Hormetic Effects of Curcumin in RPE Cells: SIRT1 and Caspase-3 Inactivation with Implications for AMD
Abstract
1. Introduction
1.1. RPE Structure and Function
1.2. Oxidative Stress and AMD Pathogenesis
1.3. SIRT1 in Cellular Homeostasis
1.4. SIRT1 Dysregulation in AMD
1.5. Curcumin as a Therapeutic Agent
1.6. Hormesis and Curcumin: A Biphasic Dose–Response Relationship
1.7. The Aim of This Study
2. Results
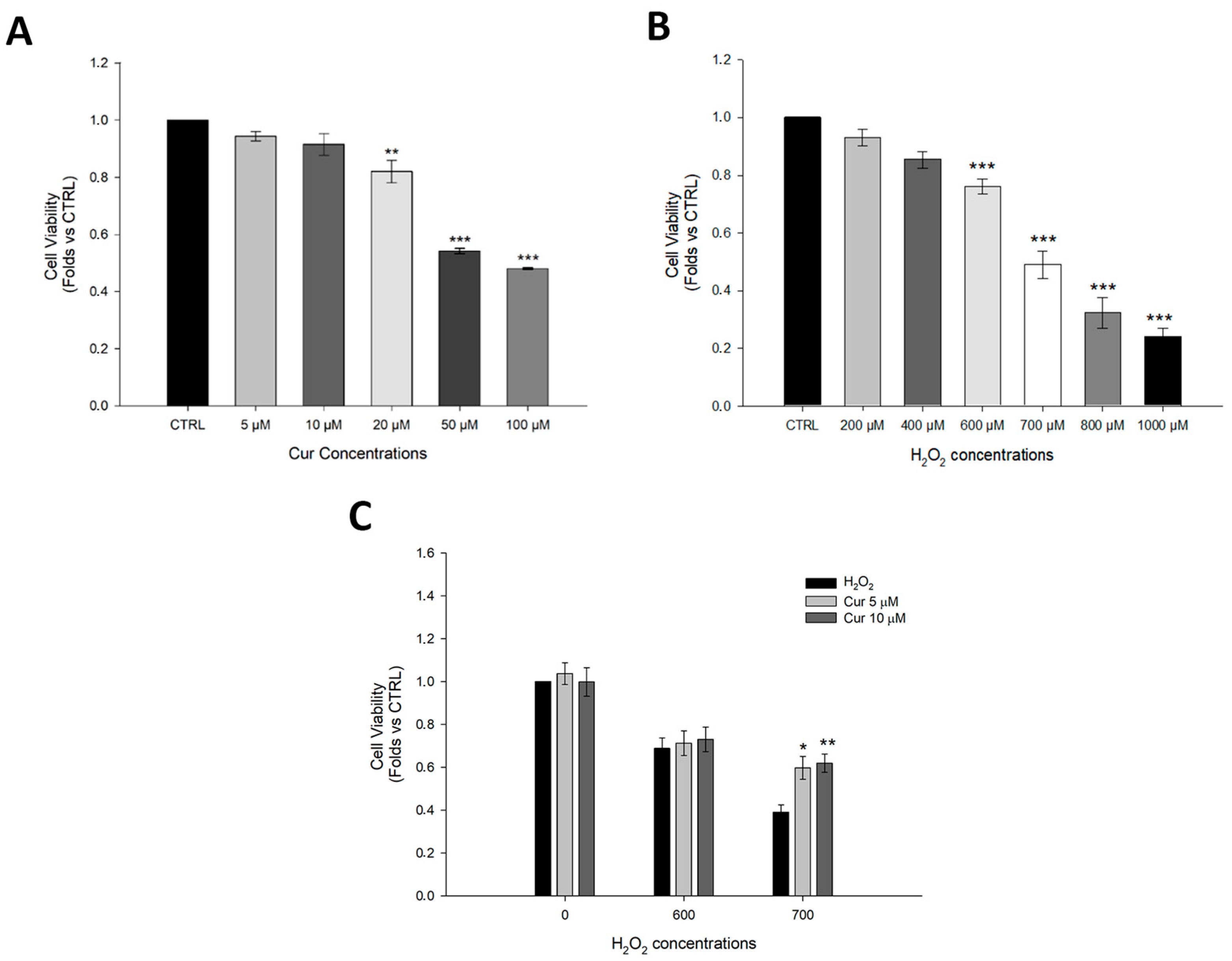
2.1. Effects of Curcumin on ARPE-19 Cell Viability Under Oxidative Stress Conditions
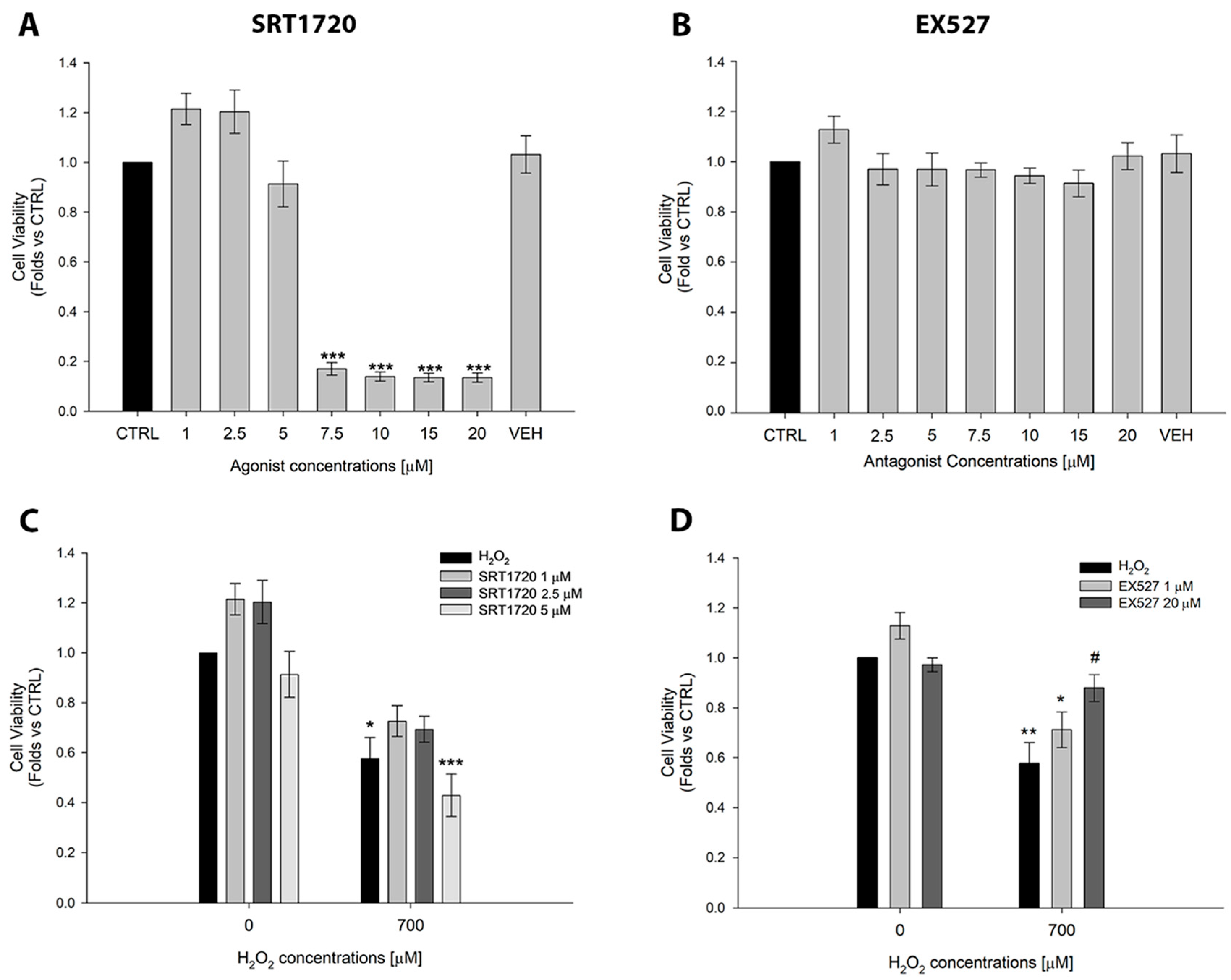
2.2. Effects of the SIRT1 Activator SRT1720 and the Inhibitor EX527 on ARPE-19 Cell Viability
2.3. Curcumin Inhibits SIRT1 Activity and Protects Against SIRT1-Mediated Cell Death in ARPE-19 Cells
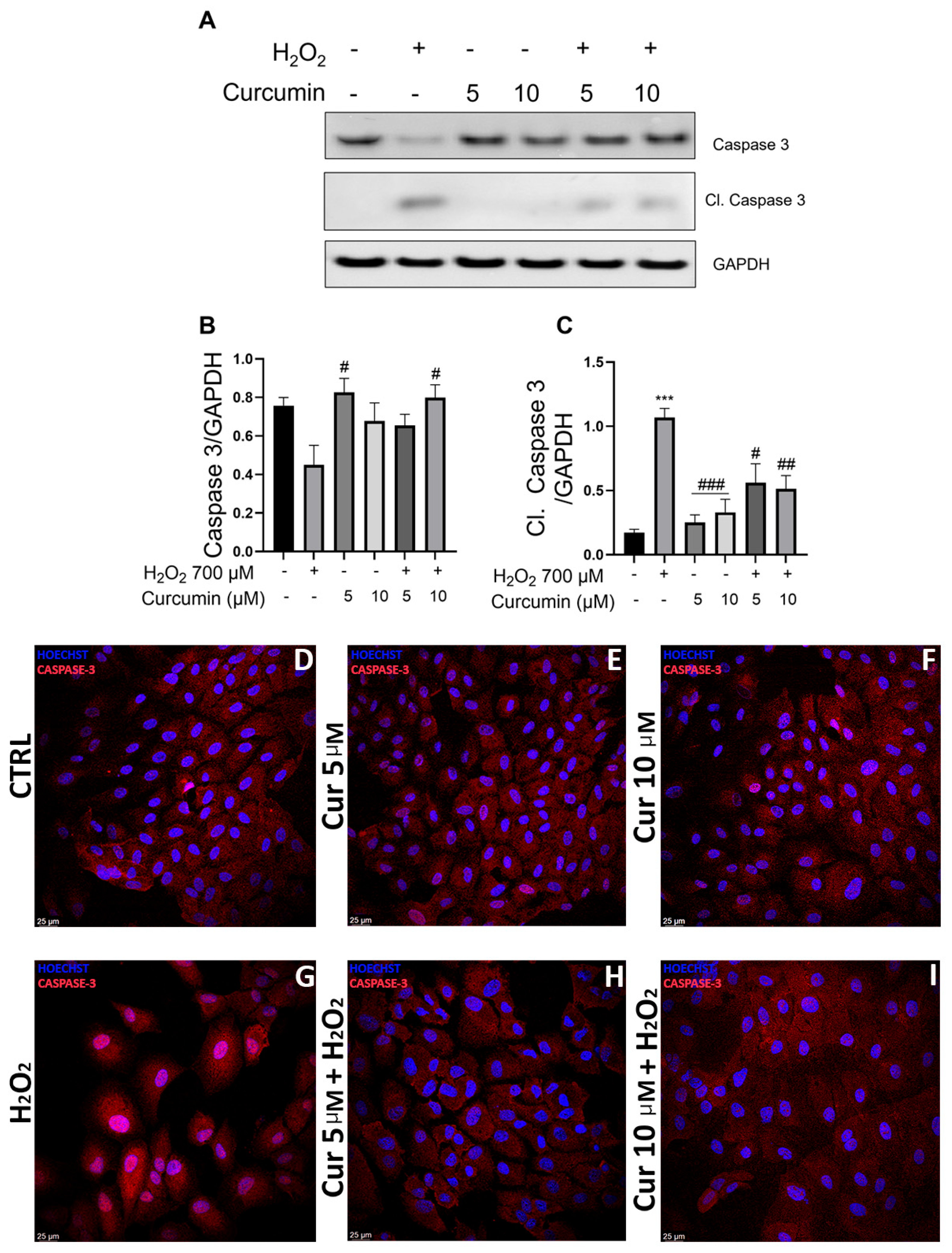
2.4. Curcumin Mitigates Oxidative Stress-Induced Apoptosis via Caspase-3 Modulation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Curcumin Treatment and Oxidative Stress Induction
4.3. SIRT1 Activator (SRT1720) and Inhibitor (EX527) Administration Under Physiological and Oxidative Stress Conditions
4.4. Cell Viability Assay
4.5. Protein Extraction and Western Blot Analysis
4.6. Immunofluorescence
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RPE | Retinal Pigment Epithelium |
| AMD | Age-related Macular Degeneration |
| ROS | Reactive Oxygen Species |
| BRB | Blood–Retinal Barrier |
| SIRT1 | Sirtuin 1 |
| HDACs | Histone Deacetylases |
| CDK | Cyclin-Dependent Kinase |
| NF-kB | Nuclear Factor kappa-light-chain-enhancer of activated B cell |
| ATCC | American Type Culture Collection |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal Bovine Serum |
| CUR | Curcumin |
| MTT | Thiazolyl Blue Tetrazolium Bromide |
| PAGE | SDS-Polyacrylamide gel electrophoresis |
| BSA | Bovine Serum Albumin |
| RT | Room Temperature |
| SE | Standard Error |
References
- Tisi, A.; Feligioni, M.; Passacantando, M.; Ciancaglini, M.; Maccarone, R. The Impact of Oxidative Stress on Blood-Retinal Barrier Physiology in Age-Related Macular Degeneration. Cells 2021, 10, 64. [Google Scholar] [CrossRef]
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- George, S.M.; Lu, F.; Rao, M.; Leach, L.L.; Gross, J.M. The Retinal Pigment Epithelium: Development, Injury Responses, and Regenerative Potential in Mammalian and Non-Mammalian Systems. Prog. Retin. Eye Res. 2021, 85, 100969. [Google Scholar] [CrossRef]
- Somasundaran, S.; Constable, I.J.; Mellough, C.B.; Carvalho, L.S. Retinal Pigment Epithelium and Age-Related Macular Degeneration: A Review of Major Disease Mechanisms. Clin. Exp. Ophthalmol. 2020, 48, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Wu, Y.; Ma, J.; Ikeda, M.; Ide, T.; Griffin, C.T.; Ding, X.Q.; Wang, S. Comparative Mechanistic Study of RPE Cell Death Induced by Different Oxidative Stresses. Redox Biol. 2023, 65, 102840. [Google Scholar] [CrossRef]
- Zhang, S.M.; Fan, B.; Li, Y.L.; Zuo, Z.Y.; Li, G.Y. Oxidative Stress-Involved Mitophagy of Retinal Pigment Epithelium and Retinal Degenerative Diseases. Cell Mol. Neurobiol. 2023, 43, 3265–3276. [Google Scholar] [CrossRef]
- Finkel, T. Signal Transduction by Reactive Oxygen Species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Chichagova, V.; Hallam, D.; Collin, J.; Zerti, D.; Dorgau, B.; Felemban, M.; Lako, M.; Steel, D.H. Cellular Regeneration Strategies for Macular Degeneration: Past, Present and Future. Eye 2018, 32, 946–971. [Google Scholar] [CrossRef] [PubMed]
- Carozza, G.; Zerti, D.; Tisi, A.; Ciancaglini, M.; Maccarrone, M.; Maccarone, R. An Overview of Retinal Light Damage Models for Preclinical Studies on Age-Related Macular Degeneration: Identifying Molecular Hallmarks and Therapeutic Targets. Rev. Neurosci. 2024, 35, 303–330. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and Aging Related Signaling Pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef]
- Mimura, T.; Kaji, Y.; Noma, H.; Funatsu, H.; Okamoto, S. The Role of SIRT1 in Ocular Aging. Exp. Eye Res. 2013, 116, 17–26. [Google Scholar] [CrossRef]
- Feldman, J.L.; Dittenhafer-Reed, K.E.; Denu, J.M. Sirtuin Catalysis and Regulation. J. Biol. Chem. 2012, 287, 42419–42427. [Google Scholar] [CrossRef]
- Lamichane, S.; Baek, S.H.; Kim, Y.J.; Park, J.H.; Lamichane, B.D.; Jang, W.B.; Ji, S.T.; Lee, N.K.; Dehua, L.; Kim, D.Y.; et al. MHY2233 Attenuates Replicative Cellular Senescence in Human Endothelial Progenitor Cells via SIRT1 Signaling. Oxid. Med. Cell. Longev. 2019, 2019, 6492029. [Google Scholar] [CrossRef]
- Pang, J.; Xiong, H.; Ou, Y.; Yang, H.; Xu, Y.; Chen, S.; Lai, L.; Ye, Y.; Su, Z.; Lin, H.; et al. SIRT1 Protects Cochlear Hair Cell and Delays Age-Related Hearing Loss via Autophagy. Neurobiol. Aging 2019, 80, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Iside, C.; Scafuro, M.; Nebbioso, A.; Altucci, L. SIRT1 Activation by Natural Phytochemicals: An Overview. Front. Pharmacol. 2020, 11, 1225. [Google Scholar] [CrossRef] [PubMed]
- Langley, E.; Pearson, M.; Faretta, M.; Bauer, U.M.; Frye, R.A.; Minucci, S.; Pelicci, P.G.; Kouzarides, T. Human SIR2 Deacetylates P53 and Antagonizes PML/P53-Induced Cellular Senescence. EMBO J. 2002, 21, 2383–2396. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, N.; Chu, Y.; Postnikova, O.; Varghese, R.; Horvath, A.; Cheema, A.K.; Golestaneh, N. Dysregulated Metabolic Pathways in Age-Related Macular Degeneration. Sci. Rep. 2020, 10, 2464. [Google Scholar] [CrossRef] [PubMed]
- Golestaneh, N.; Chu, Y.; Cheng, S.K.; Cao, H.; Poliakov, E.; Berinstein, D.M. Repressed Sirt1/Pgc-1α Pathway and Mitochondrial Disintegration in Ipsc-Derived Rpe Disease Model of Age-Related Macular Degeneration. J. Transl. Med. 2016, 14, 344. [Google Scholar] [CrossRef]
- Chou, W.W.; Chen, K.C.; Wang, Y.S.; Wang, J.Y.; Liang, C.L.; Juo, S.H.H. The Role of SIRT1/AKT/ERK Pathway in Ultraviolet B Induced Damage on Human Retinal Pigment Epithelial Cells. Toxicol. Vitr. 2013, 27, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Barchitta, M.; Mazzone, M.G.; Giuliano, F.; Basile, G.; Agodi, A. Resveratrol Modulates SIRT1 and DNMT Functions and Restores LINE-1 Methylation Levels in ARPE-19 Cells under Oxidative Stress and Inflammation. Int. J. Mol. Sci. 2018, 19, 2118. [Google Scholar] [CrossRef]
- Zhuge, C.C.; Xu, J.Y.; Zhang, J.; Li, W.; Li, P.; Li, Z.; Chen, L.; Liu, X.; Shang, P.; Xu, H.; et al. Fullerenol Protects Retinal Pigment Epithelial Cells from Oxidative Stress-Induced Premature Senescence via Activating SIRT1. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4628–4638. [Google Scholar] [CrossRef]
- Chen, W.; Lin, B.; Xie, S.; Yang, W.; Lin, J.; Li, Z.; Zhan, Y.; Gui, S.; Lin, B. Naringenin Protects RPE Cells from NaIO3-Induced Oxidative Damage in Vivo and in Vitro through up-Regulation of SIRT1. Phytomedicine 2021, 80, 153375. [Google Scholar] [CrossRef]
- Chen, Z.; Zhai, Y.; Zhang, W.; Teng, Y.; Yao, K. Single Nucleotide Polymorphisms of the Sirtuin 1 (SIRT1) Gene Are Associated with Age-Related Macular Degeneration in Chinese Han Individuals a Case-Control Pilot Study. Medicine 2015, 94, e2238. [Google Scholar] [CrossRef]
- Ren, C.; Hu, C.; Wu, Y.; Li, T.; Zou, A.; Yu, D.; Shen, T.; Cai, W.; Yu, J. Nicotinamide Mononucleotide Ameliorates Cellular Senescence and Inflammation Caused by Sodium Iodate in RPE. Oxid. Med. Cell Longev. 2022, 2022, 5961123. [Google Scholar] [CrossRef]
- Miłobȩdzka, J.; Kostanecki, S.V.; Lampe, V. Zur Kenntnis Des Curcumins. Berichte Der Dtsch. Chem. Ges. 1910, 43, 2163–2170. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and Anti-Inflammatory Properties of Curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: New York, NY, USA, 2007; Volume 595, pp. 105–125. [Google Scholar] [CrossRef]
- Buccarello, L.; Dragotto, J.; Hassanzadeh, K.; Maccarone, R.; Corbo, M.; Feligioni, M. Retinal Ganglion Cell Loss in an Ex Vivo Mouse Model of Optic Nerve Cut Is Prevented by Curcumin Treatment. Cell Death Discov. 2021, 7, 394. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, K.; Vahabzadeh, Z.; Bucarello, L.; Dragotto, J.; Corbo, M.; Maccarone, R.; Feligioni, M. Protective Effect of Curcuma Extract in an Ex Vivo Model of Retinal Degeneration via Antioxidant Activity and Targeting the SUMOylation. Oxid. Med. Cell Longev. 2022, 2022, 8923615. [Google Scholar] [CrossRef]
- Yang, L.; Shi, J.; Wang, X.; Zhang, R. Curcumin Alleviates D-Galactose-Induced Cardiomyocyte Senescence by Promoting Autophagy via the SIRT1/AMPK/MTOR Pathway. Evid. Based Complement. Altern. Med. 2022, 2022, 2990843. [Google Scholar] [CrossRef]
- Ren, B.C.; Zhang, Y.F.; Liu, S.S.; Cheng, X.J.; Yang, X.; Cui, X.G.; Zhao, X.R.; Zhao, H.; Hao, M.F.; Li, M.D.; et al. Curcumin Alleviates Oxidative Stress and Inhibits Apoptosis in Diabetic Cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt Signalling Pathways. J. Cell Mol. Med. 2020, 24, 12355–12367. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, X.; Hu, G.; Xu, C.; Jiang, H. Curcumin Attenuates Hydrogen Peroxide-Induced Premature Senescence via the Activation of SIRT1 in Human Umbilical Vein Endothelial Cells. Biol. Pharm. Bull. 2015, 38, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Carozza, G.; Tisi, A.; Capozzo, A.; Cinque, B.; Giovannelli, A.; Feligioni, M.; Flati, V.; Maccarone, R. New Insights into Dose-Dependent Effects of Curcumin on ARPE-19 Cells. Int. J. Mol. Sci. 2022, 23, 14771. [Google Scholar] [CrossRef]
- Chandrasekaran, P.R.; Madanagopalan, V.G. Role of Curcumin in Retinal Diseases—A Review. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 1457–1473. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Mattson, M.P. Author Correction: How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech. Dis. 2023, 9, 6. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Agathokleous, E. Hormesis: Transforming Disciplines That Rely on the Dose Response. IUBMB Life 2022, 74, 8–23. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis Defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef]
- Rainey, N.E.; Moustapha, A.; Petit, P.X. Curcumin, a Multifaceted Hormetic Agent, Mediates an Intricate Crosstalk between Mitochondrial Turnover, Autophagy, and Apoptosis. Oxid. Med. Cell Longev. 2020, 2020, 3656419. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Ertuğrul, A.; Özkaya, D.; Nazıroğlu, M. Curcumin Attenuates Hydroxychloroquine-Mediated Apoptosis and Oxidative Stress via the Inhibition of TRPM2 Channel Signalling Pathways in a Retinal Pigment Epithelium Cell Line. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 2829–2844. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Gu, W. SIRT1: Regulator of P53 Deacetylation. Genes Cancer 2013, 4, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fang, Y.; Yu, J.; Chang, X. Hawthorn Polyphenols Reduce High Glucose-Induced Inflammation and Apoptosis in ARPE-19 Cells by Regulating MiR-34a/SIRT1 to Reduce Acetylation. J. Food Biochem. 2021, 45, e13623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, J.; Wang, L.; Mu, L.; Xu, X.; Li, J.; Tang, G.; Chen, G.; Zhang, C.; Zhang, Y.; et al. SIRT1/P53 in Retinal Pigment Epithelial Cells in Diabetic Retinopathy: A Gene Co-Expression Analysis and He-Ying-Qing-Re Formula Treatment. Front. Mol. Biosci. 2024, 11, 1366020. [Google Scholar] [CrossRef]
- Yang, D.; Elner, S.G.; Clark, A.J.; Hughes, B.A.; Petty, H.R.; Elner, V.M. Activation of P2X Receptors Induces Apoptosis in Human Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1522–1530. [Google Scholar] [CrossRef]
- Attia, S.A.; Truong, A.T.; Phan, A.; Lee, S.J.; Abanmai, M.; Markanovic, M.; Avila, H.; Luo, H.; Ali, A.; Sreekumar, P.G.; et al. AB-Crystallin Peptide Fused with Elastin-like Polypeptide: Intracellular Activity in Retinal Pigment Epithelial Cells Challenged with Oxidative Stress. Antioxidants 2023, 12, 1817. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, D.; Wang, V.M.; Yu, C.R.; Wang, R.X.; Tuo, J.; Chan, C.C. Enhanced Apoptosis in Retinal Pigment Epithelium under Inflammatory Stimuli and Oxidative Stress. Apoptosis 2012, 17, 1144–1155. [Google Scholar] [CrossRef]
- Dunn, K.C.; Aotaki-Keen, A.E.; Putkey, F.R.; Hjelmeland, L.M. ARPE-19, a Human Retinal Pigment Epithelial Cell Line with Differentiated Properties. Exp. Eye Res. 1996, 62, 155–170. [Google Scholar] [CrossRef]
- Zingale, E.; Masuzzo, S.; Lajunen, T.; Reinisalo, M.; Rautio, J.; Consoli, V.; D’Amico, A.G.; Vanella, L.; Pignatello, R. Protective Role and Enhanced Intracellular Uptake of Curcumin in Retinal Cells Using Self-Emulsifying Drug Delivery Systems (SNEDDS). Pharmaceuticals 2025, 18, 265. [Google Scholar] [CrossRef]
- Mandal, M.N.A.; Patlolla, J.M.R.; Zheng, L.; Agbaga, M.P.; Tran, J.T.A.; Wicker, L.; Kasus-Jacobi, A.; Elliott, M.H.; Rao, C.V.; Anderson, R.E. Curcumin Protects Retinal Cells from Light-and Oxidant Stress-Induced Cell Death. Free Radic. Biol. Med. 2009, 46, 672–679. [Google Scholar] [CrossRef]
- Moghaddam, N.S.A.; Oskouie, M.N.; Butler, A.E.; Petit, P.X.; Barreto, G.E.; Sahebkar, A. Hormetic Effects of Curcumin: What Is the Evidence? J. Cell Physiol. 2019, 234, 10060–10071. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yang, B.; Najafi, M. Targeting of Cancer Cell Death Mechanisms by Curcumin: Implications to Cancer Therapy. Basic Clin. Pharmacol. Toxicol. 2021, 129, 397–415. [Google Scholar] [CrossRef]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N.; et al. Biological Activities of Curcumin and Its Analogues (Congeners) Made by Man and Mother Nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Tang, J. Exendin-4 Alleviates Diabetic Retinopathy by Activating Autophagy via Regulation of the Adenosine Monophosphate-Activated Protein Kinase/Sirtuin 1 Pathway. J. Mol. Histol. 2025, 56, 210. [Google Scholar] [CrossRef] [PubMed]
- Palabiyik, A.A.; Palabiyik, E. Pharmacological Approaches to Enhance Mitochondrial Biogenesis: Focus on PGC-1A, AMPK, and SIRT1 in Cellular Health. Mol. Biol. Rep. 2025, 52, 270. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, T.; Xian, M.; Wei, J. Therapeutic Applications of Exercise in Neurodegenerative Diseases: Focusing on the Mechanism of SIRT1. Mol. Cell Biochem. 2025. [Google Scholar] [CrossRef]
- Li, J.; Cui, S.; Li, Y.; Zhang, C.; Chang, C.; Jian, F. Sirtuin1 in Spinal Cord Injury: Regulatory Mechanisms, Microenvironment Remodeling and Therapeutic Potential. CNS Neurosci. Ther. 2025, 31, e70244. [Google Scholar] [CrossRef]
- Luo, T.; Li, C.; Zhou, L.; Sun, H.; Yang, M.M. Protein Acetylation in Age-Related Macular Degeneration: Mechanisms, Roles, and Therapeutic Perspectives. Investig. Ophthalmol. Vis. Sci. 2025, 66, 30. [Google Scholar] [CrossRef]
- Cole, G.M.; Teter, B.; Frautschy, S.A. Neuroprotective Effects of Curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: New York, NY, USA, 2007; Volume 595, pp. 197–212. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Xu, C.; Peng, A.; Qin, H.; Yao, K. Advancements in Age-Related Macular Degeneration Treatment: From Traditional Anti-VEGF to Emerging Therapies in Gene, Stem Cell, and Nanotechnology. Biochem. Pharmacol. 2025, 236, 116902. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From Kitchen to Clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Gregorio, J.; Zerti, D.; Carozza, G.; Capozzo, A.; Flati, V.; Feligioni, M.; Maccarone, R. Hormetic Effects of Curcumin in RPE Cells: SIRT1 and Caspase-3 Inactivation with Implications for AMD. Int. J. Mol. Sci. 2025, 26, 8555. https://doi.org/10.3390/ijms26178555
Di Gregorio J, Zerti D, Carozza G, Capozzo A, Flati V, Feligioni M, Maccarone R. Hormetic Effects of Curcumin in RPE Cells: SIRT1 and Caspase-3 Inactivation with Implications for AMD. International Journal of Molecular Sciences. 2025; 26(17):8555. https://doi.org/10.3390/ijms26178555
Chicago/Turabian StyleDi Gregorio, Jacopo, Darin Zerti, Giulia Carozza, Annamaria Capozzo, Vincenzo Flati, Marco Feligioni, and Rita Maccarone. 2025. "Hormetic Effects of Curcumin in RPE Cells: SIRT1 and Caspase-3 Inactivation with Implications for AMD" International Journal of Molecular Sciences 26, no. 17: 8555. https://doi.org/10.3390/ijms26178555
APA StyleDi Gregorio, J., Zerti, D., Carozza, G., Capozzo, A., Flati, V., Feligioni, M., & Maccarone, R. (2025). Hormetic Effects of Curcumin in RPE Cells: SIRT1 and Caspase-3 Inactivation with Implications for AMD. International Journal of Molecular Sciences, 26(17), 8555. https://doi.org/10.3390/ijms26178555










