation and purification of honokiol and magnolol from Magnolia officinalis Rehd. et Wils by high-speed countercurrent chromatography. Fen Xi Hua Xue Bian Ji Bu 2005, 33, 1016–1018. [Google Scholar]
- Wang, X.; Wang, Y.; Geng, Y.; Li, F.; Zheng, C. Isolation and purification of honokiol and magnolol from cortex Magnoliae officinalis by high-speed counter-current chromatography. J. Chromatogr. A. 2004, 1036, 171–175. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Q.; Yang, G.; Fan, L.; Tang, J.; Garrard, I.; Ignatova, S.; Fisher, D.; Sutherland, I.A. Rapid purification and scale-up of honokiol and magnolol using high-capacity high-speed counter-current chromatography. J. Chromatogr. A. 2007, 1142, 115–122, Erratum in: J. Chromatogr. A 2007, 1163, 337. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, X.; Fan, L.; Chen, X.; Hu, Z. Separation and determination of honokiol and magnolol in herbal medicines by flow injection-capillary electrophoresis. Anal. Bioanal. Chem. 2006, 384, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Park, C.; Hwangbo, H.; Bang, E.; Kim, S.O.; Shim, J.H.; Park, S.H.; Lee, H.; Leem, S.H.; Kim, G.Y.; et al. Activation of heme oxygenase-1 is involved in the preventive effect of honokiol against oxidative damage in human retinal pigment epithelial cells. Biotechnol. Bioprocess Eng. 2022, 27, 975–986. [Google Scholar] [CrossRef]
- Qu, C.; Li, Q.P.; Su, Z.R.; Ip, S.P.; Yuan, Q.J.; Xie, Y.L.; Xu, Q.Q.; Yang, W.; Huang, Y.F.; Xian, Y.F.; et al. Nano-Honokiol ameliorates the cognitive deficits in TgCRND8 mice of Alzheimer’s disease via inhibiting neuropathology and modulating gut microbiota. J. Adv. Res. 2022, 35, 231–243. [Google Scholar] [CrossRef]
- Mikhaevich, E.I.; Sorokin, D.V.; Scherbakov, A.M. Honokiol inhibits the growth of hormone-resistant breast cancer cells: Its promising effect in combination with metformin. Res. Pharm. Sci. 2023, 18, 580–591. [Google Scholar] [CrossRef] [PubMed]
- Aktay, I.; Bitirim, C.V.; Olgar, Y.; Durak, A.; Tuncay, E.; Billur, D.; Akcali, K.C.; Turan, B. Cardioprotective role of a magnolol and honokiol complex in the prevention of doxorubicin-mediated cardiotoxicity in adult rats. Mol. Cell. Biochem. 2024, 479, 337–350. [Google Scholar] [CrossRef]
- Liao, K.S.; Lee, Y.R.; Chao, W.Y.; Huang, Y.J.; Chung, H.C.; Chen, S.H.; Li, Y.Z.; Zhao, P.W.; Chang, H.Y. Honokiol suppresses cell proliferation and tumor migration through ros in human anaplastic thyroid cancer cells. Endocr. Metab. Immune Disord. Drug Targets 2025, 25, 251–259. [Google Scholar] [CrossRef]
- Lu, X.; Lu, X.; Zhang, Z.; Lv, H. Preparation and characterization of honokiol nanosuspensions and preliminary evaluation of anti-inflammatory effect. AAPS PharmSciTech 2020, 21, 62. [Google Scholar] [CrossRef]
- Benedé, J.L.; Rodriguez, E.; Chisvert, A.; Salvador, A. Rapid and simple determination of honokiol and magnolol in cosmetic products by liquid chromatography with ultraviolet detection. Anal. Lett. 2021, 54, 1510–1521. [Google Scholar] [CrossRef]
- Solanki, R.; Rawat, L.; Tabasum, S.; Pal, S.; Patel, S.; Sabarwal, A. A comprehensive review of anti-cancer mechanisms of polyphenol honokiol and nano carrier-based approaches to enhance its therapeutic potential. Phytochem. Rev. 2025, 1–27. [Google Scholar] [CrossRef]
- Jeong, H.U.; Kim, J.H.; Kong, T.Y.; Choi, W.G.; Lee, H.S. Comparative metabolism of honokiol in mouse, rat, dog, monkey, and human hepatocytes. Arch. Pharm. Res. 2016, 39, 516–530. [Google Scholar] [CrossRef] [PubMed]
- Talarek, S.; Listos, J.; Barreca, D.; Tellone, E.; Sureda, A.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Neuroprotective effects of honokiol: From chemistry to medicine. Biofactors 2017, 43, 760–769. [Google Scholar] [CrossRef]
- Liu, T.; Liu, H.; Wang, P.; Hu, Y.; Yang, R.; Liu, F.; Kim, H.G.; Dong, Z.; Liu, K. Honokiol inhibits melanoma growth by targeting keratin 18 in vitro and in vivo. Front. Cell Dev. Biol. 2020, 8, 603472. [Google Scholar] [CrossRef]
- Ezzeldeen, Y.; Swidan, S.; ElMeshad, A.; Sebak, A. Green synthesized honokiol transfersomes relieve the immunosuppressive and stem-like cell characteristics of the aggressive B16F10 melanoma. Int. J. Nanomed. 2021, 16, 5693–5712. [Google Scholar] [CrossRef]
- Yu, Y.; Li, M.; Su, N.; Zhang, Z.; Zhao, H.; Yu, H.; Xu, Y. Honokiol protects against renal ischemia/reperfusion injury via the suppression of oxidative stress, iNOS, inflammation and STAT3 in rats. Mol. Med. Rep. 2016, 13, 1353–1360. [Google Scholar] [CrossRef]
- Xia, S.; Lin, H.; Liu, H.; Lu, Z.; Wang, H.; Fan, S.; Li, N. Honokiol attenuates sepsis-associated acute kidney injury via the inhibition of oxidative stress and inflammation. Inflammation 2019, 42, 826–834. [Google Scholar] [CrossRef]
- Debsharma, S.; Pramanik, S.; Bindu, S.; Mazumder, S.; Das, T.; Saha, D.; Rudranil, D.; Nag, S.; Banerjee, C.; Siddiqui, A.A.; et al. Honokiol, an inducer of sirtuin-3, protects against non-steroidal anti-inflammatory drug-induced gastric mucosal mitochondrial pathology, apoptosis and inflammatory tissue injury. Br. J. Pharmacol. 2023, 180, 2317–2340. [Google Scholar] [CrossRef]
- Liu, C.; Cao, Z.; Li, L.; Li, Q.; Zhang, C.; Wang, Y.; Li, L.; Fu, P. Self-assembled Pt/honokiol nanomicelles for the treatment of sepsis-associated acute kidney injury. ACS Biomater. Sci. Eng. 2024, 11, 383–401. [Google Scholar] [CrossRef]
- Sarrica, A.; Kirika, N.; Romeo, M.; Salmona, M.; Diomede, L. Safety and toxicology of magnolol and honokiol. Planta Med. 2018, 84, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, I.; Weil, E. Intravenous honokiol in drug-resistant cancer: Two case reports. Integr. Cancer Ther. 2020, 19, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cai, Z.; Huang, Y.; Liu, X.; Liu, X.; Chen, F.; Li, W. Honokiol in glioblastoma recurrence: A case report. Front. Neurol. 2023, 14, 1172860. [Google Scholar] [CrossRef]
- Xie, B.; Song, S.S.; Wang, Y.F.; Wu, J.B.; Li, X.Y. Effects of honokiol on activation of transient receptor potential channel V1 and secretion of thymic stromal lymphopoietin in HaCaT keratinocytes. Int. J. Dermatol. Venereal. 2020, 3, 31–36. [Google Scholar] [CrossRef]
- Pasikowska-Piwko, M.; Bujak, J.; Debowska, R.; Tyszczuk, B.; Kuranc, A.; Bednarczyk, P.; Rogiewicz, A.; Eris, I. Magnolol-honokiol as a novel TRPV1 channel-modulating complex? Impact on skin sensitivity symptoms. In Proceedings of the 53rd Annual ESDR Meeting, Lisbon, Portugal, 4–7 September 2024. [Google Scholar]
- Borgonetti, V.; Galeotti, N. Honokiol-rich Magnolia officinalis bark extract attenuates trauma-induced neuropathic pain. Antioxidants 2023, 12, 1518. [Google Scholar] [CrossRef] [PubMed]
- Dominiak, K.; Gostyńska, A.; Szulc, M.; Stawny, M. The anticancer application of delivery systems for honokiol and magnolol. Cancers 2024, 16, 2257. [Google Scholar] [CrossRef]
- Nie, W.; Ding, L.F.; Lei, T.; Liu, Z.X.; Li, J.D.; Song, L.D.; Wu, X.D. Biphenyl-type neolignans with NO inhibitory activity from the fruits of Magnolia tripetala. Phytochem. Lett. 2021, 44, 222–226. [Google Scholar] [CrossRef]
- Xu, T.; Meng, J.R.; Cheng, W.; Liu, J.Z.; Chu, J.; Zhang, Q.; Ma, N.; Bai, L.P.; Guo, Y. Discovery of honokiol thioethers containing 1,3,4-oxadiazole moieties as potential α-glucosidase and SARS-CoV-2 entry inhibitors. Bioorg. Med. Chem. 2022, 67, 116838. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Al-Mana, F.A.; Mahmoud, E.A.; Ali Zin El-Abedin, T.K.; Mattar, M.A.; Ekiert, H. Phenolic compounds of Catalpa speciosa, Taxus cuspidata, and Magnolia acuminata have antioxidant and anticancer activity. Molecules 2019, 24, 412. [Google Scholar] [CrossRef]
- Esmaealzadeh, D.; Ghalibaf, A.M.; Rad, M.S.; Rezaee, R.; Razawi, B.M.; Hossein, H. Pharmacological effects of safranal: An updated review. Iran J. Basic. Med. Sci. 2023, 26, 1131–1143. [Google Scholar] [CrossRef]
- Wang, Z.; Perumalsamy, H.; Wang, X.; Ahn, Y.J. Toxicity and possible mechanisms of action of honokiol from Magnolia denudata seeds against four mosquito species. Sci. Rep. 2019, 9, 411. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. Biological potential and therapeutic effectiveness of phytoproduct ‘Fargesin’ in medicine: Focus on the potential of an active phytochemical of Magnolia fargesii. Recent Adv. Inflamm. Allergy Drug Discov. 2024, 18, 79–89. [Google Scholar] [CrossRef]
- Borah, P.; Goswami, R.C.D.; Jha, V.; Saikia, M. Phytochemical analysis and molecular identification of Magnolia hodgsonii (Hook. f. & Thomson) H. Keng from Northeast India. Biochem. Syst. Ecol. 2024, 112, 104762. [Google Scholar] [CrossRef]
- Kawahara, T.; Fujii, K.; Nakajima, K.; Fujii, R.; Inagaki, S.; Hara, K.; Yasui, H. Suppressive effects of hot-water extract of Magnolia obovata on Clostridium perfringens enterotoxin-induced cytotoxicity in human intestinal Caco-2 cells. Planta Med. 2020, 86, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Lovecká, P.; Svobodová, A.; Macůrková, A.; Vrchotová, B.; Demnerová, K.; Wimmer, Z. Decorative Magnolia plants: A comparison of the content of their biologically active components showing antimicrobial effects. Plants 2020, 9, 879. [Google Scholar] [CrossRef] [PubMed]
- Mir, A.H.; Jeri, L.; Upadhaya, K.; Bhat, N.A.; Borah, R.; Choudhury, H.; Kumar, Y. Diversity, bioprospection and commercial importance of Indian Magnolias. In Plants for Human Survival and Medicine, 1st ed.; Singh, B., Ed.; New India Publishing Agency: New Delhi, India, 2019; pp. 227–250. [Google Scholar]
- Zgórka, G.; Adamska-Szewczyk, A.; Baj, T. Response surface methodology in optimising the extraction of polyphenolic antioxidants from flower buds of Magnolia × soulangeana Soul. Bod. var. ‘Lennei’ and their detailed qualitative and quantitative profiling. Molecules 2023, 28, 6335. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.W.C. 4-O-Methylhonokiol: A lesser-known neolignan from Magnolia species with diverse and promising pharmacological properties. J. Appl. Pharm. Sci. 2022, 12, 23–29. [Google Scholar] [CrossRef]
- Fesenko, T.V.; Laguta, I.V.; Stavinskaya, O.M.; Kuzema, P.O.; Anishchenko, V.M.; Oranska, O.I.; Skorochod, I.O. Green synthesis of antibacterial cerium oxide nanoparticles using Magnolia kobus leaf extract. Chem. Phys. Lett. 2023, 14, 546–554. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, S.; Choi, Y.A.; Chung, J.M.; Kim, E.N.; Lee, B.; Kim, S.Y.; Jeong, G.S.; Kim, S.H. Magnolia kobus DC leaf ethanol extract alleviated lipopolysaccharide-induced acute lung inflammation by suppressing NF-κB and Nrf2 signaling. J. Herbmed. Pharmacol. 2024, 13, 90–100. [Google Scholar] [CrossRef]
- Poivre, M.; Duez, P. Biological activity and toxicity of the Chinese herb Magnolia officinalis Rehder & E. Wilson (Houpo) and its constituents. J. Zhejiang Univ. Sci. B 2017, 18, 194–214. [Google Scholar] [CrossRef]
- Luo, H.; Wu, H.; Yu, X.; Zhang, X.; Lu, Y.; Fan, J.; Tang, L.; Wang, Z. A review of the phytochemistry and pharmacological activities of Magnoliae officinalis cortex. J. Ethnopharmacol. 2019, 236, 412–442. [Google Scholar] [CrossRef]
- Yi, J.; Wu, J.G.; Wu, J.Y.; Wu, Y.B. Quality evaluation of the leaves of Magnolia officinalis var. biloba using high-performance liquid chromatography fingerprint analysis of phenolic compounds. J. Sep. Sci. 2016, 39, 784–792. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, T.; Guan, J.; Wang, J.; Chen, J.; Liu, X.; Qian, J.; Xu, X.; Qu, W.; Huang, Z.; et al. Oral delivery of honokiol microparticles for nonrapid eye movement sleep. Mol. Pharm. 2019, 16, 737–743. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, P.P.; Hu, K.L.; Li, L.N.; Yu, X.; Lu, Y.; Chang, H.S. Antidepressant-like effect and mechanism of action of honokiol on the mouse lipopolysaccharide (LPS) depression model. Molecules 2019, 24, 2035. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.X.; Sun, W.Y.; Li, Y.; Tang, Q.; Li, L.N.; Yu, X.; Wang, S.Y.; Fan, A.R.; Xu, X.Q.; Chang, H.S. Honokiol improves depression-like behaviors in rats by HIF-1α-VEGF signaling pathway activation. Front. Pharmacol. 2022, 13, 968124. [Google Scholar] [CrossRef]
- Srihari, P.; Kumar, Y.B.; Suresh, B. Gram scale synthesis of honokiol. Org. Prep. Proced. Int. 2022, 54, 49–55. [Google Scholar] [CrossRef]
- Loaiza-Cano, V.; Monsalve-Escudero, L.M.; Filho, C.S.M.B.; Martinez-Gutierrez, M.; de Sousa, D.P. Antiviral role of phenolic compounds against dengue virus: A review. Biomolecules 2021, 11, 11. [Google Scholar] [CrossRef]
- Amorati, R.; Zotova, J.; Baschieri, A.; Valgimigli, L. Antioxidant activity of magnolol and honokiol: Kinetic and mechanistic investigations of their reaction with peroxyl radicals. J. Org. Chem. 2015, 80, 10651–10659. [Google Scholar] [CrossRef]
- Banik, K.; Ranaware, A.M.; Deshpande, V.; Nalawade, S.P.; Padmavathi, G.; Bordoloi, D.; Sailo, B.L.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; et al. Honokiol for cancer therapeutics: A traditional medicine that can modulate multiple oncogenic targets. Pharmacol. Res. 2019, 144, 192–209. [Google Scholar] [CrossRef]
- Wu, W.; Xu, H. Construction, characterization, and bioavailability evaluation of honokiol-loaded porous starch by melting method without any solvent. Drug Deliv. 2021, 28, 2574–2581. [Google Scholar] [CrossRef]
- Mitchell, J.; Lo, K.W.H. The use of small-molecule compounds for cell adhesion and migration in regenerative medicine. Biomedicines 2023, 11, 2507. [Google Scholar] [CrossRef]
- PUBCHEM. N-(1,1-Dimethylethyl)-2-benzothiazolesulfenamide. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/7230 (accessed on 19 August 2025).
- Ding, W.; Hou, X.; Cong, S.; Zhang, Y.; Chen, M.; Lei, J.; Meng, Y.; Li, X.; Li, G. Co-delivery of honokiol, a constituent of Magnolia species, in a self-microemulsifying drug delivery system for improved oral transport of lipophilic sirolimus. Drug Deliv. 2016, 23, 2513–2523. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yang, D.; Hu, K.; Zhou, H.; Guo, Y.; Du, G.; Lu, Y. Certification of a new certified reference material of honokiol. Anal. Bioanal. Chem. 2015, 407, 5849–5855. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.L.; Cheng, W.T.; Chen, L.C.; Ho, H.O.; Lin, S.Y.; Hsieh, C.M. Honokiol/magnolol-loaded self-assembling lecithin-based mixed polymeric micelles (lb MPMs) for improving solubility to enhance oral bioavailability. Int. J. Nanomed. 2021, 16, 651–665. [Google Scholar] [CrossRef]
- Montané, X.; Kowalczyk, O.; Reig-Vano, B.; Bajek, A.; Roszkowski, K.; Tomczyk, R.; Pawliszak, W.; Giamberini, M.; Mocek-Płóciniak, A.; Tylkowski, B. Current perspectives of the applications of polyphenols and flavonoids in cancer therapy. Molecules 2020, 25, 3342. [Google Scholar] [CrossRef]
- Prasher, P.; Fatima, R.; Sharma, M.; Tynybekov, B.; Alshahrani, A.M.; Ateşşahin, D.A.; Sharifi-Rad, J.; Calina, D. Honokiol and its analogues as anticancer compounds: Current mechanistic insights and structure-activity relationship. Chem.-Biol. Interact. 2023, 386, 110747. [Google Scholar] [CrossRef]
- Kiyama, R. Biological effects induced by estrogenic activity of lignans. Trends Food Sci. Technol. 2016, 54, 186–196. [Google Scholar] [CrossRef]
- Li, D.; Luo, F.; Guo, T.; Han, S.; Wang, H.; Lin, Q. Targeting NF-κB pathway by dietary lignans in inflammation: Expanding roles of gut microbiota and metabolites. Crit. Rev. Food Sci. Nutr. 2023, 63, 5967–5983. [Google Scholar] [CrossRef] [PubMed]
- Villalaín, J. Localization and aggregation of honokiol in the lipid membrane. Antioxidants 2024, 13, 1025. [Google Scholar] [CrossRef]
- Chiu, C.S.; Tsai, C.H.; Hsieh, M.S.; Tsai, S.C.; Jan, Y.J.; Lin, W.Y.; Lai, D.W.; Wu, S.M.; Hsing, H.Y.; Arbiser, J.L.; et al. Exploiting honokiol-induced ER stress CHOP activation inhibits the growth and metastasis of melanoma by suppressing the MITF and β-catenin pathways. Cancer Lett. 2019, 442, 113–125. [Google Scholar] [CrossRef]
- Hsiao, Y.P.; Chen, H.T.; Liang, Y.C.; Wang, T.E.; Huang, K.H.; Hsu, C.C.; Liang, H.J.; Huang, C.H.; Jan, T.R. Development of nanosome-encapsulated honokiol for intravenous therapy against experimental autoimmune encephalomyelitis. Int. J. Nanomed. 2020, 15, 17–29. [Google Scholar] [CrossRef]
- Wen, J.; Wang, X.; Pei, H.; Xie, C.; Qiu, N.; Li, S.; Wang, W.; Cheng, X.; Chen, L. Anti-psoriatic effects of honokiol through the inhibition of NF-κB and VEGFR-2 in animal model of K14-VEGF transgenic mouse. J. Pharmacol. Sci. 2015, 128, 116–124. [Google Scholar] [CrossRef][Green Version]
- Hwang, T.L. Dimethylhonokiol inhibits PDE4 activity that impedes neutrophil activation and protects against imiquimod induced psoriasis. J. Dermatol. Sci. 2017, 86, e12–e13. [Google Scholar] [CrossRef]
- Mottaghi, S.; Abbaszadeh, H. Natural lignans honokiol and magnolol as potential anticarcinogenic and anticancer agents. A comprehensive mechanistic review. Nutr. Cancer 2022, 74, 761–778. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Katiyar, S.K. Honokiol, an active compound of Magnolia plant, inhibits growth, and progression of cancers of different organs. Adv. Exp. Med. Biol. 2016, 928, 245–265. [Google Scholar] [CrossRef]
- Ainbinder, D.; Protokin, R.; Chaouat, M.; Touitou, E. Effect of honokiol and 5-FU on non-melanoma skin cancer cells. J. Drug Deliv. Sci. Technol. 2009, 19, 283–287. [Google Scholar] [CrossRef]
- Cho, J.H.; Jeon, Y.J.; Park, S.M.; Shin, J.C.; Lee, T.H.; Jung, S.; Park, H.; Ryu, J.; Chen, H.; Dong, Z.; et al. Multifunctional effects of honokiol as an anti-inflammatory and anti-cancer drug in human oral squamous cancer cells and xenograft. Biomaterials 2015, 53, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.J.; Kuo, C.H.; Chen, S.H.; Lin, C.Y.; Lee, Y.R. Honokiol inhibits in vitro and in vivo growth of oral squamous cell carcinoma through induction of apoptosis, cell cycle arrest and autophagy. J. Cell Mol. Med. 2018, 22, 1894–1908. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xu, M.; Jing, X.; Qiu, J.; Huang, S.; Yan, H.; Yin, L.; Lou, J.; Zhao, L.; Fan, Y.; et al. Honokiol suppresses the aberrant interactions between renal resident macrophages and tubular epithelial cells in lupus nephritis through the NLRP3/IL-33/ST2 axis. Cell Death Dis. 2023, 14, 174. [Google Scholar] [CrossRef]
- Yi, X.; Guo, W.; Shi, Q.; Yang, Y.; Zhang, W.; Chen, X.; Kang, P.; Chen, J.; Cui, T.; Ma, J.; et al. SIRT3-dependent mitochondrial dynamics remodeling contributes to oxidative stress-induced melanocyte degeneration in vitiligo. Theranostics 2019, 9, 1614–1633. [Google Scholar] [CrossRef]
- Li, S.; Chen, J.; Chen, F.; Wang, C.; Guo, X.; Wang, C.; Guo, X.; Wang, C.; Fan, Y.; Wang, Y.; et al. Liposomal honokiol promotes hair growth via activating Wnt3a/β-catenin signaling pathway and down regulating TGF-β1 in C57BL/6N mice. Biomed. Pharmacother. 2021, 141, 111793. [Google Scholar] [CrossRef]
- Khalid, S.; Khan, A.; Shal, B.; Ali, H.; Kim, Y.S.; Khan, S. Suppression of TRPV1 and P2Y nociceptors by honokiol isolated from Magnolia officinalis in 3rd degree burn mice by inhibiting inflammatory mediators. Biomed. Pharmacother. 2019, 114, 108777, Erratum in: Biomed. Pharmacother. 2025, 186, 117988. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Tennakoon, D.S.; Jayatunga, D.P.W.; Hongsanan, S.; Xie, N. Humans vs. fungi: An overview of fungal pathogens against humans. Pathogens 2024, 13, 426. [Google Scholar] [CrossRef]
- Yang, F.; Yang, B.; Song, K.; Jin, Y.; Wang, G.; Li, P.; Yu, Q.; Ling, F. Natural product honokiol exhibits antiviral effects against Micropterus salmoides rhabdovirus (MSRV) both in vitro and in vivo. J. Fish Dis. 2024, 47, e13915. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Du, C.; Shan, S.; Zhang, Y.; Du, Z.; Han, D. Honokiol alleviates hypertrophic scar by targeting transforming growth factor-β/Smad2/3 signaling pathway. Front. Pharamcol. 2017, 8, 206. [Google Scholar] [CrossRef]
- Liu, S.; Li, L.; Tan, L.; Liang, X. Inhibition of herpes simplex virus-1 replication by natural compound honokiol. Virol. Sin. 2019, 34, 315–323. [Google Scholar] [CrossRef]
- Ouyang, Y.; Tang, X.; Zhao, Y.; Zuo, X.; Ren, X.; Wang, J.; Zou, L.; Lu, J. Disruption of bacterial thiol-dependent redox homeostasis by magnolol and honokiol as an antibacterial strategy. Antioxidants 2023, 12, 1180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, B.; Lv, Y.; Wei, S.; Zhang, S.; Hu, Y. Transcriptomic analysis shows the antifungal mechanism of honokiol against Aspergillus flavus. Int. J. Food Microbiol. 2023, 384, 109972. [Google Scholar] [CrossRef]
- Borgonetti, V.; Governa, P.; Manetti, F.; Miraldi, E.; Biagi, M.; Galeotti, N. A honokiol-enriched Magnolia officinalis Rehder & E.H. Wilson. bark extract possesses anxiolytic-like activity with neuroprotective effect through the modulation of CB1 receptor. J. Pharm. Pharmacol. 2021, 73, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.; Singh, S. Neuroprotective potential of honokiol in ICV-STZ induced neuroinflammation, Aβ (1–42) and NF-kB expression in experimental model of rats. Neurosci. Lett. 2023, 799, 137090. [Google Scholar] [CrossRef]
- Sharanya, J.; Purushothaman, A.; Janardanan, D.; Koley, K. Theoretical exploration of the antioxidant activity of honokiol and magnolol. Comput. Theor. Chem. 2024, 1232, 114460. [Google Scholar] [CrossRef]
- Elfeky, M.G.; Mantawy, E.M.; Gad, A.M.; Fawzy, H.M.; El-Demerdash, E. Mechanistic aspects of antifibrotic effects of honokiol in Con A-induced liver fibrosis in rats: Emphasis on TGF-β/SMAD/MAPK signaling pathways. Life Sci. 2020, 240, 117096. [Google Scholar] [CrossRef]
- Kataoka, S.; Umemura, A.; Okuda, K.; Taketani, H.; Seko, Y.; Nishikawa, T.; Yamaguci, K.; Moriguchi, M.; Kanbara, Y.; Arbiser, J.L.; et al. Honokiol acts as a potent anti-fibrotic agent in the liver through inhibition of TGF-β1/SMAD signaling and autophagy in hepatic stellate cells. Int. J. Mol. Sci. 2021, 22, 13354. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Liu, M.; Duan, X.H.; Hu, K.L.; Li, L.N.; Yu, X.; Chang, H.S. Antidepressant-like mechanism of honokiol in a rodent model of corticosterone-induced depression. J. Integr. Neurosci. 2020, 19, 459–467. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Grumezescu, A.M. Natural compounds for preventing ear, nose, and throat-related oral infections. Plants 2021, 10, 1847. [Google Scholar] [CrossRef]
- Rahmatullah, M.; Rahman, T.; Hasan, A.; Jahan, R.; Hossan, M.S.; Jannat, K.; Bondhon, T.A. Plants to drugs: A case study of human papilloma virus and traditional chinese medicine. In Promising Antimicrobials from Natural Products, 2nd ed.; Rai, M., Kosalec., I., Eds.; Springer: Cham, Switzerland, 2022; pp. 135–182. [Google Scholar] [CrossRef]
- Fang, C.Y.; Chen, S.J.; Wu, H.N.; Ping, Y.H.; Lin, C.Y.; Shiuan, D.; Chen, C.L.; Lee, Y.R.; Huang, K.J. Honokiol, a lignan biphenol derived from the magnolia tree, inhibits dengue virus type 2 infection. Viruses 2015, 7, 4894–4910. [Google Scholar] [CrossRef]
- Valdés-Torres, P.; Campos, D.; Bhakta, M.; Galán-Jurado, P.E.; Durant-Archibold, A.A.; González-Santamaría, J. Honokiol and alpha-Mangostin inhibit Mayaro virus replication through different mechanisms. Molecules 2022, 27, 7362. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Meng, J.R.; Liu, J.Z.; Xu, T.; Zheng, Z.Y.; Jiang, Z.H.; Bai, L.P. Synthesis and biological evaluation of honokiol derivatives bearing 3-((5-phenyl-1, 3, 4-oxadiazol-2-yl) methyl) oxazol-2 (3H)-ones as potential viral entry inhibitors against SARS-CoV-2. Pharmaceuticals 2021, 14, 885. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, T.; Hayashi, T.; Suzuki, R.; Kitamura, M.; Inoue, Y. Inhibitory effect of honokiol on furin-like activity and SARS-CoV-2 infection. J. Tradit. Complement. Med. 2022, 12, 69–72. [Google Scholar] [CrossRef]
- Salgado-Benvindo, C.; Leijs, A.A.; Thaler, M.; Tas, A.; Arbiser, J.L.; Snijder, E.J.; van Hemert, M.J. Honokiol inhibits SARS-CoV-2 replication in cell culture at a post-entry step. Microbiol. Spectr. 2023, 11, e03273-22. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Jung, E.; Park, Y.; Kim, K.; Park, B.; Jung, K.; Park, E.; Kim, J.; Park, D. In vitro antibacterial and anti-inflammatory effects of honokiol and magnolol against Propionibacterium sp. Eur. J. Pharmacol. 2004, 496, 189–195. [Google Scholar] [CrossRef]
- Guo, Y.; Hou, E.; Wen, T.; Yan, X.; Han, M.; Bai, L.P.; Fu, X.; Liu, J.; Qin, S. Development of membrane-active honokiol/magnolol amphiphiles as potent antibacterial agents against methicillin-resistant Staphylococcus aureus (MRSA). J. Med. Chem. 2021, 64, 12903–12916. [Google Scholar] [CrossRef]
- Cheng, C.; Quing-Wen, Z.; Yang, Y.E.; Li-Gen, L. Honokiol: A naturally occurring lignan with pleiotropic bioactivities. Chin. J. Nat. Med. 2021, 19, 481–490. [Google Scholar] [CrossRef]
- Choi, E.J.; Kim, H.I.; Kim, J.A.; Jun, S.Y.; Kang, S.H.; Park, D.J.; Son, S.J.; Kim, Y.; Shin, O.S. The herbal-derived honokiol and magnolol enhances immune response to infection with methicillin-sensitive Staphylococcus aureus (MSSA) and methicillin-resistant S. aureus (MRSA). Appl. Microbiol. Biotechnol. 2015, 99, 4387–4396. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, Y.J.; Park, S.N. Synergistic antimicrobial effect of Lonicera japonica and Magnolia obovata extracts and potential as a plant-derived natural preservative. J. Microbiol. Biotechnol. 2018, 28, 1814–1822. [Google Scholar] [CrossRef]
- Zuo, G.Y.; Zhang, X.J.; Han, J.; Li, Y.Q.; Wang, G.C. In vitro synergism of magnolol and honokiol in combination with antibacterial agents against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA). BMC Complement. Altern. Med. 2015, 15, 425. [Google Scholar] [CrossRef]
- Khadke, S.K.; Lee, J.H.; Woo, J.T.; Lee, J. Inhibitory effects of honokiol and magnolol on biofilm formation by Acinetobacter baumannii. Biotechnol. Bioproc. Eng. 2019, 24, 359–365. [Google Scholar] [CrossRef]
- Sakaue, Y.; Domon, H.; Oda, M.; Takenaka, S.; Kubo, M.; Fukuyama, Y.; Okiji, T.; Terao, Y. Anti-biofilm and bactericidal effects of magnolia bark-derived magnolol and honokiol on Streptococcus mutans. Microbiol. Immunol. 2016, 60, 10–16. [Google Scholar] [CrossRef]
- Chiu, K.C.; Shih, Y.H.; Wang, T.H.; Lan, W.C.; Li, P.J.; Jhuang, H.S.; Hsia, S.M.; Shen, Y.W.; Yuan-Chien Chen, M.; Shieh, T.M. In vitro antimicrobial and antipro-inflammation potential of honokiol and magnolol against oral pathogens and macrophages. J. Formos. Med. Assoc. 2021, 120, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Zhao, X.C.; Zhao, Z.W.; Huang, Y.J.; Zhu, X.Z.; Meng, R.Z.; Shi, C.; Yu, L.; Guo, N. In vitro antimicrobial activity of honokiol against Staphylococcus aureus in biofilm mode. J. Asian. Nat. Prod. Res. 2016, 18, 1178–1185. [Google Scholar] [CrossRef]
- Ghorbani, F.; Haghgoo, R.; Aramjoo, H.; Rakhshandeh, H.; Jamehdar, S.A.; Zare-Bidaki, M. The antibacterial effect of Magnolia mouthwash on the levels of salivary Streptococcus mutans in dental plaque: A randomized, single-blind, placebo-controlled trial. Iran J. Microbiol. 2021, 13, 104–111. [Google Scholar] [CrossRef]
- Zhan, L.; Peng, X.; Lin, J.; Zhang, Y.; Gao, H.; Zhu, Y.; Huan, Y.; Zhao, G. Honokiol reduces fungal load, toll-like receptor-2, and inflammatory cytokines in Aspergillus fumigatus keratitis. Investig. Ophthalmol. Vis. Sci. 2020, 61, 48. [Google Scholar] [CrossRef]
- Zhan, L.; Tian, X.; Lin, J.; Zhang, Y.; Zheng, H.; Peng, X.; Zhao, G. Honokiol reduces fungal burden and ameliorate inflammation lesions of Aspergillus fumigatus keratitis via Dectin-2 down-regulation. Int. Immunopharmacol. 2023, 118, 109849. [Google Scholar] [CrossRef] [PubMed]
- Nurdin, R.S.C.; Vitayani, S.; Amin, S.; Kadir, D.; Djamaluddin, W.; Adriani, A. Cutaneous candidiasis caused by Candida kefyr. Pan Afr. Med. J. 2021, 38, 178. [Google Scholar] [CrossRef]
- Palese, E.; Nudo, M.; Zino, G.; Devirgiliis, V.; Carbotti, M.; Cinelli, E.; Rodio, D.M.; Bressan, A.; Prezioso, C.; Ambrosi, C.; et al. Cutaneous candidiasis caused by Candida albicans in a young non-immunosuppressed patient: An unusual presentation. Int. J. Immunopathol. Pharmacol. 2018, 32, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liao, K. The effect of honokiol on ergosterol biosynthesis and vacuole function in Candida albicans. J. Microbiol. Biotechnol. 2020, 30, 1835–1842. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. Environmental air pollutants affecting skin functions with systemic implications. Int. J. Mol. Sci. 2023, 24, 10502. [Google Scholar] [CrossRef] [PubMed]
- Neuberger, A.; Nadezhdin, K.D.; Sobolevsky, A.I. Chapter Two—TRPV3 expression and purification for structure determination by Cryo-EM. Methods Enzymol. 2021, 652, 31–48. [Google Scholar] [CrossRef]
- Cai, X.; Jiang, X.; Zhao, M.; Su, K.; Tang, M.; Hong, F.; Ye, N.; Zhang, R.; Li, N.; Wang, L.; et al. Identification of the target protein and molecular mechanism of honokiol in anti-inflammatry action. Phytomedicine 2023, 109, 154617. [Google Scholar] [CrossRef]
- Rickert, U.; Cossais, F.; Heimke, M.; Arnold, P.; Preuße-Prange, A.; Wilms, H.; Lucius, R. Antiinflammatory properties of honokiol in activated primary microglia and astrocytes. J. Neuroimmunol. 2018, 323, 78–86. [Google Scholar] [CrossRef]
- Wijesuriya, Y.K.; Lappas, M. Potent anti-inflammatory effects of honokiol in human fetal membranes and myometrium. Phytomedicine 2018, 49, 11–22. [Google Scholar] [CrossRef]
- Khalid, S.; Ullah, M.Z.; Khan, A.U.; Afridi, R.; Rasheed, H.; Khan, A.; Ali, H.; Kim, Y.S.; Khan, S. Antihyperalgesic properties of honokiol in inflammatory pain models by targeting of NF-κB and Nrf2 signaling. Front. Pharmacol. 2018, 9, 140. [Google Scholar] [CrossRef]
- Guillermo, R.F.; Chilampalli, C.; Zhang, X.; Zeman, D.; Fahmy, H.; Dwivedi, C. Time and dose-response effects of honokiol on UVB-induced skin cancer development. Drug Discov. Ther. 2012, 6, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Singh, T.; Pal, H.C.; Prasad, R. Honokiol stimulates immune reactivity in UV-irradiated skin through DNA demethylation-dependent functional activation of dendritic cells in mice. Cancer Res. 2017, 77 (Suppl. S13), 2221. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Singh, T.; Prasad, R. Honokiol, a phytochemical from Magnolia plant, prevents UV radiation-induced immune suppression in mice through inhibition of PGE2 and DNA methylation. Cancer Res. 2016, 76 (Suppl. S14), 2607. [Google Scholar] [CrossRef]
- Vaid, M.; Sharma, S.D.; Katiyar, S.K. Honokiol, a phytochemical from the Magnolia plant, inhibits photocarcinogenesis by targeting UVB-induced inflammatory mediators and cell cycle regulators: Development of topical formulation. Carcinogenesis 2010, 31, 2004–2011. [Google Scholar] [CrossRef]
- Chilampalli, S.; Zhang, X.; Fahmy, H.; Kaushik, R.S.; Zeman, D.; Hildreth, M.B.; Dwivedi, C. Chemopreventive effects of honokiol on UVB-induced skin cancer development. Anticancer Res. 2010, 30, 777–783. [Google Scholar]
- Dummer, R.; Hauschild, A.; Lindenblatt, N.; Pentheroudakis, G.; Keilholz, U. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v126–v132. [Google Scholar] [CrossRef]
- Lee, Y.H.; Jeong, E.Y.; Kim, Y.H.; Park, J.H.; Yoon, J.H.; Lee, Y.J.; Lee, S.H.; Nam, Y.K.; Cha, S.Y.; Park, J.S.; et al. Identification of senescence rejuvenation mechanism of Magnolia officinalis extract including honokiol as a core ingredient. Aging 2025, 17, 497–523. [Google Scholar] [CrossRef]
- Liu, X.; Gu, Y.; Bian, Y.; Cai, D.; Li, Y.; Zhao, Y.; Zhang, Z.; Xue, M.; Zhang, L. Honokiol induces paraptosis-like cell death of acute promyelocytic leukemia via mTOR & MAPK signaling pathways activation. Apoptosis 2021, 26, 195–208. [Google Scholar] [CrossRef]
- Lai, X.; Sun, Y.; Zhang, X.; Wang, D.; Wang, J.; Wang, H.; Zhao, Y.; Liu, X.; Xu, X.; Song, H.; et al. Honokiol induces ferroptosis by upregulating HMOX1 in acute myeloid leukemia cells. Front. Pharmacol. 2022, 13, 897791. [Google Scholar] [CrossRef]
- Mędra, A.; Witkowska, M.; Majchrzak, A.; Cebula-Obrzut, B.; Bonner, M.Y.; Robak, T.; Arbiser, J.L.; Smolewski, P. Pro-apoptotic activity of new honokiol/triphenylmethane analogues in B-cell lymphoid malignancies. Molecules 2016, 21, 995. [Google Scholar] [CrossRef]
- Xia, L.; Kang, D.; Wan, D.; Chu, C.; Chen, M.; Zhang, S.; Li, X.; He, L.; Yan, J.; Liu, T.; et al. Honokiol-chlorambucil co-prodrugs selectively enhance the killing effect through STAT3 binding on lymphocytic leukemia cells in vitro and in vivo. ACS Omega 2020, 5, 19844–19852. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Hueng, D.Y.; Huang, H.Y.; Chen, J.Y.; Chen, Y. Magnolol and honokiol exert a synergistic anti-tumor effect through autophagy and apoptosis in human glioblastomas. Oncotarget. 2016, 7, 29116–29130. [Google Scholar] [CrossRef]
- Fan, Y.; Xue, W.; Schachner, M.; Zhao, W. Honokiol eliminates glioma/glioblastoma stem cell-like cells via JAK-STAT3 signaling and inhibits tumor progression by targeting epidermal growth factor receptor. Cancers 2019, 11, 22. [Google Scholar] [CrossRef]
- Lee, J.S.; Sul, J.Y.; Park, J.B.; Lee, M.S.; Cha, E.Y.; Ko, Y.B. Honokiol induces apoptosis and suppresses migration and invasion of ovarian carcinoma cells via AMPK/mTOR signaling pathway. Int. J. Mol. Med. 2019, 43, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Songjang, W.; Jiraviriyakul, A. Honokiol and magnolol induce apoptosis and cell cycle arrest in human ovarian cancer cells. Pharmacogn. J. 2019, 11, 1114–1123. [Google Scholar] [CrossRef]
- Huang, J.S.; Yao, C.J.; Chuang, S.E.; Yeh, C.T.; Lee, L.M.; Chen, R.M.; Chao, W.J.; Whang-Peng, J.; Lai, G.M. Honokiol inhibits sphere formation and xenograft growth of oral cancer side population cells accompanied with JAK/STAT signaling pathway suppression and apoptosis induction. BMC Cancer 2016, 16, 245. [Google Scholar] [CrossRef]
- Wang, T.; Wang, H.; Yang, F.; Gao, K.; Luo, S.; Bai, L.; Ma, K.; Liu, M.; Wu, S.; Wang, H.; et al. Honokiol inhibits proliferation of colorectal cancer cells by targeting anoctamin 1/TMEM16A Ca2+-activated Cl− channels. Br. J. Pharmacol. 2021, 178, 4137–4154. [Google Scholar] [CrossRef]
- Yang, B.; Ni, X.; Chen, L.; Zhang, H.; Ren, P.; Feng, Y.; Chen, Y.; Fu, S.; Wu, J. Honokiol-loaded polymeric nanoparticles: An active targeting drug delivery system for the treatment of nasopharyngeal carcinoma. Drug Deliv. 2017, 24, 660–669. [Google Scholar] [CrossRef]
- Balan, M.; Chakraborty, S.; Flynn, E.; Zurakowski, D.; Pal, S. Honokiol inhibits c-Met-HO-1 tumor-promoting pathway and its cross-talk with calcineurin inhibitor-mediated renal cancer growth. Sci. Rep. 2017, 7, 5900. [Google Scholar] [CrossRef]
- Hsiao, C.H.; Yao, C.J.; Lai, G.M.; Lee, L.M.; Whang-Peng, J.; Shih, P.H. Honokiol induces apoptotic cell death by oxidative burst and mitochondrial hyperpolarization of bladder cancer cells. Exp. Ther. Med. 2019, 17, 4213–4222. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Lee, Y.; Zhang, Q.; Xiong, D.; Wan, T.C.; Wang, Y.; You, M. Honokiol decreases lung cancer metastasis through inhibition of the STAT3 signaling pathway. Cancer Prev. Res. 2017, 10, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yang, Y.; Huang, N.; Zhao, W. Updated progression of honokiol in lung cancer treatment. J. Pharm. Pharmacol. 2025, rgaf007. [Google Scholar] [CrossRef]
- Xie, L.; Jiang, F.; Zhang, X.; Alitongbieke, G.; Shi, X.; Meng, M.; Xu, Y.; Ren, A.; Wang, J.; Cai, L.; et al. Honokiol sensitizes breast cancer cells to TNF-α induction of apoptosis by inhibiting Nur77 expression. Br. J. Pharmacol. 2016, 173, 344–356. [Google Scholar] [CrossRef]
- Hahm, E.R.; Singh, K.B.; Singh, S.V. c-Myc is a novel target of cell cycle arrest by honokiol in prostate cancer cells. Cell Cycle 2016, 15, 2309–2320. [Google Scholar] [CrossRef]
- Halasi, M.; Hitchinson, B.; Shah, B.N.; Váraljai, R.; Khan, I.; Benevolenskaya, E.V.; Gaponenko, V.; Arbiser, J.L.; Gartel, A.L. Honokiol is a FOXM1 antagonist. Cell Death. Dis. 2018, 9, 84. [Google Scholar] [CrossRef]
- Lei, Y. Analysis of the Molecular Mechanisms of Inhibition of Cell Migration in Response to Honokiol. Master’s Thesis, Edinburgh Napier University, Edinburgh, Scotland, 2021. [Google Scholar] [CrossRef]
- Lu, C.H.; Chen, S.H.; Chang, Y.S.; Liu, Y.W.; Wu, J.Y.; Lim, Y.P.; Yu, H.I.; Lee, Y.R. Honokiol, a potential therapeutic agent, induces cell cycle arrest and program cell death in vitro and in vivo in human thyroid cancer cells. Pharmacol. Res. 2017, 115, 288–298. [Google Scholar] [CrossRef]
- Chou, H.C.; Lu, C.H.; Su, Y.C.; Lin, L.H.; Yu, H.I.; Chuang, H.H.; Tsai, Y.T.; Liao, E.C.; Wei, Y.S.; Yang, Y.T.; et al. Proteomic analysis of honokiol-induced cytotoxicity in thyroid cancer cells. Life Sci. 2018, 207, 184–204. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Li, J.; Xiao, Y.; Wang, X.; Gong, M.; Wang, Q.; Zhu, Z.; Zhang, S.; Zhang, W.; Cao, F.; et al. Honokiol suppresses perineural invasion of pancreatic cancer by inhibiting SMAD2/3 signaling. Front. Oncol. 2021, 11, 728583. [Google Scholar] [CrossRef]
- Okuda, K.; Umemura, A.; Umemura, S.; Kataoka, S.; Taketani, H.; Seko, Y.; Nishikawa, T.; Yamaguchi, K.; Moriguchi, M.; Kanbara, Y.; et al. Honokiol prevents non-alcoholic steatohepatitis-induced liver cancer via EGFR degradation through the glucocorticoid receptor—MIG6 axis. Cancers 2021, 13, 1515. [Google Scholar] [CrossRef]
- Qu, D.; Qu, S.; Wang, Y. FTO and Smad6 involved in honokiol-induced osteosarcoma cell apoptosis. Int. J. Clin. Exp. Med. 2019, 12, 9871–9880. [Google Scholar]
- Zhang, X.; Chen, H.; Zhang, Y.; Huang, Q.; Feng, J.; Xing, H.; Fu, X.; Yan, X.; Zhang, Y.; Xu, Q.; et al. HA-DOPE-modified honokiol-loaded liposomes targeted therapy for osteosarcoma. Int. J. Nanomed. 2022, 1, 5137–5151. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Gupta, N.A.; Xu, S.; Prasad, R.; Velu, S.E.; Katiyar, S.K. Honokiol inhibits the growth of head and neck squamous cell carcinoma by targeting epidermal growth factor receptor. Oncotarget 2015, 6, 21268–21282. [Google Scholar] [CrossRef]
- Eida, S.; Fukuda, M.; Katayama, I.; Takagi, Y.; Sasaki, M.; Mori, H.; Kawakami, M.; Nishino, T.; Ariji, Y.; Sumi, M. Metastatic lymph node detection on ultrasound images using YOLOv7 in patients with head and neck squamous cell carcinoma. Cancers 2024, 16, 274. [Google Scholar] [CrossRef]
- Liew, Y.X.; Karen-Ng, L.P.; Vincent-Chong, V.K. A comprehensive review of natural products as therapeutic or chemopreventive agents against head and neck squamous cell carcinoma cells using preclinical models. Biomedicines 2023, 11, 2359. [Google Scholar] [CrossRef]
- Ali, M.L.; Roky, A.H.; Azad, S.M.A.K.; Shaikat, A.H.; Meem, J.N.; Hoque, E.; Ahasan, A.M.F.; Islam, M.M.; Arif, S.R.; Mostaq, M.S.; et al. Autophagy as a targeted therapeutic approach for skin cancer: Evaluating natural and synthetic molecular interventions. Cancer Pathog. Ther. 2024, 2, 231–245. [Google Scholar] [CrossRef]
- Garbe, C.; Forsea, A.M.; Amaral, T.; Arenberger, P.; Autier, P.; Berwick, M.; Boonen, B.; Bylaite, M.; del Marmol, V.; Dreno, B.; et al. Skin cancers are the most frequent cancers in fair-skinned populations, but we can prevent them. Eur. J. Cancer 2024, 204, 114074. [Google Scholar] [CrossRef]
- Mosbah, S.A.; Elsayed, M.; Abd-Alhafez, A.; Zouair, M. Chemopreventive efficacy of honokiol on experimentally induced hamster buccal pouch squamous cell carcinoma. Al-Azhar J. Dent. Sci. 2022, 25, 463–476. [Google Scholar] [CrossRef]
- Rakshit, S.; Chitra, V. Role of Pi3k/Akt/Mtor pathways behind pathogenesis of skin cancer: A brief review. NeuroQuantology 2022, 20, 412–436. [Google Scholar] [CrossRef]
- Yapar, E.A.; Özdemir, M.N.; Durgun, M.E.; Dağistan, Ö.A.; Cavulu, S.; Özsoy, Y.; Kartal, M. Nanodelivery approaches of phytoactives for skin cancers: Current and future perspectives. Curr. Pharm. Biotechnol. 2025, 26, 631–653. [Google Scholar] [CrossRef]
- Pisani, D.; Micallef, D.; Scerri, J.; Betts, A.; Degaetano, J.; Baldacchino, S. Neuroendocrine transdifferentiation in cutaneous melanoma: A case report and review of the literature. Am. J. Dermatopathol. 2023, 45, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Cassalia, F.; Danese, A.; Tudurachi, I.; Federico, S.; Zambello, A.; Guidotti, A.; Franceschin, L.; Bolzon, A.; Naldi, L.; Fortina, A.B. PRAME updated: Diagnostic, prognostic, and therapeutic role in skin cancer. Int. J. Mol. Sci. 2024, 25, 1582. [Google Scholar] [CrossRef]
- Dziankowska-Zaborszczyk, E.; Maniecka-Bryła, I.; Pikala, M. Mortality trends due to skin melanoma in Poland in the years 2000–2020. Int. J. Environ. Res. Public Health 2022, 19, 16118. [Google Scholar] [CrossRef] [PubMed]
- Danciu, C.; Soica, C.; Antal, D.; Alexa, E.; Pavel, I.Z.; Ghiulai, R.; Ardelean, F.; Babuta, R.M.; Popescu, A.; Dehelean, C.A. Natural compounds in the chemoprevention of malignant melanoma. Anticancer Agents Med. Chem. 2018, 18, 631–644. [Google Scholar] [CrossRef]
- Bonner, M.Y.; Karlsson, I.; Rodolfo, M.; Arnold, R.S.; Vergani, E.; Arbiser, J.L. Honokiol bis-dichloroacetate (honokiol DCA) demonstrates activity in vemurafenib-resistant melanoma in vivo. Oncotarget 2016, 7, 12857–12868. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G.; Venugopal, A.; Ramamoorthy, P.; Standing, D.; Subramaniam, D.; Umar, S.; Jensen, R.A.; Anant, S.; Mammen, J.M.V. Honokiol inhibits melanoma stem cells by targeting notch signaling. Mol. Carcinog. 2015, 54, 1710–1721. [Google Scholar] [CrossRef]
- Guillermo-Lagae, R.; Santha, S.; Thomas, M.; Zoelle, E.; Stevens, J.; Kaushik, R.S.; Dwivedi, C. Antineoplastic effects of honokiol on melanoma. BioMed Res. Int. 2017, 2017, 5496398. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Katiyar, S.K. Honokiol inhibits the invasive potential of melanoma cells by targeting NADPH oxidase 1 and decreasing the binding of core proteins. Cancer Res. 2015, 75 (Suppl. S15), 2823. [Google Scholar] [CrossRef]
- Prasad, R.; Kappes, J.C.; Katiyar, S.K. Inhibition of NADPH oxidase 1 activity and blocking the binding of cytosolic and membrane-bound proteins by honokiol inhibit migratory potential of melanoma cells. Oncotarget 2016, 7, 7899–7912. [Google Scholar] [CrossRef] [PubMed]
- Boyle, D.P. Non-melanocytic skin cancers. In Histopathology Reporting Guidelines for Surgical Cancer, 2nd ed.; Boyle, D.P., Allen, D.C., Eds.; Springer: Cham, Switzerland, 2020; pp. 231–246. [Google Scholar] [CrossRef]
- Smith, H.; Wernham, A.; Patel, A. When to suspect a non-melanoma skin cancer. BMJ 2020, 368, m692. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Ciążyńska, M.; Kamińska-Winciorek, G.; Lange, D.; Lewandowski, B.; Reich, A.; Sławińska, M.; Pabianek, M.; Szczepaniak, K.; Hankiewicz, A.; Ułańska, M.; et al. The incidence and clinical analysis of non-melanoma skin cancer. Sci. Rep. 2021, 11, 4337. [Google Scholar] [CrossRef]
- Massone, C.; Hofman-Wellenhof, R.; Chiodi, S.; Sola, S. Dermoscopic criteria, histopathological correlates and genetic findings of thin melanoma on non-volar skin. Genes 2021, 12, 1288. [Google Scholar] [CrossRef]
- Muntyanu, A.; Ghazawi, F.M.; Nedjar, H.; Rahme, E.; Alakel, A.; Zubarev, A.; Netchiporouk, E.; Litvinov, I.V. Non-melanoma skin cancer distribution in the Russian Federation. Dermatology 2021, 237, 1007–1015. [Google Scholar] [CrossRef]
- Nalgiev, T.M.; Dagaeva, F.K.; Khubetsova, Z.O.; Majidov, A.S.; Dadasheva, B.M.; Kadieva, M.T.; Kostylev, I.A.; Latyrova, M.Y.; Kurbanov, A.A.; Arzumanyan, S.G. Pathogenetic features and spread of non-pigmented (amelanotic) melanoma of the skin. Int. Trans. J. Eng. Manag. Appl. Sci. Technol. 2022, 13, 1–9. [Google Scholar] [CrossRef]
- Sharquie, K.E.; Abdulwahhab, W.S. Pigmented basal cell carcinoma develops de nova without pre-existing solar keratosis in skin type III and IV. J. Pak. Assoc. Dermatol. 2023, 33, 832–840. [Google Scholar]
- Davodabadi, F.; Sajjadi, S.F.; Sarhadi, M.; Mirghasemi, S.; Hezaveh, M.N.; Khosravi, S.; Andani, M.K.; Cordani, M.; Basiri, M.; Ghavami, S. Cancer chemotherapy resistance: Mechanisms and recent breakthrough in targeted drug delivery. Eur. J. Pharmacol. 2023, 958, 176013. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, F.; Qiao, Y.; Chen, T.; Fan, L.; Shen, X.; Yu, D.; Huang, Y.; Wei, M. Honokiol inhibits interleukin-induced angiogenesis in the NSCLC microenvironment through the NF-κB signaling pathway. Chem. Biol. Interact. 2023, 370, 110295. [Google Scholar] [CrossRef] [PubMed]
- Foltz, E.A.; Witkowski, A.; Becker, A.L.; Latour, E.; Lim, J.Y.; Hamilton, A.; Ludzik, J. Artificial intelligence applied to non-invasive imaging modalities in identification of nonmelanoma skin cancer: A systematic review. Cancers 2024, 16, 629. [Google Scholar] [CrossRef] [PubMed]
- Verdaguer-Faja, J.; Toll, A.; Boada, A.; Guerra-Amor, Á.; Ferrándiz-Pulido, C.; Jaka, A. Management of cutaneous squamous cell carcinoma of the scalp: The role of imaging and therapeutic approaches. Cancers 2024, 16, 664. [Google Scholar] [CrossRef]
- Cocuz, I.G.; Popelea, M.C.; Niculescu, R.; Manea, A.; Sabău, A.H.; Tinca, A.C.; Szoke, A.R.; Budin, C.E.; Stoian, A.; Morariu, S.H.; et al. Pathophysiology, histopathology, and differential diagnostics of basal cell carcinoma and cutaneous squamous cell carcinoma—An update from the pathologist’s point of view. Int. J. Mol. Sci. 2024, 25, 2220. [Google Scholar] [CrossRef]
- Mathieu, O.; Chaine, A.; Benassarou, M.; Combes, F.; Debelmas, A.; Lanciaux, S.; Bertolus, C.; Bouaoud, J. Epidemiology of facial skin cancers managed in a French ambulatory surgical center. J. Stomatol. Oral Maxillofac. Surg. 2024, 125, 101726. [Google Scholar] [CrossRef]




| Magnolia Species | Organ | Honokiol Content (mg·g−1 D.W.) | Reference | |
|---|---|---|---|---|
| M. champaca | bark | 5.09 | [52] | |
| flower | 4.09 | |||
| M. denudata | bark | 5.89 | ||
| flower | 2.10 | |||
| M. grandiflora | bark | 6.61 | ||
| flower | 18.92 | |||
| M. obovata | leaf | 0.70 | [94] | |
| flower | petals | 1.74 | ||
| sexual parts | 4.70 | |||
| M. officinalis | flower | 13.20 | [52] | |
| bark | 56.79 | |||
| trunk | 0.08–25.28 | [79] | ||
| branches | 6.64 | |||
| root | 87.56–96.51 | |||
| M. officinalis var. biloba | trunk | 13.12–18.37 | ||
| bark | 3.18–18.37 | [102] | ||
| leaf | 0.06–9.76 | |||
| Magnolia × pruhoniciana | leaf | 0.11–8.26 | [94] | |
| flower | petals | 7.25–18.90 | ||
| sexual parts | 17.13–55.34 | |||
| M. tripetala | leaf | 191.62 | ||
| flower | petals | 252.26 | ||
| sexual parts | 230.82 | |||
| Name/Parameter | Characteristics | Reference |
|---|---|---|
| IUPAC name | 3′,5-Di(prop-2-en-1-yl)[1,1′-biphenyl]-2,4′-diol | [2,109] |
| Synonym name | 3,5′-diallilo-4,2′-dihydroxybiphenyl | [112] |
| 4-allyl-2-(3-allyl-4-hydroxy-phenyl)phenol | ||
| Molecular formula | C18H18O2 | [2,89,113] |
| Physical state 20 °C | White crystalline solid | [114] |
| Molecular weight | 266.334 g·mol | [3] |
| Affinity for water | hydrophobic | [16] |
| Melting point | 86–86.5 °C | [109,114] |
| Boiling point | 400 °C | [109] |
| Heat of evaporation | 67.7 kJ·mol−1 | [109] |
| Solubility | dimethyl sulfoxide: 36 mg·mL−1 | [110,112,115] |
| water: practically insoluble | ||
| methanol, ethanol: very soluble 33 mg·mL | ||
| soluble in chloroform, benzene, and caustic alkali | ||
| Odour | spicy and fragrant | [109,116] |
| Honokiol/Extract/Other Chemical Compounds | Virus | Cell Lines | Honokiol Activity | Reference |
|---|---|---|---|---|
| Honokiol: 0, 10, 20 µM | DENV-2 | BHK, Huh-7 | Honokiol (HK) inhibited DENV-2 replication and endocytosis, as well as the expression of viral genes and NS1, NS3, and dsRNA proteins; it also disrupted the co-localisation of DENV envelope glycoproteins and early endosomes. Hence, honokiol may be used as a therapeutic agent in the chemotherapy of DENV infections. | [148] |
| Honokiol extract: 0, 5, 10, 15, 25, and 50 μg mL−1, acyclovir, | HSV-1, MHV68, KSHV | Vero, MEF | HK reduced the replication of the DNA of HSV-1, MHV68, and KSHV viruses and blocked the expression of HSV-1 viral genes at the levels of RNA and proteins; this activity increased when combined with acyclovir. HK may be a new ingredient in the treatment of HSV-1 and other herpes viruses. | [137] |
| Honokiol and α-mangostin at concentrations of 0, 5, and 10 µM) | MAYV | Vero-E6, HDF, HeLa | HK and α-mangostin shielded Vero-E6 cells against MAYV virus and reduced viral RNA replication in HeLa. They limited the infection with MAYV, Una, Chikungunya, and Zika viruses, inhibited the expression of viral proteins E1 and nsP1, and increased the expression of type I IFNs (IFN-α/β) and interferon-stimulated genes (IFN α, IFN β, TNF α, ISG15, MxA, MDA-5, OAS2, and IL-1β). Both these compounds exhibit wide-ranging anti-arbovirus activity involving different mechanisms. | [149] |
| Honokiol derivatives 3-((5-phenyl-1,3,4-oxadiazol-2-yl)methyl)oxazol-2(3 H)-ones | SARS-CoV-2 | HEK-293-T-ACE2 | HK derivatives were effective agents against SARS-CoV-2 as blockers of human ACE2 and were safe for host cells. | [150] |
| Honokiol thioethers containing 1,3,4-oxadiazole fragments | Some honokiol thioethers blocked SARS-CoV-2, binding to the ACE2 receptor via a specific immunological phenomenon involving T cell receptors called dual recognition of the SARS-CoV-2 RBD spike and human ACE2 and were not cytotoxic; hence, they may be inhibitors of α-D-glucoside glucohydrolase and SARS-CoV-2 entry. | [87] | ||
| Honokiol, magnolol, trans-3-Phenylacrylic (cinnamic) acid (concentration of each: 100 µM), furin inhibitor 25 µM | VeroE6 | HK slowed down SARS-CoV-2 infection and an analogue site in SARS-CoV-2 S protein and limited SARS-CoV-2 infection via inhibition of furin-like activity and blocking PI3K/Akt signalling. | [151] | |
| Honokiol: 0, 7, 8 μM | VeroE6, A549 | HK prevented the SARS-CoV-2-mediated cytopathic effect (50% effective concentration 7.8 μM), reduced viral RNA copies and infectious progeny titres, and inhibited SARS-CoV-2 replication, including its variant Omicron, and other human coronaviruses at a post-entry step of the replication cycle via the expression of transmembrane protease serine 2 and angiotensin-converting enzyme 2. Honokiol is strongly recommended for animal and clinical studies to evaluate its effect on virus replication and pathogen–host (inflammatory) interplay. | [152] |
| Honokiol/Extract/Other Compounds | Bacterial Species/Strain | Minimal Inhibitory Concentration (MIC) | Main Conclusions | Reference |
|---|---|---|---|---|
| in vitro | ||||
| Honokiol, magnolol: 0, 50, 90% | Methicillin-resistant Staphylococcus aureus Methicillin-susceptible S. aureus | MIC/MBC at 16–64 mg·L−1 against the susceptible strain MIC90/MBC90 of 64/64 (magnolol) 32/64 mg·L−1 (honokiol) against the resistant strain | Honokiol (HK) showed similar activity to that of amikacin, gentamicin, and levofloxacin against the methicillin-susceptible S. aureus strain. It exhibited lesser anti-methicillin resistant potency than vancomycin and etilmicin but stronger efficacy than magnolol, gentamicin, amikacin, levofloxacin, piperacillin, fosfomicin, ciprofloxacin, or norfloxacin. Honokiol and magnolol exerted a synergistic effect, enhancing the activity of conventional antibiotics. The resistance reversal effects, especially those of amikacin and gentamicin against S. aureus, are related to the modulation of bacterial cell membrane penetration and cell wall damage. | [158] |
| Honokiol, magnolol: 10 mg·mL−1 | Methicillin-resistant S. aureus | 1 μg·mL−1 | In murine phagocytes, honokiol and magnolol enhanced antioxidant activity in S. aureus infection by reducing ROS and inflammatory cytokines/chemokines, increased interferon type I and III mRNA expression, and inhibited S. aureus internalisation by human lung alveolar epithelial cells, which confirms the immunomodulatory and antimicrobial activity. Studies on the non-parasitic nematode Caenorhabditis elegans suggest antimicrobial activity of both neolignans from Magnolia bark in vivo. | [156] |
| Lonicera japonica ethanol extract, ethyl acetate fraction (50%), Magnolia obovata, ethanol extract, ethyl acetate fraction (70%) | B. subtilis, E. coli, P. aeruginosa, S. aureus | MIC (μg·mL−1) of magnolol and honokiol 31.2 (S. aureus) 10,000.0 (E. coli, B. subtilis) 1250.0 (P. aeruginosa) | L. japonica 50% ethyl alcohol extract was effective against S. aureus and P. aeruginosa, while L. japonica ethyl acetate fraction, as well as M. obovata 70% ethanol and ethyl extracts, were effective against all tested bacterial strains. This activity was related to the presence of neolignans (honokiol and magnolol) in M. obovata and polyphenols (luteolin and caffeic acid) in L. japonica. Synergistic antibacterial activity against B. subtilis and S. aureus was found using a combination of both ethyl acetate fractions, which suggests their potential use as natural preservatives in cosmetics. | [157] |
| Honokiol, magnolol (98%) | Acetinobacter baumannii ATCC 17978 | 500 μg·mL−1 (honokiol) 1000 μg·mL−1 (magnolol) | Honokiol and magnolol inhibited biofilm formation in five and four clinical isolates of A. baumannii, respectively. They reduced pellicle development and the gilding motility of this pathogen and extended lifespan of infected nematodes Caenorhabditis elegans; therefore, they may be useful in combating infections with this bacterium. | [159] |
| Honokiol and its derivatives | S. aureus, S. pyogenes, S. albus, S. epidermidis, E. faecalis, E. coli, K. Pneumoniae, P. aeruginosa | 256 μg·mL−1 (E. coli and P. aeruginosa), 8–32 μg·mL−1 (K. pneumoniae and S. pyogenes), 16 μg·mL−1 (S. aureus and S. epidermidis), 16–32 μg·mL−1 (S. faecalis) | Among the 12 honokiol derivatives, the 7c derivative containing the piperidine ring showed a particularly broad spectrum of antibacterial efficacy, including monoderm and diderm species, which was more potent than that of linezolid, vancomycin, honokiol, and the other derivatives of this compound. This derivative probably destroyed the cell walls of the bacteria (S. aureus ATCC25923) at 1 × MIC and 4 × MIC. | [11] |
| Honokiol and magnolol (10, 20 and 50 µg·mL−1, magnolia bark methanol extract (30, 40, and 50 µg·mL−1. DMSO was used as the control | Streptococcus mutans | MIC of magnolia bark extract: 40 µg·mL−1, MIC of honokiol and magnolol: 10 µg·mL−1 in relation to chlorhexidine: 0.25 µg·mL−1 | Honokiol exhibited time- and dose-dependent germ-killing activity against S. mutans planktonic cells and restrained the multi-step and complex biofilm formation process where free-floating bacteria attach to a surface, multiply, and create a protective matrix. Due to poor penetration, the biofilm is resistant to antibiotics. Magnolia bark extract exerted anti-biofilm action at concentrations higher than 30 µg·mL−1. Honokiol (50 30 µg·mL−1) and chlorhexidine (500 µg·mL−1) were characterised by lower penetration potential and bactericidal effects on S. mutans biofilm than magnolol (50 µg·mL−1) at 5 min post-exposure. All these agents exhibited scant biofilm-inhibiting activity after 30 s. In contrast to chlorhexidine, honokiol showed low toxicity towards gingival epithelial cells Ca9-22. | [160] |
| Honokiol and magnolol (0, 2.5, and 5 µg·mL−1) | Oral pathogens A. actinomycetemcomitans; S. mutans; S. aureus; methicillin-resistant S. aureus MRSA; E. coli | Honokiol MIC/MBC (µg·mL−1) A. actinomycetemcomitans 10/10; S. mutans 10/20; MRSA 10/20; S. aureus 10/90; E. coli > 100 > 100 Magnolol MIC/MBC (µg·mL−1) A. actinomycetemcomitans 10/20; S. mutans 10/20; MRSA 20/30; S. aureus 10/90; E. coli > 100 > 100 | Honokiol decreased microbial biofilm formation and reduced pathogen antibiotic resistance. Honokiol at 5 µg·mL−1 repressed the femA and femB gene expression, essential for the formation of the cell wall peptidoglycan layer and methicillin resistance, and the expression of the mecA and mecR1 genes. The mecA gene encodes the unique protein PBP2a with transpeptidase activity, which is not inhibited by β-lactam antibiotics (methacyclin), while the mecR1 gene encodes the β-lactam-sensing transmembrane signalling protein MecR1. Magnolol had no impact on the femA and femB expression but upregulated the mecR1 expression and repressed the mecA and mecI expression. The latter gene encodes a transcription repressor protein, MecI. The biofilm formation and expression of genes that contribute to antibiotic resistance were consistent with the respective phenotypes. Both honokiol and magnolol (2.5–10 μg·mL) efficiently repressed the expression of pro-inflammatory genes (IL-6 IL-1β, COX-2, and iNOS mRNA) in RAW264.7 cells. Honokiol and magnolol concentrations of 2.5–5 µg·mL−1 upregulated, while the dose of 10 µg·mL−1 downregulated, the TNF-α mRNA. | [161] |
| DMSO-dissolved honokiol or magnolol (200, 500, and 1000 μg·mL−1) and equivalent DMSO concentrations as the control | A. baumannii 17978 and other clinical A. baumannii isolates, antibiotic resistant ATCC BAA 1709 and A 571, as well as multidrug-resistant strains: A 550, A553, A 556, A 564, A 578, and A 580) | Not applicable | Both honokiol and magnolol efficiently disrupted biofilm formation dose-dependently and dispersed matured (established) A. baumannii ATCC 17978 biofilms via triggering ROS formation. Both neolignans greatly restricted biofilm formation by high biofilm formers ATCC BAA 1709 and A550 isolates, intermediate biofilm formers A564 and A580, and low biofilm-forming strain A571 but did not exert an anti-biofilm effect on two other less efficient biofilm formers, A553 and A556, and an intermediate biofilm former, A578. Honokiol and magnolol efficiently repressed the formation of a thin surface-attached layer (a pellicle) at a liquid–air interface, suppressed the surface motilities of A. baumannii, and extended in vivo the survival of infected nematodes Caenorhabditis elegans. Therefore, these major bioactive polyphenols of Magnolia officinalis may be useful for preventing the spread and managing existing A. baumannii infections. | [159] |
| Honokiol (0.5, 1, 2, 4, and 8 μg·mL−1) | S. aureus ATCC 29213 and 9 clinical S. aureus isolates (SA 56, SA 121, SA 1987, SA 1862, SA 2985, SA 3015, SA 3101, SA 3303, and SA 3629) | MIC values against the tested strains in the suspension ranging within 4–16 μg·mL−1 MBC values ranging within 8-32 μg·mL−1 | S. aureus isolates produce various types of biofilms mediated by the main component of the bacterial biofilm extracellular matrix (extracellular DNA, eDNA) or partially deacetylated N-acetylglucosamine polymer polysaccharide intercellular adhesion (PIA) with high importance for cell-to-cell adhesion and biofilm accumulation produced by the icaADBC operon. Honokiol was recognised as an efficient agent against S. aureus biofilm cultures, as its MBICs for strains grown in the biofilm cultures oscillated between 32 and 128 μg·mL−1. Honokiol detaches mature biofilms, destroys bacteria in biofilms, prevents mRNA production from genes icaA, cidA, and sarA, and therefore inhibits eDNA release by S. aureus and downregulates PIA expression. Gene icaA, encoding N-acetylglucosaminyl-transferase, is essential for PIA synthesis and regulates the synthesis of exopolysaccharide matrix components. Gene CidA, related to cell lysis and eDNA release, enhances extracellular murein hydrolase activity. Staphylococcal-specific SarA gene family transcription regulators control large numbers of target genes involved in, e.g., biofilm formation. The SarA gene plays a pivotal role in biofilm formation capacity, acting as a regulator for virulence factors and biofilm development. | [162] |
| Honokiol and magnolol dissolved in 10% DMSO | Propionibacterium acnes ATCC 6919 and Propionibacterium granulosum ATCC 25564 | MIC against both tested strains: honokiol 3–4 μg·mL−1 (11.3–15 μM), magnolol 9 μg·mL−1 (33.8 μM) | Honokiol and magnolol may be regarded as promising acne-alleviating candidates for cutaneous application without causing any adverse reactions. Both neolignans killed P. acnes swiftly, with 105 organisms/mL eliminated within 10 min upon either honokiol (20 μg·mL−1; 75.2 μM) or magnolol (45 μg·mL−1; 169.2 μM) treatment. Honokiol showed higher cytotoxicity than magnolol in human normal fibroblasts and HaCaT cell lines, but both these neolignans exhibited similar cytotoxicity in co-administration with an acne-mitigating agent triclosan. Honokiol and magnolol (concentrations of 5, 10, and 20 μM) lowered the secretion of P. acnes-induced pro-inflammatory cytokines (TNF-α, IL-8) in human leukaemia monocytic cell line (THP-1) cells. | [153] |
| in vivo | ||||
| Amphiphiles of honokiol and magnolol: 0,2 mM; 53.3 mg | Methicillin-resistant S. aureus | 0.5–2.0 μg·mL−1 | Among various new honokiol/magnolol amphipaths that mimic the molecular structures and germ-killing properties of cationic amphipathic peptides, compound 5i, with low cytotoxicity (CC50 > 128 μg·mL−1), haemolytic activities (HC50 = 789.2 μg·mL−1), and high membrane selectivity (SI = 789.2), showed strong activity against low-drug-resistance S. aureus isolates. 5i disrupts biofilm and damages bacterial cell membranes, has strong anti-infective efficacy in a mouse model of sepsis (female BALB/c mice) with S. aureus isolate, and may be a safe antibacterial agent in the therapy of bacterial infections. | [150] |
| 0.3% Water-ethanol extract from M. grandiflora bark | S. mutans | 0.3 mg·mL−1 | Clinical studies conducted on a group of volunteers (18–35 years old) not using any mouthwash or antibiotics showed that Magnolia bark extract used as mouthwash had an antibacterial effect against S. mutans in dental plaque, reduced the concentration of S. mutans on teeth, and decreased the volume of microbial plaque on dental surfaces and the plaque index. | [163] |
| Honokiol/Extract/Other Compounds | Fungi/Strains | MIC/MFC Minimum Fungicidal Concentration | Main Conclusions | Reference |
|---|---|---|---|---|
| in vitro | ||||
| Magnolia obovata, ethanol extract, ethyl acetate fraction (70% each), Lonicera japonica ethanol extract, ethyl acetate fraction (50% each) | A. brasiliensis—mould C. albicans—yeast | 70% ethyl acetate fraction from M. obovata MIC (μg·mL−1): 625 (C. albicans), 2500 (A. brasilensis);—70% ethanol extract MIC (μg·mL−1): 1250 (C. albicans), 1000 (A. brasilensis); magnolol and honokiol MIC (μg·mL−1): 5000 (C. albicans), 39 (A. brasilensis) | Both the 70% alcoholic and ethyl acetate M. obovata extracts exhibited microbicidal activity against A. brasilensis and C. albicans. A synergistic effect against C. albicans was shown for the combination of two ethyl acetate fractions. Magnolol and honokiol were responsible for the antibacterial activity in M. obovata. These two neolignans exhibited strong activity against A. brasilensis. The combination of extracts can be a promising natural plant preservative. | [157] |
| Honokiol 16 μM | C. albicans | 16 μg·mL−1 | Honokiol (HK) inhibited the biosynthesis of ergosterol (a steroid playing a key role in fungal cell membranes), blocked the activity of plasma membrane proton-ATPase (PM H+-ATPase or Pma1p), thus inducing cytosolic acidification of the cytosol, caused abnormalities in vacuole function and morphology, and disrupted intracellular calcium homeostasis. Amiodarone-induced calcium influx lessened honokiol toxicity against C. albicans via stimulation of the calcineurin signalling pathway participating in honokiol tolerance. | [168] |
| Honokiol and magnolol (8, 16, 32, and 64 μg·mL−1) | Clinical isolates of C. albicans strains (SC5314, CA1, CA2, CA3, CA4, CA10, CA127, CA129, CA132, CA135, and CA137) | MICs 16–32 μg·mL−1 | Honokiol restricted adhesion to the HSC-T6 cells and free-floating state of C. albicans cells in a liquid medium (planktonic growth), protected against yeast-to-hypha transition, and inhibited biofilm growth at the early, developmental, and biofilm maturation stages via the Ras1-cAMP-Efg1 pathway. This critical regulator of C. albicans biofilms is activated by extracellular environmental factors that stimulate the Ras1 protein, which in turn induces adenylate cyclase (Cyr1) to synthesise the second messenger cAMP from ATP. Honokiol and magnolol (16 μg·mL−1) in vivo extended the lifespan of C. albicans-infected nematodes Caenorhabditis elegans. | [59] |
| ex vivo; in vivo | ||||
| Honokiol, magnolol: 0, 12.5, 25, 50 µM | Trichophyton ajelloi, T. gypseum, T. mentagrophyte, T. rubrum, Microsporum canis | MIC 8 mg·mL−1 (honokiol, magnolol) MFC 16 mg·mL−1 (honokiol, magnolol) | Both neolignans had strong anti-dermatophyte activity as a result of ergosterol biosynthesis suppression with simultaneous enhancement of squalene accumulation in fungal cells due to limitation of squalene conversion into 2,3-oxidosqualene. They did not have cytotoxic effects on human neutrophils in an ex vivo assay but influenced the generation of lipopolysaccharide (LPS)-stimulated cytokines in human neutrophils: magnolol inhibited the secretion of IL-1β, IL-8, and TNF, while honokiol inhibited IL-1β. Synergy was found for the magnolol–terbinafine combinations (FICI = 0.50), while the mixtures of HK with terbinafine (positive control) displayed an additive effect (FICI = 0.56). HK and magnolol, which act via impairment of ergosterol biosynthesis, can be effective fungicidal agents against dermatophytes. | [7] |
| Honokiol: 0, 2, 4, 8, 16 μg·mL˗1. Honokiol concentrations no more than 8 µg/mL were considered nontoxic and were used in the experiments | A. fumigatus | MFC 12 μg·mL−1 | Honokiol exhibited fungistatic (at 6 h) and fungicidal action (at 24 h). HK inhibited A. fumigatus growth, lessened its adhesion ability, diminished biofilm formation, and increased membrane permeability. In an in vivo A. fumigatus keratitis murine model (8-week-old females), HK reduced fungal loading, diminished the number and vitality of neutrophils, and repressed TLR-2 action, inflammatory cytokines (TNF-α, IL-1β), and amphoterin, also known as HMGB1. HK may be a powerful antimycotic and anti-inflammatory agent for A. fumigatus keratitis. | [164] |
| Honokiol (5 μL) and mannan (10 mg·mL˗1), Dectin-2siRNA (10 μM) or honokiol (8 μg/mL) three times a day (9:00 a.m., 1:00 p.m., 5:00 p.m.) | A. fumigatus | MIC 8 µg·mL−1 | Compared to the honokiol-supplemented group, 383 downregulated and 1175 upregulated genes were activated in C57 black 6 mice keratomycosis with PBS treatment. Proteins encoded by these genes are involved in fungal defence and immune activation. HK inhibited fungal growth through delaying germination and alterations in membrane permeability in vitro and reducing hyphal load in vivo. The antiphlogistic HK action was based on reduced expression of multiprotein complex NLRP3 releasing IL-1β as well as the pattern recognition receptor for fungal Dectin-2 that initiates inflammatory responses and signal transmitting in innate immune cells. HK may be effective and safe in mitigation of fungal keratitis symptoms. | [165] |
| Honokiol | Research Model | Experimental Groups (G) | Duration | Main Conclusions | Reference |
|---|---|---|---|---|---|
| Honokiol | Human foreskin fibroblasts [HFF-1; Culture Collection Type SCRC-1041] and human keratinocyte cell line (HaCat). | G1 and G2—two control groups of HFF-1 and HaCat cell lines. G3 and G4—cigarette-smoke-exposed HFF-1 and HaCat cultures. Cultures of human fibroblasts and keratinocytes incubated with two non-cytotoxic HK concentrations, 10 and 20 μM, and exposed to cigarette smoke. | 48 h | Honokiol (HK) was a strong and safe anti-inflammatory, anticollagenolytic, anti-apoptotic, and anti-senescence agent against cigarette smoke. In cell cultures exposed to tobacco smoke, HK decreased IL-8 and IL-1α production in keratinocytes, prevented degradation of fibroblast-produced collagen I and IV via enhancement of the activity of matrix proteinases inhibitor TIMP-2, shielded fibroblasts from apoptosis, and diminished β-galactosidase expression in the fibroblasts. HK may be recommended for basic science and clinical use. | [9] |
| Honokiol dissolved in 0.1% DMSO | Primary cultures of normal buccal mucosal fibroblasts (BMFs) known as normal cheek fibroblasts 2. Patient-derived fibrotic buccal mucosal fibroblasts (fBMFs) derived from precancerous oral submucous fibrosis (OSF) tissues. | 1G: control; 2G: arecoline; 3G: arecoline + 5 μM HK; 4G: arecoline + 10 μM HK; 5G: arecoline + 20 μM HK. Normal BMFs seeded at 1 × 104 cells/well and fBMFs treated with HK for 48 h (5 replicates for each HK concentration). | 48 h | HK is a promising safe agent inhibiting oral fibrogenesis progression and preventing oral mucosa transformation into cancer. HK suppressed arecoline-induced myofibroblast activities, e.g., collagen gel shrinkage, cellular motility, and wound repair capacities. The anti-fibrotic effect of HK resulted from arecoline-elicited improvement of myofibroblast activities via TGF-β pathway suppression. Increasing HK concentrations in BMFs suppressed the arecoline-induced TGF-β secretion. HK inhibited arecoline-stimulated Smad2 phosphorylation together with α-1 type I collagen and α-SMA downregulation. HK attenuated tubulointerstitial fibrosis by restraining the ECM and inflammatory mediators. | [23] |
| Honokiol | Normal human epidermal keratinocytes (NHEKs). | 1G: control 100 µM 2-aminoethoxydiphenyl borate (2-ABP); 2G: 2-ABP 100 µM + 10 µM HK; 3G: 2-ABP 100 µM + 30 µM HK; 4G: 2-ABP 100 µM + magnolol 10 µM; 5G: 2-ABP 100 µM + magnolol 30 µM; 6G: DMSO; 7G: HK 10 µM; 8G: magnolol 10 µM. | 24–48 h | Honokiol and magnolol strongly inhibited TRPV3 ion channels produced by keratinocytes and mitigated the TRPV3-channel agonists (carvacol) or GOF mutation-induced cytotoxicity. HK and magnolol obstructed the TRPV3 current (ITRPV3) and the rise in cytoplasmic calcium in NHEKs and HEK293T cells—derived from HEK cells—overexpressing hTRPV3 and its GOF mutants (G573S and G573C) linked with Olmstead syndrome (mutilating palmoplantar keratoderma). HK and magnolol suppressed the TRPV3 agonist-stimulated release of inflammatory cytokines (IL-6 and IL-8) from keratinocytes and may be potentially used to treat inflammatory skin diseases. | [21] |
| Honokiol | A human monocytic leukaemia cell line (THP-1) originating from the peripheral blood of an acute monocytic leukaemia (AML-M5) patient was cultured on RPMI 1640 medium with supplementation of 10% FBS. Differentiated THP-1 cells were obtained via culturing two types of cells together with p-methoxyamphetamine (100 ng·ml−1 PMA for twenty-four hours). Then, BMDMs were cultured in DMEM with 10% FBS and 30% fibroblast-like cell line derived from mouse tissue (L929) culture supernatants were obtained from C57BL/6 mouse femora and shinbones. | In studies on HK-suppressed NLRP3 inflammasome priming and activation processes, HK was applied for 40 min to LPS-primed and ATP (5 mM)-stimulated THP-1 cells for an hour. In order to probe possible HK targets, the LPS-primed THP-1-cell lysates were incubated for 45 min with HK at concentrations of 10, 100, and 1000 μM. Pronase (Actinase E) was administered for additional 30 min for hydrolysis. In experiments concerning HK-promoted SLC3A2 degradation via the proteasome pathway, HK was applied to LPS-treated and then ATP-stimulated THP-1 cells. Prior to the LPS exposure, the cells were treated for an hour with either 5 mM autophagy blocker 3-MA or 10 µM strong proteasome blocker MG132. The LPS-stimulated THP-1 cells were additionally energised or not by ATP in the absence or presence of HK. | 7 days | Honokiol exerted severe anti-inflammatory effects in vitro. It considerably diminished IL-1β release and caspase-1 (interleukin-1 converting enzyme) activation at various time points and therefore suppressed the key component of the nonspecific (innate) immune system, the Nod-like receptor pyrin domain-containing 3 (NLRP3) inflammasome priming, and inhibited cell activation processes. | [171] |
| Honokiol | Research Model | Experimental Groups (G) | Duration | Main Conclusions | Reference |
|---|---|---|---|---|---|
| Honokiol 18 mg | Human neutrophil model | G1: LPS-stimulated (10 ng·mL−1) human neutrophils (LPS+); G2: negative control cells (LPS–); G4–6: HK 12.5, 25, and 50 μM in LPS-stimulated neutrophils; G7–9: magnolol 12.5, 25, and 50 μM in LPS-stimulated neutrophils. Both neolignans were applied 30 min prior to neutrofil LPS-stimulation. | 24 h | Honokiol (HK) and magnolol as dual activity therapeutic agents, i.e., with selective fungistatic and anti-inflammatory activity, can be used to modulate the balance between anti- and pro-inflammatory signals in human host–tinea interactions. Their anti-inflammatory properties support tissue repair and lesion regeneration process and alleviate ringworm symptoms. The tested concentrations of both neolignans did not induce cytotoxicity in polymorphonuclear leukocytes, compared to the LPS-negative control. HK and magnolol affected the ex vivo LPS–cytokine production in human neutrophils: HK decreased IL-1β, while magnolol showed an inhibitory effect towards IL-8, IL-1β, and TNF-α. | [7] |
| Honokiol | Microglia (Hortega cells) and astroglia (star shaped cells) dissected from midbrain and cerebral hemispheres of 2-day-old Sprague Dawley or Wistar rats | Control: unstimulated cells. Treatments: HK between 1 μM and 100 μM. | 48 h | Honokiol markedly reduced the LPS-induced inflammatory response of astroglia and microglia primary cultures via the inhibition of inflammatory molecules (IL-1β, IL-6, iNOS, and TNF-α), as well as anti-inflammatory cytokine (IL-10) stimulation. HK mitigated the LPS-induced expression of epithelial zinc finger protein, also known as zinc finger transcription factor KLF4, in microgliocytes and astroglia. HK shows great potential in early research for further development as a drug candidate for the treatment of neuroinflammatory disorders. | [172] |
| Honokiol | Foetal (extraembryonic) membranes, tunica muscularis (myometrium or muscular coat), freshly isolated amniotic epithelial cells, and myocytes (primary myometrial cells or uterine smooth muscle cells) | 1G: control; 2G: lipopolysaccharide (LPS); 3G: LPS + 100 µM HKu; 4G: fibroblast-stimulating lipopeptide-1 (fsl-1); 5G: fsl-1 + 100µM HK; 6G: 20 µg·ml−1 poly(I:C) (poly(I) • poly(C))—immunostimulant polyinosinic–polycytidylic acid; 7G: poly(I:C) +100µM HKu | G1, 2, 4, 6 60 min G. 3, 5, 7 20 h - | Honokiol severely reduced the inflammation-triggering substance (IL6, IL1A) and chemotactic cytokine (CCL2, CXCL1, CXCL8) mRNA expression and secretion from extraembryonic membranes (amnion and the amnion-surrounding layer choriodecidua) and the muscular middle layer called myometrium stimulated with poly(I:C), LPS, or fsl-1. In the amniotic epithelial cells, HK diminished the expression and secretion of the extracellular enzyme gelatinase B (matrix degrading enzyme MMP9). In the myometrium, HK severely inhibited the contraction-associated protein PTGFR expression, as well as the secretion of uterotonic prostaglandins PGE2 and PGF2α, but blocked TNF-induced uterine muscle contraction. In primary amnion and the muscular middle layer of the uterine wall (myometrial) cells, HK suppressed the pro-inflammatory cytokine IL1B- and TNF-induced transcriptional activity of NF-κB RelA, which is a strong transcriptional activator of pro-inflammatory genes. HK reduced the expression of pro-labour and inflammatory mediators in human amnion, choriodecidua, and tunica mascularis through suppressed activation of NF-κB. HK was recognised as a strong therapeutic agent preventing preterm birth. | [173] |
| Honokiol | Research Model | Experimental Groups (G) | Duration | Main Conclusions | Reference |
|---|---|---|---|---|---|
| Honokiol (a specific neolignan type) dissolved in 2% DMSO and physiological saline given before carrageenan- or CFA-induced inflammation | adult male 20–30 g BALB/c mice | I. experiment: carrageenan-induced (100 µL/paw) biological inflammation model groups: 1G: normal—saline with 2% dimethyl sulfoxide (DMSO); 2G: carrageenan; 3G: piroxicam (10 mg·kg−1 i.p.); 4G–6G: carrageenan + HK 0.1, 5, and 10 mg·kg−1 (i.p.). Carrageenan and piroxicam were administered intraperitoneally 4 h post carrageenan (100 µL/paw) administration. II. experiment: Freund’s complete adjuvant (FCA-induced skin inflammation) groups: 1G: vehicle control; 2G: CFA (20 µL/paw) injected into the back foot plantar surface; 3G: dexamethasone Dexa 5 and 10 mg·kg−1 (i.p.), 4G. HK 10 mg·kg−1 (i.p.) | I experiment 4 h II experiment 0–6 days | Honokiol (HK) exerted anti-allodynic and anti-hyperalgesic effects in sharp and persistent pain models induced by CFA and carrageenan and attenuated pain induced by thermal hyperalgesia. HK reduced the expression of CFA- and carrageenan-induced inflammatory cytokine mRNA (IL-1β, IL-6, TNF-α, and VEGF—a signal protein involved in angiogenesis). HK upregulated genes linked to antioxidants (SOD2) and enhanced HO-1 and Nrf2 expression, while reducing plasma nitrite levels. Studies with HK agonists [gabapentin (5 mg·kg−1), piroxicam (5 mg·kg−1), tramadol hydrochloride (50 mg·kg−1) i.p.] and antagonists [flumazenil (0.2 mg·kg−1), naloxone (4 mg·kg−1) and olanzapine (10 mg·kg−1), i.p.] revealed that HK exerted analgesic activity via inhibition of anti-inflammatory mediators. Muscle activity and liver function tests revealed that HK is reasonably safe and may be a promising candidate for ongoing development as an intervention option for chronic and acute pain. | [174] |
| Honokiol | 7-week-old Bagg Albino BALB/c mice (males) | 1G: PBS-treated controls (phosphate-buffered saline (PBS) (pH 7.4); 2G: 1-chloro-2, 4-dinitrobenzene (DNCB); 3G: HK (5 mg·kg−1) + DNCB; 4G: HK (10 mg·kg−1) + DNCB; 5G: dexamethasone (DEX 10 mg·kg−1) + DNCB. | 24 h | Honokiol (10 mg·kg−1) administration suppressed DNCB-induced mastocyte accumulation and inflammation. HK reversed DNCB-induced enhancement in serum immunoglobulin E levels. HK ameliorated the DNCB-induced rise in pro-inflammatory cytokines (interferon-γ, IL-4, 13 and 17) in the skin and lymph glands. Simultaneously, HK reversed the DNCB-induced enlargement in the lymph node size. HK (3,5′-diallyl-4,2′-dihydroxybiphenyl) markedly reduced DNCB-induced atopic reactions in the ears and lymph glands. HK may be a prospective therapeutic agent in eczema treatment. | [3] |
| Honokiol | 6-week-old SKH-1 hairless mice (females) | I. Time-effect experiments with a topical low dose of HK application before and after UVB exposure on tumour incidence groups: 1G: control acetone; 2.G: HK 2 h before UVB exposure; 3G: HK 1 h before UVB exposure; 4G: HK 30 min before UVB exposure; 5G: HK immediately after UVB exposure; 6G: HK 30 min after UVB exposure; 7G: HK 30 min after UVB exposure. Topical HK treatment—30 μg of HK in 200 μL of acetone UVB exposure 30 mJ·cm−2. II. Experiments concerning the effects of pretreatment with HK on tumour incidence in UVB-induced skin carcinogenesis groups: 1G: control (200 μL of acetone); 2G: HK 30 μg; 3G: HK 45 μg; 4G: HK 60 μg. Topical HK treatment: applications of 30, 45, and 60 μg of HK in 200 μL of acetone, respectively. HK was administered one hour before UVB exposure (30 mJ/cm2, Monday–Friday). | 27 weeks—induction of cancer in mouse cells (experiment I); 25 weeks—HK treatment (experiment II) | In the time-reaction and dose-response experiments, HK severely supressed skin tumour multiplicity by 49–58% and 36–78%, respectively, and diminished tumour volumes by 70–89% and 76–94% in a dose-related manner. Moreover, HK dose 60 μg diminished tumour incidence by 40%. Low HK doses (30 μg), when applied either prior to or after UVB rays, exhibited cancer-preventive effects. HK arrested UVB-induced skin cancer in a dose-related manner. HK was recognised as a safe and effective chemoprotective agent against skin cancer. The study of skin tumours revealed that HK induced apoptosis via extrinsic and intrinsic pathways, impeded UVB-induced inflammation and inflammatory factors, lowered cell survival cues and proliferation markers, and upregulated cell cycle inhibitor proteins, but downregulated cell cycle promoter proteins. | [175] |
| Honokiol in 0.5% CMC-Na | Specific pathogen free (SPF) grade, 22–25 g adult male ICR mice | 1G, Control, 2G. LPS (1 mg·kg−1, i.p.), 3G. LPS + HK (10 mg ·kg−1, per os). Each experimental group consisted of 10 individuals. | 11 days | Honokiol inhibited the inflammatory responses showing antidepressant effects. This compound ameliorated the LPS-induced NF-κB activation in the hippocampus and lowered the peripheral blood levels of inflammatory cytokines (TNF-α, IL-1β, and brain IFN-γ). It affected tryptophan metabolism, decreasing IDO expression, reduced the level of toxic quinolinic acid, reduced brain tissue free calcium, in consequence inhibiting calcium overload, and increased neuroprotective metabolites. | [104] |
| Honokiol | 8-week-old 20–22 g C57BL/6 mice (males) | I. LPS-induced septic shock groups: 1G, LPS (20 mg·kg−1 i.p.) alone, 2G. LPS +HK (35 mg/kg per os p.o.), 3G, LPS + HK (70 mg·kg−1 per os). II. Dextran sulphate sodium (DSS)-induced colitis “prevention model”) groups: 1G, The vehicle group fed regular water received the vehicle once a day per os; 2G, Model group fed with dextran sulphate sodium (DSS) water received vehicle once a day per os; 3G and 4G. HK (35 and 70 mg·kg−1) groups fed DSS water and received HK (35 or 70 mg·kg−1 p.o. daily). III. “therapy model” groups: 1G, Vehicle-administered group (p.o. once a day) drinking regular water; 2G, Model group fed DSS water for a week; 3G. and 4G. HK (35 and 70 mg·kg−1) groups pre-drinking DSS water for a week and next receiving HK (35 or 70 mg·kg−1 daily p.o.) for 5 days; 5G, Positive group pre-drinking DSS water for a week receiving 5-aminosalicylic acid (100 mg·kg−1 daily p.o.) for 5 days | 40 min | Honokiol exerted severe anti-inflammatory efficacy in vivo. HK mitigated LPS-induced inflammation, a severe, life-threatening stage of sepsis, and DSS-induced colon inflammation in vivo (“prevention and therapy model”) and suppressed adaptor apoptosis speck-like protein oligomerisation. The SLC3A2 protein coding gene was identified as a direct target of HK in the THP-1 cells. HK directly bound to SLC3A2 and downregulated its expression via enhanced proteasome-mediated proteolysis. HK triggered SLC3A2 to suppress cryopyrin (NLRP3) inflammasome activation via lowering L-leucine content transported into cells and lysosomes to block the mTORC1 pathway. Therefore, HK was recognised as a potential anti-inflammatory agent acting via the SLC3A2/L-leucine/mTORC1/NLRP3 pathway. | [171] |
| Honokiol /Extract | Research Model | Experimental Groups (G) | Duration | Main Conclusions | References |
|---|---|---|---|---|---|
| in vitro | |||||
| M. officinalis extract. Bark mixed with 70% ethanol in a volume ratio of 1:8 was heated at 60° C for 3 h. | Cell lines: 1G: immortalised human keratinocytes (HaCaT); 2G: human dermal fibroblasts (PCS–201–010); 3G: normal human epidermal keratinocytes (HEKn). Senescent fibroblasts with a doubling time of 14 days and young fibroblast (positive control) with a doubling time of less than 2 days. | 1G: M. officinalis extract (0.625 and 10 μg·mL−1) with a strong antioxidant resveratrol (100 µM)—a positive control and DMSO control (0.001%); 2G: honokiol (0.1; 1, and 10 μM) with M. officinalis extract (0.625 μg·mL−1) as a positive control and DMSO control (0.001%). | Extract (0.625 or 10 μg·mL−1) was administered to senescent fibroblasts at the specified concentrations for 12 days. For measurement of skin moisture retention, keratinocytes were exposed to DMSO (0.01%) or M. officinalis extract (0.625, 1.25, and 2.5 µg·mL−1) for 72 h | Honokiol (HK) is the major anti-oxidation agent in M. officinalis extracts. The non-toxic doses of this neolignan 1 and 10 μM reduced mitochondrial ROS generation more efficiently than the M. officinalis extract dose of 0.625 μg·mL−1. The HK treatment of aged fibroblasts promoted a cell proliferation comparable to the M. officinalis extract. HK reduced mitochondrial ROS generation through mitochondrial functional recovery due to a rise in MMP, resulting in lower mitochondrial mass, lower basal levels of glycolysis, and reduced post 2–DG acidification, suggesting a less intense residual glycolysis process that was not blocked by 2–DG. HK, similar to the M. officinalis extract, reduced the dependence on glycolysis as an energy source, indicating restoration of mitochondrial function by HK. Honokiol functioned as an oxygen radical scavenger via disproportionation reactions, limited ROS levels due to revitalisation of mitochondrial activity, and consequently improved senescence-associated phenotypes and enhanced the skin barrier function. HK reduced the SA–β–gal positive cells, lowered intracellular lipofuscin levels, increased SLIT2 expression, indicating honokiol-mediated enhancement of skin regeneration, inhibited the expression of MMP1 responsible for collagen degradation, decreased the expression of CCL2 and 5, and lowered IL-6 and 8, thereby mitigating skin inflammation. | [181] |
| in vivo | |||||
| Honokiol (30 μg in 0.2 mL acetone) | Highly susceptible to UVR-induced skin cancer SKH-1 hairless five-week-old female mice | Two groups of mice: G1: control (200 μL of acetone topically); G2: HK 30 μg in 200 μL of acetone administered topically 1 h before UVB exposure-initiated carcinogenesis (30 mJ/cm2/day) 5 days/week for 30 weeks. | 30 weeks | Honokiol did not affect weight gain; it maintained normal growth and development of mice and resulted in a reduction in tumour multiplicity and a decrease in the mean ratio of tumour area to total back area. Mechanistic studies revealed the proapoptotic potential of honokiol as a consequence of DNA fragmentation induction through the extrinsic (death receptor) pathway, in which the binding ligand–cell surface receptor activates caspases 8 and 3 and the intrinsic (mitochondrial) pathway via up-regulation of caspases 9 and 3, PARP, and tumour suppressor p53. This is crucial for maintaining homeostasis and determines the anti-photocarcinogenic action, making honokiol a strong topical chemopreventive, anti-skin-cancer agent. | [179] |
| Honokiol in a hydrophilic cream topical preparation | Highly susceptible to UVR-induced skin cancer SKH-1 hairless five-week-old mice | Three groups of mice with 7 individuals per group. 1G: control; 2G: UVB (180 mJ/cm2) treatment; 3G: honokiol applied externally prior or subsequent to irradiation. | 48 h | Honokiol administered topically prior to and after UVB irradiation led to (1) enhanced photoprotection in the context of tumour multiplicity and volume per tumour-transplanted mouse, (2) suppressed and reduced malignant progression of non-cancerous papillary tumour to carcinomas, (3) inhibited UVB-stimulated gene expression of PGE2, COX-2, PCNA, and inflammation-promoting molecules (TNF-α, IL-1β, IL-6) (4) decreased levels of cyclins (D1, D2, E2) and associated CDK2, CDK4 and CDK6, (5) upregulated cyclin-dependent kinase inhibitor Cip/p21, also known as p21 WAF1 or Kip/p21, crucial for cell cycle arrest, (6) suppressed PI3K activity crucial in cellular signalling and inhibited Akt at Ser473 phosphorylation responsible for Akt activation in UVB radiation-induced skin tumours. HK is a promising agent for the photoprotection through targeting inflammatory mediators, cell cycle control molecules, and cell survival signals in UVB-irradiated skin. | [178] |
| Hydrophilic ointment with honokiol (0.5 and 1.0 mg/cm2 skin area). The hydrophilic ointment base served as a vehicle for this topical formulation. | Female 5–6-week-old C3H/HeN mice homozygous for the Pde6brd1 allele. Normal—compared to wild type littermates with any gross phenotypic differences, COX-2 knockout (+/−) mice reproductive pairs kept within the 129 Ola/C57BL/6 mixed genetic background. | Five groups of mice. 1G: normal control; 2G: HK alone; 3G: UVB radiation (150 mJ/cm2) for four consecutive days); 4G: HK (0.5 mg/cm2 of skin area) + UVB; 5G: HK (1.0 mg/cm2 of skin surface) + UVB. HK was applied to shaved mouse skin in a hydrophilic cream-based topical formulation starting 3 days prior to the start of the UVB exposure and thereafter at 30 min preceding each exposure to 2 and 4 mg honokiol (4% and 8% (w/w) in the topical preparation) per mouse/50 mg vehicle. | 24 h | Honokiol prevented UVB-induced suppression of the allergic contact dermatitis response via the COX-2 synthesised principal potent lipid inflammatory mediator PGE2 but did not restrict UVB-induced cutaneous hypersensitivity suppression in Ptgs2-/- (COX-2 knockout) mice. Honokiol inhibited UVB-induced DNA hypermethylation, stimulated TET enzyme (methylcytosine dioxygenase) activity responsible for DNA dimethylation, and elevated the activity of large (~180- to 230-kDa) TET multidomain proteins in UVB-irradiated mouse skin. The DNA hypomethylating agent nucleoside analogue decitabine (5-aza-2′- deoxycytidine) and the potent COX-2 inhibitor nonsteroidal anti-inflammatory drug indomethacin enhance the contact hypersensitivity response in UVB-exposed mice. Comparative studies of the effects of topical application and gavage feeding of honokiol (2 mg per mouse; equal to 100 mg·kg−1 body weight) on UVB-mediated immunosuppression in a murine model of allergic contact dermatitis revealed quite similar inhibition of contact hypersensitivity between the two administration routes of this neolignan. The local application of equal concentrations (18.8 mM) of HK and two widely available drugs (imiquimod and 5-fluorouracil) used in the topical chemotherapy or immunotherapy did not significantly affect the UVB-mediated immunosuppression. | [10] |
| Honokiol | Research Model | Experimental Groups (G) | Duration | Main Conclusions | Reference |
|---|---|---|---|---|---|
| in vitro | |||||
| Honokiol | Human melanoma cell lines: G1—A375 cells with epithelial morphology isolated from the skin of a 54-year-old cutaneous melanoma female patient; G2—Hs294t cell line established from a human melanoma metastasis to the lymph node; groups 3 and 4—SK-Mel119 and SK-Mel28 melanocytes from the skin of a 51-year-old malignant melanoma male patient with unspecified ethnic origin. | Honokiol-treated melanoma cell lines treated (0, 5, 10, and 20 μM for 42 h) | 24 h | Honokiol (HK)-treated metastatic melanoma-derived cell line cultures revealed a dose-dependent limitation of cell migration due to Nox1 downregulation and relieved oxidative stress. Diphenyleneiodonium chloride, a Nox1 inhibitor, decreased the migration of SK-Mel28 and Hs294t cells with high and low expression, respectively, of SIRT3, a protein serving as a treatment target of both tumour-suppressive and oncogenic function. Honokiol enhanced the build-up of the NCF1 gene-encoded cytosolic p47phox protein but diminished the membrane-bound p22phox protein level, thus completely preventing their interaction and blocking Nox1 activation in the SK-Mel28 and Hs294t cells. | [223] |
| Honokiol | Melanoma cell lines: group 1—SKMEL-2 with polygonal morphology from the skin of a 60-year-old cutaneous melanoma white male patient; group 2—UACC-62 derived from a 41-year-old male with pancreatic ductal adenocarcinoma metastatic to the peritoneal mass. | Honokiol-treated SKMEL-2 and UACC-62 cells (0, 25, 50, 70, and 100 μM for 12, 24, 48, and 72 h) | 12–72 h | Honokiol (1) reduced cell viability, cell multiplication, and cell cycle arrest but enhanced programmed cell death and modulation of cell cycle regulatory and apoptotic proteins in both cell lines; (2) induced cell cycle quiescence in UACC-62 and G2/M checkpoint arrest in SKMEL-2 cells; (3) elevated the level of caspases and PARP responsible for maintaining viability in the cell lines but lowered the expression of cell cycle regulatory proteins. | [221] |
| Honokiol | Malignant melanoma cell lines: G1—B16F10 originating from C57BL/6J mice; G2—A375 with epithelial morphology from the skin of a 54-year-old malignant melanoma female patient; G3—MeWo with fibroblast morphology from the skin of a 78-year-old white malignant melanoma male patient; G4—A2058 with epithelial morphology from the skin of a 43-year-old white metastatic melanoma male patient. | Honokiol-treated malignant melanoma cells (0–100 μM) | 24–92 h | Honokiol suppressed the growth and metastasis of melanoma. This neolignan caused ER stress CHOP activation and lowered β-catenin, CDK2, and MITF expression in mouse melanoma tissues, thus increasing the survival likelihood. Honokiol effectively prevented the progression of advanced melanoma using the Wnt/β-catenin-MITF pathway (β-catenin/MITF axis or β-catenin-dependent MITF regulation pathway) via prompt calcium-dependent non-lysosomal cysteine protease CAPN10) and CCAAT-enhancer-binding protein (C/EBP) homologous protein (CHOP/GADD153) regulated cascades. | [121] |
| Honokiol | Human melanoma cell lines: G1—SK-MEL-5 (with high metastatic potential, more sensitive to some chemotherapeutics than SK-MEL-28; used for invasion and migration tests; G2—SK-MEL-28 (used in studies on targeted therapy; lines with moderate resistance to apoptosis with BRAF V600E mutation with glutamic acid at amino acid instead of valine at position 600). | Honokiol-treated (0, 10, 20, 30, and 40 µM) SK-MEL-5 and SK-MEL-28 cell lines | 24–96 h | Honokiol effectively suppressed the growth (anchorage-independent growth, cell proliferation, colony forming ability) of melanoma cells in a dose- and time-varying manner. Honokiol interacted directly with a key for malignant status type I cytokeratin KRT18 protein and induced its ubiquitin-mediated degradation. Increase in the level of KRT18 expression promoted melanoma cell multiplication and growth. | [73] |
| in vivo | |||||
| Honokiol | 4–6-week-old athymic hairless female mice as a tumour cell invasion model | Three groups of mice. Honokiol administration: 1G—100 mg·kg−1 body weight supplemented with 0.5% carmellose dissolved in 200 μL of sterilised water/mouse; 2G—vehicle administered via oral gavage for 7 weeks 5x per week. 3G—melanoma A375 cells (2.5 × 106) i.v. injected intravenously into the tail vein of mice either not provided with or orally supplied with honokiol. | Honokiol prevented the migration/extravasation and growth of intravenously injected melanoma cells in the liver, kidney, and lung of athymic mice as a consequence of a suppressing effect on Nox1 activity in the above-mentioned organs. | [223] | |
| Honokiol | Five–six-week-old heterozygous Nu+ thymus-less, thus unable to produce T-cells, immunodeficient hairless (nude) male mice NU/NU (NU-FOXN1(NU)). They have no rejection response, can receive multiple tissue types and tumour grafts, and are widely used for tumour biology and xenograft research. | Two groups of mice. 1G—receiving a nontoxic honokiol dose of 50 mg·kg−1 dissolved in gingelly oil (i.p.); 2G—control group, injected with an equal volume of gingelly oil (i.p.). | Animal treatment for 2–7 weeks three times per week | Honokiol potently accelerated tumour regression in UACC-62 and SKMEL-2 melanoma xenografts in the athymic mice. It is recommended to be further studied as an effective supportive therapy for malignant melanoma. | [221] |
| Honokiol | Severe combined immunodeficient (SCID) CB-17 mice with recessive autosomal mutation characterised by an absence of functional T and B cells, lymphopaenia, hypogammaglobulinaemia, and an unchanged hematopoietic environment (normal NK cells, macrophages, and granulocytes). | Groups receiving honokiol at 30–140 mg·kg−1 oral or i.p. | Six weeks of treatment three times per week | Honokiol prevented UVB-induced skin cancer via targeting chemical mediators of inflammation (lowering PGE2, COX-2, PCNA, IL-1β, IL-6, and TNF-α levels), cell cycle regulators (increased expression the CDK inhibitor), and cell survival signals (suppression of cyclins D1, D2, E2 and CDKs2, CDK4, and CDK6). It induced programmed cell death via death receptor (extrinsic) and mitochondrial (intrinsic) pathways, activating proapoptotic proteins, including upregulation of caspases 8, 9, and 3 and enzymatic PARP breakdown (PARP cleavage)—an apoptosis marker that facilitates cellular disassembly through DNA shearing and suppressing DNA repair. Honokiol inhibited the melanoma cell growth promoter KRT18 and prevented advanced melanoma progression using the β-catenin-dependent MITF regulation pathway via prompt calpain-10 and CCAAT-enhancer-binding proteins (C/EBPs) homologous protein (CHOP/GADD153) transcription factor-regulated cascades. | [121] |
| Honokiol | SK-MEL-5 or SK-MEL-28 melanoma cell-derived xenograft mouse models. Five–six-week-old severe combined immunodeficient, i.e., “living in the bubble” syndrome or “bubble boy disease,” female CB 17 mice. | Three G of mice. 1G—vehicle control (1% carmellose sodium in physiological saline), 2G—honokiol 30 mg·kg−1, and 3G—50 mg·kg−1 (i.p.). | 20 days | Honokiol decreased KRT18 expression and suppressed the growth of melanoma cell lines implanted into immunodeficient mice (cell derived xenograft models). It reduced neoplasia marker Ki-67 expression. Honokiol acts as an inhibitor of the oncogenic protein KRT18 in melanoma. | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chwil, M.; Dzida, K.; Terlecka, P.; Gruľová, D.; Matraszek-Gawron, R.; Terlecki, K.; Kasprzyk, A.; Kostryco, M. Antibacterial, Photoprotective, Anti-Inflammatory, and Selected Anticancer Properties of Honokiol Extracted from Plants of the Genus Magnolia and Used in the Treatment of Dermatological Problems—A Review. Int. J. Mol. Sci. 2025, 26, 8737. https://doi.org/10.3390/ijms26178737
Chwil M, Dzida K, Terlecka P, Gruľová D, Matraszek-Gawron R, Terlecki K, Kasprzyk A, Kostryco M. Antibacterial, Photoprotective, Anti-Inflammatory, and Selected Anticancer Properties of Honokiol Extracted from Plants of the Genus Magnolia and Used in the Treatment of Dermatological Problems—A Review. International Journal of Molecular Sciences. 2025; 26(17):8737. https://doi.org/10.3390/ijms26178737
Chicago/Turabian StyleChwil, Mirosława, Katarzyna Dzida, Paulina Terlecka, Daniela Gruľová, Renata Matraszek-Gawron, Karol Terlecki, Anna Kasprzyk, and Mikołaj Kostryco. 2025. "Antibacterial, Photoprotective, Anti-Inflammatory, and Selected Anticancer Properties of Honokiol Extracted from Plants of the Genus Magnolia and Used in the Treatment of Dermatological Problems—A Review" International Journal of Molecular Sciences 26, no. 17: 8737. https://doi.org/10.3390/ijms26178737
APA StyleChwil, M., Dzida, K., Terlecka, P., Gruľová, D., Matraszek-Gawron, R., Terlecki, K., Kasprzyk, A., & Kostryco, M. (2025). Antibacterial, Photoprotective, Anti-Inflammatory, and Selected Anticancer Properties of Honokiol Extracted from Plants of the Genus Magnolia and Used in the Treatment of Dermatological Problems—A Review. International Journal of Molecular Sciences, 26(17), 8737. https://doi.org/10.3390/ijms26178737








