Deletion of the Epidermal Protease KLK5 Aggravates the Symptoms of Congenital Ichthyosis CDSN-nEDD
Abstract
1. Introduction
2. Results
2.1. Production and Validation of the HEE Model for CDSN-nEDD
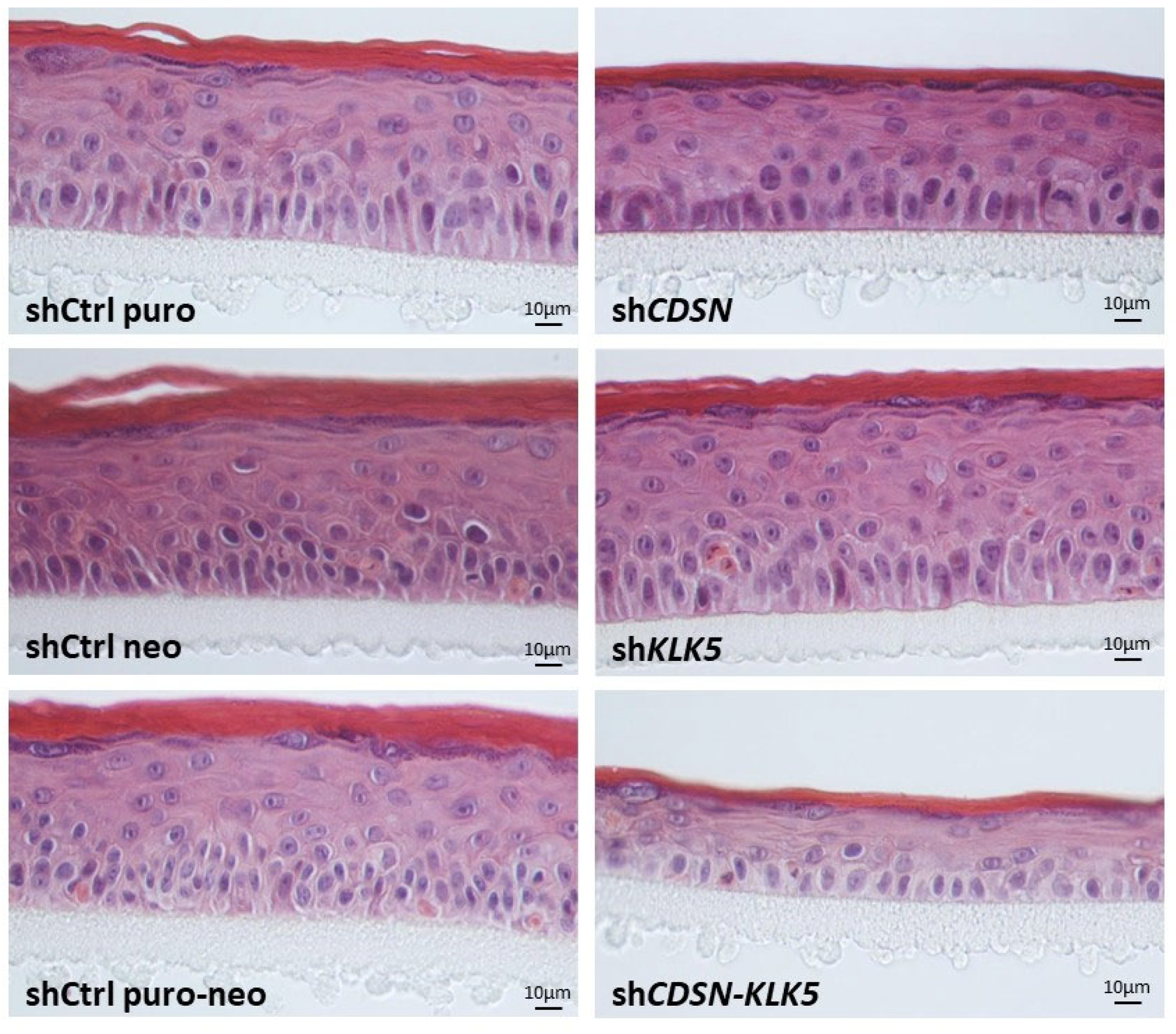
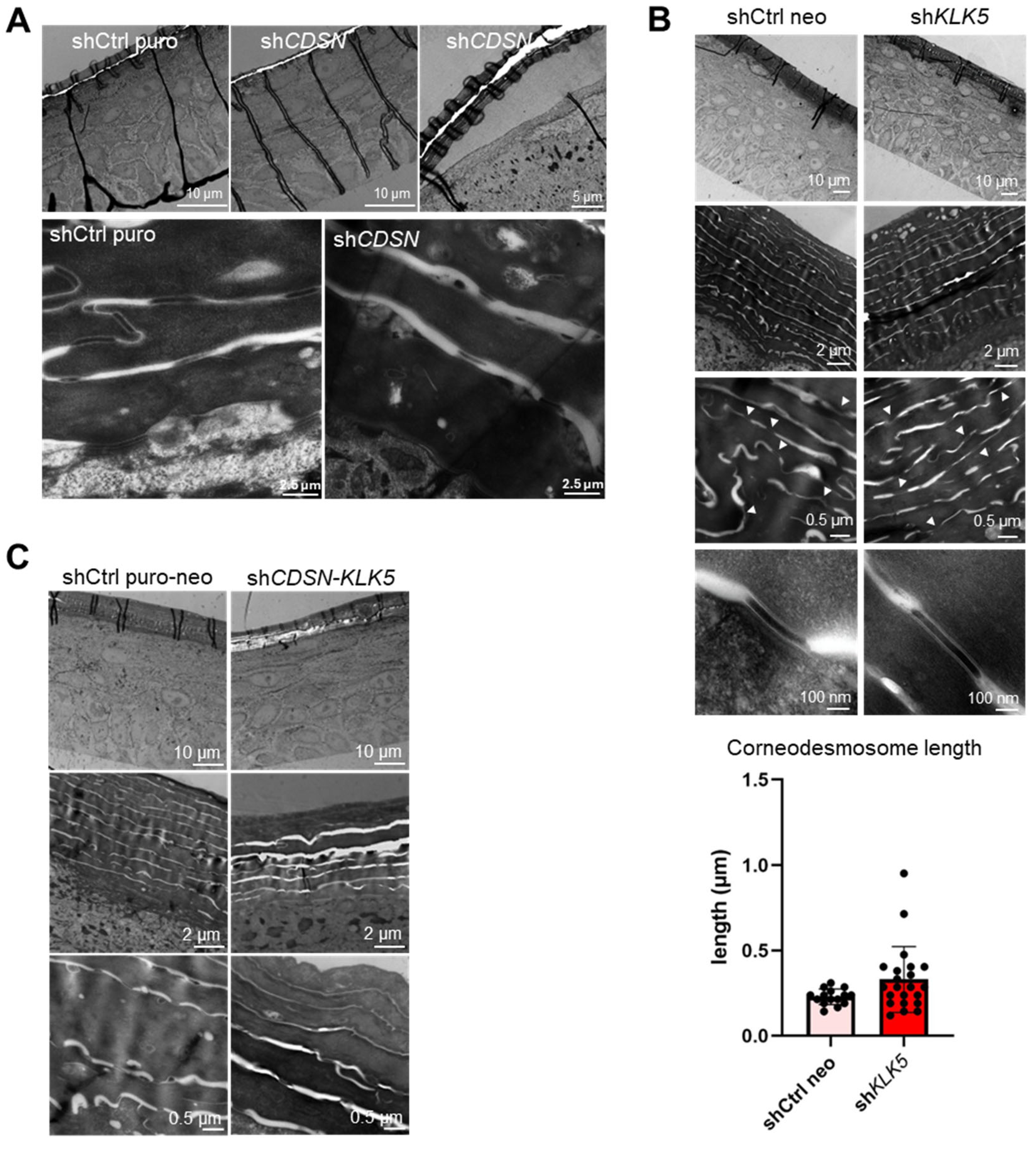
2.2. KLK5 Gene Silencing Exacerbates the Histological and Ultrastructural Abnormalities Observed in shCDSN HEE
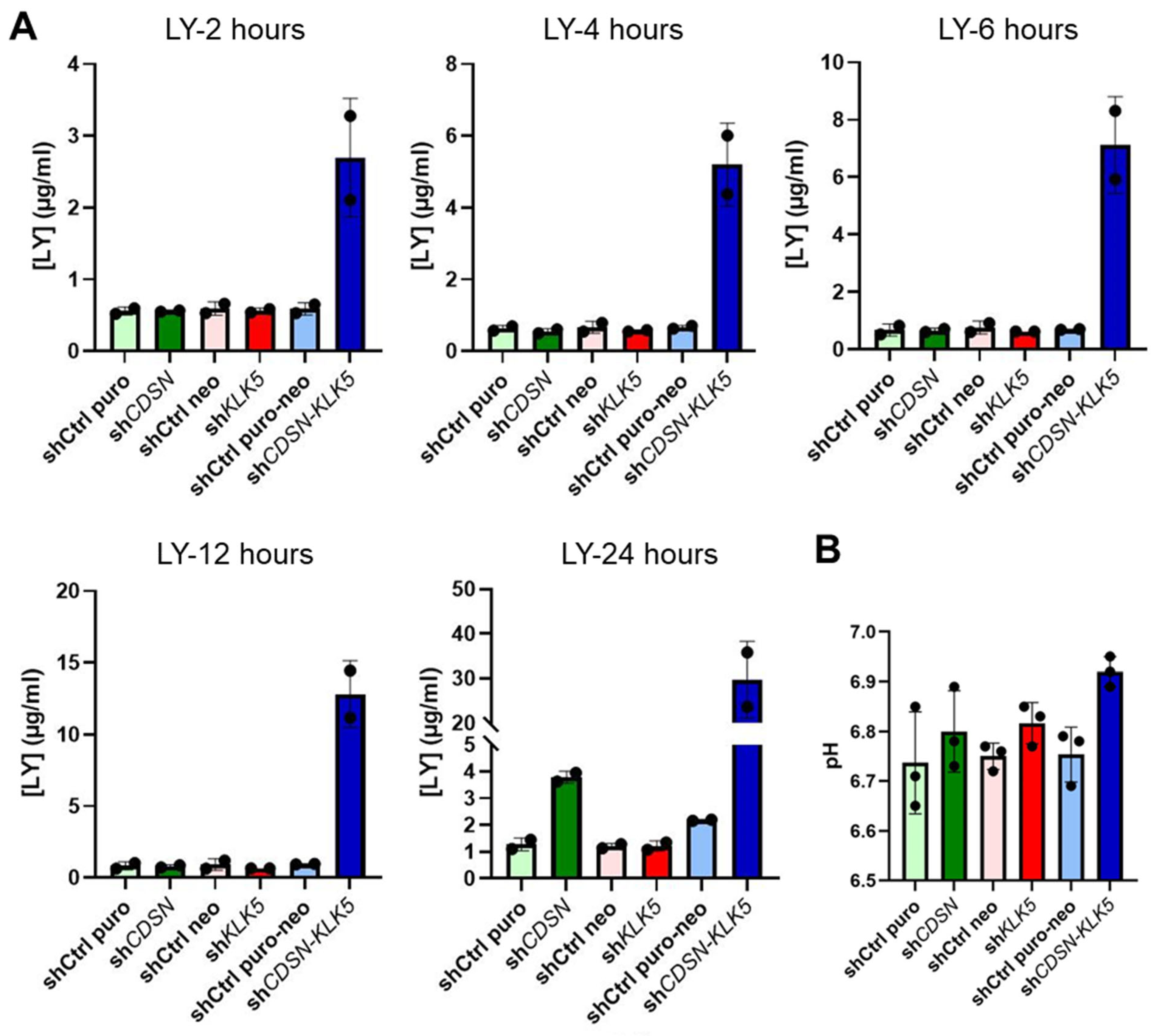
2.3. KLK5 Gene Silencing Worsens the Impaired Permeability Barrier Observed in the shCDSN HEEs
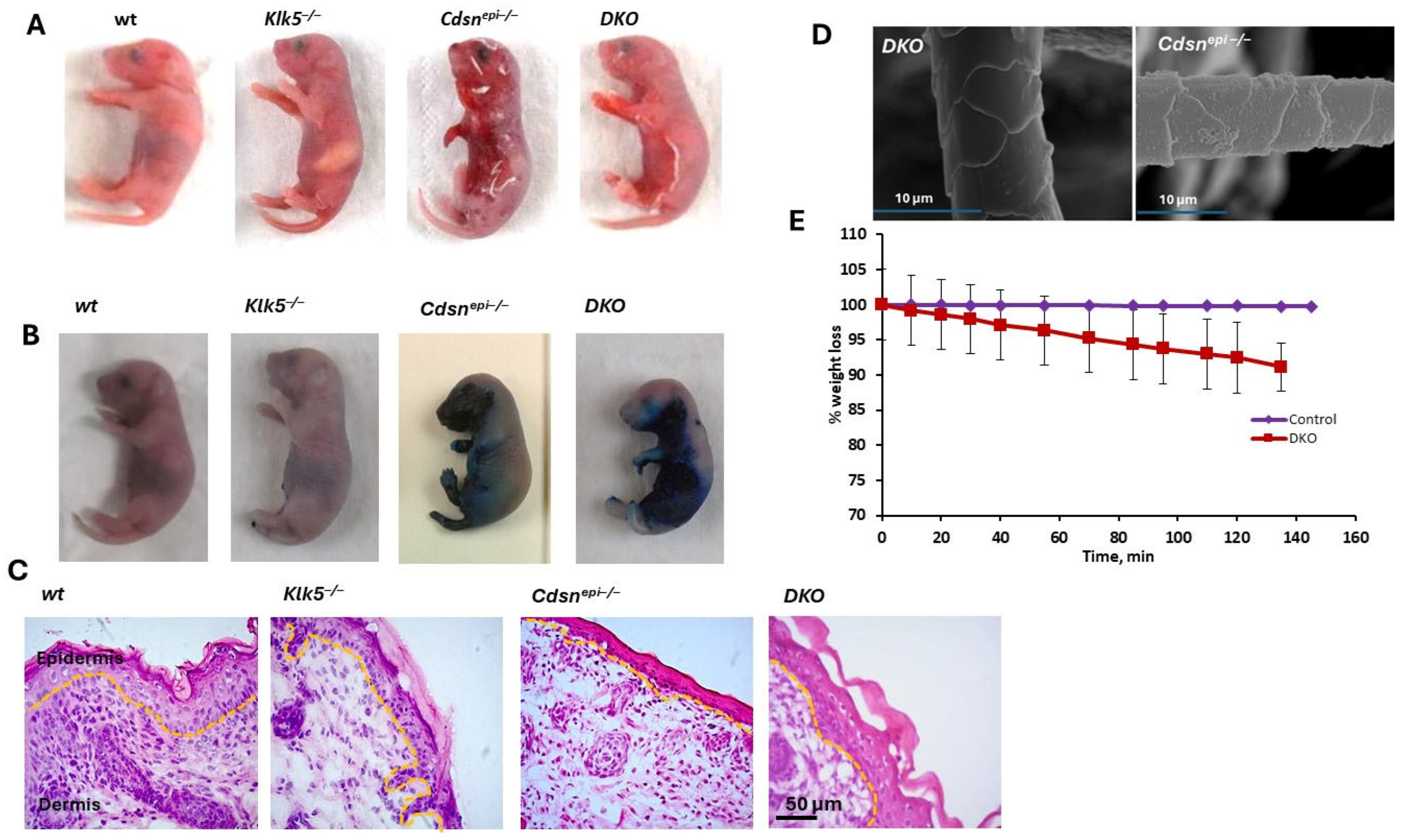
2.4. Deletion of Klk5 in Cdsnepi−/− Mice Does Not Improve the Phenotype of CSDN-nEDD
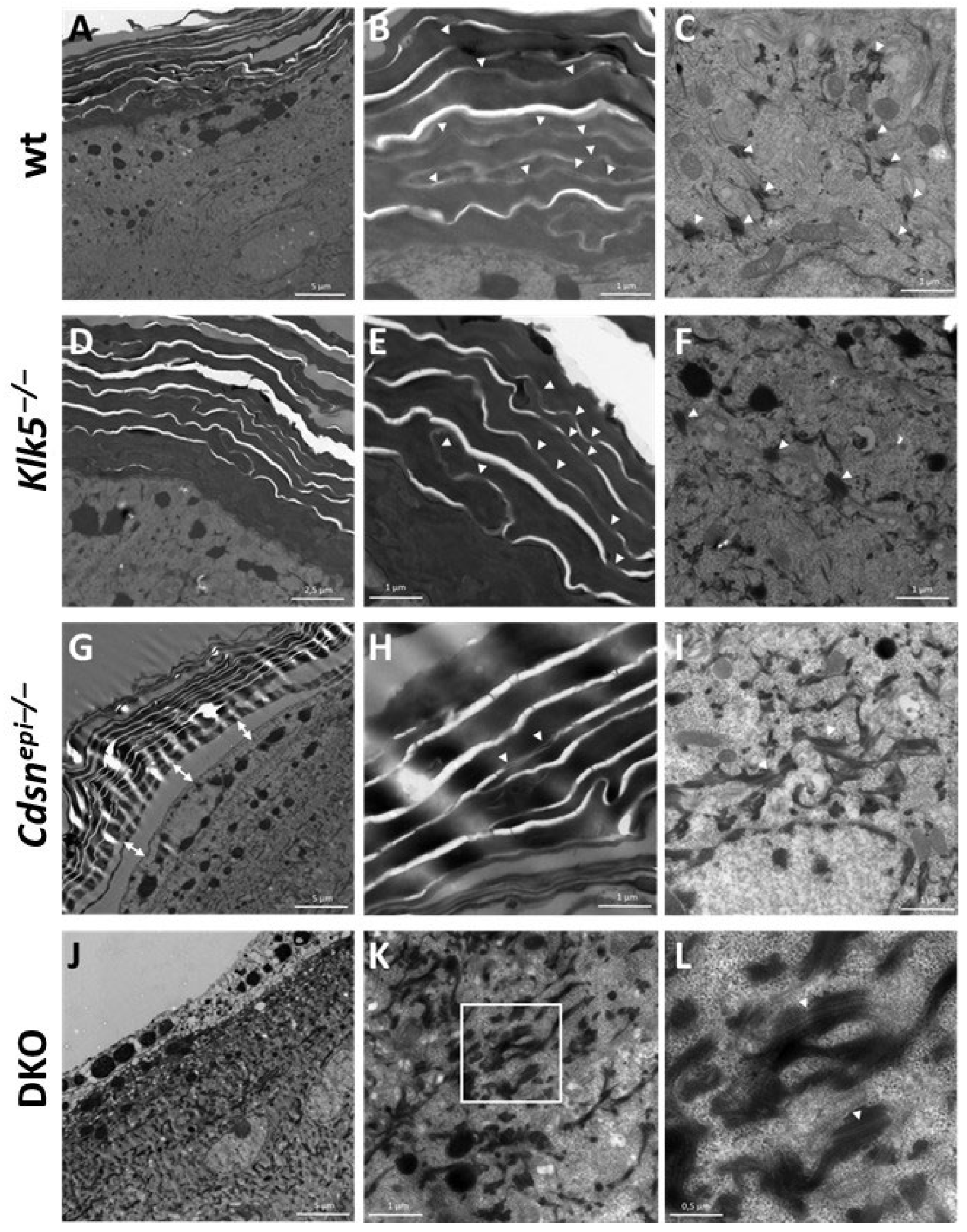
2.5. DKO Mice Display Ultrastructural Alteration Similar to Those Observed in shCDSN-KLK5 HEEs
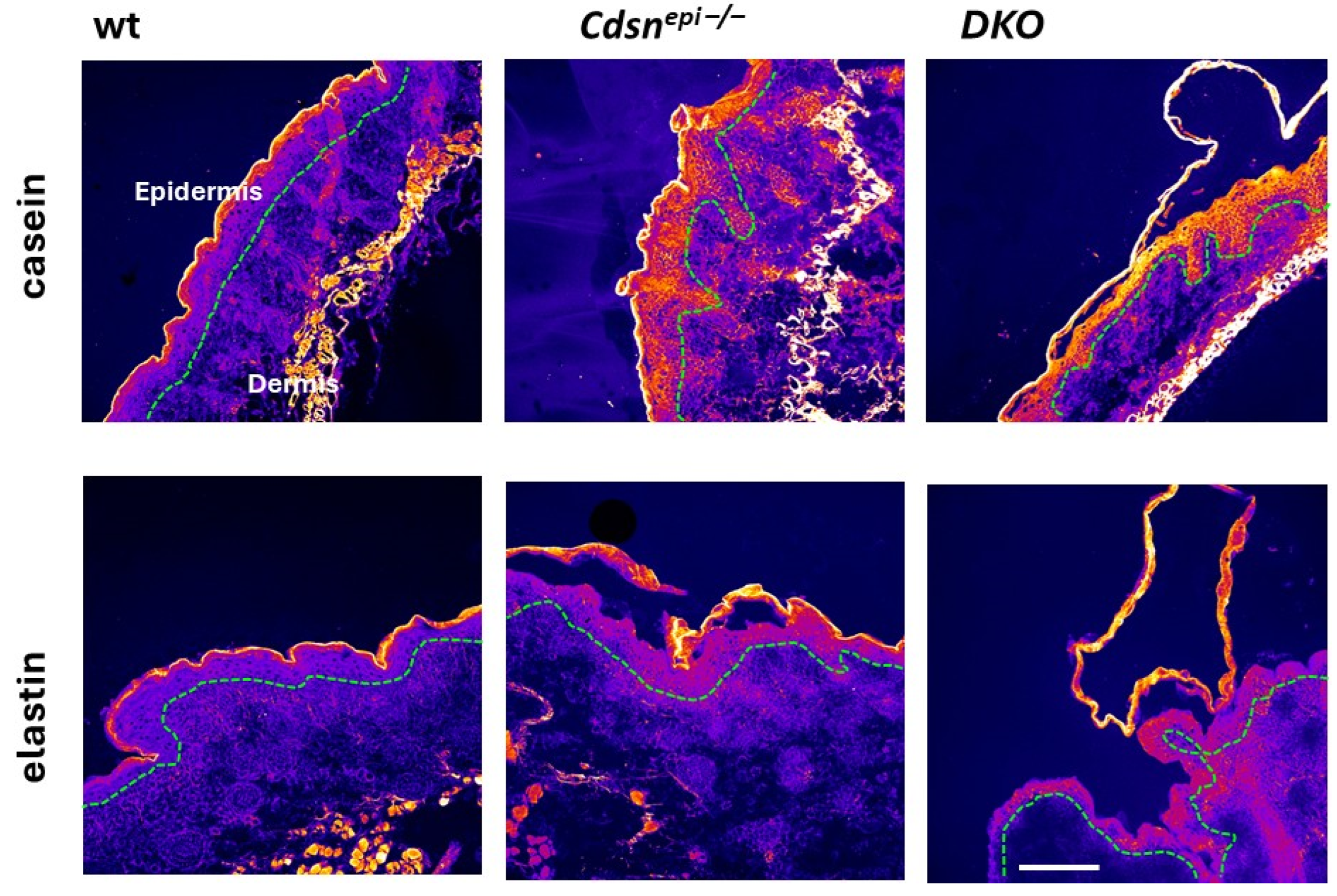
2.6. Aberrant Overall Proteolytic Activities in DKO Epidermis
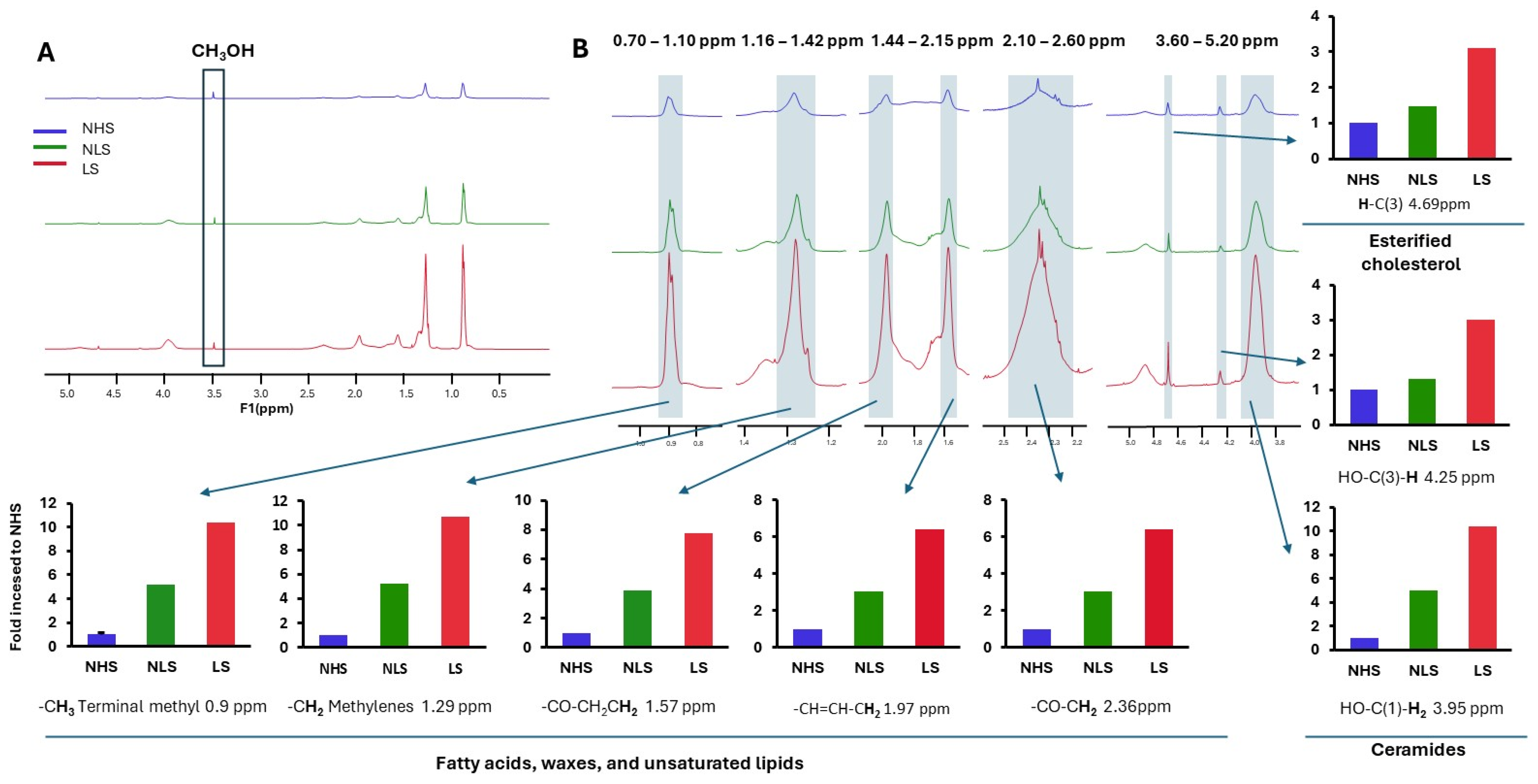
2.7. Lipid Characterization of the DKO Epidermis and Human SC Sample
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Human Samples
4.3. Mice
4.4. Cell Culture and Generation of HEE
4.5. shRNA Lentiviral Particles and Keratinocyte Transduction
4.6. In Situ Zymography
4.7. Western Blot
4.8. Transmission Electron Microscopy (TEM)
4.9. Scanning Electron Microscopy (SEM)
4.10. Toluidine Blue Penetration and Dehydration Assay
4.11. Lucifer Yellow Permeability Assay
4.12. Histology and Immunohistochemistry (IHC)
4.13. Lipid Extraction and 1H-NMR
4.14. Stratum Corneum pH Measurement
4.15. Oil Red Staining of HEE Cryosections
4.16. Cornified Envelopes Maturation Assay
4.17. RNA Extraction and RT-qPCR
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDSN | corneodesmosin |
| DKO | double knockout |
| EDD | epidermal differentiation disorder |
| HEE | human epidermal equivalent |
| SC | stratum corneum |
| SG | stratum granulosum |
| TEM | transmission electron microscopy |
References
- Hernández-Martín, A.; Paller, A.S.; Sprecher, E.; Akiyama, M.; Granier Tournier, C.; Aldwin-Easton, M.; Bodemer, C.; Choate, K.; Fischer, J.; Gostynski, A.; et al. Proposal for a new pathogenesis-guided classification for inherited epidermal differentiation disorders. Br. J. Dermatol. 2025, 193, 544–548. [Google Scholar] [CrossRef]
- Oji, V.; Eckl, K.M.; Aufenvenne, K.; Nätebus, M.; Tarinski, T.; Ackermann, K.; Seller, N.; Metzer, D.; Nürnberg, G.; Fölster-Holst, R.; et al. Loss of corneodesmosin leads to severe skin barrier defect, pruritis, and atopy: Unraveling the peeling skin disease. Am. J. Hum. Genet. 2010, 87, 274–281. [Google Scholar] [CrossRef]
- Gutiérrez-Cerrajero, C.; Sprecher, E.; Paller, A.S.; Akiyama, M.; Mazereeuw-Hautier, J.; Hernández-Martín, A.; González-Sarmiento, R. Ichthysosis. Nat. Rev. Dis. Primers 2023, 9, 2. [Google Scholar] [CrossRef]
- Jonca, N.; Leclerc, E.A.; Caubet, C.; Simon, M.; Guerrin, M.; Serre, G. Corneodesmosomes and corneodesmosin: From the stratum corneum cohesion to the pathophysiology of genodermatoses. Eur. J. Dermatol. 2011, 21 (Suppl. S2), 35–42. [Google Scholar] [CrossRef]
- Gordon, H.; Yap, P.; Hsiao, K.-C.; Watson, M.; Purvis, D. A novel pathogenic variant in the corneodesmosin gene causing generalized inflammatory peeling skin syndrome with marked eosinophilia and trichorrhexis invaginata. Pediatr. Dermatol. 2022, 39, 268–272. [Google Scholar] [CrossRef]
- Leclerc, E.A.; Huchenq, A.; Mattiuzzo, N.R.; Metzger, D.; Chambon, P.; Ghyselinck, N.B.; Serre, G.; Jonca, N.; Guerrin, M. Corneodesmosin gene ablation induces lethal skin-barrier disruption and hair-follicle degeneration related to desmosome dysfunction. J. Cell Sci. 2009, 122, 2699–2709. [Google Scholar] [CrossRef]
- Matsumoto, M.; Zhou, Y.; Matsuo, S.; Nakanishi, H.; Hirose, K.; Oura, H.; Arase, S.; Ishida-Yamamoto, A.; Bando, Y.; Izumi, K.; et al. Targeted deletion of the murine corneodesmosin gene delineates its essential role in skin and hair physiology. Proc. Natl. Acad. Sci. USA 2008, 105, 6720–6724. [Google Scholar] [CrossRef]
- Süβmuth, K.; Traupe, H.; Metze, D.; Oji, V. Ichthyoses in everyday practice: Management of a rare group of diseases. J. Dtsch. Dermatol. Ges. 2020, 18, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Diociaiuti, A.; El Hachem, M.; Pisaneschi, E.; Giancristoforo, S.; Genovese, S.; Sirleto, P.; Boldrini, R.; Angioni, A. Role of molecular testing in the multidisciplinary diagnostic approach of ichthyosis. Orphanet J. Rare Dis. 2016, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, G.; Zingkou, E.; Pampalakis, G. Reconstructing the epidermal proteolytic cascades in health and disease. J. Pathol. 2022, 257, 545–560. [Google Scholar] [CrossRef]
- Nauroy, P.; Nyström, A. Kallikreins: Essential epidermal messengers for regulation of the skin microenvironment during homeostasis, repair and disease. Matrix Biol. Plus 2020, 6–7, 100019. [Google Scholar]
- Furio, L.; Hovnanian, A. Netherton syndrome: Defective kallikrein inhibition in the skin leads to skin inflammation and allergy. Biol. Chem. 2014, 395, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Furio, L.; Pampalakis, G.; Michael, I.P.; Nagy, A.; Sotiropoulou, G.; Hovnanian, A. KLK5 inactivation reverses cutaneous hallmarks of Netherton syndrome. PLoS Genet. 2015, 11, e1005389. [Google Scholar] [CrossRef]
- White, G.V.; Edgar, E.V.; Holmes, D.S.; Lewell, X.Q.; Liddle, J.; Polyakova, O.; Smith, K.J.; Thorpe, J.H.; Walker, A.L.; Wang, Y.; et al. Kallikrein 5 inhibitors identified through structure based drug design in search for a treatment of Netherton syndrome. Bioorg. Med. Chem. Lett. 2019, 29, 821–825. [Google Scholar] [PubMed]
- Tan, X.; Soualmia, F.; Furio, L.; Renard, J.F.; Kempen, I.; Qin, L.; Pagano, M.; Pirotte, B.; El Amri, C.; Hovnanian, A.; et al. Toward the first class of suicide inhibitors of kallikreins involved in skin diseases. J. Med. Chem. 2015, 58, 598–612. [Google Scholar] [PubMed]
- Gonschorek, P.; Zorzi, A.; Maric, T.; Le Jeune, M.; Schüttel, M.; Montagnon, M.; Gómez-Ojea, R.; Vollmar, D.P.; Whitfield, C.; Reymond, L.; et al. Phage display selected cyclic peptide inhibitors of kallikrein-related peptidase 5 and 7 and their in vivo delivery to the skin. J. Med. Chem. 2022, 65, 9735–9749. [Google Scholar] [CrossRef]
- De Veer, S.J.; Swedberg, J.E.; Brattsand, M.; Clements, J.A.; Harris, J.M. Exploring the active site binding specificity of kallikrein-related peptidase 5 (KLK5) guides the design of new peptide substrates and inhibitors. Biol. Chem. 2016, 397, 1237–1249. [Google Scholar] [CrossRef]
- Walker, A.L.; Bingham, R.P.; Edgar, E.V.; Ferrie, A.; Holmes, D.S.; Liddle, J.; Polyakova, O.; Rella, M.; Smith, K.J.; Thorpe, J.H.; et al. Structure guided drug design to develop kallikrein 5 inhibitors to treat Netherton syndrome. Bioorg. Med. Chem. Lett. 2019, 29, 1454–1458. [Google Scholar]
- Walker, A.L.; Denis, A.; Bingham, R.P.; Bouillot, A.; Edgar, E.V.; Ferrie, A.; Holmes, D.S.; Laroze, A.; Liddle, J.; Fouchet, M.H.; et al. Design and development of a series of borocycles as selective, covalent kallikrein 5 inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 126675, Erratum in Bioorg Med Chem Lett. 2020. [Google Scholar]
- Liddle, J.; Beneton, V.; Benson, M.; Bingham, R.; Bouillot, A.; Boullay, A.B.; Brook, E.; Cryan, J.; Denis, A.; Edgar, E.; et al. A potent and selective kallikrein-5 inhibitor delivers high pharmacological activity in skin from patients with Netherton syndrome. J. Investig. Dermatol. 2021, 141, 2272–2279. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Di Nardo, A.; Bardan, A.; Murakami, M.; Ohtake, T.; Coda, A.; Dorschner, R.A.; Bonnart, C.; Descargues, P.; Hovnanian, A.; et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat. Med. 2007, 13, 975–980. [Google Scholar] [CrossRef]
- Canbolat, P.; Wilzopolski, J.; Kaessmeyer, S.; Filor, V.; Vidak, J.; Rüger, M.; Mägert, H.-J.; Forssmann, W.-G.; Bäumer, W. LEKTI domain 6 displays anti-inflammatory action in vitro and in a murine atopic dermatitis model. J. Dermatol. Sci. 2024, 115, 13–20. [Google Scholar] [CrossRef]
- Chavarria-Smith, J.; Chiu, C.P.C.; Jackman, J.K.; Yin, J.; Zhang, J.; Hackney, J.A.; Lin, W.-Y.; Tyagi, T.; Sun, Y.; Tao, J.; et al. Dual antibody inhibition of KLK5 and KLK7 for Netherton syndrome and atopic dermatitis. Sci. Transl. Med. 2022, 14, eabp9159. [Google Scholar] [CrossRef]
- Komatsu, N.; Suga, Y.; Saijoh, K.; Liu, A.C.; Khan, S.; Mizuno, Y.; Ikeda, S.; Wu, H.K.; Jayakumar, A.; Clayman, G.L.; et al. Elevated human tissue kallikrein levels in the stratum corneum and serum of peeling skin syndrome-type B patients suggests and over-desquamation of corneocytes. J. Investig. Dermatol. 2006, 126, 2338–2342. [Google Scholar] [CrossRef]
- Lundström, A.; Serre, G.; Haftek, M.; Egelrud, T. Evidence for a role of corneodesmosin, a protein which may serve to modify desmosomes during cornification, in stratum corneum cohesion and desquamation. Arch. Dermatol. Res. 1994, 286, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Pampalakis, G.; Zingkou, E.; Kaklamanis, L.; Spella, M.; Stathopoulos, G.T.; Sotiropoulou, G. Elimination of KLK5 inhibits early skin tumorigenesis by reducing epidermal proteolysis and reinforcing epidermal microstructure. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 165520. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, K.P.; Mukherjee, S.; Chandar, P. Stratum corneum fatty acids: Their critical role in preserving barrier integrity during cleansing. Int. J. Cosmet. Sci. 2013, 35, 337–345. [Google Scholar] [CrossRef]
- Mojumdar, E.H.; Helder, R.W.J.; Gooris, G.S.; Bouwstra, J.A. Monounsaturated fatty acids reduce the barrier of stratum corneum lipid membranes by enhancing the formation of a hexagonal lateral packing. Langmuir 2014, 30, 6534–6543. [Google Scholar] [CrossRef] [PubMed]
- Janssens, M.; van Smeden, J.; Gooris, G.S.; Bras, W.; Portale, G.; Caspers, P.J.; Vreeken, R.J.; Hankemeier, T.; Kezic, S.; Wolterbeek, R.; et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J. Lipid Res. 2012, 53, 2755–2766. [Google Scholar] [CrossRef]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef]
- Wang, S.; Olt, S.; Schoefmann, N.; Stuetz, A.; Winiski, A.; Wolff-Wininski, B. SPINK5 knockdown in organotypic human skin culture as a model system for Netherton syndrome: Effect of genetic inhibition of serine proteases kallikrein 5 and kallikrein 7. Exp. Dermatol. 2014, 23, 524–526. [Google Scholar] [CrossRef]
- Yeo, S.Y.; Low, T.; Fernandis, A.Z. Chemical suppression of KLK5 and KLK7 rescues barrier integrity in a Netherton syndrome model. Exp. Dermatol. 2025, 34, e70055. [Google Scholar] [CrossRef]
- Valentin, F.; Wiegmann, H.; Tarinski, T.; Nikolenko, H.; Traupe, H.; Liebau, E.; Dathe, M.; Oji, V. Development of a pathogenesis-based therapy for peeling skin syndrome type 1. Br. J. Dermatol. 2021, 184, 1123–1131. [Google Scholar] [CrossRef]
- Kasparek, P.; Ileninova, Z.; Zbodakova, O.; Kanchev, I.; Benada, O.; Chalupsky, K.; Brattsand, M.; Beck, I.M.; Sedlacek, R. KLK5 and KLK7 ablation fully rescues lethality of Netherton Syndrome-like phenotype. PLoS Genet. 2017, 13, e1006566. [Google Scholar] [CrossRef] [PubMed]
- Zaafouri, S.; Pichery, M.; Huchenq, A.; Valentin, F.; Oji, V.; Mazereeuw-Hautier, J.; Serre, J.; Jonca, N. Transcriptomic analysis of two Cdsn-deficient mice shows gene signatures biologically relevant for peeling skin disease. J. Investig. Dermatol. 2018, 138, 1431–1435. [Google Scholar] [CrossRef]
- Williams, M.R.; Nakatsuji, T.; Sanford, J.A.; Vrbanac, A.F.; Gallo, R.L. Staphylococcus aureus induces increased serine protease activity in keratinocytes. J. Investig. Dermatol. 2017, 137, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Canbay, V.; Auf dem Keller, U. New strategies to identify protease substrates. Curr. Opin. Chem. Biol. 2021, 60, 89–96. [Google Scholar] [CrossRef]
- de Veer, S.J.; Furio, L.; Harris, J.M.; Hovnanian, A. Proteases: Common culprits in human skin disorders. Trends Mol. Med. 2014, 20, 166–178. [Google Scholar] [CrossRef]
- Yoon, H.; Blaber, S.I.; Li, W.; Scarisbrick, I.A.; Blaber, M. Activation profiles of human kallikrein-related peptidases by matric metalloproteinases. Biol. Chem. 2013, 394, 137–147. [Google Scholar] [CrossRef]
- Hachem, J.-P.; Man, M.-Q.; Crumrine, D.; Uchida, Y.; Brown, B.E.; Rogiers, V.; Roseeuw, D.; Feingold, K.R.; Elias, P.M. Sustained serine proteases activity by prolonged increase in pH leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J. Investig. Dermatol. 2005, 125, 510–520. [Google Scholar] [CrossRef]
- Kasparek, P.; Ileninova, Z.; Haneckova, R.; Kanchev, I.; Jenickova, I.; Sedlacek, R. A viable mouse model for Netherton syndrome based on mosaic inactivation of the Spink5 gene. Biol. Chem. 2016, 397, 1287–1292. [Google Scholar] [CrossRef]
- Reynier, M.; Allart, S.; Gaspard, E.; Moga, A.; Goudounèche, D.; Serre, G.; Simon, M.; Leprince, C. Rab11a is essential for lamellar body biogenesis in the human epidermis. J. Investig. Dermatol. 2016, 136, 1199–1209. [Google Scholar] [CrossRef]
- Kriat, M.; Vion-Dury, J.; Confort-Gouny, S.; Favre, R.; Viout, P.; Sciaky, M.; Sari, H.; Cozzone, P.J. Analysis of plasma lipids by NMR spectroscopy: Application to modifications induced by malignant tumors. J. Lipid Res. 1993, 34, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Adosraku, R.K.; Choi, G.T.; Constantinou-Kokotos, V.; Anderson, M.M.; Gibbons, W.A. NMR lipid profiles of cells, tissues, and body fluids: Proton NMR analysis of human erythrocyte lipids. J. Lipid Res. 1994, 35, 1925–1931. [Google Scholar] [CrossRef] [PubMed]
- Tynkkynen, T. 1H NMR Analysis of Serum Lipids. Ph.D. Thesis, University of Eastern Finland, Kuopio, Finland, 2012. [Google Scholar]
- Pancarte, M.; Leignadier, J.; Courrech, S.; Serre, G.; Attia, J.; Jonca, N. Strengthening the skin barrier by using a late cornified envelope 6A-derived biomimetic. Exp. Dermatol. 2024, 33, e15191. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zingkou, E.; Reynier, M.; Pampalakis, G.; Serre, G.; Jonca, N.; Sotiropoulou, G. Deletion of the Epidermal Protease KLK5 Aggravates the Symptoms of Congenital Ichthyosis CDSN-nEDD. Int. J. Mol. Sci. 2025, 26, 8605. https://doi.org/10.3390/ijms26178605
Zingkou E, Reynier M, Pampalakis G, Serre G, Jonca N, Sotiropoulou G. Deletion of the Epidermal Protease KLK5 Aggravates the Symptoms of Congenital Ichthyosis CDSN-nEDD. International Journal of Molecular Sciences. 2025; 26(17):8605. https://doi.org/10.3390/ijms26178605
Chicago/Turabian StyleZingkou, Eleni, Marie Reynier, Georgios Pampalakis, Guy Serre, Nathalie Jonca, and Georgia Sotiropoulou. 2025. "Deletion of the Epidermal Protease KLK5 Aggravates the Symptoms of Congenital Ichthyosis CDSN-nEDD" International Journal of Molecular Sciences 26, no. 17: 8605. https://doi.org/10.3390/ijms26178605
APA StyleZingkou, E., Reynier, M., Pampalakis, G., Serre, G., Jonca, N., & Sotiropoulou, G. (2025). Deletion of the Epidermal Protease KLK5 Aggravates the Symptoms of Congenital Ichthyosis CDSN-nEDD. International Journal of Molecular Sciences, 26(17), 8605. https://doi.org/10.3390/ijms26178605




