Treatment of Dyslipidemia in Patients with Type 1 Diabetes Mellitus: A Review of Current Evidence and Knowledge Gaps
Abstract
1. Introduction
2. Results
3. Current Guidelines
4. Clinical Implications
5. Limitations
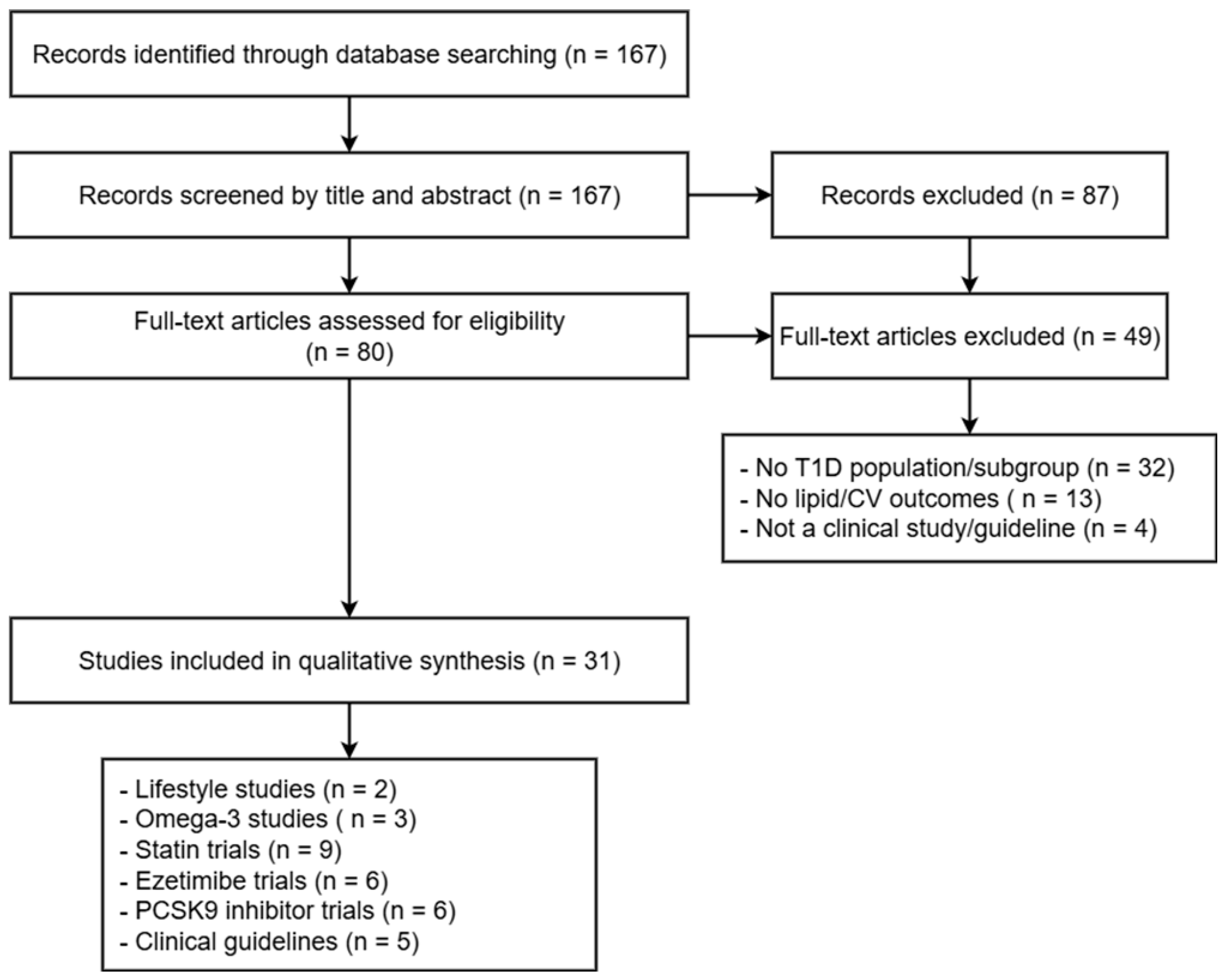
6. Methods
- Studies were included if they met the following criteria:
- Investigating lipid management in patients with T1D, T2D, or both;
- Clinical trials (randomized or non-randomized), cohort studies, meta-analyses, or systematic reviews;
- Containing data on lipid parameters, cardiovascular outcomes, or treatment efficacy;
- Clinical practice guidelines from major international organizations, such as the American Diabetes Association (ADA), the European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS), the American College of Cardiology (ACC), the American Heart Association (AHA), and the International Society for Pediatric and Adolescent Diabetes (ISPAD).
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogle, G.D.; Wang, F.; Haynes, A.; Gregory, G.A.; King, T.W.; Deng, K.; Dabelea, D.; James, S.; Jenkins, A.J.; Li, X.; et al. Global type 1 diabetes prevalence, incidence, and mortality estimates 2025: Results from the International diabetes Federation Atlas, 11th Edition, and the T1D Index Version 3.0. Diabetes Res. Clin. Pract. 2025, 225, 112277. [Google Scholar] [CrossRef]
- Tell, S.; Nadeau, K.J.; Eckel, R.H. Lipid management for cardiovascular risk reduction in type 1 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; Eckel, R.H.; Vrablik, M.; Zambon, A. Lipid-lowering in diabetes: An update. Atherosclerosis 2024, 394, 117313. [Google Scholar] [CrossRef] [PubMed]
- Vergès, B. Lipid disorders in type 1 diabetes. Diabetes Metab. 2009, 35, 353–360. [Google Scholar] [CrossRef]
- Wu, L.; Parhofer, K.G. Diabetic dyslipidemia. Metabolism 2014, 63, 1469–1479. [Google Scholar] [CrossRef]
- Taskinen, M.R. Diabetic dyslipidemia: From basic research to clinical practice. Diabetologia 2003, 46, 733–749. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, I.; Matsuda, M.; Hammer, R.E.; Bashmakov, Y.; Brown, M.S.; Goldstein, J.L. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol. Cell 2000, 6, 77–86. [Google Scholar] [CrossRef]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Yan, S.F.; Ramasamy, R.; Schmidt, A.M. The RAGE axis: A fundamental mechanism signaling danger to the vulnerable vasculature. Circ. Res. 2010, 106, 842–853. [Google Scholar] [CrossRef]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef]
- Nagareddy, P.R.; Murphy, A.J.; Stirzaker, R.A.; Hu, Y.; Yu, S.; Miller, R.G.; Ramkhelawon, B.; Distel, E.; Westerterp, M.; Huang, L.S.; et al. Hyperglycemia promotes myelopoiesis and impairs the resolution of atherosclerosis. Cell Metab. 2013, 17, 695–708. [Google Scholar] [CrossRef]
- Nagareddy, P.R.; Kraakman, M.; Masters, S.L.; Stirzaker, R.A.; Gorman, D.J.; Grant, R.W.; Dragoljevic, D.; Hong, E.S.; Abdel-Latif, A.; Smyth, S.S.; et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014, 19, 821–835. [Google Scholar] [CrossRef]
- Maahs, D.M.; Daniels, S.R.; de Ferranti, S.D.; Dichek, H.L.; Flynn, J.; Goldstein, B.I.; Kelly, A.S.; Nadeau, K.J.; Martyn-Nemeth, P.; Osganian, S.K.; et al. Cardiovascular disease risk factors in youth with diabetes mellitus: A scientific statement from the American Heart Association. Circulation 2014, 130, 1532–1558. [Google Scholar] [CrossRef]
- Perez, A.; Caixas, A.; Carreras, G.; Mauricio, D.; Pou, J.M.; Serrat, J.; Gómez-Gerique, J.; de Leiva, A. Lipoprotein compositional abnormalities in type I diabetes: Effect of improved glycaemic control. Diabetes Res. Clin. Pract. 1997, 36, 83–90. [Google Scholar] [CrossRef]
- Rawshani, A.; Rawshani, A.; Sattar, N.; Franzén, S.; McGuire, D.K.; Eliasson, B.; Svensson, A.M.; Zethelius, B.; Miftaraj, M.; Rosengren, A.; et al. Relative Prognostic Importance and Optimal Levels of Risk Factors for Mortality and Cardiovascular Outcomes in Type 1 Diabetes Mellitus. Circulation 2019, 139, 1900–1912. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; DCCT/EDIC Research Group. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study at 30 Years: Overview. Diabetes Care 2014, 37, 9–16. [Google Scholar] [CrossRef]
- Miller, R.G.; Costacou, T.; Orchard, T.J. Risk factor modeling for cardiovascular disease in type 1 diabetes in the pittsburgh epidemiology of diabetes complications (EDC) study: A comparison with the diabetes control and complications trial/epidemiology of diabetes interventions and complications study (DCCT/EDIC). Diabetes 2019, 68, 409–419. [Google Scholar] [CrossRef]
- Orchard, T.J.; Forrest, K.Y.Z.; Kuller, L.H.; Becker, D.J. Lipid and blood pressure treatment goals for type 1 diabetes: 10-year incidence data from the pittsburgh epidemiology of diabetes complications study. Diabetes Care 2001, 24, 1053–1059. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Care in Diabetes. Available online: https://professional.diabetes.org/standards-of-care (accessed on 26 June 2025).
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary Circulation. J. Am. Coll. Cardiol. 2019, 73, 3168–3209, Correction in J. Am. Coll. Cardiol. 2024, 84, 1772. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Znayenko-Miller, T.; Smith, K.; Khambatta, C.; Barbaro, R.; Sutton, M.; Holtz, D.N.; Sklar, M.; Pineda, D.; Holubkov, R.; et al. Effect of a Dietary Intervention on Insulin Requirements and Glycemic Control in Type 1 Diabetes: A 12-Week Randomized Clinical Trial. Clin. Diabetes 2024, 42, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Jaacks, L.M.; Liu, W.; Ji, L.; Mendez, M.A.; Du, S.; Crandell, J.; Rosamond, W.; Mayer-Davis, E.J. Diabetes nutrition therapy and dietary intake among individuals with Type 1 diabetes in China. Diabet. Med. 2015, 32, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Moran, J.; Forsblom, C.; Harjutsalo, V.; Thorn, L.; Ahola, A.; Wadén, J.; Tolonen, N.; Saraheimo, M.; Gordin, D.; et al. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care 2011, 34, 861–866. [Google Scholar] [CrossRef]
- Colhoun, H.M.; Betteridge, D.J.; Durrington, P.N.; A Hitman, G.; Neil, H.A.W.; Livingstone, S.J.; Thomason, M.J.; I Mackness, M.; Charlton-Menys, V.; Fuller, J.H. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): Multicentre randomised placebo-controlled trial. Lancet 2004, 364, 685–696. [Google Scholar] [CrossRef]
- Collins, R.; Armitage, J.; Parish, S.; Sleight, P.; Peto, R. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebocontrolled trial. Lancet 2002, 360, 7–22. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaborators; Kearney, P.M.; Blackwell, L.; Collins, R.; Keech, A.; Simes, J.; Peto, R.; Armitage, J.; Baigent, C. Efficacy of cholesterol-lowering therapy in 18 686 people with diabetes in 14 randomised trials of statins: A meta-analysis. Lancet 2008, 371, 117–125. [Google Scholar] [CrossRef]
- Nathan, D.M.; Cleary, P.A.; Backlund, J.Y.; Genuth, S.M.; Lachin, J.M.; Orchard, T.J.; Raskin, P.; Zinman, B.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive Diabetes Treatment and Cardiovascular Disease in Patients with Type 1 Diabetes. N. Engl. J. Med. 2005, 353, 2643–2653. [Google Scholar] [CrossRef]
- Hero, C.; Rawshani, A.; Svensson, A.M.; Franzén, S.; Eliasson, B.; Eeg-Olofsson, K.; Gudbjörnsdottir, S. Association between use of lipid-lowering therapy and cardiovascular diseases and death in individuals with type 1 diabetes. Diabetes Care 2016, 39, 996–1003. [Google Scholar] [CrossRef]
- Marcovecchio, M.L.; Chiesa, S.T.; Bond, S.; Daneman, D.; Dawson, S.; Donaghue, K.C.; Jones, T.W.; Mahmud, F.H.; Marshall, S.M.; Neil, H.A.W.; et al. ACE Inhibitors and Statins in Adolescents with Type 1 Diabetes. N. Engl. J. Med. 2017, 377, 1733–1745. [Google Scholar] [CrossRef]
- De Vries, F.M.; Kolthof, J.; Postma, M.J.; Denig, P.; Hak, E. Efficacy of Standard and Intensive Statin Treatment for the Secondary Prevention of Cardiovascular and Cerebrovascular Events in Diabetes Patients: A Meta-Analysis. PLoS ONE 2014, 9, e111247. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Jeon, J.; Baek, M.; Song, S.O.; Kim, J. Impact of statin treatment on cardiovascular risk in patients with type 1 diabetes: A population-based cohort study. J. Transl. Med. 2023, 21, 806. [Google Scholar] [CrossRef] [PubMed]
- Haller, M.; Stein, J.; Shuster, J.; Theriaque, D.; Samyn, M.; Pepine, C.; Silverstein, J. Pediatric Atorvastatin in Diabetes Trial (PADIT): A Pilot Study to Determine the Effect of Atorvastatin on Arterial Stiffness and Endothelial Function in Children with Type 1 Diabetes Mellitus. J. Pediatr. Endocrinol. Metab. 2009, 22, 65–68. [Google Scholar] [CrossRef]
- Martin, S.; Herder, C.; Schloot, N.C.; Koenig, W.; Heise, T.; Heinemann, L.; Kolb, H.; on behalf of the DIATOR Study Group; Song, Y. Residual Beta Cell Function in Newly Diagnosed Type 1 Diabetes after Treatment with Atorvastatin: The Randomized DIATOR Trial. PLoS ONE 2011, 6, e17554. [Google Scholar] [CrossRef]
- de Zeeuw, D.; A Anzalone, D.; A Cain, V.; Cressman, M.D.; Heerspink, H.J.L.; A Molitoris, B.; Monyak, J.T.; Parving, H.-H.; Remuzzi, G.; Sowers, J.R.; et al. Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): A randomised clinical trial. Lancet Diabetes Endocrinol. 2015, 3, 181–190. [Google Scholar] [CrossRef]
- Reith, C.; Preiss, D.; Blackwell, L.; Emberson, J.; Spata, E.; Davies, K.; Halls, H.; Harper, C.; Holland, L.; Wilson, K.; et al. Effects of statin therapy on diagnoses of new-onset diabetes and worsening glycaemia in large-scale randomised blinded statin trials: An individual participant data meta-analysis. Lancet Diabetes Endocrinol. 2024, 12, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P.; Cannon, C.P.; Blazing, M.A.; Nicolau, J.C.; Corbalán, R.; Špinar, J.; Park, J.-G.; White, J.A.; Bohula, E.A.; Braunwald, E.; et al. Benefit of Adding Ezetimibe to Statin Therapy on Cardiovascular Outcomes and Safety in Patients with Versus Without Diabetes Mellitus Results from IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Effcacy International Trial). Circulation 2018, 137, 1571–1582. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Cho, J.Y.; You, S.C.; Yun, K.H.; Cho, Y.-H.; Shin, W.-Y.; Im, S.W.; Kang, W.C.; Park, Y.; Lee, S.Y.; et al. Moderate-intensity statin with ezetimibe vs. high-intensity statin in patients with diabetes and atherosclerotic cardiovascular disease in the RACING trial. Eur. Heart J. 2023, 44, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-K.; Hong, S.-J.; Lee, Y.-J.; Hong, S.J.; Yun, K.H.; Hong, B.-K.; Heo, J.H.; Rha, S.-W.; Cho, Y.-H.; Lee, S.-J.; et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): A randomised, open-label, non-inferiority trial. Lancet 2022, 400, 380–390. [Google Scholar] [CrossRef]
- Yunoki, K.; Nakamura, K.; Miyoshi, T.; Enko, K.; Kohno, K.; Morita, H.; Kusano, K.F.; Ito, H. Ezetimibe improves postprandial hyperlipemia and its induced endothelial dysfunction. Atherosclerosis 2011, 217, 486–491. [Google Scholar] [CrossRef]
- Ciriacks, K.; Coly, G.; Krishnaswami, S.; Patel, S.B.; Kidambi, S. Effects of simvastatin and ezetimibe in lowering low-density lipoprotein cholesterol in subjects with type 1 and type 2 diabetes mellitus. Metab. Syndr. Relat. Disord. 2015, 13, 84–90. [Google Scholar] [CrossRef]
- Semova, I.; Levenson, A.E.; Krawczyk, J.; Bullock, K.; Williams, K.A.; Wadwa, R.P.; Shah, A.S.; Khoury, P.R.; Kimball, T.R.; Urbina, E.M.; et al. Type 1 diabetes is associated with an increase in cholesterol absorption markers but a decrease in cholesterol synthesis markers in a young adult population. J. Clin. Lipidol. 2019, 13, 940–946. [Google Scholar] [CrossRef]
- Levenson, A.E.; Wadwa, R.P.; Shah, A.S.; Khoury, P.R.; Kimball, T.R.; Urbina, E.M.; de Ferranti, S.D.; Bishop, F.K.; Maahs, D.M.; Dolan, L.M.; et al. PCSK9 Is Increased in Youth with Type 1 Diabetes. Diabetes Care 2017, 40, e85–e87. [Google Scholar] [CrossRef] [PubMed]
- Bojanin, D.; Vekic, J.; Milenkovic, T.; Vukovic, R.; Zeljkovic, A.; Stefanovic, A.; Janac, J.; Ivanisevic, J.; Mitrovic, K.; Miljkovic, M.; et al. Association between proprotein convertase subtilisin/kexin 9 (PCSK9) and lipoprotein subclasses in children with type 1 diabetes mellitus: Effects of glycemic control. Atherosclerosis 2019, 280, 14–20. [Google Scholar] [CrossRef]
- Leiter, L.A.; Cariou, B.; Müller-Wieland, D.; Colhoun, H.M.; Del Prato, S.; Tinahones, F.J.; Ray, K.K.; Bujas-Bobanovic, M.; Domenger, C.; Mandel, J.; et al. Efficacy and safety of alirocumab in insulin-treated individuals with type 1 or type 2 diabetes and high cardiovascular risk: The ODYSSEY DM-INSULIN randomized trial. Diabetes Obes. Metab. 2017, 19, 1781–1792. [Google Scholar] [CrossRef]
- American Diabetes Association. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43 (Suppl. 1), S111–S134. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, M.L.; Fazio, S.; Giugliano, R.P.; Stroes, E.S.G.; Kanevsky, E.; Gouni-Berthold, I.; Im, K.; Pineda, A.L.; Wasserman, S.M.; Češka, R.; et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk insights from the FOURIER trial. Circulation 2019, 139, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Giugliano, R.P.; Ran, X.; Deedwania, P.; De Ferrari, G.M.; George, J.T.; Gouni-Berthold, I.; Lima, G.P.d.S.; Handelsman, Y.; Lewis, B.S.; et al. Cardiovascular Outcomes and Efficacy of the PCSK9 Inhibitor Evolocumab in Individuals with Type 1 Diabetes: Insights From the FOURIER Trial. Diabetes Care 2025, 48, 1512–1516. [Google Scholar] [CrossRef]
- Bittner, V.A.; Szarek, M.; Aylward, P.E.; Bhatt, D.L.; Diaz, R.; Edelberg, J.M.; Fras, Z.; Goodman, S.G.; Halvorsen, S.; Hanotin, C.; et al. Effect of Alirocumab on Lipoprotein(a) and Cardiovascular Risk After Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2020, 75, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Colhoun, H.M.; Leiter, L.A.; Müller-Wieland, D.; Cariou, B.; Ray, K.K.; Tinahones, F.J.; Domenger, C.; Letierce, A.; Israel, M.; Samuel, R.; et al. Effect of alirocumab on individuals with type 2 diabetes, high triglycerides, and low high-density lipoprotein cholesterol. Cardiovasc. Diabetol. 2020, 19, 14. [Google Scholar] [CrossRef]
- Cariou, B.; Leiter, L.; Müller-Wieland, D.; Bigot, G.; Colhoun, H.; Del Prato, S.; Henry, R.; Tinahones, F.; Letierce, A.; Aurand, L.; et al. Efficacy and safety of alirocumab in insulin-treated patients with type 1 or type 2 diabetes and high cardiovascular risk: Rationale and design of the ODYSSEY DM–INSULIN trial. Diabetes Metab. 2017, 43, 453–459. [Google Scholar] [CrossRef]
- The ASCEND Study Collaborative Group. Effects of n−3 Fatty Acid Supplements in Diabetes Mellitus. N. Engl. J. Med. 2018, 379, 1540–1550. [Google Scholar] [CrossRef]
- The ORIGIN Trial Investigators. n–3 Fatty Acids and Cardiovascular Outcomes in Patients with Dysglycemia. N. Engl. J. Med. 2012, 367, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- American College of Cardiology. 2019 ESC/EAS Guidelines for Management of Dyslipidemia. Available online: https://www.acc.org/latest-in-cardiology/ten-points-to-remember/2019/09/12/15/13/2019-esc-eas-guidelines-for-dyslipidaemias (accessed on 26 June 2025).
- ISPAD Clinical Practice Guidelines 2024: Editorial. Available online: https://www.ispad.org/resource/icpcg--editorial-2024-pdf.html (accessed on 26 June 2025).
- De Ferranti, S.D.; Steinberger, J.; Ameduri, R.; Baker, A.; Gooding, H.; Kelly, A.S.; Mietus-Snyder, M.; Mitsnefes, M.M.; Peterson, A.L.; St-Pierre, J.; et al. Cardiovascular Risk Reduction in High-Risk Pediatric Patients: A Scientific Statement From the American Heart Association. Circulation 2019, 139, E603–E634. [Google Scholar] [CrossRef]
- Elian, V.; Popovici, V.; Ozon, E.-A.; Musuc, A.M.; Fița, A.C.; Rusu, E.; Radulian, G.; Lupuliasa, D. Current Technologies for Managing Type 1 Diabetes Mellitus and Their Impact on Quality of Life—A Narrative Review. Life 2023, 13, 1663. [Google Scholar] [CrossRef]
- Jun, J.E.; Lee, S.; Lee, Y.; Ahn, J.Y.; Kim, G.; Hur, K.Y.; Lee, M.; Jin, S.; Kim, J.H. Continuous glucose monitoring defined glucose variability is associated with cardiovascular autonomic neuropathy in type 1 diabetes. Diabetes Metab. Res. Rev. 2019, 35, e3092. [Google Scholar] [CrossRef] [PubMed]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
| SN | Author and Year | Title | Primary and Secondary Objectives | Population/Participants | Sample Size | Intervention/Exposure | Outcome Measures | Findings and Conclusions |
|---|---|---|---|---|---|---|---|---|
| 1 | Colhoun, H.M. et al., 2004 [24] | Primary prevention of cardiovascular disease with atorvastatin in T2D in the Collaborative Atorvastatin Diabetes Study (CARDS) | Primary: to assess the effect of atorvastatin on cardiovascular events in T2D. Secondary: to evaluate safety and specific cardiovascular outcomes. | Type 2 diabetes with no prior cardiovascular disease | 2838 | Atorvastatin (10 mg/day) vs. placebo | Major cardiovascular events (acute coronary events, stroke, revascularization) | Atorvastatin reduced major cardiovascular events by 37% (HR 0.63, 95% CI 0.48–0.83), with a favorable safety profile. |
| 2 | Collins, R. et al., 2002 [25] | MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals | Primary: to assess the effect of simvastatin on major vascular events in high-risk individuals. Secondary: to assess effects in subgroups, including patients with diabetes. | High-risk individuals, including T1D and T2D | 20,536 | Simvastatin (40 mg/day) vs. placebo | Major vascular events (coronary events, stroke, revascularization) | Simvastatin reduced major vascular events by 22% in patients with diabetes (RR 0.78, 95% CI 0.67–0.91) vs. placebo. |
| 3 | Downs, J.R. et al., 2008 [26] | Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomized trials of statins: a meta-analysis | Primary: to assess the effect of statins on CV outcomes in patients with diabetes. Secondary: to evaluate effects on specific vascular events and mortality. | Diabetes patients (type 1 and 2) from 14 randomized trials | 18,686 | Statin therapy vs. control | Major vascular events, coronary events, stroke, mortality | Statins reduced major vascular events by 21% per 1 mmol/L of LDL-C reduction (RR 0.79, 95% CI 0.72–0.86), with no significant difference between type 1 and type 2 diabetes. |
| 4 | Nathan, D.M. et al., 2005 [27] | Intensive diabetes treatment and cardiovascular disease in patients with T1D | Primary: to assess the effect of intensive diabetes treatment on CV disease in T1D. Secondary: to assess long-term complications. | Type 1 diabetes patients | 1441 | Intensive vs. conventional diabetes therapy | Cardiovascular events (myocardi-al infarction, stroke, cardiovascular death) | Intensive treatment reduced cardiovascular events by 42% (HR 0.58, 95% CI 0.39–0.86) in type 1 diabetes patients. |
| 5 | Martin, S. et al., 2011 [33] | Residual beta cell function in newly diagnosed type 1 diabetes after treatment with atorvastatin: the Randomized DIATOR Trial | Primary: to assess the effect of atorvastatin on residual beta-cell function in newly diagnosed T1D. Secondary: to evaluate inflammatory markers. | Newly diagnosed patients with T1D | 89 | Atorvastatin (80 mg/day) vs. placebo | C-peptide levels, inflammatory markers (CRP, IL-6) | Atorvastatin did not significantly preserve beta-cell function (C-peptide levels) or reduce inflammatory markers |
| 6. | de Zeeuw, D. et al., 2015 [34] | Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I) | Primary: to compare the renal effects of atorvastatin and rosuvastatin in diabetes patients with proteinuria. Secondary: to assess lipid-lowering efficacy. | Diabetes patients with progressive renal disease | 353 | Atorvastatin (80 mg/day) vs. rosuvastatin (10 or 40 mg/day) | Urinary protein/creatinine ratio, glomerular filtration rate, LDL-C | Atorvastatin reduced proteinuria more effectively than rosuvastatin, with similar LDL-C reduction but better renal outcomes. |
| 7 | De Vries, F.M. et al., 2014 [30] | Efficacy of Standard and Intensive Statin Treatment for the Secondary Prevention of Cardiovascular and Cerebrovascular Events in Diabetes Patients: A Meta-Analysis | Primary: to compare standard vs. intensive statin therapy for secondary cardiovascular prevention in diabetes. Secondary: to assess cerebrovascular events. | Diabetes patients with prior cardiovascular disease | 12,563 | Standard vs. intensive statin therapy | Cardiovascular and cerebrovascular events | Intensive statin therapy reduced cardiovascular events by 9% (RR 0.90, 95% CI 0.85–0.96) compared to standard therapy in diabetes patients. |
| 8 | Yoo, J. et al., 2023 [31] | Impact of statin treatment on cardiovascular risk in patients with type 1 diabetes: a population-based cohort study | Primary: to evaluate the effect of statins on CV risk in T1D. Secondary: to assess specific CV outcomes. | Type 1 diabetes patients | 11,009 | Statin use vs. non-use | Major adverse cardiovascular events (MACEs) | Statin use was associated with a 40% reduction in MACEs (HR 0.60, 95% CI 0.45–0.80) |
| 9. | Marcovecchio, M.L. et al., 2017 [29] | ACE Inhibitors and Statins in Adolescents with Type 1 Diabetes Adolescent type 1 Diabetes cardio-renal Intervention Trial (AdDIT) | Primary: to evaluate the change in albumin excretion in adolescents with T1D. Secondary: to assess the development of microalbuminuria, progression of retinopathy, changes in eGFR, lipid levels, and CV risk. | Adolescents with T1D | 443 | Statins and/or ACE inhibitors vs. placebo | Carotid intima–media thickness, albuminuria, lipid levels | Statin use resulted in significant reductions in TC, LDL-C, and non-HDL-C, in triglyceride levels, and in the ratio of apo B to apo A1. |
| 10. | Haller, M.J. et al., 2009 [32] | Pediatric Atorvastatin in Diabetes Trial (PADIT): a pilot study to determine the effect of atorvastatin on arterial stiffness and endothelial function in children with type 1 diabetes | Primary: to assess atorvastatin’s effect on arterial stiffness and endothelial function in children with T1D. Secondary: to evaluate safety and lipid profile changes. | Children and adolescents (10–21 years old) with T1D | 51 | Atorvastatin (20 mg/day) vs. placebo | Pulse wave velocity (arterial stiffness), FMD (endothelial function), lipid profiles | Atorvastatin improved endothelial function (p = 0.04) but did not significantly affect arterial stiffness; it was safe and reduced LDL-C levels by 25%. |
| 11 | Hero, C. et al., 2016 [28] | Association between use of lipid-lowering therapy and cardiovascular diseases and death in individuals with type 1 diabetes | Primary: to investigate the association between lipid-lowering therapy and cardiovascular events/death in T1D. Secondary: to assess specific cardiovascular outcomes and mortality rates. | Individuals with type 1 diabetes from the Swedish National Diabetes Register | 24,230 | Lipid-lowering therapy vs. no lipid-lowering therapy | Cardiovascular events (myocardial infarction, stroke, coronary heart disease), all-cause mortality | Lipid-lowering therapy was associated with a 22–44% reduction in cardiovascular events and death (HR 0.78 for cardiovascular disease, 95% CI 0.68–0.89; HR 0.56 for mortality, 95% CI 0.48–0.66). |
| SN | Author and Year | Title | Primary and Secondary Objectives | Population/Participants | Sample Size | Intervention/Exposure | Outcome Measures | Findings and Conclusions |
|---|---|---|---|---|---|---|---|---|
| 1 | Giugliano, R.P. et al., 2018 [36] | Benefit of Adding Ezetimibe to Statin Therapy on Cardiovascular Outcomes and Safety in Patients with Versus Without Diabetes Mellitus | Primary: to assess if ezetimibe plus simvastatin reduces cardiovascular events compared to simvastatin alone. Secondary: to evaluate safety and efficacy in diabetes vs. non-diabetes patients. | Patients with acute coronary syndrome, with or without diabetes mellitus | 18,144 | Ezetimibe plus simvastatin vs. simvastatin alone | Primary: Composite of cardiovascular death, myocardial infarction, stroke, or revascularization. Secondary: Safety endpoints (adverse events). | Ezetimibe plus simvastatin significantly reduced cardiovascular events in patients with diabetes (HR 0.86, 95% CI 0.78–0.94) compared to simvastatin alone, with consistent safety profiles. |
| 2 | Lee, Y.J. et al., 2023 [37] | Moderate-intensity statin with ezetimibe vs. high-intensity statin in patients with diabetes and atherosclerotic cardiovascular disease in the RACING trial | Primary: to compare cardiovascular outcomes of moderate-intensity statin plus ezetimibe vs. high-intensity statin. Secondary: to assess safety and LDL-C reduction. | Patients with diabetes and atherosclerotic cardiovascular disease | 3780 | Moderate-intensity statin plus ezetimibe vs. high-intensity statin | Primary: Composite of cardiovascular death, major cardiovascular events, or stroke. Secondary: LDL-C levels, adverse events. | Moderate-intensity statin plus ezetimibe was non-inferior to high-intensity statin in reducing cardiovascular events (HR 0.94, 95% CI 0.82–1.09), with better LDL-C reduction and fewer adverse events. |
| 3 | Kim, B.K. et al., 2022 [38] | Long-term efficacy and safety of moderate-intensity statin with ezetimibe versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING) | Primary: to assess the non-inferiority of moderate-intensity statin plus ezetimibe vs. high-intensity statin for cardiovascular outcomes. Secondary: to assess long-term safety and tolerability. | Patients with atherosclerotic cardiovascular disease | 3780 | Moderate-intensity statin plus ezetimibe vs. high-intensity statin monotherapy | Primary: Composite of cardiovascular death, major cardiovascular events, or non-fatal stroke. Secondary: Adverse events, LDL-C levels. | Moderate-intensity statin plus ezetimibe was non-inferior (HR 0.92, 95% CI 0.80–1.05), with lower rates of intolerance-related discontinuations. |
| 4 | Yunoki, K. et al., 2011 [39] | Ezetimibe improves postprandial hyperlipemia and its induced endothelial dysfunction | Primary: to assess the effect of ezetimibe on postprandial hyperlipidemia and endothelial function. Secondary: to assess changes in lipid profiles. | Patients with dyslipidemia | 20 | Ezetimibe (10 mg/day) vs. placebo | Postprandial triglyceride levels, flow-mediated dilatation (FMD), lipid profiles | Ezetimibe significantly reduced postprandial triglyceride levels and improved FMD, indicating better endothelial function. |
| 5 | Ciriacks, K. et al., 2015 [40] | Effects of simvastatin and ezetimibe in lowering low-density lipoprotein cholesterol in subjects with type 1 and type 2 diabetes mellitus | Primary: to compare LDL-C reduction with simvastatin vs. simvastatin plus ezetimibe. Secondary: to assess effects on other lipid parameters. | Patients with T1D and T2D | 40 | Simvastatin (40 mg/day) vs. simvastatin plus ezetimibe (10 mg/day) | LDL-C levels, other lipid parameters (HDL-C, triglycerides) | Simvastatin plus ezetimibe resulted in greater LDL-C reduction (p < 0.05) compared to simvastatin alone in both patients with T1D and T2D. |
| 6 | Semova, I. et al., 2019 [41] | Type 1 diabetes is associated with an increase in cholesterol absorption markers but a decrease in cholesterol synthesis markers in a young adult population | Primary: to assess cholesterol metabolism in T1D. Secondary: to compare absorption and synthesis markers between T1D and controls. | Young adults with T1D and healthy controls | 200 | Observational (no intervention) | Cholesterol absorption (campesterol, sitosterol) and synthesis (lathosterol) markers | Type 1 diabetes patients had higher cholesterol absorption markers and lower synthesis markers compared to controls, suggesting altered cholesterol metabolism. |
| SN | Author and Year | Title | Primary and Secondary Objectives | Population/Participants | Sample Size | Intervention/Exposure | Outcome Measures | Findings and Conclusions |
|---|---|---|---|---|---|---|---|---|
| 1 | Kang, Y.M. et al., 2025 [47] | Cardiovascular Outcomes and Efficacy of the PCSK9 Inhibitor Evolocumab in Individuals With Type 1 Diabetes: Insights From the FOURIER Trial | Primary: to assess the effect of evolocumab on cardiovascular outcomes in T1D. Secondary: to assess safety and LDL-C reduction. | Patients with T1D and high CV risk | 27,564 participants, of which 10,834 had T2D and 197 had T1D | Evolocumab vs. placebo | MACEs (CV death, myocardial infarction, stroke, hospitalization for unstable angina, or coronary revascularization), LDL-C levels, safety endpoints | Evolocumab reduced MACEs in patients with T1D by 34% (HR = 0.66, CI = 0.32–1.38) with significant LDL-C reduction, and a similar safety profile to the overall cohort. |
| 2 | Cariou, B. et al., 2017 [50] | Efficacy and safety of alirocumab in insulin-treated patients with type 1 or type 2 diabetes and high cardiovascular risk: Rationale and design of the ODYSSEY DM–INSULIN trial | Primary: to assess the efficacy of alirocumab in reducing LDL-C in insulin-treated diabetes patients. Secondary: to evaluate safety and other lipid parameters. | Insulin-treated patients with type 1 or type 2 diabetes with high CV risk | 517 (planned) | Alirocumab vs. placebo | LDL-C reduction, adverse events, other lipid parameters | Ongoing trial. |
| 3 | Levenson, A.E. et al., 2017 [42] | PCSK9 Is Increased in Youth With Type 1 Diabetes | Primary: to compare PCSK9 levels in youth with type 1 diabetes vs. controls. Secondary: to assess associations with glycemic control and lipids. | Youth with T1D and healthy controls | 70 | Observational (no intervention) | PCSK9 levels, HbA1c, lipid profiles | PCSK9 levels were significantly higher in youth with T1D (p < 0.01) and correlated with HbA1c, suggesting a link to glycemic control. |
| 4 | Bojanin, D. et al., 2019 [43] | Association between PCSK9 and lipoprotein subclasses in children with T1D: effects of glycemic control | Primary: to investigate PCSK9′s association with lipoprotein subclasses in children with T1D. Secondary: to evaluate the effects of glycemic control. | Children with T1D | 60 | Observational (no intervention) | PCSK9 levels, lipoprotein subclasses, HbA1c | Higher PCSK9 levels were associated with adverse lipoprotein profiles in T1D, with better glycemic control linked to lower PCSK9 levels. |
| 5 | O’Donoghue, M.L. et al., 2019 [46] | Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk insights from the FOURIER trial | Primary: to assess the effect of evolocumab on lipoprotein (a) and CV risk. Secondary: to evaluate outcomes in subgroups, including diabetes. | Patients with ASCVD (subgroup with diabetes) | 27,564 | Evolocumab vs. placebo | Lipoprotein (a) levels, CV events | Evolocumab reduced lipoprotein (a) by 26.9% and cardiovascular events, with consistent benefits in patients with diabetes. |
| 6 | Bittner, V.A. et al., 2020 [48] | Effect of Alirocumab on Lipoprotein(a) and Cardiovascular Risk After Acute Coronary Syndrome | Primary: to evaluate the effect of alirocumab on lipoprotein (a) and CV risk post-acute coronary syndrome Secondary: to assess outcomes in diabetes subgroups. | Patients with post-acute coronary syndrome, including those with diabetes | 18,924 | Alirocumab vs. placebo | Lipoprotein (a) levels, major adverse CV events | Alirocumab reduced lipoprotein (a) by 23% and CV events by 15% (HR 0.85, 95% CI 0.78–0.93), with benefits observed in patients with diabetes. |
| Adult Guidelines | ||||||
|---|---|---|---|---|---|---|
| Guideline | Organization | Age Group | Risk Classification | Statin Indication | LDL-C Target | Additional Therapy |
| 2025 ADA SOMC [19] | American Diabetes Association | 20–39 years | Same as T2D under 40 | If other CV risk factors are associated | Not specified | Ezetimibe and PCSK9 inhibitors if targets not reached |
| 40–75 years without ASCVD | Same as T2D | Moderate-high dose statins recommended | Not specified | |||
| High CV risk or established ASCVD | Same as T2D | High-dose statins recommended | <70 mg/dL or <55 mg/dL | |||
| 2019 ESC/EAS [54] | European Society of Cardiology/European Atherosclerosis Society | All adults | Moderately high or very high CV risk (depending on diabetes duration, complications, other CV risk factors) | Statins as initial therapy | Risk-stratified targets | Ezetimibe and PCSK9 inhibitors if targets not reached |
| 2018 ACC/AHA [20] | American College of Cardiology/American Heart Association | 20–39 years | Similar to the 2025 ADA | If T1D duration > 20 years, nephropathy markers, microvascular complications, or ABI < 0.9 | Same as ESC/EAS (strict targets) | Similar to other guidelines |
| >40 years | Similar to the 2025 ADA | Similar to the 2025 ADA | ||||
| Pediatric Guidelines | ||||||
|---|---|---|---|---|---|---|
| Guideline | Organization | Age Group | Screening | Statin Indication | LDL-C Target | Initial Strategy |
| 2025 ADA SOMC [19] | American Diabetes Association | Pediatric | Not specified | When LDL-C > 130 mg/dL | <100 mg/dL | Glycemic control and lipid-lowering nutritional therapy |
| 2019 ESC/EAS [54] | European Society of Cardiology/European Atherosclerosis Society | 10–11+ years | Not specified | If LDL-C > 130 mg/dL or other risk factors are present | Not specified | Statins from age 10 to 11 |
| 2018 ACC/AHA [20] | American College of Cardiology/American Heart Association | Pediatric | Similar to the 2025 ADA | Similar to the 2025 ADA | Similar to the 2025 ADA | Similar to the 2025 ADA |
| 2024 ISPAD [55] | International Society of Pediatric and Adolescent Diabetes | 11+ years (earlier if risk factors are associated) | Annual screening if values have changed | Children > 10 years with LDL-C > 130 mg/dL | <100 mg/dL | Screening starting at age 11 |
| 2019 AHA Scientific Statement [56] | American Heart Association | High-risk pediatric patients | Not specified | CV risk reduction focus | Not specified | Risk reduction strategies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elian, V.; Dorita, A.; Stegaru, D.; Vinereanu, D. Treatment of Dyslipidemia in Patients with Type 1 Diabetes Mellitus: A Review of Current Evidence and Knowledge Gaps. Int. J. Mol. Sci. 2025, 26, 8558. https://doi.org/10.3390/ijms26178558
Elian V, Dorita A, Stegaru D, Vinereanu D. Treatment of Dyslipidemia in Patients with Type 1 Diabetes Mellitus: A Review of Current Evidence and Knowledge Gaps. International Journal of Molecular Sciences. 2025; 26(17):8558. https://doi.org/10.3390/ijms26178558
Chicago/Turabian StyleElian, Viviana, Alina Dorita, Daniela Stegaru, and Dragos Vinereanu. 2025. "Treatment of Dyslipidemia in Patients with Type 1 Diabetes Mellitus: A Review of Current Evidence and Knowledge Gaps" International Journal of Molecular Sciences 26, no. 17: 8558. https://doi.org/10.3390/ijms26178558
APA StyleElian, V., Dorita, A., Stegaru, D., & Vinereanu, D. (2025). Treatment of Dyslipidemia in Patients with Type 1 Diabetes Mellitus: A Review of Current Evidence and Knowledge Gaps. International Journal of Molecular Sciences, 26(17), 8558. https://doi.org/10.3390/ijms26178558







