The Function of Chitinases CmCH1 and CmCH10 in the Interaction of Coniothyrium minitans and Sclerotinia sclerotiorum
Abstract
1. Introduction
2. Results
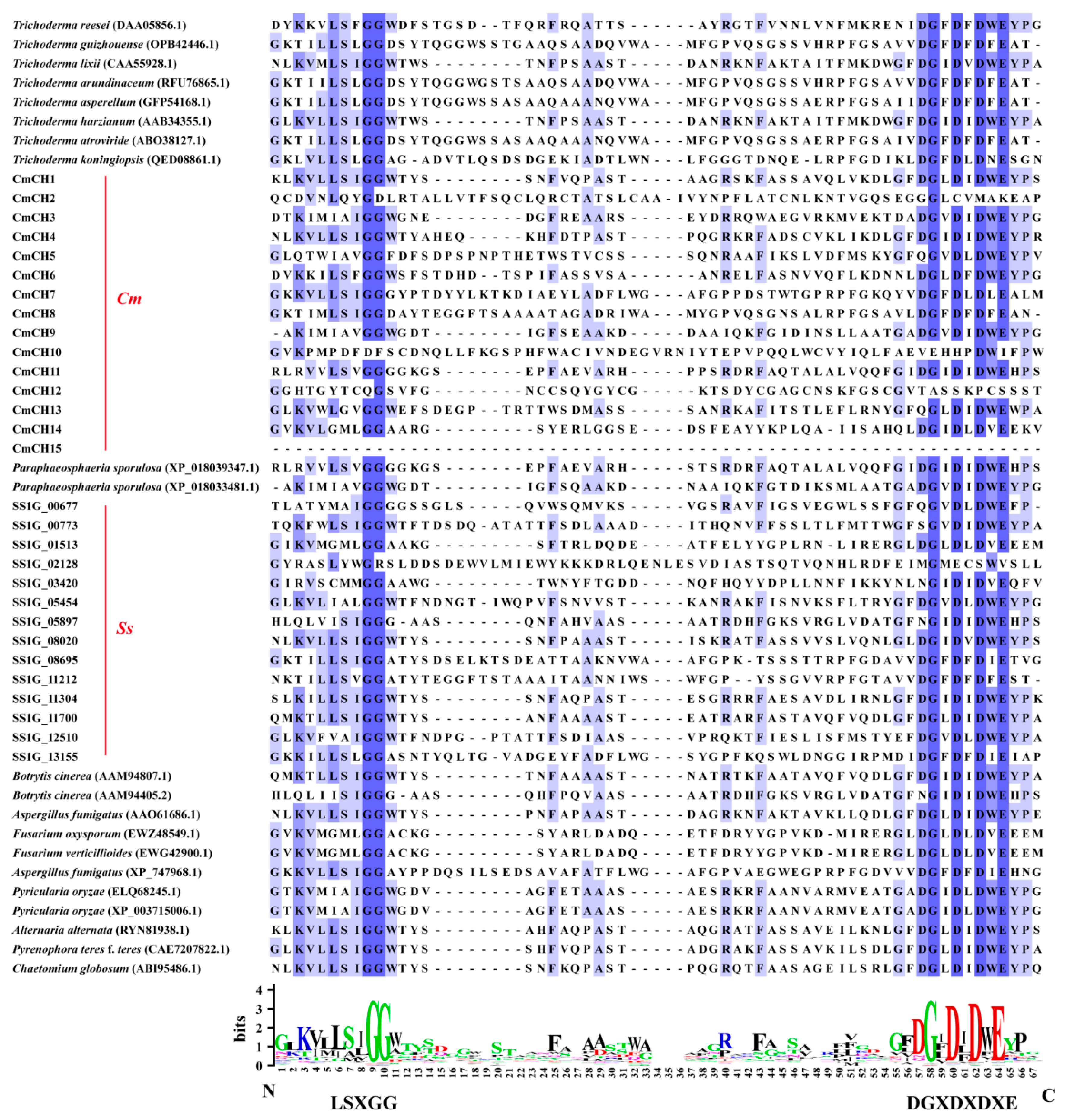
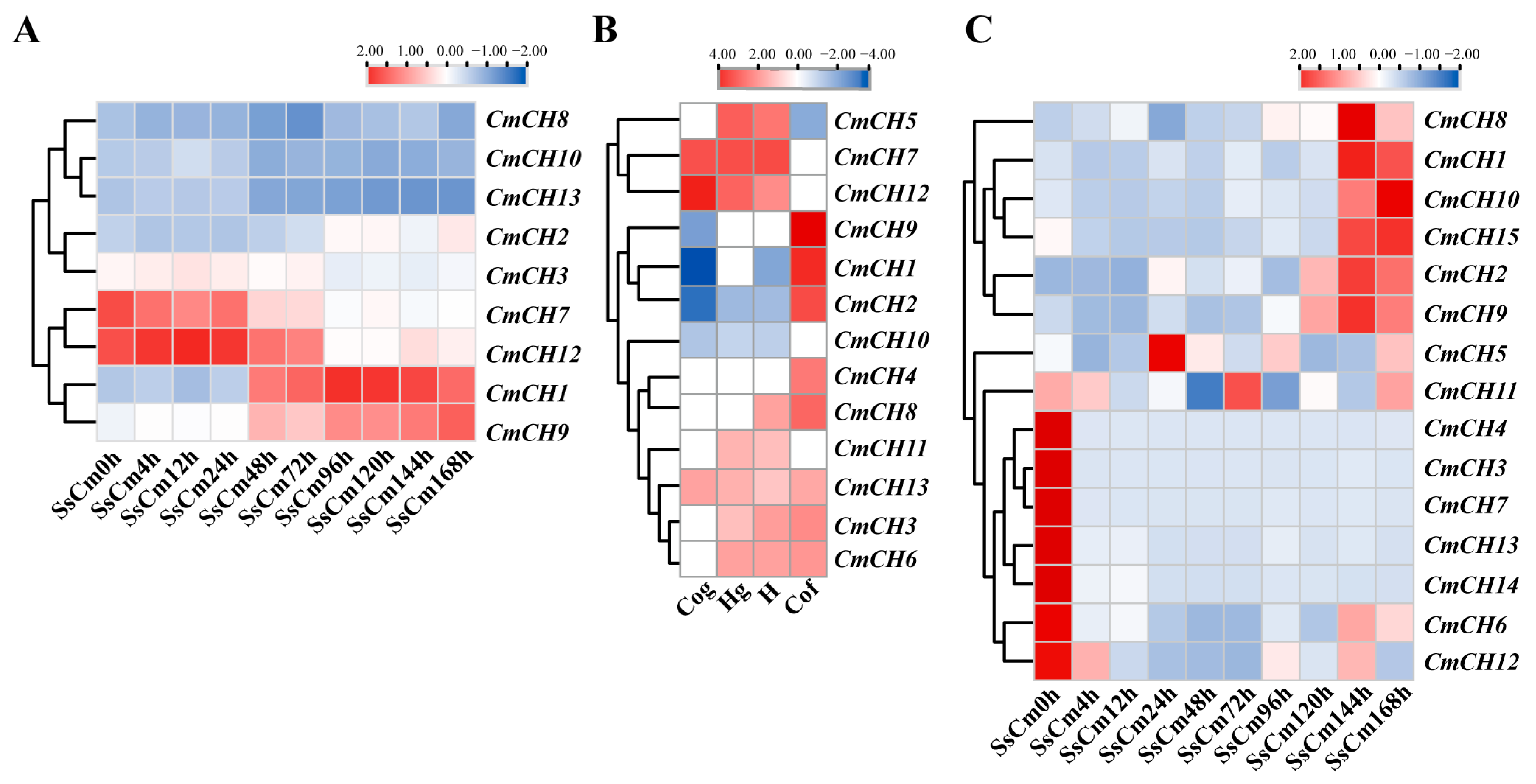
2.1. Identification of Chitinase-Encoding Genes in C. minitans
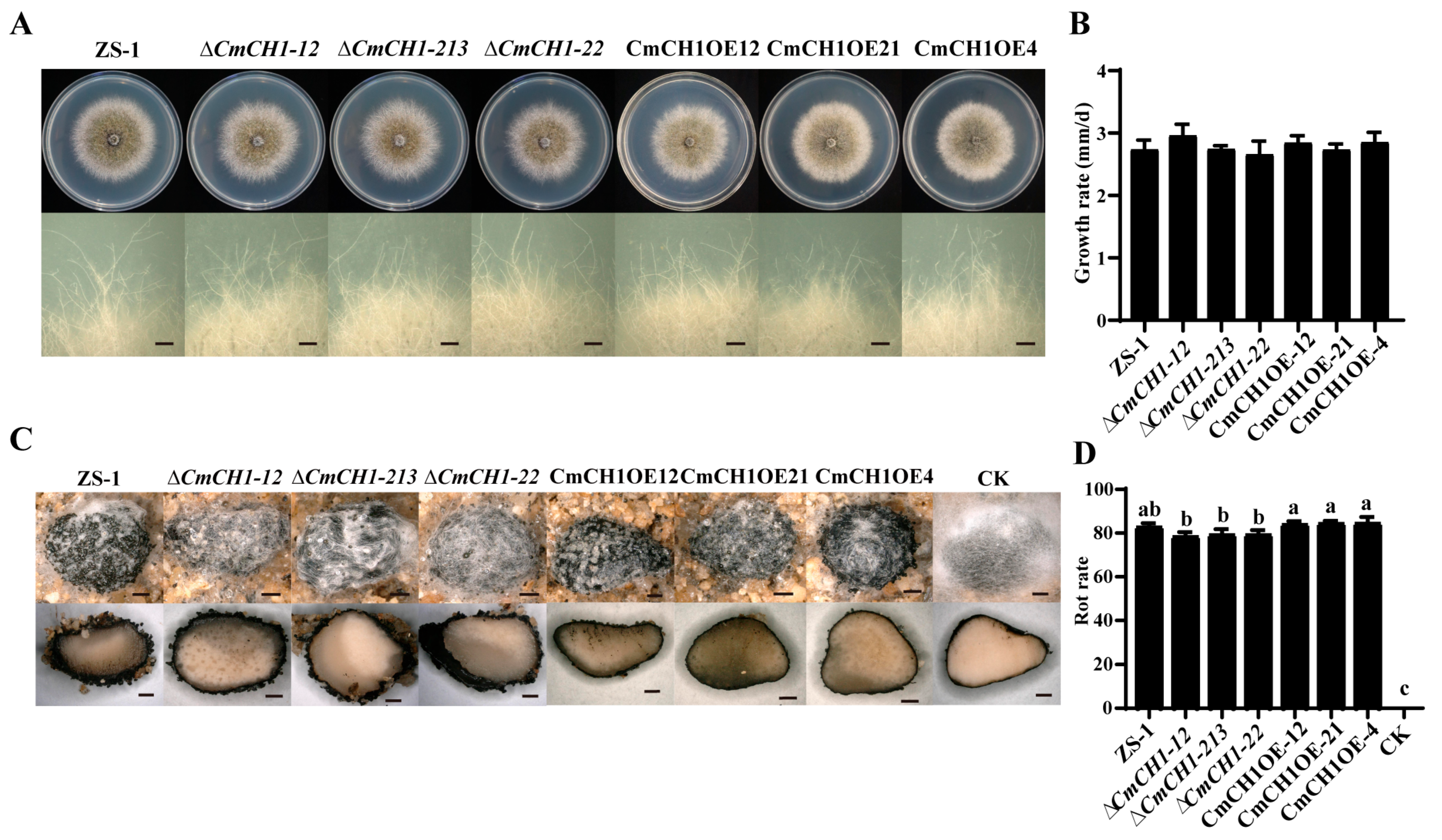
2.2. Disruption of CmCH1 Has No Influence on Mycelial Growth and Mycoparasitism of C. minitans
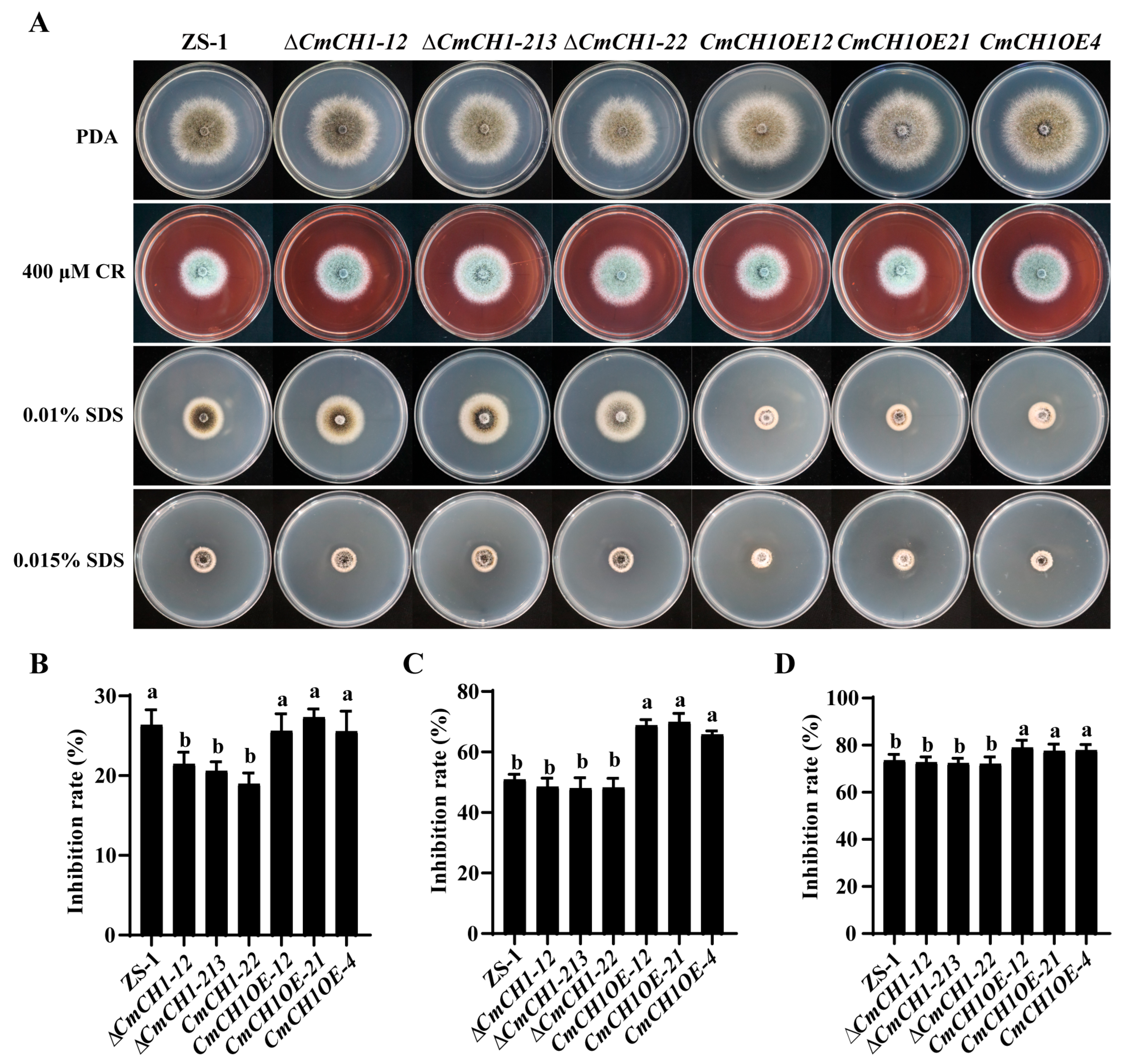
2.3. CmCH1 Compromises Cell Wall Integrity of C. minitans
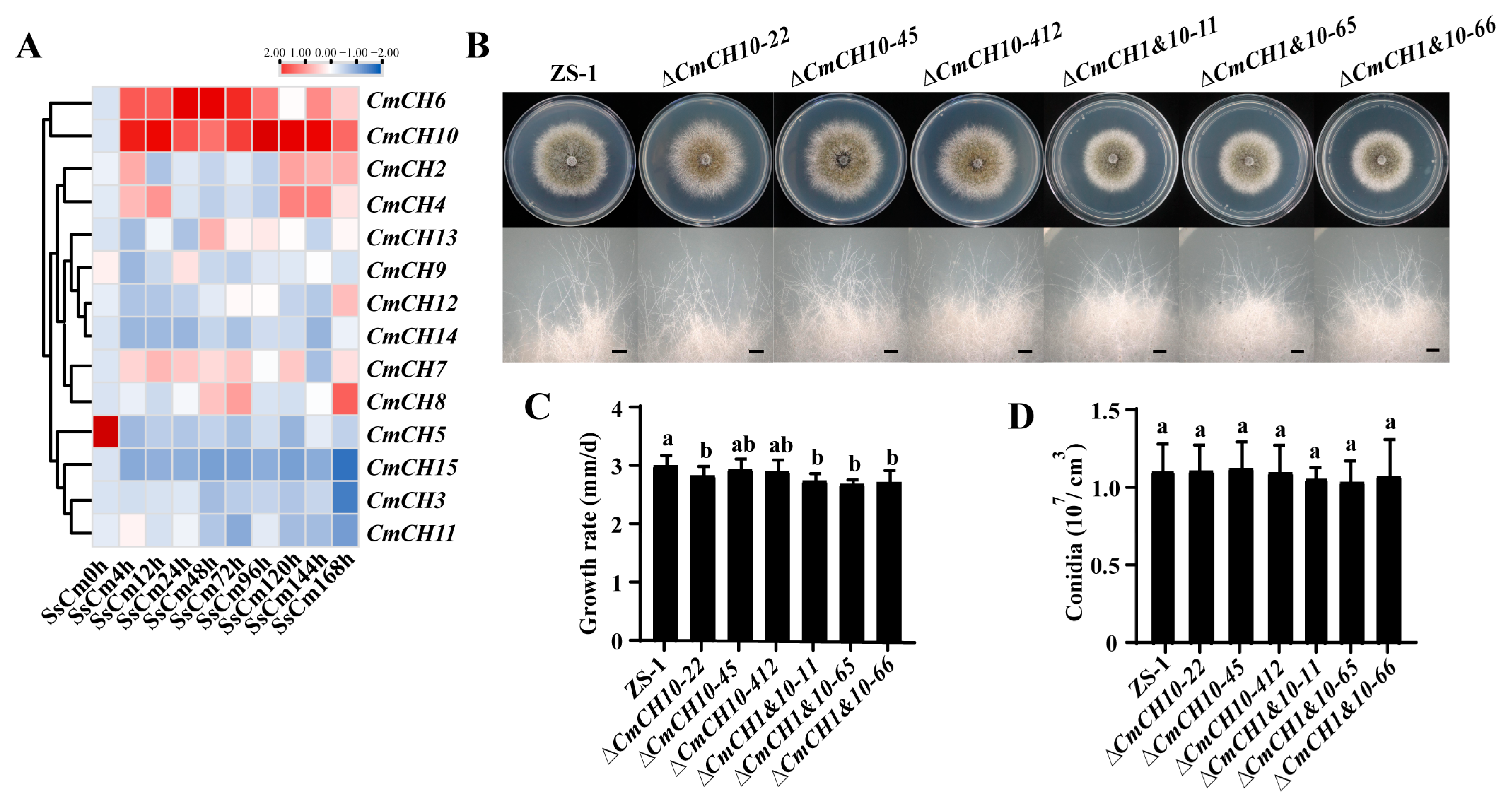
2.4. Simultaneous Deletion of CmCH1 and CmCH10 Leads to Growth Delay in C. minitans
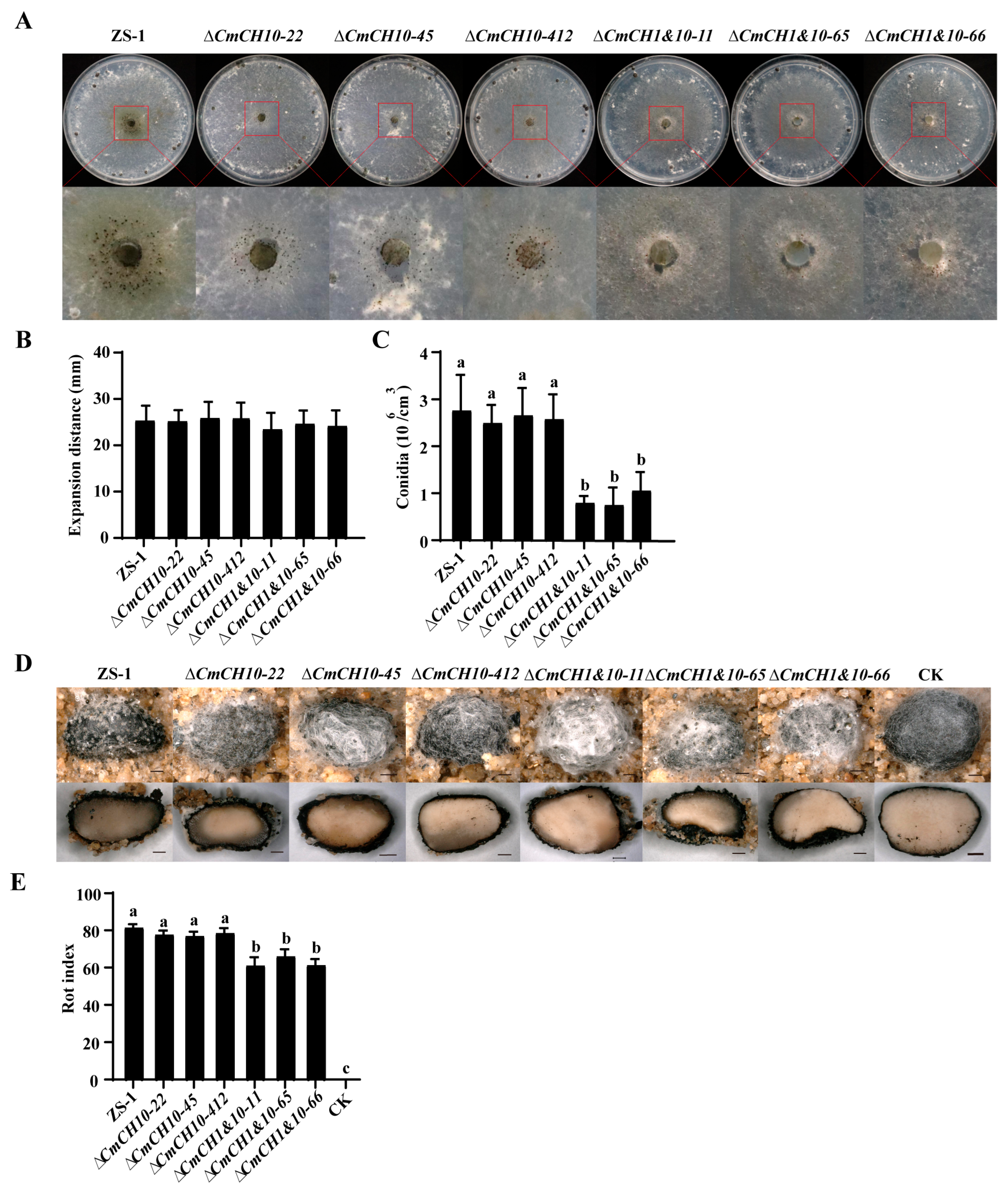
2.5. Double Deletion of CmCH1 and CmCH10 Decreases Parasitic Ability of C. minitans
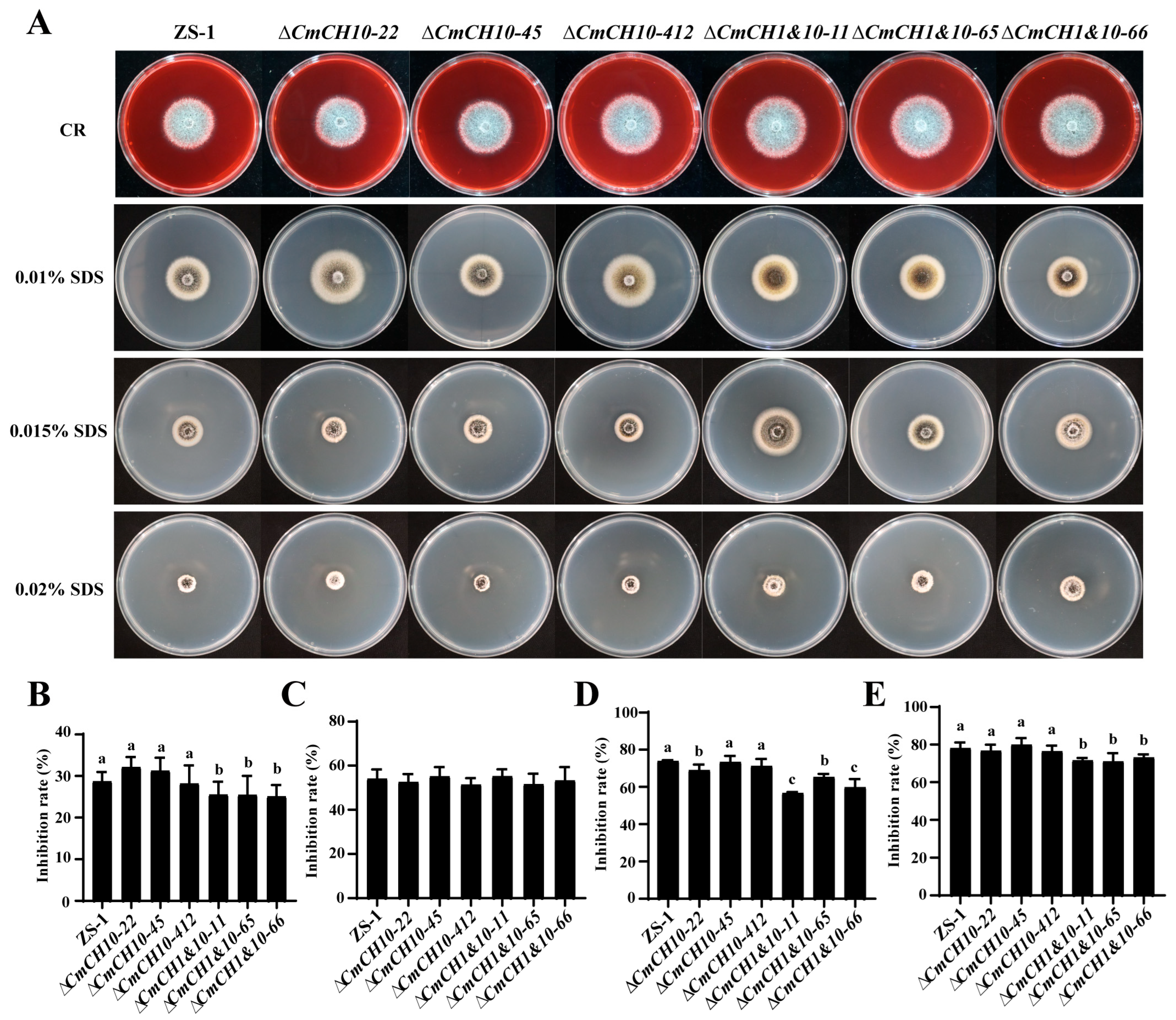
2.6. Double Deletion of CmCH1 and CmCH10 Enhances Tolerance of C. minitans to Abiotic Stress
3. Discussion
4. Materials and Methods
4.1. Strain and Growth Conditions
4.2. Sequence Analysis
4.3. Yeast Secretion Trap Screen Assay
4.4. Gene Deletion and Overexpression
4.5. Phenotypic Analysis of Mutants
4.6. Mycoparasitic Ability Assay
4.7. Sample Collection, RNA Extraction, and Quantitative Real-Time PCR Analysis
4.8. Transcriptomic Analysis
4.9. Microscopic Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boland, G.J.; Hall, R. Index of plant hosts of Sclerotinia sclerotiorum. Can. J. Plant Pathol. 1994, 16, 93–108. [Google Scholar] [CrossRef]
- Singh, J.; Yadav, P.; Budhlakoti, N.; Mishra, D.C.; Bhardwaj, N.R.; Rao, M.; Sharma, P.; Gupta, N.C. Exploration of the Sclerotinia sclerotiorum-Brassica pathosystem: Advances and perspectives in omics studies. Mol. Biol. Rep. 2024, 51, 1097. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Jiang, D.; Xie, J.; Cheng, J.; Xiao, X. The schizotrophic lifestyle of Sclerotinia sclerotiorum. Mol. Plant Pathol. 2024, 25, e13423. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Rollins, J.A. Mechanisms of broad host range necrotrophic pathogenesis in Sclerotinia sclerotiorum. Phytopathology 2018, 108, 1128–1140. [Google Scholar] [CrossRef]
- Campbell, W.A. A new species of Coniothyrium parasitic on sclerotia. Mycologia 1947, 39, 190–195. [Google Scholar] [CrossRef]
- Huang, H.C.; Erickson, R.S. Factors affecting biological control of Sclerotinia sclerotiorum by fungal antagonists. J. Phytopathol. 2010, 156, 628–634. [Google Scholar] [CrossRef]
- Huang, H.C. Importance of Coniothyrium minitans in survival of sclerotia of Sclerotinia sclerotiorum in wilted sunflower. Can. J. Bot. 1977, 55, 289–295. [Google Scholar] [CrossRef]
- McQuilken, M.P.; Chalton, D. Potential for biocontrol of sclerotinia rot of carrot with foliar sprays of Contans? WG (Coniothyrium minitans). Biocontrol Sci. Technol. 2009, 19, 229–235. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, Y.; Jia, J.; Xie, J.; Cheng, J.; Liu, H.; Jiang, D.; Fu, Y. Uninterrupted expression of CmSIT1 in a sclerotial parasite Coniothyrium minitans leads to reduced growth and enhanced antifungal ability. Front. Microbiol. 2017, 8, 2208. [Google Scholar] [CrossRef]
- Yang, R.; Han, Y.C.; Li, G.Q.; Jiang, D.H.; Huang, H.C. Suppression of Sclerotinia sclerotiorum by antifungal substances produced by the mycoparasite Coniothyrium minitans. Eur. J. Plant Pathol. 2007, 119, 411–420. [Google Scholar] [CrossRef]
- Zeng, F.; Gong, X.; Hamid, M.I.; Fu, Y.; Jiatao, X.; Cheng, J.; Li, G.; Jiang, D. A fungal cell wall integrity-associated MAP kinase cascade in Coniothyrium minitans is required for conidiation and mycoparasitism. Fungal Genet. Biol. 2012, 49, 347–357. [Google Scholar] [CrossRef]
- Wei, W.; Zhu, W.; Cheng, J.; Xie, J.; Jiang, D.; Li, G.; Chen, W.; Fu, Y. Nox Complex signal and MAPK cascade pathway are cross-linked and essential for pathogenicity and conidiation of mycoparasite Coniothyrium minitans. Sci. Rep. 2016, 6, 24325. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Watson, D. Parasitism and lysis by soil fungi of Sclerotinia sclerotiorum (Lib.) de Bary, a phytopathogenic fungus. Nature 1969, 224, 287–288. [Google Scholar] [CrossRef]
- Giczey, G.B.; Kerényi, Z.N.; Fülöp, L.S.; Hornok, L.S. Expression of cmg1, an exo-β-1,3-Glucanase Gene from Coniothyrium minitans, Increases during Sclerotial Parasitism. Appl. Environ. Microbiol. 2001, 67, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, H.; Luo, C.; Du, L.; Cheng, J.; Xie, J.; Jiang, D.; Fu, Y. CmAim24 is essential for mitochondrial morphology, conidiogenesis, and mycoparasitism in Coniothyrium minitans. Appl. Environ. Microbiol. 2020, 86, e02291-19. [Google Scholar] [CrossRef]
- Rogers, C.W.; Challen, M.P.; Muthumeenakshi, S.; Sreenivasaprasad, S.; Whipps, J.M. Disruption of the Coniothyrium minitans PIF1 DNA helicase gene impairs growth and capacity for sclerotial mycoparasitism. Microbiology 2008, 154, 1628–1636. [Google Scholar] [CrossRef][Green Version]
- Singh, G.; Bhalla, A.; Bhatti, J.S.; Ch, S.; Rajput, A.; Abdullah, A.; Andrabi, W.; Kaur, P. Potential of chitinases as a biopesticide against agriculturally harmful fungi and insects. Res. Rev. 2014, 3, 27–32. [Google Scholar][Green Version]
- Huang, Q.-S.; Xie, X.-L.; Liang, G.; Gong, F.; Wang, Y.; Wei, X.-Q.; Wang, Q.; Ji, Z.-L.; Chen, Q.-X. The GH18 family of chitinases: Their domain architectures, functions and evolutions. Glycobiology 2012, 22, 23–34. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Herrera-Estrella, A.; Seidl-Seiboth, V.; Martinez, D.A.; Druzhinina, I.S.; Thon, M.; Zeilinger, S.; Casas-Flores, S.; Horwitz, B.A.; Mukherjee, P.K.; et al. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biol. 2011, 12, R40. [Google Scholar] [CrossRef]
- Ulhoa, C.J.; Peberdy, J.F. Regulation of chitinase synthesis in Trichoderma harzianum. J. Gen. Microbiol. 1991, 137, 2163–2169. [Google Scholar] [CrossRef]
- García, I.; Lora, J.M.; Cruz, J.d.l.; Benítez, T.; Pintor-Toro, J.A. Cloning and characterization of a chitinase (CHIT42) cDNA from the mycoparasitic fungus Trichoderma harzianum. Curr. Genet. 1994, 27, 83–89. [Google Scholar] [CrossRef]
- Haran, S.; Schickler, H.; Oppenheim, A.; Chet, I. New components of the chitinolytic system of Trichoderma harzianum. Mycol. Res. 1995, 99, 441–446. [Google Scholar] [CrossRef]
- Dana, M.D.L.M.; Limón, M.C.; Mejías, R.; Mach, R.L.; Kubicek, C.P. Regulation of chitinase 33 (chit33) gene expression in Trichoderma harzianum. Curr. Genet. 2001, 38, 335–342. [Google Scholar] [CrossRef]
- Carsolio, C.; Gutierrez, A.; Jimenez, B.; Van Montagu, M.; Herrera-Estrella, A. Characterization of ech-42, a Trichoderma harzianum endochitinase gene expressed during mycoparasitism. Proc. Natl. Acad. Sci. USA 1994, 91, 10903–10907. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, J.; Han, Y.-C.; Yang, L.; Wu, M.-d.; Jiang, D.-H.; Chen, W.; Li, G.-Q. Degradation of oxalic acid by the mycoparasite Coniothyrium minitans plays an important role in interacting with Sclerotinia sclerotiorum. Environ. Microbiol. 2014, 16, 2591–2610. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, T.; Xie, J.; Cheng, J.; Chen, T.; Jiang, D.; Fu, Y. Mycoparasitism illuminated by genome and transcriptome sequencing of Coniothyrium minitans, an important biocontrol fungus of the plant pathogen Sclerotinia sclerotiorum. Microb. Genom. 2020, 6, e000345. [Google Scholar] [CrossRef]
- Abeles, F.B.; Bosshart, R.P.; Forrence, L.E.; Habig, W.H. Preparation and purification of glucanase and chitinase from bean leaves. Plant Physiol. 1971, 47, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, C.; Chen, X.; Cheng, J.; Xie, J.; Lin, y.; Fu, Y.; Jiang, D.; Chen, T. Overexpression of chitinase PbChia1 from Plasmodiophora brassicae improves broad-spectrum disease resistance of Arabidopsis. Virulence 2023, 14, 2233147. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, J.; Li, H.; Niu, L.; Xing, G.; Zhang, Y.; Xu, W.; Zhao, Q.; Li, Q.; Dong, Y. Overexpression of the chitinase gene CmCH1 from Coniothyrium minitans renders enhanced resistance to Sclerotinia sclerotiorum in soybean. Transgenic Res. 2020, 29, 187–198. [Google Scholar] [CrossRef]
- Chupp, G.; Lee, C.-M.; Jarjour, N.N.; Shim, Y.M.; Holm, C.; He, S.; Dziura, J.; Reed, J.L.; Coyle, A.J.; Kiener, P.A.; et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N. Engl. J. Med. 2007, 357, 2016–2027. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, H. Evolution of digestive enzymes and dietary diversification in birds. PeerJ 2019, 7, e6840. [Google Scholar] [CrossRef]
- Merzendorfer, H.; Zimoch, L. Chitin metabolism in insects: Structure, function and regulation of chitin synthases and chitinases. J. Exp. Biol. 2003, 206, 4393–4412. [Google Scholar] [CrossRef]
- Gama, M.d.V.F.; Moraes, C.S.; Gomes, B.; Díaz-Albiter, H.; Mesquita, R.D.; Seabra-Junior, E.S.; Azambuja, P.; Garcia, E.d.S.; Genta, F.A. Structure and expression of Rhodnius prolixus GH18 chitinases and chitinase-like proteins: Characterization of the physiological role of RpCht7, a gene from subgroup VIII, in vector fitness and reproduction. Front. Physiol. 2022, 13, 861620. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.; Gehri, A.; Mauch, F.; Vögeli, U. Chitinase in bean leaves: Induction by ethylene, purification, properties, and possible function. Planta 1983, 157, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Schlumbaum, A.; Mauch, F.; Vögeli, U.; Boller, T. Plant chitinases are potent inhibitors of fungal growth. Nature 1986, 324, 365–367. [Google Scholar] [CrossRef]
- Liang, D.; Andersen, C.B.; Vetukuri, R.R.; Dou, D.; Grenville-Briggs, L.J. Horizontal gene transfer and tandem duplication shape the unique CAZyme complement of the mycoparasitic oomycetes Pythium oligandrum and Pythium periplocum. Front. Microbiol. 2020, 11, 581698. [Google Scholar] [CrossRef]
- Hawtin, R.E.; Arnold, K.; Ayres, M.D.; Zanotto, P.M.d.A.; Howard, S.C.; Gooday, G.W.; Chappell, L.H.; Kitts, P.A.; King, L.A.; Possee, R.D. Identification and preliminary characterization of a chitinase gene in the Autographa californica nuclear polyhedrosis virus genome. Virology 1995, 212, 673–685. [Google Scholar] [CrossRef]
- Seidl, V.; Huemer, B.; Seiboth, B.; Kubicek, C.P. A complete survey of Trichoderma chitinases reveals three distinct subgroups of family 18 chitinases. FEBS J. 2005, 272, 5923–5939. [Google Scholar] [CrossRef]
- Seidl, V. Chitinases of filamentous fungi: A large group of diverse proteins with multiple physiological functions. Fungal Biol. Rev. 2008, 22, 36–42. [Google Scholar] [CrossRef]
- Gruber, S.; Seidl-Seiboth, V. Self versus non-self: Fungal cell wall degradation in Trichoderma. Microbiology 2012, 158, 26–34. [Google Scholar] [CrossRef]
- Hartl, L.; Zach, S.; Seidl-Seiboth, V. Fungal chitinases: Diversity, mechanistic properties and biotechnological potential. Appl. Microbiol. Biotechnol. 2011, 2012, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Reichard, U.; Hung, C.-Y.; Thomas, P.W.; Cole, G.T. Disruption of the gene which encodes a serodiagnostic antigen and chitinase of the human fungal pathogen Coccidioides immitis. Infect. Immun. 2000, 68, 5830–5838. [Google Scholar] [CrossRef] [PubMed]
- Jaques, A.K.; Fukamizo, T.; Hall, D.; Barton, R.C.; Escott, G.M.; Parkinson, T.; Hitchcock, C.A.; Adams, D.J. Disruption of the gene encoding the ChiB1 chitinase of Aspergillus fumigatus and characterization of a recombinant gene product. Microbiology 2003, 149, 2931–2939. [Google Scholar] [CrossRef] [PubMed]
- Dünkler, A.; Walther, A.; Specht, C.A.; Wendland, J. Candida albicans CHT3 encodes the functional homolog of the Cts1 chitinase of Saccharomyces cerevisiae. Fungal Genet. Biol. 2005, 42, 935–947. [Google Scholar] [CrossRef]
- Kämper, J.; Kahmann, R.; Bölker, M.; Ma, L.J.; Brefort, T.; Saville, B.J.; Banuett, F.; Kronstad, J.W.; Gold, S.E.; Müller, O.; et al. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 2006, 444, 97–101. [Google Scholar] [CrossRef]
- López-Mondéjar, R.; Catalano, V.; Kubicek, C.P.; Seidl, V. The beta-N-acetylglucosaminidases NAG1 and NAG2 are essential for growth of Trichoderma atroviride on chitin. FEBS J. 2009, 276, 5137–5148. [Google Scholar] [CrossRef]
- van den Burg, H.A.; Harrison, S.J.; Joosten, M.H.A.J.; Vervoort, J.; de Wit, P.J.G.M. Cladosporium fulvum Avr4 protects fungal cell walls against hydrolysis by plant chitinases accumulating during infection. Mol. Plant-Microbe Interact. 2006, 19, 1420–1430. [Google Scholar] [CrossRef]
- Rosado, I.V.; Rey, M.; Codón, A.C.; Govantes, J.; Moreno-Mateos, M.A.; Benítez, T. QID74 Cell wall protein of Trichoderma harzianum is involved in cell protection and adherence to hydrophobic surfaces. Fungal Genet. Biol. 2007, 44, 950–964. [Google Scholar] [CrossRef]
- Cheng, J.; Jiang, D.; Yi, X.; Fu, Y.; Li, G.; Whipps, J.M. Production, survival and efficacy of Coniothyrium minitans conidia produced in shaken liquid culture. FEMS Microbiol. Lett. 2003, 227, 127–131. [Google Scholar] [CrossRef]
- Gietz, R.D.; Schiestl, R.H. Large-scale high-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2007, 2, 38–41. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.S.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.; Clamp, M.; Barton, G.J. Jalview Version 2--a multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Lee, S.; Kim, B.-D.; Rose, J.K.C. Identification of eukaryotic secreted and cell surface proteins using the yeast secretion trap screen. Nat. Protoc. 2006, 1, 2439–2447. [Google Scholar] [CrossRef]
- Catlett, N.L.; Lee, B.; Yoder, O.C.; Turgeon, B.G. Split-marker recombination for efficient targeted deletion of fungal genes. Fungal Genet. Rep. 2003, 50, 9–11. [Google Scholar]
- Kohn, L.M.; Stasovski, E.; Carbone, J.; Anderson, J. Mycelial incompatibility and molecular markers identify genetic variability in field populations of Sclerotinia sclerotiorum. Phytopathology 1991, 81, 480–485. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, Z.; Xu, Y.; Wang, H.; Xie, J.; Cheng, J.; Jiang, D.; Fu, Y. SsNEP2 plays a role in the interaction between Sclerotinia sclerotiorum and Coniothyrium minitans. J. Fungi 2025, 11, 151. [Google Scholar] [CrossRef]
| Strain | Protoplasts (105/cm3) | Conidial Germination (%) |
|---|---|---|
| ZS-1 | 3.10 ± 1.02 ab | 61.33 ± 0.94 ab |
| ∆CmCH1-12 | 2.40 ± 1.02 b | 57.66 ± 1.25 b |
| ∆CmCH1-213 | 2.50 ± 0.89 b | 58.00 ± 0.82 b |
| ∆CmCH1-22 | 2.30 ± 0.40 b | 58.00 ± 1.63 b |
| CmCH1OE-12 | 4.90 ± 1.11 a | 64.00 ± 0.82 a |
| CmCH1OE-21 | 4.50 ± 0.32 a | 63.67 ± 1.25 a |
| CmCH1OE-4 | 4.80 ± 0.68 a | 64.67 ± 0.47 a |
| Strain | Protoplasts (105/cm3) | Conidial Germination (%) |
|---|---|---|
| ZS-1 | 3.00 ± 0.65 a | 69.67 ± 4.82 a |
| ∆CmCH10-22 | 2.92 ± 1.30 a | 63.00 ± 12.42 a |
| ∆CmCH10-45 | 3.25 ± 1.18 a | 62.67 ± 6.50 a |
| ∆CmCH10-412 | 2.47 ± 1.72 a | 60.67 ± 9.91 a |
| ∆CmCH1&10-11 | 0.83 ± 0.75 b | 47.67 ± 9.55 b |
| ∆CmCH1&10-65 | 0.67 ± 0.55 b | 52.33 ± 5.09 b |
| ∆CmCH1&10-66 | 0.75 ± 0.69 b | 53.67 ± 3.73 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhao, H.; Zhu, Z.; Lin, Y.; Xie, J.; Cheng, J.; Jiang, D.; Fu, Y. The Function of Chitinases CmCH1 and CmCH10 in the Interaction of Coniothyrium minitans and Sclerotinia sclerotiorum. Int. J. Mol. Sci. 2025, 26, 8706. https://doi.org/10.3390/ijms26178706
Wang H, Zhao H, Zhu Z, Lin Y, Xie J, Cheng J, Jiang D, Fu Y. The Function of Chitinases CmCH1 and CmCH10 in the Interaction of Coniothyrium minitans and Sclerotinia sclerotiorum. International Journal of Molecular Sciences. 2025; 26(17):8706. https://doi.org/10.3390/ijms26178706
Chicago/Turabian StyleWang, Haixuan, Huizhang Zhao, Zihang Zhu, Yang Lin, Jiatao Xie, Jiasen Cheng, Daohong Jiang, and Yanping Fu. 2025. "The Function of Chitinases CmCH1 and CmCH10 in the Interaction of Coniothyrium minitans and Sclerotinia sclerotiorum" International Journal of Molecular Sciences 26, no. 17: 8706. https://doi.org/10.3390/ijms26178706
APA StyleWang, H., Zhao, H., Zhu, Z., Lin, Y., Xie, J., Cheng, J., Jiang, D., & Fu, Y. (2025). The Function of Chitinases CmCH1 and CmCH10 in the Interaction of Coniothyrium minitans and Sclerotinia sclerotiorum. International Journal of Molecular Sciences, 26(17), 8706. https://doi.org/10.3390/ijms26178706









