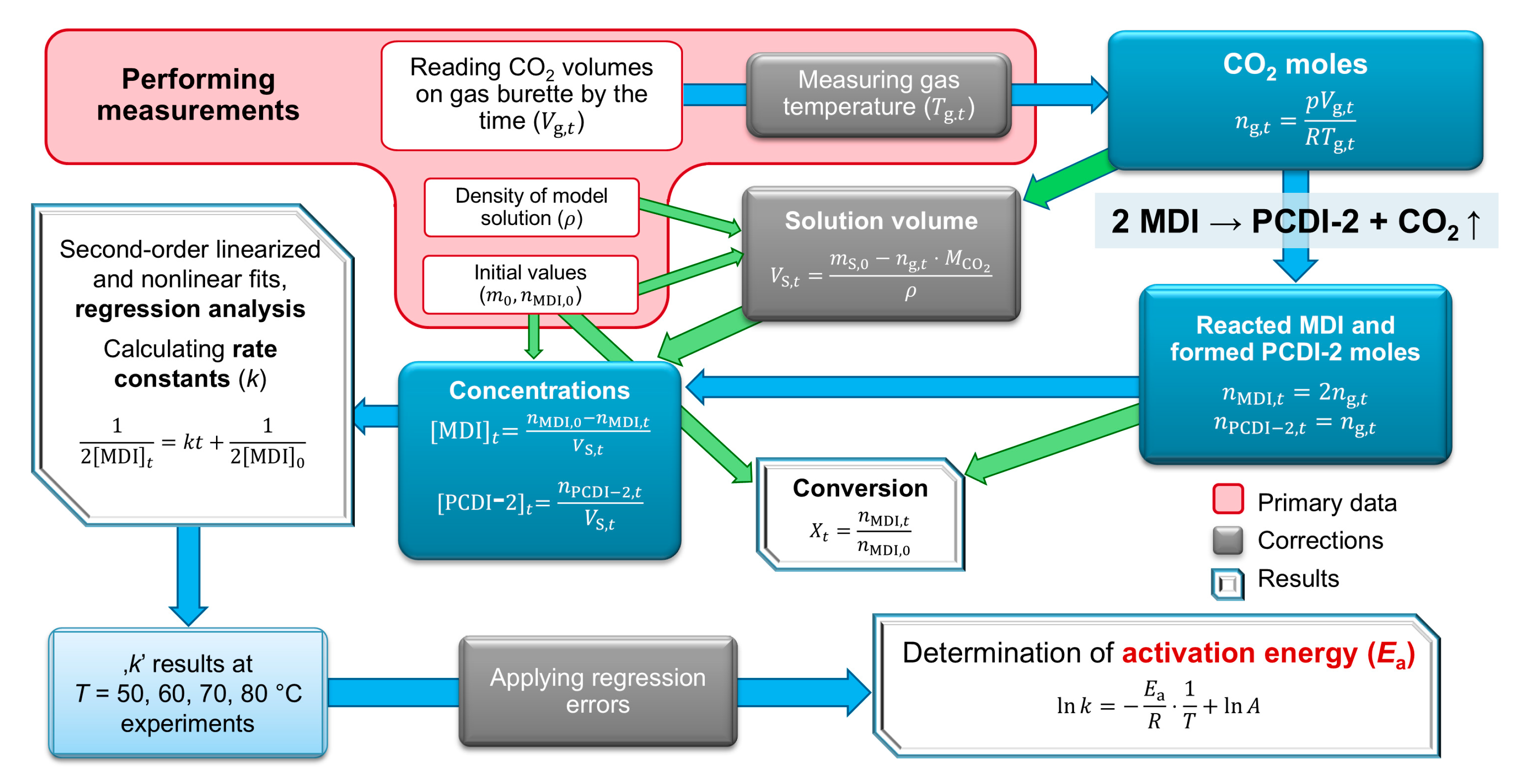
In the results section, the kinetic theory of the complex carbodiimide formation from MDI is described, as is data processing and evaluation, including experimental and theoretical considerations. Diagrams show the properties and determine the kinetic parameters of the supposed reaction.
2.1. Reaction Kinetics Background
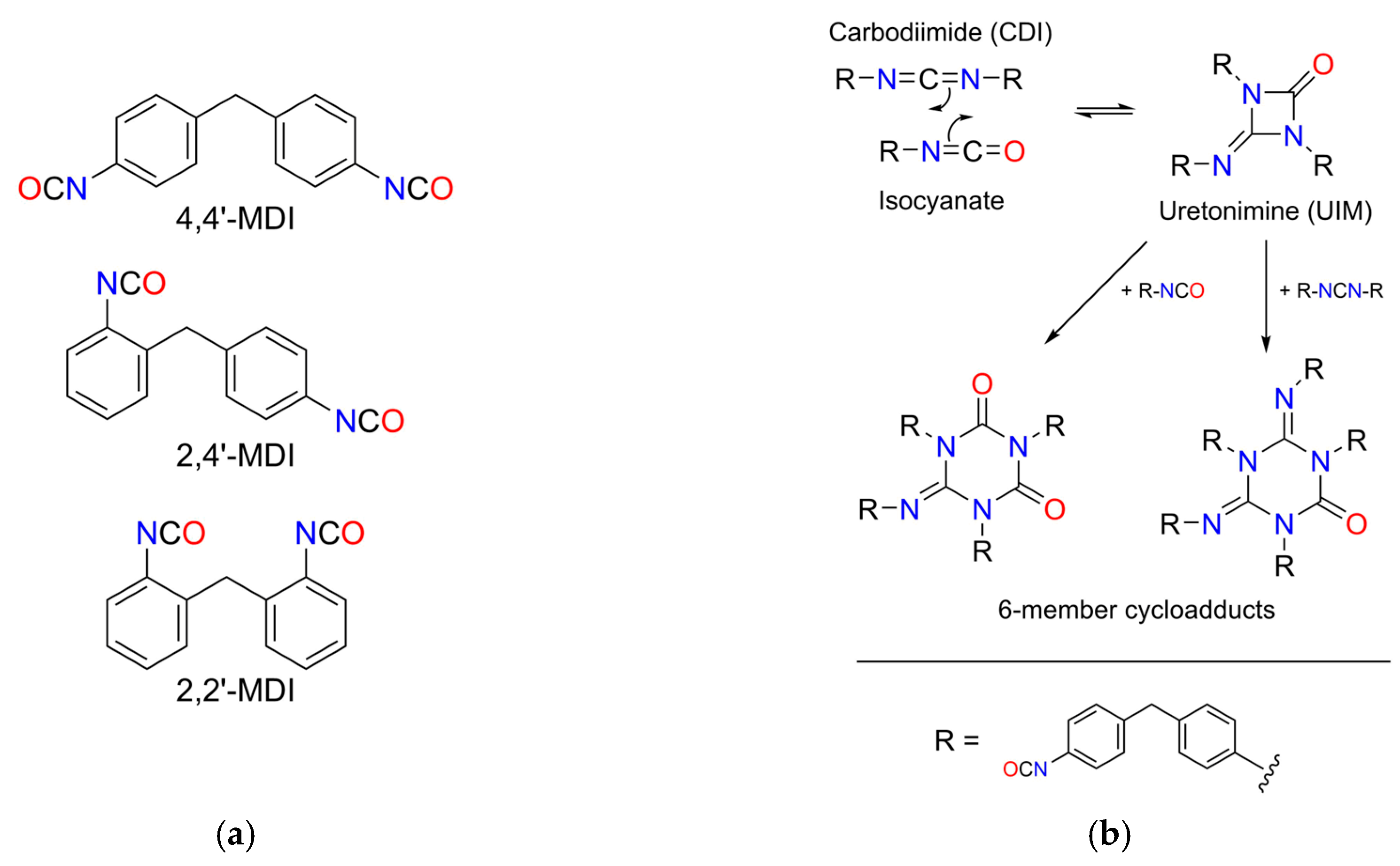
The reaction chamber initially contains a bifunctional reactant molecule, MDI, where the separate NCO groups react independently with the MPPO catalyst and form carbodiimide. Since the product also has free NCO groups, they also react to different chain-like oligomers, which is schematically depicted in
Figure 2b. Furthermore, uretonimine and 6-member ring cycloadduct formation also occurs in addition to carbodiimidization, and thus the amount of reactant MDI is significantly decreased throughout the reaction. In kinetic terms, MDI is involved in parallel reactions, and CDI oligomers take part in consecutive reactions. The separate, most possible gross reactions are shown in
Table 1.
Regarding all probable reactions, gross differential kinetic equations can be described to the decrease in MDI (Equation (1)) and increase of CO
2 (Equation (2)); moreover, to the change in PCDI-2 (Equation (3)). Since uretonimine and 6-ring cycloadduct formation do not generate CO
2, Equation (2) shows only the MPPO catalyzed carbodiimidization reactions. Reactions that are relevant in terms of the following kinetic evaluation are indicated with bold letters.
where square brackets show actual concentrations,
,
etc., are the corresponding rate constants according to
Table 1. Since the occurrence probability of the possible reactions and exact rate constants cannot be determined, the gross differential equation to MDI can be simplified if applying the method of initial rates. Oligomer formation has the same apparent rate constants (
) due to the principle of equal reactivity [
32]. Nevertheless, the concentration of oligomers larger than PCDI-2 is negligible at the beginning of the reaction ([PCDI-3] ≈ [PCDI-
n] ≈ 0), and reaction rate is as follows
…; thus, the dominant reaction is 2 MDI → PCDI-2 + CO
2 (
Figure 2a) at the initial stage of reactions. This way, simplification can be applied to the overall process, resulting in second-order kinetics, which is in contrast with Campbell’s pseudo-zero-order [
15] but in agreement with our similar second-order observations [
21]. Results discussed later will also confirm this assumption. This final differential rate equation is shown in Equation (4), where
k is the observed rate constant, which describes the decrease in MDI and formation of the PCDI-2 product. For plotting MDI decrease on diagram, the well-known integrated version is used (Equation (5)), where
is the dependent variable and
t, time, is the independent variable. The slope of the line gives the desired rate constant
k, while intercept contains the initial concentration of MDI. Equation (6) shows the predicted half-life of the second-order approximation on the decrease in MDI.
The formation of PCDI-2 is described assuming stoichiometric formation with the formula of Equation (7). Rate constants can be derived from both the decrease in reactant MDI and the increase in product PCDI-2, respectively.
It is important to highlight again that these equations are applicable only at the beginning of the reaction with all the above-mentioned simplifications. The real reaction order is probably a fractional order due to the step-growth polymerization, which is not object of this study.
2.2. Kinetic Results
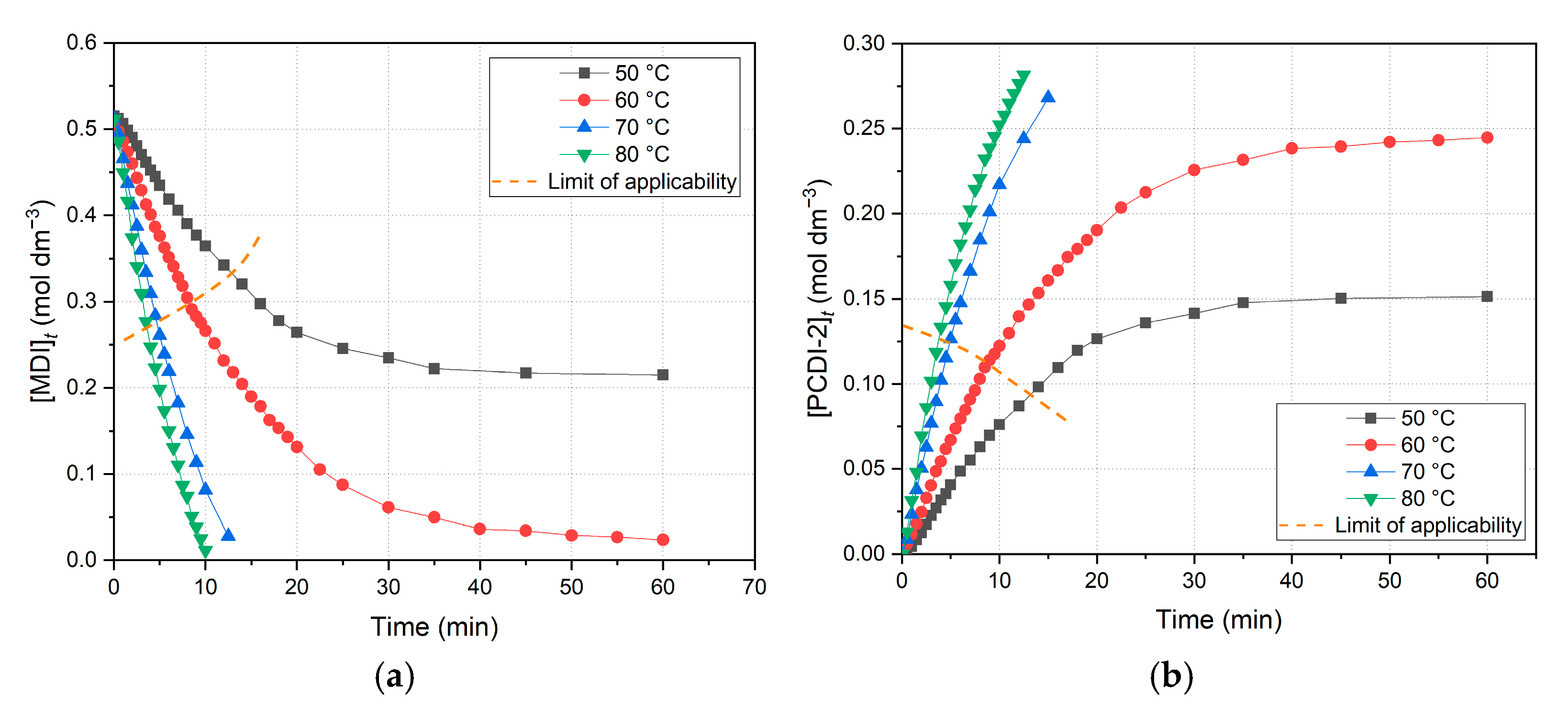
The experimental results section includes diagrams of the primary data (
Figure 4a), conversion (
Figure 4b), dependence of concentration by time (
Figure 5), linearized (
Figure 6) and nonlinearized second-order kinetics (
Figure 7), their regression analysis and the Arrhenius diagrams (
Figure 8).
Table S1 shows all sets of raw data and the calculated intermediate data of the measurements.
At first, primary data are processed, which are CO
2 gas volumes over time. Volume data by temperatures are depicted in a combined gas evolution diagram on
Figure 4a. At the beginning of the experiments, the weighed amounts of reactants, i.e., MDI and MPPO, were adjusted to the theoretical maximum of CO
2, which was calculated to be about 100 cm
3 due to physical limitations. Nonetheless, due to multiple reactions occurring (oligomerization), after the initial part of the curve, more gas evolutes than the theoretical 100% MDI conversion, as can be seen on
Figure 4, especially in the case of 70 and 80 °C measurements. Because of this phenomenon, a notation was applied on the following diagrams, which is an orange dashed line (
Figure 4b,
Figure 5 and
Figure 7), and called it the
limit of applicability. Above this limit, data points plotted do not show the accurate values (which are unknown with this measuring method), but rather smaller or larger values due to systematic errors of the calculation method. This accompanies the whole calculation process, and the limit for each following diagrams is adjusted to the data intervals of the linear regression. In the following investigation, concentrations of reacted MDI were converted into conversion values to describe the extent of reaction. Conversion vs. time diagram is also plotted below (
Figure 4b).
Comparing these diagrams, they pretend to be the same; however, conversion values are calculated from independent initial concentrations, thus the benchmark is slightly different. As a result, conversion diagram is not identical to gas volume diagram, even though in the case of ideally accurate initial weighing and neglected side-reactions, the theoretical maximum of CO
2 volume would be 100 cm
3 at 100% MDI conversion. As can be deducted from conversion diagram, the values are overestimated and the two measurements seem to exceed 100%, which occurs due to the mentioned phenomenon and the applied stoichiometric calculation method (
Figure 2a, Equation (4)). The assumption is that more MDI is consumed and reacted in the later stage of the reaction than the initial amount, [MDI]
0, and every mole of CO
2 comes from the reaction of 2 moles of MDI, which is only valid within the limit of applicability, at the initial stage. Specifically, it seems like that the 60 °C experiment converges to 100%; however, data points are also over this limit, and thus those values are overestimated and are actually much less than 100%. Moreover, the 80 °C measurement is supposed to be the closest to full conversion in the observed temperature range.
The most significant observations during evaluation were performed via calculating concentrations. The derived concentration vs. time plots of the decrease in MDI (
Figure 5a) and increase in PCDI-2 (
Figure 5b) are depicted below.
Regarding the overall data points, reactant (MDI) concentration decreases, while main product (PCDI-2) increases over time. Comparing these diagrams, the ordinate scale is exactly the half in the case of PCDI-2 (
Figure 5b) when compared to MDI (
Figure 5a), which consequently indicates that the maximum amount of product cannot exceed the half of the initial MDI concentration, according to
Figure 2a and Equation (7). On the other hand, the limit of applicability is highlighted again, owing to the unreliability of further data points; for example, the 70 and 80 °C points in
Figure 5a tend to continue below the zero concentration, which is impossible.
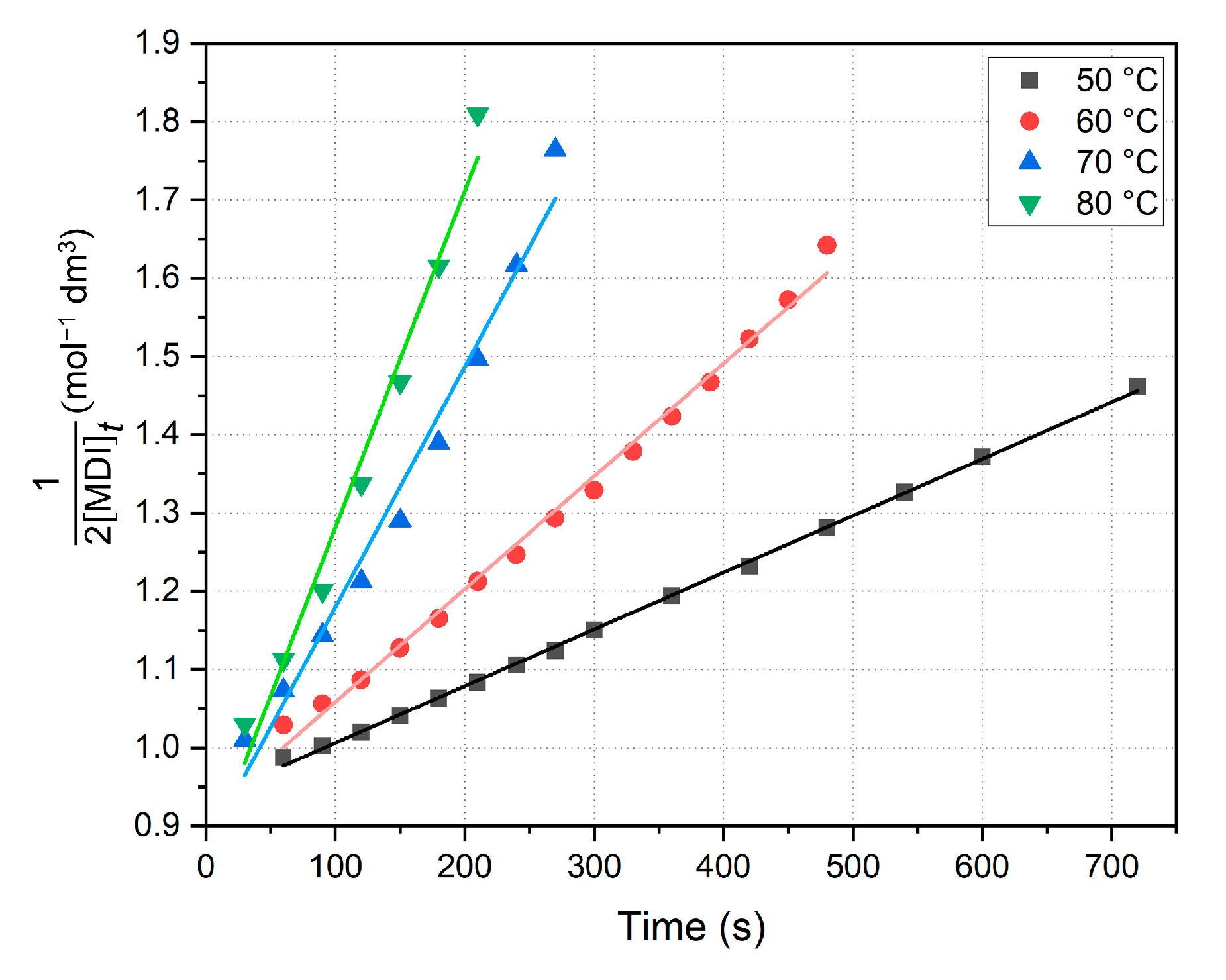
Both linearized and nonlinearized fits were performed on the sections of concentration vs. time plots to implement the appropriate analysis of regression. The rate constants are obtained first by plotting the second-order linearized formula via Equation (5) and fitting a line to the initial section (
Figure 6), applying the method of initial rates and the method of least squares. Linearized kinetic results are summarized in
Table 2, including the coefficient of determination (COD,
R2,
R-square); furthermore, theoretical half-lives were also calculated from the decrease in MDI via Equation (6).
Table 2.
Line fitting parameters of the linearized kinetic diagram. The corresponding symbol colours are the same as in
Figure 6. Error bars are predicted according to regression software [
34].
Table 2.
Line fitting parameters of the linearized kinetic diagram. The corresponding symbol colours are the same as in
Figure 6. Error bars are predicted according to regression software [
34].
| Temperature (T) | Slope, Rate Constant
(k) (10−4 mol−1 dm3 s−1) | Intercept
(mol−1 dm3) | Coefficient of Determination (R2) | Calculated, Theoretical Half-Life (s) |
|---|
| ━ 50 °C | 7.26 ± 0.059 | 0.9334 ± 0.0022 | 0.9991 | 1360 ± 11 |
| ━ 60 °C | 14.4 ± 0.319 | 0.9140 ± 0.0095 | 0.9937 | 715 ± 15 |
| ━ 70 °C | 30.7 ± 1.680 | 0.8724 ± 0.0284 | 0.9795 | 329 ± 18 |
| ━ 80 °C | 42.9 ± 2.632 | 0.8515 ± 0.0353 | 0.9816 | 240 ± 15 |
As shown in
Table 2, the initial parts of the curves yield well-fit straight lines (
R2 > 0.979), and thus the assumed second-order kinetics are proven to be applicable to this section of the reaction. Data show that the reaction rate is six times bigger at 80 °C than at 40 °C, and in addition, error intervals only indicate the slight uncertainty error of line fits. Since the initial concentrations of MDI were about 0.5 mol dm
−3 for all measurements, as a result, the derived intercepts on the diagram (
Figure 6) are also roughly the same (Equation (5)). Half-life analysis resulted in values decreasing as temperature increased, and interestingly, they are they are mostly close to the end point of linear fits or, in other words, concentrations are halved at the end of the dominant PCDI-2 formation reaction. Although calculations do not provide the exact time when MDI is the half of the initial amount (due to the applied second-order approximation), but suggests a loose relation between the concentration change and the polymerization process.
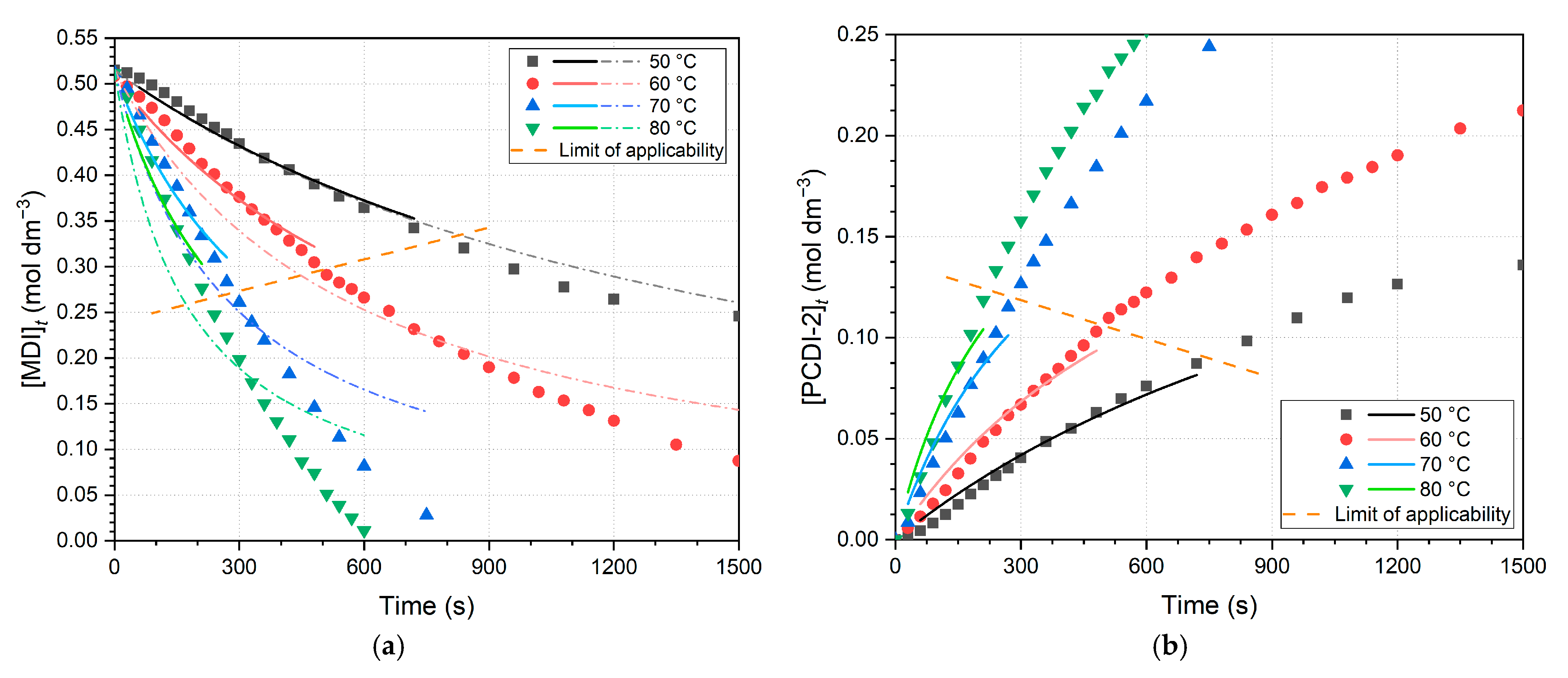
Nonlinear regressions are also performed to investigate whether rate constants obtained this way can validate linear regression results, whilst the error of linearization also disappears. For the decrease in MDI concentrations, nonlinear fits are found in
Figure 7a. According to Equation (7), a short comparison was carried out on the product formation; thus, the fits for PCDI-2 are also shown in
Figure 7b. It should be emphasized that the data intervals of these fits exactly correspond to the linear regressions (
Figure 6) and these kinetic results (
Table 3) are not used in the further Arrhenius analysis.
Table 3.
Nonlinear regression parameters in the case of fitting interval are the same as those applied to the linear regressions and in the case of the full dataset being fitted. Derived parameters were the rate constants with error bars, which are calculated by regression software [
34]. The parameter [MDI]
0 of the fitting equations was chosen to be the fixed initial concentrations for each measurement, not the regression parameter. MDI—4,4′-methylenediphenyl diisocyanate, PCDI-2—polycarbodiimide dimer.
Table 3.
Nonlinear regression parameters in the case of fitting interval are the same as those applied to the linear regressions and in the case of the full dataset being fitted. Derived parameters were the rate constants with error bars, which are calculated by regression software [
34]. The parameter [MDI]
0 of the fitting equations was chosen to be the fixed initial concentrations for each measurement, not the regression parameter. MDI—4,4′-methylenediphenyl diisocyanate, PCDI-2—polycarbodiimide dimer.
| Observed Molecule | Number of Regression Points | Temperatures (T) | Derived Rate Constants (k)
(10−4 mol−1 dm3 s−1) | Coefficients of Determination (R2) |
|---|
| MDI | 7–15 points, below the limit of applicability | ━ 50 °C | 6.20 ± 0.18 | 0.9751 |
| ━ 60 °C | 11.9 ± 0.36 | 0.9671 |
| ━ 70 °C | 23.6 ± 1.39 | 0.9451 |
| ━ 80 °C | 32.0 ± 2.38 | 0.9348 |
| Full dataset | ![Ijms 26 08570 i001 Ijms 26 08570 i001]() 50 °C 50 °C | 6.32 ± 0.24 | 0.9718 |
![Ijms 26 08570 i002 Ijms 26 08570 i002]() 60 °C 60 °C | 16.8 ± 0.90 | 0.9452 |
![Ijms 26 08570 i003 Ijms 26 08570 i003]() 70 °C 70 °C | 34.2 ± 3.60 | 0.8852 |
![Ijms 26 08570 i004 Ijms 26 08570 i004]() 80 °C 80 °C | 56.0 ± 6.83 | 0.8582 |
| PCDI-2 | 7–15 points, below the limit of applicability | ━ 50 °C | 6.52 ± 0.20 | 0.9739 |
| ━ 60 °C | 13.4 ± 0.44 | 0.9626 |
| ━ 70 °C | 25.9 ± 1.62 | 0.9407 |
| ━ 80 °C | 36.8 ± 2.99 | 0.9273 |
Comparing nonlinear regression data of PCDI-2 and MDI plots, at lower temperatures, rate constants deviate minimally (0.57–0.65 × 10
−4 mol
−1 dm
3 s
−1), while at higher temperatures the difference is marginally bigger (2.34–2.58 × 10
−4 mol
−1 dm
3 s
−1). Nonetheless, in the case of PCDI-2, the reliability of nonlinear regression is limited, as CODs decreasing by increasing temperature. Furthermore, regressions were also performed for the full dataset, which demonstrated that the derived rate constants deviate more from expected values for narrower fitting intervals and CODs show worse fits. The reason for this is the change from second-order approximation above the limit of applicability. This is particularly eveident in the case of the 70 and 80 °C measurements in
Figure 7a, where second-order curves start to become horizontal and converge on the abscissa, in contrast to the trend of data points. It was also revealed that the rate constants in the case of linearized fits (
Table 2) are slightly bigger than the nonlinearized ones (
Figure 7a), and the differences in the corresponding temperatures are negligible, varying between 0.09 and 3.48 × 10
−4 dm
3 mol
−1 s
−1.
The connection between rate constants and temperature is given by Arrhenius equation. Mostly, the linearized formula is used to plot the correlation between rate constants determined at different temperatures. This ln(
k) vs. 1/
T diagram and the linear regression weighted with error bars of rate constants are shown in
Figure 8a. The less common, but more accurate nonlinearized Arrhenius diagram is also depicted in
Figure 8b, and the equation of fitted Arrhenius curves obtained are also shown in Equations (8) and (9), respectively. (See
Figure S1).
Figure 8.
Arrhenius diagrams made from rate constants obtained from linear regressions (to be consistent with our previous results [
21]). (
a) Linearized diagram (logarithm of rate constants vs. reciprocal temperatures in K) with error bars from
Table 2. Equation (8) and the coefficient of determination (
R2) is also presented. (
b) Nonlinear Arrhenius diagram with nonlinear regression, also regarding error bars. Fitted parameters are shown in Equation (9).
Figure 8.
Arrhenius diagrams made from rate constants obtained from linear regressions (to be consistent with our previous results [
21]). (
a) Linearized diagram (logarithm of rate constants vs. reciprocal temperatures in K) with error bars from
Table 2. Equation (8) and the coefficient of determination (
R2) is also presented. (
b) Nonlinear Arrhenius diagram with nonlinear regression, also regarding error bars. Fitted parameters are shown in Equation (9).
where
k is the temperature dependent rate constant in mol
−1 dm
3 s
−1 and
T is the temperature in K. Activation energy is calculated from slope of the linear fit on the diagram (
Figure 8a) and based on the linearized Arrhenius equation, the energy is given to 60.4 ± 3.0 kJ mol
−1. It should be noted, that the data are heteroscedastic due to the error considerations, which are mostly significant at 70 and 80 °C (best seen at
Figure 8b); thus, this method of determination is subject to greater uncertainty regarding these higher temperature measurements. By analyzing the results, a linearized Arrhenius plot (
Figure 8a) predicted the pre-exponential factor with significant errors:
× 10
6 mol
−1 dm
3 s
−1 (from Equation (8); positive error is especially significant), due to exponential dependence, which is more symmetrical in the case of nonlinear plot (
Figure 8b):
mol
−1 dm
3 s
−1 (from Equation (9)). This phenomenon also reflects on the more favoured use of nonlinear Arrhenius fitting.
Moreover, preliminary calculations were performed in order to investigate whether the observed solution phase reaction is diffusion-controlled or activation-controlled. The value of activation energy is significantly high and the viscosity also does not exert an important influence on rate, considering the formation reaction of the shortest oligomer, PCDI-2. Consequently, the initial part of the reaction is not diffusion-controlled, because results do not show the temperature dependence of the diffusion constant, and the chemical reaction is the determining factor. In the following, the results of the theoretical calculations are presented.
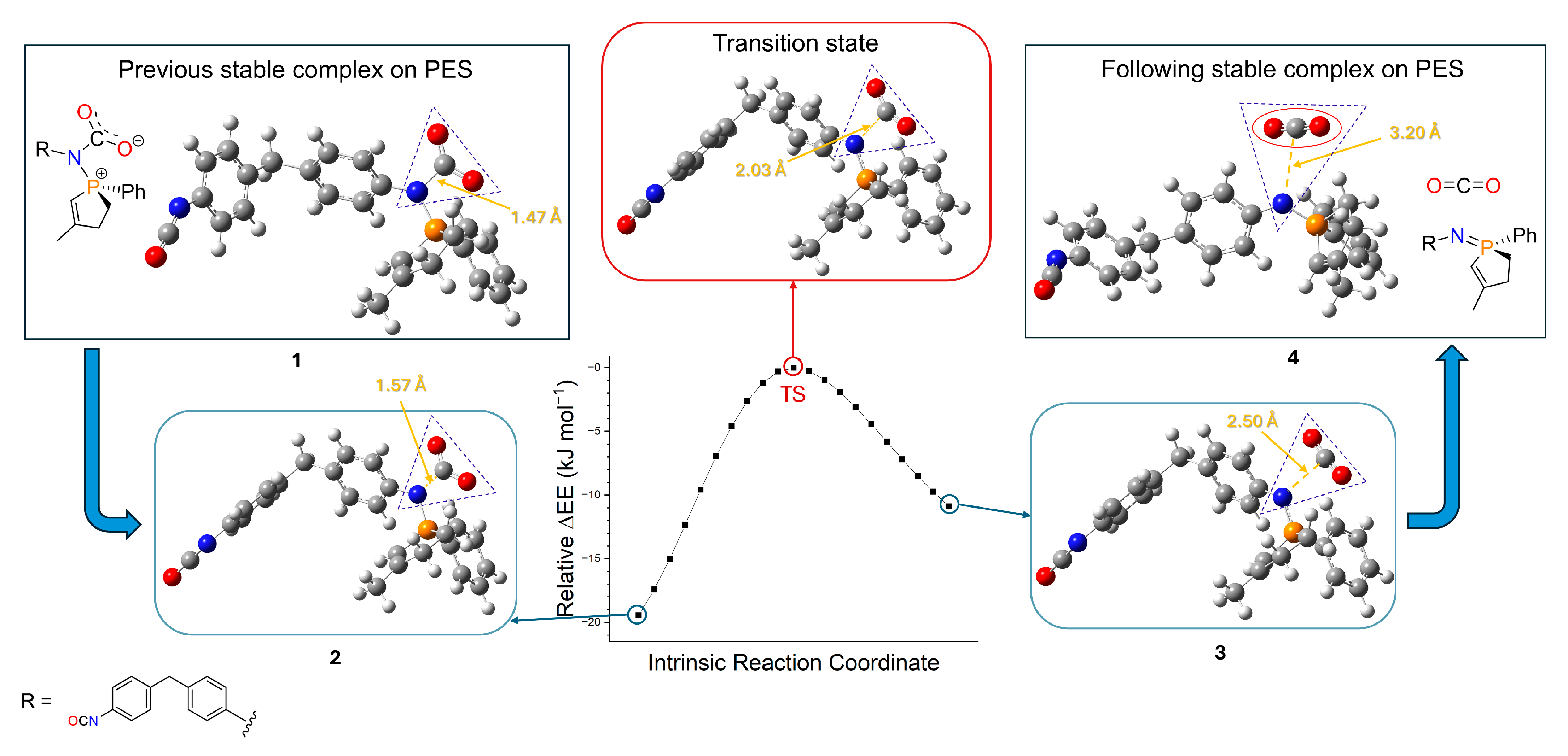
2.3. Theoretical Calculations
Computational chemistry calculations were carried out to support the experimental results and obtain preliminary information about the mechanism of polycarbodiimide formation. In our first article about carbodiimides [
21], the full mechanism of the CDI formation is discussed, regarding the two subprocesses (
Figure 3), complexes, transition states and thermochemical results (
Table S2) calculated by Gaussian, using B3LYP/6-31G(d)[SMD(ODCB)] level of theory. Calculations showed that the rate-determining step is the TS3 transition state in the first subprocess, where CO
2 separates from the complex and exits from the system (
Figure 3) [
21]. The driving force here is this leaving molecule, which increases the entropy of the system, thus lowering Gibbs free energy and letting the reaction occur. Current calculations in case of MDI were also performed at the same level of theory, but indicating only the significant step, the initial molecules (MDI, MPPO) and the rate-determining step (TS, which refers to the previously called TS3 [
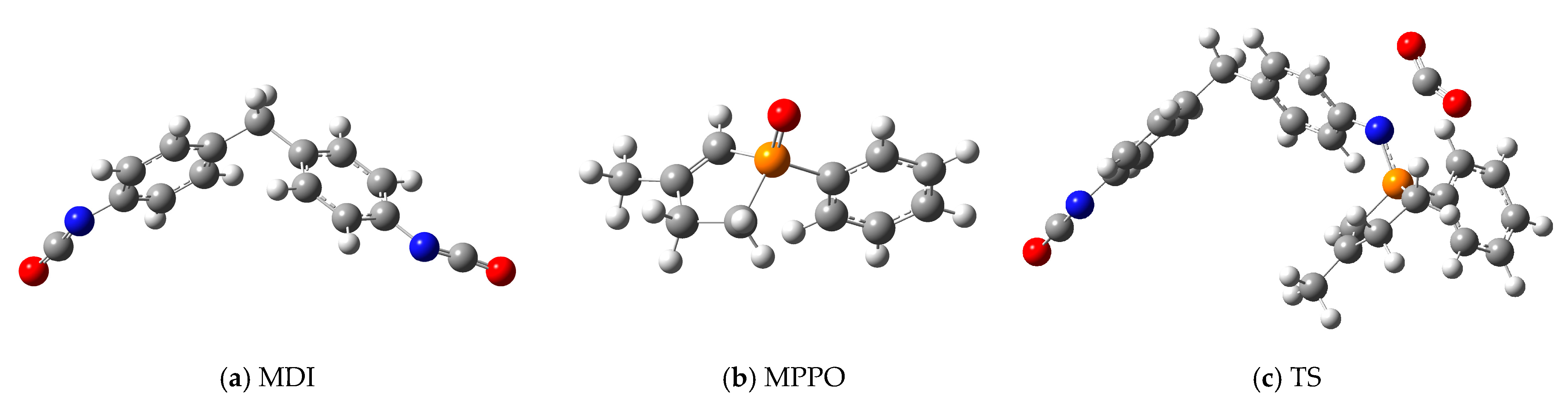
21]). Structures (
Figure 9 and
Figure S1;
Table S3) were geometrically optimized followed by frequency calculations and intrinsic reaction coordinate (IRC) analysis (
Figure 10,
Tables S4 and S5), which ensured that the results were adequate. The thermochemical evaluation showed that the relative enthalpy of activation of the rate-determining transition state (TS) compared to the initial energy of reactants (MDI + MPPO) was 52.89 kJ mol
−1 (
Table S2), and the further analysis of the intermediate steps was omitted.
Furthermore, knowing the theoretically determined enthalpy and entropy of activation of the reaction (
Table S2) at different temperatures, transition state theory (TST) can be applied to determine theoretical rate constants and the activation energy, which are comparable with experimental values. The modified Eyring—Polányi equation is shown below (Equation (10)):
where
k is the second-order rate constant (mol dm
−3 s
−1),
is the transmission coefficient (
= 1),
is a correction factor we applied,
kB is the Boltzmann constant,
T is the absolute temperature (K),
h is the Planck constant,
is the standard concentration (
= 1 mol dm
−3),
is the entropy of activation at
T temperature (J mol
−1 K
−1),
is the enthalpy of activation at
T temperature (J mol
−1), and
R is the gas constant. Energies were determined from the 298.15 K standard computational calculations, using the heat capacity of activation (
) on the same temperature range as the experiments, i.e., 50–80 °C. Results showed that the rate constants that were derived from Equation (10) and corrected with heat capacity, were plotted with the corresponding temperatures, yielding a new Arrhenius diagram, and the predicted activation energy was 56.3 kJ mol
−1 and the pre-exponential factor was 3.80 × 10
3 mol
−1 dm
3 s
−1 (fitting errors here are negligible since the
k values are roughly multiples of each other and
R2 = 1.00). However, this determination method is subject to the errors of assuming the ideal TST (
= 1) and a harmonic oscillator model, as well as a low level of theory and improper entropy calculations. Since the pre-exponential factors obtained from DFT calculations omit the solvent effect, an empirical
factor was applied as an additional correction to Equation (10) by comparing the experimental results. The corrected pre-exponential factor and the comparison of results are presented in the discussion.
Inspections reveal that TST is also applicable to predicting theoretical activation parameters from experimentally measured rate constants, as well as Arrhenius theory. Although, this case is not discussed in detail, a brief comparison (
Figure S2) showed that both the enthalpy and entropy of activation derived from linearized Eyring equation reproduced the theoretical energies (at 298.15 K), using the data of
Table 2. Values are
= 57.6 kJ mol
−1 and
= −127.4 J mol
−1 K
−1, respectively.
2.4. Discussion of Kinetic Results
The comparison of different experimental regressions and theoretical methods with each other and the bifunctional MDI with the monofunctional phenyl isocyanate (PhNCO) [
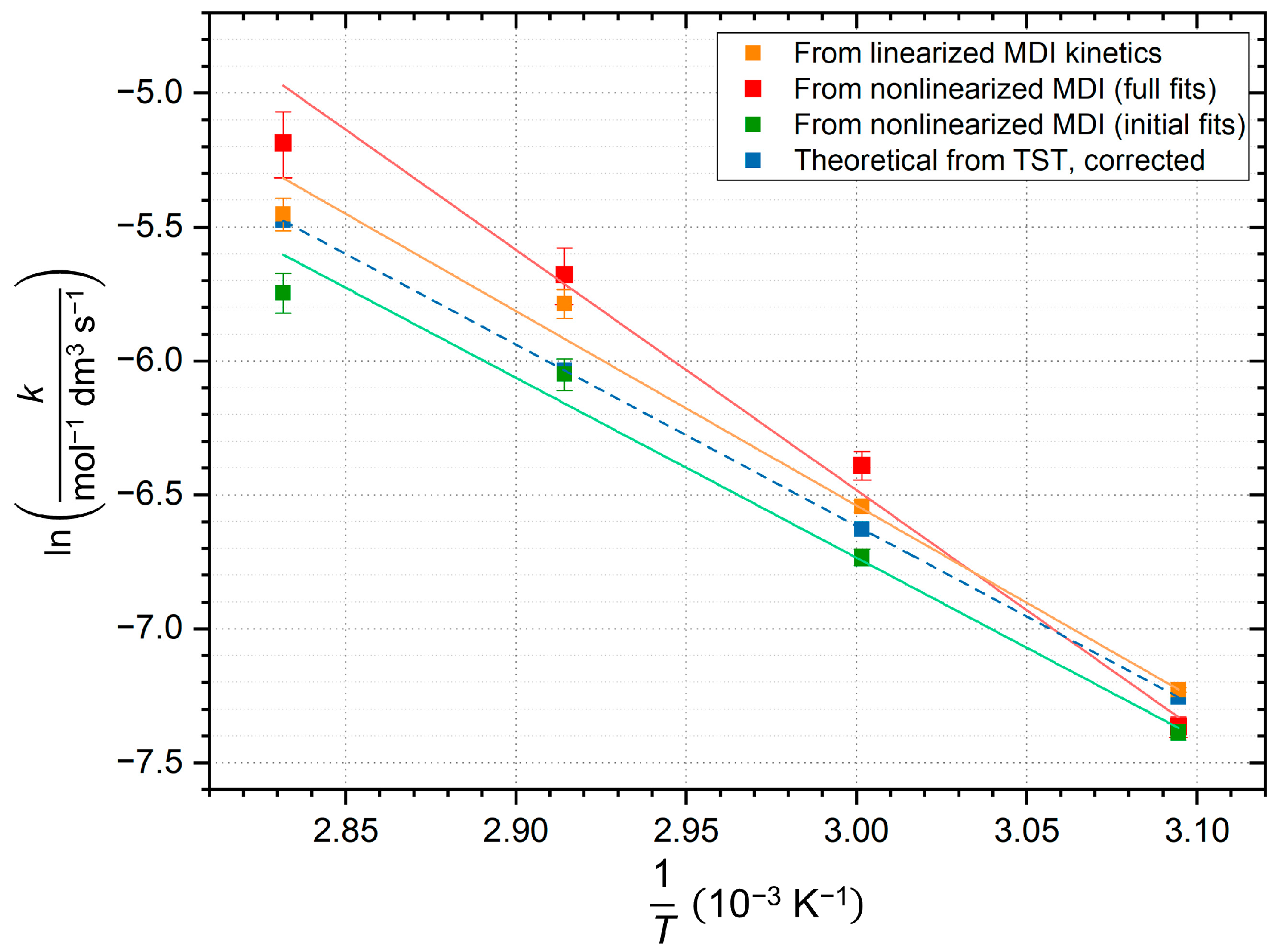
21] represent a perfect base for comparison. Due to the various results, a combined Arrhenius diagram of different fits (
Figure 11), its derived regression parameters (
Table 4), and a comparison matrix are also presented (
Table 5).
Table 4.
Line fitting parameters of the combined Arrhenius diagram. The corresponding symbol colours are the same as in
Figure 11. Error bars are predicted according to regression software [
34]. TST—transition state theory.
Table 4.
Line fitting parameters of the combined Arrhenius diagram. The corresponding symbol colours are the same as in
Figure 11. Error bars are predicted according to regression software [
34]. TST—transition state theory.
| Source of Rate Constants | Activation Energy, Derived from Slope
(kJ mol−1) | Pre-Exponential Factor Derived from Intercept
(mol−1 dm3 s−1) | Coefficient of Determination (R2) |
|---|
| ━ Linearized fit | 60.4 ± 3.0 | × 106 | 0.9950 |
| ━ Nonlinearized fit (full dataset) | 74.6 ± 6.3 | × 108 | 0.9858 |
| ━ Nonlinearized fit (initial part) | 55.9 ± 4.0 | × 105 | 0.9900 |
![Ijms 26 08570 i005 Ijms 26 08570 i005]() Theoretical, TST Theoretical, TST | 56.3 | 8.8 × 105 * | 1.0000 |
Figure 11 compares kinetic data from linearized and nonlinearized regressions and theoretical results too. The high deviation of nonlinearized fit for the full dataset in all parameters indicates that the reaction is not second-order throughout the full progress, as oligomerization changes it. Conversely, the rate constants derived from the nonlinear fits to the initial section of the kinetic curve show better correlation with the ones from the linear regression and the theoretical ones, demonstrating that the second-order approximation is true at the beginning of the reaction, which also applies to the CODs. The data suggest that slight uncertainty in the intercepts plays major role in the value of pre-exponential factors derived from them, which appears in asymmetric error bars, but mainly in the upper deviation. Theoretical calculations include the
k constants obtained from Eyring equation, followed by empirical correction, where the
factor was considered to be
to ensure consistent comparison. As a result, this pre-exponential factor reproduces the result of nonlinear initial fit in the same order of magnitude. The following comparison is made in order to draw conclusions using literature data.
Table 5.
Comparison matrix of summarized results, including experimental data from linear regressions for both MDI (4,4′-methylenediphenyl diisocyanate) and our previously published PhNCO (phenyl isocyanate) [
21]. The comparison of the theoretically obtained barrier of enthalpy of activation at 298.15 K is also shown. TS3 refers to the same rate-determining step for MDI (TS in
Figure 9 and
Figure 10) that was examined for PhNCO.
Table 5.
Comparison matrix of summarized results, including experimental data from linear regressions for both MDI (4,4′-methylenediphenyl diisocyanate) and our previously published PhNCO (phenyl isocyanate) [
21]. The comparison of the theoretically obtained barrier of enthalpy of activation at 298.15 K is also shown. TS3 refers to the same rate-determining step for MDI (TS in
Figure 9 and
Figure 10) that was examined for PhNCO.
| | | PhNCO (“mono”) | MDI (“di”) |
|---|
| Experimental measurements | Activation energy, Ea (kJ mol−1) | 55.8 ± 2.1 [21] | 60.4 ± 3.0 |
Pre-exponential factor, A
(mol−1 dm3 s−1) | (4.2 ± 2.7) × 105 [21] | × 106 |
| Theoretical calculations | R–TS3 enthalpy of activation, (kJ mol−1) | 52.87 [21] | 52.89 |
Table 5 reflects on the strongly similar results of the activation energies (average difference is 4.6 kJ mol
−1 in relation to PhNCO), as well as the pre-exponential factors with similar orders of magnitude (difference is explained by the fact that the initial molar concentration is approx. half in the case of MDI, in contrast to the case of PhNCO). This observation shows that the molecular interactions take place similarly according to collision theory and TST [
29], which reflects the previously discussed principle of equal reactivity and the effect of group additivity, as the results are independent of the type of observed isocyanate and also not regarding the number of NCO functionalities. Moreover, the more popular
second-order kinetic formula could also be used during the analysis, instead of the applied, more accurate Equation (5). This formula neglects the exact stoichiometry according to the gross equation (
Figure 2a); however, the Arrhenius parameters calculated this way would remain the same.
Highlighting theoretical calculations, the TS enthalpy of activation for MDI (52.89 kJ mol
−1) was compared with the case of PhNCO, which was 52.87 kJ mol
−1 [
21]. The two values have a good correlation, as the difference is only 0.02 kJ mol
−1, which is negligible; thus the theoretical calculations also confirm that the carbodiimidization reaction is independent of the type of isocyanate. This phenomenon is similar to the experimentally compared results (
Table 5), and the activation energy is also in good agreement with the theoretical one. Furthermore, if we apply a temperature correction to the obtained activation enthalpy, according to the equation
[
29] (p. 114), then activation energy is finally given as 55.37 kJ mol
−1. This is even closer to the experimental value, 60.4 ± 3.0 kJ mol
−1 (difference is only 5.0 kJ mol
−1), than the enthalpy, even though theoretical calculations were performed at 298.15 K. Based on this latter observation, it can be seen that the theoretical calculations support and confirm our developed experimental method.

 50 °C
50 °C 60 °C
60 °C 70 °C
70 °C 80 °C
80 °C
 Theoretical, TST
Theoretical, TST