1. Introduction
Sarcomas represent a rare category of cancers primarily comprising bone and soft tissue malignancies, accounting for approximately 1% of all diagnosed malignancies [
1]. The standard treatment for resectable sarcomas is surgical resection with adequate margins, with or without adjuvant chemotherapy or radiotherapy [
2]. However, for unresectable cases, effective local therapeutic options are limited to conventional photon radiotherapy, which often yields suboptimal results owing to the inherent radioresistance of sarcomas [
3]. Heavy-ion radiotherapy has emerged as a revolutionary approach for treating unresectable sarcomas, as it enables the delivery of high-dose irradiation to the tumor while minimizing exposure to the surrounding normal tissues [
4]. This modality has demonstrated promising outcomes in terms of local control [
5]. However, heavy-ion radiotherapy is not indicated for advanced cases with metastases in Japan [
6,
7], and, currently, no effective radiotherapy options are available for metastatic sarcomas.
Targeted alpha therapy (TAT) has recently gained attention as a promising strategy for treating various malignancies, particularly those resistant to traditional therapies [
8]. Alpha particles possess high linear energy transfer properties that induce double-strand breaks in the DNA, resulting in potent cytotoxic effects [
9]. In addition, these particles have an extremely short range (50–100 μm) within living tissues, maximizing their tumor-killing effect while reducing damage to the surrounding normal tissues [
9]. TAT facilitates systemic administration of alpha-emitting radionuclides as an internal radiation therapy, allowing simultaneous targeting of multiple foci, including metastatic sites [
10]. These characteristics make TAT particularly well-suited for treating disseminated or multi-focal disease. Among the radionuclides being studied for TAT, astatin-211 (
211At) has received considerable attention owing to its adequate half-life of 7.2 h, high energy alpha emission, and feasibility of production using accelerator-based technology [
11]. One of the promising molecular targets for nuclear medicine therapy is the L-type amino acid transporter 1 (LAT1), a transmembrane protein responsible for the uptake of essential amino acids [
12]. LAT1 is highly overexpressed in several malignancies, including malignant bone and soft tissue sarcomas, but is minimally expressed in normal tissues [
13,
14].
We have previously developed LAT1-targeted radiopharmaceuticals using 3-[
211At] Astato-α-methyl-L-tyrosine (
211At-AAMT-OH-L), a novel alpha-emitting therapeutic agent specifically designed to target LAT1-expressing tumors [
15]. Compared with other TAT candidates,
211At-AAMT-OH-L has been reported to exert rapid effects that may reduce the burden on patients and rapid elimination that may reduce side effects, along with high affinity to LAT1 and strong cytotoxicity of alpha rays [
15]. More recently, we further optimized this compound by introducing structural modifications, including methyl, ethyl, and propyl substitutions, to improve its stability and tumor retention and decrease nonspecific accumulation [
16]. Among the variants developed, the methyl-substituted derivative
211At-AAMT-O-Me-L (
211At-AAMT) exhibited the highest tumor uptake and retention with minimal off-target accumulation, making it the most promising candidate for clinical application [
16]. The chemical structures of
211At-AAMT-OH-L and
211At-AAMT-O-Me-L are shown in
Supplementary Figure S1. Preclinical xenograft studies using a human pancreatic cancer cell line have confirmed its efficacy in inhibiting tumor growth with limited accumulation in normal tissue [
16].
In this study, we aimed to evaluate the antitumor effects of this novel TAT (211At-AAMT-O-Me-L) in malignant bone and soft tissue tumor cells compared with first-line chemotherapeutic agents currently used for bone and soft tissue sarcomas.
3. Discussion
In this study, we employed both in vitro and in vivo models to investigate the therapeutic efficacy and safety of
211At-AAMT, a LAT1-targeted alpha-releasing compound, in bone and soft tissue sarcomas. Although these malignancies are rare, they present significant therapeutic challenges owing to their resistance to conventional chemotherapy and radiation therapy as well as limited treatment options for unresectable and metastatic cases [
17]. The findings presented in this study suggest that
211At-AAMT may serve as an effective and minimally invasive treatment for this disease.
211At-labeled agents have been investigated in a variety of malignancies, including glioblastoma, pancreatic cancer, prostate cancer, differentiated thyroid cancer, neuroblastoma, and pheochromocytoma, and have demonstrated significant antitumor effects, suggesting their potential clinical applications [
18,
19,
20,
21,
22,
23]. In Japan, multiple clinical trials are currently underway for various cancer types involving
211At-labeled compounds targeting disease-specific molecular transporters. For example, [
211At] NaAt, which targets the sodium/iodide symporter, is been under investigation in a human phase I clinical trial for advanced differentiated thyroid cancer at Osaka University Hospital since November 2021 [
24]. In addition, [
211At] meta-astatobenzylguanidine, which is taken up via the norepinephrine transporter, is being evaluated at Fukushima Medical University in patients with malignant pheochromocytomas and paraganglioma [
25]. In June 2024, a clinical trial of the prostate-specific membrane antigen (PSMA)-targeted radiotherapeutic agent [
211At] PSMA-5 was initiated in patients with castration-resistant prostate cancer [
26]. These studies highlight the growing promise of alpha-particle therapy in Japan and reinforce the potential of
211At-based targeted approaches for treatment-resistant cancers.
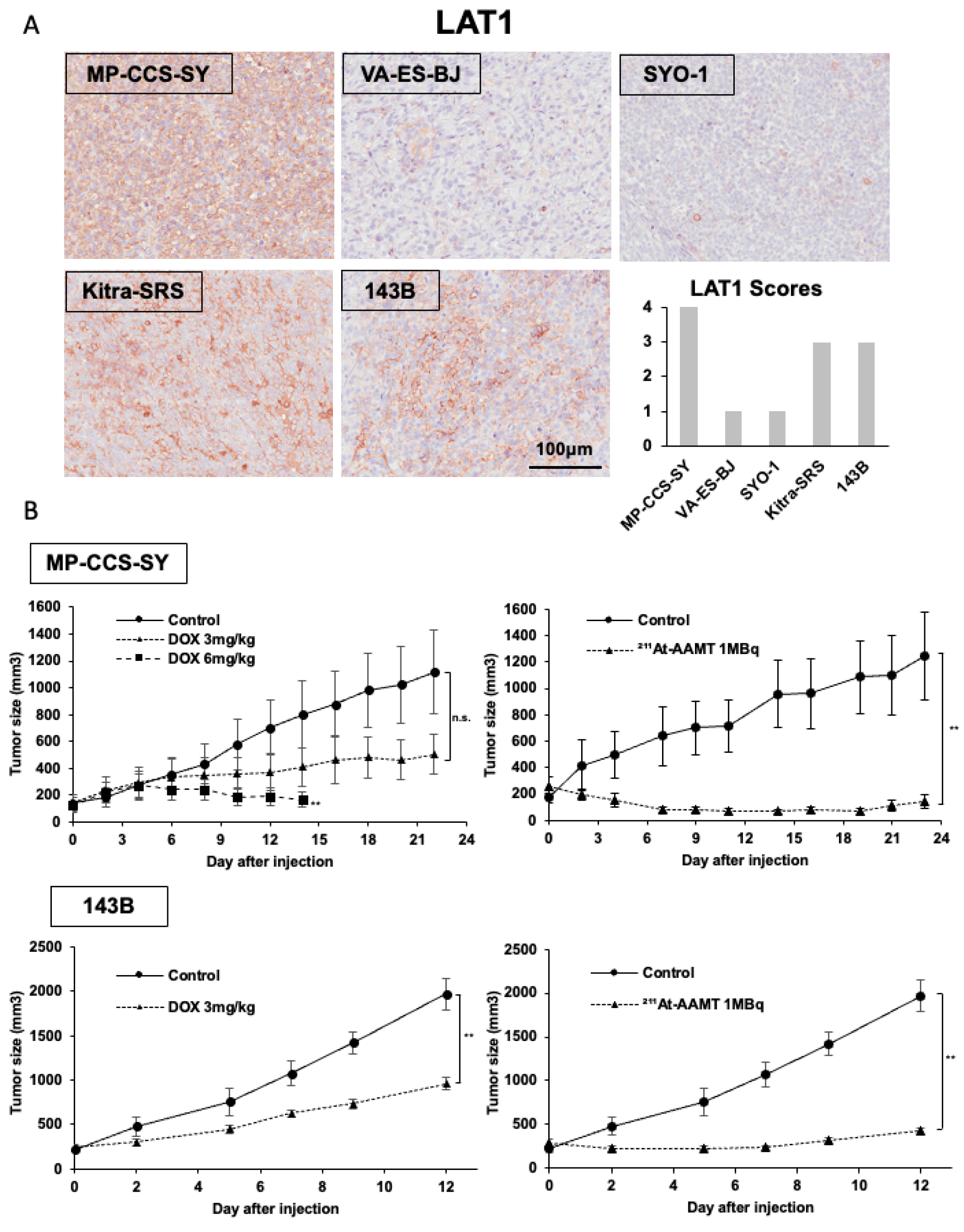
Several reports have described the application of alpha-particle radiation therapy to synovial sarcoma (SS) using
211At and
225Ac [
27,
28]. Li et al. reported that
211At-anti-Frizzled homolog 10 (FZD10) antibodies efficiently suppressed the growth of SS xenografts [
27]. FZD10—a transmembrane protein member of the Frizzled family that serves as a putative receptor in the Wnt signaling pathway—is highly expressed in SS tumors but largely absent in other types of sarcomas and most normal tissues, suggesting its potential as a promising subtype-specific molecular target [
27]. However, to date, no studies have reported the use of alpha therapy in other types of sarcomas, highlighting the need for further investigation in this area. In the present study, we demonstrated that LAT1 is overexpressed in multiple sarcoma subtypes and confirmed the antitumor efficacy of LAT1-targeted alpha therapy, which may be broadly applicable across diverse sarcoma subtypes. Furthermore, the small-molecule LAT1-targeted agent
211At-AAMT offers several advantages over antibody-targeted alpha therapies, including high specificity and affinity, favorable tumor permeability with rapid accumulation, and rapid clearance from most normal tissues [
29,
30]. Additionally, the relatively simple chemical structure of AAMT facilitates efficient radiolabeling and scalable production, both of which are important for clinical applications [
16].
Fujimoto et al. also reported high LAT1 expression in tumor tissues from patients with clear cell sarcoma (CCS) and demonstrated that boron neutron capture therapy using LAT1-mediated uptake of p-borono-l-phenylalanine achieved complete local control of CCS [
31]. This clinical case report further supports the potential utility of LAT1-target radiotherapy for bone and soft tissue sarcomas.
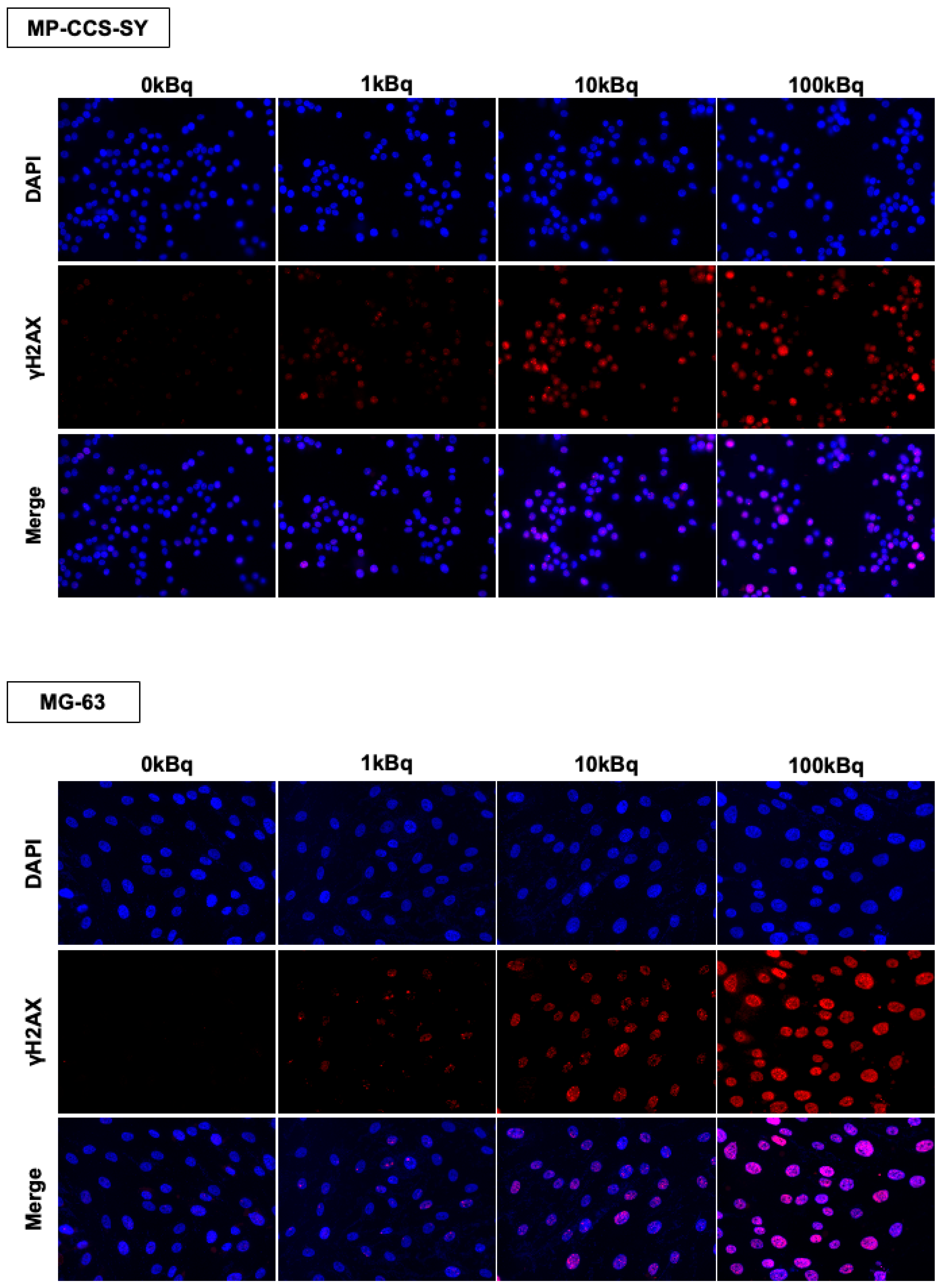
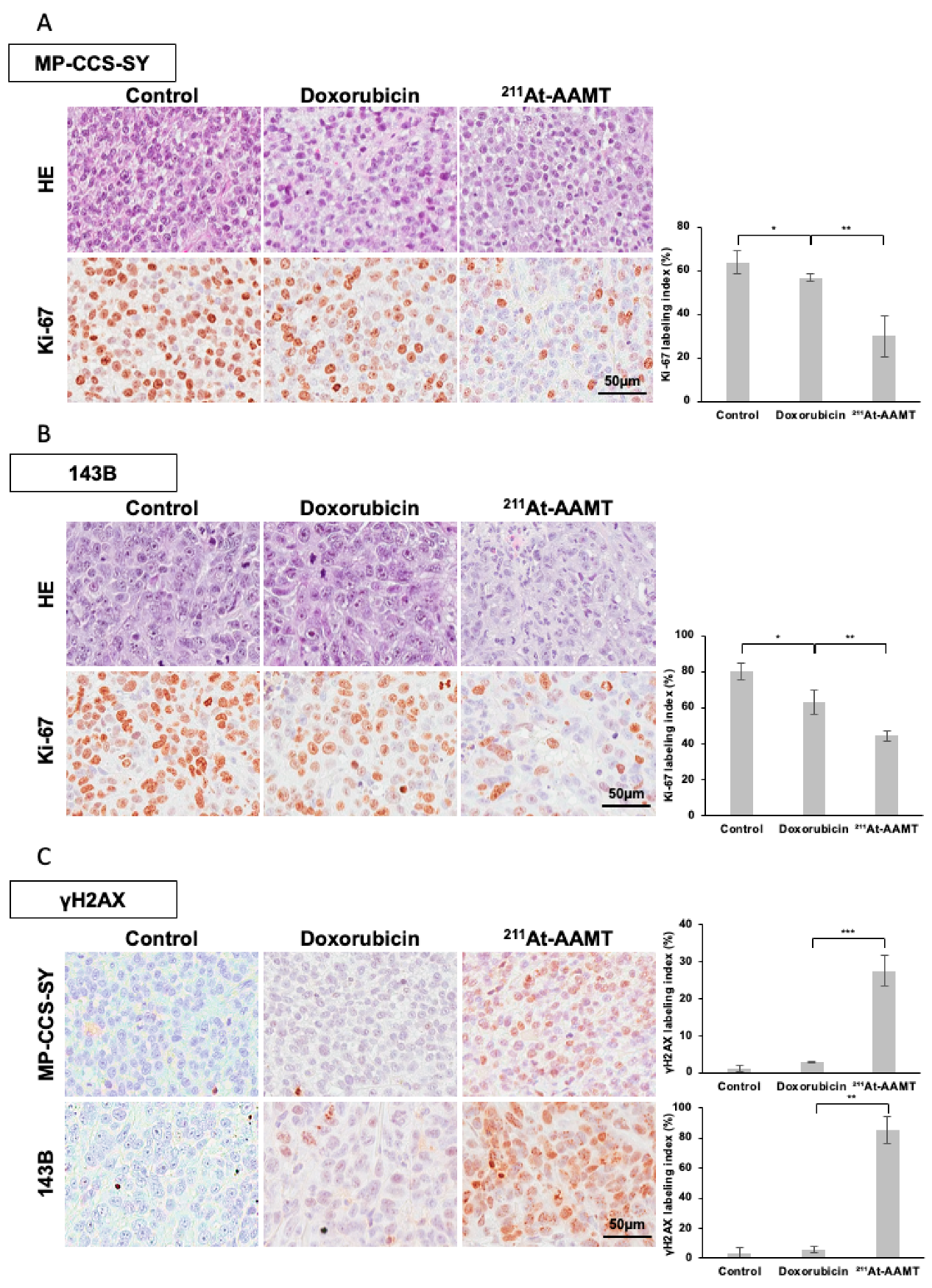
In this study, immunohistochemical (IHC) and immunofluorescence analyses showed that
211At-AAMT induced significant DSBs (increased γH2AX foci) both in vitro and in vivo. The potent DNA-damaging effect of alpha particles, coupled with their selective delivery to LAT1-expressing tumor cells, may contribute to the tumor suppressive effect. Notably, the antiproliferative effect persisted after a single administration, suggesting the possibility of biological effects beyond the physical half-life of
211At (half-life 7.2 h), such as the induction of tumor cell senescence or remodeling of the tumor microenvironment [
32]. Importantly, LAT1 expression persisted in residual tumor tissues following
211At-AAMT treatment, suggesting the potential for re-irradiation or combination therapy. Further investigation is warranted to assess the radiosensitivity of LAT1-expressing cells after
211At-AAMT treatment.
Although the adverse events associated with
211At-based alpha therapy in humans have not yet been comprehensively reported, previous toxicity studies of [
211At]PSMA-5 demonstrated transient changes, with no irreversible toxicity observed 14 days after administration [
18]. Consistently, our previous toxicity study of
211At-AAMT, with follow-up for 28 days, showed transient hematopoietic suppression that subsequently recovered, with no pathological abnormalities in major organs [
16]. Notably, in the present study, although doxorubicin induced significant weight loss at effective doses, a single dose of
211At-AAMT suppressed tumor growth without causing weight loss.
Despite these promising results, this study has some limitations. First, the xenograft model used in this study did not fully recapitulate the complex tumor microenvironment and immune system of human sarcomas, and thus the findings, including the retention of LAT1 expression after 211At-AAMT treatment, may not be entirely extrapolated to clinical settings. Confirmation in more advanced preclinical systems, such as patient-derived xenografts, will be necessary. Second, the therapeutic sensitivity of 211At-AAMT varied among sarcoma cell lines, and this variability could not be explained solely by LAT1 expression levels. Other biological factors—including the functional activity of the LAT1–CD98 complex, intracellular retention and efflux of radiolabeled compounds, proliferative capacity, and DNA damage repair proficiency—may contribute. Further experimental studies are required to elucidate these mechanisms. Third, to more firmly establish the LAT1 specificity of 211At-AAMT, additional studies using shLAT1 sarcoma cells are warranted, as these models will enable a direct demonstration of the causal link between LAT1 expression and therapeutic efficacy. Fourth, although a significant effect was demonstrated with a single dose, the optimal dosing schedule, including the possibility of divided dosing, remains to be established. Fifth, comprehensive toxicity assessments, including off-target effects at LAT1 expression sites in normal tissues such as the blood–brain barrier and placenta, as well as long-term follow-up to detect potential delayed adverse events, are essential prior to clinical application.
4. Materials and Methods
4.1. Production and Isolation of 211At
211At was produced via α-particle irradiation of bismuth-209 targets using an AVF cyclotron at the Research Center for Nuclear Physics, Osaka University (Ibaraki, Japan), through a nuclear reaction
209Bi(α,2n)
211At [
33]. The bismuth layer, deposited onto an aluminum substrate, was irradiated with an α-beam, and
211At was subsequently isolated from the irradiated target via dry distillation.
Dry distillation and purification were performed using COSMiC-Mini VTRSC2 (Nihon Mediphysics Business Support, Hyogo, Japan), an automated distillation system, and the purified
211At was obtained as an aqueous solution. This system was developed based on previously reported dry separation protocols [
34].
4.2. Synthesis of 211At-AAMT-O-Me-L
211At-AAMT-O-Me-L was synthesized via the Shirakami reaction using a boron-containing precursor (AAMT-O-Me-L) following the method described by Kaneda et al. [
16]. The precursor was custom-synthesized by Kishida Chemical Co., Ltd. (Osaka, Japan). For precursors containing a pinacolborane moiety, a 7% sodium bicarbonate solution (Meylon
®, Otsuka Pharmaceutical, Tokyo, Japan) was used as the reaction solvent, whereas water was used as a boronic acid derivative. For radiolabeling, approximately 10 MBq of aqueous
211At was mixed with the precursor compound and potassium iodide as the carrier. The reaction was performed at 50 °C for 50 min. The crude product was purified using an Oasis HLB column (Waters, Milford, MA, USA). The radiochemical purity and identity of the labeled compounds were confirmed by HPLC and TLC. TLC analysis was performed on silica gel G60 plates (Merck Millipore, Burlington, MA, USA) using a mobile phase of n-butanol/acetic acid/water (4:1:1). Radiolabeled spots were visualized using the Typhoon FLA-7000 biomolecular imager (GE Healthcare, Milwaukee, WI, USA). The final
211At-AAMT-O-Me-L product was adjusted to a concentration of 5 MBq/mL and mixed with 1.0% (
w/
v) ascorbic acid (pH 6.0), which served as reducing and stabilizing agents, respectively.
4.3. Cell Culture
The human cell line Kitra-SRS (CIC-DUX4sarcoma; RRID:CVCL_YI69) was established in our laboratory. MP-CCS-SY (CCS; RRID:CVCL_0J33) and SYO-1 (synovial sarcoma; RRID:CVCL_7146) were kindly provided by Dr. Moritake (Miyazaki University, Miyazaki, Japan) and Dr. Ozaki (Okayama University, Okayama, Japan), respectively. VAESBJ (epithelioid sarcoma; RRID:CVCL_1785), HT-1080 (fibrosarcoma; RRID:CVCL_0317), 143B (osteosarcoma; RRID:CVCL_2270), MG-63 (osteosarcoma; RRID:CVCL_0426), and U2OS (osteosarcoma; RRID:CVCL_0042) were purchased from ATCC. Saos-2 cells (osteosarcoma; RRID:CVCL_0548) were obtained from the Riken Cell Bank. NHDFs were purchased from Kurabo (Osaka, Japan). All cell lines were maintained in DMEM (Nacalai Tesque, Tokyo, Japan), which was supplemented with 10% FBS (Sigma-Aldrich, St. Louis, MO, USA) and 1% penicillin–streptomycin (100 IU/mL of penicillin and 100 μg/mL of streptomycin) at 37 °C in a humidified incubator with 5% CO2. Cell line authentication was performed based on morphological assessments, PCR-based genotyping, and the evaluation of growth characteristics. All experiments used cells between passages 10 and 30. Prior to experimentation, mycoplasma contamination was routinely tested and confirmed to be negative using the PCR Mycoplasma Detection Set (Takara Bio Inc., Shiga, Japan).
4.4. Western Blotting
For lysate preparation, cultured cells were washed with PBS and lysed using RIPA lysis and an extraction buffer (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with a 1% protease and phosphatase inhibitor cocktail (Cell Signaling Technology). Tumor tissues were homogenized and lysed using the T-PER Tissue Protein Extraction Reagent (Thermo Fisher Scientific). Protein concentrations were determined using a bicinchoninic acid (BCA) assay (Thermo Fisher Scientific).
Equal amounts of protein lysates were separated by SDS-PAGE using 4–12% Bis-Tris gels (Life Technologies, Waltham, MA, USA) and transferred onto PVDF membranes (Nippon Genetics, Tokyo, Japan). The membranes were blocked with 5% skim milk in TBS with Tween 20 at room temperature and incubated overnight at 4 °C, with the primary antibodies diluted in Can Get Signal Solution 1 (TOYOBO, Osaka, Japan). After washing, the membranes were incubated for 1 h at room temperature, with the secondary antibodies diluted in Can Get Signal Solution 2 (TOYOBO). Protein bands were visualized using the ChemiDOC Touch Imaging System (Bio-Rad, Hercules, CA, USA). The primary antibodies used in the experiments are listed in
Supplementary Table S2.
Western blot images were acquired as 8-bit TIFFs, and band intensities of LAT1 and β-actin were quantified using Fiji/ImageJ (version 1.54p, National Institutes of Health, Bethesda, MD, USA). LAT1 signals were background-corrected and normalized to the corresponding β-actin band. For soft tissue sarcoma cell lines, values were expressed relative to HT-1080 (=1.0) within each blot, and for osteosarcoma cell lines, values were expressed relative to U-2 OS (=1.0).
4.5. Cellular Uptake Assay
To investigate the cellular uptake of 211At-AAMT via LAT1, various cell lines were seeded in 24-well plates at a density of 2.5 × 103 cells per well and cultured for 48 h. Following 30 min incubation in HBSS, the cells were treated with 1 kBq of 211At-AAMT in the presence or absence of 200 mmol/L of the dual LAT1/LAT2 inhibitor BCH (Sigma-Aldrich) and 10 µmol/L of the LAT1-selective inhibitor nanvuranlat (JPH203; GlpBio Technology Inc., Montclair, CA, USA). The cells were subsequently washed twice with PBS(−) and lysed in 0.1 N NaOH, with the radioactivity measured using a gamma counter (2480 Wizard2; PerkinElmer, Waltham, MA, USA). The total protein content was quantified using a BCA protein assay kit (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) and a microplate reader.
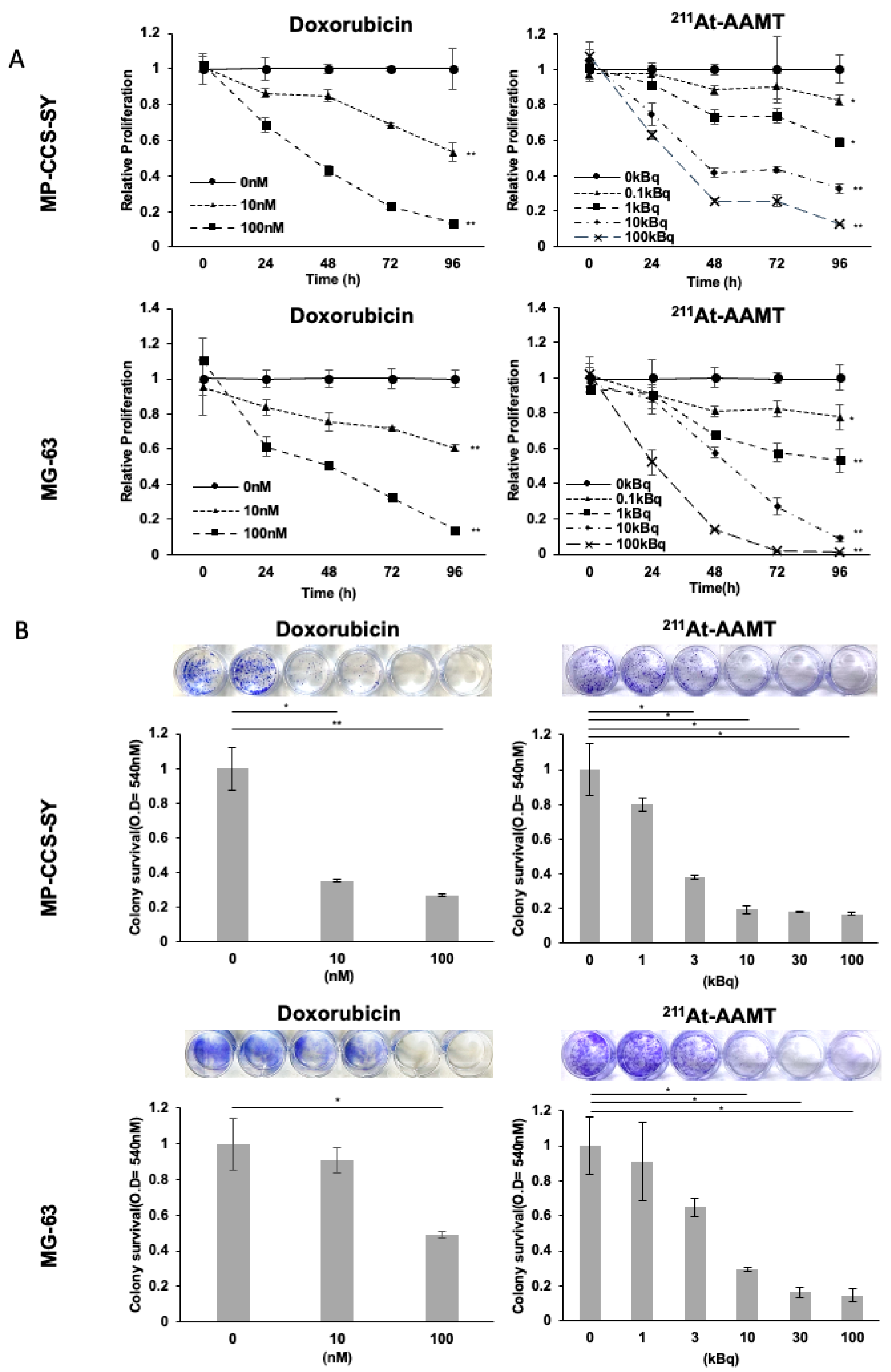
4.6. Cell Proliferation Assay
Cells were seeded in 96-well plates at a density of 2 × 103 cells per well in triplicates. After overnight incubation, the cells were treated with either 211At-AAMT or doxorubicin hydrochloride (TCI, Tokyo, Japan) for the indicated durations. The vehicle control for 211At-AAMT was its formulation buffer (0.2% acetic acid with 1.0% ascorbic acid, pH 6.0), whereas DMSO was used as the vehicle control for doxorubicin.
Cell proliferation was evaluated using the WST-8 assay with Cell Count Reagent SF (Nacalai Tesque) following the manufacturer’s instructions. The absorbance was measured at 450 and 690 nm (reference wavelength) using a spectrophotometer (MultiskanTM FC, Thermo Fisher Scientific). Relative proliferation rates were calculated by normalizing the absorbance values to the average of each respective control group, which was set to 1. IC50 values were log-transformed for statistical analysis; geometric means and 95% confidence intervals are reported.
4.7. Colony Formation Assay
Cells were seeded in 24-well plates at a density of 1 × 103 cells per well in quadruplicates. After overnight incubation, the cells were treated with various doses of 211At-AAMT or doxorubicin for 48 h, followed by replacement with a fresh medium. When the control cells reached approximately 80% confluence, all wells were washed twice with PBS(−) and stained with 0.2% crystal violet for 30 min. The plates were then washed with water and photographed. For quantification, the dye was extracted using a solution of 50% ethanol and 0.02 mol/L hydrochloric acid, and the absorbance was measured at 540 nm using a spectrophotometer.
4.8. Cellular Immunofluorescence Staining
Cells were seeded in an 8-well slide chamber (WATSON Slide Chamber 8 Well; WATSON Co., Ltd., Tokyo, Japan) and incubated overnight to allow cell attachment. The next day, the cells were treated with 211At-AAMT for 24 h. After treatment, the cells were washed twice with PBS and fixed with 4% paraformaldehyde at room temperature for 30 min. The cells were permeabilized with 0.1% Triton X-100 in PBS for 15 min and blocked with 3% BSA in PBS for 30 min.
The cells were incubated overnight at 4 °C with a rabbit monoclonal anti-phospho-histone γH2AX (Ser139) antibody (clone 20E3, Cell Signaling Technology, #9718) diluted at 1:800 in a blocking buffer. The following day, the cells were washed three times with PBS and incubated for 2 h at room temperature in the dark with the Alexa Fluor 594-conjugated goat anti-rabbit IgG (H + L), F(ab’)2 fragment (Cell Signaling Technology, #8889, 1:1000 dilution). After staining, the upper part of the slide chamber was removed, and the slides were mounted using ProLongTM Gold Antifade Mountant with DAPI (Thermo Fisher Scientific, #S36939). Fluorescence images were acquired using a BZ-X810 fluorescence microscope (Keyence Corporation, Osaka, Japan).
For quantification, γH2AX fluorescence intensity per nucleus was measured using Fiji/ImageJ. Regions of interest (ROIs) were defined around individual nuclei based on DAPI staining, and the integrated density was calculated. Background correction was performed by subtracting the product of the ROI area and the mean background intensity. The corrected integrated density values were normalized to the 0 kBq control (=1). For foci analysis, γH2AX foci were manually counted 50 nuclei per condition, and the average number of foci per nucleus was calculated.
4.9. In Vivo Xenograft Models
Five-week-old female BALB/c nu/nu athymic mice (n = 64, body weight range: 17.1–22.6 g) were housed under standard laboratory conditions at the Institute of Experimental Animal Sciences of Osaka University Medical School. All animal experiments were performed in accordance with the protocols approved by the Institutional Animal Care and Use Committee of the Graduate School of Medicine, Osaka University Graduate School of Medicine. To establish subcutaneous xenograft tumor models, 1 × 107 MP-CCS-SY or 143B cells were injected into the left dorsal flank of each mouse. When the tumors reached an average volume of approximately 200 mm3, treatment was initiated. In the MP-CCS-SY model, mice were randomly assigned to four groups (n = 5 per group): 3 mg/kg or 6 mg/kg doxorubicin, 1 MBq of 211At-AAMT, or vehicle control. By contrast, the 143B model included three groups (n = 5 per group): 3 mg/kg doxorubicin, 1 MBq of 211At-AAMT, and vehicle control. The 6 mg/kg doxorubicin group was not used in this model. Doxorubicin was administered intraperitoneally every 4 days, whereas 211At-AAMT was administered intravenously via the tail vein. The tumor size and body weight were measured every alternate day. Tumor volumes were calculated using caliper measurements according to the following formula: tumor volume (mm3) = (length × width2)/2. When the tumor volume exceeded 2000 mm3, the mice were humanely euthanized by CO2 asphyxiation and tumors were excised and weighed. To assess histological changes 24 h post-treatment, MP-CCS-SY and 143B xenograft models (n = 4 per group) were treated with 3 mg/kg doxorubicin or 1 MBq 211At-AAMT, followed by tissue collection for histological analysis.
4.10. Immunohistochemistry
Excised xenograft tumors were fixed in 10% neutral-buffered formalin and processed for paraffin embedding. Serial sections were prepared at a thickness of 3.5 μm and subjected to histological evaluation by hematoxylin and eosin (HE) staining and IHC analysis. For IHC staining, paraffin sections were deparaffinized and rehydrated using a graded alcohol series. Antigen retrieval was performed by heating the sections in 10 mM citrate buffer (pH 6.0) at 95 °C for 30 min. For LAT1-specific staining, antigen retrieval was performed by autoclaving the sections in HistoFine antigen retrieval solution (pH 9.0, Nichirei Biosciences, Tokyo, Japan) for 5 min. To block endogenous peroxidase activity, the sections were incubated with methanol containing 3% hydrogen peroxide for 10 min. Nonspecific binding was blocked by incubating the sections in TBS containing 2% BSA for 1 h at room temperature. Primary antibodies were then applied, and the slides were incubated overnight at 4 °C. The following day, the sections were incubated with HRP-conjugated secondary antibodies for 1 h. Color development was achieved using 3,3′-diaminobenzidine tetrahydrochloride (Dako, Carpinteria, CA, USA), followed by nuclear counterstaining with hematoxylin. The details of the primary antibodies used in this study are presented in
Supplementary Table S2. All IHC analyses were performed using Aperio CS2 (Leica, Wetzlar, Germany), and staining intensities were compared at ×200. LAT1 expression was confirmed at ×50 for each preparation, and LAT1 expression was scored as follows: 0 = no expression; 1 = <10% positive cells; 2 = 10–30%, 3 = 30–50%, and 4 = >50% of tumor cells showing positive staining.
4.11. Statistical Analysis
All results are presented as means ± standard deviation (SD). Statistical comparisons for in vitro assays were performed using Student’s t-test, whereas differences in animal study data were evaluated using the Mann–Whitney U test. A two-tailed p-value of <0.05 was considered statistically significant.