Pyruvate Kinase Deficiency: Markedly Decreased Reticulocyte PK Activity and Limited Specificity of the PK/HK Ratio
Abstract
1. Introduction
- The actual level of PK activity in the reticulocytes of PKD patients and the impact of reticulocytosis on the total PK activity (activity in erythrocytes isolated from whole blood purified from leukocytes);
- The reliability of PK activity and the PK/HK ratio for differential diagnosis between PKD and other anemias with similar clinical presentations and reduced PK activity;
- The critical level of residual PK activity associated with clinically relevant hemolysis and anemia.
2. Results
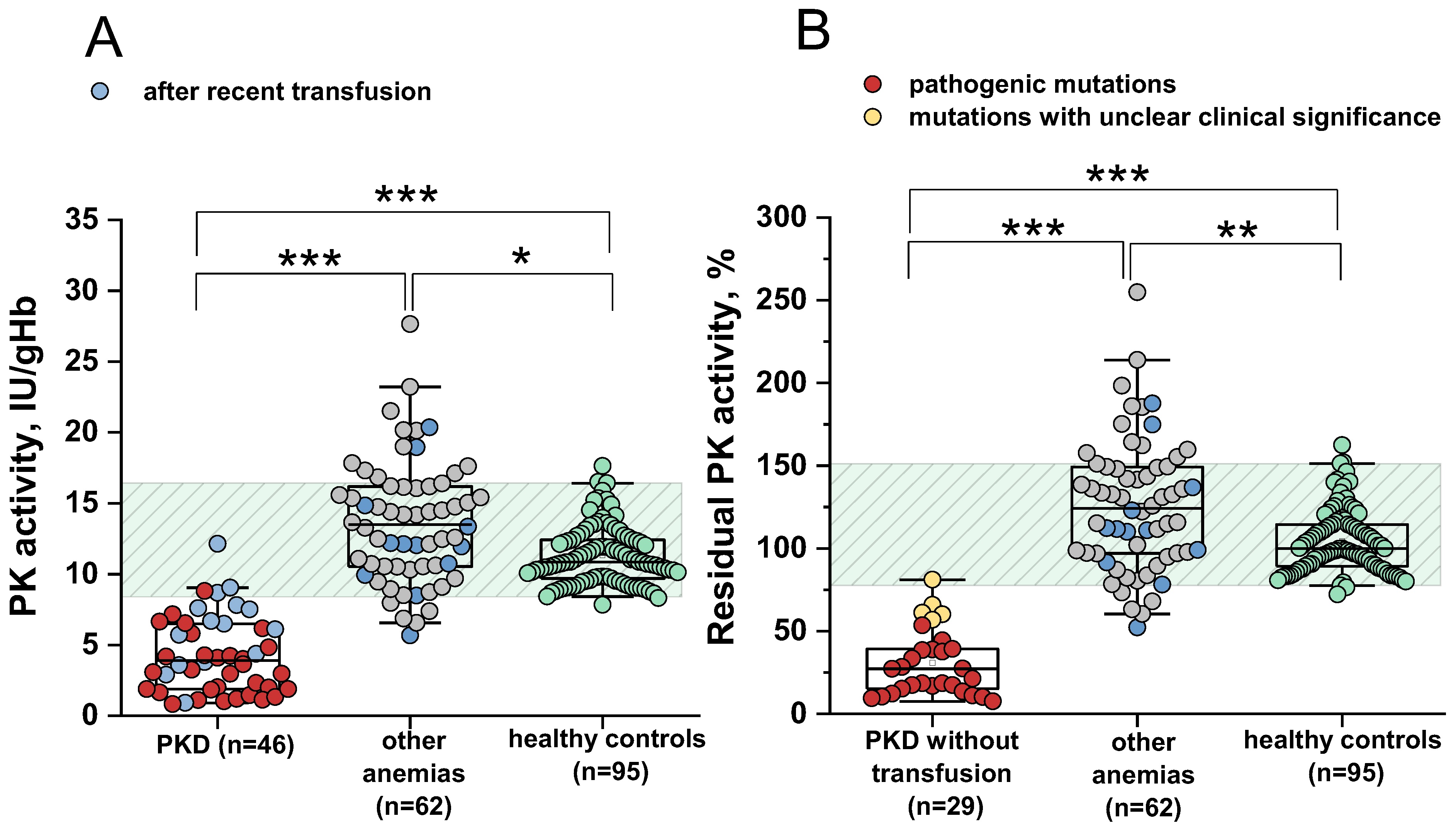
2.1. Sensitivity and Specificity of the PK Activity Assay for the Differential Diagnosis of PK Deficiency
2.2. Comparison of Sensitivity and Specificity of PK Activity and the PK/HK Ratio in the Diagnosis of PKD
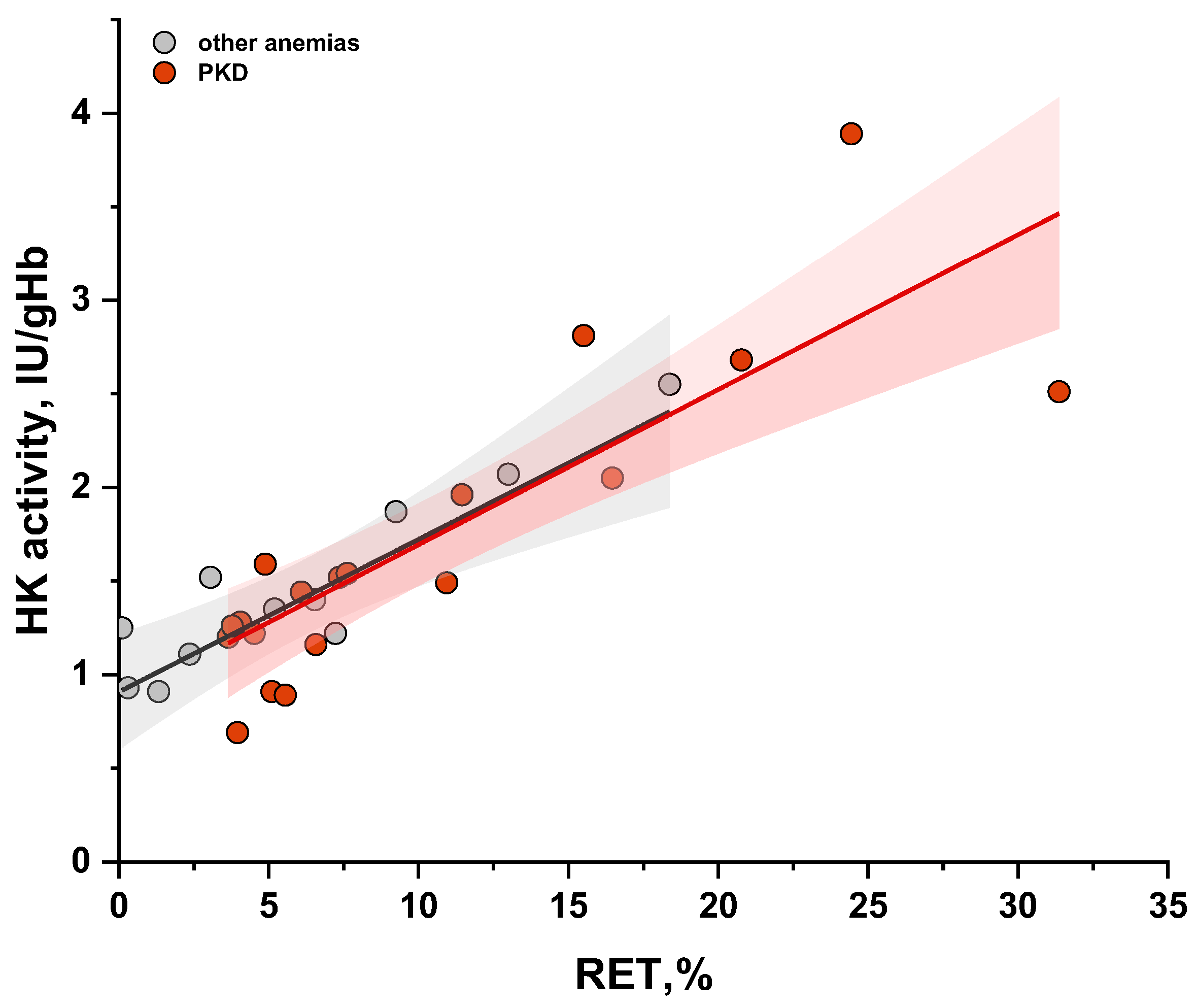
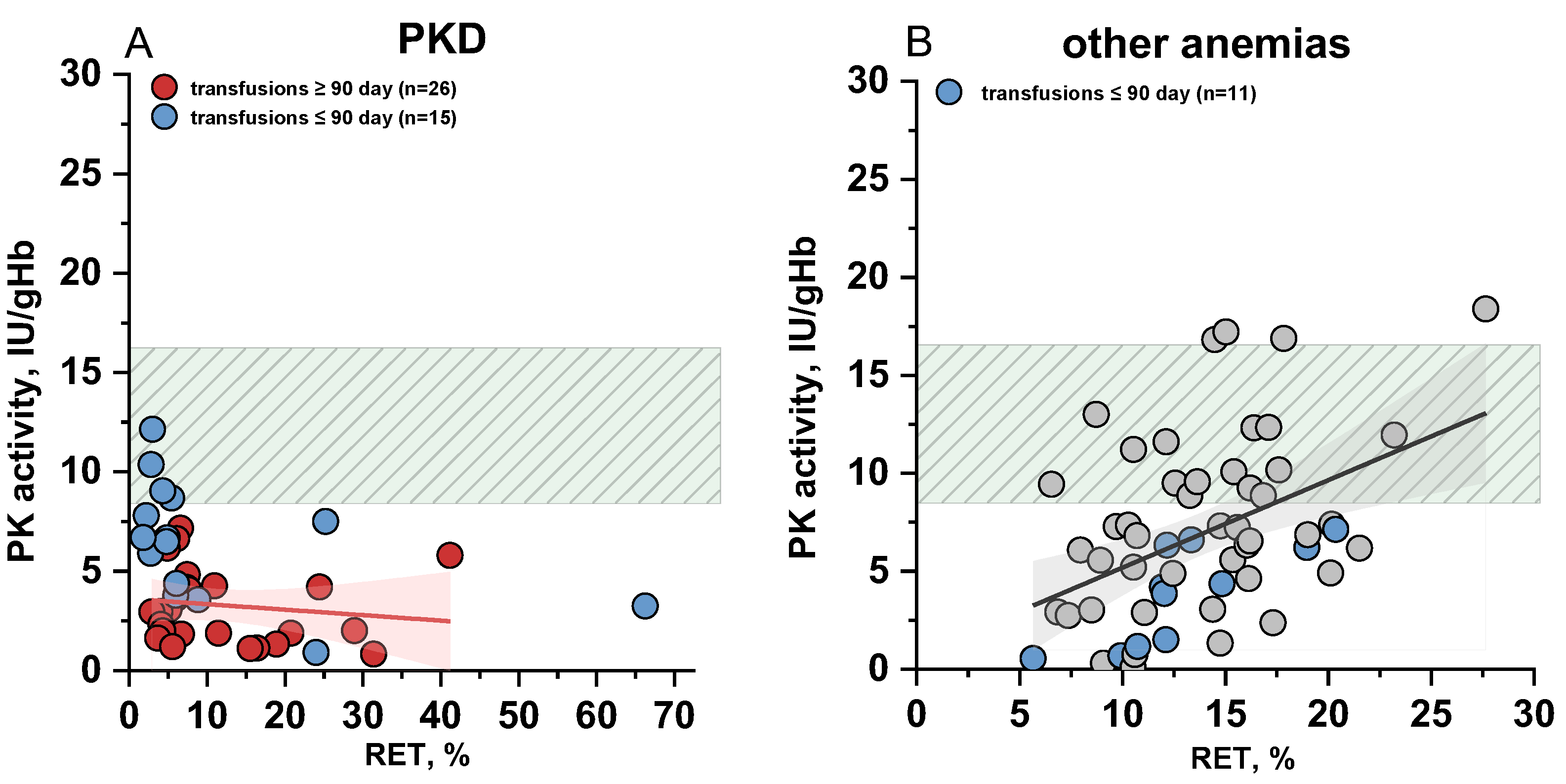
2.3. The Relationship Between Measured PK Activity and Reticulocyte Count in the Blood
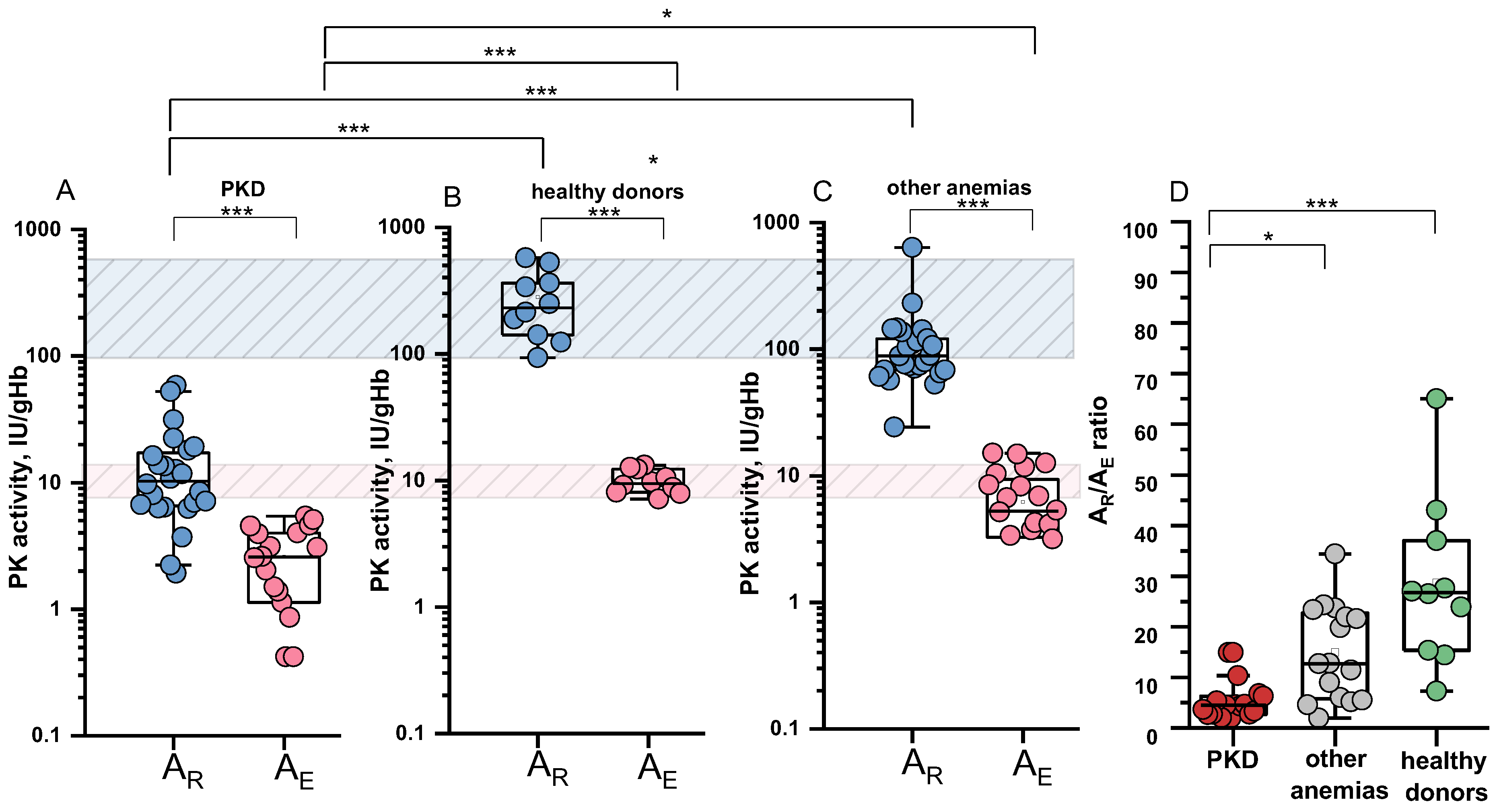
2.4. Specific PK Activities in the Reticulocyte and Erythrocyte and Their Ratio
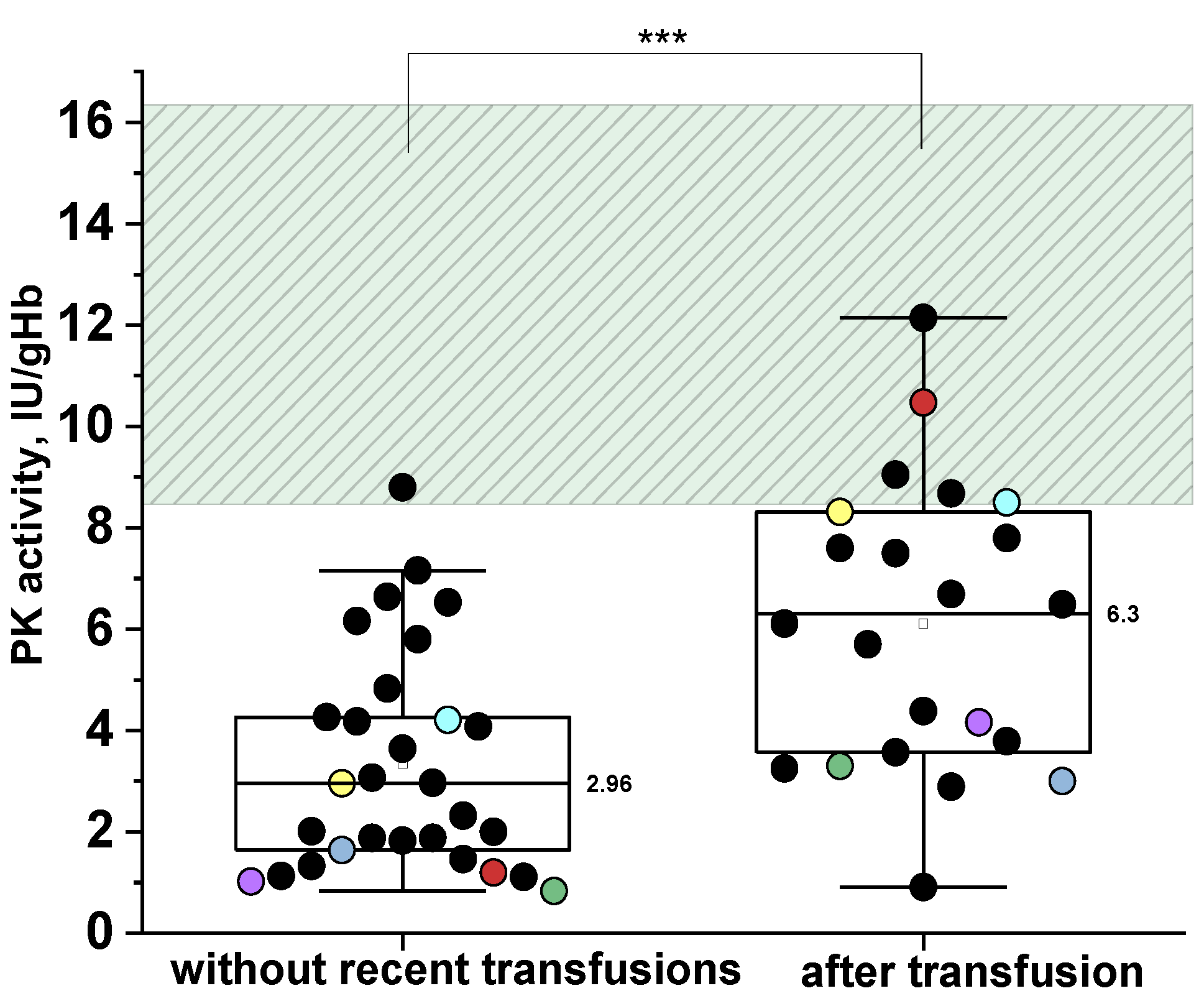
2.5. The Impact of Donor Red Blood Cells Transfusions on the PKD Diagnosis
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Reagents
4.3. Routine Hematological Studies
4.4. Genetic Analysis
4.5. Blood Preparation for Studies
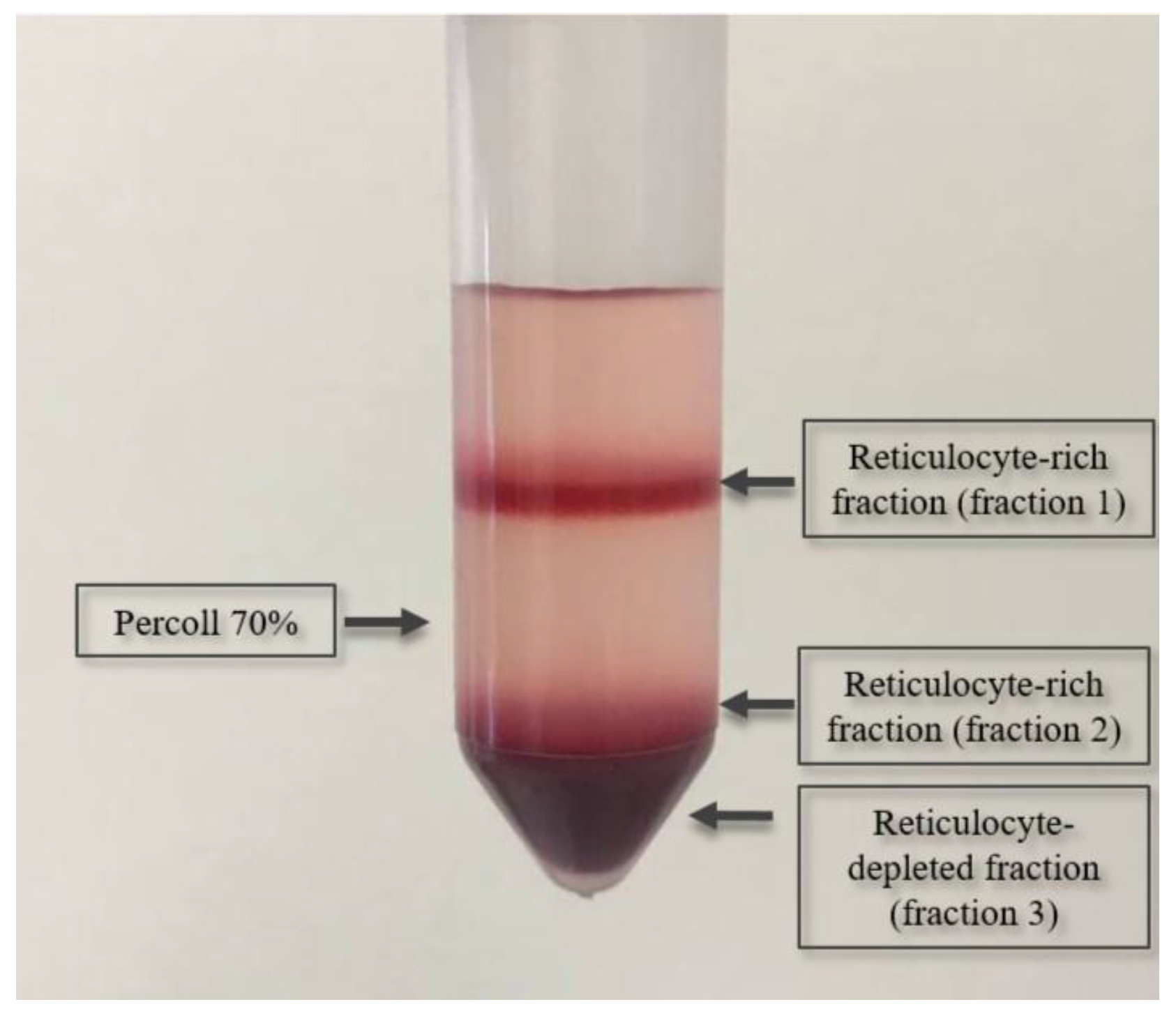
4.6. Isolation of Reticulocytes
4.7. Measurement of Enzyme Activity in Erythrocytes
4.8. Determination of Specific PK Activity in Erythrocytes and Reticulocytes
4.9. Statistical Analysis
5. Conclusions
- The data from our study suggest that reticulocytosis does not significantly contribute to the observed total PK activity. This is due to the markedly reduced (23-fold) PK activity in reticulocytes of PKD patients compared to normal reticulocyte activity.
- A distinct difference in the ratio of specific PK activities between reticulocytes and erythrocytes is observed in PKD patients and healthy donors. Healthy donors show a more pronounced difference in PK activity between these two cell types than patients.
- Key factors influencing the sensitivity and specificity of PK activity include transfusions of donor erythrocytes and decreased PK activity in other types of anemia, including within reticulocytes.
- In patients receiving regular transfusions (every 1–3 months), PK deficiency can be diagnosed by isolating reticulocytes and measuring specific PK activity in the reticulocytes.
- Slightly decreased PK activity is insufficient for diagnosing PK deficiency. In 97% of cases, residual PK activity greater than 66% in patients without recent transfusions indicates anemia not related to PK deficiency.
- The PK/HK ratio demonstrates higher sensitivity than PK activity when diagnosing PKD relative to healthy donors. However, it is less specific for the differential diagnosis of PKD and other anemias due to potential reduced PK activity and frequently observed reticulocytosis in other anemias. For this ratio to be used effectively, it is necessary to establish a reference range that accounts for the PK/HK ratio in patients with other types of anemia.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Secrest, M.H.; Storm, M.; Carrington, C.; Casso, D.; Gilroy, K.; Pladson, L.; Boscoe, A.N. Prevalence of Pyruvate Kinase Deficiency: A Systematic Literature Review. Eur. J. Haematol. 2020, 105, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Forman, L.; Rios-Larrain, E. Elevated Pyruvate Kinase Activity in Patients with Hemolytic Anemia Due to Red Cell Pyruvate Kinase “Deficiency”. Am. J. Med. 1987, 83, 899–904. [Google Scholar] [CrossRef]
- Martinov, M.V.; Plotnikov, A.G.; Vitvitsky, V.M.; Ataullakhanov, F.I. Deficiencies of Glycolytic Enzymes as a Possible Cause of Hemolytic Anemia. Biochim. Biophys. Acta Gen. Subj. 2000, 1474, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Grace, R.F.; Bianchi, P.; van Beers, E.J.; Eber, S.W.; Glader, B.; Yaish, H.M.; Despotovic, J.M.; Rothman, J.A.; Sharma, M.; McNaull, M.M.; et al. Clinical Spectrum of Pyruvate Kinase Deficiency: Data from the Pyruvate Kinase Deficiency Natural History Study. Blood 2018, 131, 2183–2192. [Google Scholar] [CrossRef]
- Fermo, E.; Bianchi, P.; Chiarelli, L.R.; Cotton, F.; Vercellati, C.; Writzl, K.; Baker, K.; Hann, I.; Rodwell, R.; Valentini, G.; et al. Red Cell Pyruvate Kinase Deficiency: 17 New Mutations of the PK-LR Gene. Br. J. Haematol. 2005, 129, 839–846. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Shehata, N.; Lang-Robertson, K.; Bianchi, P.; Glenthøj, A.; Sheth, S.; Neufeld, E.J.; Rees, D.C.; Chonat, S.; Kuo, K.H.M.; et al. Diagnosis and Management of Pyruvate Kinase Deficiency: International Expert Guidelines. Lancet Haematol. 2024, 11, e228–e239. [Google Scholar] [CrossRef]
- Bianchi, P.; Fermo, E.; Glader, B.; Kanno, H.; Agarwal, A.; Barcellini, W.; Eber, S.; Hoyer, J.D.; Kuter, D.J.; Maia, T.M.; et al. Addressing the Diagnostic Gaps in Pyruvate Kinase Deficiency: Consensus Recommendations on the Diagnosis of Pyruvate Kinase Deficiency. Am. J. Hematol. 2019, 94, 149–161. [Google Scholar] [CrossRef]
- Yozgat, A.K.; Erdem, A.Y.; Kaçar, D.; Özbek, N.Y.; Yaralı, N. Pyruvate Kinase Deficiency Mimicking Congenital Dyserythropoietic Anemia Type I. Turk. J. Pediatr. 2022, 64, 951–955. [Google Scholar] [CrossRef]
- Addonizio, K.; Al-Samkari, H.; Glader, B.; Morton, D.H.; Chonat, S.; Thompson, A.A.; Kuo, K.H.M.; Ravindranath, Y.; Wang, H.; Rothman, J.A.; et al. Pyruvate Kinase (PK) Protein and Enzyme Levels in the Diagnosis and Clinical Phenotype of PK Deficiency. Blood 2019, 134, 3515. [Google Scholar] [CrossRef]
- Lezon-Geyda, K.; Rose, M.J.; McNaull, M.A.; Knoll, C.M.; Yaish, H.M.; Pastore, Y.D.; Fermi, E.; Glader, B.; Bianchi, P.; Grace, R.F.; et al. Pklr Intron Splicing-Associated Mutations and Alternate Diagnoses Are Common in Pyruvate Kinase Deficient Patients with Single or No Pklr Coding Mutations. Blood 2018, 132, 3607. [Google Scholar] [CrossRef]
- Fattizzo, B.; Cavallaro, F.; Marcello, A.P.M.L.; Vercellati, C.; Barcellini, W. Pyruvate Kinase Deficiency: Current Challenges and Future Prospects. J. Blood Med. 2022, 13, 461–471. [Google Scholar] [CrossRef]
- Lakomek, M.; Schröter, W.; De Maeyer, G.; Winkler, H. On the Diagnosis of Erythrocyte Enzyme Defects in the Presence of High Reticulocyte Counts. Br. J. Haematol. 1989, 72, 445–451. [Google Scholar] [CrossRef]
- Zimran, A.; Torem, S.; Beutler, E. The in Vivo Ageing of Red Cell Enzymes: Direct Evidence of Biphasic Decay from Polycythaemic Rabbits with Reticulocytosis. Br. J. Haematol. 1988, 69, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Jansen, G.; Koenderman, L.; Rijksen, G.; Cats, B.P.; Staal, G.E. Characteristics of Hexokinase, Pyruvate Kinase, and Glucose-6-Phosphate Dehydrogenase during Adult and Neonatal Reticulocyte Maturation. Am. J. Hematol. 1985, 20, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Zanella, A.; Fermo, E.; Bianchi, P.; Valentini, G. Red Cell Pyruvate Kinase Deficiency: Molecular and Clinical Aspects. Br. J. Haematol. 2005, 130, 11–25. [Google Scholar] [CrossRef]
- Gök, V.; Leblebisatan, G.; Gürlek Gökçebay, D.; Güler, S.; Doğan, M.E.; Tuğ Bozdoğan, S.; Koca Yozgat, A.; Özcan, A.; Pekpak Şahinoğlu, E.; Tokgöz, H.; et al. Pyruvate Kinase Deficiency in 29 Turkish Patients with Two Novel Intronic Variants. Br. J. Haematol. 2024, 205, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Al-Samkari, H.; Addonizio, K.; Glader, B.; Morton, D.H.; Chonat, S.; Thompson, A.A.; Kuo, K.H.M.; Ravindranath, Y.; Wang, H.; Rothman, J.A.; et al. The Pyruvate Kinase (PK) to Hexokinase Enzyme Activity Ratio and Erythrocyte PK Protein Level in the Diagnosis and Phenotype of PK Deficiency. Br. J. Haematol. 2021, 192, 1092–1096. [Google Scholar] [CrossRef]
- Andres, O.; Loewecke, F.; Morbach, H.; Kraus, S.; Einsele, H.; Eber, S.; Speer, C.P. Hereditary Spherocytosis Is Associated with Decreased Pyruvate Kinase Activity Due to Impaired Structural Integrity of the Red Blood Cell Membrane. Br. J. Haematol. 2019, 187, 386–395. [Google Scholar] [CrossRef]
- de Wilde, J.R.A.; Ruiter, T.J.J.; Bos, J.; Traets, M.J.M.; van Oirschot, B.A.; Doeven, T.; Jans, J.J.M.; Wind-Rotolo, M.; Glenthøj, A.; van Solinge, W.W.; et al. Ex Vivo Activation of Pyruvate Kinase Improves Red Blood Cell Metabolism and Hydration in Hereditary Spherocytosis. Blood Red Cells Iron 2025, 1, 100005. [Google Scholar] [CrossRef]
- Rab, M.A.E.; van Oirschot, B.A.; van Straaten, S.; Biemond, B.J.; Bos, J.; Kosinski, P.A.; Kung, C.; van Beers, E.J.; van Wijk, R. Decreased Activity and Stability of Pyruvate Kinase in Hereditary Hemolytic Anemia: A Potential Target for Therapy By AG-348 (Mitapivat), an Allosteric Activator of Red Blood Cell Pyruvate Kinase. Blood 2019, 134 (Suppl. S1), 3506. [Google Scholar] [CrossRef]
- Traets, M.J.M.; Bos, J.F.; van der Veen, S.; van Pelt, L.; van Dijk, M.J.; van Oirschot, B.A.; de Wilde, J.R.A.; Jans, J.J.; van Solinge, W.W.; Schols, S.E.M.; et al. Pyruvate Kinase Function Correlates With Red Blood Cell Properties and Clinical Manifestations in Sickle Cell Disease. Am. J. Hematol. 2025, 100, 785–796, Correction in Am. J. Hematol. 2025, 100, 1471. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rab, M.A.E.; Bos, J.; van Oirschot, B.A.; van Straaten, S.; Kosinski, P.A.; Chubukov, V.; Kim, H.; Mangus, H.; Schutgens, R.E.G.; Pasterkamp, G.; et al. Decreased Activity and Stability of Pyruvate Kinase in Sickle Cell Disease: A Novel Target for Mitapivat Therapy. Blood 2021, 137, 2997–3001. [Google Scholar] [CrossRef] [PubMed]
- Fattizzo, B.; Vercellati, C.; Marcello, A.; Patel, P.; Wind-Rotolo, M.; Pettine, L.; Bonanomi, M.; Gaglio, D.; Bortolotti, M.; Leoni, C.; et al. Glycolytic Activity and in Vitro Effect of the Pyruvate Kinase Activator AG-946 in Red Blood Cells from Low-Risk Myelodysplastic Syndromes Patients: A Proof-of-Concept Study. Am. J. Hematol. 2024, 99, 1201–1204. [Google Scholar] [CrossRef]
- Utsugisawa, T.; Uchiyama, T.; Toki, T.; Shimojima-Yamamoto, K.; Ohga, S.; Ito, E.; Kanno, H. Enzymatic Changes in Red Blood Cells of Diamond-Blackfan Anemia. Tohoku J. Exp. Med. 2021, 255, 49–55. [Google Scholar] [CrossRef]
- Grace, R.F.; Barcellini, W. Management of Pyruvate Kinase Deficiency in Children and Adults. Blood 2020, 136, 1241–1249. [Google Scholar] [CrossRef]
- Piomelli, S.; Seaman, C.; Corash, L.; Beutler, E. How Do Red Cell Enzymes Age? Hypothesis and Facts. Br. J. Haematol. 1986, 64, 407–409. [Google Scholar] [CrossRef]
- Lakomek, M.; Neubauer, B.; von der Lühe, A.; Hoch, G.; Schröter, W.; Winkler, H. Erythrocyte Pyruvate Kinase Deficiency: Relations of Residual Enzyme Activity, Altered Regulation of Defective Enzymes and Concentrations of High-energy Phosphates with the Severity of Clinical Manifestation. Eur. J. Haematol. 1992, 49, 82–92. [Google Scholar] [CrossRef]
- van Dijk, M.J.; de Wilde, J.R.A.; Bartels, M.; Kuo, K.H.M.; Glenthøj, A.; Rab, M.A.E.; van Beers, E.J.; van Wijk, R. Activation of Pyruvate Kinase as Therapeutic Option for Rare Hemolytic Anemias: Shedding New Light on an Old Enzyme. Blood Rev. 2023, 61, 101103, Corrigendum in Blood Rev. 2024, 64, 101160. [Google Scholar] [CrossRef] [PubMed]
- Lenzner, C.; Nürnberg, P.; Jacobasch, G.; Gerth, C.; Thiele, B.-J. Molecular Analysis of 29 Pyruvate Kinase–Deficient Patients From Central Europe With Hereditary Hemolytic Anemia. Blood 1997, 89, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Rijksen, G.; Schipper-Kester, G.P.M.; Staal, G.E.J.; Veerman, A.J.P. Diagnosis of Pyruvate Kinase Deficiency in a Transfusion-dependent Patient with Severe Hemolytic Anemia. Am. J. Hematol. 1990, 35, 187–193. [Google Scholar] [CrossRef]
- Beutler, E. Red Cell Metabolism, 2nd ed.; Gruen & Stratton Inc: Orlando, FL, USA, 1984. [Google Scholar]
- Russell, B.; Suwanarusk, R.; Borlon, C.; Costa, F.T.M.; Chu, C.S.; Rijken, M.J.; Sriprawat, K.; Warter, L.; Koh, E.G.L.; Malleret, B.; et al. A Reliable Ex Vivo Invasion Assay of Human Reticulocytes by Plasmodium Vivax. Blood 2011, 118, e74–e81. [Google Scholar] [CrossRef] [PubMed]

| Patient | Gene Mutation | cDNA Nucleotide Substitution | Total PK Activity IU/gHb | AR, IU/gHb | AE, IU/gHb | AR/AE | PK/HK Ratio | |
|---|---|---|---|---|---|---|---|---|
| Allele1 | Allele2 | |||||||
| Patients with pyruvate kinase deficiency | ||||||||
| Severe condition | ||||||||
| 1. * | PKLR | hom c.101-1G>A | 7.8 | 14 ± 5 | 7.7 ± 0.7 | 1.8 ± 0.7 | n/d | |
| 2. | PKLR | hom c.401T>A | 5.8 ± 0.4 | n/d | n/d | n/d | n/d | |
| 3. | PKLR | hom c.695-2A>C | 2.01 ± 0.07 | n/d | n/d | n/d | n/d | |
| 4. * | PKLR | hom c.1079G>A | 7.60 | 23 ± 11 | 7.52 ± 0.36 | 3.1 ± 1.5 | 10.3 | |
| 5. * | PKLR | hom c.1269+1G>A | 12.1 | n/d | n/d | n/d | n/d | |
| 6. | PKLR | hom c.1529G>A | 1.65 | 6.2 ± 0.7 | 1.39 ± 0.09 | 4.5 ± 0.6 | 1.37 | |
| 6. * | 3 | n/d | n/d | n/d | n/d | |||
| 7. | PKLR | hom c.1529G>A | 1.20 | 2.23 ± 0.08 | 1.13 ± 0.01 | 1.98 ± 0.04 | 1.35 | |
| 7. * | 10.47 | n/d | n/d | n/d | n/d | |||
| 8. | PKLR | hom c.1529G>A | 1.83 | 7.0 ± 0.3 | 1.50 ± 0.03 | 4.64 ± 0.11 | n/d | |
| 9. | PKLR | hom c.1529G>A | 1.13 | 3.7 ± 2.3 | 0.86 ± 0.43 | 4.3 ± 3.4 | 0.39 | |
| 10. | PKLR | hom c.1529G>A | 1.88 | n/d | n/d | n/d | 0.96 | |
| 11. | PKLR | hom c.1529G>A | 1.88 | n/d | n/d | n/d | 0.70 | |
| 12. * | PKLR | c.101-1G>A | c.1318G>T | 5.7 ± 0.2 | n/d | n/d | n/d | n/d |
| 13. * | PKLR | c.948C>A | c.848T>A | 6.11 | 53 ± 7 | 5.08 ± 0.17 | 10.4 ± 1.4 | 7.53 |
| 14. | PKLR | c.1130T>C | c.1318G>T | 8.8 ± 0.02 | n/d | n/d | n/d | n/d |
| 15. * | PKLR | c.1174G>A | c.1456C>T | 7.51 ± 0.08 | n/d | n/d | n/d | n/d |
| 16. * | PKLR | c.1174G>A | c.1456C>T | 9.05 | 11.7 ± 0.3 | 8.80 ± 0.14 | 1.33 ± 0.04 | 11.8 |
| 17. * | PKLR | c.1436G>A | c.487C>T | 2.89 ± 0.2 | n/d | n/d | n/d | n/d |
| 18. | PKLR | c.1456C>T | c.1157C>T | 4.18 | 8 ± 3 | 4.0 ± 0.8 | 2.0 ± 0.9 | 2.75 |
| 19. * | PKLR | Ex 1-2 del | c.1529G>A | 3.25 ± 0.09 | n/d | n/d | n/d | n/d |
| 20. * | PKLR | Ex 1-2 del | c.1529G>A | 3.3 | 12.69 ± 2.16 | 2.8 ± 0.25 | 4.5 ± 0.9 | n/d |
| 20. | 0.83 | n/d | n/d | n/d | 0.41 | |||
| 21. * | PKLR | Ex 1-2 del | c.1529G>A | 4.16 | 6.39 ± 2.38 | 4.3 ± 0.3 | 1.5 ± 0.7 | n/d |
| 21. | 1.02 | n/d | n/d | n/d | 0.68 | |||
| 22. * | PKLR | c.-63G>A | c.1529G>A | 6.69 | n/d | n/d | n/d | 8.92 |
| 23. * | PKLR | c.101-1G>A | c.1529G>A | 3.6 ± 0.2 | n/d | n/d | n/d | n/d |
| 24. * | PKLR | c.460G>A | c.1529G>A | 4.38 | n/d | n/d | n/d | 1.65 |
| 25. * | PKLR | c.994G>A | c.1529G>A | 3.8 ± 0.4 | n/d | n/d | n/d | n/d |
| 26. | PKLR | c.1079G>A | c.1529G>A | 0.91 | 1.9 ± 0.9 | 0.4 ± 0.3 | 5 ± 3 | n/d |
| 27. | PKLR | c.1223C>T | c.1529G>A | 4 | n/d | n/d | n/d | n/d |
| 28. * | PKLR | c.1583A>T | c.1436G>A | 6.49 | n/d | n/d | n/d | n/d |
| 29. * | PKLR | c.1637T>C | c.1529G>A | 8.5 | 13.5 ± 0.12 | 8.27± 0.02 | 1.63 ± 0.015 | n/d |
| 29. | 4.21 | n/d | n/d | n/d | 1.08 | |||
| Moderate condition | ||||||||
| 30. | PKLR | hom c.1318G>A | 1.11 | 9.8 ± 0.9 | 0 | n/d | 0.4 | |
| 31. | PKLR | c.932T>C | c.1456C>T | 2.97 | 7.1 ± 0.96 | 2.6 ± 0.096 | 2.7 ± 0.4 | 4.31 |
| 32. | PKLR | c.1231G>T | c.1456C>T | 4.08 | 19.2 ± 3.2 | 3.06 ± 0.25 | 6.3 ± 1.2 | 2.65 |
| 33. | PKLR | c.1231G>T | c.1456C>T | 4.26 | 8.46 ± 4 | 3.1 ± 0.6 | 2.9 ± 1.4 | 2.84 |
| 34. * | PKLR | c.1130T>C | c.1456C>T | 8.7 ± 0.5 | n/d | n/d | n/d | n/d |
| 35. | PKLR | c.1594C>T | c.1456C>T | 2.96 | n/d | n/d | n/d | 2.35 |
| 35. * | 8.31 | n/d | n/d | n/d | n/d | |||
| 36. | PKLR | c.1130T>C | c.1456C>T | 6.64 | 16 ± 6 | 4.5 ± 1.2 | 3.6 ± 1.6 | n/d |
| Mild condition | ||||||||
| 37. | PKLR | c.932T>C | c.1456C>T | 1.46 ± 0.17 | n/d | n/d | n/d | n/d |
| 38. | PKLR | c.1076G>A | c.1456C>T | 4.83 ± 0.3 | n/d | n/d | n/d | n/d |
| 39. | PKLR | c.1181C>T | c.1456C>T | 6.16 | 31.3 ± 1.0 | 4.61 ± 0.19 | 6.8 ± 0.4 | 3.88 |
| 40. | PKLR | c.1181C>T | c.1456C>T | 7.16 | 18 ± 3 | 5.4 ± 0.6 | 3.3 ± 0.7 | 6.15 |
| 41. | PKLR | c.1195del | c.1456C>T | 3.07 | 7 ± 6 | 2.6 ± 0.4 | 2.6 ± 1.0 | 3.38 |
| 42. | PKLR | c.1291G>A | c.1529G>A | 6.53 | 59 ± 6 | 3.9 ± 0.4 | 15 ± 2 | 5.35 |
| 43. | PKLR | c.665G>A | c.1429A>G | 3.64 | n/d | n/d | n/d | n/d |
| 44. | PKLR | c.1072G>T | c.1529G>A | 1.33 | 6.27 ± 0.5 | 0.42 ± 0.2 | 15 ± 8 | n/d |
| 45. | PKLR | c.1583A>T | c.1510C>T | 2.32 | 10.8 ± 0.4 | 2.03 ± 0.03 | 5.3 ± 0.2 | 1.81 |
| 46. | PKLR | hom c.1529G>A | 2 | n/d | n/d | n/d | n/d | |
| Median | 3.9 | 10.27 | 2.57 | 4.5 | 2.75 | |||
| Patients with other anemias | ||||||||
| 47. | ALAD | het c.375del | 14.38 | n/d | n/d | n/d | 9.46 | |
| 48. | ANK1 | het c.5097-33G>A | 19 | 76.1 | 14.8 | 5.15 | n/d | |
| 49. | ANK1 | het c.596dup frameshift ter | 15.0 | 74.5 ± 1.1 | 0 | n/d | n/d | |
| 50. | ANK1 | het c.4104+4A>G | 8.50 | 120 ± 20 | 5.2 ± 0.8 | 23 ± 6 | n/d | |
| PIEZO1 | het c.3284A>C | |||||||
| 51. | ANK1 | het c.3329_3336 delinsACAAG | 16.13 | 71 ± 9 | 11.7 ± 1.6 | 6.1 ± 1.1 | n/d | |
| 52. * | ANK1 | het c.3778T>C | 14.8 | 110 ± 30 | 8 ± 2 | 14 ± 5 | n/d | |
| 53. * | ANK1 | het c.1814del | 13.3 | 65 ± 17 | 8.7 ± 1.5 | 7.5 ± 1.3 | 14.50 | |
| 54. | ANK1 | het c.596dup frameshift ter | 15.4 | 61 ± 4 | 5.3 ± 0.9 | 11.5 ± 2.1 | n/d | |
| 55. * | ANK1 | het c.4153C>T | 11.9 | 136 ± 14 | 6.9 ± 0.8 | 20 ± 3 | n/d | |
| 56. | ANK1 | het c.2325dupG | 13.3 | 115 | 3.36 | 34.4 | n/d | |
| 57. | GPI | c.1039C>T | c.1612C>A | 27.7 ± 0.5 | n/d | n/d | n/d | 10.8 |
| 58. | HBB | het c.193G>T | 10.5 | n/d | n/d | n/d | 8.41 | |
| 59. * | HK1 | c.1951G>A | c.2128G>A | 12.2 | 79.1 ± 1.1 | 9.3 ± 0.3 | 8.5 ± 0.3 | 18.22 |
| 60. * | HK1 | homo c.34C>T | 5.66 | 144 ± 3 | 5.5 ± 0.4 | 26.2 ± 2.0 | 14.2 | |
| HBB | homo c.316-106 C>G | |||||||
| 61. | KCNN4 | het c.940T>C | 16.2 | 130 ± 40 | 8 ± 4 | 17 ± 10 | 11.6 | |
| 62. | PIEZO1 | het c.7483_7488dup | 9.60 | 68 ± 3 | 3.2 ± 1.0 | 21 ± 7 | n/d | |
| 63. | PIEZO1 | het c.7483_7489dup | 10.5 | 53 ± 2 | 4.1 ± 0.7 | 13 ± 2 | n/d | |
| 64. | PIGA | c.264delA | c.715+1G>A | 16.4 | 56.8 | 10.3 | 5.49 | n/d |
| 65. * | RPL11 | het c.45delT | 10.7 | n/d | n/d | n/d | 7.84 | |
| 66. * | RPS19 | het c.3G>A | 9.92 | 80 ± 2 | 6.4 ± 0.2 | 12.5 ± 0.6 | n/d | |
| 67. | SLC4A1 | het c.1030C>T | 15.6 | 74 ± 12 | 8.2 ± 1.6 | 9 ± 2 | 12.8 | |
| 68. | SLC4A1 | het c.1030C>T | 10.5 | 88.3 ± 1.3 | 3.7 ± 0.3 | 24 ± 2 | 7.81 | |
| 69. | SLC4A1 | het c.749del | 20.2 | 230 ± 50 | 0 | n/d | n/d | |
| 70. | SPTA1 | het c.82C>T | 16.1 | 600 ± 400 | 0 | n/d | n/d | |
| 71. | SPTA1 | het c.2222A>T | 11.1 | 150 ± 60 | 6.7 ± 1.9 | 22 ± 10 | n/d | |
| 72. | SPTA1 | het c.4019C>A | 14.7 | n/d | n/d | n/d | 16.2 | |
| NF1 | het c.4614G>A | |||||||
| KDM6A | het c.2308C>T | |||||||
| 73. | SPTA1 | het c.5645_5647del | 17.3 | n/d | n/d | n/d | 15.5 | |
| FLNA | het c.1790_1791delTT | |||||||
| COL3A1 | het c.689A>T | |||||||
| CFH | het c.2407T>A | |||||||
| 74. | SPTA1 | c.4339-99C>T | c.6421C>T | 13.6 | 24.1 | 12.6 | 1.91 | n/d |
| 75. | SPTA1 | het c.2671C>T | 23.2 | 68.8 | 15.2 | 4.54 | n/d | |
| HBB | het c.364G>C | |||||||
| 76. | SPTB | het c.566+1G>A | 17.1 | 104 ± 3 | 4.3 ± 0.8 | 24 ± 5 | n/d | |
| 77. | SPTB | het c.1912C>T | 14.5 | 89 ± 13 | 0 | n/d | n/d | |
| PKLR | het c.1456C>T | |||||||
| 78. | SPTB | het c.5800_5801insCAGG | 8.72 | 65 ± 6 | 0 | n/d | 4.21 | |
| 79. | ANK1 | het c.4462C>T | 16.2 | n/d | n/d | n/d | 8.65 | |
| 80. | SF3B1 | n/d | 9.08 | 107 ± 10 | 8.4 ± 0.3 | 12.7 ± 1.3 | 9.75 | |
| Median | 13.5 | 88.3 | 5.2 | 12.7 | 10.8 | |||
| Healthy donors (n = 95) | ||||||||
| Median | 10.85 | 231.5 | 9.4 | 26.7 | 13.5 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koleva, L.; Dolgikh, I.A.; Kryukova, A.V.; Prudinnik, D.S.; Bovt, E.A.; Shakhidzhanov, S.S.; Mann, S.G.; Smetanina, N.S.; Ataullakhanov, F.I.; Sinauridze, E.I. Pyruvate Kinase Deficiency: Markedly Decreased Reticulocyte PK Activity and Limited Specificity of the PK/HK Ratio. Int. J. Mol. Sci. 2025, 26, 8606. https://doi.org/10.3390/ijms26178606
Koleva L, Dolgikh IA, Kryukova AV, Prudinnik DS, Bovt EA, Shakhidzhanov SS, Mann SG, Smetanina NS, Ataullakhanov FI, Sinauridze EI. Pyruvate Kinase Deficiency: Markedly Decreased Reticulocyte PK Activity and Limited Specificity of the PK/HK Ratio. International Journal of Molecular Sciences. 2025; 26(17):8606. https://doi.org/10.3390/ijms26178606
Chicago/Turabian StyleKoleva, Larisa, Ivan A. Dolgikh, Aleksandra V. Kryukova, Dmitry S. Prudinnik, Elizaveta A. Bovt, Soslan S. Shakhidzhanov, Svetlana G. Mann, Nataliya S. Smetanina, Fazoil I. Ataullakhanov, and Elena I. Sinauridze. 2025. "Pyruvate Kinase Deficiency: Markedly Decreased Reticulocyte PK Activity and Limited Specificity of the PK/HK Ratio" International Journal of Molecular Sciences 26, no. 17: 8606. https://doi.org/10.3390/ijms26178606
APA StyleKoleva, L., Dolgikh, I. A., Kryukova, A. V., Prudinnik, D. S., Bovt, E. A., Shakhidzhanov, S. S., Mann, S. G., Smetanina, N. S., Ataullakhanov, F. I., & Sinauridze, E. I. (2025). Pyruvate Kinase Deficiency: Markedly Decreased Reticulocyte PK Activity and Limited Specificity of the PK/HK Ratio. International Journal of Molecular Sciences, 26(17), 8606. https://doi.org/10.3390/ijms26178606







