Impact of Smoking, Steroid Use and Immunosuppression on Anti-dsDNA Antibodies in Systemic Lupus Erythematosus
Abstract
1. Introduction
Background
2. Results
3. Discussion
Limitations
- (1)
- First, the retrospective study design limits the ability to establish causal relationships between smoking, therapy, and anti-dsDNA levels. While significant associations were identified, prospective longitudinal studies are needed to confirm these findings and further explore the temporal dynamics of these factors in systemic lupus erythematosus (SLE) progression.
- (2)
- Second, smoking status was assessed using cumulative pack-years (PPYs), which provides an estimate of exposure but does not capture potential variations in smoking intensity, duration, or cessation periods. More detailed data, including biomarkers of tobacco exposure, could help refine the analysis.
- (3)
- Third, while anti-dsDNA levels were measured using fluorescent enzyme immunoassays (FEIAs) and confirmed via indirect immunofluorescence (IFA), variability among different laboratory methods may have influenced the results. Future studies could benefit from a standardized multi-platform assessment of autoantibody levels.
- (4)
- Additionally, the cohort was monocentric, potentially limiting the generalizability of the findings to broader SLE populations with different genetic, environmental, or treatment backgrounds. Larger multicenter studies would help validate these observations.
- (5)
- Another key limitation is the temporal sequencing of therapies. Some patients may have received multiple treatments over time, and in some cases, concomitant use of different immunosuppressants could have influenced anti-dsDNA levels. This variability makes it difficult to determine the isolated effect of each therapy. Future studies with longitudinal treatment data and stratified analyses of sequential or overlapping therapies could help clarify these effects.
- (6)
- Finally, while this study focused on anti-dsDNA levels, other disease activity markers (e.g., complement levels, interferon signatures, or specific cytokines) were not included in the analysis; a comprehensive immunological assessment could provide a more detailed understanding of how these factors interact with disease pathophysiology.
4. Materials and Methods
4.1. Population and Study Design
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
References
- Nandakumar, K.S.; Nündel, K. Systemic lupus erythematosus-predisposition factors, pathogenesis, diagnosis, treatment and disease models. Front. Immunol. 2022, 13, 1118180. [Google Scholar] [CrossRef]
- Ceppellini, R.; Polli, E.; Celada, F. A DNA-Reacting Factor in Serum of a Patient with Lupus Erythematosus Diffusus. Exp. Biol. Med. 1957, 96, 572–574. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.S. IgA anti-dsDNA antibodies: A neglected serological parameter in systemic lupus erythematosus. Lupus 2022, 31, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Rekvig, O.P. The Anti-DNA Antibodies: Their Specificities for Unique DNA Structures and Their Unresolved Clinical Impact—A System Criticism and a Hypothesis. Front. Immunol. 2022, 12, 808008. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Tong, Y.; Liu, Y.; Hu, H. Self-dsDNA in the pathogenesis of systemic lupus erythematosus. Clin. Exp. Immunol. 2017, 191, 1–10. [Google Scholar] [CrossRef]
- Narayanan, K.; Marwaha, V.; Shanmuganandan, K.; Shankar, S. Correlation between Systemic Lupus Erythematosus Disease Activity Index, C3, C4 and Anti-dsDNA Antibodies. Med. J. Armed Forces India 2010, 66, 102–107. [Google Scholar] [CrossRef]
- Bouts, Y.M.; Wolthuis, D.F.; Dirkx, M.F.; Pieterse, E.; Simons, E.M.; Van Boekel, A.M.; Dieker, J.W.; Van Der Vlag, J. Apoptosis and NET formation in the pathogenesis of SLE. Autoimmunity 2012, 45, 597–601. [Google Scholar] [CrossRef]
- Aringer, M.; Johnson, S.R. 2019 EULAR/ACR Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019, 71, 1400–1412. [Google Scholar] [CrossRef]
- Hochberg, M.C. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef]
- Petri, M.; Orbai, A.; Alarcón, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef]
- Nicola, S.; Borrelli, R.; Corradi, F.; Sardo, L.L.; Badiu, I.; Romito, A.; Rashidy, N.; Quinternetto, A.; Mazzola, M.; Meli, F.; et al. Relationship between clinical manifestations and serological profile in patients affected by Systemic Lupus Erythematosus. Front. Immunol. 2024, 15, 1390642. [Google Scholar] [CrossRef] [PubMed]
- Font, J.; Cervera, R. 1982 Revised Criteria for Classification of Systemic Lupus Erythematosus—Ten Years Later. Lupus 1993, 2, 339–341. [Google Scholar] [CrossRef]
- Barbhaiya, M.; Tedeschi, S.K.; Lu, B.; Malspeis, S.; Kreps, D.; A Sparks, J.; Karlson, E.W.; Costenbader, K.H. Cigarette smoking and the risk of systemic lupus erythematosus, overall and by anti-double stranded DNA antibody subtype, in the Nurses’ Health Study cohorts. Ann. Rheum. Dis. 2018, 77, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Arnson, Y.; Shoenfeld, Y.; Amital, H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J. Autoimmun. 2010, 34, J258–J265. [Google Scholar] [CrossRef] [PubMed]
- Ezra, N.; Jorizzo, J. Hydroxychloroquine and smoking in patients with cutaneous lupus erythematosus: Hydroxychloroquine and smoking in patients with CLE. Clin. Exp. Dermatol. 2012, 37, 327–334. [Google Scholar] [CrossRef]
- Parodis, I.; Sjöwall, C.; Jönsen, A.; Ramsköld, D.; Zickert, A.; Frodlund, M.; Sohrabian, A.; Arnaud, L.; Rönnelid, J.; Malmström, V.; et al. Smoking and pre-existing organ damage reduce the efficacy of belimumab in systemic lupus erythematosus. Autoimmun. Rev. 2017, 16, 343–351. [Google Scholar] [CrossRef]
- Parodis, I.; Gomez, A.; Frodlund, M.; Jönsen, A.; Zickert, A.; Sjöwall, C.; A Bengtsson, A.; Gunnarsson, I. Smoking reduces the efficacy of belimumab in mucocutaneous lupus. Expert Opin. Biol. Ther. 2018, 18, 911–920. [Google Scholar] [CrossRef]
- Tseng, C.; Buyon, J.P.; Kim, M.; Belmont, H.; Mackay, M.; Diamond, B.; Marder, G.; Rosenthal, P.; Haines, K.; Ilie, V.; et al. The effect of moderate-dose corticosteroids in preventing severe flares in patients with serologically active, but clinically stable, systemic lupus erythematosus: Findings of a prospective, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006, 54, 3623–3632. [Google Scholar] [CrossRef]
- Duvvuri, B.; Lood, C. Cell-Free DNA as a Biomarker in Autoimmune Rheumatic Diseases. Front. Immunol. 2019, 10, 502. [Google Scholar] [CrossRef]
- Wang, F.; Miao, H.; Pei, Z.; Chen, Z. Serological, fragmentomic, and epigenetic characteristics of cell-free DNA in patients with lupus nephritis. Front. Immunol. 2022, 13, 1001690. [Google Scholar] [CrossRef]
- Deocharan, B.; Qing, X.; Lichauco, J.; Putterman, C. α-Actinin Is a Cross-Reactive Renal Target for Pathogenic Anti-DNA Antibodies. J. Immunol. 2002, 168, 3072–3078. [Google Scholar] [CrossRef]
- Cervera, R.; Khamashta, M.; Hughes, G. The Euro-lupus project: Epidemiology of systemic lupus erythematosus in Europe. Lupus 2009, 18, 869–874. [Google Scholar] [CrossRef]
- Barber, M.R.W.; Drenkard, C.; Falasinnu, T.; Hoi, A.; Mak, A.; Kow, N.Y.; Svenungsson, E.; Peterson, J.; Clarke, A.E.; Ramsey-Goldman, R. Global epidemiology of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2021, 17, 515–532, Correction in Nat. Rev. Rheumatol. 2021, 17, 645. [Google Scholar] [CrossRef]
- Gergianaki, I.; Bortoluzzi, A.; Bertsias, G. Update on the epidemiology, risk factors, and disease outcomes of systemic lupus erythematosus. Best Pract. Res. Clin. Rheumatol. 2018, 32, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Al Maimouni, H.; Gladman, D.D.; Ibañez, D.; Urowitz, M.B. Switching Treatment Between Mycophenolate Mofetil and Azathioprine in Lupus Patients: Indications and Outcomes. Arthritis Care Res. 2014, 66, 1905–1909. [Google Scholar] [CrossRef] [PubMed]
- Tani, C.; Zucchi, D.; Cardelli, C.; Elefante, E.; Signorini, V.; Schilirò, D.; Cascarano, G.; Gualtieri, L.; Valevich, A.; Puccetti, G.; et al. Analysis of belimumab prescription and outcomes in a 10-year monocentric cohort: Is there an advantage with early use? RMD Open 2024, 10, e003981. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Jägerback, S.; Sjöwall, C.; Parodis, I. Belimumab and antimalarials combined against renal flares in patients treated for extra-renal systemic lupus erythematosus: Results from 4 phase III clinical trials. Rheumatology 2024, 63, 338–348. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; E Pope, J.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18, Correction in Ann. Rheum. Dis. 2025, 82, 3–18. [Google Scholar] [CrossRef]
- Buchman, A.L. Side Effects of Corticosteroid Therapy. J. Clin. Gastroenterol. 2001, 33, 289–294. [Google Scholar] [CrossRef]
- Rice, J.B.; White, A.G.; Scarpati, L.M.; Wan, G.; Nelson, W.W. Long-term Systemic Corticosteroid Exposure: A Systematic Literature Review. Clin. Ther. 2017, 39, 2216–2229. [Google Scholar] [CrossRef]
- Stojan, G.; Petri, M. Atherosclerosis in Systemic Lupus Erythematosus. J. Cardiovasc. Pharmacol. 2013, 62, 255–262. [Google Scholar] [CrossRef]
- Shipa, M.; Embleton-Thirsk, A.; Parvaz, M.; Santos, L.R.; Muller, P.; Chowdhury, K.; Isenberg, A.D.; Doré, C.J.; Gordon, C.; Ehrenstein, M.R. Effectiveness of Belimumab After Rituximab in Systemic Lupus Erythematosus: A Randomized Controlled Trial. Ann. Intern. Med. 2021, 174, 1647–1657. [Google Scholar] [CrossRef]
- Feng, L.; Deng, J.; Huo, D.; Wu, Q.; Liao, Y. Mycophenolate mofetil versus azathioprine as maintenance therapy for lupus nephritis: A meta-analysis. Nephrology 2013, 18, 104–110. [Google Scholar] [CrossRef]
- Thanou, A.; James, J.A.; Arriens, C.; Aberle, T.; Chakravarty, E.; Rawdon, J.; Stavrakis, S.; Merrill, J.T.; Alskanase, A. Scoring systemic lupus erythematosus (SLE) disease activity with simple, rapid outcome measures. Lupus Sci. Med. 2019, 6, e000365. [Google Scholar] [CrossRef]
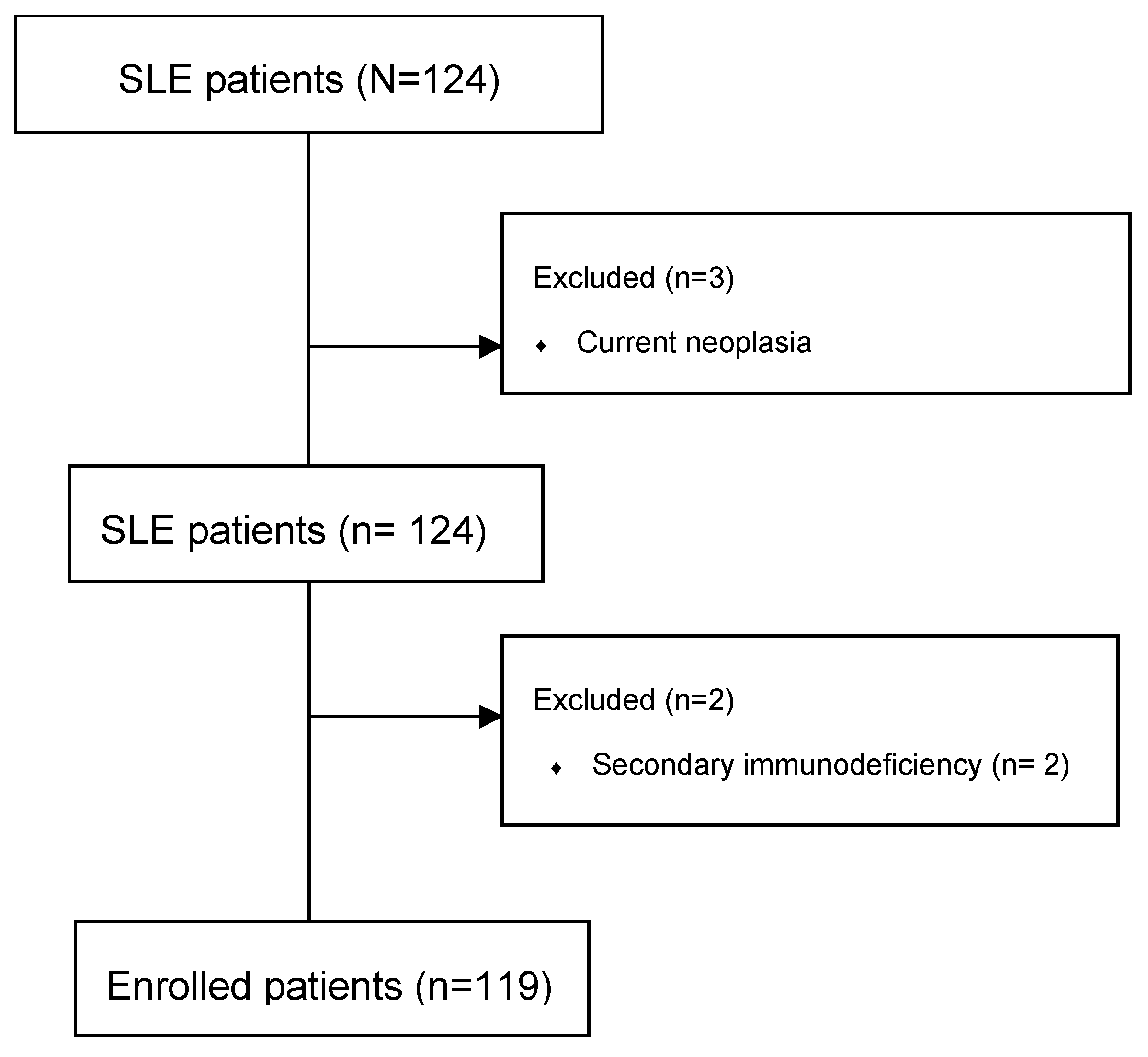
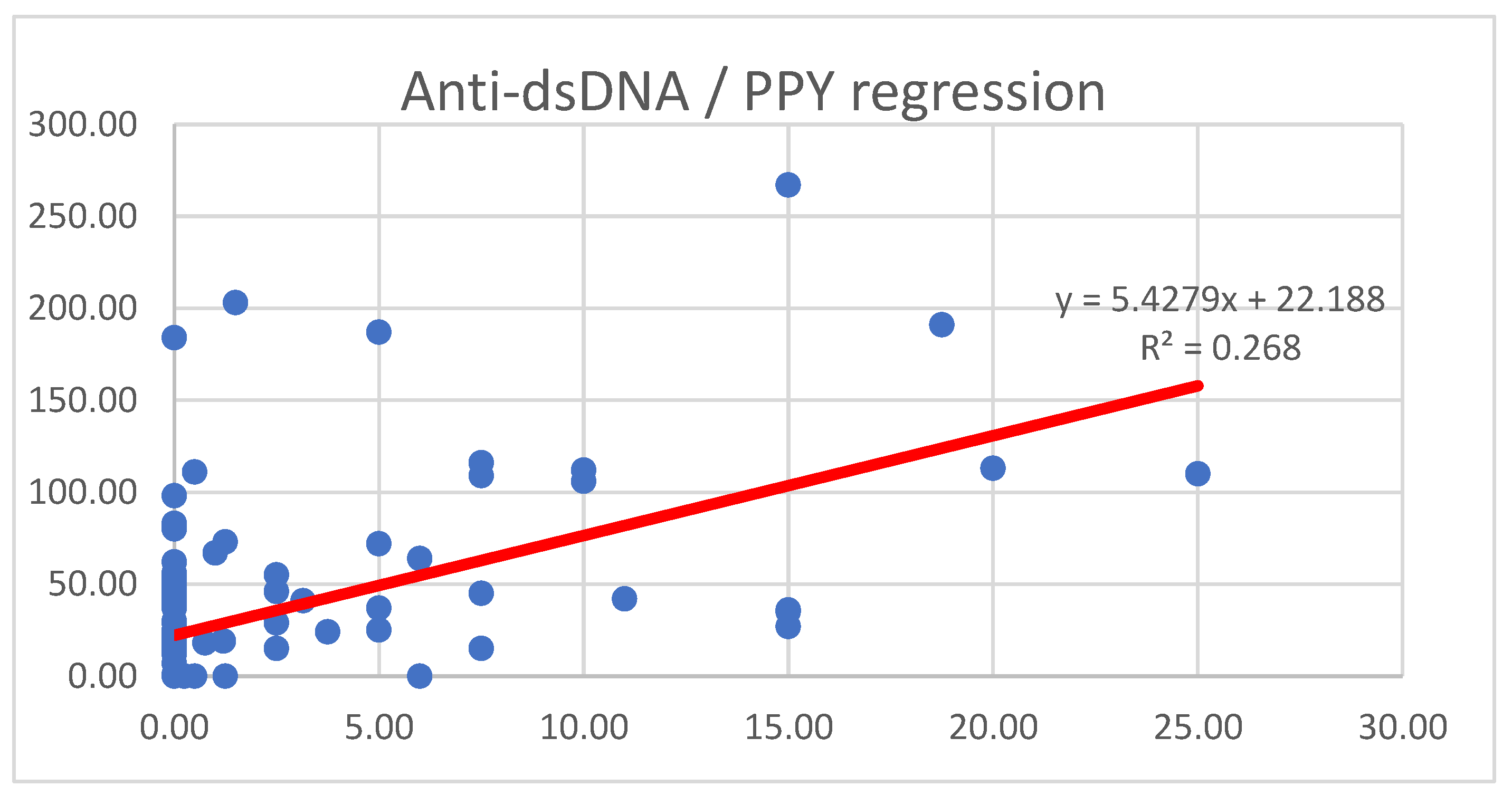
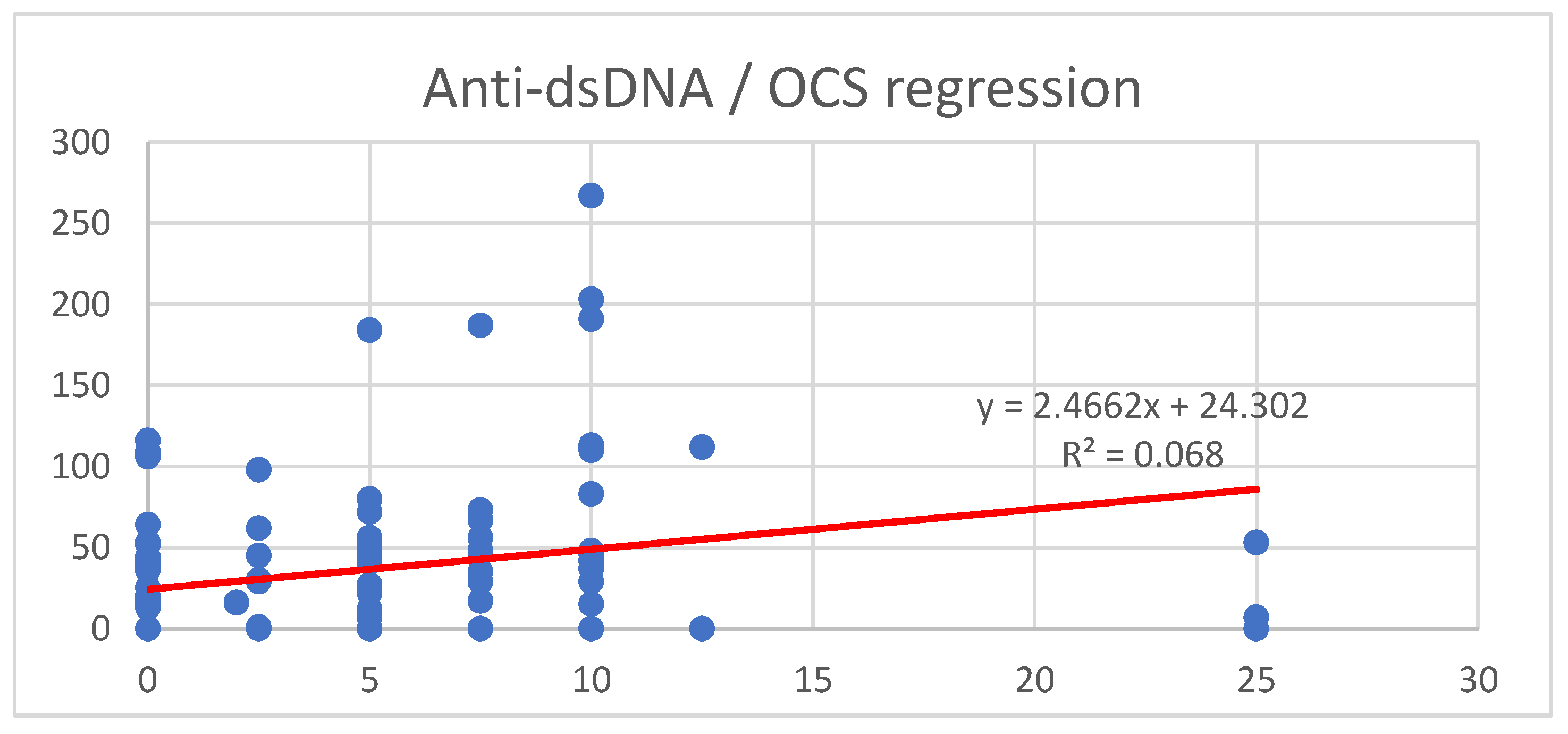
| n | Age | SD | Disease Duration (y) | SD | |
|---|---|---|---|---|---|
| TOT | 119 | 54.62 | 13.61 | 25.60 | 13.54 |
| FEMALE | 107 | 54.3 | 13.62 | 25.30 | 13.62 |
| MALE | 12 | 57.25 | 13.56 | 17.98 | 9.47 |
| Comorbidity | n (%) |
|---|---|
| Cardiovascular Disease | 32 |
| Endocrine/Metabolic | 11 |
| Others/Unclassified | 5 |
| Hematology | 2 |
| Gastrointestinal Disease | 6 |
| Neurologic/Psychiatric | 1 |
| Musculoskeletal | 1 |
| Parameter | n (%) | Median (Q25; Q75) |
|---|---|---|
| Anti-dsDNA positivity | 68 (57.14) | 44.5 (24.75; 68.25) |
| SELENA-SLEDAI | 119 (100) | 2.0 (0.0; 4.0) |
| Therapy | n (%) | Concomitant to BEL (n) | Duration of Therapy, Median (Q25; Q75) |
|---|---|---|---|
| BEL | 29 (24.37) | NA | 4.10 (2.31; 6.58) |
| AZA | 45 (37.82) | 7 | 11.32 (3.24; 17.44) |
| MMF | 36 (30.25) | 4 | 14.22 (5.25; 17.73) |
| MTX | 5 (4.20) | 1 | 3.21 (1.87; 6.93) |
| RTX | 4 (3.36) | 0 | 2.87 (1.17; 3.81) |
| HCQ | 85 (71.42) | 27 | 24.16 (23.11; 25.42) |
| Patients | Anti-dsDNA + | Anti-dsDNA − | Comparison vs. Active Smokers (p-Value) |
|---|---|---|---|
| Total, N = 119 (100) | 68 (57.14) | 51 (42.86) | NA |
| Active smokers | 32 (26.89) | 4 (3.36) | NA |
| Previous smokers | 9 (7.56) | 7 (5.88) | 0.023 |
| Nonsmokers | 27 (22.69) | 40 (33.61) | <0.001 |
| Therapy No. 1 | Therapy No. 2 | p-Value |
|---|---|---|
| AZA | MMF | 0.27 |
| AZA | MTX | 0.004 |
| AZA | CTX | 0.40 |
| MMF | MTX | 0.04 |
| MMF | CTX | 0.26 |
| BEL | AZA | 0.04 |
| BEL | MMF | 0.17 |
| BEL | MTX | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrelli, R.; Nicola, S.; Corradi, F.; Lo Sardo, L.; Badiu, I.; Quinternetto, A.; Vitali, I.; Negrini, S.; Brussino, L. Impact of Smoking, Steroid Use and Immunosuppression on Anti-dsDNA Antibodies in Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2025, 26, 8705. https://doi.org/10.3390/ijms26178705
Borrelli R, Nicola S, Corradi F, Lo Sardo L, Badiu I, Quinternetto A, Vitali I, Negrini S, Brussino L. Impact of Smoking, Steroid Use and Immunosuppression on Anti-dsDNA Antibodies in Systemic Lupus Erythematosus. International Journal of Molecular Sciences. 2025; 26(17):8705. https://doi.org/10.3390/ijms26178705
Chicago/Turabian StyleBorrelli, Richard, Stefania Nicola, Federica Corradi, Luca Lo Sardo, Iuliana Badiu, Anna Quinternetto, Ilaria Vitali, Simone Negrini, and Luisa Brussino. 2025. "Impact of Smoking, Steroid Use and Immunosuppression on Anti-dsDNA Antibodies in Systemic Lupus Erythematosus" International Journal of Molecular Sciences 26, no. 17: 8705. https://doi.org/10.3390/ijms26178705
APA StyleBorrelli, R., Nicola, S., Corradi, F., Lo Sardo, L., Badiu, I., Quinternetto, A., Vitali, I., Negrini, S., & Brussino, L. (2025). Impact of Smoking, Steroid Use and Immunosuppression on Anti-dsDNA Antibodies in Systemic Lupus Erythematosus. International Journal of Molecular Sciences, 26(17), 8705. https://doi.org/10.3390/ijms26178705







