5-Hydroxylysine Captures the Suicidally-Inactivated Conformational State of Lysine 5,6-Aminomutase
Abstract
1. Introduction
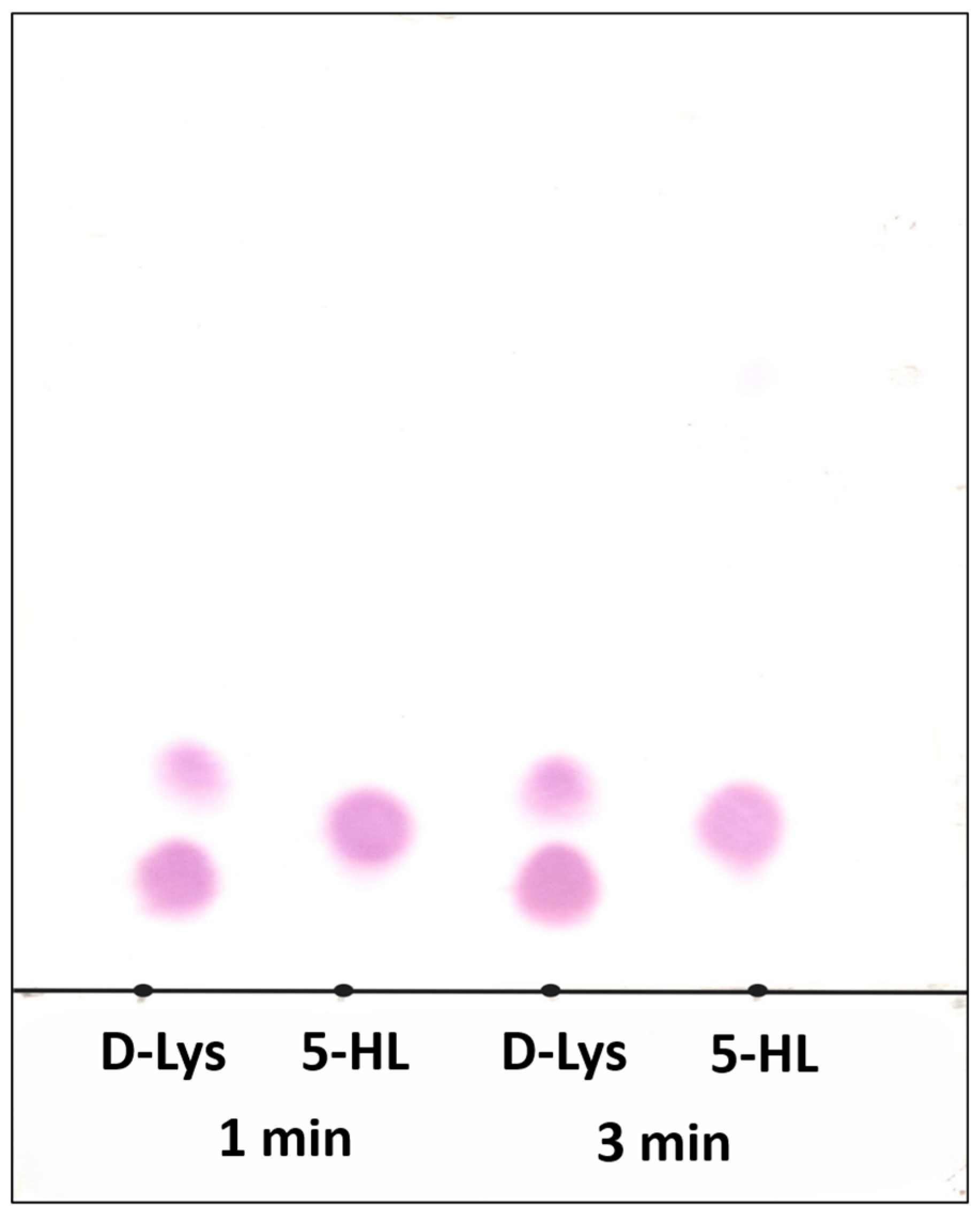
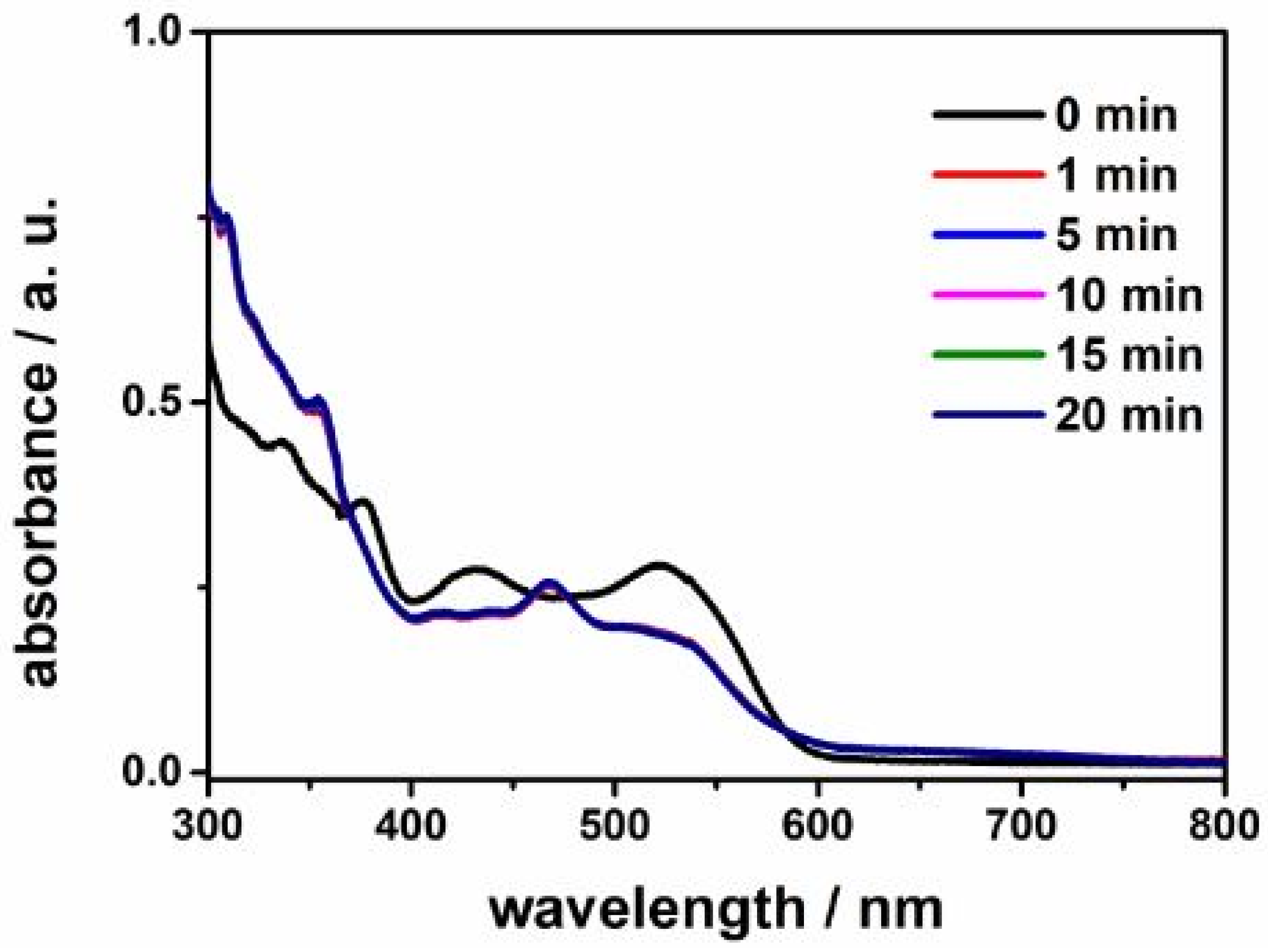
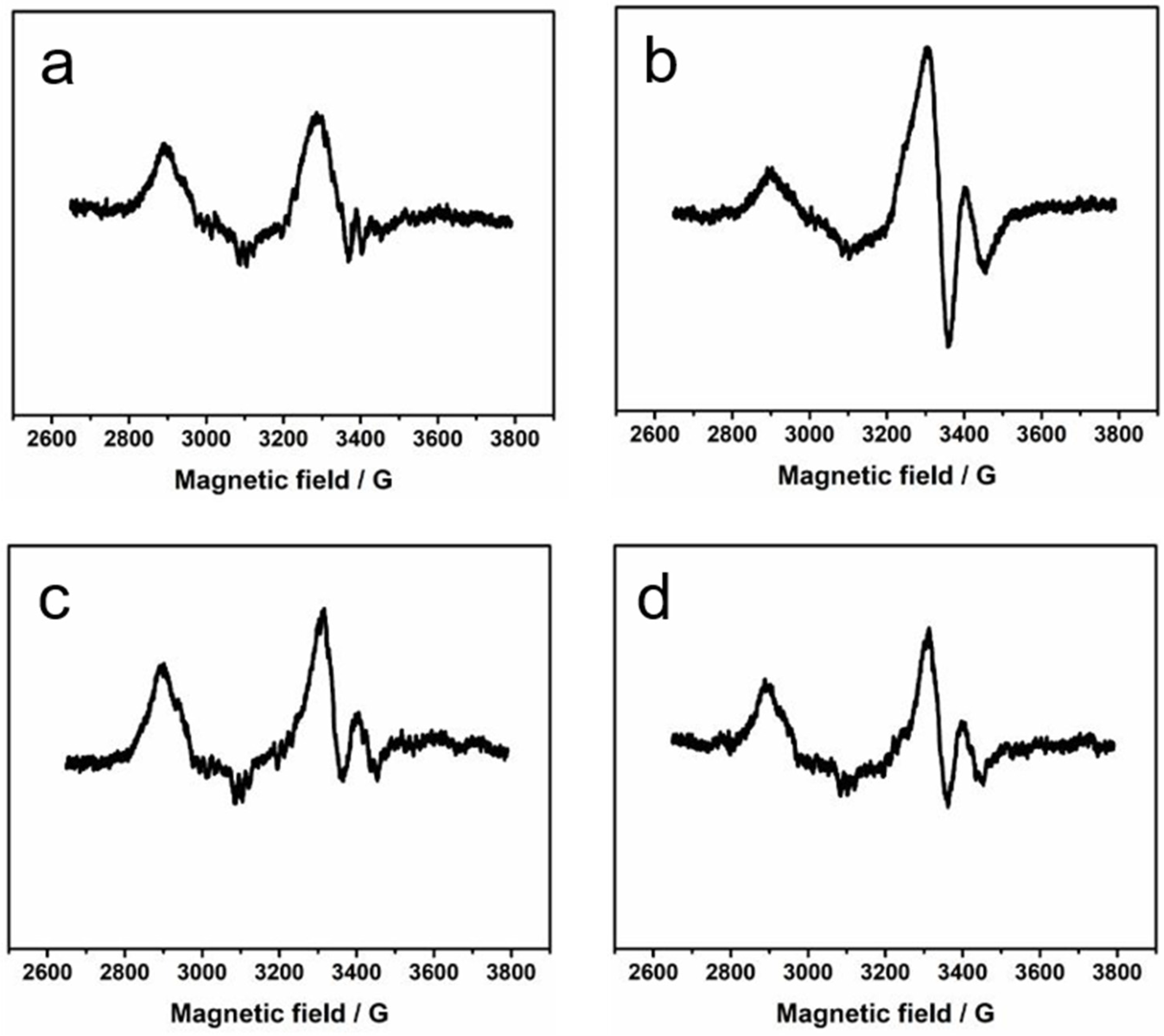
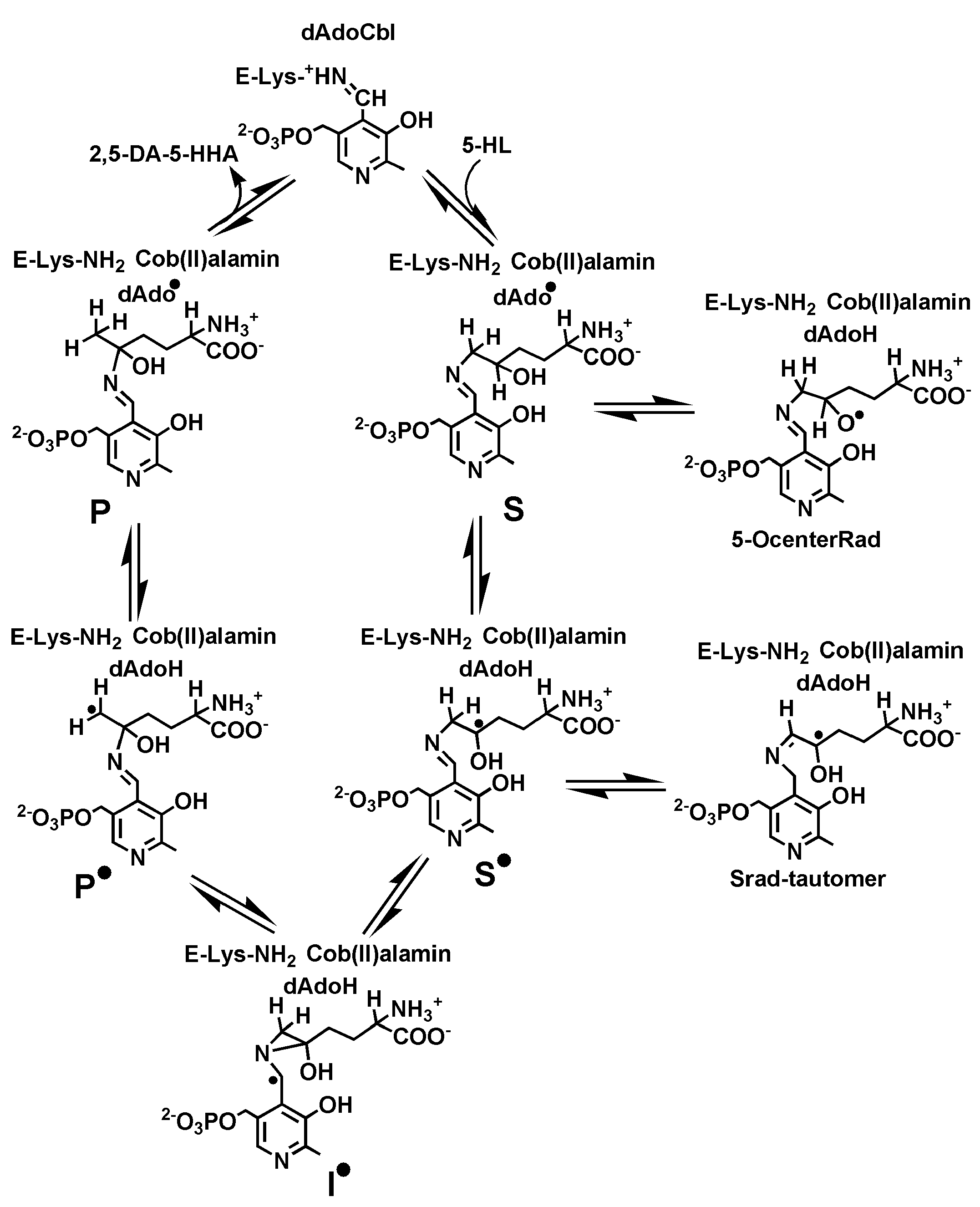
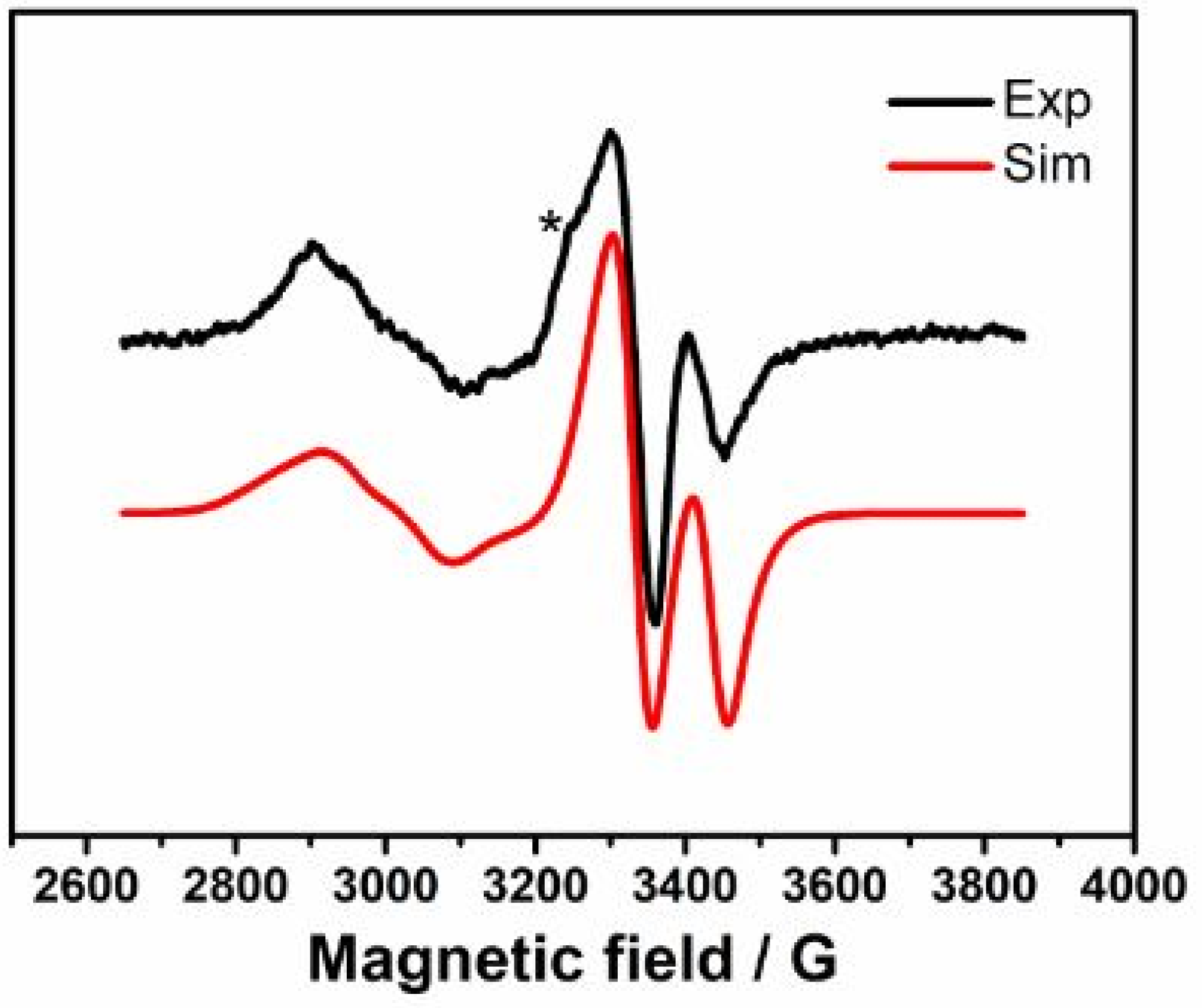
2. Results and Discussion
3. Materials and Methods
3.1. UV–Visible Spectroscopy
3.2. EPR Spectroscopy
3.3. Computational Details
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5,6-LAM. | Lysine 5,6-aminomutase |
| PLP | Pyridoxal 5′-phosphate |
| dAdoCbl | 5′-Deoxyadenosylcobalamin |
| EPR | Electron paramagnetic resonance |
| 5-HL | DL-5-hydroxylysine |
| TLC | Thin-layer chromatographic |
| DFT | Density functional theory |
| dAdoH | 5′-Deoxyadenosine |
References
- Honig, M.; Sondermann, P.; Turner, N.J.; Carreira, E.M. Enantioselective Chemo- and Biocatalysis: Partners in Retrosynthesis. Angew. Chem. Int. Edit. 2017, 56, 8942–8973. [Google Scholar] [CrossRef]
- Maity, A.N.; Chen, Y.H.; Ke, S.C. Large-scale domain motions and pyridoxal-5′-phosphate assisted radical catalysis in coenzyme B12-dependent aminomutases. Int. J. Mol. Sci. 2014, 15, 3064–3087. [Google Scholar] [CrossRef] [PubMed]
- Maity, A.N.; Lin, H.H.; Chiang, H.S.; Lo, H.H.; Ke, S.C. Reaction of pyridoxal-5′-phosphate-N-oxide with lysine 5,6-aminomutase: Enzyme flexibility toward cofactor analog. ACS Catal. 2015, 5, 3093–3099. [Google Scholar] [CrossRef]
- Wu, B.; Szymanski, W.; Heberling, M.M.; Feringa, B.L.; Janssen, D.B. Aminomutases: Mechanistic diversity, biotechnological applications and future perspectives. Trends. Biotechnol. 2011, 29, 352–362. [Google Scholar] [CrossRef]
- Maity, A.N.; Chen, J.-R.; Li, Q.-Y.; Ke, S.-C. The Nitrogen Atom of Vitamin B6 Is Essential for the Catalysis of Radical Aminomutases. Int. J. Mol. Sci. 2022, 23, 5210. [Google Scholar] [CrossRef]
- Berkovitch, F.; Behshad, E.; Tang, K.-H.; Enns, E.A.; Frey, P.A.; Drennan, C.L. A locking mechanism preventing radical damage in the absence of substrate, as revealed by the x-ray structure of lysine 5,6-aminomutase. Proc. Natl. Acad. Sci. USA 2004, 101, 15870–15875. [Google Scholar] [CrossRef]
- Lo, H.H.; Lin, H.H.; Maity, A.N.; Ke, S.C. The molecular mechanism of the open-closed protein conformational cycle transitions and coupled substrate binding, activation and product release events in lysine 5,6-aminomutase. Chem. Commun. 2016, 52, 6399–6402. [Google Scholar] [CrossRef]
- Chen, J.-R.; Ke, T.-X.; Frey, P.A.; Ke, S.-C. Electron Spin Echo Envelope Modulation Spectroscopy Reveals How Adenosylcobalamin-Dependent Lysine 5,6-Aminomutase Positions the Radical Pair Intermediates and Modulates Their Stabilities for Efficient Catalysis. ACS Catal. 2021, 11, 14352–14368. [Google Scholar] [CrossRef]
- Hoffman, B.M.; Broderick, W.E.; Broderick, J.B. Mechanism of Radical Initiation in the Radical SAM Enzyme Superfamily. Annu. Rev. Biochem. 2023, 92, 333–349. [Google Scholar] [CrossRef]
- Banerjee, R. Chemistry and Biochemistry of B12; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Reed, G.H.; Mansoorabadi, S.O. The positions of radical intermediates in the active sites of adenosylcobalamin-dependent enzymes. Curr. Opin. Struct. Biol. 2003, 13, 716–721. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maity, A.N.; Chen, J.-R.; Ke, S.-C. Exploring the mechanism of action of lysine 5,6-aminomutase using EPR and ENDOR spectroscopies. Methods Enzymol. 2022, 669, 197–228. [Google Scholar][Green Version]
- Ruetz, M.; Campanello, G.C.; Purchal, M.; Shen, H.Y.; McDevitt, L.; Gouda, H.; Wakabayashi, S.; Zhu, J.H.; Rubin, E.J.; Warncke, K.; et al. Itaconyl-CoA forms a stable biradical in methylmalonyl-CoA mutase and derails its activity and repair. Science 2019, 366, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Wolthers, K.R.; Levy, C.; Scrutton, N.S.; Leys, D. Large-scale domain dynamics and adenosylcobalamin reorientation orchestrate radical catalysis in ornithine 4,5-aminomutase. J. Biol. Chem. 2010, 285, 13942–13950. [Google Scholar] [CrossRef]
- Ghosh, A.P.; Toda, M.J.; Kozlowski, P.M. What Triggers the Cleavage of the Co-C, Bond in Coenzyme B-Dependent Itaconyl-CoA Methylmalonyl-CoA Mutase? ACS Catal. 2021, 11, 7943–7955. [Google Scholar] [CrossRef]
- Maity, A.N.; Hsieh, C.P.; Huang, M.H.; Chen, Y.H.; Tang, K.H.; Behshad, E.; Frey, P.A.; Ke, S.C. Evidence for conformational movement and radical mechanism in the reaction of 4-thia-L-lysine with lysine 5,6-aminomutase. J. Phys. Chem. B 2009, 113, 12161–12163. [Google Scholar] [CrossRef]
- Menon, B.R.K.; Fisher, K.; Rigby, S.E.J.; Scrutton, N.S.; Leys, D. A Conformational Sampling Model for Radical Catalysis in Pyridoxal Phosphate- and Cobalamin-dependent Enzymes. J. Biol. Chem. 2014, 289, 34161–34174. [Google Scholar] [CrossRef][Green Version]
- Fraser, J.S.; Clarkson, M.W.; Degnan, S.C.; Erion, R.; Kern, D.; Alber, T. Hidden alternative structures of proline isomerase essential for catalysis. Nature 2009, 462, 669–673. [Google Scholar] [CrossRef]
- Hart, K.M.; Ho, C.M.W.; Dutta, S.; Gross, M.L.; Bowman, G.R. Modelling proteins’ hidden conformations to predict antibiotic resistance. Nat. Commun. 2016, 7, 12965. [Google Scholar] [CrossRef]
- Curado-Carballada, C.; Feixas, F.; Iglesias-Fernández, J.; Osuna, S. Hidden Conformations in Monoamine Oxidase are Key for Catalytic Efficiency. Angew. Chem. Int. Edit. 2019, 58, 3097–3101. [Google Scholar] [CrossRef] [PubMed]
- Willkomm, S.; Jakob, L.; Kramm, K.; Graus, V.; Neumeier, J.; Meister, G.; Grohmann, D. Single-molecule FRET uncovers hidden conformations and dynamics of human Argonaute 2. Nat. Commun. 2022, 13, 3825. [Google Scholar] [CrossRef] [PubMed]
- Sharon, E.M.; Henderson, L.W.; Clemmer, D.E. Resolving Hidden Solution Conformations of Hemoglobin Using IMS-IMS on a Cyclic Instrument. J. Am. Soc. Mass. Spectr. 2023, 34, 1559–1568. [Google Scholar] [CrossRef] [PubMed]
- Maria-Solano, M.A.; Serrano-Hervas, E.; Romero-Rivera, A.; Iglesias-Fernandez, J.; Osuna, S. Role of conformational dynamics in the evolution of novel enzyme function. Chem. Commun. 2018, 54, 6622–6634. [Google Scholar] [CrossRef]
- Tang, K.H.; Chang, C.H.; Frey, P.A. Electron transfer in the substrate-dependent suicide inactivation of lysine 5,6-aminomutase. Biochemistry 2001, 40, 5190–5199. [Google Scholar] [CrossRef] [PubMed]
- Abend, A.; Bandarian, V.; Reed, G.H.; Frey, P.A. Identification of cis-ethanesemidione as the organic radical derived from glycolaldehyde in the suicide inactivation of dioldehydrase and of ethanolamine ammonia-lyase. Biochemistry 2000, 39, 6250–6257. [Google Scholar] [CrossRef]
- Schwartz, P.; LoBrutto, R.; Reed, G.H.; Frey, P.A. Suicide inactivation of dioldehydrase by 2-chloroacetaldehyde: Formation of the ‘cis-ethanesemidione’ radical, and the role of a monovalent cation. Helv. Chim. Acta 2003, 86, 3764–3775. [Google Scholar] [CrossRef]
- Sandala, G.M.; Smith, D.M.; Coote, M.L.; Golding, B.T.; Radom, L. Insights into the hydrogen-abstraction reactions of diol dehydratase: Relevance to the catalytic mechanism and suicide inactivation. J. Am. Chem. Soc. 2006, 128, 3433–3444. [Google Scholar] [CrossRef]
- Frey, P.A.; Hegeman, A.D.; Reed, G.H. Free radical mechanisms in enzymology. Chem. Rev. 2006, 106, 3302–3316. [Google Scholar] [CrossRef]
- Tunçkanat, T.; Gendron, A.; Sadler, Z.; Neitz, A.; Byquist, S.; Lie, T.J.; Allen, K.D. Lysine 2,3-Aminomutase and a Newly Discovered Glutamate 2,3-Aminomutase Produce β-Amino Acids Involved in Salt Tolerance in Methanogenic Archaea. Biochemistry 2022, 61, 1077–1090. [Google Scholar] [CrossRef]
- Ruzicka, F.J.; Frey, P.A. Glutamate 2,3-aminomutase: A new member of the radical SAM superfamily of enzymes. Biochim. Biophys. Acta-Proteins Proteom. 2007, 1774, 286–296. [Google Scholar] [CrossRef]
- Frey, P.A.; Reed, G.H. Pyridoxal-5′-phosphate as the catalyst for radical isomerization in reactions of PLP-dependent aminomutases. Biochim. Biophys. Acta 2011, 1814, 1548–1557. [Google Scholar] [CrossRef]
- Frey, P.A. Comprehensive Natural Products II: Chemistry and Biology; Elsevier: Oxford, UK, 2010; Volume 7, pp. 501–546. [Google Scholar]
- Frey, P.A. Travels with Carbon-Centered Radicals. 5′-Deoxyadenosine and 5′-Deoxyadenosine-5′-yl in Radical Enzymology. Acc. Chem. Res. 2014, 47, 540–549. [Google Scholar] [CrossRef]
- Yang, H.; Ho, M.B.; Lundahl, M.N.; Mosquera, M.A.; Broderick, W.E.; Broderick, J.B.; Hoffman, B.M. ENDOR Spectroscopy Reveals the “Free”5′-Deoxyadenosyl Radicalin a Radical SAM Enzyme Active Site Actually is Chaperoned by Close Interaction with the Methionine-Bound [4Fe-4S]2+ Cluster. J. Am. Chem. Soc. 2024, 146, 3710–3720. [Google Scholar] [CrossRef]
- Yang, H.; McDaniel, E.C.; Impano, S.; Byer, A.S.; Jodts, R.J.; Yokoyama, K.; Broderick, W.E.; Broderick, J.B.; Hoffman, B.M. The elusive 5′-deoxyadenosyl radical: Captured and characterized by electron paramagnetic resonance and electron nuclear double resonance spectroscopies. J. Am. Chem. Soc. 2019, 141, 12139–12146. [Google Scholar] [CrossRef] [PubMed]
- Sayler, R.I.; Stich, T.A.; Joshi, S.; Cooper, N.; Shaw, J.T.; Begley, T.P.; Tantillo, D.J.; Britt, R.D. Trapping and electron paramagnetic resonance characterization of the 5’dAdo. radical in a radical S-adenosyl methionine enzyme reaction with a non-native substrate. ACS Cent. Sci. 2019, 5, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Sin, Y.C.; Park, M.; Griffin, T.J.; Yong, J.S.; Chen, Y. A constitutional isomer selective chemical proteomic strategy for system-wide profiling of protein lysine 5-hydroxylation. Chem. Sci. 2024, 15, 18395–18404. [Google Scholar] [CrossRef]
- Hayashi, G.; Sakamoto, R.; Okamoto, A. 2-Oxazoline Formation for Selective Chemical Labeling of 5-Hydroxylysine. Chem. Asian. J. 2015, 10, 1138–1141. [Google Scholar] [CrossRef]
- Yamauchi, M.; Sricholpech, M. Lysine post-translational modifications of collagen. Essays Biochem. 2012, 52, 113–133. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, A.; Knight, C.; Xu, L.Y.; Cooper, G.J. Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity. J. Biol. Chem. 2002, 277, 19521–19529. [Google Scholar] [CrossRef]
- Unoki, M.; Masuda, A.; Dohmae, N.; Arita, K.; Yoshimatsu, M.; Iwai, Y.; Fukui, Y.; Ueda, K.; Hamamoto, R.; Shirakawa, M.; et al. Lysyl 5-hydroxylation, a novel histone modification, by Jumonji domain containing 6 (JMJD6). J. Biol. Chem. 2013, 288, 6053–6062. [Google Scholar] [CrossRef]
- Kawai, S.; Sugaya, Y.; Hagihara, R.; Tomita, H.; Katsuyama, Y.; Ohnishi, Y. Complete Biosynthetic Pathway of Alazopeptin, a Tripeptide Consisting of Two Molecules of 6-Diazo-5-oxo-l-norleucine and One Molecule of Alanine. Angew. Chem. Int. Edit. 2021, 60, 10319–10325. [Google Scholar] [CrossRef]
- Kawai, S.; Moriga, K.; Nirdnoy, W.; Hara, R.; Ogawa, J.; Katsuyama, Y.; Ohnishi, Y. Identification of Two Distinct Stereoselective Lysine 5-Hydroxylases by Genome Mining Based on Alazopeptin Biosynthetic Enzymes. Chem. Eur. J. 2025, 31, e202404790. [Google Scholar]
- Shan, M.J.; Liu, H.; Hao, Y.; Song, K.X.; Meng, T.; Feng, C.; Wang, Y.B.; Huang, Y.S. Metabolomic Profiling Reveals That 5-Hydroxylysine and 1-Methylnicotinamide Are Metabolic Indicators of Keloid Severity. Front. Genet. 2022, 12, 804248. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.H.; Mansoorabadi, S.O.; Reed, G.H.; Frey, P.A. Radical triplets and suicide inhibition in reactions of 4-thia-D- and 4-thia-L-lysine with lysine 5,6-aminomutase. Biochemistry 2009, 48, 8151–8160. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.H.; Maity, A.N.; Pan, Y.C.; Frey, P.A.; Ke, S.C. Radical stabilization is crucial in the mechanism of action of lysine 5,6-aminomutase: Role of tyrosine-263α as revealed by electron paramagnetic resonance spectroscopy. J. Am. Chem. Soc. 2011, 133, 17152–17155. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Frey, P.A. Cloning, sequencing, heterologous expression, purification, and characterization of adenosylcobalamin-dependent D-lysine 5,6-aminomutase from Clostridium sticklandii. J. Biol. Chem. 2000, 275, 106–114. [Google Scholar] [CrossRef][Green Version]
- Maity, A.N.; Ke, S.C. 5-Fluorolysine as alternative substrate of lysine 5,6-aminomutase: A computational study. Comput. Theor. Chem. 2013, 1022, 1–5. [Google Scholar] [CrossRef]
- Maity, A.N.; Ke, S.C. 4′-CyanoPLP presents better prospect for the experimental detection of elusive cyclic intermediate radical in the reaction of lysine 5,6-aminomutase. Biochem. Biophys. Res. Commun. 2015, 457, 161–164. [Google Scholar] [CrossRef]
- Sandala, G.M.; Smith, D.M.; Radom, L. In search of radical intermediates in the reactions catalyzed by lysine 2,3-aminomutase and lysine 5,6-aminomutase. J. Am. Chem. Soc. 2006, 128, 16004–16005. [Google Scholar] [CrossRef]
- Marin, J.; Didierjean, C.; Aubry, A.; Briand, J.P.; Guichard, G. Diastereoselective hydroxylation of 6-substituted piperidin-2-ones: An efficient synthesis of (2S,5R)-5-hydroxylysine and related α-amino acids. J. Org. Chem. 2002, 67, 8440–8449. [Google Scholar] [CrossRef]
- Allevi, P.; Anastasia, M. Synthesis of all four possible stereoisomers of 5-hydroxylysine. Tetrahedron Asymmetry 2000, 11, 3151–3160. [Google Scholar] [CrossRef]
- Maity, A.N.; Ke, S.C. Synthesis of 4-thia[5-13C]lysine. J. Label. Compd. Radiopharm. 2011, 54, 589–590. [Google Scholar]
- Maity, A.N.; Shaikh, A.C.; Srimurugan, S.; Wu, C.J.; Chen, C.P.; Ke, S.C. Synthesis of 4-thia-[6-13C]lysine from [2-13C]glycine: Access to site-directed isotopomers of 2-aminoethanol, 2-bromoethylamine and 4-thialysine. Amino Acids 2012, 42, 309–315. [Google Scholar]
- Bagneris, C.; Naylor, C.E.; McCusker, E.C.; Wallace, B.A. Structural model of the open-closed-inactivated cycle of prokaryotic voltage-gated sodium channels. J. Gen. Physiol. 2015, 145, 5–16. [Google Scholar] [PubMed]
- Pang, J.; Scrutton, N.S.; Sutcliffe, M.J. Quantum mechanics/molecular mechanics studies on the mechanism of action of cofactor pyridoxal 5′-phosphate in ornithine 4,5-aminomutase. Chem. Eur. J. 2014, 20, 11390–11401. [Google Scholar] [CrossRef] [PubMed]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.P.; Radom, L. Harmonic vibrational frequencies: An evaluation of Hartree-Fock, Moller-Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J. Phys. Chem. 1996, 100, 16502–16513. [Google Scholar] [CrossRef]
- Wetmore, S.D.; Smith, D.M.; Radom, L. Enzyme catalysis of 1,2-amino shifts: The cooperative action of B6, B12, and aminomutases. J. Am. Chem. Soc. 2001, 123, 8678–8689. [Google Scholar] [CrossRef]
- Wetmore, S.D.; Smith, D.M.; Golding, B.T.; Radom, L. Interconversion of (S)-glutamate and (2S,3S)-3-methylaspartate: A distinctive B12-dependent carbon-skeleton rearrangement. J. Am. Chem. Soc. 2001, 123, 7963–7972. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
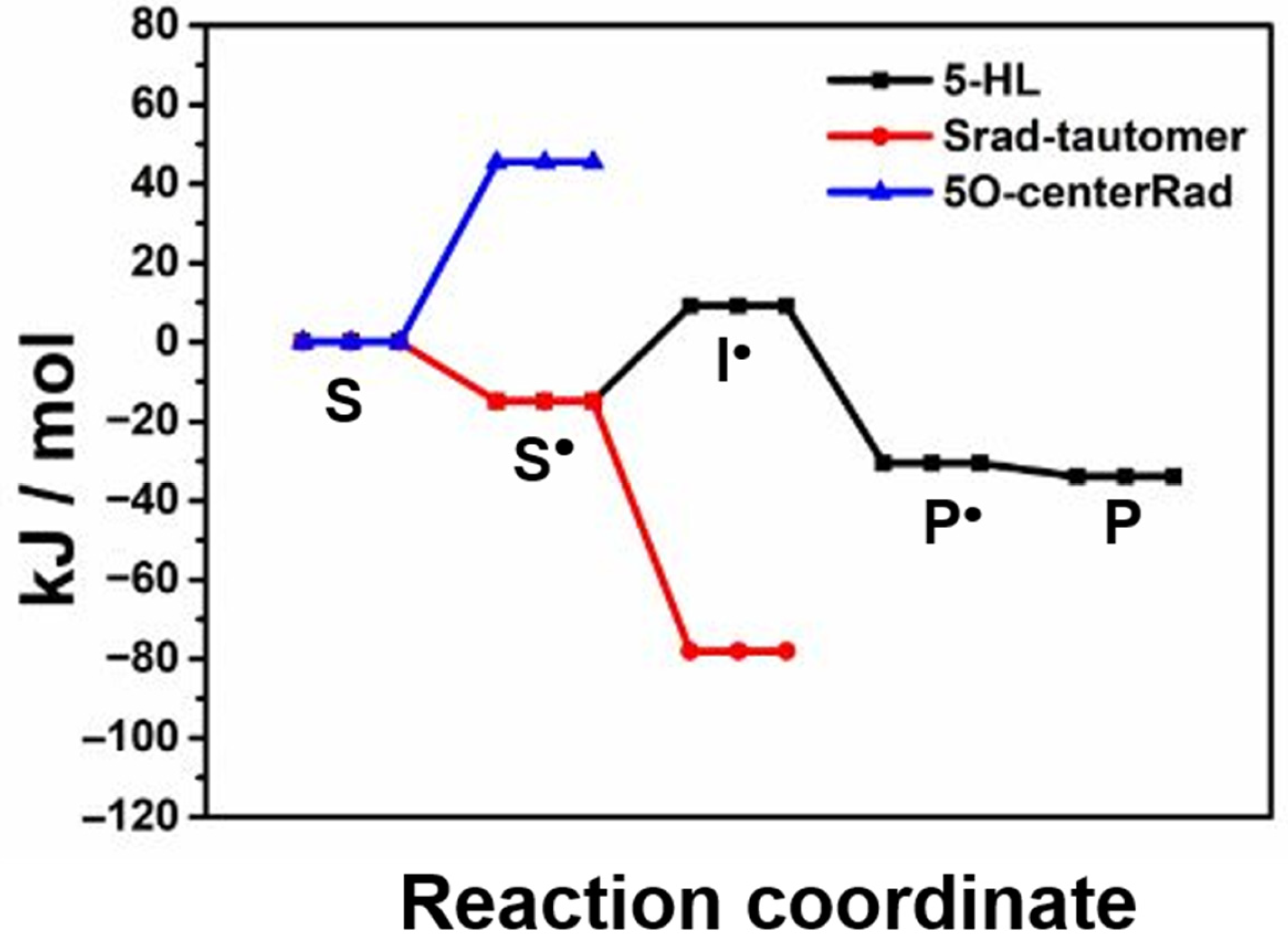
| Relative Energy (kJ/mol) | |||
|---|---|---|---|
| 5-HL | Lysine a | 4-Thialysine a | |
| S | 0 | 0 | 0 |
| S• | −14.9 | −10.4 | −37.7 |
| I• | 9.1 | 16.9 | 12.3 |
| P• | −30.6 | −15.7 | −22.9 |
| P | −34.0 | −17.1 | n. a. |
| Srad-tautomer | −78.1 | n. a. | −64.1 b |
| 5O-centerRad | 45.4 | n. a. | n. a. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maity, A.N.; Chen, J.-R.; Ke, T.-X.; Ke, S.-C. 5-Hydroxylysine Captures the Suicidally-Inactivated Conformational State of Lysine 5,6-Aminomutase. Int. J. Mol. Sci. 2025, 26, 8561. https://doi.org/10.3390/ijms26178561
Maity AN, Chen J-R, Ke T-X, Ke S-C. 5-Hydroxylysine Captures the Suicidally-Inactivated Conformational State of Lysine 5,6-Aminomutase. International Journal of Molecular Sciences. 2025; 26(17):8561. https://doi.org/10.3390/ijms26178561
Chicago/Turabian StyleMaity, Amarendra Nath, Jun-Ru Chen, Ting-Xi Ke, and Shyue-Chu Ke. 2025. "5-Hydroxylysine Captures the Suicidally-Inactivated Conformational State of Lysine 5,6-Aminomutase" International Journal of Molecular Sciences 26, no. 17: 8561. https://doi.org/10.3390/ijms26178561
APA StyleMaity, A. N., Chen, J.-R., Ke, T.-X., & Ke, S.-C. (2025). 5-Hydroxylysine Captures the Suicidally-Inactivated Conformational State of Lysine 5,6-Aminomutase. International Journal of Molecular Sciences, 26(17), 8561. https://doi.org/10.3390/ijms26178561






