Multifunctional Activity of Syzygium aromaticum Extracts Against Candida albicans: Free Radicals, Membrane Permeabilization and Cdr1p Localization
Abstract
1. Introduction
2. Results
2.1. Composition of Eugenol Extracts
2.1.1. HPLC-UV Analysis
2.1.2. ATR-FTIR Analysis
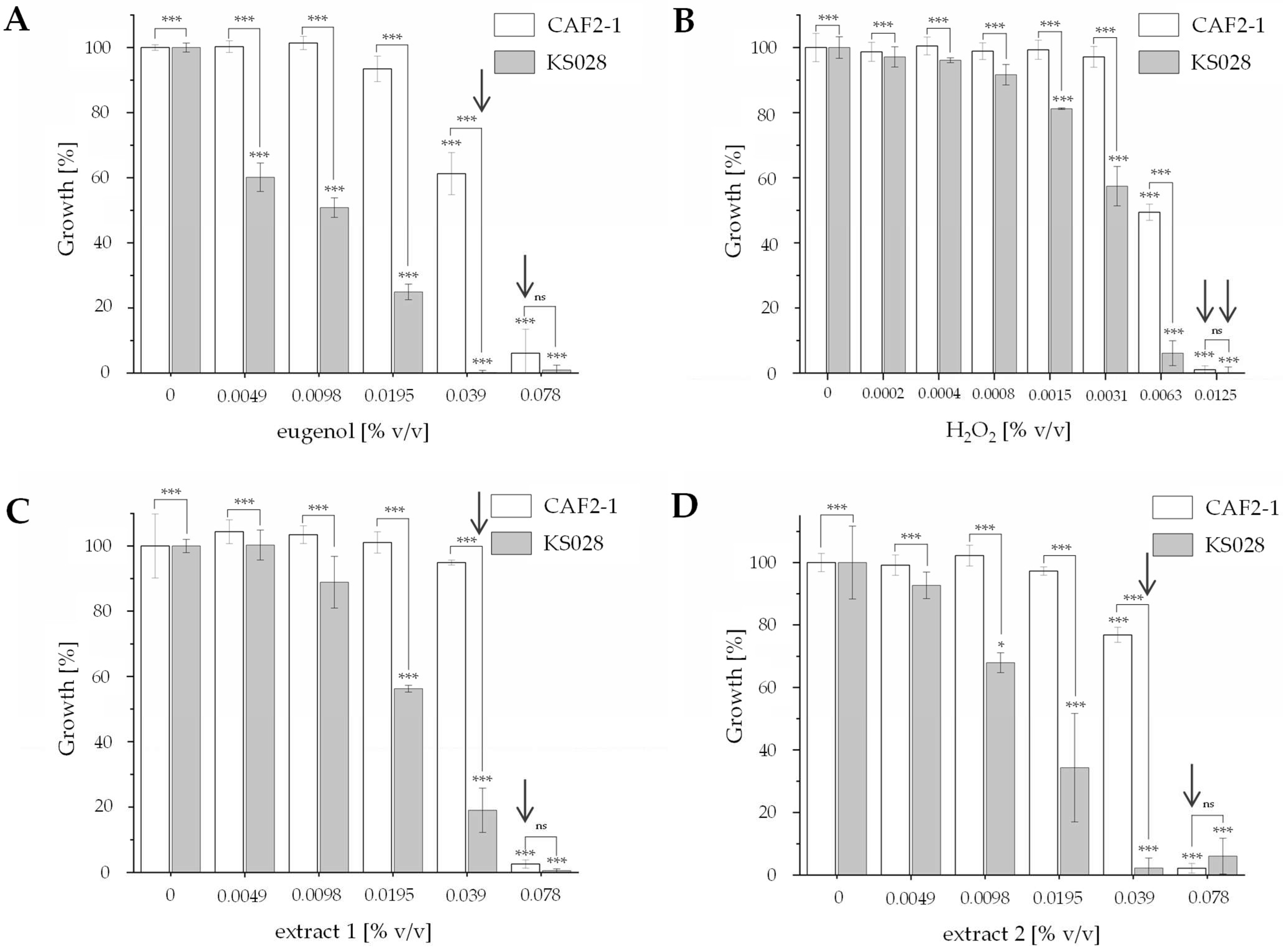
2.2. Antifungal Activity of Eugenol Extracts Against Candida albicans
2.3. Oxidative Stress Induced in Response to Eugenol Extracts
2.3.1. DPPH Free Radical Scavenging
2.3.2. Cellular ROS Level
2.4. Candida albicans PM Integrity Maintenance in Response to Eugenol Extracts
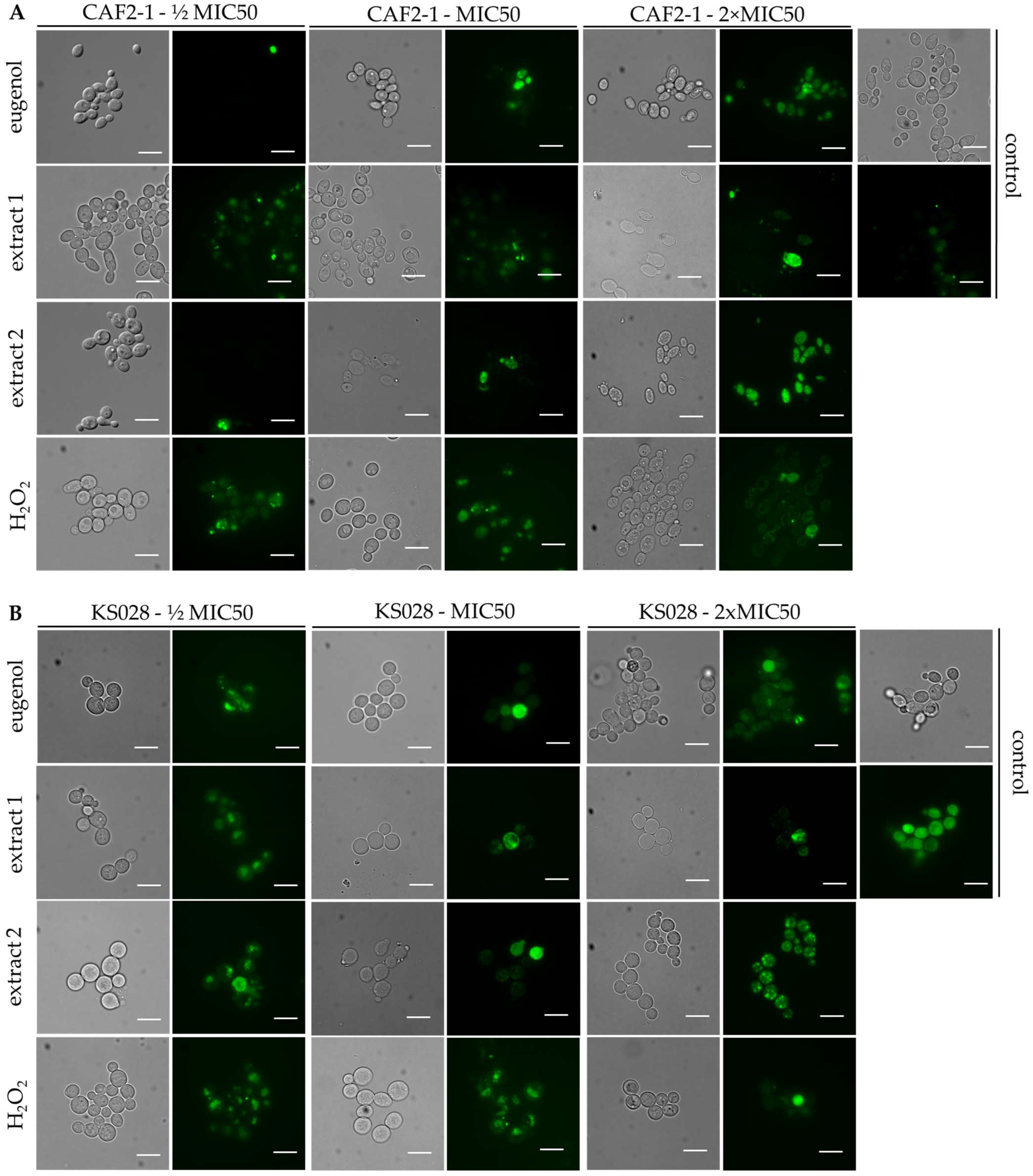
2.4.1. Permeabilization of Fungal PM
2.4.2. Fluidity of Fungal PM
2.4.3. PM Localization of CaCdr1p
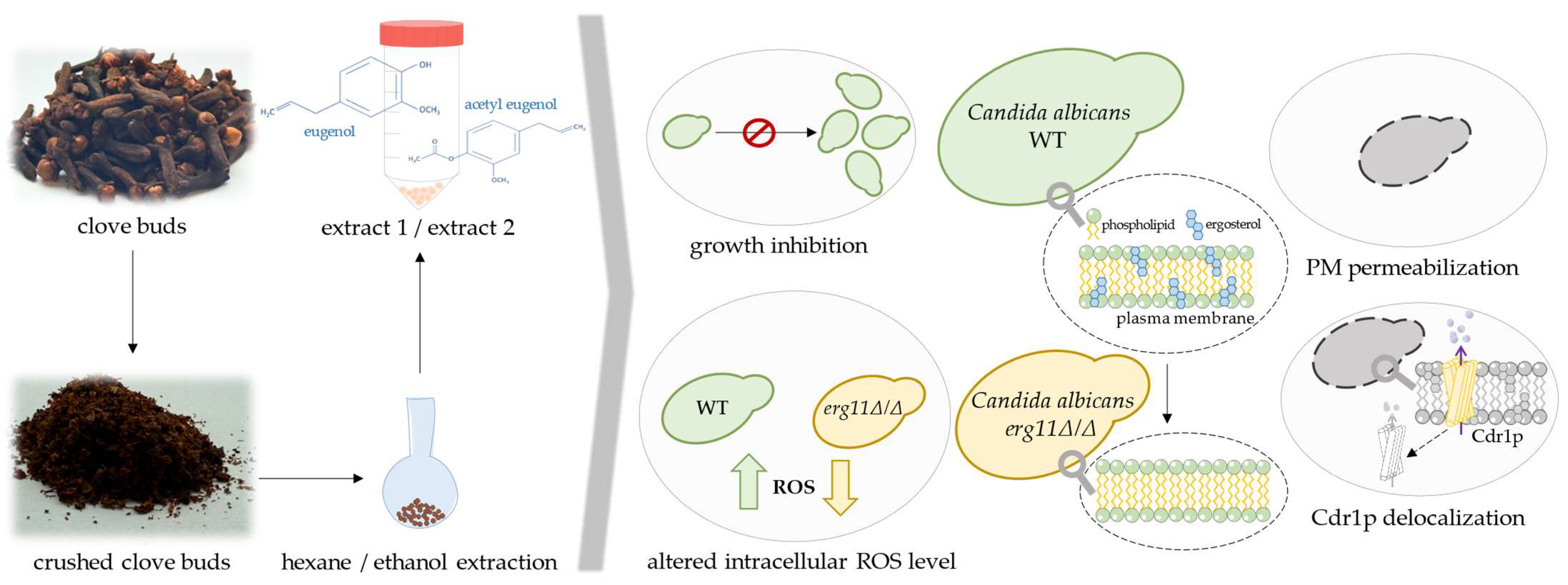
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Chemicals
4.3. HPLC-UV Analysis of Eugenol Extracts
4.4. ATR-FTIR Analysis of Eugenol and Extracts
4.5. Strains and Culture Conditions
4.6. Susceptibility Testing and Determination of Minimal Inhibitory Concentrations
4.7. Analysis of DPPH Free Radical Scavenging Potential
4.8. Analysis of Intracellular ROS Production
4.9. Analysis of Membrane Permeability
4.10. Analysis of Membrane Fluidity
4.11. Observations of CaCdr1-GFP Localization in PM
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bezerra, D.P.; Militão, G.C.G.; de Morais, M.C.; de Sousa, D.P. The Dual Antioxidant/Prooxidant Effect of Eugenol and Its Action in Cancer Development and Treatment. Nutrients 2017, 9, 1367. [Google Scholar] [CrossRef] [PubMed]
- Nisar, M.F.; Khadim, M.; Rafiq, M.; Chen, J.; Yang, Y.; Wan, C.C. Pharmacological Properties and Health Benefits of Eugenol: A Comprehensive Review. Oxidative Med. Cell. Longev. 2021, 2021, 2497354. [Google Scholar] [CrossRef]
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol—A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant activity of eugenol: A structure-activity relationship study. J. Med. Food 2011, 14, 975–985. [Google Scholar]
- Tammannavar, P.; Pushpalatha, C.; Jain, S.; Sowmya, S.V. An unexpected positive hypersensitive reaction to eugenol. BMJ Case Rep. 2013, 2013, bcr2013009464. [Google Scholar] [CrossRef]
- Didehdar, M.; Chegini, Z.; Shariati, A. Eugenol: A novel therapeutic agent for the inhibition of Candida species infection. Front. Pharmacol. 2022, 13, 872127. [Google Scholar] [CrossRef] [PubMed]
- Campaniello, D.; Corbo, M.R.; Sinigaglia, M. Antifungal Activity of Eugenol against Penicillium, Aspergillus, and Fusarium Species. J. Food Prot. 2010, 73, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, G.E.; Lawrence, R. Mechanisms of bactericidal action of Eugenol against Escherichia coli. J. Herb. Med. 2020, 26, 100406. [Google Scholar]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, S.; Mukherjee, D.; Joshi, S.J.; Ray, R.R. Antibiofilm and anti-quorum sensing activities of eugenol and linalool from Ocimum tenuiflorum against Pseudomonas aeruginosa biofilm. J. Appl. Microbiol. 2021, 131, 2821–2837. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Khan, L.A.; Manzoor, N. In vitro synergy of eugenol and methyleugenol with fluconazole against clinical Candida isolates. J. Med. Microbiol. 2010, 59, 1178–1184. [Google Scholar]
- Bezerra, S.R.; Bezerra, A.H.; de Sousa Silveira, Z.; Macedo, N.S.; Dos Santos Barbosa, C.R.; Muniz, D.F.; Sampaio Dos Santos, J.F.; Melo Coutinho, H.D.; Bezerra da Cunha, F.A. Antibacterial activity of eugenol on the IS-58 strain of Staphylococcus aureus resistant to tetracycline and toxicity in Drosophila melanogaster. Microb. Pathog. 2022, 164, 105456. [Google Scholar] [CrossRef]
- Campos Péret, V.A.; Reis, R.C.F.M.; Braga, S.F.P.; Benedetti, M.D.; Caldas, I.S.; Carvalho, D.T.; Santana, L.F.A.; Johann, S.; Souza, T.B. New miconazole-based azoles derived from eugenol show activity against Candida spp. and Cryptococcus gattii by inhibiting the fungal ergosterol biosynthesis. Eur. J. Med. Chem. 2023, 256, 115436. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Manzoor, N.; Khan, L.A. Evolution of ergosterol biosynthesis inhibitors as fungicidal against Candida. Microb. Pathog. 2010, 48, 35–41. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.F.; Al-Askar, A.A. In vitro anticandidal potency of Syzygium aromaticum (clove) extracts against vaginal candidiasis. Complement. Med. Ther. 2020, 20, 25. [Google Scholar] [CrossRef]
- Ahmed, I.A.M.; Babiker, E.E.; Al-Juhaimi, F.Y.; Bekhit, A.E.A. Clove Polyphenolic Compounds Improve the Microbiological Status, Lipid Stability, and Sensory Attributes of Beef Burgers during Cold Storage. Antioxidants 2022, 11, 1354. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi Nejad, S.; Özgüneş, H.; Başaran, N. Pharmacological and Toxicological Properties of Eugenol. Turk. J. Pharm. Sci. 2017, 14, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chai, Y.; Zhou, D.; Yao, X.; Ji, H. Mechanism for efficient separation of eugenol and eugenol acetate with β-cyclodextrin as a selective solvent. Supramol. Chem. 2019, 31, 767–775. [Google Scholar] [CrossRef]
- Batiha, G.E.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef]
- Shahina, Z.; Ndlovu, E.; Persaud, O.; Sultana, T.; Dahms, T.E.S. Candida albicans Reactive Oxygen Species (ROS)-Dependent Lethality and ROS-Independent Hyphal and Biofilm Inhibition by Eugenol and Citral. Microbiol. Spectr. 2022, 10, e0318322. [Google Scholar] [CrossRef]
- Biernasiuk, A.; Baj, T.; Malm, A. Clove Essential Oil and Its Main Constituent, Eugenol, as Potential Natural Antifungals against Candida spp. Alone or in Combination with Other Antimycotics Due to Synergistic Interactions. Molecules 2022, 28, 215. [Google Scholar] [CrossRef] [PubMed]
- Jordá, T.; Barba-Aliaga, M.; Rozès, N.; Alepuz, P.; Martínez-Pastor, M.T.; Puig, S. Transcriptional regulation of ergosterol biosynthesis genes in response toiron deficiency. Environ. Microbiol. 2022, 24, 5248–5260. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Tóth Hervay, N.; Bujdos, M.; Gbelska, Y. Essential Role of CgErg6p in Maintaining Oxidative Stress Tolerance and Iron Homeostasis in Candida glabrata. J. Fungi 2023, 9, 579. [Google Scholar]
- Pinto, E.; Vale-Silva, L.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2009, 58, 1454–1462. [Google Scholar] [CrossRef]
- Kowalewska, A.; Majewska-Smolarek, K. Eugenol-Based Polymeric Materials-Antibacterial Activity and Applications. Antibiotics 2023, 12, 1570. [Google Scholar] [CrossRef]
- Suchodolski, J.; Muraszko, J.; Bernat, P.; Krasowska, A. A Crucial Role for Ergosterol in Plasma Membrane Composition, Localisation, and Activity of Cdr1p and H+-ATPase in Candida albicans. Microorganism 2019, 7, 378. [Google Scholar]
- de Paula, S.B.; Bartelli, T.F.; Di Raimo, V.; Santos, J.P.; Morey, A.T.; Bosini, M.A.; Nakamura, C.V.; Yamauchi, L.M.; Yamada-Ogatta, S.F. Effect of Eugenol on Cell Surface Hydrophobicity, Adhesion, and Biofilm of Candida tropicalis and Candida dubliniensis Isolated from Oral Cavity of HIV-Infected Patients. Evid.-Based Complement. Altern. Med. 2014, 2014, 505204. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Wani, M.Y.; Khan, A.; Manzoor, N.; Molepo, J. Synergistic Interactions of Eugenol-tosylate and Its Congeners with Fluconazole against Candida albicans. PLoS ONE 2015, 10, e0145053. [Google Scholar]
- Prajapati, J.; Goswami, D.; Dabhi, M.; Acharya, D.; Rawal, R.M. Potential dual inhibition of SE and CYP51 by eugenol conferring inhibition of Candida albicans: Computationally curated study with experimental validation. Comput. Biol. Med. 2022, 151, 106237. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, A.; Ramezanian, A.; Shekarforoush, S.; Niakousari, M.; Eshghi, S. Antifungal activity of thymol against the main fungi causing pomegranate fruit rot by suppressing the activity of cell wall degrading enzymes. LWT 2022, 161, 113303. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Vermaak, I.; Viljoen, A.M. Eugenol—From the remote Maluku Islands to the international market place: A review of a remarkable and versatile molecule. Molecules 2012, 17, 6953–6981. [Google Scholar] [CrossRef]
- Inam, F.; Deo, S.U.J.A.T.A.; Narkhede, N.E.H.A. HPLC–UV method development and quantification of eugenol from methanolic extracts of some spices. Int. J. Chem. Phys. Sci. 2014, 3, 96–102. [Google Scholar]
- Fonzi, W.A.; Irwin, M.Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics 1993, 134, 717–728. [Google Scholar] [CrossRef]
- Szczepaniak, J.; Łukaszewicz, M.; Krasowska, A. Estimation of Candida albicans ABC Transporter Behavior in Real-Time via Fluorescence. Front. Microbiol. 2015, 6, 1382. [Google Scholar] [CrossRef] [PubMed]
- NCCLS. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts; Approved Guideline. In NCCLS Document M44-A; NCCLS: Wayne, PA, USA, 2004; ISBN 1-56238-532-1. [Google Scholar]
- CLSI M27-A3 28; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeast. 3rd ed. CLSI: Annapolis Junction, MD, USA, 2008; p. 604.
- Urbanek, A.K.; Muraszko, J.; Derkacz, D.; Łukaszewicz, M.; Bernat, P.; Krasowska, A. The Role of Ergosterol and Sphingolipids in the Localization and Activity of Candida albicans’ Multidrug Transporter Cdr1p and Plasma Membrane ATPase Pma1p. Int. J. Mol. Sci. 2022, 23, 9975. [Google Scholar] [CrossRef] [PubMed]
- Derkacz, D.; Bernat, P.; Krasowska, A. K143R Amino Acid Substitution in 14-α-Demethylase (Erg11p) Changes Plasma Membrane and Cell Wall Structure of Candida albicans. Int. J. Mol. Sci. 2022, 23, 1631. [Google Scholar] [CrossRef] [PubMed]
- Derkacz, D.; Grzybowska, M.; Cebula, L.; Krasowska, A. Surfactin and Capric Acid Affect the Posaconazole Susceptibility of Candida albicans Strains with Altered Sterols and Sphingolipids Biosynthesis. Int. J. Mol. Sci. 2023, 24, 17499. [Google Scholar] [CrossRef]
- Ishmayana, S.; Kennedy, U.J.; Learmonth, R.P. Further investigation of relationships between membrane fluidity and ethanol tolerance in Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2017, 33, 218. [Google Scholar] [CrossRef]
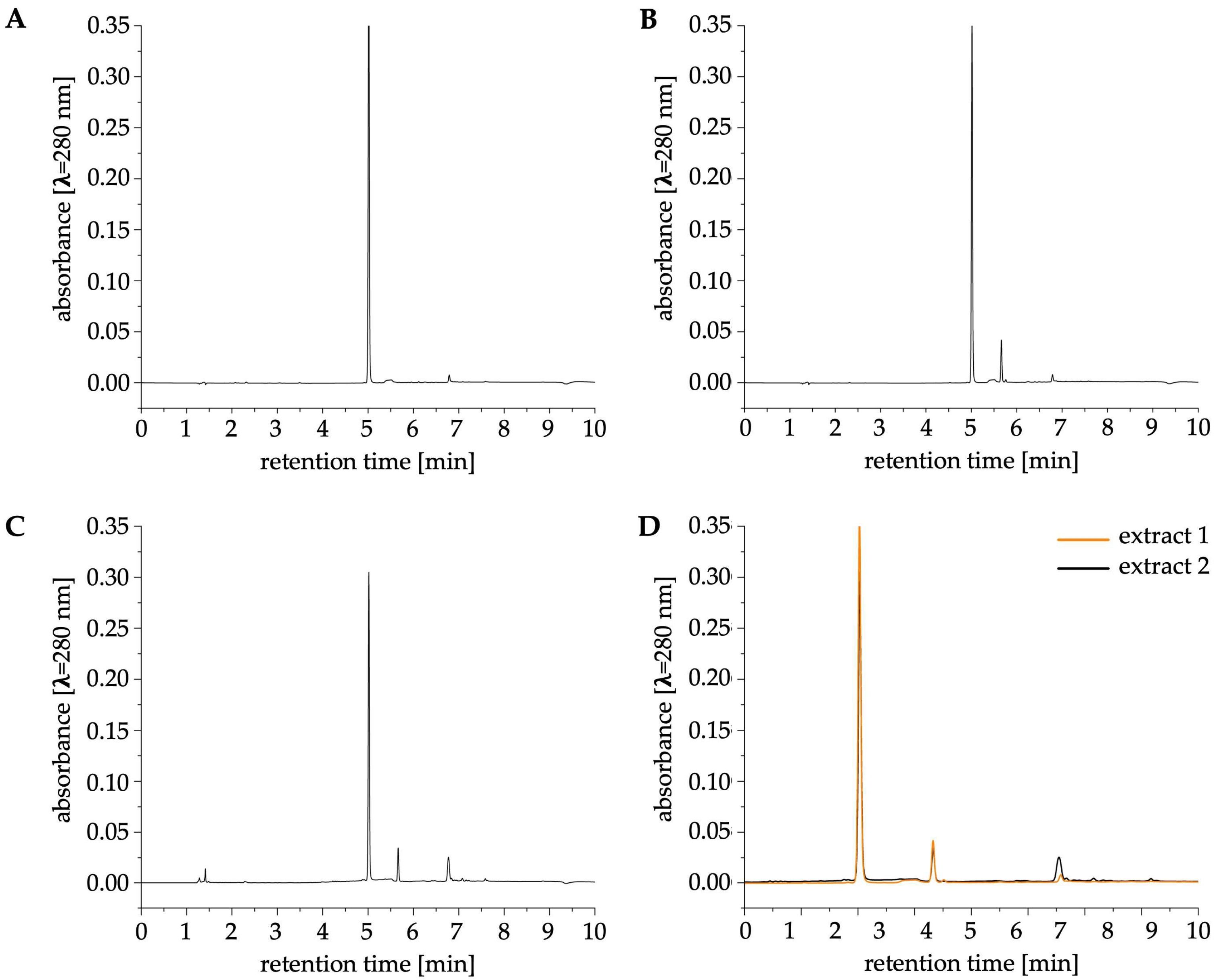
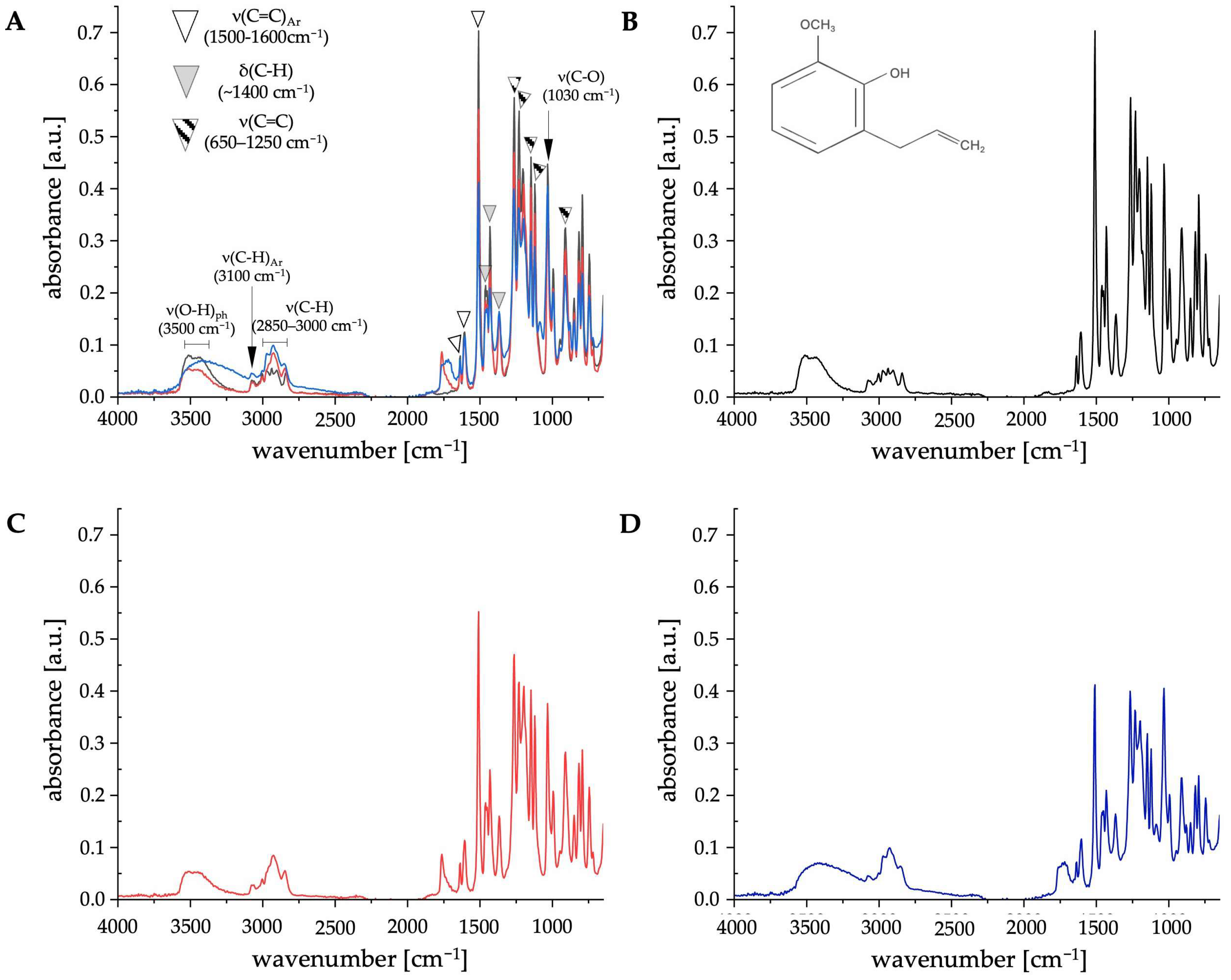


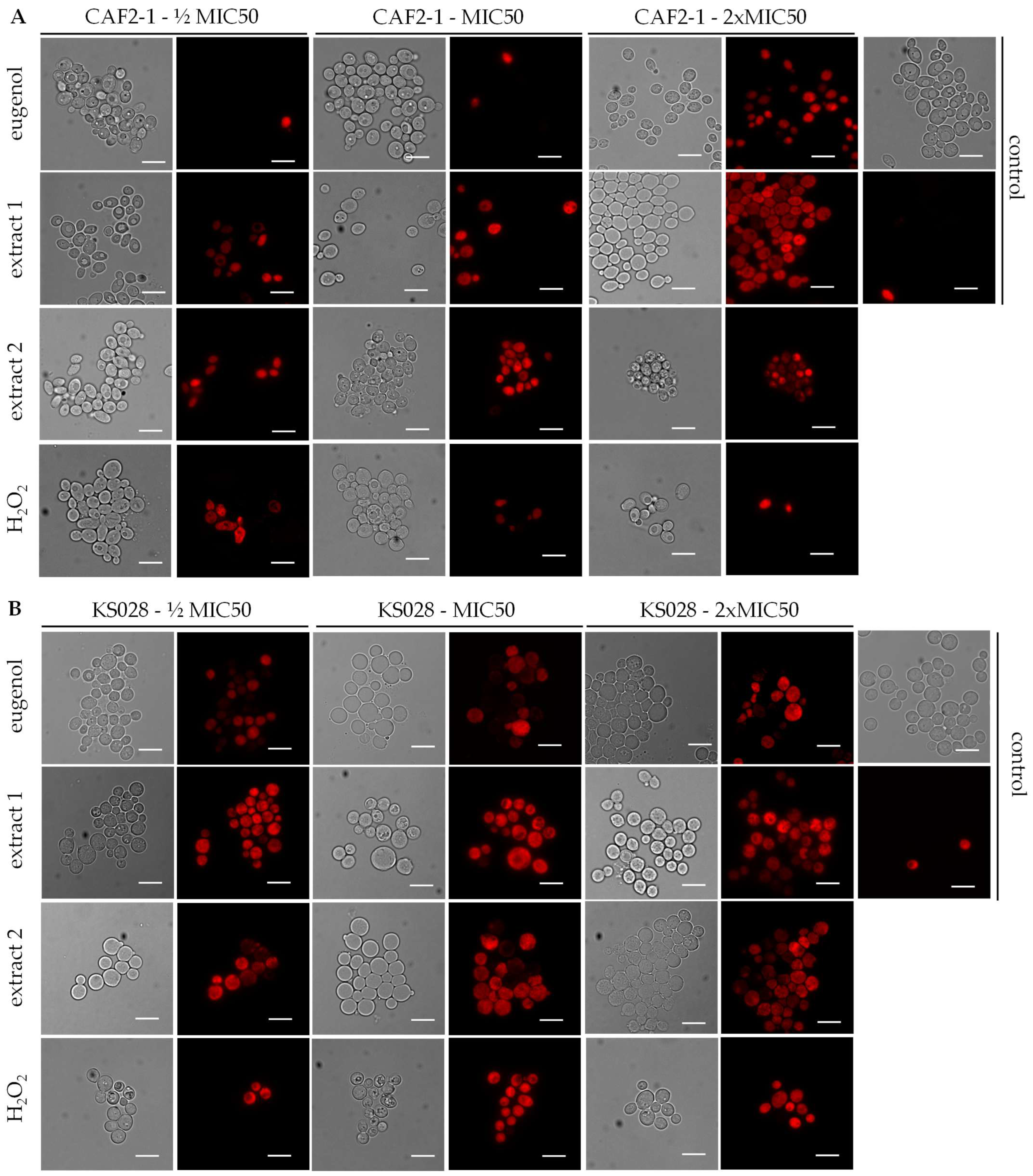


| Extract 1 | Extract 2 | |
|---|---|---|
| eugenol [mg/mL] | 662.706 | 553.178 |
| t = 0 [min] | t = 10 [min] | t = 60 [min] | t = 90 [min] | |
|---|---|---|---|---|
| trolox [500 μg/mL] | 97.46 ± 0.29 *** | 97.27 ± 0.29 | 97.01 ± 0.31 | 96.92 ± 0.32 |
| H2O2 [0.0125% v/v] | 15.38 ± 0.00 *** | 14.21 ± 0.00 *** | 10.96 ± 0.00 *** | 8.79 ± 0.00 *** |
| eugenol [0.039% v/v] | 79.22 ± 0.27 | 95.80 ± 0.54 | 96.13 ± 0.69 | 96.14 ± 0.64 |
| extract 1 [0.039% v/v] | 22.96 ± 1.73 *** | 94.41 ± 0.39 * | 95.61 ± 0.55 | 95.60 ± 0.56 |
| extract 2 [0.039% v/v] | 21.18 ± 0.57 *** | 86.89 ± 0.17 *** | 95.31 ± 0.40 | 95.22 ± 0.33 |
| ergosterol [400 μg/mL] | 3.23 ± 2.40 *** | 10.46 ± 4.33 *** | 32.55 ± 7.42 ** | 39.14 ± 8.75 ** |
| Concentration | CAF2-1 [%] | KS028 [%] | Significance (CAF2-1 vs. KS028) | |
|---|---|---|---|---|
| control | - | 6.70 ± 0.74 | 15.07 ± 1.74 *** | ns |
| eugenol | ½xMIC50 | 8.79 ± 1.00 | 57.61 ± 2.69 *** | *** |
| MIC50 | 9.76 ±1.32 | 55.51 ± 2.32 *** | *** | |
| 2xMIC50 | 82.23 ± 3.94 *** | 58.24 ± 1.45 *** | *** | |
| extract 1 | ½xMIC50 | 41.13 ± 1.11 *** | 87.46 ± 2.34 *** | *** |
| MIC50 | 45.67 ± 2.83 *** | 82.89 ± 3.55 *** | *** | |
| 2xMIC50 | 90.98 ± 1.96 *** | 88.26 ± 2.26 *** | ns | |
| extract 2 | ½xMIC50 | 35.32 ± 1.57 *** | 70.96 ± 2.23 *** | *** |
| MIC50 | 35.67 ± 1.40 *** | 67.28 ± 3.05 *** | *** | |
| 2xMIC50 | 78.83 ± 5.82 *** | 68.01 ± 2.39 *** | ** | |
| H2O2 | ½xMIC50 | 28.32 ± 4.01 *** | 25.84 ± 1.59 ** | ns |
| MIC50 | 15.30 ± 3.22 | 69.54 ± 2.38 *** | *** | |
| 2xMIC50 | 30.96 ± 4.33 *** | 82.35 ± 1.12 *** | *** |
| Concentration | CAF2-1 | KS028 | |
|---|---|---|---|
| control | - | −0.338 ± 0.028 | −0.278 ± 0.029 |
| eugenol | ¼xMIC50 | −0.240 ± 0.040 *** | −0.236 ± 0.032 * |
| ½xMIC50 | −0.259 ± 0.052 * | −0.265 ± 0.043 | |
| MIC50 | −0.324 ± 0.008 | −0.301 ± 0.043 | |
| extract 1 | ¼xMIC50 | −0.253 ± 0.015 *** | −0.251 ± 0.019 * |
| ½xMIC50 | −0.264 ± 0.016 *** | −0.273 ± 0.034 | |
| MIC50 | −0.290 ± 0.024 ** | −0.308 ± 0.017 * | |
| extract 2 | ¼xMIC50 | −0.242 ± 0.026 *** | −0.232 ± 0.043 * |
| ½xMIC50 | −0.256 ± 0.033 *** | −0.245 ± 0.034 | |
| MIC50 | −0.281 ± 0.013 *** | −0.279 ± 0.022 | |
| H2O2 | ¼xMIC50 | −0.295 ± 0.022 ** | −0.301 ± 0.006 * |
| ½xMIC50 | −0.245 ± 0.070 * | −0.272 ± 0.021 | |
| MIC50 | −0.194 ± 0.047 *** | −0.240 ± 0.041 |
| Time | Water + 0.1% HCOOH (%) | MeCN + 0.1% HCOOH (%) |
|---|---|---|
| Initial | 75 | 25 |
| 1.0 | 75 | 25 |
| 5.0 | 5 | 95 |
| 7.0 | 5 | 95 |
| 7.1 | 75 | 25 |
| C. albicans Strain | Genotype | Source |
|---|---|---|
| CAF2-1 | ura3Δ::imm434/URA3; wild-type | [32] |
| AsCa1 | ura3Δ::imm434/ura3Δ::imm434 CDR1/CDR1-yEGFP-URA3 | [33] |
| KS023 | ura3Δ::imm434/ura3Δ::imm434 CDR1/CDR1-yEGFP-URA3 erg11Δ::SAT1-FLIP/erg11Δ::FRT (parental strain: AscCa1) | [25] |
| KS028 | ura3Δ::imm434/URA3 erg11Δ::SAT1-FLIP/erg11Δ::FRT (parental strain: CAF2-1) | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derkacz, D.; Cebula, L.; Krasowska, A. Multifunctional Activity of Syzygium aromaticum Extracts Against Candida albicans: Free Radicals, Membrane Permeabilization and Cdr1p Localization. Int. J. Mol. Sci. 2025, 26, 8571. https://doi.org/10.3390/ijms26178571
Derkacz D, Cebula L, Krasowska A. Multifunctional Activity of Syzygium aromaticum Extracts Against Candida albicans: Free Radicals, Membrane Permeabilization and Cdr1p Localization. International Journal of Molecular Sciences. 2025; 26(17):8571. https://doi.org/10.3390/ijms26178571
Chicago/Turabian StyleDerkacz, Daria, Liliana Cebula, and Anna Krasowska. 2025. "Multifunctional Activity of Syzygium aromaticum Extracts Against Candida albicans: Free Radicals, Membrane Permeabilization and Cdr1p Localization" International Journal of Molecular Sciences 26, no. 17: 8571. https://doi.org/10.3390/ijms26178571
APA StyleDerkacz, D., Cebula, L., & Krasowska, A. (2025). Multifunctional Activity of Syzygium aromaticum Extracts Against Candida albicans: Free Radicals, Membrane Permeabilization and Cdr1p Localization. International Journal of Molecular Sciences, 26(17), 8571. https://doi.org/10.3390/ijms26178571






