Spectroscopic Profile of Metabolome Dynamics During Rat Cortical Neuronal Differentiation
Abstract
1. Introduction
2. Results
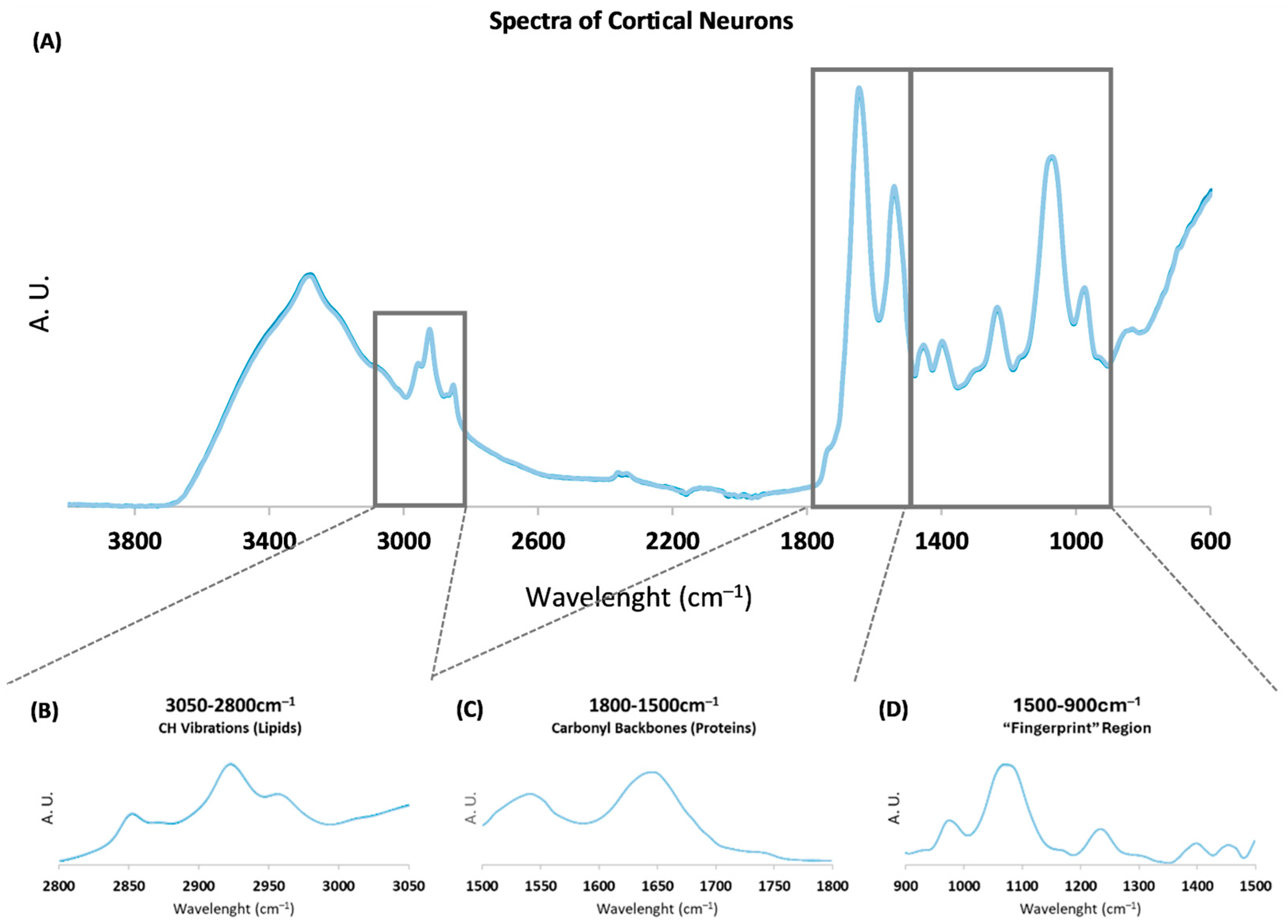
2.1. PLS-R Multivariate Analysis of Spectroscopic Profile
2.2. Peak Intensity Analysis Related to Protein Conformation
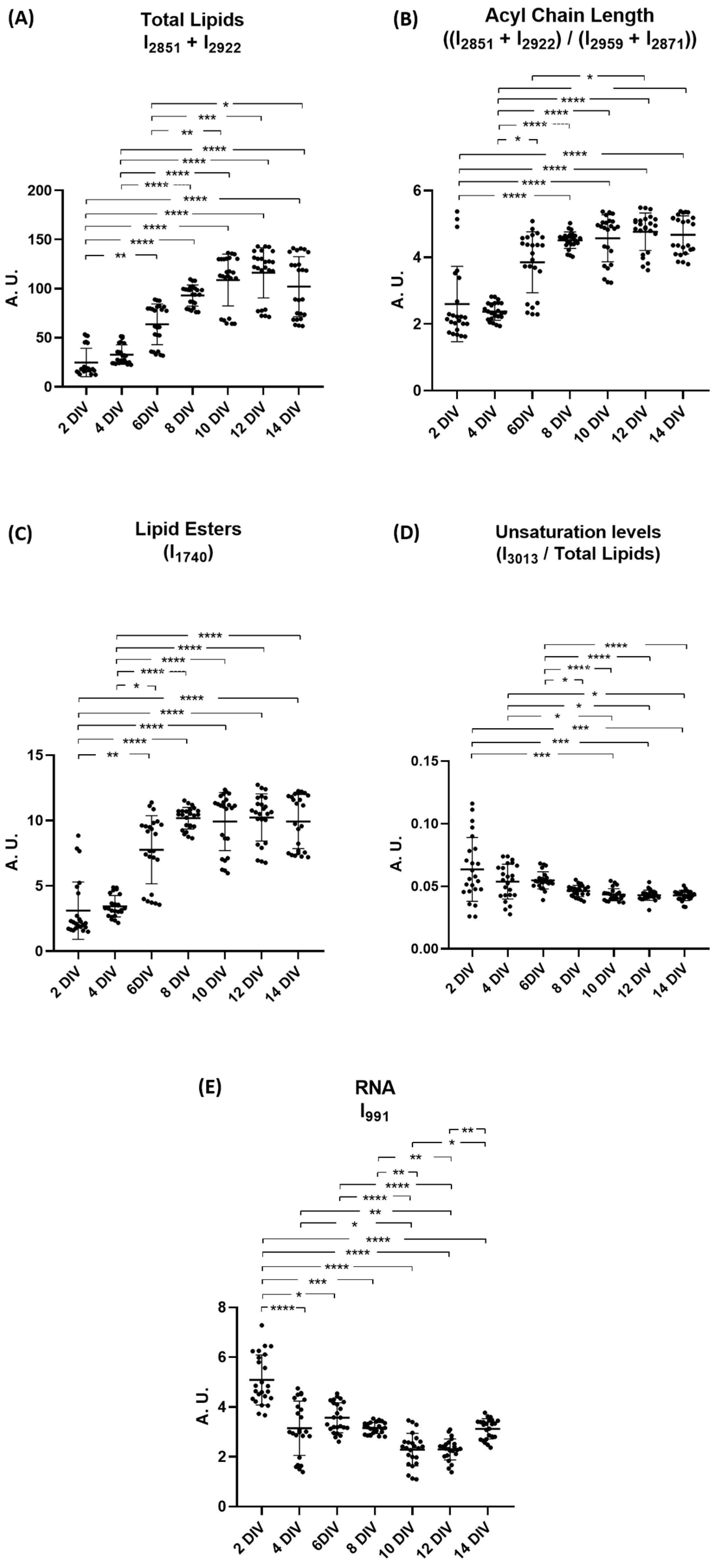
2.3. Peak Intensity Analysis of Spectroscopic Signals Related to Lipids and Nucleic Acids
3. Discussion
4. Materials and Methods
4.1. Neuronal Primary Culture Establishment
4.2. Cell Collection for FTIR Analysis
4.3. FTIR Spectra Measurements
4.4. Spectra Pre-Processing
4.5. PLS-R Multivariate Analysis
4.6. Peak Intensity Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fukata, Y.; Kimura, T.; Kaibuchi, K. Axon specification in hippocampal neurons. Neurosci. Res. 2002, 43, 305–315. [Google Scholar] [CrossRef]
- Dotti, C.; Sullivan, C.; Banker, G. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 1988, 8, 1454–1468. [Google Scholar] [CrossRef]
- Barnes, A.P.; Polleux, F. Establishment of Axon-Dendrite Polarity in Developing Neurons. Annu. Rev. Neurosci. 2009, 32, 347–381. [Google Scholar] [CrossRef]
- Mattson, M.P. Establishment and plasticity of neuronal polarity. J. Neurosci. Res. 1999, 57, 577–589. [Google Scholar] [CrossRef]
- Noctor, S.C.; Martínez-Cerdeño, V.; Ivic, L.; Kriegstein, A.R. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 2004, 7, 136–144. [Google Scholar] [CrossRef]
- Zolessi, F.R.; Poggi, L.; Wilkinson, C.J.; Chien, C.B.; Harris, W.A. Polarization and orientation of retinal ganglion cells in vivo. Neural Develop. 2006, 1, 2. [Google Scholar] [CrossRef]
- Govek, E.-E.; Newey, S.E.; Van Aelst, L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005, 19, 1–49. [Google Scholar] [CrossRef]
- Tahirovic, S.; Bradke, F. Neuronal Polarity. Cold Spring Harb. Perspect. Biol. 2009, 1, a001644. [Google Scholar] [CrossRef]
- Da Silva, J.S.; Dotti, C.G. Breaking the neuronal sphere: Regulation of the actin cytoskeleton in neuritogenesis. Nat. Rev. Neurosci. 2002, 3, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.C. The cytoskeleton and neurite initiation. BioArchitecture 2013, 3, 86–109. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Romero, M.E.; Slater, P.G. Unraveling Axon Guidance during Axotomy and Regeneration. Int. J. Mol. Sci. 2021, 22, 8344. [Google Scholar] [CrossRef]
- Sainath, R.; Gallo, G. Cytoskeletal and signaling mechanisms of neurite formation. Cell Tissue Res. 2015, 359, 267–278. [Google Scholar] [CrossRef]
- Hoff, K.J.; Neumann, A.J.; Moore, J.K. The molecular biology of tubulinopathies: Understanding the impact of variants on tubulin structure and microtubule regulation. Front. Cell. Neurosci. 2022, 16, 1023267. [Google Scholar] [CrossRef]
- Knossow, M.; Campanacci, V.; Khodja, L.A.; Gigant, B. The Mechanism of Tubulin Assembly into Microtubules: Insights from Structural Studies. iScience 2020, 23, 101511. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.W.; Merriam, E.B.; Hu, X. The dynamic cytoskeleton: Backbone of dendritic spine plasticity. Curr. Opin. Neurobiol. 2011, 21, 175–181. [Google Scholar] [CrossRef]
- Alberts, B.; Heald, R.; Johnson, A.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 7th ed.; Garland Science: New York, NY, USA, 2022. [Google Scholar]
- Konstantinides, N.; Desplan, C. Neuronal differentiation strategies: Insights from single-cell sequencing and machine learning. Development 2020, 147, dev193631. [Google Scholar] [CrossRef] [PubMed]
- Slanzi, A.; Iannoto, G.; Rossi, B.; Zenaro, E.; Constantin, G. In vitro Models of Neurodegenerative Diseases. Front. Cell Dev. Biol. 2020, 8, 328. [Google Scholar] [CrossRef] [PubMed]
- Luhmann, H.J.; Fukuda, A. Can we understand human brain development from experimental studies in rodents? Pediatr. Int. 2020, 62, 1139–1144. [Google Scholar] [CrossRef]
- Gordon, J.; Amini, S.; White, M.K. General Overview of Neuronal Cell Culture. Neuronal Cell Cult. 2013, 1078, 1–8. [Google Scholar] [CrossRef]
- Rebelo, S.; Domingues, S.C.; Santos, M.; Fardilha, M.; Esteves, S.L.C.; Vieira, S.I.; Vintém, A.P.B.; Wu, W.; Da Cruz E Silva, E.F.; Silva, O.A.B. Identification of a Novel Complex AβPP:Fe65:PP1 that Regulates AβPP Thr668 Phosphorylation Levels. J. Alzheimers Dis. 2013, 35, 761–775. [Google Scholar] [CrossRef]
- Almeida, I.; Magalhães, S.; Nunes, A. Lipids: Biomarkers of healthy aging. Biogerontology 2021, 22, 273–295. [Google Scholar] [CrossRef]
- Neto, V.; Esteves-Ferreira, S.; Inácio, I.; Alves, M.; Dantas, R.; Almeida, I.; Guimarães, J.; Azevedo, T.; Nunes, A. Metabolic Profile Characterization of Different Thyroid Nodules Using FTIR Spectroscopy: A Review. Metabolites 2022, 12, 53. [Google Scholar] [CrossRef]
- Lim, M.S.; Elenitoba-Johnson, K.S.J. Proteomics in pathology research. Lab. Investig. 2004, 84, 1227–1244. [Google Scholar] [CrossRef]
- Colantonio, D.A.; Chan, D.W. The clinical application of proteomics. Clin. Chim. Acta 2005, 357, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Amess, B.; Rohlff, C.; Stubberfield, C.; Parekh, R. Proteomics: A major new technology for the drug discovery process. Drug Discov. Today 1999, 4, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Barallobre-Barreiro, J.; Chung, Y.L.; Mayr, M. Proteomics and Metabolomics for Mechanistic Insights and Biomarker Discovery in Cardiovascular Disease. Rev. Esp. Cardiol. Engl. Ed. 2013, 66, 657–661. [Google Scholar] [CrossRef]
- Lopes, J.; Correia, M.; Martins, I.; Henriques, A.G.; Delgadillo, I.; Da Cruz E Silva, O.; Nunes, A. FTIR and Raman Spectroscopy Applied to Dementia Diagnosis Through Analysis of Biological Fluids. J. Alzheimers Dis. 2016, 52, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Boccard, J.; Veuthey, J.; Rudaz, S. Knowledge discovery in metabolomics: An overview of MS data handling. J. Sep. Sci. 2010, 33, 290–304. [Google Scholar] [CrossRef]
- Magalhães, S.; Goodfellow, B.J.; Nunes, A. FTIR spectroscopy in biomedical research: How to get the most out of its potential. Appl. Spectrosc. Rev. 2021, 56, 869–907. [Google Scholar] [CrossRef]
- Vaz, M.; Soares Martins, T.; Leandro, K.; De Almeida, L.P.; Da Cruz E Silva, O.A.B.; Nunes, A.; Henriques, A.G. Fourier Transform Infrared Spectroscopy Analysis as a Tool to Address Aβ Impact on Extracellular Vesicles. Molecules 2025, 30, 258. [Google Scholar] [CrossRef]
- Magalhães, S.; Almeida, I.; Martins, F.; Camões, F.; Soares, A.R.; Goodfellow, B.J.; Rebelo, S.; Nunes, A. FTIR Spectroscopy as a Tool to Study Age-Related Changes in Cardiac and Skeletal Muscle of Female C57BL/6J Mice. Molecules 2021, 26, 6410. [Google Scholar] [CrossRef]
- Mateus, T.; Almeida, I.; Costa, A.; Viegas, D.; Magalhães, S.; Martins, F.; Herdeiro, M.T.; Da Cruz E Silva, O.A.B.; Fraga, C.; Alves, I.; et al. Fourier-Transform Infrared Spectroscopy as a Discriminatory Tool for Myotonic Dystrophy Type 1 Metabolism: A Pilot Study. Int. J. Environ. Res. Public. Health 2021, 18, 3800. [Google Scholar] [CrossRef]
- Yonar, D.; Ocek, L.; Tiftikcioglu, B.I.; Zorlu, Y.; Severcan, F. Relapsing-Remitting Multiple Sclerosis diagnosis from cerebrospinal fluids via Fourier transform infrared spectroscopy coupled with multivariate analysis. Sci. Rep. 2018, 8, 1025. [Google Scholar] [CrossRef] [PubMed]
- Oleszko, A.; Olsztyńska-Janus, S.; Walski, T.; Grzeszczuk-Kuć, K.; Bujok, J.; Gałecka, K.; Czerski, A.; Witkiewicz, W.; Komorowska, M. Application of FTIR-ATR Spectroscopy to Determine the Extent of Lipid Peroxidation in Plasma during Haemodialysis. BioMed Res. Int. 2015, 2015, 245607. [Google Scholar] [CrossRef]
- Magalhães, S.; Almeida, I.; Pereira, C.D.; Rebelo, S.; Goodfellow, B.J.; Nunes, A. The Long-Term Culture of Human Fibroblasts Reveals a Spectroscopic Signature of Senescence. Int. J. Mol. Sci. 2022, 23, 5830. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Dichter, M.A. Rat cortical neurons in cell culture: Culture methods, cell morphology, electrophysiology, and synapse formation. Brain Res. 1978, 149, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.I.; Rebelo, S.; Esselmann, H.; Wiltfang, J.; Lah, J.; Lane, R.; Small, S.A.; Gandy, S.; Da Cruz E Silva, E.F.; Da Cruz E Silva, O.A. Retrieval of the Alzheimer’s amyloid precursor protein from the endosome to the TGN is S655 phosphorylation state-dependent and retromer-mediated. Mol. Neurodegener. 2010, 5, 40. [Google Scholar] [CrossRef]
- Rebelo, S.; Vieira, S.I.; Da Cruz E Silva, O.A.B.; Esselmann, H.; Wiltfang, J.; Da Cruz E Silva, E.F. Tyr687 dependent APP endocytosis and abeta production. J. Mol. Neurosci. 2007, 32, 1–8. [Google Scholar] [CrossRef]
- Lesslich, H.M.; Klapal, L.; Wilke, J.; Haak, A.; Dietzel, I.D. Adjusting the neuron to astrocyte ratio with cytostatics in hippocampal cell cultures from postnatal rats: A comparison of cytarabino furanoside (AraC) and 5-fluoro-2′-deoxyuridine (FUdR). PLoS ONE 2022, 17, e0265084. [Google Scholar] [CrossRef] [PubMed]
- Frese, C.K.; Mikhaylova, M.; Stucchi, R.; Gautier, V.; Liu, Q.; Mohammed, S.; Heck, A.J.R.; Altelaar, A.F.M.; Hoogenraad, C.C. Quantitative Map of Proteome Dynamics during Neuronal Differentiation. Cell Rep. 2017, 18, 1527–1542. [Google Scholar] [CrossRef]
- Zhang, T.; Gygi, S.P.; Paulo, J.A. Temporal Proteomic Profiling of SH-SY5Y Differentiation with Retinoic Acid Using FAIMS and Real-Time Searching. J. Proteome Res. 2021, 20, 704–714. [Google Scholar] [CrossRef]
- Murillo, J.R.; Goto-Silva, L.; Sánchez, A.; Nogueira, F.C.S.; Domont, G.B.; Junqueira, M. Quantitative proteomic analysis identifies proteins and pathways related to neuronal development in differentiated SH-SY5Y neuroblastoma cells. EuPA Open Proteom. 2017, 16, 1–11. [Google Scholar] [CrossRef]
- Tanthanuch, W.; Thumanu, K.; Lorthongpanich, C.; Parnpai, R.; Heraud, P. Neural differentiation of mouse embryonic stem cells studied by FTIR spectroscopy. J. Mol. Struct. 2010, 967, 189–195. [Google Scholar] [CrossRef]
- Ami, D.; Neri, T.; Natalello, A.; Mereghetti, P.; Doglia, S.M.; Zanoni, M.; Zuccotti, M.; Garagna, S.; Redi, C.A. Embryonic stem cell differentiation studied by FT-IR spectroscopy. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2008, 1783, 98–106. [Google Scholar] [CrossRef]
- Hashimoto, K.; Andriana, B.B.; Matsuyoshi, H.; Sato, H. Discrimination analysis of excitatory and inhibitory neurons using Raman spectroscopy. Analyst 2018, 143, 2889–2894. [Google Scholar] [CrossRef]
- Feng, L.; Zhu, S.; Chen, S.; Bao, Y.; He, Y. Combining Fourier Transform Mid-Infrared Spectroscopy with Chemometric Methods to Detect Adulterations in Milk Powder. Sensors 2019, 19, 2934. [Google Scholar] [CrossRef]
- Aizenman, Y.; De Vellis, J. Brain neurons develop in a serum and glial free environment: Effects of transferrin, insulin- insulin-like growth factor-I and thyroid hormone on neuronal survival, growth and differentiation. Brain Res. 1987, 406, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Van Oostrum, M.; Campbell, B.; Seng, C.; Müller, M.; Tom Dieck, S.; Hammer, J.; Pedrioli, P.G.A.; Földy, C.; Tyagarajan, S.K.; Wollscheid, B. Surfaceome dynamics reveal proteostasis-independent reorganization of neuronal surface proteins during development and synaptic plasticity. Nat. Commun. 2020, 11, 4990, Erratum in Nat. Commun. 2020, 11, 5741. [Google Scholar] [CrossRef] [PubMed]
- Penazzi, L.; Bakota, L.; Brandt, R. Microtubule Dynamics in Neuronal Development, Plasticity, and Neurodegeneration. Int. Rev. Cell Mol. Biol. 2016, 321, 89–169. [Google Scholar] [CrossRef] [PubMed]
- Nowick, J.S. Exploring β-Sheet Structure and Interactions with Chemical Model Systems. Acc. Chem. Res. 2008, 41, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.N.; Pham, J.D.; Nowick, J.S. The Supramolecular Chemistry of β-Sheets. J. Am. Chem. Soc. 2013, 135, 5477–5492. [Google Scholar] [CrossRef]
- Hubin, E.; Deroo, S.; Schierle, G.K.; Kaminski, C.; Serpell, L.; Subramaniam, V.; Van Nuland, N.; Broersen, K.; Raussens, V.; Sarroukh, R. Two distinct β-sheet structures in Italian-mutant amyloid-beta fibrils: A potential link to different clinical phenotypes. Cell. Mol. Life Sci. 2015, 72, 4899–4913. [Google Scholar] [CrossRef]
- Yassine, W.; Taib, N.; Federman, S.; Milochau, A.; Castano, S.; Sbi, W.; Manigand, C.; Laguerre, M.; Desbat, B.; Oda, R.; et al. Reversible transition between α-helix and β-sheet conformation of a transmembrane domain. Biochim. Biophys. Acta BBA—Biomembr. 2009, 1788, 1722–1730. [Google Scholar] [CrossRef]
- Khan, R.; Kulasiri, D.; Samarasinghe, S. Functional repertoire of protein kinases and phosphatases in synaptic plasticity and associated neurological disorders. Neural Regen. Res. 2021, 16, 1150–1157. [Google Scholar] [CrossRef]
- Grant, P.; Pant, H.C. Pant Neurofilament protein synthesis and phosphorylation. J. Neurocytol. 2000, 29, 843–872. [Google Scholar] [CrossRef] [PubMed]
- Noble, W.; Hanger, D.P.; Miller, C.C.J.; Lovestone, S. The Importance of Tau Phosphorylation for Neurodegenerative Diseases. Front. Neurol. 2013, 4, 83. [Google Scholar] [CrossRef]
- DeGiosio, R.A.; Needham, P.G.; Andrews, O.A.; Tristan, H.; Grubisha, M.J.; Brodsky, J.L.; Camacho, C.; Sweet, R.A. Differential regulation of MAP2 by phosphorylation events in proline-rich versus C-terminal domains. FASEB J. 2023, 37, e23194. [Google Scholar] [CrossRef] [PubMed]
- Albert-Gascó, H.; Ros-Bernal, F.; Castillo-Gómez, E.; Olucha-Bordonau, F.E. MAP/ERK Signaling in Developing Cognitive and Emotional Function and Its Effect on Pathological and Neurodegenerative Processes. Int. J. Mol. Sci. 2020, 21, 4471. [Google Scholar] [CrossRef]
- Long, H.Z.; Cheng, Y.; Zhou, Z.W.; Luo, H.Y.; Wen, D.D.; Gao, L.C. PI3K/AKT Signal Pathway: A Target of Natural Products in the Prevention and Treatment of Alzheimer’s Disease and Parkinson’s Disease. Front. Pharmacol. 2021, 12, 648636. [Google Scholar] [CrossRef]
- Rodríguez-Berdini, L.; Caputto, B.L. Lipid Metabolism in Neurons: A Brief Story of a Novel c-Fos-Dependent Mechanism for the Regulation of Their Synthesis. Front. Cell. Neurosci. 2019, 13, 198. [Google Scholar] [CrossRef]
- Roy, D.; Tedeschi, A. The Role of Lipids, Lipid Metabolism and Ectopic Lipid Accumulation in Axon Growth, Regeneration and Repair after CNS Injury and Disease. Cells 2021, 10, 1078. [Google Scholar] [CrossRef]
- Knobloch, M.; Jessberger, S. Metabolism and neurogenesis. Curr. Opin. Neurobiol. 2017, 42, 45–52. [Google Scholar] [CrossRef]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Imran, A.; Qasim, M.; Zafar, S.; Kamran, S.K.S.; Razzaq, A.; Aziz, N.; et al. Role of cholesterol and sphingolipids in brain development and neurological diseases. Lipids Health Dis. 2019, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Mencarelli, C.; Martinez–Martinez, P. Ceramide function in the brain: When a slight tilt is enough. Cell. Mol. Life Sci. 2013, 70, 181–203. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.F.; Ellis, J.M. Acyl-CoA synthetases as regulators of brain phospholipid acyl-chain diversity. Prostaglandins Leukot. Essent. Fatty Acids 2020, 161, 102175. [Google Scholar] [CrossRef] [PubMed]
- Ramosaj, M.; Madsen, S.; Maillard, V.; Scandella, V.; Sudria-Lopez, D.; Yuizumi, N.; Telley, L.; Knobloch, M. Lipid droplet availability affects neural stem/progenitor cell metabolism and proliferation. Nat. Commun. 2021, 12, 7362. [Google Scholar] [CrossRef]
- Ralhan, I.; Chang, C.L.; Lippincott-Schwartz, J.; Ioannou, M.S. Lipid droplets in the nervous system. J. Cell Biol. 2021, 220, e202102136. [Google Scholar] [CrossRef]
- Gopalan, A.B.; Van Uden, L.; Sprenger, R.R.; Fernandez-Novel Marx, N.; Bogetofte, H.; Neveu, P.A.; Meyer, M.; Noh, K.M.; Diz-Muñoz, A.; Ejsing, C.S. Lipotype acquisition during neural development is not recapitulated in stem cell–derived neurons. Life Sci. Alliance 2024, 7, e202402622. [Google Scholar] [CrossRef]
- Marszalek, J.R.; Kitidis, C.; Dararutana, A.; Lodish, H.F. Acyl-CoA Synthetase 2 Overexpression Enhances Fatty Acid Internalization and Neurite Outgrowth. J. Biol. Chem. 2004, 279, 23882–23891. [Google Scholar] [CrossRef]
- Petermann, A.B.; Reyna-Jeldes, M.; Ortega, L.; Coddou, C.; Yévenes, G.E. Roles of the Unsaturated Fatty Acid Docosahexaenoic Acid in the Central Nervous System: Molecular and Cellular Insights. Int. J. Mol. Sci. 2022, 23, 5390. [Google Scholar] [CrossRef]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Dec, K.; Alsaqati, M.; Morgan, J.; Deshpande, S.; Wood, J.; Hall, J.; Harwood, A.J. A high ratio of linoleic acid (n-6 PUFA) to alpha-linolenic acid (n-3 PUFA) adversely affects early stage of human neuronal differentiation and electrophysiological activity of glutamatergic neurons in vitro. Front. Cell Dev. Biol. 2023, 11, 1166808. [Google Scholar] [CrossRef]
- Naudí, A.; Jové, M.; Ayala, V.; Portero-Otín, M.; Barja, G.; Pamplona, R. Membrane lipid unsaturation as physiological adaptation to animal longevity. Front. Physiol. 2013, 4, 372. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Miyazaki, H.; Watanabe, H.; Sasaki, T.; Maehama, T.; Frohman, M.A.; Kanaho, Y. Phosphatidylinositol 4-Phosphate 5-Kinase Is Essential for ROCK-mediated Neurite Remodeling. J. Biol. Chem. 2002, 277, 17226–17230. [Google Scholar] [CrossRef]
- Liu, T.; Lee, S.Y. Phosphatidylinositol 4-phosphate 5-kinase α negatively regulates nerve growth factor-induced neurite outgrowth in PC12 cells. Exp. Mol. Med. 2013, 45, e16. [Google Scholar] [CrossRef]
- Viljetić, B.; Blažetić, S.; Labak, I.; Ivić, V.; Zjalić, M.; Heffer, M.; Balog, M. Lipid Rafts: The Maestros of Normal Brain Development. Biomolecules 2024, 14, 362. [Google Scholar] [CrossRef]
- Kaizuka, T.; Takumi, T. Alteration of synaptic protein composition during developmental synapse maturation. Eur. J. Neurosci. 2024, 59, 2894–2914. [Google Scholar] [CrossRef]
- Westra, M.; Gutierrez, Y.; MacGillavry, H.D. Contribution of Membrane Lipids to Postsynaptic Protein Organization. Front. Synaptic Neurosci. 2021, 13, 790773. [Google Scholar] [CrossRef]
- Mitiku, N.; Baker, J.C. Genomic Analysis of Gastrulation and Organogenesis in the Mouse. Dev. Cell 2007, 13, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Martinez, I.; Schurch, N.; Li, R.A.; Song, J.; Halley, P.A.; Das, R.M.; Burt, D.W.; Barton, G.J.; Storey, K.G. Major transcriptome re-organisation and abrupt changes in signalling, cell cycle and chromatin regulation at neural differentiation in vivo. Development 2014, 141, 3266–3276. [Google Scholar] [CrossRef]
- Blair, J.D.; Hockemeyer, D.; Doudna, J.A.; Bateup, H.S.; Floor, S.N. Widespread Translational Remodeling during Human Neuronal Differentiation. Cell Rep. 2017, 21, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Hamby, M.E.; Coskun, V.; Sun, Y.E. Transcriptional regulation of neuronal differentiation: The epigenetic layer of complexity. Biochim. Biophys. Acta BBA—Gene Regul. Mech. 2008, 1779, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Gaughwin, P.; Ciesla, M.; Yang, H.; Lim, B.; Brundin, P. Stage-Specific Modulation of Cortical Neuronal Development by Mmu-miR-134. Cereb. Cortex 2011, 21, 1857–1869. [Google Scholar] [CrossRef] [PubMed]
- Bicker, S.; Lackinger, M.; Weiß, K.; Schratt, G. MicroRNA-132, -134, and -138: A microRNA troika rules in neuronal dendrites. Cell. Mol. Life Sci. 2014, 71, 3987–4005. [Google Scholar] [CrossRef]
- Wang, C.; Hui, J.; Zhu, X.; Cui, S.; Cui, Z.; Xu, D. Lobetyolin Efficiently Promotes Angiogenesis and Neuronal Development in Transgenic Zebrafish. Nat. Prod. Commun. 2020, 15, 1–7. [Google Scholar] [CrossRef]
- Xia, M.; Zhang, Y.; Wu, H.; Zhang, Q.; Liu, Q.; Li, G.; Zhao, T.; Liu, X.; Zheng, S.; Qian, Z.; et al. Forsythoside B attenuates neuro-inflammation and neuronal apoptosis by inhibition of NF-κB and p38-MAPK signaling pathways through activating Nrf2 post spinal cord injury. Int. Immunopharmacol. 2022, 111, 109120. [Google Scholar] [CrossRef]
- Geng, Y.N.; Zhao, M.; Yang, J.L.; Cheng, X.; Han, Y.; Wang, C.B.; Jiang, X.-F.; Fan, M.; Zhu, L.L. GP-14 protects against severe hypoxia-induced neuronal injury through the AKT and ERK pathways and its induced transcriptome profiling alteration. Toxicol. Appl. Pharmacol. 2022, 448, 116092. [Google Scholar] [CrossRef]
- Guo, B.; Qi, M.; Luo, X.; Guo, L.; Xu, M.; Zhang, Y.; Li, Z.; Li, M.; Wu, R.; Guan, T.; et al. GIP attenuates neuronal oxidative stress by regulating glucose uptake in spinal cord injury of rat. CNS Neurosci. Ther. 2024, 30, e14806. [Google Scholar] [CrossRef]
- Jiang, H.; Lu, C.; Wu, H.; Ding, J.; Li, J.; Ding, J.; Gao, Y.; Wang, G.; Luo, Q. Decreased cold-inducible RNA-binding protein (CIRP) binding to GluRl on neuronal membranes mediates memory impairment resulting from prolonged hypobaric hypoxia exposure. CNS Neurosci. Ther. 2024, 30, e70059. [Google Scholar] [CrossRef]
- Martins, F.; Serrano, J.B.; Müller, T.; Da Cruz E Silva, O.A.B.; Rebelo, S. BRI2 Processing and Its Neuritogenic Role Are Modulated by Protein Phosphatase 1 Complexing. J. Cell. Biochem. 2017, 118, 2752–2763. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Marfell, B.J.; Christensen, M.E.; Waterhouse, N.J. Measuring Cell Death by Trypan Blue Uptake and Light Microscopy. Cold Spring Harb. Protoc. 2016, 2016, pdb-prot087155. [Google Scholar] [CrossRef] [PubMed]


| Calibration | Validation | |
|---|---|---|
| Correlation | 0.683 | 0.677 |
| RMSEC/RMSECV | 2.251 | 2.283 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, I.; Martins, F.; Goodfellow, B.J.; Nunes, A.; Rebelo, S. Spectroscopic Profile of Metabolome Dynamics During Rat Cortical Neuronal Differentiation. Int. J. Mol. Sci. 2025, 26, 8027. https://doi.org/10.3390/ijms26168027
Almeida I, Martins F, Goodfellow BJ, Nunes A, Rebelo S. Spectroscopic Profile of Metabolome Dynamics During Rat Cortical Neuronal Differentiation. International Journal of Molecular Sciences. 2025; 26(16):8027. https://doi.org/10.3390/ijms26168027
Chicago/Turabian StyleAlmeida, Idália, Filipa Martins, Brian J. Goodfellow, Alexandra Nunes, and Sandra Rebelo. 2025. "Spectroscopic Profile of Metabolome Dynamics During Rat Cortical Neuronal Differentiation" International Journal of Molecular Sciences 26, no. 16: 8027. https://doi.org/10.3390/ijms26168027
APA StyleAlmeida, I., Martins, F., Goodfellow, B. J., Nunes, A., & Rebelo, S. (2025). Spectroscopic Profile of Metabolome Dynamics During Rat Cortical Neuronal Differentiation. International Journal of Molecular Sciences, 26(16), 8027. https://doi.org/10.3390/ijms26168027









