Self-Assembly Strategies in Upconversion Nanoparticle-Based Nanocomposites: Structure Designs and Applications
Abstract
1. Introduction
2. Self-Assembly Mechanisms for UCNP-Based Nanocomposites
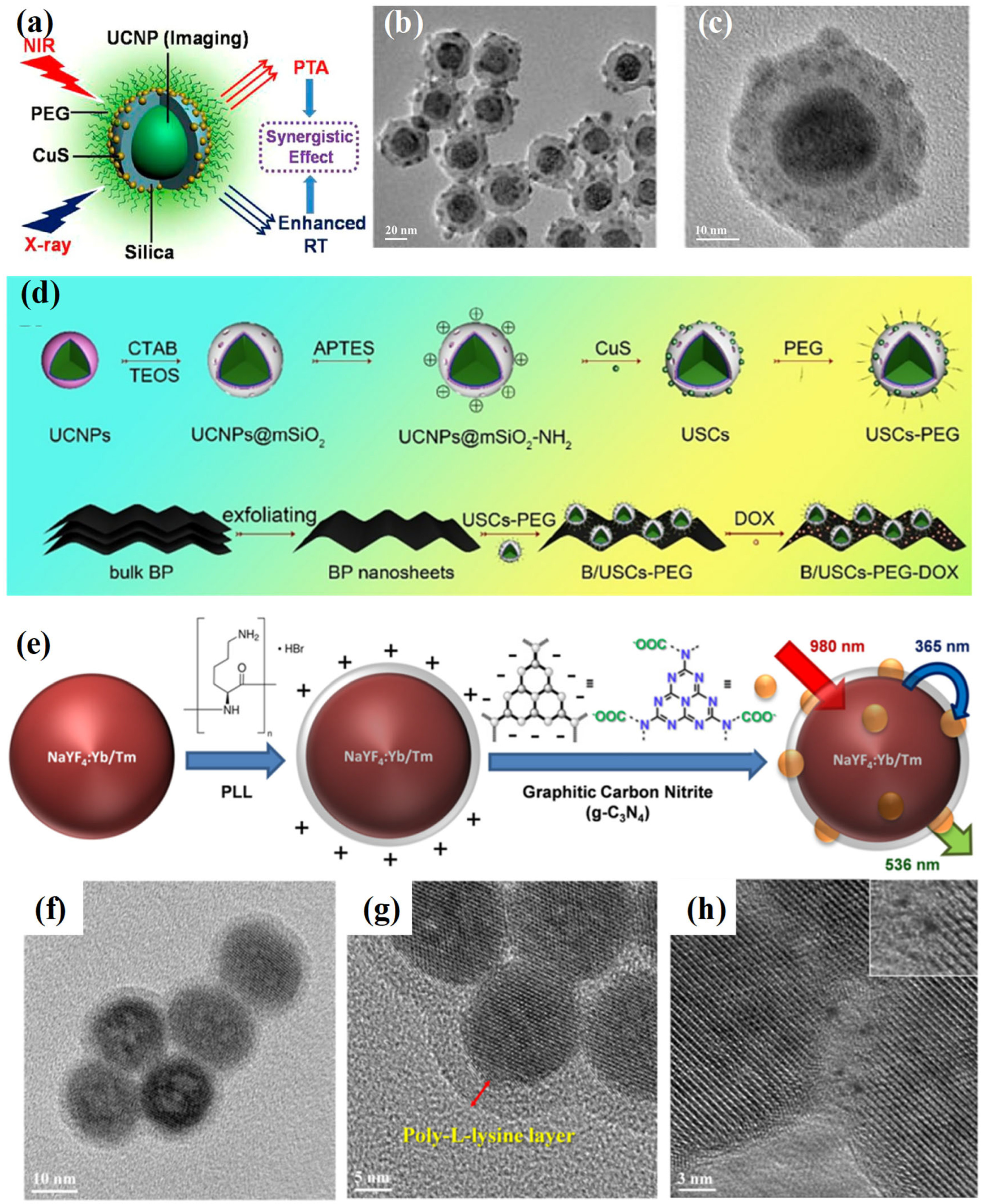
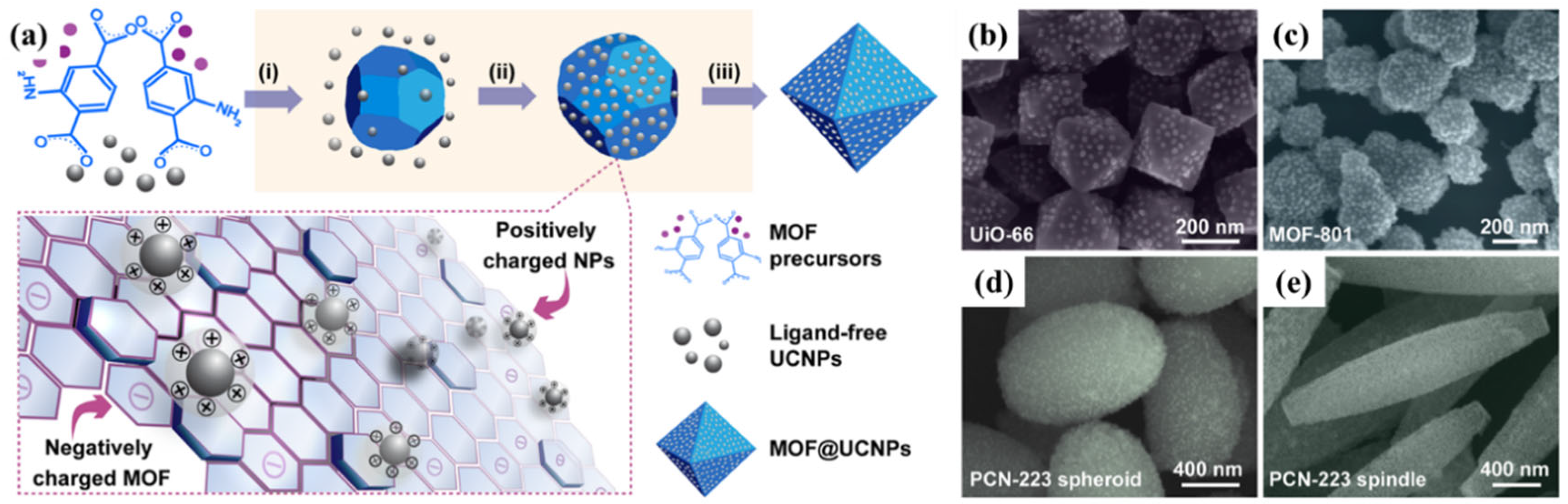
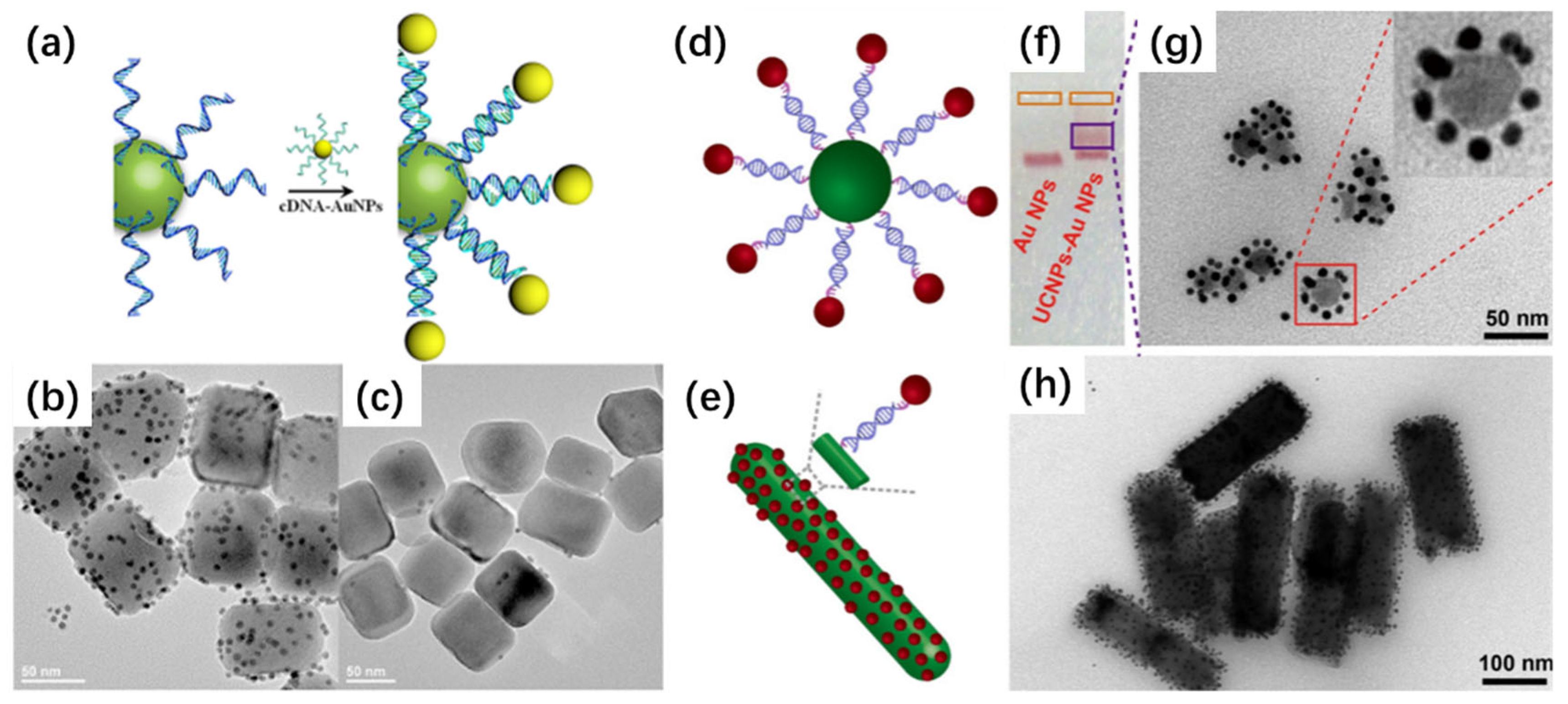
2.1. Electrostatic Interactions
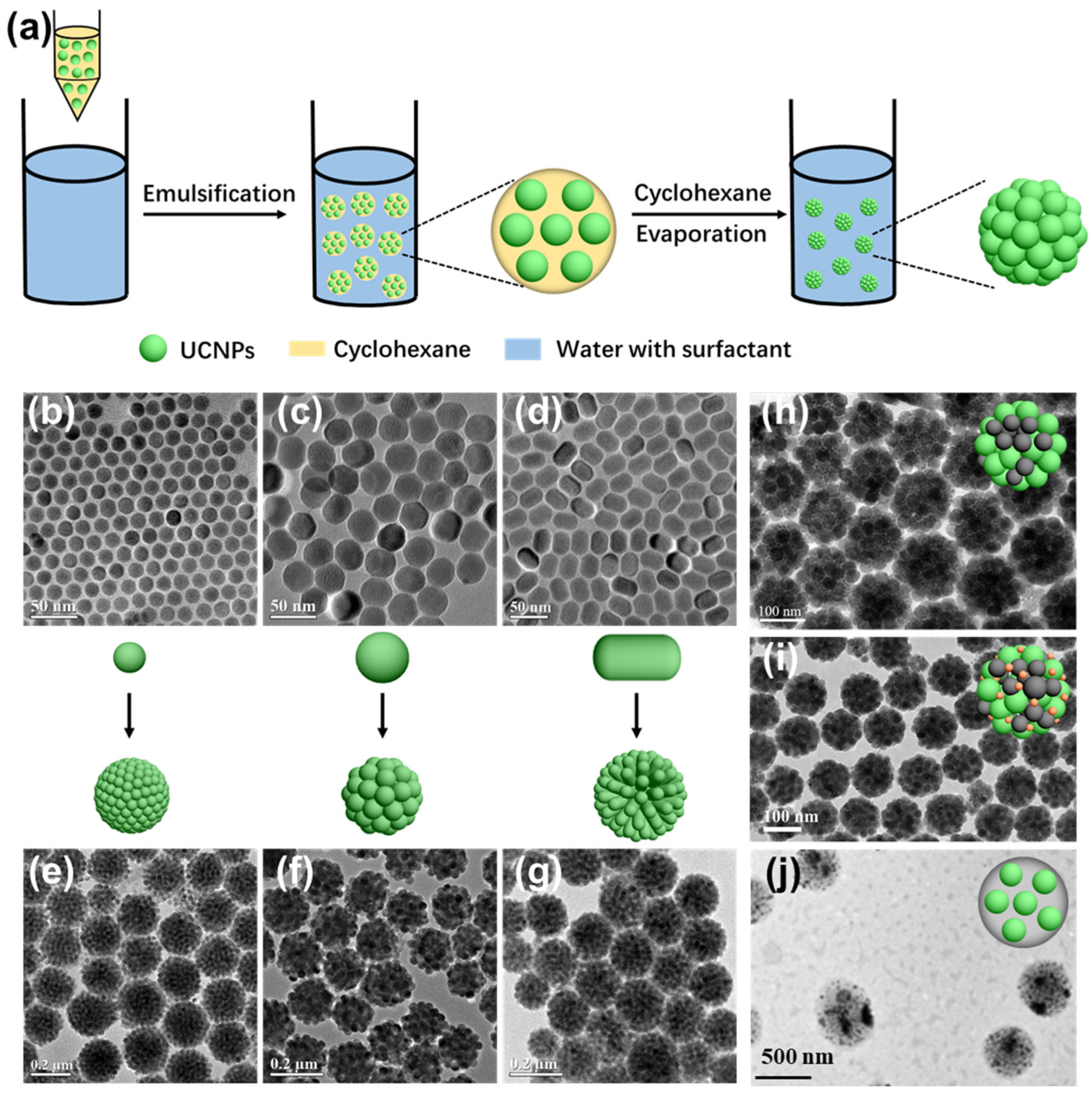
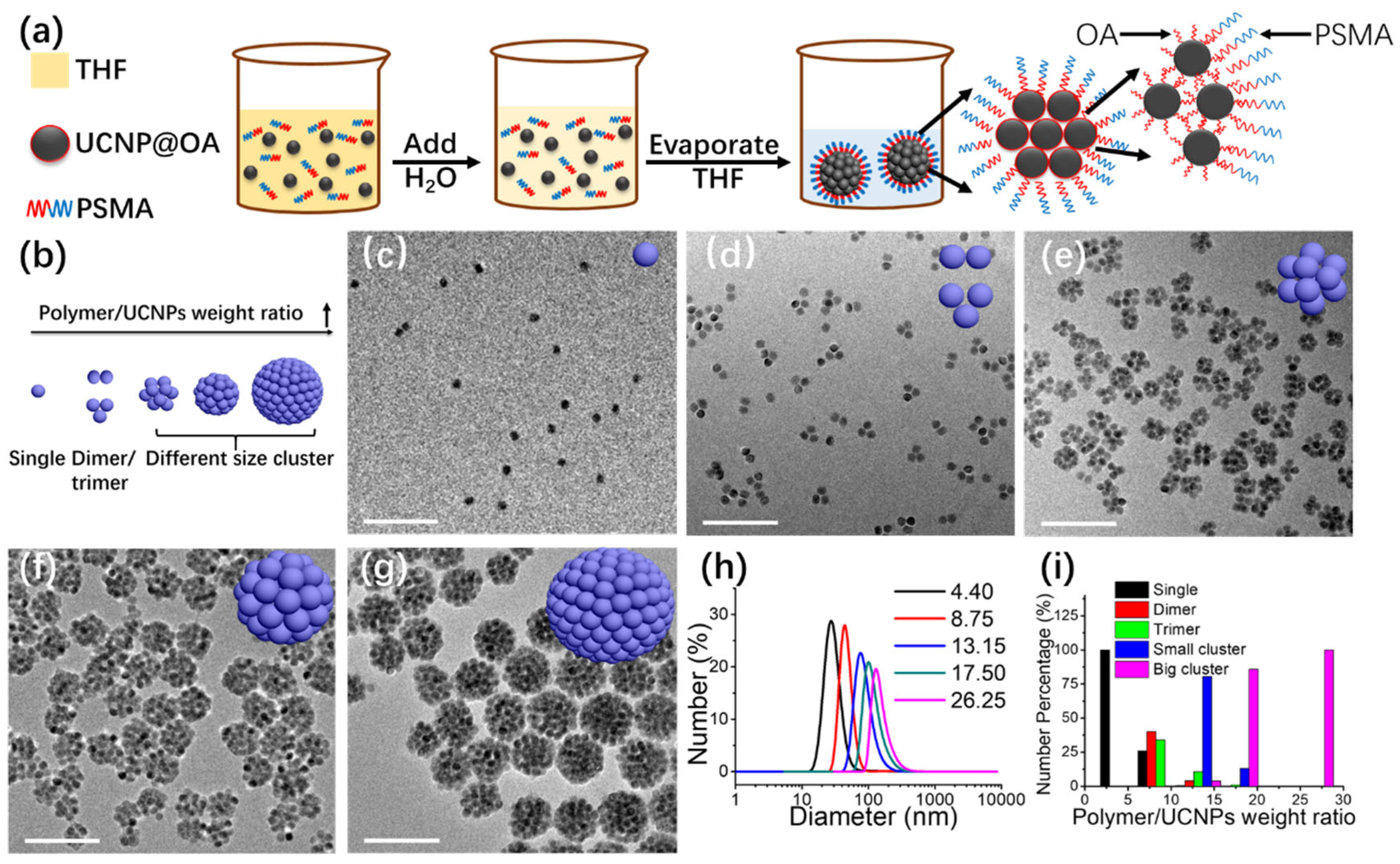
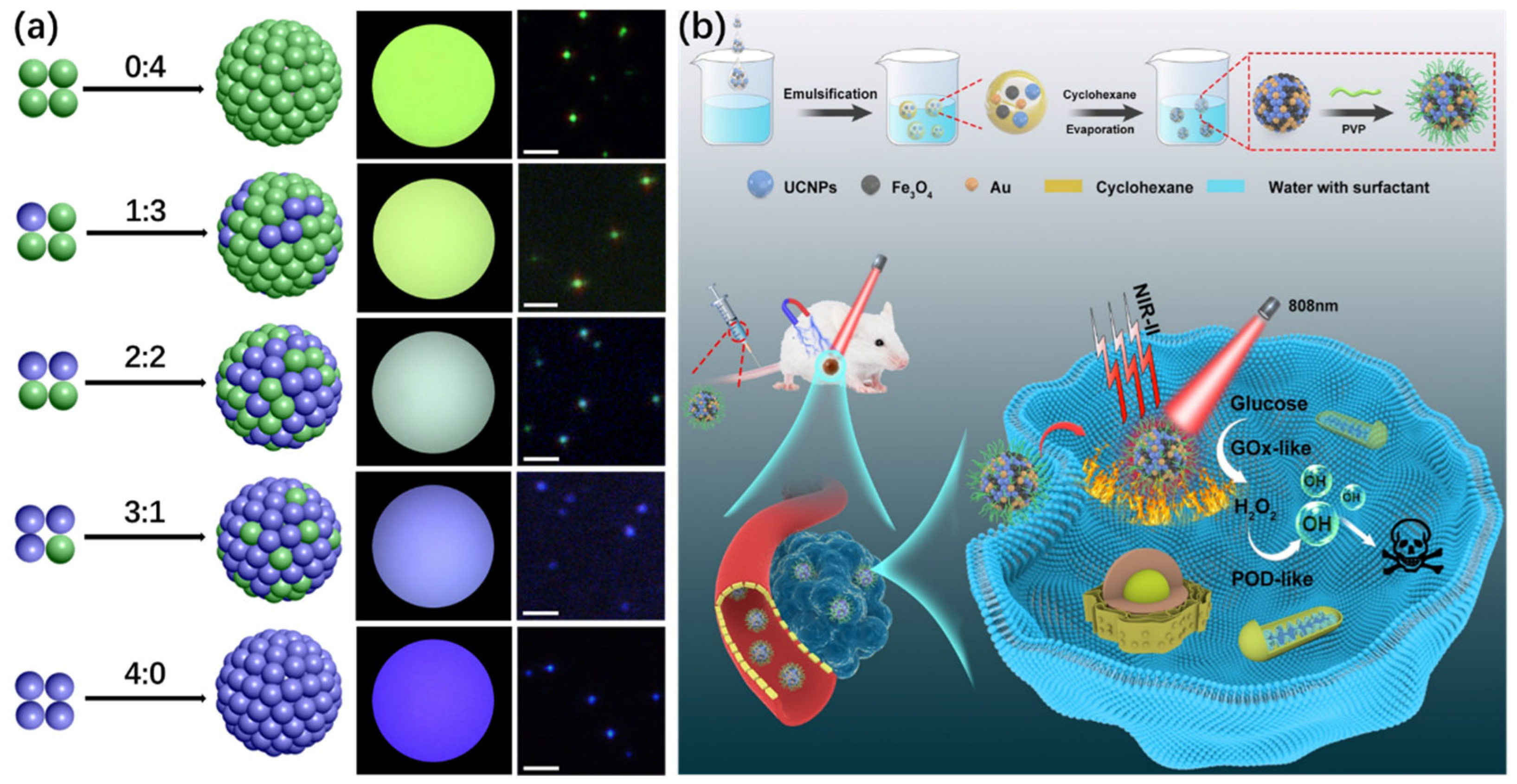
2.2. Hydrophobic Interaction
2.3. Covalent Coupling
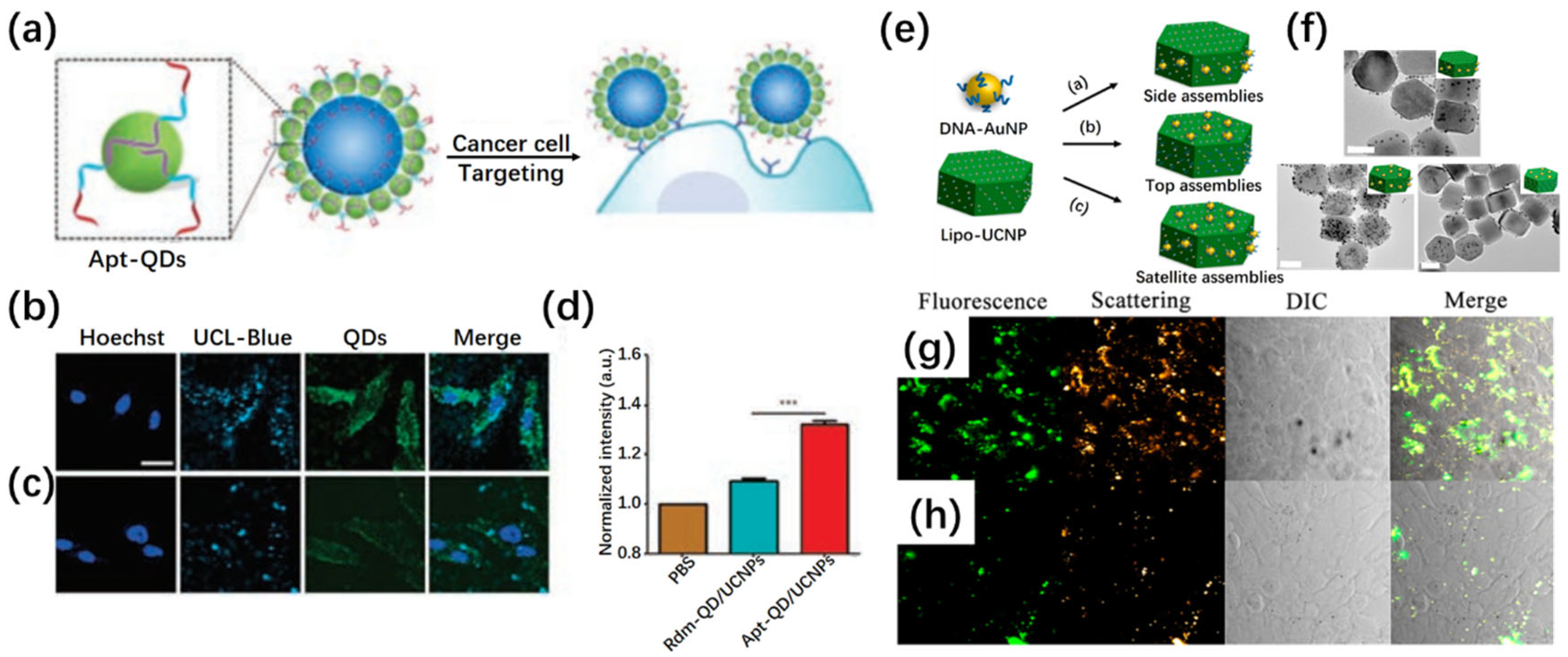
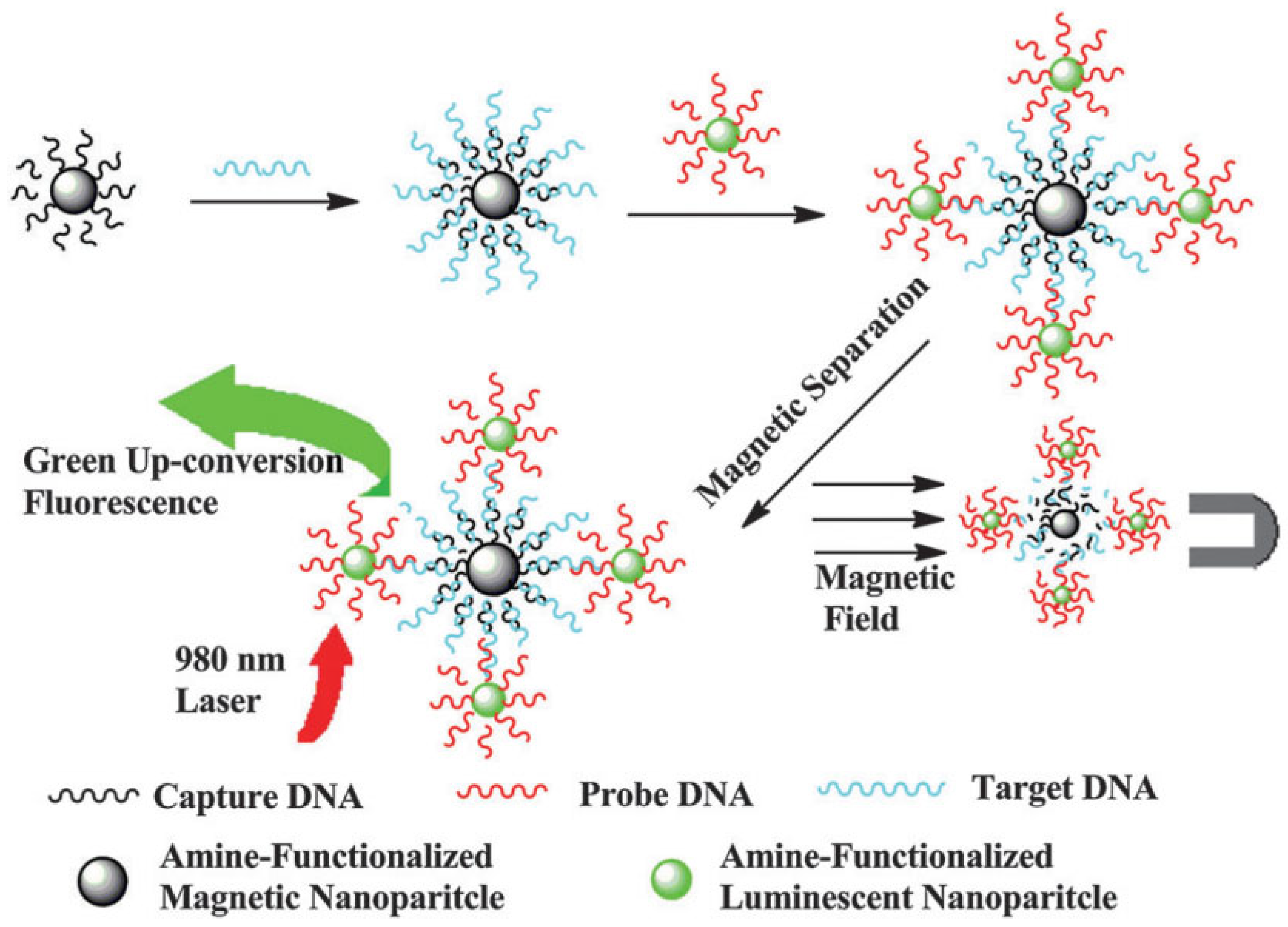
2.4. Biorecognition-Mediated Assembly
3. Applications of Self-Assembled UCNP Composites
3.1. Multimodal Imaging
3.2. Advanced Biosensing
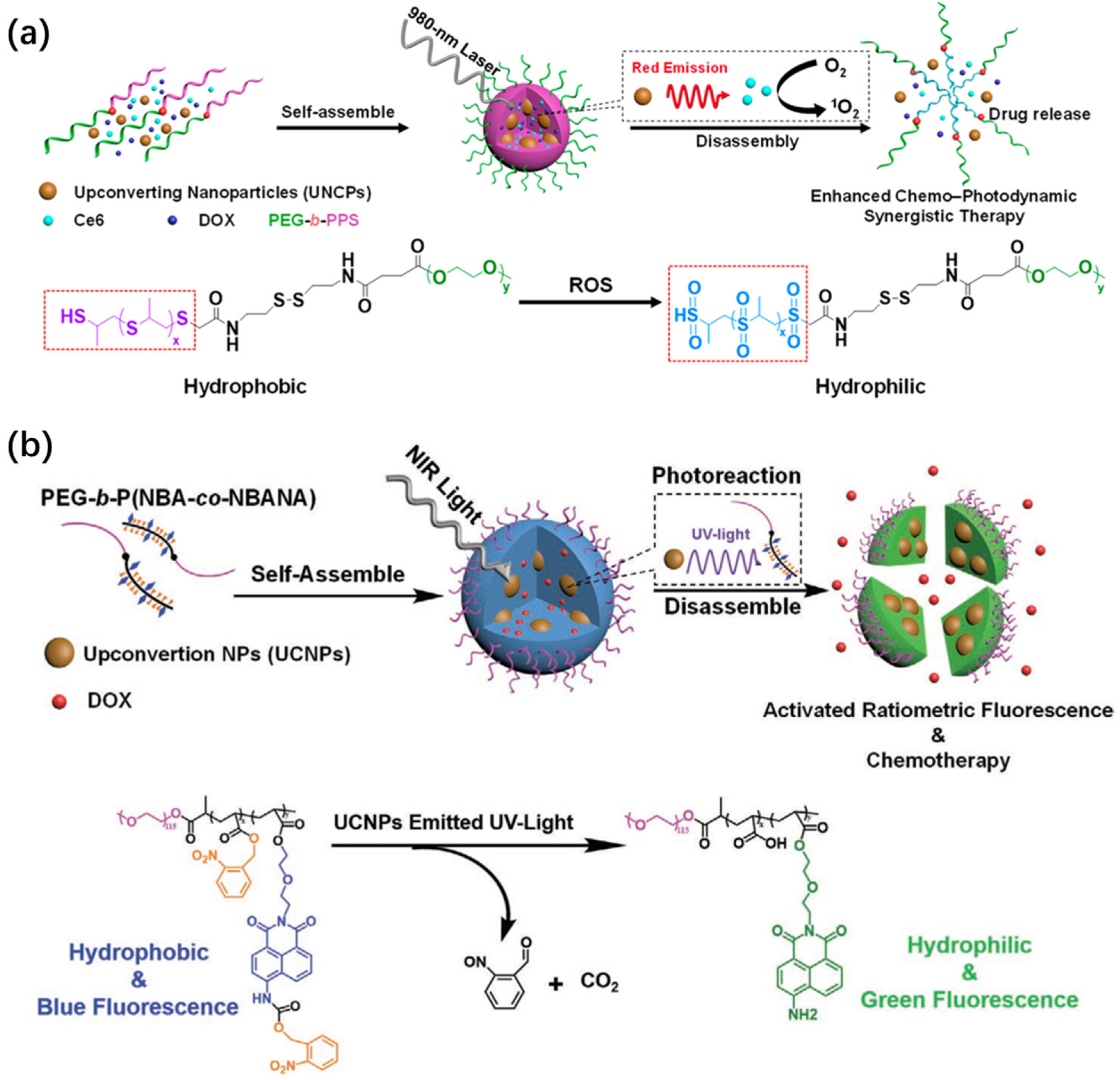
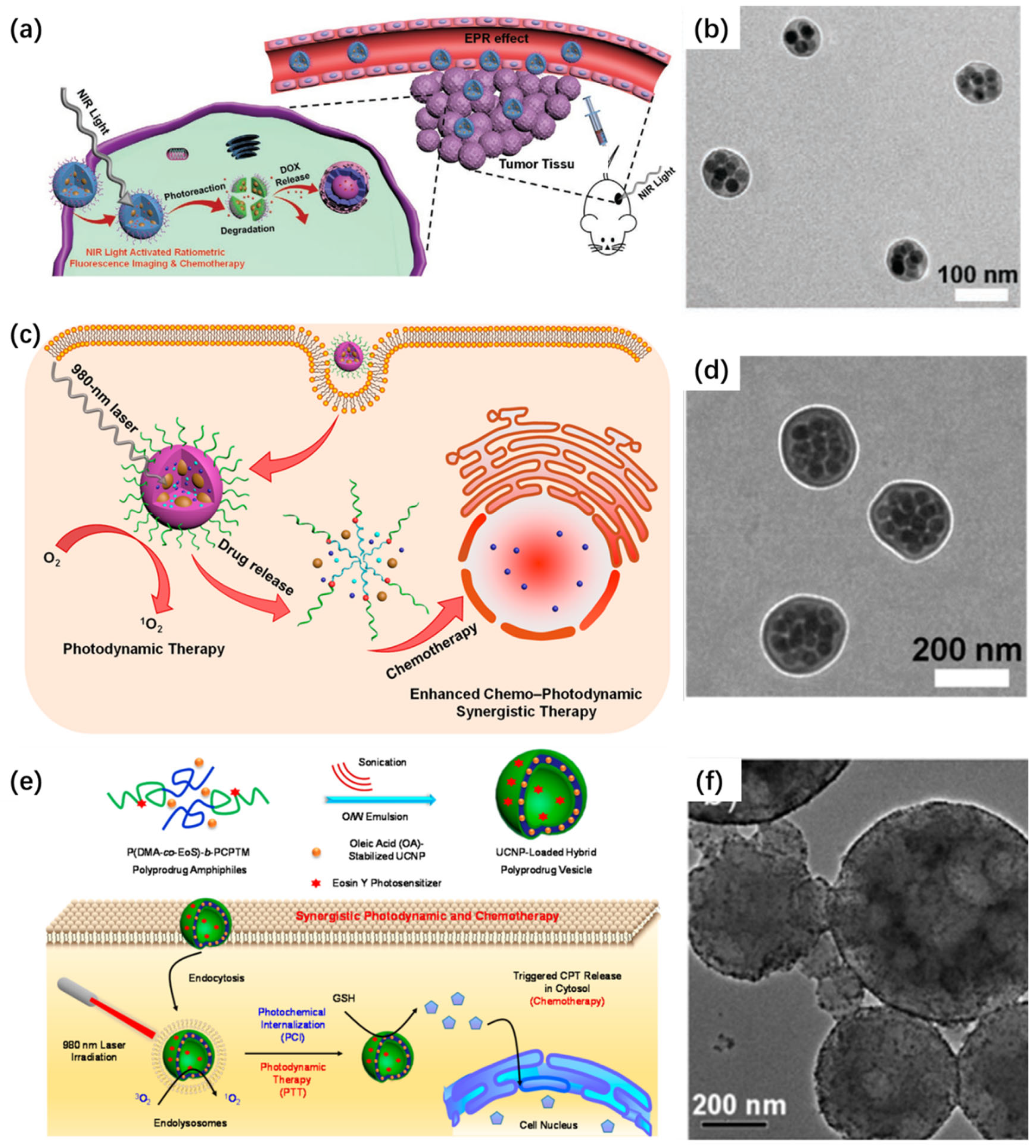
3.3. Smart Nanocarriers for Controlled Molecular Delivery
3.4. Orthogonal Photoactivation for Programmable Therapy
4. Challenges and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, K.; Feng, J.; Gao, X.; Zhang, H. Nanocomposites Based on Lanthanide-Doped Upconversion Nanoparticles: Diverse Designs and Applications. Light Sci. Appl. 2022, 11, 222. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, J.; Yue, J.; Wei, Y.; Gao, C.; Xie, X.; Huang, L. Recent Development in Sensitizers for Lanthanide-Doped Upconversion Luminescence. Chem. Rev. 2022, 122, 15998–16050. [Google Scholar] [CrossRef]
- Liu, S.; Yan, L.; Huang, J.; Zhang, Q.; Zhou, B. Controlling Upconversion in Emerging Multilayer Core–Shell Nanostructures: From Fundamentals to Frontier Applications. Chem. Soc. Rev. 2022, 51, 1729–1765. [Google Scholar] [CrossRef]
- Zhang, Y.; Mei, Q.; Zhang, Z. Advancements in Microemulsion-Based Fabrication of Upconversion-Mediated Multifunctional Materials. Front. Photonics 2024, 5, 1363223. [Google Scholar] [CrossRef]
- Bharmoria, P.; Bildirir, H.; Moth-Poulsen, K. Triplet-Triplet Annihilation Based Near Infrared to Visible Molecular Photon Upconversion. Chem. Soc. Rev. 2020, 49, 6529–6554. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.; Zhang, Y. Exploring Heterostructured Upconversion Nanoparticles: From Rational Engineering to Diverse Applications. ACS Nano 2021, 15, 3709–3735. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, J.; Liu, J.; Zhang, Y. Recent Progress of Rare-Earth Doped Upconversion Nanoparticles: Synthesis, Optimization, and Applications. Adv. Sci. 2019, 6, 1901358. [Google Scholar] [CrossRef]
- Zhang, Z.; Shikha, S.; Liu, J.; Zhang, J.; Mei, Q.; Zhang, Y. Upconversion Nanoprobes: Recent Advances in Sensing Applications. Anal. Chem. 2019, 91, 548–568. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, X.; Bu, W. Upconversion-Based Photodynamic Cancer Therapy. Coord. Chem. Rev. 2019, 379, 82–98. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Zhang, Y. Self-Assembly of Upconversion Nanoparticles Based Materials and Their Emerging Applications. Small 2022, 18, 2103241. [Google Scholar] [CrossRef] [PubMed]
- Ghorpade, K.B.; Kumar, M.; Tiwari, S. A Review on Composites Based on Upconversion Nanoparticles and Graphene Oxide: Development and Theranostic Applications Centered at Solid Tumors. J. Mater. Sci. Mater. Eng. 2024, 19, 48. [Google Scholar] [CrossRef]
- Du, J.; Jia, T.; Zhang, J.; Chen, G. Heterostructures Combining Upconversion Nanoparticles and Metal-Organic Framework: Fundamental, Classification, and Theranostic Applications. Adv. Opt. Mater. 2023, 11, 2202122. [Google Scholar] [CrossRef]
- Huang, J.; Yan, L.; Liu, S.; Tao, L.; Zhou, B. Expanding the Toolbox of Photon Upconversion for Emerging Frontier Applications. Mater. Horiz. 2022, 9, 1167–1195. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Ke, J.; Li, X.; Tu, D.; Chen, X. Luminescent Nano-Bioprobes Based on NIR Dye/Lanthanide Nanoparticle Composites. Aggregate 2021, 2, e59. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, L.; Zhang, F. Exploiting Lanthanide-Doped Upconversion Nanoparticles with Core/Shell Structures. Nano Today 2019, 25, 68–84. [Google Scholar] [CrossRef]
- Wen, S.; Zhou, J.; Zheng, K.; Bednarkiewicz, A.; Liu, X.; Jin, D. Advances in Highly Doped Upconversion Nanoparticles. Nat. Commun. 2018, 9, 2415. [Google Scholar] [CrossRef]
- Chen, G.; Ågren, H.; Ohulchanskyy, T.Y.; Prasad, P.N. Light Upconverting Core-Shell Nanostructures: Nanophotonic Control for Emerging Applications. Chem. Soc. Rev. 2015, 44, 1680–1713. [Google Scholar] [CrossRef]
- Alkahtani, M.; Alzahrani, Y.A.; Alromaeh, A.; Hemmer, P. Novel Nanocomposites for Luminescent Thermometry with Two Different Modalities. Molecules 2024, 29, 1350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Peng, R.; Liu, W.; Donovan, M.J.; Wang, L.; Ismail, I.; Li, J.; Li, J.; Qu, F.; Tan, W. Engineering DNA on the Surface of Upconversion Nanoparticles for Bioanalysis and Therapeutics. ACS Nano 2021, 15, 17257–17274. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Ghosh, S.; Thakur, M.K.; Zhou, J.; Ostrikov, K.; Jin, D.; Chattopadhyay, S. Up-Conversion Hybrid Nanomaterials for Light- and Heat-Driven Applications. Prog. Mater. Sci. 2021, 121, 100838. [Google Scholar] [CrossRef]
- Tian, G.; Zhang, X.; Gu, Z.; Zhao, Y. Recent Advances in Upconversion Nanoparticles-Based Multifunctional Nanocomposites for Combined Cancer Therapy. Adv. Mater. 2015, 27, 7692–7712. [Google Scholar] [CrossRef]
- Cheng, Z.; Lin, J. Synthesis and Application of Nanohybrids Based on Upconverting Nanoparticles and Polymers. Macromol. Rapid Commun. 2015, 36, 790–827. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Chen, Y. Recent Progress in Utilizing Upconversion Nanoparticles with Switchable Emission for Programmed Therapy. Adv. Therap. 2021, 5, 2100172. [Google Scholar] [CrossRef]
- Xiao, Q.; Zheng, X.; Bu, W.; Ge, W.; Zhang, S.; Chen, F.; Xing, H.; Ren, Q.; Fan, W.; Zhao, K.; et al. A Core/Satellite Multifunctional Nanotheranostic for in Vivo Imaging and Tumor Eradication by Radiation/Photothermal Synergistic Therapy. J. Am. Chem. Soc. 2013, 135, 13041–13048. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Yang, D.; Yang, P.; Xu, J.; He, F.; Gai, S.; Li, C.; Dai, Y.; Yang, G.; Lin, J. Integration of Upconversion Nanoparticles and Ultrathin Black Phosphorus for Efficient Photodynamic Theranostics under 808 Nm near-Infrared Light Irradiation. Chem. Mater. 2016, 28, 4724–4734. [Google Scholar] [CrossRef]
- Liang, L.; Everest-Dass, A.V.; Kostyuk, A.B.; Khabir, Z.; Zhang, R.; Trushina, D.B.; Zvyagin, A.V. The Surface Charge of Polymer-Coated Upconversion Nanoparticles Determines Protein Corona Properties and Cell Recognition in Serum Solutions. Cells 2022, 11, 3644. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, P.; Li, S.; Zhang, X.; Hu, J.; Yang, M.; Yao, S.; Gao, F.; Xia, A.; Shen, J.; et al. Layer-by-Layer Assembled Pei-Based Vector with the Upconversion Luminescence Marker for Gene Delivery. Biochem. Biophys. Res. Commun. 2018, 503, 2504–2509. [Google Scholar] [CrossRef]
- Guller, A.E.; Nadort, A.; Generalova, A.N.; Khaydukov, E.V.; Nechaev, A.V.; Kornienko, I.A.; Petersen, E.V.; Liang, L.; Shekhter, A.B.; Qian, Y.; et al. Rational Surface Design of Upconversion Nanoparticles with Polyethylenimine Coating for Biomedical Applications: Better Safe Than Brighter? ACS Biomater. Sci. 2018, 4, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.H.; Chen, C.W.; Lee, I.J.; Chan, Y.C.; Tu, D.; Hsiao, M.; Chen, C.H.; Chen, X.; Liu, R.S. Near-Infrared Light-Mediated Photodynamic Therapy Nanoplatform by the Electrostatic Assembly of Upconversion Nanoparticles with Graphitic Carbon Nitride Quantum Dots. Inorg. Chem. 2016, 55, 10267–10277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, Y.; Yang, X.; Pan, Y.; Sun, L.; Zhang, W.; Shao, Y.; Shen, J.; Lin, J.; Li, L.; et al. Upconverted/Downshifted Nalnf4 and Metal-Organic Framework Heterostructures Boosting NIR-II Imaging-Guided Photodynamic Immunotherapy toward Tumors. Nano Today 2022, 43, 101439. [Google Scholar] [CrossRef]
- Shao, Y.; Liu, B.; Di, Z.; Zhang, G.; Sun, L.D.; Li, L.; Yan, C.H. Engineering of Upconverted Metal–Organic Frameworks for near-Infrared Light-Triggered Combinational Photodynamic/Chemo-/Immunotherapy against Hypoxic Tumors. J. Am. Chem. Soc. 2020, 142, 3939–3946. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, B.; Zhao, J.; Di, Z.; Chen, D.; Gu, Z.; Li, L.; Zhao, Y. Nd3+-Sensitized Upconversion Metal-Organic Frameworks for Mitochondria-Targeted Amplified Photodynamic Therapy. Angew. Chem. Int. Ed. 2020, 59, 2634–2638. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Ni, Q.; Mu, J.; Fan, W.; Liu, L.; Wang, Z.; Li, L.; Tang, W.; Liu, Y.; Cheng, Y.; et al. Solvent-Assisted Self-Assembly of a Metal-Organic Framework Based Biocatalyst for Cascade Reaction Driven Photodynamic Therapy. J. Am. Chem. Soc. 2020, 142, 6822–6832. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, L.; Li, S.; Zhang, W.; Lu, M.; Pan, Y.; Xie, X.; Huang, L.; Huang, W. Paving Metal-Organic Frameworks with Upconversion Nanoparticles Via Self-Assembly. J. Am. Chem. Soc. 2018, 140, 15507–15515. [Google Scholar] [CrossRef]
- Medishetty, R.; Zaręba, J.K.; Mayer, D.; Samoć, M.; Fischer, R.A. Nonlinear Optical Properties, Upconversion and Lasing in Metal-Organic Frameworks. Chem. Soc. Rev. 2017, 46, 4976–5004. [Google Scholar] [CrossRef]
- Wang, T.; LaMontagne, D.; Lynch, J.; Zhuang, J.; Cao, Y.C. Colloidal Superparticles from Nanoparticle Assembly. Chem. Soc. Rev. 2013, 42, 2804–2823. [Google Scholar] [CrossRef]
- Zhang, Z.; Jayakumar, M.K.G.; Shikha, S.; Zhang, Y.; Zheng, X.; Zhang, Y. Modularly Assembled Upconversion Nanoparticles for Orthogonally Controlled Cell Imaging and Drug Delivery. ACS Appl. Mater. Interfaces 2020, 12, 12549–12556. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Hu, Z.; Wang, Z.; Li, Y.; Tormo, A.D.; Le Thomas, N.; Wang, B.; Gianneschi, N.C.; Shawkey, M.D.; Dhinojwala, A. Bioinspired Bright Noniridescent Photonic Melanin Supraballs. Sci. Adv. 2017, 3, e1701151. [Google Scholar] [CrossRef]
- Boles, M.A.; Engel, M.; Talapin, D.V. Self-Assembly of Colloidal Nanocrystals: From Intricate Structures to Functional Materials. Chem. Rev. 2016, 116, 11220–11289. [Google Scholar] [CrossRef]
- Lee, N.; Yoo, D.; Ling, D.; Cho, M.H.; Hyeon, T.; Cheon, J. Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev. 2015, 115, 10637–10689. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Yin, Y. Colloidal Nanoparticle Clusters: Functional Materials by Design. Chem. Soc. Rev. 2012, 41, 6874–6887. [Google Scholar] [CrossRef]
- Bai, F.; Wang, D.; Huo, Z.; Chen, W.; Liu, L.; Liang, X.; Chen, C.; Wang, X.; Peng, Q.; Li, Y. A Versatile Bottom-up Assembly Approach to Colloidal Spheres from Nanocrystals. Angew. Chem. Int. Ed. 2007, 46, 6650–6653. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Lei, P.; Zhang, Z.; Chen, Y. Multifunctional Superparticles for Magnetically Targeted NIR-II Imaging and Photodynamic Therapy. Adv. Sci. 2022, 10, 2203669. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, Y.; Lei, P.; Zhang, Z.; Zhang, H. Tumor Microenvironment-Responsive Modular Integrated Nanocomposites for Magnetically Targeted and Photothermal Enhanced Catalytic Therapy. Nano Res. 2023, 16, 9826–9834. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Zhao, J.; Dai, J. One-Step Self-Assembly of ZnPc/NaGdF4:Yb, Er Nanoclusters for Simultaneous Fluorescence Imaging and Photodynamic Effects on Cancer Cells. J. Mater. Chem. B 2013, 1, 4637–4643. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, H.; Li, J.; Wang, Y.; Wang, X. Fabrication of Ph-Responsive PlGA(UCNPs/Dox) Nanocapsules with Upconversion Luminescence for Drug Delivery. Sci. Rep. 2017, 7, 18014. [Google Scholar] [CrossRef]
- Wang, D.; Yang, D.; Huang, C.; Huang, Y.; Yang, D.; Zhang, H.; Liu, Q.; Tang, T.; Gamal El-Din, M.; Kemppi, T.; et al. Stabilization Mechanism and Chemical Demulsification of Water-in-Oil and Oil-in-Water Emulsions in Petroleum Industry: A Review. Fuel 2021, 286, 119390. [Google Scholar] [CrossRef]
- Jiang, H.; Sheng, Y.; Ngai, T. Pickering Emulsions: Versatility of Colloidal Particles and Recent Applications. Curr. Opin. Colloid Interface Sci. 2020, 49, 1–15. [Google Scholar] [CrossRef]
- Iqbal, M.; Zafar, N.; Fessi, H.; Elaissari, A. Double Emulsion Solvent Evaporation Techniques Used for Drug Encapsulation. Int. J. Pharm. 2015, 496, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fan, Q.; Yin, Y. Colloidal Self-Assembly Approaches to Smart Nanostructured Materials. Chem. Rev. 2022, 122, 4976–5067. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Yang, Y.; Liu, B.; He, J.; Nie, Z. Polymer-Guided Assembly of Inorganic Nanoparticles. Chem. Soc. Rev. 2020, 49, 465–508. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, J.; Wang, L.; Zhang, L.; Cai, C. Self-Assembly of Copolymer Micelles: Higher-Level Assembly for Constructing Hierarchical Structure. Chem. Rev. 2020, 120, 4111–4140. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Shi, Y.; Chen, Y. Molecular Bottlebrushes as Emerging Nanocarriers: Material Design and Biomedical Application. Langmuir 2024, 40, 7286–7299. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ke, F.; Li, Y.; Shi, Y.; Zhang, Z.; Chen, Y. Emulsion Confined Block Copolymer Self-Assembly: Recent Progress and Prospect. Nano Res. 2022, 16, 564–582. [Google Scholar] [CrossRef]
- Hou, W.; Zhang, Z.; Shi, Y.; Chen, Y. Co-Assembly of Diblock Copolymers and Molecular Bottlebrushes. Macromolecules 2022, 55, 6364–6371. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Huang, X.; Chen, D. Solution-Based Thermodynamically Controlled Conversion from Diblock Copolymers to Janus Nanoparticles. ACS Macro Lett. 2017, 6, 580–585. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, C.; Dong, H.; Chen, D. Solution-Based Fabrication of Narrow-Disperse Abc Three-Segment and Θ-Shaped Nanoparticles. Angew. Chem. Int. Ed. 2016, 55, 6182–6186. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, Y.; Chen, Y. Hairy Nanoparticles Via Self-Assembled Linear Block Copolymers. In Hairy Nanoparticles: From Synthesis to Applications; Wiley: Hoboken, NJ, USA, 2023; pp. 49–72. [Google Scholar] [CrossRef]
- Zheng, Y.; Oz, Y.; Gu, Y.; Ahamad, N.; Shariati, K.; Chevalier, J.; Kapur, D.; Annabi, N. Rational design of Polymeric Micelles for Targeted Therapeutic Delivery. Nano Today 2024, 55, 102147. [Google Scholar] [CrossRef]
- Ma, C.; Li, G.; Xu, W.; Qu, H.; Zhang, H.; Bahojb Noruzi, E.; Li, H. Recent Advances in Stimulus-Responsive Nanocarriers for Pesticide Delivery. J. Agric. Food Chem. 2024, 72, 8906–8927. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Sun, Y.; Zhu, H.; Fu, Q. Stimuli-Responsive Polymer-Based Nanosystems for Cancer Theranostics. ACS Nano 2023, 17, 23223–23261. [Google Scholar] [CrossRef]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef]
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-Responsive Polymeric Nanogels as Smart Drug Delivery Systems. Acta Biomater. 2019, 92, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-Responsive Bio-Based Polymeric Systems and Their Applications. J. Mater. Chem. B 2019, 7, 709–729. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, J.; Tian, D.; Li, Y.; Jiang, H.; Zhu, J. Polymer-Upconverting Nanoparticle Hybrid Micelles for Enhanced Synergistic Chemo-Photodynamic Therapy: Effects of Emission-Absorption Spectral Match. Biomacromolecules 2019, 20, 4044–4052. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, T.; Liu, P.; Ren, J.; Li, Y.; Jiang, H.; Zhang, L.; Zhu, J. NIR-Light-Activated Ratiometric Fluorescent Hybrid Micelles for High Spatiotemporally Controlled Biological Imaging and Chemotherapy. Small 2020, 16, 2005667. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, Q.; Qin, S.; Fu, Y.; Liu, R.; Zhi, J.; Shan, C. Nanodiamonds Conjugated Upconversion Nanoparticles for Bio-Imaging and Drug Delivery. J. Colloid Interface Sci. 2019, 537, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lv, R.; He, F.; Qu, F.; Gai, S.; Du, S.; Wei, Z.; Yang, P. A Core/Shell/Satellite Anticancer Platform for 808 NIR Light-Driven Multimodal Imaging and Combined Chemo-/Photothermal Therapy. Nanoscale 2015, 7, 13747–13758. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yang, G.; Wang, X.; Lv, R.; Gai, S.; He, F.; Gulzar, A.; Yang, P. Y2O3:Yb, Er@mSiO2-CuxS Double-Shelled Hollow Spheres for Enhanced Chemo-/Photothermal Anti-Cancer Therapy and Dual-Modal Imaging. Nanoscale 2015, 7, 12180–12191. [Google Scholar] [CrossRef]
- Wang, Y.; Song, S.; Liu, J.; Liu, D.; Zhang, H. Zno-Functionalized Upconverting Nanotheranostic Agent: Multi-Modality Imaging-Guided Chemotherapy with on-Demand Drug Release Triggered by Ph. Angew. Chem. Int. Ed. 2015, 54, 536–540. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S. Cross-Linking Agents in Three-Component Materials Dedicated to Biomedical Applications: A Review. Polymers 2024, 16, 2679. [Google Scholar] [CrossRef]
- Adamiak, K.; Sionkowska, A. Current Methods of Collagen Cross-Linking: Review. Int. J. Biol. Macromol. 2020, 161, 550–560. [Google Scholar] [CrossRef]
- Paeth, M.; Stapleton, J.; Dougherty, M.L.; Fischesser, H.; Shepherd, J.; McCauley, M.; Falatach, R.; Page, R.C.; Berberich, J.A.; Konkolewicz, D. Chapter Nine—Approaches for Conjugating Tailor-Made Polymers to Proteins. In Methods in Enzymology; Kumar, C.V., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 590, pp. 193–224. [Google Scholar]
- Zhu, Y.; Zhao, J.; Li, X.; Xu, X.; Huang, J.; Ji, X.; Yang, G.; Pan, G. Stable and Efficient Upconversion Single Red Emission from Cspbi3 Perovskite Quantum Dots Triggered by Upconversion Nanoparticles. Inorg. Chem. 2021, 60, 2649–2655. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; D’Amario, C.; Gee, A.; Duong, H.T.T.; Shimoni, O.; Valenzuela, S.M. Dispersion Stability and Biocompatibility of Four Ligand-Exchanged NaYF4: Yb, Er Upconversion Nanoparticles. Acta Biomater. 2020, 102, 384–393. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Ye, S.; Zhang, W.; Li, S.; Nie, Z.; Xu, X.; Li, W.; Abdukayum, A.; Chen, W.T.; Hu, G. Synthesis of Magnetic Mesoporous Silica Adsorbents by Thiol-Ene Click Chemistry with Optimised Lewis Base Properties through Molecular Imprinting for the Rapid and Effective Capture of Pb(II). Chem. Eng. J. 2024, 489, 151294. [Google Scholar] [CrossRef]
- Taiariol, L.; Chaix, C.; Farre, C.; Moreau, E. Click and Bioorthogonal Chemistry: The Future of Active Targeting of Nanoparticles for Nanomedicines? Chem. Rev. 2022, 122, 340–384. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, H.; Ceper, E.B.; Li, X.; Yang, J.; Dong, W.; Ozmen, M.M.; Theato, P. Sulfur Chemistry in Polymer and Materials Science. Macromol. Rapid Commun. 2019, 40, 1800650. [Google Scholar] [CrossRef]
- Wang, M.; Hou, W.; Mi, C.; Wang, W.; Xu, Z.; Teng, H.; Mao, C.; Xu, S. Immunoassay of Goat Antihuman Immunoglobulin G Antibody Based on Luminescence Resonance Energy Transfer between near-Infrared Responsive NaYF4:Yb, Er Upconversion Fluorescent Nanoparticles and Gold Nanoparticles. Anal. Chem. 2009, 81, 8783–8789. [Google Scholar] [CrossRef]
- Wang, L.; Yan, R.; Huo, Z.; Wang, L.; Zeng, J.; Bao, J.; Wang, X.; Peng, Q.; Li, Y. Fluorescence Resonant Energy Transfer Biosensor Based on Upconversion-Luminescent Nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 6054–6057. [Google Scholar] [CrossRef]
- Li, Q.F.; Ren, S.Y.; Wang, Y.; Bai, J.L.; Peng, Y.; Ning, B.A.; Lyu, Q.J.; Gao, Z.X. Efficient Detection of Environmental Estrogens Bisphenol a and Estradiol by Sensing System Based on AuNP-AuNP-UCNP Triple Structure. Chin. J. Anal. Chem. 2018, 46, 486–492. [Google Scholar] [CrossRef]
- Kong, J.; Zhu, J.; Chen, K.; Keyser, U.F. Specific Biosensing Using DNA Aptamers and Nanopores. Adv. Funct. Mater. 2019, 29, 1807555. [Google Scholar] [CrossRef]
- Li, L.; Wu, P.; Hwang, K.; Lu, Y. An Exceptionally Simple Strategy for DNA-Functionalized up-Conversion Nanoparticles as Biocompatible Agents for Nanoassembly, DNA Delivery, and Imaging. J. Am. Chem. Soc. 2013, 135, 2411–2414. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Wang, D.; Pan, Y.; Guo, Y.; Li, H.; Zhang, F.; Zhu, X.; Li, Y.; Zhang, C.; Huang, L. Sequence-Dependent DNA Functionalization of Upconversion Nanoparticles and Their Programmable Assemblies. Angew. Chem. Int. Ed. 2020, 59, 8133–8137. [Google Scholar] [CrossRef]
- Li, S.; Xu, L.; Sun, M.; Wu, X.; Liu, L.; Kuang, H.; Xu, C. Hybrid Nanoparticle Pyramids for Intracellular Dual Micrornas Biosensing and Bioimaging. Adv. Mater. 2017, 29, 1606086. [Google Scholar] [CrossRef]
- Ding, L.; Liu, B.; Peil, A.; Fan, S.; Chao, J.; Liu, N. DNA-Directed Assembly of Photonic Nanomaterials for Diagnostic and Therapeutic Applications. Adv. Mater. 2025, 2500086. [Google Scholar] [CrossRef]
- Zhao, Z.; Han, Y.; Liu, Y. DNA Origami Enabled Assembly of Nanophotonic Structures and Their Applications. Opt. Mater. Express 2022, 12, 284–307. [Google Scholar] [CrossRef]
- Dey, S.; Fan, C.; Gothelf, K.V.; Li, J.; Lin, C.; Liu, L.; Liu, N.; Nijenhuis, M.A.D.; Saccà, B.; Simmel, F.C.; et al. DNA Origami. Nat. Rev. Methods Primers 2021, 1, 13. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhang, T.; Kang, Y.F.; Qi, C.B.; Zheng, B.; Xu, C.M.; Lin, Y.; Pang, D.W.; Tang, H.W. Incorporating Luminescence-Concentrating Upconversion Nanoparticles and DNA Walkers into Optical Tweezers Assisted Imaging: A Highly Stable and Ultrasensitive Bead Supported Assay. Chem. Commun. 2020, 56, 6997–7000. [Google Scholar] [CrossRef]
- Lin, Z.; Ji, X.; Liu, J.; Liu, Y.; Zhang, L.; Ji, J.; Xiao, X.; Guo, J.; Ke, F.; Zhang, K.; et al. Upconversion-Based Photodynamic Therapy for Psoriatic Dermatitis. ACS Appl. Bio Mater. 2025, 8, 4084–4092. [Google Scholar] [CrossRef]
- Ke, F.; Liu, S.; Ji, X.; Chen, N.; Zhang, K.; Huang, W.; Liu, Y.; Chen, Y.; Lei, P.; Zhang, Z.; et al. Glutathione-Depleting and Reactive Oxygen Species-Amplifying Upconversion Nanoplatform for Synergistic Ferroptosis. ACS Appl. Nano Mater. 2025, 8, 12505–12513. [Google Scholar] [CrossRef]
- Hlaváček, A.; Farka, Z.; Mickert, M.J.; Kostiv, U.; Brandmeier, J.C.; Horák, D.; Skládal, P.; Foret, F.; Gorris, H.H. Bioconjugates of Photon-Upconversion Nanoparticles for Cancer Biomarker Detection and Imaging. Nat. Protoc. 2022, 17, 1028–1072. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, F. Combating Concentration Quenching in Upconversion Nanoparticles. Acc. Chem. Res. 2020, 53, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gulzar, A.; Yang, P.; Bi, H.; Yang, D.; Gai, S.; He, F.; Lin, J.; Xing, B.; Jin, D. Recent Advances in near-Infrared Emitting Lanthanide-Doped Nanoconstructs: Mechanism, Design and Application for Bioimaging. Coord. Chem. Rev. 2019, 381, 104–134. [Google Scholar] [CrossRef]
- Yao, J.; Yu, Z.; Gao, Y.; Wang, B.; Wang, Z.; Zhong, T.; Pan, B.; Li, H.; Hui, H.; Zheng, W.; et al. Deep-Penetrating and High-Resolution Continuous-Wave Nonlinear Microscopy Based on Homologous Dual-Emission Upconversion Adaptive Optics. Nano Lett. 2025, 25, 5485–5492. [Google Scholar] [CrossRef]
- Rajchel-Mieldzioć, P.; Bednarkiewicz, A.; Prorok, K.; Fita, P. Strong Emission Enhancement Via Dual-Wavelength Coexcitation in Ybtm-Doped Upconverting Nanoparticles for near-Infrared and Subdiffraction Imaging. ACS Nano 2025, 19, 26932–26941. [Google Scholar] [CrossRef]
- Lu, F.; Wang, X.; Ge, Y.; Sun, X.; Zhao, T.; Lu, X.; Fan, Q. Nd3+-Sensitized Multilayered Rare-Earth Nanocrystals with Enhanced NIR-IIb Luminescence for High Resolution Optical Imaging. Ceram. Int. 2024, 50, 25060–25067. [Google Scholar] [CrossRef]
- Akabe, Y.; Shinohara, K.; Cadatal-Raduban, M.; Yoshikawa, A.; Shimizu, T.; Kato, K.; Agulto, V.C.; Nakajima, M.; Sarukura, N.; Damdee, B.; et al. Near-Infrared Imaging Via Upconversion in Er3+:CaF2 Crystal with Dual Wavelength Excitation. Opt. Lett. 2024, 49, 3998–4001. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, Y.; Liu, M.; Pan, S.; Liu, Z.; Xu, D.; Lin, H. High-Intensity First near-Infrared Emission through Energy Migration in Multilayered Upconversion Nanoparticles. Phys. Chem. Chem. Phys. 2023, 25, 19923–19931. [Google Scholar] [CrossRef]
- Malhotra, K.; Hrovat, D.; Kumar, B.; Qu, G.; Houten, J.V.; Ahmed, R.; Piunno, P.A.E.; Gunning, P.T.; Krull, U.J. Lanthanide-Doped Upconversion Nanoparticles: Exploring a Treasure Trove of NIR-Mediated Emerging Applications. ACS Appl. Mater. Interfaces 2023, 15, 2499–2528. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Liu, J.; Wang, K.; Zhang, Y. Efficient Upconversion and Downshifting Luminescence of CaIn2O4: Yb3+/Tm3+/Re3+ (Re = Er/Ho) Phosphor: Temperature Sensing Performance in the Visible and near-Infrared Range. Ceram. Int. 2023, 49, 30510–30521. [Google Scholar] [CrossRef]
- Bi, S.; Deng, Z.; Huang, J.; Wen, X.; Zeng, S. NIR-II Responsive Upconversion Nanoprobe with Simultaneously Enhanced Single-Band Red Luminescence and Phase/Size Control for Bioimaging and Photodynamic Therapy. Adv. Mater. 2023, 35, 2207038. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Di, Z.; Zhao, Y.; Zhang, A.; Li, L. DNA-Mediated Coordinative Assembly of Upconversion Hetero-Nanostructures for Targeted Dual-Modality Imaging of Cancer Cells. Chin. Chem. Lett. 2019, 30, 899–902. [Google Scholar] [CrossRef]
- Li, L.; Lu, Y. Regiospecific Hetero-Assembly of DNA-Functionalized Plasmonic Upconversion Superstructures. J. Am. Chem. Soc. 2015, 137, 5272–5275. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Kaur, N.; Swami, K.; Abhijith, T.S.; Moun, N.; Kumar, P.; Khatri, M.; Shanmugam, V. Upconversion Enabled Innovation: Transfer of Lab Sensor to Smartphone Based Field Device. Food Res. Int. 2025, 213, 116547. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Jin, D. Responsive Sensors of Upconversion Nanoparticles. ACS Sens. 2021, 6, 4272–4282. [Google Scholar] [CrossRef] [PubMed]
- Mahata, M.K.; Bae, H.; Lee, K.T. Upconversion Luminescence Sensitized pH-Nanoprobes. Molecules 2017, 22, 2064. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y. Green Upconversion Nanocrystals for DNA Detection. Chem. Commun. 2006, 2557–2559. [Google Scholar] [CrossRef]
- Esmaeili, S.; Rajil, N.; Hazrathosseini, A.; Neuman, B.W.; Alkahtani, M.H.; Sen, D.; Hu, Q.; Wu, H.J.; Yi, Z.; Brick, R.W.; et al. Quantum-Enhanced Detection of Viral cDNA Via Luminescence Resonance Energy Transfer Using Upconversion and Gold Nanoparticles. Nanophotonics 2025. [Google Scholar] [CrossRef]
- Zhu, K.; Liu, G.; Hu, J.; Liu, S. Near-Infrared Light-Activated Photochemical Internalization of Reduction-Responsive Polyprodrug Vesicles for Synergistic Photodynamic Therapy and Chemotherapy. Biomacromolecules 2017, 18, 2571–2582. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y. Orthogonal Emissive Upconversion Nanoparticles: Material Design and Applications. Small 2021, 17, 2004552. [Google Scholar] [CrossRef]
- Lei, Z.; Ling, X.; Mei, Q.; Fu, S.; Zhang, J.; Zhang, Y. An Excitation Navigating Energy Migration of Lanthanide Ions in Upconversion Nanoparticles. Adv. Mater. 2020, 32, 1906225. [Google Scholar] [CrossRef]
- Mei, Q.; Bansal, A.; Jayakumar, M.K.G.; Zhang, Z.; Zhang, J.; Huang, H.; Yu, D.; Ramachandra, C.J.A.; Hausenloy, D.J.; Soong, T.W.; et al. Manipulating Energy Migration within Single Lanthanide Activator for Switchable Upconversion Emissions Towards Bidirectional Photoactivation. Nat. Commun. 2019, 10, 4416. [Google Scholar] [CrossRef]
- Zhou, B.; Tao, L.; Chai, Y.; Lau, S.P.; Zhang, Q.; Tsang, Y.H. Constructing Interfacial Energy Transfer for Photon up- and Down-Conversion from Lanthanides in a Core-Shell Nanostructure. Angew. Chem. Int. Ed. 2016, 55, 12356–12360. [Google Scholar] [CrossRef]
- Lai, J.; Zhang, Y.; Pasquale, N.; Lee, K.B. An Upconversion Nanoparticle with Orthogonal Emissions Using Dual Nir Excitations for Controlled Two-Way Photoswitching. Angew. Chem. Int. Ed. 2014, 53, 14419–14423. [Google Scholar] [CrossRef]
- Zhang, Z.; Jayakumar, M.K.G.; Zheng, X.; Shikha, S.; Zhang, Y.; Bansal, A.; Poon, D.J.J.; Chu, P.L.; Yeo, E.L.L.; Chua, M.L.K.; et al. Upconversion Superballs for Programmable Photoactivation of Therapeutics. Nat. Commun. 2019, 10, 4586. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, R.; Zhaocheng, L.; Dayu, Z.; Andrew, K.; Ekaterina, P.; Augustine, U.; Wenshan, C. Photonic Upconversion Maximization for Nonlinear Meta-Material Enabled by Deep Learning. In Proceedings of the Nonlinear Frequency Generation and Conversion: Materials and Devices XXII, San Francisco, CA, USA, 28 January–3 February 2023; p. 124050F. [Google Scholar]
- Wang, Q.; Makarenko, M.; Burguete Lopez, A.; Getman, F.; Fratalocchi, A. Advancing Statistical Learning and Artificial Intelligence in Nanophotonics Inverse Design. Nanophotonics 2022, 11, 2483–2505. [Google Scholar] [CrossRef] [PubMed]
- So, S.; Badloe, T.; Noh, J.; Bravo-Abad, J.; Rho, J. Deep Learning Enabled Inverse Design in Nanophotonics. Nanophotonics 2020, 9, 1041–1057. [Google Scholar] [CrossRef]
- Barth, C.; Becker, C. Machine Learning Classification for Field Distributions of Photonic Modes. Commun. Phys. 2018, 1, 58. [Google Scholar] [CrossRef]


| Assembly Route | Operating Conditions | Inter-Particle Spacing/Structure | Optical Outcomes | Colloidal Stability | Biocompatibility |
|---|---|---|---|---|---|
| Electrostatic interactions | Highly dependent on pH, ionic strength, and surface charge | Tunable; typically a few nm; often flexible | Moderate quantum yield (QY); possible FRET enhancement due to close spacing | Sensitive to salt concentration; limited in physiological media | Moderate; surface charge may induce cytotoxicity |
| Hydrophobic interactions | Requires nonpolar solvents, amphiphiles, or surfactants | Compact, dense packing; anisotropic possible | Can enhance energy transfer via close packing; moderate photoluminescence (PL) control | Poor in aqueous media without further modification | Limited unless further coated; not inherently biocompatible |
| Covalent coupling | Requires functional ligands with reactive groups | Strong, permanent linkages; rigid structures | Stable PL efficiency; minimal quenching; better reproducibility | High stability due to irreversible bonding | Tunable with linkers; it depends on the chemistry used |
| Biorecognition-mediated assembly | Mild aqueous conditions; relies on biomolecular interactions | Highly specific, programmable, nm-scale precision | High control over FRET; precise PL modulation; multifunctional outputs | High if biomolecular linkers stabilized | High; especially with DNA, peptides, antibodies |
| Aspect | Epitaxial Growth | Self-Assembly |
|---|---|---|
| Interface | Atomically coherent, lattice-matched epitaxial interfaces; defect suppression | Noncovalent/covalent linkages; non-crystallographic, often flexible or reversible |
| Structure control | High precision at the atomic level; uniform shells and heterostructures | Versatile architectures with tunable interparticle spacing |
| Quantum yield | Typically high in optimized core@shell systems | Moderate, but enables enhanced functionalities (e.g., FRET, multi-modal imaging, catalysis) |
| Photostability | Excellent; strong suppression of surface quenching | Variable; depends on linker stability and environmental conditions |
| Scalability | Limited; requires high temperature, precise conditions, and complex synthesis | More scalable; solution-based, mild conditions, but reproducibility may vary with the environment |
| Biocompatibility | Requires surface modification (e.g., PEGylation) for aqueous/biological applications | Readily tunable via biomolecules, polymers, or responsive ligands |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Ji, X.; Huang, W.; Mai, Q.; Cheng, D. Self-Assembly Strategies in Upconversion Nanoparticle-Based Nanocomposites: Structure Designs and Applications. Int. J. Mol. Sci. 2025, 26, 8671. https://doi.org/10.3390/ijms26178671
Zhang Z, Ji X, Huang W, Mai Q, Cheng D. Self-Assembly Strategies in Upconversion Nanoparticle-Based Nanocomposites: Structure Designs and Applications. International Journal of Molecular Sciences. 2025; 26(17):8671. https://doi.org/10.3390/ijms26178671
Chicago/Turabian StyleZhang, Zhen, Xiaoyu Ji, Weijia Huang, Qizhen Mai, and Du Cheng. 2025. "Self-Assembly Strategies in Upconversion Nanoparticle-Based Nanocomposites: Structure Designs and Applications" International Journal of Molecular Sciences 26, no. 17: 8671. https://doi.org/10.3390/ijms26178671
APA StyleZhang, Z., Ji, X., Huang, W., Mai, Q., & Cheng, D. (2025). Self-Assembly Strategies in Upconversion Nanoparticle-Based Nanocomposites: Structure Designs and Applications. International Journal of Molecular Sciences, 26(17), 8671. https://doi.org/10.3390/ijms26178671






