Baricitinib and Infliximab Mitigate the Endothelial-to-Mesenchymal Transition (EndMT) Induced by Cytokines in HUVECs
Abstract
1. Introduction
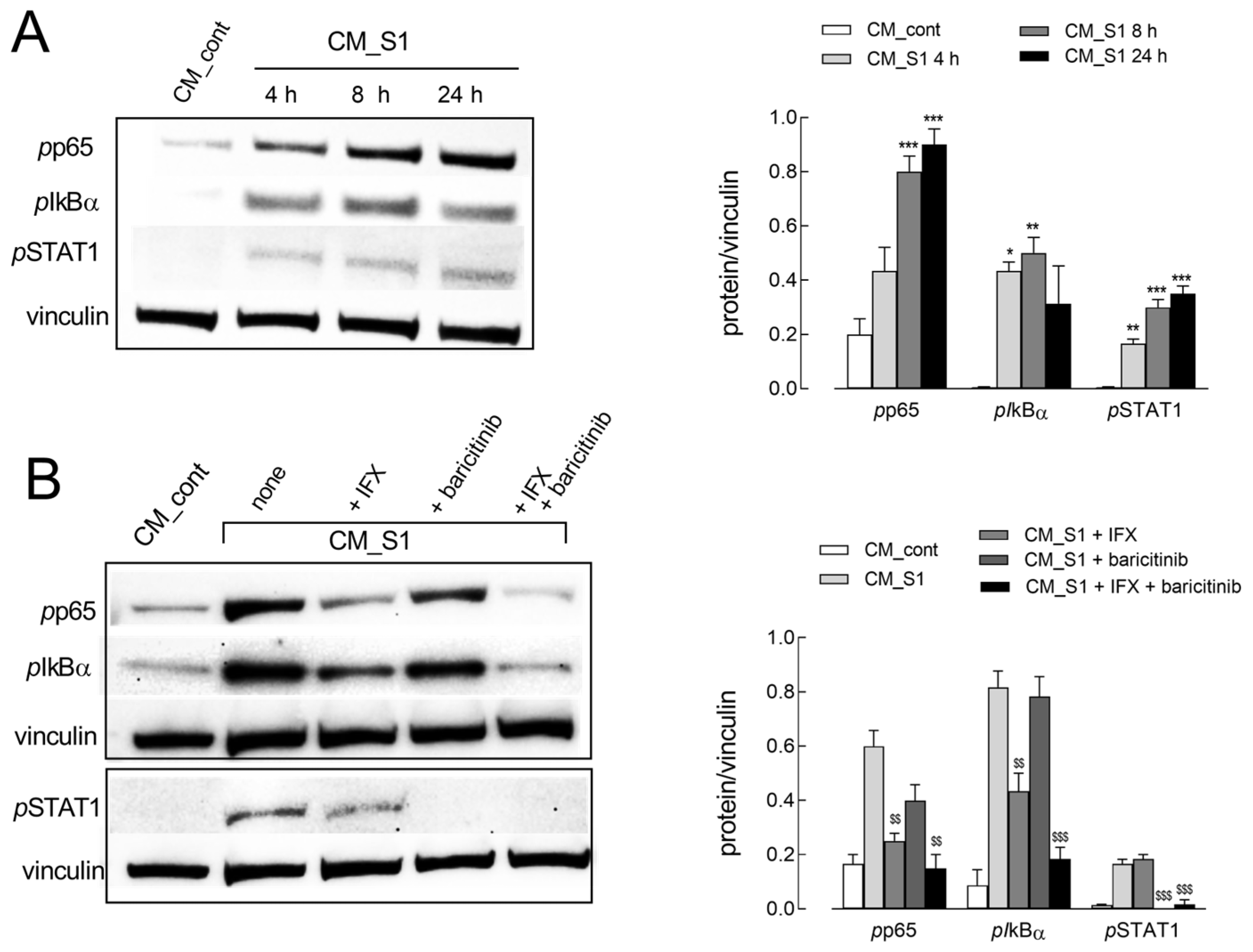
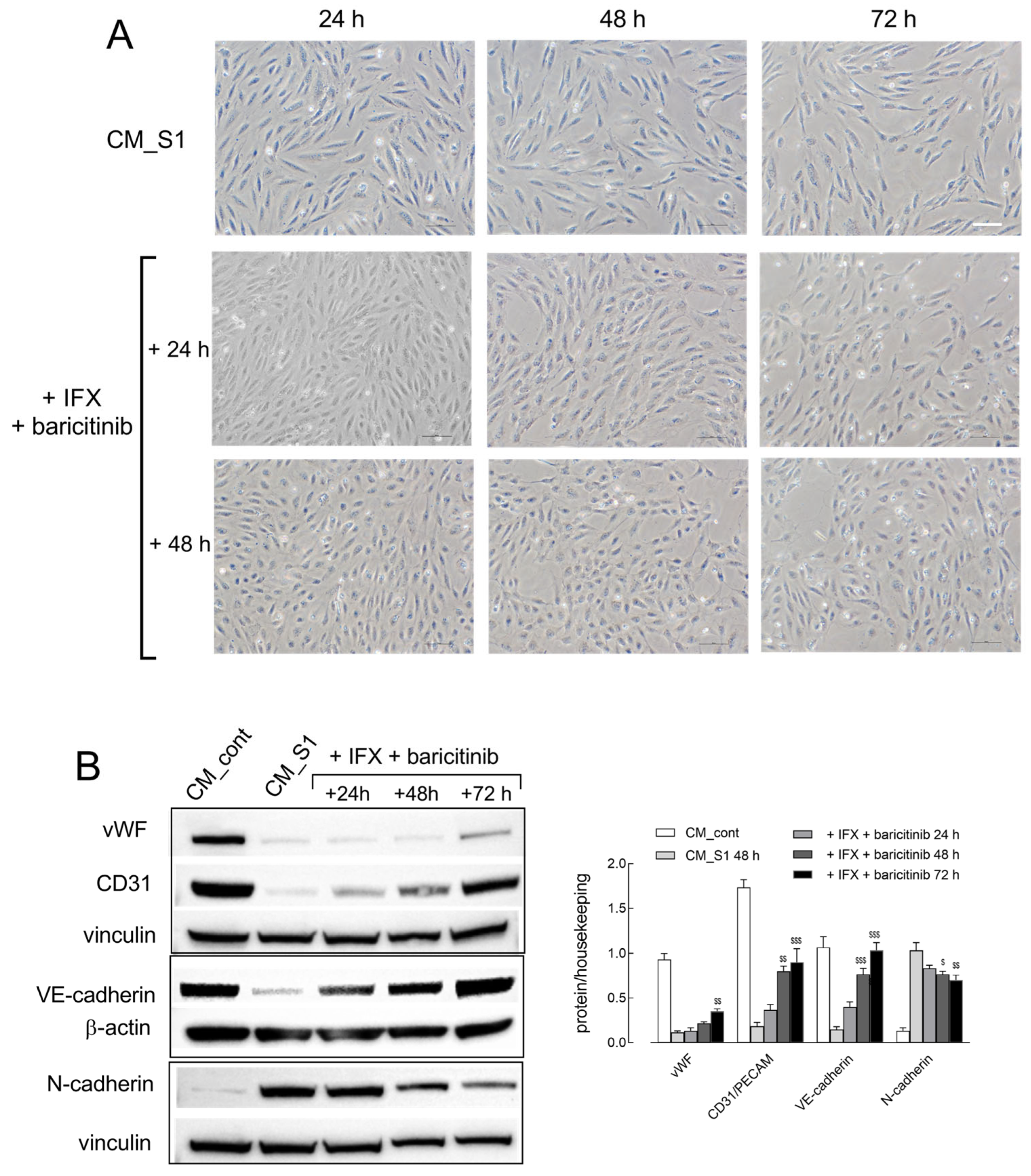
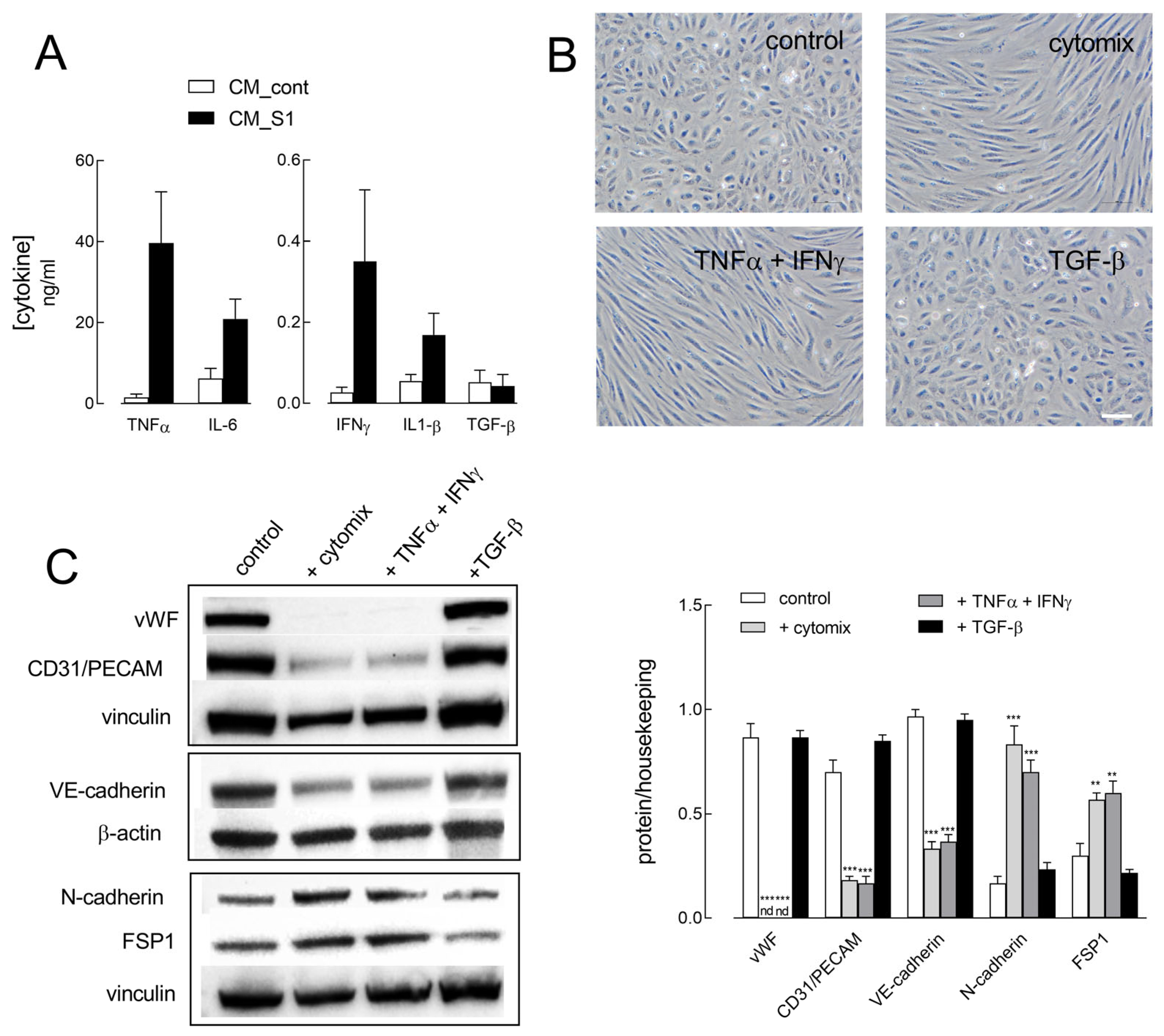
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Experimental Treatments
4.2. RT-qPCR Analysis
4.3. Western Blot Analysis
4.4. Cytokine Analysis
4.5. Statistical Analysis
4.6. Materials
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makhluf, H.; Madany, H.; Kim, K. Long covid: Long-term impact of SARS-CoV-2. Diagnostics 2024, 14, 711. [Google Scholar]
- Karuturi, S. Long COVID-19: A systematic review. J. Assoc. Physicians India 2023, 71, 82–94. [Google Scholar] [PubMed]
- Desai, A.D.; Lavelle, M.; Boursiquot, B.C.; Wan, E.Y. Long-term complications of COVID-19. Am. J. Physiol. Cell Physiol. 2022, 322, C1–C11. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long covid: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146, Correction in Nat. Rev. Microbiol. 2023, 21, 408. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.D.; da Silva, T.S.; Mendes, C.G.; Valbao, M.C.M.; Badu-Tawiah, A.K.; Laurindo, L.F.; Barbalho, S.M.; Direito, R.; Miglino, M.A. Advances in understanding long covid: Pathophysiological mechanisms and the role of omics technologies in biomarker identification. Mol. Diagn. Ther. 2025. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.J.; Liu, S.H.; Manachevakul, S.; Lee, T.A.; Kuo, C.T.; Bello, D. Biomarkers in long COVID-19: A systematic review. Front. Med. 2023, 10, 1085988. [Google Scholar] [CrossRef]
- Espin, E.; Yang, C.; Shannon, C.P.; Assadian, S.; He, D.; Tebbutt, S.J. Cellular and molecular biomarkers of long covid: A scoping review. EBioMedicine 2023, 91, 104552. [Google Scholar] [CrossRef]
- Hawley, H.B. Long covid: Clinical findings, pathology, and endothelial molecular mechanisms. Am. J. Med. 2025, 138, 91–97. [Google Scholar] [CrossRef]
- Charfeddine, S.; Ibn Hadj Amor, H.; Jdidi, J.; Torjmen, S.; Kraiem, S.; Hammami, R.; Bahloul, A.; Kallel, N.; Moussa, N.; Touil, I.; et al. Long COVID-19 syndrome: Is it related to microcirculation and endothelial dysfunction? Insights from tun-endcov study. Front. Cardiovasc. Med. 2021, 8, 745758. [Google Scholar]
- Xu, Y.; Kovacic, J.C. Endothelial to mesenchymal transition in health and disease. Annu. Rev. Physiol. 2023, 85, 245–267. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Y.; Soaita, I.; Lee, H.W.; Bae, H.; Boutagy, N.; Bostwick, A.; Zhang, R.M.; Bowman, C.; Xu, Y.; et al. Acetate controls endothelial-to-mesenchymal transition. Cell Metab. 2023, 35, 1163–1178.e10. [Google Scholar] [CrossRef]
- Ma, J.; Sanchez-Duffhues, G.; Goumans, M.J.; Ten Dijke, P. Tgf-beta-induced endothelial to mesenchymal transition in disease and tissue engineering. Front. Cell Dev. Biol. 2020, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, A.; Heldin, C.H. Mechanisms of tgfbeta-induced epithelial-mesenchymal transition. J. Clin. Med. 2016, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Qin, L.; Li, G.; Wang, Z.; Dahlman, J.E.; Malagon-Lopez, J.; Gujja, S.; Cilfone, N.A.; Kauffman, K.J.; Sun, L.; et al. Endothelial tgf-beta signalling drives vascular inflammation and atherosclerosis. Nat. Metab. 2019, 1, 912–926. [Google Scholar] [CrossRef] [PubMed]
- Piera-Velazquez, S.; Jimenez, S.A. Endothelial to mesenchymal transition: Role in physiology and in the pathogenesis of human diseases. Physiol. Rev. 2019, 99, 1281–1324. [Google Scholar] [CrossRef]
- Cho, J.G.; Lee, A.; Chang, W.; Lee, M.S.; Kim, J. Endothelial to mesenchymal transition represents a key link in the interaction between inflammation and endothelial dysfunction. Front. Immunol. 2018, 9, 294. [Google Scholar] [CrossRef]
- Good, R.B.; Gilbane, A.J.; Trinder, S.L.; Denton, C.P.; Coghlan, G.; Abraham, D.J.; Holmes, A.M. Endothelial to mesenchymal transition contributes to endothelial dysfunction in pulmonary arterial hypertension. Am. J. Pathol. 2015, 185, 1850–1858. [Google Scholar] [CrossRef]
- Perez, L.; Munoz-Durango, N.; Riedel, C.A.; Echeverria, C.; Kalergis, A.M.; Cabello-Verrugio, C.; Simon, F. Endothelial-to-mesenchymal transition: Cytokine-mediated pathways that determine endothelial fibrosis under inflammatory conditions. Cytokine Growth Factor Rev. 2017, 33, 41–54. [Google Scholar] [CrossRef]
- Baldassarro, V.A.; Alastra, G.; Cescatti, M.; Quadalti, C.; Lorenzini, L.; Giardino, L.; Calza, L. SARS-CoV-2-related peptides induce endothelial-to-mesenchymal transition in endothelial capillary cells derived from different body districts: Focus on membrane (m) protein. Cell Tissue Res. 2024, 397, 241–262. [Google Scholar] [CrossRef]
- Recchia Luciani, G.; Visigalli, R.; Dall’Asta, V.; Rotoli, B.M.; Barilli, A. Cytokines from macrophages activated by spike s1 of SARS-CoV-2 cause enos/arginase imbalance in endothelial cells. Int. J. Mol. Sci. 2025, 26, 5916. [Google Scholar] [CrossRef]
- Amini-Farsani, Z.; Yadollahi-Farsani, M.; Arab, S.; Forouzanfar, F.; Yadollahi, M.; Asgharzade, S. Prediction and analysis of micrornas involved in COVID-19 inflammatory processes associated with the nf-kb and jak/stat signaling pathways. Int. Immunopharmacol. 2021, 100, 108071. [Google Scholar] [CrossRef]
- Recchia Luciani, G.; Barilli, A.; Visigalli, R.; Dall’Asta, V.; Rotoli, B.M. Cytokines from SARS-CoV-2 spike-activated macrophages hinder proliferation and cause cell dysfunction in endothelial cells. Biomolecules 2024, 14, 927. [Google Scholar] [CrossRef]
- Mori, M.; Sakamoto, A.; Kawakami, R.; Guo, L.; Slenders, L.; Mosquera, J.V.; Ghosh, S.K.B.; Wesseling, M.; Shiraki, T.; Bellissard, A.; et al. Cd163(+) macrophages induce endothelial-to-mesenchymal transition in atheroma. Circ. Res. 2024, 135, e4–e23. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Herranz, L.; Sahun-Espanol, A.; Paredes, A.; Gonzalo, P.; Gkontra, P.; Nunez, V.; Clemente, C.; Cedenilla, M.; Villalba-Orero, M.; Inserte, J.; et al. Macrophages promote endothelial-to-mesenchymal transition via mt1-mmp/tgfbeta1 after myocardial infarction. Elife 2020, 9, e57920. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Luo, N.S.; Ying, R.; Xie, Y.; Chen, J.Y.; Wang, X.Q.; Gu, Z.J.; Mai, J.T.; Liu, W.H.; Wu, M.X.; et al. Macrophage-derived foam cells impair endothelial barrier function by inducing endothelial-mesenchymal transition via ccl-4. Int. J. Mol. Med. 2017, 40, 558–568. [Google Scholar] [CrossRef]
- Hall, I.F.; Kishta, F.; Xu, Y.; Baker, A.H.; Kovacic, J.C. Endothelial to mesenchymal transition: At the axis of cardiovascular health and disease. Cardiovasc. Res. 2024, 120, 223–236. [Google Scholar] [CrossRef]
- Mobus, L.; Yla-Outinen, L.; Mannino, L.; Migliaccio, G.; Kosunen, K.; D’Alessandro, N.; Serra, A.; Greco, D. Endothelial sensitivity to pro-fibrotic signals links systemic exposure to pulmonary fibrosis. Cell Death Dis. 2025, 16, 500. [Google Scholar] [CrossRef] [PubMed]
- Goltseva, Y.D.; Dergilev, K.V.; Boldyreva, M.A.; Parfyonova, E.V.; Beloglazova, I.B. Tgfbeta1 regulates cellular composition of in vitro cardiac perivascular niche based on cardiospheres. Bull. Exp. Biol. Med. 2024, 177, 115–123. [Google Scholar] [CrossRef]
- Poggioli, G.; Laureti, S.; Campieri, M.; Pierangeli, F.; Gionchetti, P.; Ugolini, F.; Gentilini, L.; Bazzi, P.; Rizzello, F.; Coscia, M. Infliximab in the treatment of crohn’s disease. Ther. Clin. Risk Manag. 2007, 3, 301–308. [Google Scholar] [CrossRef]
- Caraba, A.; Stancu, O.; Crisan, V.; Georgescu, D. Anti tnf-alpha treatment improves microvascular endothelial dysfunction in rheumatoid arthritis patients. Int. J. Mol. Sci. 2024, 25, 9925. [Google Scholar] [CrossRef]
- Booth, A.D.; Jayne, D.R.; Kharbanda, R.K.; McEniery, C.M.; Mackenzie, I.S.; Brown, J.; Wilkinson, I.B. Infliximab improves endothelial dysfunction in systemic vasculitis: A model of vascular inflammation. Circulation 2004, 109, 1718–1723. [Google Scholar] [CrossRef]
- Son, M.B.; Gauvreau, K.; Ma, L.; Baker, A.L.; Sundel, R.P.; Fulton, D.R.; Newburger, J.W. Treatment of kawasaki disease: Analysis of 27 us pediatric hospitals from 2001 to 2006. Pediatrics 2009, 124, 1–8. [Google Scholar] [CrossRef]
- Shimizu, C.; Kim, J.; He, M.; Tremoulet, A.H.; Hoffman, H.M.; Shyy, J.Y.; Burns, J.C. Rna sequencing reveals beneficial effects of atorvastatin on endothelial cells in acute kawasaki disease. J. Am. Heart Assoc. 2022, 11, e025408. [Google Scholar] [CrossRef]
- Almoallim, H.M.; Omair, M.A.; Ahmed, S.A.; Vidyasagar, K.; Sawaf, B.; Yassin, M.A. Comparative efficacy and safety of jak inhibitors in the management of rheumatoid arthritis: A network meta-analysis. Pharmaceuticals 2025, 18, 178. [Google Scholar] [CrossRef]
- Hou, Z.; Su, X.; Han, G.; Xue, R.; Chen, Y.; Chen, Y.; Wang, H.; Yang, B.; Liang, Y.; Ji, S. Jak1/2 inhibitor baricitinib improves skin fibrosis and digital ulcers in systemic sclerosis. Front. Med. 2022, 9, 859330, Erratum in Front. Med. 2023, 5, 1125836. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Gomez, C.A. Baricitinib: The immunomodulator of choice for severe COVID-19-the verdict is in. Crit. Care Med. 2025, 53, e186–e189. [Google Scholar] [CrossRef]
- Sweeney, D.A.; Kalil, A.C. Guidelines without borders: The case for jak inhibitors as the first-line immunomodulator COVID-19 treatment. Lancet Respir. Med. 2025, 13, 478–480. [Google Scholar] [CrossRef]
- Barilli, A.; Visigalli, R.; Ferrari, F.; Recchia Luciani, G.; Soli, M.; Dall’Asta, V.; Rotoli, B.M. The jak1/2 inhibitor baricitinib mitigates the spike-induced inflammatory response of immune and endothelial cells in vitro. Biomedicines 2022, 10, 2324. [Google Scholar] [CrossRef] [PubMed]
- Barilli, A.; Visigalli, R.; Ferrari, F.; Bianchi, M.G.; Dall’Asta, V.; Rotoli, B.M. Immune-mediated inflammatory responses of alveolar epithelial cells: Implications for COVID-19 lung pathology. Biomedicines 2022, 10, 618. [Google Scholar] [CrossRef]
- Barilli, A.; Visigalli, R.; Ferrari, F.; Recchia Luciani, G.; Soli, M.; Dall’Asta, V.; Rotoli, B.M. Growth arrest of alveolar cells in response to cytokines from spike s1-activated macrophages: Role of ifn-gamma. Biomedicines 2022, 10, 3085. [Google Scholar] [CrossRef] [PubMed]


| Gene/Protein Name | Forward Primer | Reverse Primer |
|---|---|---|
| VWF/von Willebrand factor | TGGAGGGAGGAGAGATTGAG | CCCAGCAGCAGAATGATGTA |
| PECAM/CD31 | AACAGTGTTGACATGAAGAGCC | TGTAAAACAGCACGTCATCCTT |
| CDH5/VE-CAD | AAGCGTGAGTCGCAAGAATG | TCTCCAGGTTTTCGCCAGTG |
| S100A4/FSP1 | GATGAGCAACTTGGACAGCAA | CTGGGCTGCTTATCTGGGAAG |
| CDH2/N-CAD | TCAGGCGTCTGTAGAGGCTT | ATGCACATCCTTCGATAAGACTG |
| FN-1/fibronectin | GAGAATAAGCTGTACCATCGCAA | CGACCACATAGGAAGTCCCAG |
| VIM/vimentin | TGCCGTTGAAGCTGCTAACTA | CCAGAGGGAGTGAATCCAGATTA |
| ACTA1/αSMA | GCTGTTTTCCCATCCATTGT | TTTGCTCTGTGCTTCGTCAC |
| RPL15/RPL15 | GCAGCCATCAGGTAAGCCAAG | AGCGGACCCTCAGAAGAAAGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barilli, A.; Visigalli, R.; Recchia Luciani, G.; Crescini, E.; Dall’Asta, V.; Rotoli, B.M. Baricitinib and Infliximab Mitigate the Endothelial-to-Mesenchymal Transition (EndMT) Induced by Cytokines in HUVECs. Int. J. Mol. Sci. 2025, 26, 8672. https://doi.org/10.3390/ijms26178672
Barilli A, Visigalli R, Recchia Luciani G, Crescini E, Dall’Asta V, Rotoli BM. Baricitinib and Infliximab Mitigate the Endothelial-to-Mesenchymal Transition (EndMT) Induced by Cytokines in HUVECs. International Journal of Molecular Sciences. 2025; 26(17):8672. https://doi.org/10.3390/ijms26178672
Chicago/Turabian StyleBarilli, Amelia, Rossana Visigalli, Giulia Recchia Luciani, Eleonora Crescini, Valeria Dall’Asta, and Bianca Maria Rotoli. 2025. "Baricitinib and Infliximab Mitigate the Endothelial-to-Mesenchymal Transition (EndMT) Induced by Cytokines in HUVECs" International Journal of Molecular Sciences 26, no. 17: 8672. https://doi.org/10.3390/ijms26178672
APA StyleBarilli, A., Visigalli, R., Recchia Luciani, G., Crescini, E., Dall’Asta, V., & Rotoli, B. M. (2025). Baricitinib and Infliximab Mitigate the Endothelial-to-Mesenchymal Transition (EndMT) Induced by Cytokines in HUVECs. International Journal of Molecular Sciences, 26(17), 8672. https://doi.org/10.3390/ijms26178672






