Activation of Specific Reagents in Molecular Films by Sub-Ionization Electrons: Chlorobenzene/Water Films
Abstract
1. Introduction
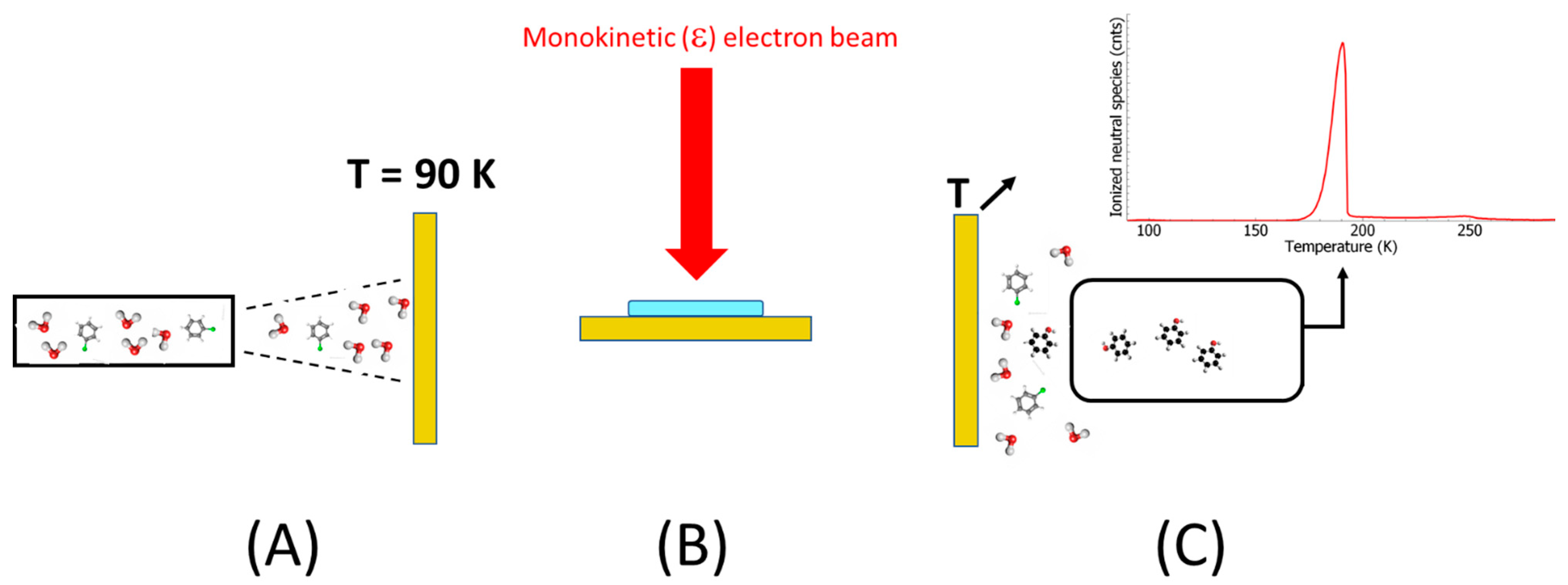
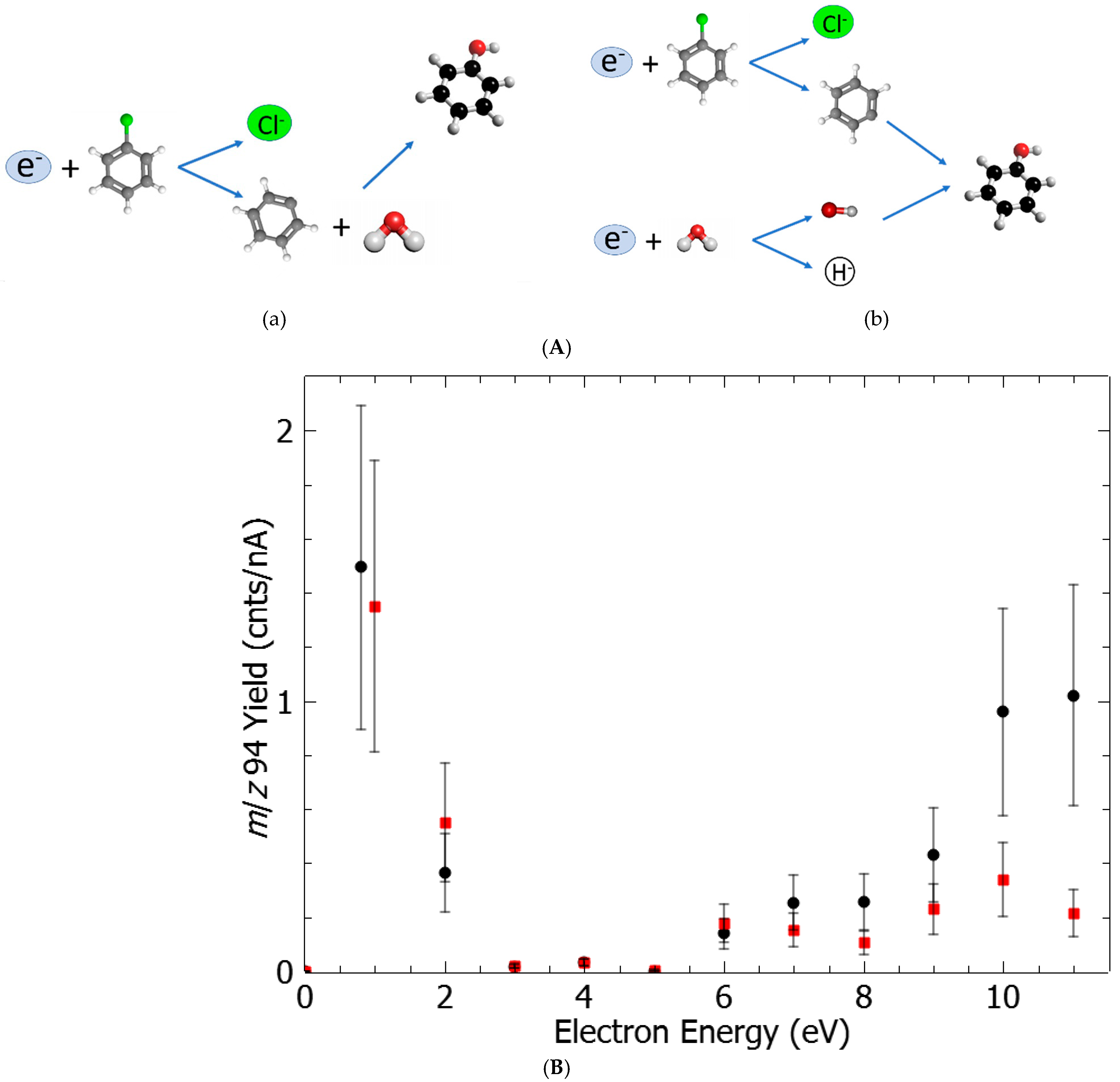
2. Results and Discussion
3. Material and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aubert, S.; Bezagu, M.; Spivey, A.C.; Arseniyadis, S. Spatial and temporal control of chemical processes. Nat. Rev. Chem. 2019, 3, 707–722. [Google Scholar] [CrossRef]
- Calegari, F.; Martin, F. Open question in attochemistry. Commun. Chem. 2023, 6, 183. [Google Scholar] [CrossRef] [PubMed]
- Nisoli, M.; Decleva, P.; Calegari, F.; Palacios, A.; Martín, F. Attosecond electron dynamics in molecule. Chem. Rev. 2017, 117, 10760–10825. [Google Scholar] [CrossRef] [PubMed]
- Böhler, E.; Warneke, J.; Swiderek, P. Control of chemical reactions and synthesis by low energy electrons. Chem. Soc. Rev. 2013, 42, 9219–9231. [Google Scholar] [CrossRef]
- Abdoul-Carime, H.; Bald, I.; Illenberger, E.; Kopyra, J. Selective synthesis of ethylene and acetylene from dimethyl sulfide cold films controlled by slow electrons. J. Phys. Chem. C 2019, 122, 24137–24142. [Google Scholar] [CrossRef]
- Au-Yeung, K.H.; Kühne, T.; Aiboudi, O.; Sarkar, S.; Guskova, O.; Ryndyk, D.A.; Heine, T.; Lissel, F.; Moresco, F. STM-induced ring closure of vinylheptafulvene molecular dipole switches on Au(111). Nanoscale Adv. 2022, 4, 4351–4357. [Google Scholar] [CrossRef]
- Albrecht, F.; Fatayer, S.; Pozo, I.; Tavernelli, I.; Repp, J.; Peña, D.; Gross, L. Selectivity in single molecule reactions by tip-induced redox chemistry. Science 2022, 377, 298–301. [Google Scholar] [CrossRef]
- Anggara, K.; Huang, K.; Leung, L.; Chatterjee, A.; Cheng, F.; Polanyi, J.C. Polanyi. Bond selectivity in electron induced reaction due to direct recoil on an anitrotropic substrate. Nat. Commun. 2016, 7, 13690. [Google Scholar] [CrossRef]
- Illenberger, E.; Momigny, J. Gaseous Molecular Ions: An Introduction to Elementary Processes Induced by Ionization; Baümgartel, H., Franck, E.U., Grünbein, W., Eds.; Springer: New York, NY, USA, 1992. [Google Scholar]
- Ingolfsson, O. (Ed.) Low Energy Electrons: Fundamental and Applications; Jenny Stanford Publishing: Singapore, 2019; ISBN 978-9814800006. [Google Scholar]
- Khatymov, R.V.; Muftakhov, M.V.; Mazunov, V.A. Phenol, chlorobenzene and chlorophenol isomers: Resonant states and dissociative electron attachment. Rapid Commun. Mass Spectrom. 2003, 17, 2327–2336. [Google Scholar] [CrossRef]
- Burrow, P.D.; Modelli, A.; Jordan, K.D. Temporary anion states of the chlorobenzenes. Chem. Phys. Lett. 1986, 132, 441–447. [Google Scholar] [CrossRef]
- Prajapati, D.; Yadav, H.; Vinodkumar, M.; Limbachiya, C.; Vinodkumar, P.C. Electron impact scattering study on chlorobenzene. Mol. Phys. 2018, 117, 1838–1849. [Google Scholar] [CrossRef]
- Barbosa, A.S.; Varella, M.T.D.N.; Sanchez, S.D.; Ameixa, J.; Blanco, F.; García, G.; Limão-Vieira, P.; da Silva, F.F.; Bettega, M.H.F. Theoretical and experimental study on electron interaction with chlorobenzene: Shape resonance and differential cross sections. J. Chem. Phys. 2016, 145, 084311. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-L.; White, J.M. Photon and electron induced chemistry of chlorobenzene on Ag(111). J. Chem. Phys. 1990, 92, 5612–5621. [Google Scholar] [CrossRef]
- Rawat, P.; Prabhudesai, V.S.; Aravind, G.; Rahman, M.A.; Krishnakumar, E. Absolute cross section for dissociative electron attachment to H2O and D2O. J. Phys. B At. Mol. Opt. Phys. 2007, 40, 4625. [Google Scholar] [CrossRef]
- Pan, X.; Abdoul-Carime, H.; Cloutier, P.; Bass, A.D.; Sanche, L. D−, O− and OD− desorption induced by low energy (0-20 eV) electron impact on amorphous D2O films. Radiat. Phys. Chem. 2005, 72, 139–199. [Google Scholar] [CrossRef]
- Fabrikant, I.I.; Eden, S.; Mason, N.J.; Fedor, J. Recent progress in dissociative electron attachment: From diatomic to biomolecules. Adv. Atom. Mol. Opt. Phys. 2017, 66, 545–657. [Google Scholar]
- Rabilloud, F. (Université Claude Bernard Lyon 1, Institut Lumière Matière, Villeurbanne, France). Private communication, 2025.
- Pan, X.; Bass, A.D.; Jay-Gerin, J.-P.; Sanche, L. A mechanism for the production of hydrogen peroxide and the hydroperoxyl radical on icy satellites by low energy electrons. Icarus 2004, 172, 521–525. [Google Scholar] [CrossRef]
- Annan, M.; Vouros, P. A comparison of the reactions of the oxide radical anion (O-) with simple aromatic compounds at atmospheric and rediced pressure conditions. J. Am. Soc. Mass Spectrom. 1994, 5, 367–376. [Google Scholar] [CrossRef]
- Chang, Y.; Yu, Y.; An, F.; Luo, Z.; Quan, D.; Zhang, X.; Hu, X.; Li, Q.; Yang, J.; Chen, Z.; et al. Three body photodissociation of the water molecule and its implications for prebiotic oxygen production. Nat. Commun. 2021, 12, 2476. [Google Scholar] [CrossRef]
- Engel, V.; Staemmler, V.; Wal, R.L.V.; Crim, F.F.; Sension, R.J.; Hudson, B.; Andresen, P.; Hennig, S.; Weide, K.; Schinke, R. Photodissociation of water in the first absorption band: A prototype for dissociation on a repulsive potential energy surface. J. Phys. Chem. 1992, 96, 3201–3213. [Google Scholar] [CrossRef]
- Blanksby, S.J.; Ellison, G.B. Bond dissociation energy of organic molecules. Acc. Chem. Res. 2003, 36, 255–263. [Google Scholar] [CrossRef]
- Rienstra-Kiracofe, J.C.; Tschumper, G.S.; Schaefer, H.F.; Nandi, S.; Ellison, G.B. Atomic and molecular electron affinities: Photoelectron experiments and theoretical computations. Chem. Rev. 2002, 102, 231–282. [Google Scholar] [CrossRef]
- Mardyukov, A.; Sanchez-Garcia, E.; Crespo-Otero, R.; Sander, W. Interaction and reaction of the phenyl radical with water: A source of OH radicals. Angew. Chem. Int. Ed. Engl. 2009, 48, 4804–4807. [Google Scholar] [CrossRef]
- Abdoul-Carime, H.; Lathuilière, B.; Nedelec, P.; Kopyra, J. Synthesis of benzene and phenol from the irradiation of benzonitrile:water ice by (<10 eV) electrons: Application to the planets and meteorite surface chemistry. J. Geophys. Res. Planets 2024, 129, e203JE007151. [Google Scholar]
- Singh, S.; Naghma, R.; Kaur, J.; Antony, B. Calculation of total cross sections for electron scattering by primary benzene compound. J. Chem. Phys. 2016, 145, 034309. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, G.; Goulet, T.; Cloutier, P.; Jay-Gerin, J.P.; Sanche, L. Low-energy (0–10 eV) electron transmission spectra of multilayer tryptophan films. J. Phys. Chem. A 1987, 91, 4999–5001. [Google Scholar] [CrossRef]
- Sanche, L. Transmission of 0–15 eV monoenergetic electrons through thin-film molecular solids. J. Phys. Chem. 1979, 71, 4860–4882. [Google Scholar] [CrossRef]
- Lu, J.-M.; Wang, H.-F.; Guo, Q.-H.; Wang, J.-W.; Li, T.-T.; Chen, K.-X.; Zhang, M.-T.; Chen, J.-B.; Shi, Q.-N.; Huang, Y.; et al. Roboticized AI-assisted microfluidic photocatalytic synthesis and screening up to 10,000 reactions per days. Nat. Commun. 2024, 15, 8826. [Google Scholar] [CrossRef]
- Zhan, C.; Chen, X.-J.; Yi, J.; Li, J.-F.; Wu, D.-Y.; Tian, Z.-Q. From plasmon enhanced molecular spectroscopy to plasmon mediated chemical reactions. Nat. Rev. 2018, 2, 216–230. [Google Scholar] [CrossRef]
- Studer, A.; Curran, D.P. The electron is a catalyst. Nat. Chem. 2014, 6, 765–773. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdoul-Carime, H.; Kopyra, J. Activation of Specific Reagents in Molecular Films by Sub-Ionization Electrons: Chlorobenzene/Water Films. Int. J. Mol. Sci. 2025, 26, 8751. https://doi.org/10.3390/ijms26178751
Abdoul-Carime H, Kopyra J. Activation of Specific Reagents in Molecular Films by Sub-Ionization Electrons: Chlorobenzene/Water Films. International Journal of Molecular Sciences. 2025; 26(17):8751. https://doi.org/10.3390/ijms26178751
Chicago/Turabian StyleAbdoul-Carime, Hassan, and Janina Kopyra. 2025. "Activation of Specific Reagents in Molecular Films by Sub-Ionization Electrons: Chlorobenzene/Water Films" International Journal of Molecular Sciences 26, no. 17: 8751. https://doi.org/10.3390/ijms26178751
APA StyleAbdoul-Carime, H., & Kopyra, J. (2025). Activation of Specific Reagents in Molecular Films by Sub-Ionization Electrons: Chlorobenzene/Water Films. International Journal of Molecular Sciences, 26(17), 8751. https://doi.org/10.3390/ijms26178751





