Myopia and Metabolomics: A Comparative Study of Aqueous Humor and Serum Metabolites in Myopic Adults Undergoing Cataract Surgery
Abstract
1. Introduction
2. Results
2.1. Patients’ Characteristics
2.2. Aqueous Humor
2.3. Serum
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Study Participants
4.2. Metabolomic Analysis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Diez, P.S.; Ohlendorf, A.; Barraza-Bernal, M.J.; Kratzer, T.; Wahl, S. Evaluating the impact of COVID-19 pandemic-related home confinement on the refractive error of school-aged children in Germany: A cross-sectional study based on data from 414 eye care professional centres. BMJ Open 2023, 13, e071833. [Google Scholar] [CrossRef]
- AlShamlan, F.T.; Bubshait, L.K.; A AlAhmad, E.; AlOtaibi, B.S.; A AlShakhs, A.; A AlHammad, F. Myopia progression in school children with prolonged screen time during the coronavirus disease confinement. Med. Hypothesis Discov. Innov. Ophthalmol. 2023, 12, 90–97. [Google Scholar] [CrossRef]
- Pršová, L.; Halička, J.; Kozár, M.; Kuderavá, Z.; Pršo, M.; Jakušová, Ľ.; Bánovčin, P.; Žiak, P. The Prevalence of Myopia in School-Age Children in Slovakia and the Covid-19 Pandemic. Czech Slovak Ophthalmol. 2023, 79, 186–190. [Google Scholar] [CrossRef]
- Rodriguez, N.M.O.; Acevedo, A.O.; Torres, V.O.P.; Romero, A.F.O. Refractive Error Changes Due to COVID-19 Pandemic Confinement in Children from Puerto Rico: A Retrospective Study. Optom. Vis. Sci. 2023, 100, 638–644. [Google Scholar] [CrossRef]
- Wallman, J.; Winawer, J. Homeostasis of Eye Growth and the Question of Myopia. Neuron 2004, 43, 447–468. [Google Scholar] [CrossRef]
- Jaddoe, V.W.V.; van Duijn, C.M.; Franco, O.H.; van der Heijden, A.J.; van Iizendoorn, M.H.; de Jongste, J.C.; van der Lugt, A.; Mackenbach, J.P.; Moll, H.A.; Raat, H.; et al. The Generation R Study: Design and cohort update 2012. Eur. J. Epidemiol. 2012, 27, 739–756. [Google Scholar] [CrossRef]
- Tideman, J.W.L.; Polling, J.R.; Vingerling, J.R.; Jaddoe, V.W.V.; Williams, C.; Guggenheim, J.A.; Klaver, C.C.W. Axial length growth and the risk of developing myopia in European children. Acta Ophthalmol. 2018, 96, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Jong, M.; Jonas, J.B.; Wolffsohn, J.S.; Berntsen, D.A.; Cho, P.; Clarkson-Townsend, D.; Flitcroft, D.I.; Gifford, K.L.; Haarman, A.E.G.; Pardue, M.T.; et al. IMI 2021 Yearly Digest. Investig. Opthalmol. Vis. Sci. 2021, 62, 7. [Google Scholar] [CrossRef]
- Lam, C.S.Y.; Tang, W.C.; Tse, D.Y.-Y.; Lee, R.P.K.; Chun, R.K.M.; Hasegawa, K.; Qi, H.; Hatanaka, T.; To, C.H. Defocus Incorporated Multiple Segments (DIMS) spectacle lenses slow myopia progression: A 2-year randomised clinical trial. Br. J. Ophthalmol. 2019, 104, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.; Tang, W.C.; Lee, P.H.; Zhang, H.Y.; Qi, H.; Hasegawa, K.; To, C.H. Myopia control effect of defocus incorporated multiple segments (DIMS) spectacle lens in Chinese children: Results of a 3-year follow-up study. Br. J. Ophthalmol. 2021, 106, 1110–1114. [Google Scholar] [CrossRef]
- Lanca, C.; Pan, C.-W.; Grzybowski, A. Will the new anti-myopia spectacles be the standard of care in future? Am. J. Ophthalmol. 2024, 263, xi–xiii. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-C.; Tsai, P.-C.; Lee, S.-H.; Wang, J.-H.; Chiu, C.-J. Systematic Review of Myopia Progression after Cessation of Optical Interventions for Myopia Control. J. Clin. Med. 2023, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-R.; Wang, J.-H.; Huang, H.-K.; Chen, T.-L.; Chen, P.-W.; Chiu, C.-J. Efficacy of atropine, orthokeratology, and combined atropine with orthokeratology for childhood myopia: A systematic review and network meta-analysis. J. Formos. Med. Assoc. 2022, 121, 2490–2500. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, N.; Rui, Y.; Xia, Y.; Xiong, S.; Xia, X. New insight of metabolomics in ocular diseases in the context of 3P medicine. EPMA J. 2023, 14, 53–71. [Google Scholar] [CrossRef]
- Dai, L.; Yang, W.; Qin, X.; Li, Y.; Cao, H.; Zhou, C.; Wang, Y. Serum metabolomics profiling and potential biomarkers of myopia using LC-QTOF/MS. Exp. Eye Res. 2019, 186, 107737. [Google Scholar] [CrossRef]
- Du, B.; Jin, N.; Zhu, X.; Lu, D.; Jin, C.; Li, Z.; Han, C.; Zhang, Y.; Lai, D.; Liu, K.; et al. A prospective study of serum metabolomic and lipidomic changes in myopic children and adolescents. Exp. Eye Res. 2020, 199, 108182. [Google Scholar] [CrossRef]
- Ke, C.; Xu, H.; Chen, Q.; Zhong, H.; Pan, C.-W. Serum metabolic signatures of high myopia among older Chinese adults. Eye 2020, 35, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Fang, J.; Jin, J.; Zhu, S.; Xu, X.; Xu, Y.; Ye, B.; Lin, S.-H.; Xu, X. Serum Metabolomics Reveals Personalized Metabolic Patterns for Macular Neovascular Disease Patient Stratification. J. Proteome Res. 2019, 19, 699–707. [Google Scholar] [CrossRef]
- Kearney, S.; O’DOnoghue, L.; Pourshahidi, L.K.; Cobice, D.; Saunders, K.J. Myopes have significantly higher serum melatonin concentrations than non-myopes. Ophthalmic Physiol. Opt. 2017, 37, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Barbas-Bernardos, C.; Armitage, E.G.; García, A.; Mérida, S.; Navea, A.; Bosch-Morell, F.; Barbas, C. Looking into aqueous humor through metabolomics spectacles—Exploring its metabolic characteristics in relation to myopia. J. Pharm. Biomed. Anal. 2016, 127, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Rao, J.; Rong, X.; Lou, S.; Zheng, Z.; Lu, Y. Metabolic characterization of human aqueous humor in relation to high myopia. Exp. Eye Res. 2017, 159, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Zhang, Z.; Cai, X.; Wu, X.; Huang, B.; Shen, Y.; Tong, J. Correlation analysis of aqueous humor metabolomics with myopic axial length and choroidal parameters. BMC Ophthalmol. 2023, 23, 356. [Google Scholar] [CrossRef]
- Tang, Y.-P.; Zhang, X.-B.; Hu, Z.-X.; Lin, K.; Lin, Z.; Chen, T.-Y.; Wu, R.-H.; Chi, Z.-L. Vitreous metabolomic signatures of pathological myopia with complications. Eye 2023, 37, 2987–2993. [Google Scholar] [CrossRef]
- Wu, W.; Song, Y.; Sun, M.; Li, Y.; Xu, Y.; Xu, M.; Yang, Y.; Li, S.; Zhang, F. Corneal metabolic biomarkers for moderate and high myopia in human. Exp. Eye Res. 2023, 237, 109689. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, L.; Chen, S.; Xie, C.; Tong, J.; Shen, Y. The potential role of amino acids in myopia: Inspiration from metabolomics. Metabolomics 2024, 21, 6. [Google Scholar] [CrossRef]
- Grochowski, E.T.; Pietrowska, K.; Kowalczyk, T.; Mariak, Z.; Kretowski, A.; Ciborowski, M.; Dmuchowska, D.A. Omics in Myopia. J. Clin. Med. 2020, 9, 3464. [Google Scholar] [CrossRef]
- Grochowski, E.T.; Pietrowska, K.; Godlewski, A.; Gosk, W.; Buczynska, A.; Wojnar, M.; Konopinska, J.; Kretowski, A.; Ciborowski, M.; Dmuchowska, D.A. Simultaneous Comparison of Aqueous Humor and Serum Metabolic Profiles of Diabetic and Nondiabetic Patients Undergoing Cataract Surgery—A Targeted and Quantitative Metabolomics Study. Int. J. Mol. Sci. 2023, 24, 12671. [Google Scholar] [CrossRef] [PubMed]
- Rinsky, B.; Beykin, G.; Grunin, M.; Amer, R.; Khateb, S.; Tiosano, L.; Almeida, D.; Hagbi-Levi, S.; Elbaz-Hayoun, S.; Chowers, I. Analysis of the Aqueous Humor Proteome in Patients With Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2021, 62, 18. [Google Scholar] [CrossRef] [PubMed]
- Midena, E.; Frizziero, L.; Midena, G.; Pilotto, E. Intraocular fluid biomarkers (liquid biopsy) in human diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 3549–3560. [Google Scholar] [CrossRef] [PubMed]
- Kunikata, H.; Ida, T.; Sato, K.; Aizawa, N.; Sawa, T.; Tawarayama, H.; Murayama, N.; Fujii, S.; Akaike, T.; Nakazawa, T. Metabolomic profiling of reactive persulfides and polysulfides in the aqueous and vitreous humors. Sci. Rep. 2017, 7, srep41984. [Google Scholar] [CrossRef]
- Agrawal, R.; Balne, P.K.; Wei, X.; Bijin, V.A.; Lee, B.; Ghosh, A.; Narayanan, R.; Agrawal, M.; Connolly, J. Cytokine Profiling in Patients With Exudative Age-Related Macular Degeneration and Polypoidal Choroidal Vasculopathy. Investig. Opthalmol. Vis. Sci. 2019, 60, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Oguchi, Y.; Omori, T.; Shintake, H.; Tomita, R.; Kasai, A.; Ogasawara, M.; Sugano, Y.; Itagaki, K.; Ojima, A.; et al. Complement Activation Products and Cytokines in Pachychoroid Neovasculopathy and Neovascular Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2020, 61, 39. [Google Scholar] [CrossRef]
- Noma, H.; Funatsu, H.; Yamasaki, M.; Tsukamoto, H.; Mimura, T.; Sone, T.; Hirayama, T.; Tamura, H.; Yamashita, H.; Minamoto, A.; et al. Aqueous humour levels of cytokines are correlated to vitreous levels and severity of macular oedema in branch retinal vein occlusion. Eye 2006, 22, 42–48. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Hsu, C.-N. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins 2017, 9, 92. [Google Scholar] [CrossRef]
- Chang, K.; Gao, D.; Yan, J.; Lin, L.; Cui, T.; Lu, S. Critical Roles of Protein Arginine Methylation in the Central Nervous System. Mol. Neurobiol. 2023, 60, 6060–6091. [Google Scholar] [CrossRef] [PubMed]
- Oliva-Damaso, E.; Oliva-Damaso, N.; Rodriguez-Esparragon, F.; Payan, J.; Baamonde-Laborda, E.; Gonzalez-Cabrera, F.; Santana-Estupiñan, R.; Rodriguez-Perez, J.C. Asymmetric (ADMA) and Symmetric (SDMA) Dimethylarginines in Chronic Kidney Disease: A Clinical Approach. Int. J. Mol. Sci. 2019, 20, 3668. [Google Scholar] [CrossRef]
- Firat, P.G.; Demirel, E.E.; Demirel, S.; Dikci, S.; Turkoz, Y.; Ozyalın, F. Increased Aqueous Humor Symmetric Dimethylarginine Level in Patients with Primary Open Angle Glaucoma. Curr. Eye Res. 2019, 44, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Obayashi, K.; Miyata, K.; Saeki, K.; Ogata, N. Association Between the Asymmetric Dimethylarginine Levels and Glaucoma Severity: A Cross-Sectional Analysis of the LIGHT Study. Investig. Opthalmol. Vis. Sci. 2021, 62, 7. [Google Scholar] [CrossRef]
- Imaki, H.; Jacobson, S.G.; Kemp, C.M.; Knighton, R.W.; Neuringer, M.; Sturman, J. Retinal morphology and visual pigment levels in 6-and 12-month-old rhesus monkeys fed a taurine-free human infant formula. J. Neurosci. Res. 1993, 36, 290–304. [Google Scholar] [CrossRef]
- Chesney, R.W.; Helms, R.A.; Christensen, M.; Budreau, A.M.; Han, X.; Sturman, J.A. The role of taurine in infant nutrition. In Taurine 3. Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1998; Volume 442, pp. 463–476. [Google Scholar] [CrossRef]
- Verner, A.M.; McGuire, W.; Craig, J.S. Effect of taurine supplementation on growth and development in preterm or low birth weight infants. Cochrane Database Syst. Rev. 2007, 2010, CD006072. [Google Scholar] [CrossRef]
- Froger, N.; Sahel, J.A.; Picaud, S. Chapter 51—Taurine Deficiency and the Eye. In Handbook of Nutrition, Diet and the Eye; Academic Press: Cambridge, MA, USA, 2014; pp. 505–513. [Google Scholar] [CrossRef]
- Francisco, B.-M.; Salvador, M.; Amparo, N. Oxidative stress in Myopia. Oxidative Med. Cell. Longev. 2015, 2015, 750637. [Google Scholar] [CrossRef]
- Heinämäki, A.A.; Muhonen, A.S.H.; Piha, R.S. Taurine and other free amino acids in the retina, vitreous, lens, irisciliary body, and cornea of the rat eye. Neurochem. Res. 1986, 11, 535–542. [Google Scholar] [CrossRef]
- El-Sherbeny, A.; Naggar, H.; Miyauchi, S.; Ola, M.S.; Maddox, D.M.; Martin, P.M.; Ganapathy, V.; Smith, S.B. Osmoregulation of taurine transporter function and expression in retinal pigment epithelial, ganglion, and müller cells. Investig. Opthalmol. Vis. Sci. 2004, 45, 694–701. [Google Scholar] [CrossRef][Green Version]
- Warskulat, U.; Heller-Stilb, B.; Oermann, E.; Zilles, K.; Haas, H.; Lang, F.; Häussinger, D. Phenotype of the taurine transporter knockout mouse. Methods Enzymol. 2007, 428, 439–458. [Google Scholar] [CrossRef]
- Heller-Stilb, B.; Roeyen, C.; Rascher, K.; Hartwig, H.-G.; Huth, A.; Seeliger, M.W.; Warskulat, U.; Häussinger, D. Disruption of the taurine transporter gene (taut) leads to retinal degeneration in mice. FASEB J. 2001, 16, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Pasantes-Morales, H.; Domínguez, L.; Campomanes, M.A.; Pacheco, P. Retinal degeneration induced by taurine deficiency in light-deprived cats. Exp. Eye Res. 1986, 43, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Li, F.; Gu, C.; Xie, L.; Yan, W.; Wang, X.; Shi, R.; Linghu, S.; Liu, T. Metabolomic profiling of ocular tissues in rabbit myopia: Uncovering differential metabolites and pathways. Exp. Eye Res. 2024, 240, 109796. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Gollapalli, K.; Mangiola, S.; Schranner, D.; Yusuf, M.A.; Chamoli, M.; Shi, S.L.; Bastos, B.L.; Nair, T.; Riermeier, A.; et al. Taurine deficiency as a driver of aging. Science 2023, 380, eabn9257. [Google Scholar] [CrossRef]
- Ansar, M.; Ranza, E.; Shetty, M.; A Paracha, S.; Azam, M.; Kern, I.; Iwaszkiewicz, J.; Farooq, O.; Pournaras, C.J.; Malcles, A.; et al. Taurine treatment of retinal degeneration and cardiomyopathy in a consanguineous family with SLC6A6 taurine transporter deficiency. Hum. Mol. Genet. 2019, 29, 618–623. [Google Scholar] [CrossRef]
- Pietrowska, K.; Dmuchowska, D.A.; Godlewski, A.; Grochowski, E.T.; Wojnar, M.; Gosk, W.; Konopinska, J.; Kretowski, A.; Ciborowski, M. Extent of interocular (a)symmetry based on the metabolomic profile of human aqueous humor. Front. Mol. Biosci. 2023, 10, 1166182. [Google Scholar] [CrossRef] [PubMed]
- Fecke, A.; Saw, N.M.M.T.; Kale, D.; Kasarla, S.S.; Sickmann, A.; Phapale, P. Quantitative Analytical and Computational Workflow for Large-Scale Targeted Plasma Metabolomics. Metabolites 2023, 13, 844. [Google Scholar] [CrossRef] [PubMed]
- Burdukiewicz, M.; Chilimoniuk, J.; Grzesiak, K.; Krętowski, A.; Ciborowski, M. ML-based clinical decision support models based on metabolomics data. TrAC Trends Anal. Chem. 2024, 178, 117819. [Google Scholar] [CrossRef]
- Pietrowska, K.; Godlewski, A.; Grochowski, E.; Gosk, W.; Konopinska, J.; Kretowski, A.; Ciborowski, M.; Dmuchowska, D. Adaptation of the AbsoluteIDQ p180 kit to the analysis of metabolites in the human aqueous humor. J. Chromatogr. B 2023, 1229, 123880. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Myopia | Control | p-Value |
|---|---|---|---|
| Number of patients | 52 | 41 | |
| Sex, female, n (%) | 28 (54) | 27 (66) | 0.16 |
| Age, mean ± SD (years) | 68.14 ± 10.36 | 70.46 ± 8.52 | 0.25 |
| BMI, mean ± SD | 29.81 ± 5.82 | 28.08 ± 4.57 | 0.12 |
| AL, median [Q1;Q3] (mm) | 24.85 [24.3; 26.25] | 22.9 [22.5; 23.4] | <0.0001 |
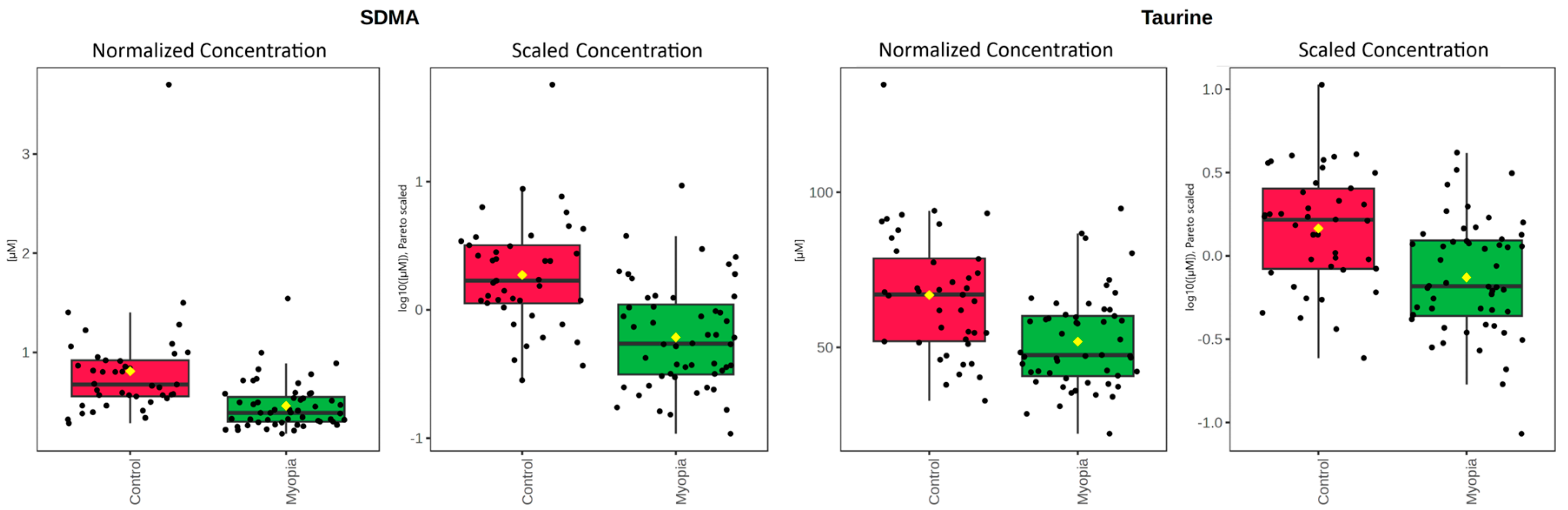
| Myopia (µM) [SD] | Control (µM) [SD] | p-Value | FDR | |
|---|---|---|---|---|
| SDMA | 0.462 [0.25] | 0.810 [0.55] | <0.0001 | <0.0001 |
| Taurine | 51.865 [15.4] | 66.872 [20.25] | <0.0001 | 0.002 |
| p-Value | Adjusted p-Value | B | |
|---|---|---|---|
| SDMA | <0.0001 | <0.0001 | 6.8453 |
| Taurine | <0.0001 | 0.002 | 0.85187 |
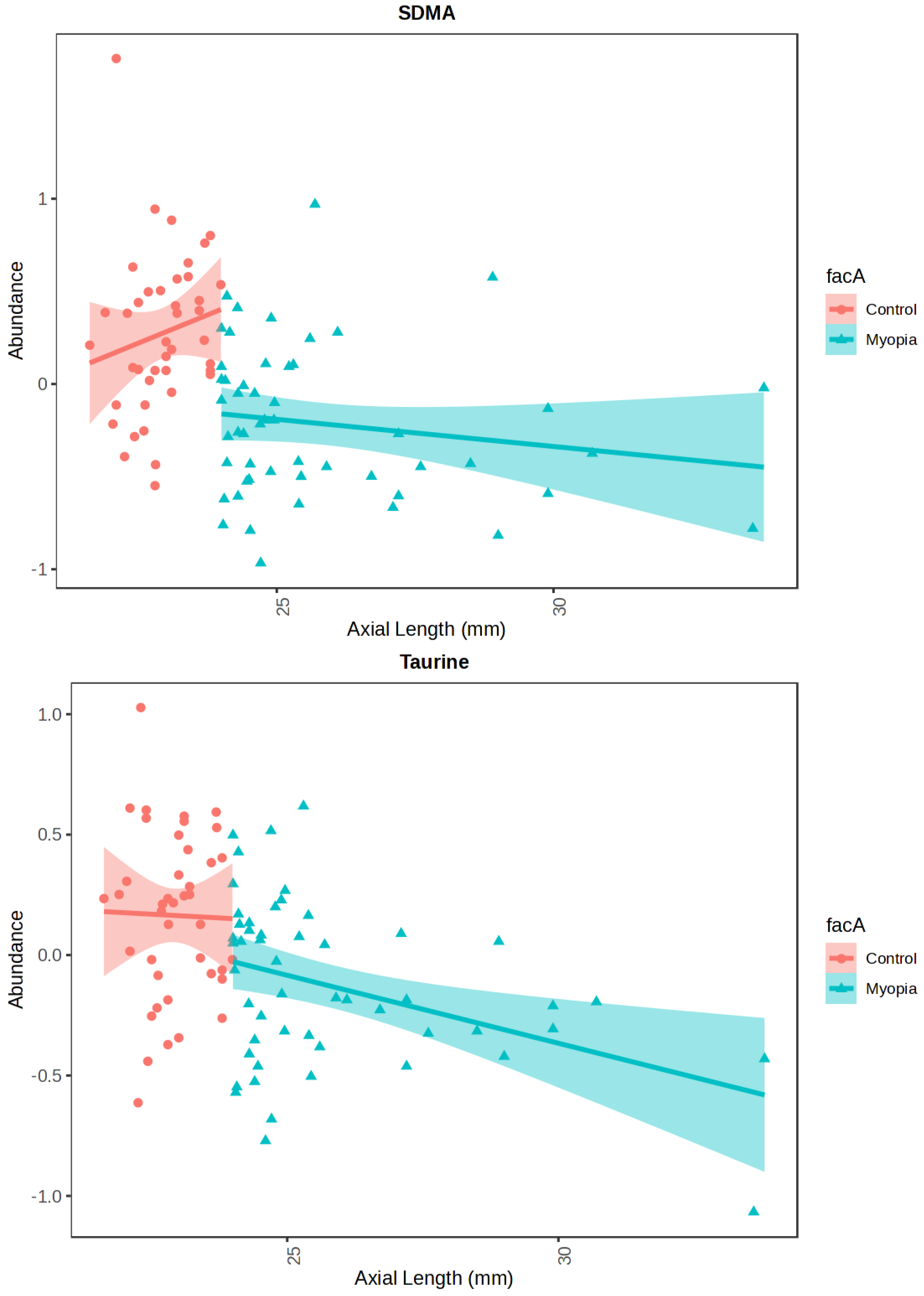
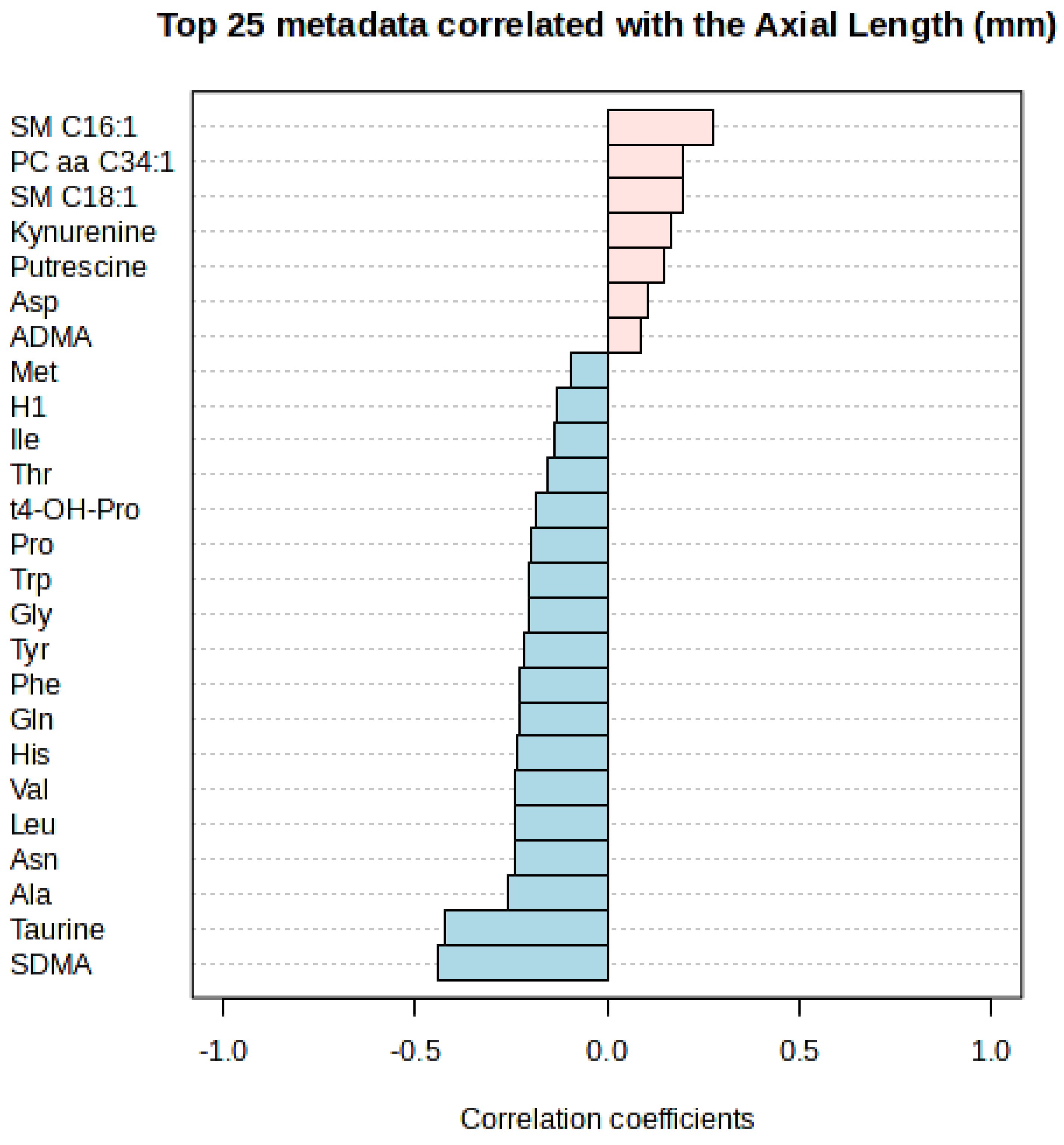
| Correlation | t-Stat | p-Value | FDR | |
|---|---|---|---|---|
| SDMA | −0.4401 | 193,040.0 | <0.0001 | <0.0001 |
| Taurine | −0.4212 | 190,500.0 | <0.0001 | <0.0001 |
| Correlation | p-Value | |
|---|---|---|
| Myopia | −0.1796 | 0.202662 |
| Control | 0.008671 | 0.95709 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grochowski, E.T.; Godlewski, A.; Pietrowska, K.; Gosk, W.; Wojnar, M.; Konopinska, J.; Kretowski, A.; Ciborowski, M.; Dmuchowska, D.A. Myopia and Metabolomics: A Comparative Study of Aqueous Humor and Serum Metabolites in Myopic Adults Undergoing Cataract Surgery. Int. J. Mol. Sci. 2025, 26, 8557. https://doi.org/10.3390/ijms26178557
Grochowski ET, Godlewski A, Pietrowska K, Gosk W, Wojnar M, Konopinska J, Kretowski A, Ciborowski M, Dmuchowska DA. Myopia and Metabolomics: A Comparative Study of Aqueous Humor and Serum Metabolites in Myopic Adults Undergoing Cataract Surgery. International Journal of Molecular Sciences. 2025; 26(17):8557. https://doi.org/10.3390/ijms26178557
Chicago/Turabian StyleGrochowski, Emil Tomasz, Adrian Godlewski, Karolina Pietrowska, Wioleta Gosk, Malgorzata Wojnar, Joanna Konopinska, Adam Kretowski, Michal Ciborowski, and Diana Anna Dmuchowska. 2025. "Myopia and Metabolomics: A Comparative Study of Aqueous Humor and Serum Metabolites in Myopic Adults Undergoing Cataract Surgery" International Journal of Molecular Sciences 26, no. 17: 8557. https://doi.org/10.3390/ijms26178557
APA StyleGrochowski, E. T., Godlewski, A., Pietrowska, K., Gosk, W., Wojnar, M., Konopinska, J., Kretowski, A., Ciborowski, M., & Dmuchowska, D. A. (2025). Myopia and Metabolomics: A Comparative Study of Aqueous Humor and Serum Metabolites in Myopic Adults Undergoing Cataract Surgery. International Journal of Molecular Sciences, 26(17), 8557. https://doi.org/10.3390/ijms26178557








