PAC1 Receptor Knockout Mice Reveal Critical Links Between ER Stress, Myelin Homeostasis, and Neurodegeneration
Abstract
1. Introduction
2. Results
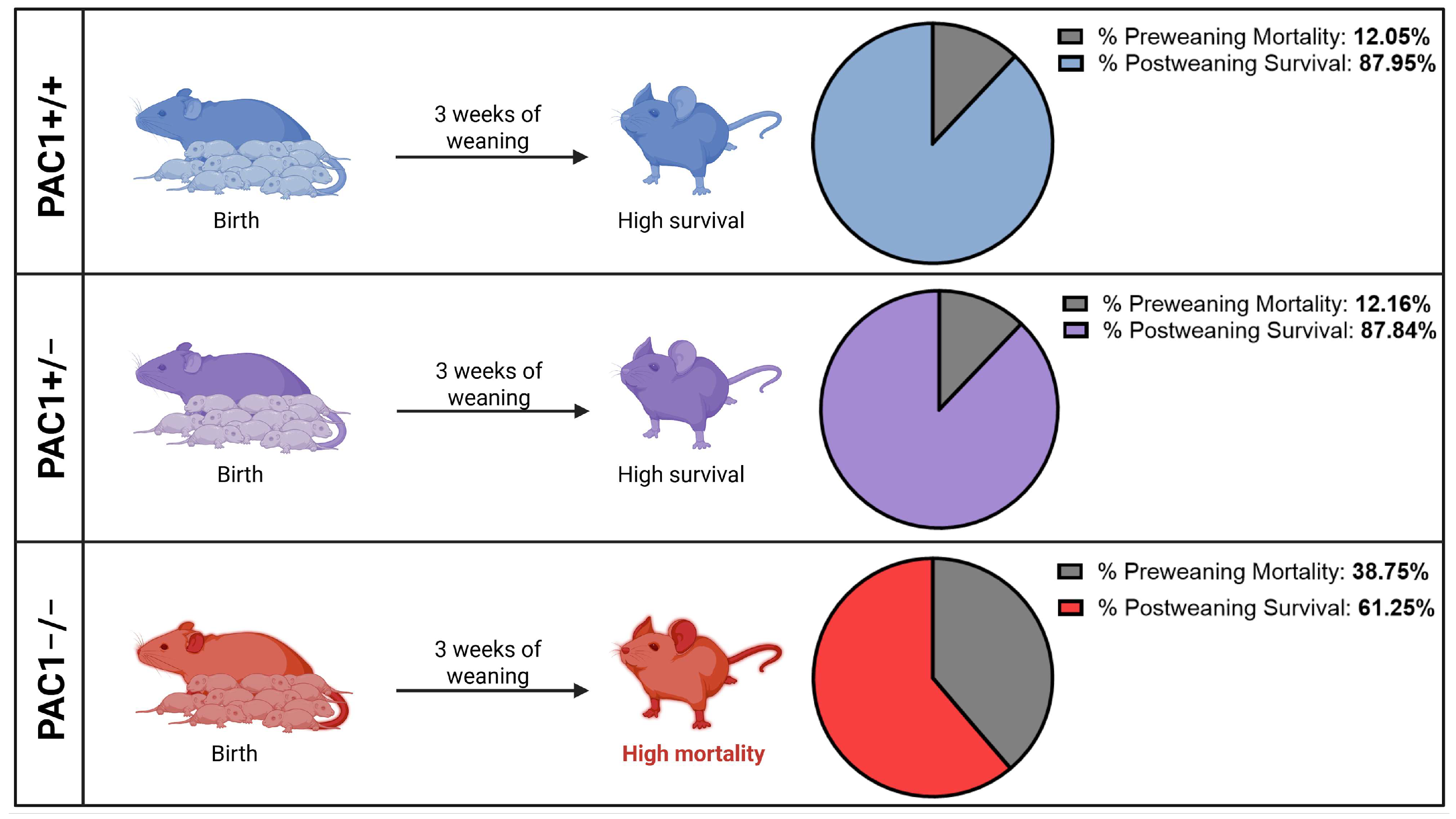
2.1. Complete Loss of PAC1 Elevates the Percentage of Pre-Weaning Deaths
2.2. PAC1 Deletion Alters Locomotor Activity and Anxiety-Related Behaviours
2.3. PAC1 Receptor Deletion Disrupts Oligodendrocyte-Associated Gene Expression and Reduces Mature Oligodendrocyte Density
2.4. Effects of Global PAC1 Deletion on the Expression of ER Stress Markers in the White Matter and Motor Cortex
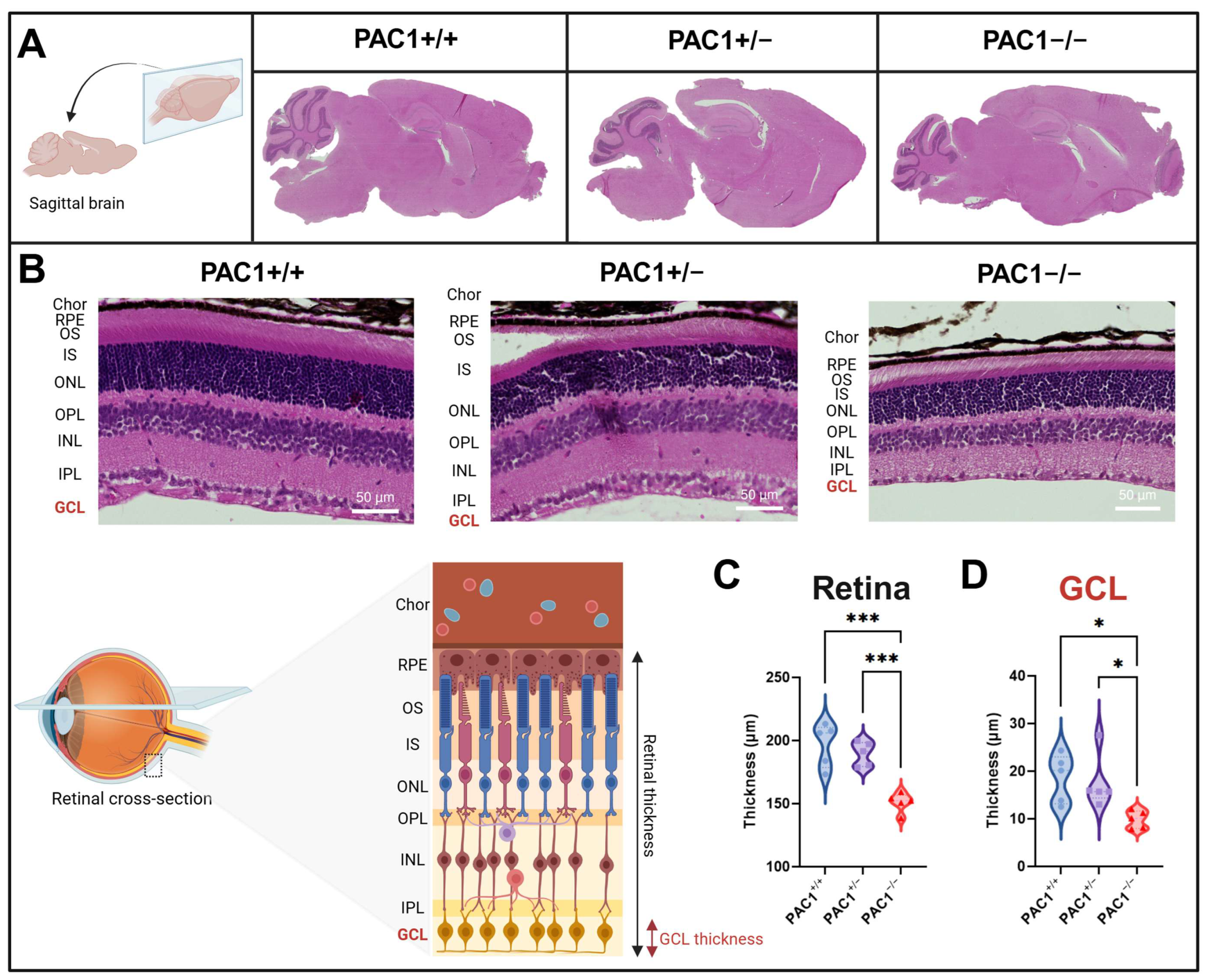
2.5. PAC1 Deletion Reduces the Thickness of Both the Retinal Ganglion Cell Layer and the Overall Retina
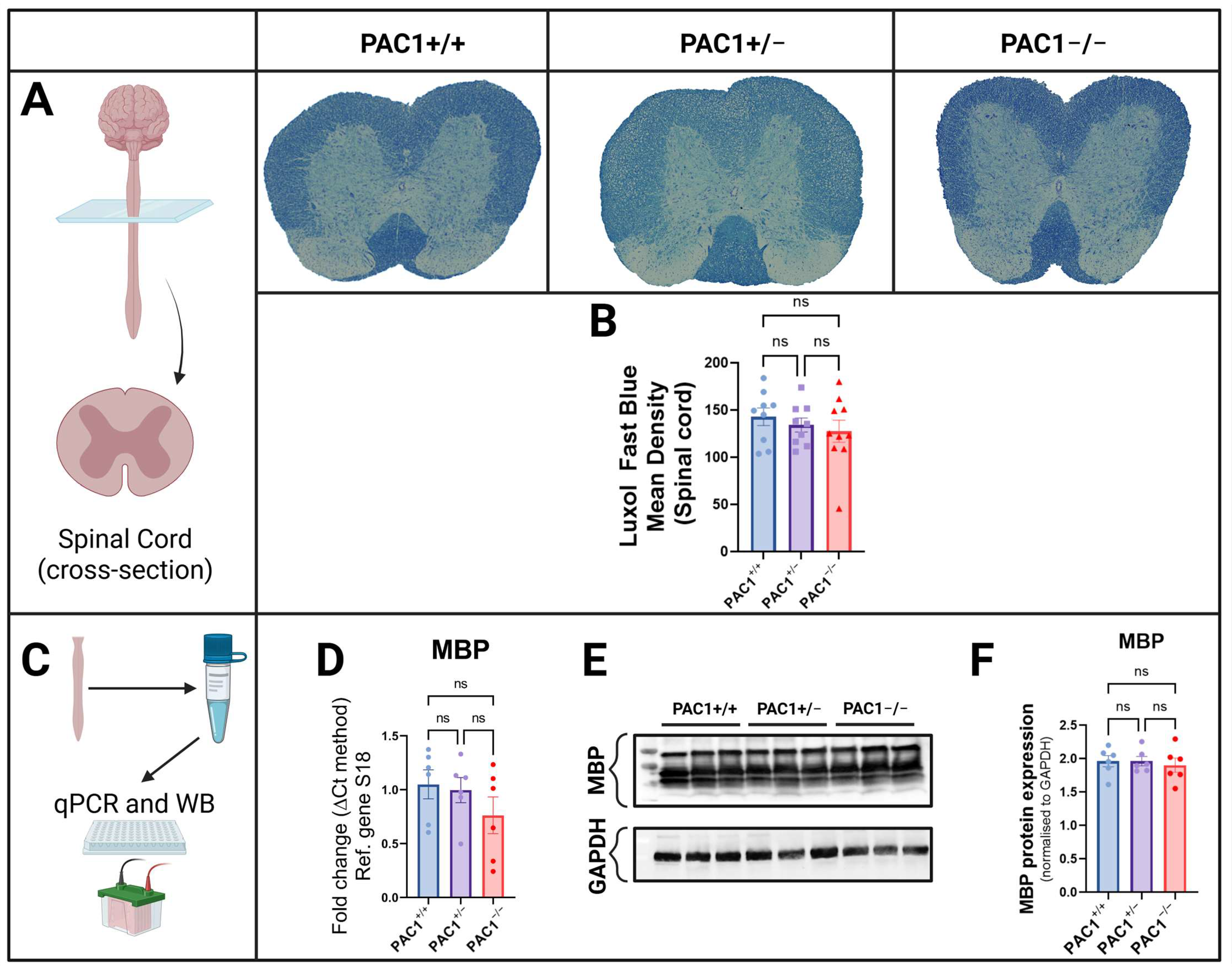
2.6. PAC1 Ablation Causes No Changes in Spinal Cord Myelin Intensity and Gene Expression
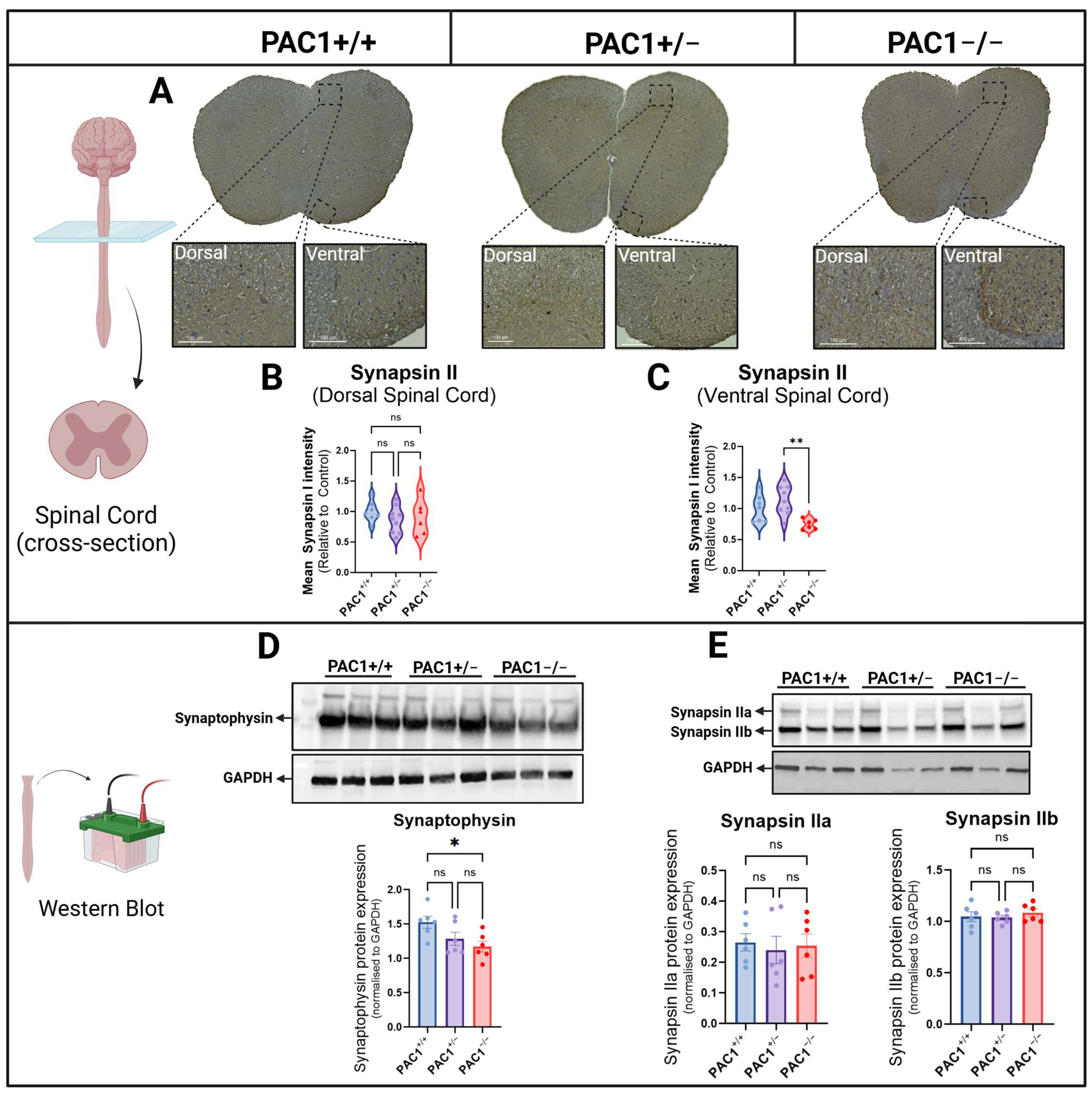
2.7. PAC1 Deficiency Alters Presynaptic Markers in the Spinal Cord
3. Discussion
4. Materials and Methods
4.1. Animals and Study Design
4.2. Open Field Test
4.3. Rotarod Test
4.4. Luxol Fast Blue Staining
4.5. Hematoxylin and Eosin (H&E) Staining
4.6. Immunofluorescence
4.7. Image Analysis
4.8. Immunohistochemistry
4.9. Real-Time Quantitative Polymerase Chain Reaction (qPCR)
4.10. Tissue Protein Extraction and Western Blots
4.11. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef] [PubMed]
- Lassmann, H. Multiple Sclerosis Pathology. Cold Spring Harb. Perspect. Med. 2018, 8, a028936. [Google Scholar] [CrossRef] [PubMed]
- Klineova, S.; Lublin, F.D. Clinical Course of Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a028928. [Google Scholar] [CrossRef]
- Elliott, C.; Belachew, S.M.; Wolinsky, J.S.; Hauser, S.L.; Kappos, L.; Barkhof, F.; Bernasconi, C.; Fecker, J.; Model, F.; Wei, W.; et al. Chronic white matter lesion activity predicts clinical progression in primary progressive multiple sclerosis. Brain 2019, 142, 2787–2799. [Google Scholar] [CrossRef]
- Dhaiban, S.; Al-Ani, M.; Elemam, N.M.; Al-Aawad, M.H.; Al-Rawi, Z.; Maghazachi, A.A. Role of Peripheral Immune Cells in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis. Sci 2021, 3, 12. [Google Scholar] [CrossRef]
- Cencioni, M.T.; Mattoscio, M.; Magliozzi, R.; Bar-Or, A.; Muraro, P.A. B cells in multiple sclerosis—From targeted depletion to immune reconstitution therapies. Nat. Rev. Neurol. 2021, 17, 399–414. [Google Scholar] [CrossRef]
- Duddy, M.; Niino, M.; Adatia, F.; Hebert, S.; Freedman, M.; Atkins, H.; Kim, H.J.; Bar-Or, A. Distinct Effector Cytokine Profiles of Memory and Naive Human B Cell Subsets and Implication in Multiple Sclerosis. J. Immunol. 2007, 178, 6092–6099. [Google Scholar] [CrossRef]
- van Langelaar, J.; Rijvers, L.; Smolders, J.; van Luijn, M.M. B and T Cells Driving Multiple Sclerosis: Identity, Mechanisms and Potential Triggers. Front. Immunol. 2020, 11, 760. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Nociti, V.; Romozzi, M. The Importance of Managing Modifiable Comorbidities in People with Multiple Sclerosis: A Narrative Review. J. Pers. Med. 2023, 13, 1524. [Google Scholar] [CrossRef] [PubMed]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Kettel, P.; Karagöz, G.E. Endoplasmic reticulum: Monitoring and maintaining protein and membrane homeostasis in the endoplasmic reticulum by the unfolded protein response. Int. J. Biochem. Cell Biol. 2024, 172, 106598. [Google Scholar] [CrossRef]
- Wang, M.; Kaufman, R.J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 2016, 529, 326–335. [Google Scholar] [CrossRef]
- Szegezdi, E.; Logue, S.E.; Gorman, A.M.; Samali, A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Maurel, M.; McGrath, E.P.; Mnich, K.; Healy, S.; Chevet, E.; Samali, A. Controlling the unfolded protein response-mediated life and death decisions in cancer. Semin. Cancer Biol. 2015, 33, 57–66. [Google Scholar] [CrossRef]
- Hetz, C.; Papa, F.R. The Unfolded Protein Response and Cell Fate Control. Mol. Cell 2018, 69, 169–181. [Google Scholar] [CrossRef]
- Zinszner, H.; Kuroda, M.; Wang, X.; Batchvarova, N.; Lightfoot, R.T.; Remotti, H.; Stevens, J.L.; Ron, D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998, 12, 982–995. [Google Scholar] [CrossRef]
- Ghemrawi, R.; Khair, M. Endoplasmic Reticulum Stress and Unfolded Protein Response in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 6127. [Google Scholar] [CrossRef]
- Mháille, A.N.; McQuaid, S.; Windebank, A.; Cunnea, P.; McMahon, J.; Samali, A.; FitzGerald, U. Increased expression of endoplasmic reticulum stress-related signaling pathway molecules in multiple sclerosis lesions. J. Neuropathol. Exp. Neurol. 2008, 67, 200–211. [Google Scholar] [CrossRef]
- Huang, W.; Chen, W.-W.; Zhang, X. Multiple sclerosis: Pathology, diagnosis and treatments. Exp. Ther. Med. 2017, 13, 3163–3166. [Google Scholar] [CrossRef]
- Hirabayashi, T.; Nakamachi, T.; Shioda, S. Discovery of PACAP and its receptors in the brain. J. Headache Pain 2018, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, D.M.; Proctor, M.D. Developmental regulation of pituitary adenylate cyclase-activating polypeptide and PAC(1) receptor mRNA expression in the rat central nervous system. Brain Res. Dev. Brain Res. 2000, 120, 27–39. [Google Scholar] [CrossRef]
- Waschek, J.A. VIP and PACAP: Neuropeptide modulators of CNS inflammation, injury, and repair. Br. J. Pharmacol. 2013, 169, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Abad, C.; Tan, Y.-V. Immunomodulatory Roles of PACAP and VIP: Lessons from Knockout Mice. J. Mol. Neurosci. 2018, 66, 102–113. [Google Scholar] [CrossRef]
- Jansen, M.I.; Thomas Broome, S.; Castorina, A. Exploring the Pro-Phagocytic and Anti-Inflammatory Functions of PACAP and VIP in Microglia: Implications for Multiple Sclerosis. Int. J. Mol. Sci. 2022, 23, 4788. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Jiang, X.; Ji, R.; Meng, L.; Liu, F.; Chen, X.; Xin, Y. Therapeutic potential of PACAP for neurodegenerative diseases. Cell. Mol. Biol. Lett. 2015, 20, 265–278. [Google Scholar] [CrossRef]
- Rivnyak, A.; Kiss, P.; Tamas, A.; Balogh, D.; Reglodi, D. Review on PACAP-Induced Transcriptomic and Proteomic Changes in Neuronal Development and Repair. Int. J. Mol. Sci. 2018, 19, 1020. [Google Scholar] [CrossRef]
- Baranowska-Bik, A.; Kochanowski, J.; Uchman, D.; Wolinska-Witort, E.; Kalisz, M.; Martynska, L.; Baranowska, B.; Bik, W. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) in humans with multiple sclerosis. J. Neuroimmunol. 2013, 263, 159–161. [Google Scholar] [CrossRef]
- Kato, H.; Ito, A.; Kawanokuchi, J.; Jin, S.; Mizuno, T.; Ojika, K.; Ueda, R.; Suzumura, A. Pituitary adenylate cyclase-activating polypeptide (PACAP) ameliorates experimental autoimmune encephalomyelitis by suppressing the functions of antigen presenting cells. Mult. Scler. 2004, 10, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.I.; Mahmood, Y.; Lee, J.; Broome, S.T.; Waschek, J.A.; Castorina, A. Targeting the PAC1 receptor mitigates degradation of myelin and synaptic markers and diminishes locomotor deficits in the cuprizone demyelination model. J. Neurochem. 2024, 168, 3250–3267. [Google Scholar] [CrossRef] [PubMed]
- Withana, M.; Castorina, A. Potential Crosstalk between the PACAP/VIP Neuropeptide System and Endoplasmic Reticulum Stress-Relevance to Multiple Sclerosis Pathophysiology. Cells 2023, 12, 2633. [Google Scholar] [CrossRef]
- London, A.; Benhar, I.; Schwartz, M. The retina as a window to the brain-from eye research to CNS disorders. Nat. Rev. Neurol. 2013, 9, 44–53. [Google Scholar] [CrossRef]
- D’Agata, V.; Cavallaro, S. Functional and molecular expression of PACAP/VIP receptors in the rat retina. Brain Res. Mol. Brain Res. 1998, 54, 161–164. [Google Scholar] [CrossRef]
- Dénes, V.; Czotter, N.; Lakk, M.; Berta, G.; Gábriel, R. PAC1-expressing structures of neural retina alter their PAC1 isoform splicing during postnatal development. Cell Tissue Res. 2014, 355, 279–288. [Google Scholar] [CrossRef]
- Patko, E.; Szabo, E.; Toth, D.; Tornoczky, T.; Bosnyak, I.; Vaczy, A.; Atlasz, T.; Reglodi, D. Distribution of PACAP and PAC1 Receptor in the Human Eye. J. Mol. Neurosci. 2022, 72, 2176–2187. [Google Scholar] [CrossRef]
- Hannibal, J.; Georg, B.; Fahrenkrug, J. Altered Circadian Food Anticipatory Activity Rhythms in PACAP Receptor 1 (PAC1) Deficient Mice. PLoS ONE 2016, 11, e0146981. [Google Scholar] [CrossRef] [PubMed]
- Webb, I.C.; Coolen, L.M.; Lehman, M.N. NMDA and PACAP receptor signaling interact to mediate retinal-induced scn cellular rhythmicity in the absence of light. PLoS ONE 2013, 8, e76365. [Google Scholar] [CrossRef]
- Van, C.; Condro, M.C.; Ko, H.H.; Hoang, A.Q.; Zhu, R.; Lov, K.; Ricaflanca, P.T.; Diep, A.L.; Nguyen, N.N.M.; Lipshutz, G.S.; et al. Targeted deletion of PAC1 receptors in retinal neurons enhances neuron loss and axonopathy in a model of multiple sclerosis and optic neuritis. Neurobiol. Dis. 2021, 160, 105524. [Google Scholar] [CrossRef]
- Otto, C.; Martin, M.; Wolfer, D.P.; Lipp, H.P.; Maldonado, R.; Schütz, G. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res. Mol. Brain Res. 2001, 92, 78–84. [Google Scholar] [CrossRef]
- Li, Y.; Huang, S.; Wang, J.; Dai, J.; Cai, J.; Yan, S.; Huang, Z.; He, S.; Wang, P.; Liu, J.; et al. Phosphorylation at Ser(724) of the ER stress sensor IRE1α governs its activation state and limits ER stress-induced hepatosteatosis. J. Biol. Chem. 2022, 298, 101997. [Google Scholar] [CrossRef] [PubMed]
- Marzagalli, R.; Leggio, G.M.; Bucolo, C.; Pricoco, E.; Keay, K.A.; Cardile, V.; Castorina, S.; Salomone, S.; Drago, F.; Castorina, A. Genetic blockade of the dopamine D3 receptor enhances hippocampal expression of PACAP and receptors and alters their cortical distribution. Neuroscience 2016, 316, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Tasma, Z.; Rees, T.A.; Guo, S.; Tan, S.; O’Carroll, S.J.; Faull, R.L.M.; Curtis, M.A.; Christensen, S.L.; Hay, D.L.; Walker, C.S. Pharmacology of PACAP and VIP receptors in the spinal cord highlights the importance of the PAC(1) receptor. Br. J. Pharmacol. 2024, 181, 2655–2675. [Google Scholar] [CrossRef]
- Petrova, N.; Nutma, E.; Carassiti, D.; Rs Newman, J.; Amor, S.; Altmann, D.R.; Baker, D.; Schmierer, K. Synaptic Loss in Multiple Sclerosis Spinal Cord. Ann. Neurol. 2020, 88, 619–625. [Google Scholar] [CrossRef]
- Ciccarelli, O.; Cohen, J.A.; Reingold, S.C.; Weinshenker, B.G. Spinal cord involvement in multiple sclerosis and neuromyelitis optica spectrum disorders. Lancet Neurol. 2019, 18, 185–197. [Google Scholar] [CrossRef]
- Nicot, A.; Ratnakar, P.V.; Ron, Y.; Chen, C.C.; Elkabes, S. Regulation of gene expression in experimental autoimmune encephalomyelitis indicates early neuronal dysfunction. Brain 2003, 126, 398–412. [Google Scholar] [CrossRef]
- Polak, T.; Schlaf, G.; Schöll, U.; Krome-Cesar, C.; Mäder, M.; Felgenhauer, K.; Weber, F. Characterization of the human T cell response against the neuronal protein synapsin in patients with multiple sclerosis. J. Neuroimmunol. 2001, 115, 176–181. [Google Scholar] [CrossRef]
- Boucher, M.N.; May, V.; Braas, K.M.; Hammack, S.E. PACAP orchestration of stress-related responses in neural circuits. Peptides 2021, 142, 170554. [Google Scholar] [CrossRef]
- Vu, J.P.; Luong, L.; Sanford, D.; Oh, S.; Kuc, A.; Pisegna, R.; Lewis, M.; Pisegna, J.R.; Germano, P.M. PACAP and VIP Neuropeptides’ and Receptors’ Effects on Appetite, Satiety and Metabolism. Biology 2023, 12, 1013. [Google Scholar] [CrossRef]
- Jansen, M.I.; Hrncir, H.; MacKenzie-Graham, A.; Waschek, J.A.; Brinkman, J.; Bradfield, L.A.; Withana, M.; Musumeci, G.; D’Agata, V.; Castorina, A. Neuronal PAC1 deletion impairs structural plasticity. Life Sci. 2025, 378, 123843. [Google Scholar] [CrossRef]
- Yu, R.; Yi, T.; Xie, S.; Hong, A. Long-term administration of maxadilan improves glucose tolerance and insulin sensitivity in mice. Peptides 2008, 29, 1347–1353. [Google Scholar] [CrossRef]
- Nakata, M.; Kohno, D.; Shintani, N.; Nemoto, Y.; Hashimoto, H.; Baba, A.; Yada, T. PACAP deficient mice display reduced carbohydrate intake and PACAP activates NPY-containing neurons in the rat hypothalamic arcuate nucleus. Neurosci. Lett. 2004, 370, 252–256. [Google Scholar] [CrossRef]
- Vincze, A.; Reglodi, D.; Helyes, Z.; Hashimoto, H.; Shintani, N.; Abrahám, H. Role of endogenous pituitary adenylate cyclase activating polypeptide (PACAP) in myelination of the rodent brain: Lessons from PACAP-deficient mice. Int. J. Dev. Neurosci. 2011, 29, 923–935. [Google Scholar] [CrossRef]
- Lelievre, V.; Ghiani, C.A.; Seksenyan, A.; Gressens, P.; de Vellis, J.; Waschek, J.A. Growth factor-dependent actions of PACAP on oligodendrocyte progenitor proliferation. Regul. Pept. 2006, 137, 58–66. [Google Scholar] [CrossRef]
- Lee, M.; Lelievre, V.; Zhao, P.; Torres, M.; Rodriguez, W.; Byun, J.Y.; Doshi, S.; Ioffe, Y.; Gupta, G.; de los Monteros, A.E.; et al. Pituitary adenylyl cyclase-activating polypeptide stimulates DNA synthesis but delays maturation of oligodendrocyte progenitors. J. Neurosci. 2001, 21, 3849–3859. [Google Scholar] [CrossRef]
- Li, Q.; Ishii, K.-a.; Kamoshita, K.; Takahashi, K.; Abuduwaili, H.; Takayama, H.; Galicia-Medina, C.M.; Tanida, R.; Ko Oo, H.; Gafiyatullina, G.; et al. PAC1 Deficiency Protects Obese Male Mice From Immobilization-Induced Muscle Atrophy by Suppressing FoxO–Atrogene Axis. Endocrinology 2023, 164, bqad065. [Google Scholar] [CrossRef]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef]
- Lu, L. Mobilizing ER IP3 receptors as a mechanism to enhance calcium signaling. Cell. Mol. Immunol. 2021, 18, 2284–2285. [Google Scholar] [CrossRef]
- Carreras-Sureda, A.; Pihán, P.; Hetz, C. Calcium signaling at the endoplasmic reticulum: Fine-tuning stress responses. Cell Calcium 2018, 70, 24–31. [Google Scholar] [CrossRef]
- Burguet Villena, F.; Cerdá-Fuertes, N.; Hofer, L.; Schädelin, S.; Sellathurai, S.; Schoenholzer, K.; D’Souza, M.; Oechtering, J.; Hanssen, H.; Gugleta, K.; et al. Retinal neuronal loss and progression independent of relapse activity in multiple sclerosis. J. Neurol. 2025, 272, 454. [Google Scholar] [CrossRef]
- Zhu, B.; Luo, L.; Moore, G.R.; Paty, D.W.; Cynader, M.S. Dendritic and synaptic pathology in experimental autoimmune encephalomyelitis. Am. J. Pathol. 2003, 162, 1639–1650. [Google Scholar] [CrossRef][Green Version]
- Jamen, F.; Persson, K.; Bertrand, G.; Rodriguez-Henche, N.; Puech, R.; Bockaert, J.; Ahrén, B.; Brabet, P. PAC1 receptor-deficient mice display impaired insulinotropic response to glucose and reduced glucose tolerance. J. Clin. Investig. 2000, 105, 1307–1315. [Google Scholar] [CrossRef]
- Jamen, F.; Rodriguez-Henche, N.; Pralong, F.; Jegou, B.; Gaillard, R.; Bockaert, J.; Brabet, P. PAC1 null females display decreased fertility. Ann. N. Y. Acad. Sci. 2000, 921, 400–404. [Google Scholar] [CrossRef]
- Ross, R.A.; Leon, S.; Madara, J.C.; Schafer, D.; Fergani, C.; Maguire, C.A.; Verstegen, A.M.; Brengle, E.; Kong, D.; Herbison, A.E.; et al. PACAP neurons in the ventral premammillary nucleus regulate reproductive function in the female mouse. eLife 2018, 7, e35960. [Google Scholar] [CrossRef]
- Sturman, O.; Germain, P.-L.; Bohacek, J. Exploratory rearing: A context- and stress-sensitive behavior recorded in the open-field test. Stress 2018, 21, 443–452. [Google Scholar] [CrossRef]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior. In Pre-Clinical Models: Techniques and Protocols; Guest, P.C., Ed.; Springer: New York, NY, USA, 2019; pp. 99–103. [Google Scholar]
- La-Vu, M.; Tobias, B.C.; Schuette, P.J.; Adhikari, A. To Approach or Avoid: An Introductory Overview of the Study of Anxiety Using Rodent Assays. Front. Behav. Neurosci. 2020, 14, 145. [Google Scholar] [CrossRef]
- Grabovskaya, S.V.; Salyha, Y.T. Do Results of the Open Field Test Depend on the Arena Shape? Neurophysiology 2014, 46, 376–380. [Google Scholar] [CrossRef]
- Gilli, F.; Royce, D.B.; Pachner, A.R. Measuring Progressive Neurological Disability in a Mouse Model of Multiple Sclerosis. J. Vis. Exp. 2016, 117, 54616. [Google Scholar] [CrossRef]
- Knight, P.; Chellian, R.; Wilson, R.; Behnood-Rod, A.; Panunzio, S.; Bruijnzeel, A.W. Sex differences in the elevated plus-maze test and large open field test in adult Wistar rats. Pharmacol. Biochem. Behav. 2021, 204, 173168. [Google Scholar] [CrossRef]
- Eltokhi, A.; Kurpiers, B.; Pitzer, C. Comprehensive characterization of motor and coordination functions in three adolescent wild-type mouse strains. Sci. Rep. 2021, 11, 6497. [Google Scholar] [CrossRef]
- Jakkamsetti, V.; Scudder, W.; Kathote, G.; Ma, Q.; Angulo, G.; Dobariya, A.; Rosenberg, R.N.; Beutler, B.; Pascual, J.M. Quantification of early learning and movement sub-structure predictive of motor performance. Sci. Rep. 2021, 11, 14405. [Google Scholar] [CrossRef]
- Rizzo, S.J.S. Rotarod Assay. Standard Operating Procedure, The Jackson Laboratory Mouse Neurobehavioral Phenotyping Facility; The Jackson Laboratory Mouse Neurobehavioral Phenotyping Facility: Bar Harbor, ME, USA, 2014. [Google Scholar]
- Crowe, A.R.; Yue, W. Semi-quantitative Determination of Protein Expression using Immunohistochemistry Staining and Analysis: An Integrated Protocol. Bio-Protocol 2019, 9, e3465. [Google Scholar] [CrossRef]
- Sen, M.K.; Almuslehi, M.S.M.; Coorssen, J.R.; Mahns, D.A.; Shortland, P.J. Behavioural and histological changes in cuprizone-fed mice. Brain Behav. Immun. 2020, 87, 508–523. [Google Scholar] [CrossRef]
- Klein, B.; Mrowetz, H.; Barker, C.M.; Lange, S.; Rivera, F.J.; Aigner, L. Age Influences Microglial Activation After Cuprizone-Induced Demyelination. Front. Aging Neurosci. 2018, 10, 278. [Google Scholar] [CrossRef]
- Mandwie, M.; Karunia, J.; Niaz, A.; Keay, K.A.; Musumeci, G.; Rennie, C.; McGrath, K.; Al-Badri, G.; Castorina, A. Metformin Treatment Attenuates Brain Inflammation and Rescues PACAP/VIP Neuropeptide Alterations in Mice Fed a High-Fat Diet. Int. J. Mol. Sci. 2021, 22, 13660. [Google Scholar] [CrossRef]
- Thomas Broome, S.; Fisher, T.; Faiz, A.; Keay, K.A.; Musumeci, G.; Al-Badri, G.; Castorina, A. Assessing the Anti-Inflammatory Activity of the Anxiolytic Drug Buspirone Using CRISPR-Cas9 Gene Editing in LPS-Stimulated BV-2 Microglial Cells. Cells 2021, 10, 1312. [Google Scholar] [CrossRef]



| Type of Investigation | PAC1+/− Mice (vs. PAC1+/+ Mice) | PAC1−/− Mice (vs. PAC1+/+ Mice) | CNS Area |
|---|---|---|---|
| Animal Phenotypes | |||
| Pre-weaning survivability | Unchanged | ↓ Increased | n/a |
| Animal weight | Unchanged | ↓ Decreased | n/a |
| Rotarod latency (Week 0) | Unchanged | ↓ Decreased | n/a |
| Open Field Test—total distance travelled (Week 0) | Unchanged | ↑ Increased | n/a |
| Open Field Test—time spent in centre (Week 0) | Unchanged | ↑ Increased | n/a |
| Gene Expression Studies | |||
| PLP1 | Unchanged | ↓ Decreased | White Matter |
| Olig2 | ↓ Decreased | ↓ Decreased | White Matter |
| MBP | ↓ Decreased | ↓ Decreased | Motor Cortex |
| Olig2 | ↑ Increased | ↑ Increased | Motor Cortex |
| PERK | Unchanged | ↑ Increased | Motor Cortex |
| ERN1 | ↓ Decreased | ↓ Decreased | Motor Cortex |
| Histology/IF | |||
| Luxol Fast Blue (Myelin Density) | Unchanged | ↓ Decreased | Corpus Callosum, Cortex and Hippocampus |
| ASPA | Unchanged | ↓ Decreased | Corpus Callosum |
| pIRE1 | Unchanged | ↑ Increased | Motor Cortex |
| Retinal thickness | Unchanged | ↓ Decreased | Retina |
| GCL thickness | Unchanged | ↓ Decreased | Retina |
| Antibody | Species | Dilution | Product Code |
|---|---|---|---|
| Recombinant Monoclonal ASPA antibody | Rabbit | 1:500 | Abcam, ab223269, EPR22072 |
| β-III tubulin (TUJ1) Monoclonal Antibody | Mouse | 1:500 | Bio Legend, Cat# 80120, San Diego, CA, USA |
| Phosphorylated Inositol-Requiring Enzyme 1 (pIRE1) Antibody (Ser 724) | Rabbit | 1:250 | Sigma Aldrich, Cat# ZRB1072-25UL |
| Gene | Gene Bank Accession Number | Primer Sequence | Length (bp) |
|---|---|---|---|
| S18 | NM_011296.2 | Fwd: CCCTGAGAAGTTCCAGCACA Rev: GGTGAGGTCGATGTCTGCTT | 145 |
| MOG | NM_010814.2 | Fwd: CTTCTTCAGAGACCACTCTTACC Rev: CCCAATAGAAGGGATCTTCCAC | 71 |
| MBP | NM_001025251.2 | Fwd: TATAAATCGGCTCACAAGGGATT Rev: TGTCTCTTCCTCCCAGCTTA | 85 |
| Olig2 | NM_016967.2 | Fwd: AAAGACAAGAAGCAGATGACTGA Rev: AGCATGAGGATGTAGTTTCGC | 200 |
| PLP1 | NM_011123.4 | Fwd: ATGCCAGAATGTATGGTGTTCT Rev: TTTAAGGACGGCGAAGTTGTAAG | 200 |
| ERN1 | NM_023913.2 | Fwd: GAGACAAAGGAGAGTGTGTGAT Rev: TCAAGTAGTTCAGCTTGCTCTT | 87 |
| PERK | NM_010121.3 | Fwd: CCTTGGTTTCATCTAGCCTCA Rev: ACTTGTAGGAAGATTCGAGCAG | 156 |
| DDIT3 (CHOP) | NM_007837.4 | Fwd: GCTCTCCAGATTCCAGTCAG Rev: CTCCTTCTCCTTCATGCGTT | 131 |
| ATF4 | NM_009716.3 | Fwd: CCTCAGACAGTGAACCCAAT Rev: AATGCTCTGGAGTGGAAGAC | 127 |
| ATF6 | NM_001081304.1 | Fwd: GAGCTGTCTGTGTGATGATAGT Rev: CTAGGTTTCACTCTTCGGGATT | 94 |
| Antibody | Dilution | Product Code |
|---|---|---|
| Myelin Basic Protein (MBP) | 1:1000 | GTX133108, GeneTex |
| Synaptophysin | 1:1000 | MA514532, ThermoFisher |
| Synapsin II | 1:500 | GTX135310, GeneTex |
| GAPDH | 1:2000 | VPA00187, BioRad |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Withana, M.; Bradfield, L.; Jansen, M.I.; Musumeci, G.; Waschek, J.A.; Castorina, A. PAC1 Receptor Knockout Mice Reveal Critical Links Between ER Stress, Myelin Homeostasis, and Neurodegeneration. Int. J. Mol. Sci. 2025, 26, 8668. https://doi.org/10.3390/ijms26178668
Withana M, Bradfield L, Jansen MI, Musumeci G, Waschek JA, Castorina A. PAC1 Receptor Knockout Mice Reveal Critical Links Between ER Stress, Myelin Homeostasis, and Neurodegeneration. International Journal of Molecular Sciences. 2025; 26(17):8668. https://doi.org/10.3390/ijms26178668
Chicago/Turabian StyleWithana, Minduli, Laura Bradfield, Margo I. Jansen, Giuseppe Musumeci, James A. Waschek, and Alessandro Castorina. 2025. "PAC1 Receptor Knockout Mice Reveal Critical Links Between ER Stress, Myelin Homeostasis, and Neurodegeneration" International Journal of Molecular Sciences 26, no. 17: 8668. https://doi.org/10.3390/ijms26178668
APA StyleWithana, M., Bradfield, L., Jansen, M. I., Musumeci, G., Waschek, J. A., & Castorina, A. (2025). PAC1 Receptor Knockout Mice Reveal Critical Links Between ER Stress, Myelin Homeostasis, and Neurodegeneration. International Journal of Molecular Sciences, 26(17), 8668. https://doi.org/10.3390/ijms26178668









