Translating Biomarker Discovery: From Bench to Bedside in Dry Eye Disease
Abstract
1. Introduction
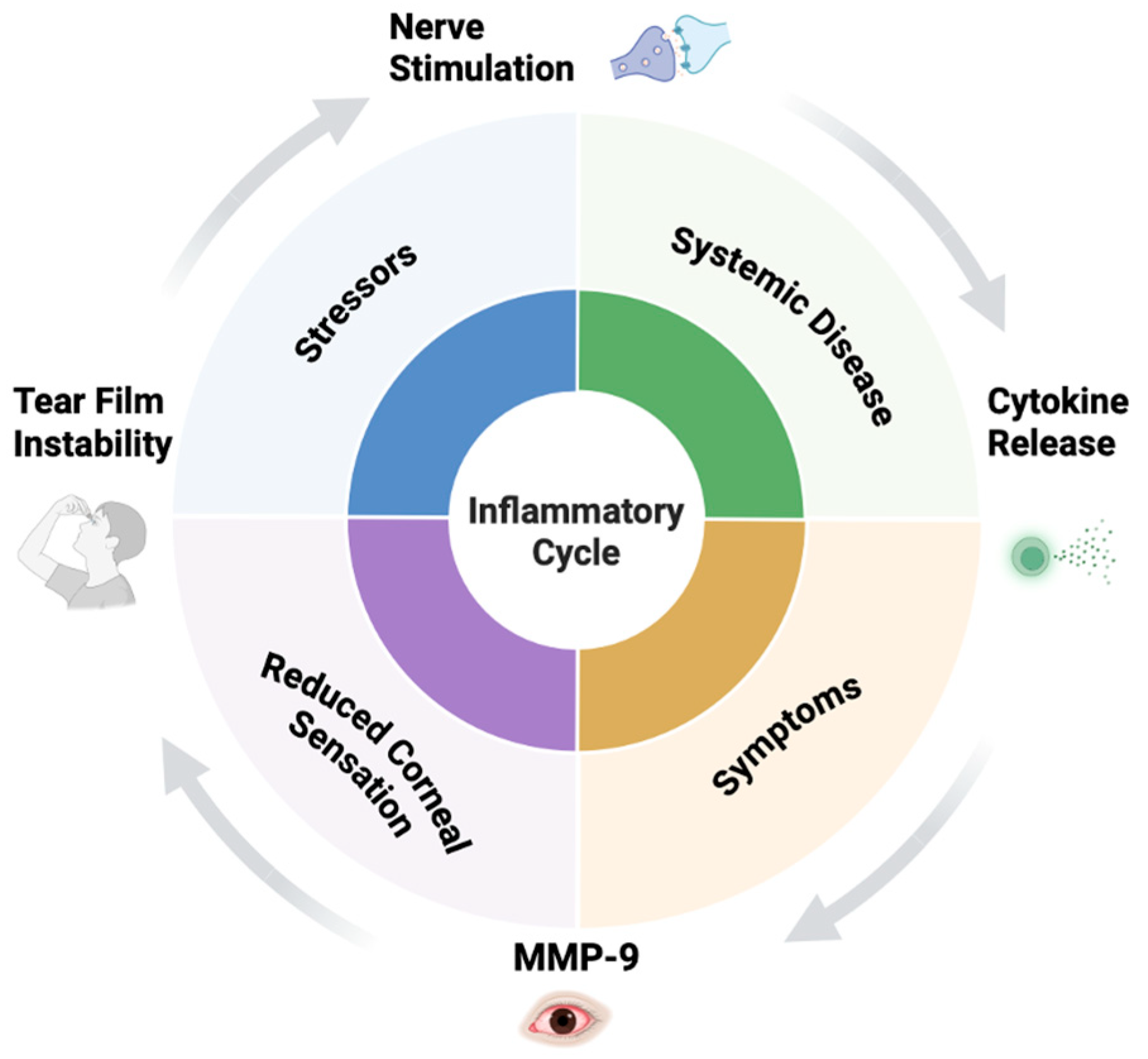
2. Pathophysiology of DED
3. Molecular Biomarkers of DED
3.1. Inflammatory Markers
3.1.1. Matrix Metalloproteinases and MMP-9
3.1.2. Cytokines and Chemokines
| Biomarker | Role | Clinical Relevance | Reference |
|---|---|---|---|
| 1. Protein Biomarkers | |||
| MMP-9 | Degrades extracellular matrix; upregulated during inflammation | Elevated in DED; used in point-of-care test (InflammaDry); marker of ocular surface inflammation | [23,24,25,26,27,29,30,31] |
| Lactoferrin | Iron-binding glycoprotein with antimicrobial and anti-inflammatory properties | Decreased in aqueous-deficient DED; indicates lacrimal gland dysfunction | [39,40,41,42] |
| Lacritin | Tear glycoprotein that promotes epithelial cell survival, autophagy, and secretion | Deficient in aqueous-deficient DED; shown to restore tear secretion and corneal integrity in preclinical models | [43,44,45,46,47] |
| Lysozyme | Antimicrobial enzyme secreted by lacrimal glands | Reduced levels suggest impaired tear secretion | [48,49,50,51,52] |
| Lipocalin-1 | Stabilizes the tear film lipid layer | Altered levels associated with tear film instability | [43,53,54,55,56] |
| MUC5AC | Secreted gel-forming mucin from conjunctival goblet cells | Decreased in DED, especially in mucin-deficient or Sjögren’s syndrome cases | [57,58,59,60,61,62,63,64,65,66,67] |
| HLA-DR | Major histocompatibility complex class II molecule | Upregulated in conjunctival epithelial cells; marker of immune activation | [68,69,70,71,72] |
| 2. Cytokines and Chemokines | |||
| IL-1β, IL-6, TNF-α | Pro-inflammatory cytokines | Elevated levels in tears of DED patients; drive ocular surface inflammation | [22,73,74,75,76,77,78,79,80,81] |
| IL-8 (CXCL8) | Neutrophil chemoattractant | Reflects active inflammation and epithelial damage | [5,17,75,82,83,84] |
| IFN-γ | Activates immune response, especially Th1-mediated | Linked to goblet cell loss and mucin downregulation | [17,75,82,84,85] |
| CCL5 (RANTES) | Recruits T cells | Found in increased levels in tears and conjunctiva of DED patients | [5,86,87] |
| 3. Lipid Biomarkers | |||
| Meibum Lipids (e.g., wax esters, cholesterol esters) | Maintain tear film stability and reduce evaporation | Altered composition in Meibomian Gland Dysfunction (MGD) contributes to evaporative DED | [88,89,90,91,92,93,94] |
| Phospholipids, sphingolipids | Inflammatory signaling molecules | Lipidomics has revealed dysregulated lipid profiles in DED associated with inflammation | [95,96,97,98,99,100,101,102] |
| 4. Metabolites and Small Molecules | |||
| Lactate, Urea | Indicators of metabolic stress | Elevated levels found in tear fluid of DED patients | [103,104,105] |
| Glutamate, Glutamine | Linked to oxidative stress and inflammation | Altered profiles can distinguish DED subtypes | [106,107,108,109] |
| Reactive oxygen species (ROS) | Oxidative stress marker | Associated with cellular damage in DED pathogenesis | [110,111,112,113] |
| 5. Nucleic Acid Biomarkers (Genomic/Epigenomic) | |||
| MicroRNAs (e.g., miR-146a, miR-155) | Post-transcriptional gene regulation of inflammation | Dysregulated in tears and conjunctiva; potential non-invasive biomarkers for DED diagnosis and subtype stratification | [114,115,116,117,118,119] |
| HLA gene polymorphisms | Immune response genes | Certain variants associated with Sjögren’s syndrome and autoimmune-related DED | [120,121,122,123] |
| 6. Functional and Imaging Biomarkers | |||
| Tear Osmolarity | Measures tear solute concentration | Elevated (>308 mOsm/L) in DED; reproducible marker for severity | [124,125,126,127,128,129] |
| Corneal Sensitivity | Assesses corneal nerve function and ocular surface integrity | Reduced in DED; associated with neurosensory abnormalities and disease severity | [130,131,132,133] |
| Tear Break-Up Time (TBUT/NITBUT) | Measures tear film stability | Decreased in DED, especially in evaporative forms | [134,135,136,137,138] |
| Meibography | Visualizes meibomian gland structure | Gland dropout seen in MGD-related DED | [139,140,141,142] |
| In vivo confocal microscopy (IVCM) | Assesses corneal nerves and immune cells | Reveals corneal nerve loss or dendritic cell activation in DED | [143,144,145,146,147,148] |
3.2. Lacrimal Gland Protein Markers
3.2.1. Lactoferrin
3.2.2. Lysosome
3.3. Lipids
3.3.1. Omega-6 and Omega-3 Fatty Acids
3.3.2. O-acyl-ω-hydroxy Fatty Acids (OAHFAs)
3.3.3. Diesters (DiEs)
3.4. MicroRNAs (miRNAs)
4. From Biomarkers to Clinical Diagnosis
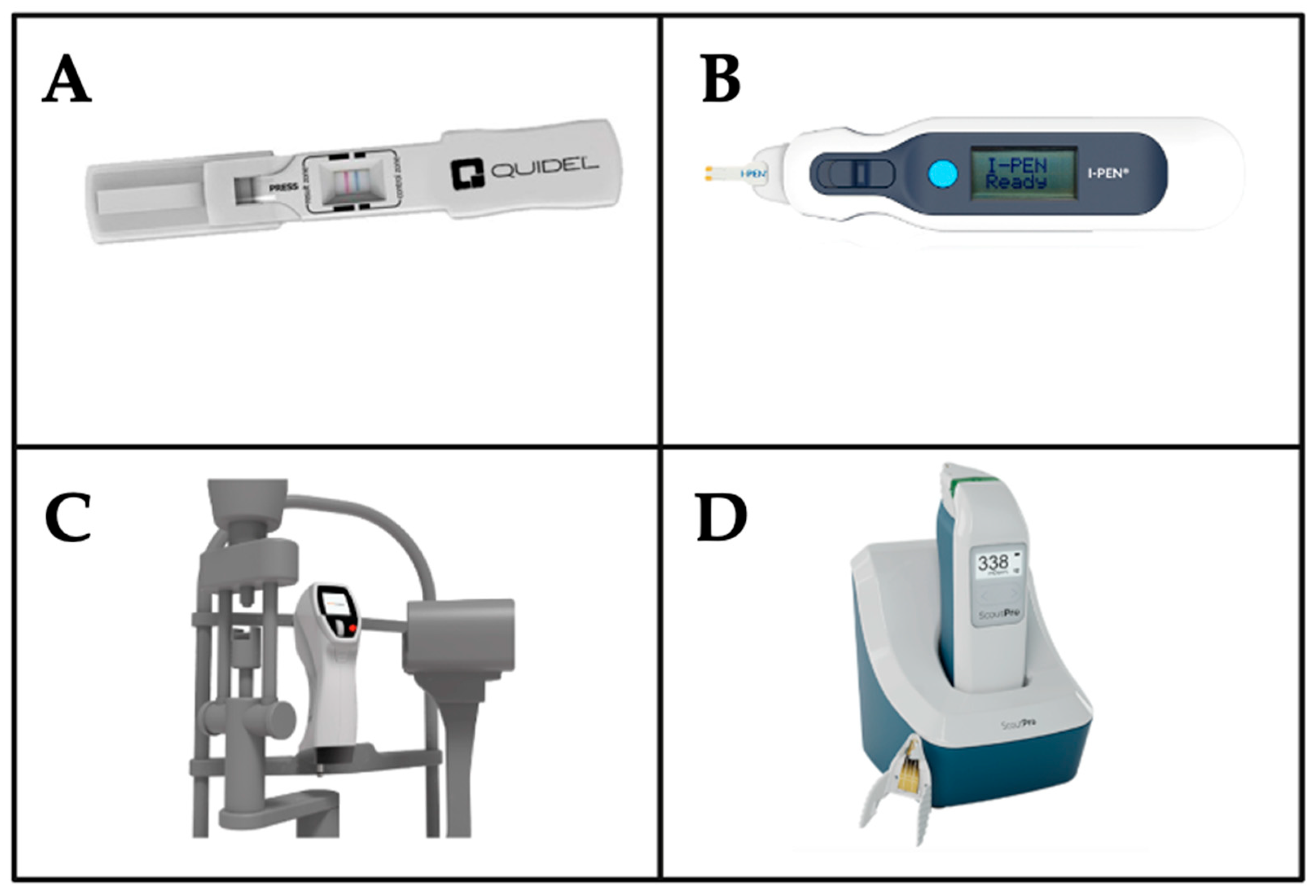
4.1. Tools for MMP-9 Measurement
4.2. Tools for Tear Osmolarity Measurement
| Device | Primary Function | Biomarkers/Parameters Measured | Role in DED Diagnosis |
|---|---|---|---|
| InflammaDry | Immunoassay for inflammation detection | MMP-9 | Detects elevated MMP-9 levels (>40 ng/mL). High sensitivity and specificity for rapid, in-clinic diagnosis [152]. |
| I-Pen | Tear osmolarity system | Tear osmolarity | Measures osmolarity using electrical impedance of the tear fluid of the palpebral conjunctiva [127,128] |
| Brill | Esthesiometry | Corneal sensitivity | Quantifies corneal sensitivity to aid in early detection of corneal dysesthesia and monitoring of treatment efficacy [129]. |
| ScoutPro | Tear osmolarity system | Tear osmolarity | Measures osmolarity using microfluidics to collect a tiny tear sample for measurement of electrical impedance of the tear fluid, which is used to calculate the osmolarity result with accuracy [162,163] |
| Corneal Topography | Maps corneal surface to detect irregularities | Corneal surface irregularities, tear film instability | Identifies corneal changes due to tear film instability, enhancing diagnostic precision for DED related ocular surface damage |
| Anterior Segment OCT | High-resolution imaging of anterior chamber structures | Tear film thickness, corneal epithelium, meibomian gland structure | Visualizes alterations in tear film and glands, correlating with DED severity and aiding in diagnosis |
| KOWA DR-1a Interferometer | Analyzes tear film lipid layer dynamics | Lipid layer thickness, tear film stability | Assesses evaporative DED by evaluating lipid layer dynamics, providing insights into tear film instability [164]. |
4.3. Corneal Esthesiometry
4.4. Other Imaging Tools
4.5. Unmet Needs
5. Current Treatment Methods and Limitations
6. Future Directions
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; de Paiva, C.S. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology 2017, 124, S4–S13. [Google Scholar] [CrossRef]
- Messmer, E.M. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch. Arztebl. Int. 2015, 112, 71–81; quiz 82. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.K.; Mohan, R.; Gokhale, N.; Matalia, H.; Mehta, P. Inflammation and dry eye disease-where are we? Int. J. Ophthalmol. 2022, 15, 820–827. [Google Scholar] [CrossRef]
- Stevenson, W.; Chauhan, S.K.; Dana, R. Dry eye disease: An immune-mediated ocular surface disorder. Arch. Ophthalmol. 2012, 130, 90–100. [Google Scholar] [CrossRef]
- Baudouin, C.; Messmer, E.M.; Aragona, P.; Geerling, G.; Akova, Y.A.; Benitez-del-Castillo, J.; Boboridis, K.G.; Merayo-Lloves, J.; Rolando, M.; Labetoulle, M. Revisiting the vicious circle of dry eye disease: A focus on the pathophysiology of meibomian gland dysfunction. Br. J. Ophthalmol. 2016, 100, 300–306. [Google Scholar] [CrossRef]
- Messmer, E.M. Pathophysiology of dry eye disease and novel therapeutic targets. Exp. Eye Res. 2022, 217, 108944. [Google Scholar] [CrossRef]
- Savini, G.; Prabhawasat, P.; Kojima, T.; Grueterich, M.; Espana, E.; Goto, E. The challenge of dry eye diagnosis. Clin. Ophthalmol. 2008, 2, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.S.; Wei, Y.; Kuklinski, E.; Asbell, P.A. The Growing Need for Validated Biomarkers and Endpoints for Dry Eye Clinical Research. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO1–BIO19. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B. Challenges in using signs and symptoms to evaluate new biomarkers of dry eye disease. Ocul. Surf. 2014, 12, 2–9. [Google Scholar] [CrossRef]
- Choi, W.; Lian, C.; Ying, L.; Kim, G.E.; You, I.C.; Park, S.H.; Yoon, K.C. Expression of Lipid Peroxidation Markers in the Tear Film and Ocular Surface of Patients with Non-Sjogren Syndrome: Potential Biomarkers for Dry Eye Disease. Curr. Eye Res. 2016, 41, 1143–1149. [Google Scholar] [CrossRef]
- Pinto-Fraga, J.; Enriquez-de-Salamanca, A.; Calonge, M.; Gonzalez-Garcia, M.J.; Lopez-Miguel, A.; Lopez-de la Rosa, A.; Garcia-Vazquez, C.; Calder, V.; Stern, M.E.; Fernandez, I. Severity, therapeutic, and activity tear biomarkers in dry eye disease: An analysis from a phase III clinical trial. Ocul. Surf. 2018, 16, 368–376. [Google Scholar] [CrossRef]
- Fong, P.Y.; Shih, K.C.; Lam, P.Y.; Chan, T.C.Y.; Jhanji, V.; Tong, L. Role of tear film biomarkers in the diagnosis and management of dry eye disease. Taiwan J. Ophthalmol. 2019, 9, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Bai, Y.; Ngo, W.; Nichols, K.K.; Wilson, L.; Barnes, S.; Nichols, J.J. Human Meibum and Tear Film Derived (O-Acyl)-Omega-Hydroxy Fatty Acids as Biomarkers of Tear Film Dynamics in Meibomian Gland Dysfunction and Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2021, 62, 13. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Cortes, T.; Merino-Inda, N.; Benitez-Del-Castillo, J.M. Tear and ocular surface disease biomarkers: A diagnostic and clinical perspective for ocular allergies and dry eye disease. Exp. Eye Res. 2022, 221, 109121. [Google Scholar] [CrossRef]
- Byambajav, M.; Collier, A.; Shu, X.; Hagan, S. Tear Fluid Biomarkers and Quality of Life in People with Type 2 Diabetes and Dry Eye Disease. Metabolites 2023, 13, 733. [Google Scholar] [CrossRef]
- Kumar, N.R.; Praveen, M.; Narasimhan, R.; Khamar, P.; D’Souza, S.; Sinha-Roy, A.; Sethu, S.; Shetty, R.; Ghosh, A. Tear biomarkers in dry eye disease: Progress in the last decade. Indian J. Ophthalmol. 2023, 71, 1190–1202. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Duan, H.; Yang, T.; Zhou, Y.; Ma, B.; Chen, Y.; Qi, H. Clinical Characteristics and Tear Film Biomarkers in Patients with Chronic Dry Eye Disease After Femtosecond Laser-Assisted Laser in Situ Keratomileusis. J. Refract. Surg. 2023, 39, 556–563. [Google Scholar] [CrossRef]
- Liu, R.; Gao, C.; Chen, H.; Li, Y.; Jin, Y.; Qi, H. Analysis of Th17-associated cytokines and clinical correlations in patients with dry eye disease. PLoS ONE 2017, 12, e0173301. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, D.; Xu, B.; Kumar, M. Biomarkers in immunology: Their impact on immune function and response. Adv. Biomark. Sci. Technol. 2025, 7, 95–110. [Google Scholar] [CrossRef]
- Chu, L.; Wang, C.; Zhou, H. Inflammation mechanism and anti-inflammatory therapy of dry eye. Front. Med. 2024, 11, 1307682. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.L.; Stern, M.E.; Pflugfelder, S.C. Inflammatory basis for dry eye disease flares. Exp. Eye Res. 2020, 201, 108294. [Google Scholar] [CrossRef]
- Gupta, S.; Shankar, S.; Bhatta, S.; Mishra, A.; Singh, A. Comparison of tear Matrix Metalloproteinase 9 (MMP-9) estimation with Schirmer’s test in Ocular Surface Disorders. Rom. J. Ophthalmol. 2024, 68, 225–232. [Google Scholar] [CrossRef]
- Lanza, N.L.; Valenzuela, F.; Perez, V.L.; Galor, A. The Matrix Metalloproteinase 9 Point-of-Care Test in Dry Eye. Ocul. Surf. 2016, 14, 189–195. [Google Scholar] [CrossRef]
- Kang, M.J.; Kim, H.S.; Kim, M.S.; Kim, E.C. The Correlation between Matrix Metalloproteinase-9 Point-of-Care Immunoassay, Tear Film Osmolarity, and Ocular Surface Parameters. J. Ophthalmol. 2022, 2022, 6132016. [Google Scholar] [CrossRef]
- Messmer, E.M.; von Lindenfels, V.; Garbe, A.; Kampik, A. Matrix Metalloproteinase 9 Testing in Dry Eye Disease Using a Commercially Available Point-of-Care Immunoassay. Ophthalmology 2016, 123, 2300–2308. [Google Scholar] [CrossRef]
- Shoari, A.; Kanavi, M.R.; Rasaee, M.J. Inhibition of matrix metalloproteinase-9 for the treatment of dry eye syndrome; a review study. Exp. Eye Res. 2021, 205, 108523. [Google Scholar] [CrossRef]
- Chotikavanich, S.; de Paiva, C.S.; Li, D.Q.; Chen, J.J.; Bian, F.; Farley, W.J.; Pflugfelder, S.C. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3203–3209. [Google Scholar] [CrossRef] [PubMed]
- Jamerson, E.C.; Elhusseiny, A.M.; ElSheikh, R.H.; Eleiwa, T.K.; El Sayed, Y.M. Role of Matrix Metalloproteinase 9 in Ocular Surface Disorders. Eye Contact Lens 2020, 46 (Suppl. 2), S57–S63. [Google Scholar] [CrossRef] [PubMed]
- Aragona, P.; Aguennouz, M.; Rania, L.; Postorino, E.; Sommario, M.S.; Roszkowska, A.M.; De Pasquale, M.G.; Pisani, A.; Puzzolo, D. Matrix metalloproteinase 9 and transglutaminase 2 expression at the ocular surface in patients with different forms of dry eye disease. Ophthalmology 2015, 122, 62–71. [Google Scholar] [CrossRef]
- Caban, M.; Owczarek, K.; Lewandowska, U. The Role of Metalloproteinases and Their Tissue Inhibitors on Ocular Diseases: Focusing on Potential Mechanisms. Int. J. Mol. Sci. 2022, 23, 4256. [Google Scholar] [CrossRef]
- Solomon, A.; Dursun, D.; Liu, Z.; Xie, Y.; Macri, A.; Pflugfelder, S.C. Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2283–2292. [Google Scholar]
- Na, K.S.; Mok, J.W.; Kim, J.Y.; Rho, C.R.; Joo, C.K. Correlations between tear cytokines, chemokines, and soluble receptors and clinical severity of dry eye disease. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5443–5450. [Google Scholar] [CrossRef]
- Zhao, C.S.; Chen, Y.; Ying, G.S.; Asbell, P.A.; Dry Eye, A.; Management Study, G. Association of Tear Cytokine Ratios with Symptoms and Signs of Dry Eye Disease: Biomarker Data from the Dry Eye Assessment and Management Study. Curr. Eye Res. 2024, 49, 16–24. [Google Scholar] [CrossRef]
- Shahini, A.; Shahini, A. Role of interleukin-6-mediated inflammation in the pathogenesis of inflammatory bowel disease: Focus on the available therapeutic approaches and gut microbiome. J. Cell Commun. Signal 2023, 17, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.C.; Jeong, I.Y.; Park, Y.G.; Yang, S.Y. Interleukin-6 and tumor necrosis factor-alpha levels in tears of patients with dry eye syndrome. Cornea 2007, 26, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Dermer, H.; Theotoka, D.; Lee, C.J.; Chhadva, P.; Hackam, A.S.; Galor, A.; Kumar, N. Total Tear IgE Levels Correlate with Allergenic and Irritating Environmental Exposures in Individuals with Dry Eye. J. Clin. Med. 2019, 8, 1627. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Tian, L.; Meng, Y.; Wu, B.; Wang, J.; He, J.; Shao, Q.; Wang, C.; Jie, Y.; Zhang, L. Total IgE in tears accurately reflects the severity and predicts the prognosis of seasonal allergic conjunctivitis. Clin. Transl. Allergy 2022, 12, e12139. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Hong, C.; Hsu, M.Y.; Lai, T.T.; Huang, C.W.; Lu, C.Y.; Chen, W.L.; Cheng, C.M. Fluorescence-based reagent and spectrum-based optical reader for lactoferrin detection in tears: Differentiating Sjogren’s syndrome from non-Sjogren’s dry eye syndrome. Sci. Rep. 2024, 14, 14505. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, C.; Zhang, J. Lactoferrin and Its Detection Methods: A Review. Nutrients 2021, 13, 2492. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, P.; Tang, H.; Zhang, J. Rapid detection of tear lactoferrin for diagnosis of dry eyes by using fluorescence polarization-based aptasensor. Sci. Rep. 2023, 13, 15179. [Google Scholar] [CrossRef]
- Connell, S.; Kawashima, M.; Nakamura, S.; Imada, T.; Yamamoto, H.; Tsubota, K.; Fukuda, S. Lactoferrin Ameliorates Dry Eye Disease Potentially through Enhancement of Short-Chain Fatty Acid Production by Gut Microbiota in Mice. Int. J. Mol. Sci. 2021, 22, 12384. [Google Scholar] [CrossRef]
- Karnati, R.; Laurie, D.E.; Laurie, G.W. Lacritin and the tear proteome as natural replacement therapy for dry eye. Exp. Eye Res. 2013, 117, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Dias-Teixeira, K.; Horton, X.; McKown, R.; Romano, J.; Laurie, G.W. The Lacritin-Syndecan-1-Heparanase Axis in Dry Eye Disease. Adv. Exp. Med. Biol. 2020, 1221, 747–757. [Google Scholar] [CrossRef]
- Georgiev, G.A.; Gh, M.S.; Romano, J.; Dias Teixeira, K.L.; Struble, C.; Ryan, D.S.; Sia, R.K.; Kitt, J.P.; Harris, J.M.; Hsu, K.L.; et al. Lacritin proteoforms prevent tear film collapse and maintain epithelial homeostasis. J. Biol. Chem. 2021, 296, 100070. [Google Scholar] [CrossRef]
- Vijmasi, T.; Chen, F.Y.; Balasubbu, S.; Gallup, M.; McKown, R.L.; Laurie, G.W.; McNamara, N.A. Topical administration of lacritin is a novel therapy for aqueous-deficient dry eye disease. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5401–5409. [Google Scholar] [CrossRef] [PubMed]
- McKown, R.L.; Wang, N.; Raab, R.W.; Karnati, R.; Zhang, Y.; Williams, P.B.; Laurie, G.W. Lacritin and other new proteins of the lacrimal functional unit. Exp. Eye Res. 2009, 88, 848–858. [Google Scholar] [CrossRef][Green Version]
- deLuise, V.P.; Tabbara, K.F. Quantitation of tear lysozyme levels in dry-eye disorders. Arch. Ophthalmol. 1983, 101, 634–635. [Google Scholar] [CrossRef] [PubMed]
- Avisar, R.; Menache, R.; Shaked, P.; Rubinstein, J.; Machtey, I.; Savir, H. Lysozyme content of tears in patients with Sjogren’s syndrome and rheumatoid arthritis. Am. J. Ophthalmol. 1979, 87, 148–151. [Google Scholar] [CrossRef]
- Jia, Z.; Wei, W.; Tu, K.; Fang, B.; Zhang, M.; Shi, L. Point-of-care monitoring of dry eye disease using lysozyme in tear based on commercial pregnancy test strips. Sens. Actuators B Chem. 2023, 378, 133179. [Google Scholar] [CrossRef]
- Berra, M.; Galperin, G.; Berra, F.; Marquez, M.I.; Mandaradoni, M.; Tau, J.; Berra, A. Tear Lysozyme in Sjogren s syndrome, Meibomian gland dysfunction, and non-dry-eye. Arq. Bras. Oftalmol. 2021, 85, 103–108. [Google Scholar] [CrossRef]
- Lee, D.; Song, S.; Cho, G.; Dalle Ore, L.C.; Malmstadt, N.; Fuwad, A.; Kim, S.M.; Jeon, T.J. Elucidating the Molecular Interactions between Lipids and Lysozyme: Evaporation Resistance and Bacterial Barriers for Dry Eye Disease. Nano Lett. 2023, 23, 9451–9460. [Google Scholar] [CrossRef]
- Yeh, P.T.; Casey, R.; Glasgow, B.J. A novel fluorescent lipid probe for dry eye: Retrieval by tear lipocalin in humans. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1398–1410. [Google Scholar] [CrossRef]
- Gasymov, O.K.; Abduragimov, A.R.; Prasher, P.; Yusifov, T.N.; Glasgow, B.J. Tear lipocalin: Evidence for a scavenging function to remove lipids from the human corneal surface. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3589–3596. [Google Scholar] [CrossRef]
- Yamada, M.; Mochizuki, H.; Kawai, M.; Tsubota, K.; Bryce, T.J. Decreased tear lipocalin concentration in patients with meibomian gland dysfunction. Br. J. Ophthalmol. 2005, 89, 803–805. [Google Scholar] [CrossRef]
- Subbaraman, L.; Mistry, R.; Thangavelu, M.; Jones, L. Quantification of lipocalin-1 in tears and contact lens deposits using a sandwich ELISA technique. Contact Lens Anterior Eye 2013, 36, e45–e46. [Google Scholar] [CrossRef]
- Gipson, I.K.; Hori, Y.; Argueso, P. Character of ocular surface mucins and their alteration in dry eye disease. Ocul. Surf. 2004, 2, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y. Secreted Mucins on the Ocular Surface. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES151–DES156. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lu, P.; Song, H.; Zheng, Q.; Nan, K. Expression of mucins MUC5AC and MUC19 on the ocular surface in dry eye syndrome model of ovariectomized female rabbits. Adv. Clin. Exp. Med. 2019, 28, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Carrington, S.D.; Goodall, C.; Myerscough, N.; Corfield, A.P. Canine ocular mucins in health and dry eye disease. Biochem. Soc. Trans. 1993, 21, 484S. [Google Scholar] [CrossRef]
- Shamloo, K.; Barbarino, A.; Alfuraih, S.; Sharma, A. Graft Versus Host Disease-Associated Dry Eye: Role of Ocular Surface Mucins and the Effect of Rebamipide, a Mucin Secretagogue. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4511–4519. [Google Scholar] [CrossRef]
- Portal, C.; Gouyer, V.; Gottrand, F.; Desseyn, J.L. Ocular mucins in dry eye disease. Exp. Eye Res. 2019, 186, 107724. [Google Scholar] [CrossRef]
- Floyd, A.M.; Zhou, X.; Evans, C.; Rompala, O.J.; Zhu, L.; Wang, M.; Chen, Y. Mucin deficiency causes functional and structural changes of the ocular surface. PLoS ONE 2012, 7, e50704. [Google Scholar] [CrossRef]
- Aldina, R.; Sujuti, H.; Permatasari, N.; Widodo, M.A. The effects of genistein on estrogen receptor-β, IL-1β levels, and MUC5AC expression in ovariectomized rats with dry eye. Clin. Nutr. Exp. 2019, 27, 21–28. [Google Scholar] [CrossRef][Green Version]
- Duan, H.; Yang, T.; Zhou, Y.; Ma, B.; Zhao, L.; Chen, J.; Qi, H. Comparison of mucin levels at the ocular surface of visual display terminal users with and without dry eye disease. BMC Ophthalmol. 2023, 23, 189. [Google Scholar] [CrossRef] [PubMed]
- Corrales, R.M.; Narayanan, S.; Fernandez, I.; Mayo, A.; Galarreta, D.J.; Fuentes-Paez, G.; Chaves, F.J.; Herreras, J.M.; Calonge, M. Ocular mucin gene expression levels as biomarkers for the diagnosis of dry eye syndrome. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8363–8369. [Google Scholar] [CrossRef]
- Xu, K.; Liu, X.N.; Zhang, H.B.; Zhu, X.P.; Zhang, X.J. Tear film instability is associated with weakened colocalization between occludin and MUC5AC in scopolamine-induced dry eye disease (DED) rats. Int. Ophthalmol. 2023, 43, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Blautain, B.; Rabut, G.; Dupas, B.; Riancho, L.; Liang, H.; Luzu, J.; Labbe, A.; Garrigue, J.S.; Brignole-Baudouin, F.; Baudouin, C.; et al. Multimodal Approach in Dry Eye Disease Combining In Vivo Confocal Microscopy and HLA-DR Expression. Transl. Vis. Sci. Technol. 2024, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.S.; Wei, Y.; Yu, Y.; Ying, G.S.; Kuklinski, E.; Barry, B.; Maguire, M.G.; Dana, R.; Brightwell-Arnold, M.; Asbell, P.A.; et al. Conjunctival HLA-DR Expression and Its Association with Symptoms and Signs in the DREAM Study. Transl. Vis. Sci. Technol. 2019, 8, 31. [Google Scholar] [CrossRef]
- Epstein, S.P.; Gadaria-Rathod, N.; Wei, Y.; Maguire, M.G.; Asbell, P.A. HLA-DR expression as a biomarker of inflammation for multicenter clinical trials of ocular surface disease. Exp. Eye Res. 2013, 111, 95–104. [Google Scholar] [CrossRef]
- Roy, N.S.; Yu, Y.; Ying, G.S.; Maguire, M.G.; Asbell, P.A.; Group, D.S. Effect of Omega-3 on HLA-DR Expression by Conjunctival Cells and Tear Cytokine Concentrations in the Dry Eye Assessment and Management Study. Eye Contact Lens 2022, 48, 384–390. [Google Scholar] [CrossRef]
- Fernandez, K.B.; Epstein, S.P.; Raynor, G.S.; Sheyman, A.T.; Massingale, M.L.; Dentone, P.G.; Landegger, L.D.; Asbell, P.A. Modulation of HLA-DR in dry eye patients following 30 days of treatment with a lubricant eyedrop solution. Clin. Ophthalmol. 2015, 9, 1137–1145. [Google Scholar] [CrossRef]
- Wu, J.; Li, G.J.; Niu, J.; Wen, F.; Han, L. Analyze interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha levels in dry eye and the therapeutic effect of cyclosporine A. World J. Clin. Cases 2024, 12, 5665–5672. [Google Scholar] [CrossRef] [PubMed]
- Mrugacz, M.; Ostrowska, L.; Bryl, A.; Szulc, A.; Zelazowska-Rutkowska, B.; Mrugacz, G. Pro-inflammatory cytokines associated with clinical severity of dry eye disease of patients with depression. Adv. Med. Sci. 2017, 62, 338–344. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.; Ma, Y.; Lin, X.; Yu, X.; He, S.; Luo, C.; Xu, W. Analysis of tear inflammatory molecules and clinical correlations in evaporative dry eye disease caused by meibomian gland dysfunction. Int. Ophthalmol. 2020, 40, 3049–3058. [Google Scholar] [CrossRef]
- Massingale, M.L.; Li, X.; Vallabhajosyula, M.; Chen, D.; Wei, Y.; Asbell, P.A. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea 2009, 28, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.P.; Yeon, C.Y.; Adhikari, N.; Neupane, S.; Kim, H.; Lee, D.C.; Son, M.J.; Lee, H.G.; Kim, J.Y.; Jun, J.H. Cyclosporine A eyedrops with self-nanoemulsifying drug delivery systems have improved physicochemical properties and efficacy against dry eye disease in a murine dry eye model. PLoS ONE 2019, 14, e0224805. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J.; Li, X.; Li, Y.; Zhao, X.; Sun, X.; Li, Y. Fenofibrate inhibits activation of cGAS-STING pathway by alleviating mitochondrial damage to attenuate inflammatory response in diabetic dry eye. Free Radic. Biol. Med. 2025, 235, 364–378. [Google Scholar] [CrossRef]
- Simmons, K.T.; Xiao, Y.; Pflugfelder, S.C.; de Paiva, C.S. Inflammatory Response to Lipopolysaccharide on the Ocular Surface in a Murine Dry Eye Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T. Inflammatory Response in Dry Eye. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES192–DES199. [Google Scholar] [CrossRef]
- Yu, L.; Yu, C.; Dong, H.; Mu, Y.; Zhang, R.; Zhang, Q.; Liang, W.; Li, W.; Wang, X.; Zhang, L. Recent Developments About the Pathogenesis of Dry Eye Disease: Based on Immune Inflammatory Mechanisms. Front. Pharmacol. 2021, 12, 732887. [Google Scholar] [CrossRef]
- Blanco-Vazquez, M.; Vazquez, A.; Fernandez, I.; Novo-Diez, A.; Martinez-Plaza, E.; Garcia-Vazquez, C.; Gonzalez-Garcia, M.J.; Sobas, E.M.; Calonge, M.; Enriquez-de-Salamanca, A. Inflammation-related molecules in tears of patients with chronic ocular pain and dry eye disease. Exp. Eye Res. 2022, 219, 109057. [Google Scholar] [CrossRef]
- Bruscolini, A.; Lambiase, A.; Segatto, M.; La Cava, M.; Nebbioso, M.; Sacchetti, M. Evaluation of IL8 pathway on the ocular surface: New insights in patients with ocular mucous membrane pemphigoid. Acta Ophthalmol. 2020, 98, e173–e177. [Google Scholar] [CrossRef]
- Galvez, B.G.; Martinez-Perez, C.; Villa-Collar, C.; Alvarez-Peregrina, C.; Sanchez-Tena, M.A. Influence of Cytokines on Inflammatory Eye Diseases: A Citation Network Study. J. Clin. Med. 2022, 11, 661. [Google Scholar] [CrossRef]
- Lopez-Miguel, A.; Teson, M.; Martin-Montanez, V.; Enriquez-de-Salamanca, A.; Stern, M.E.; Gonzalez-Garcia, M.J.; Calonge, M. Clinical and Molecular Inflammatory Response in Sjogren Syndrome-Associated Dry Eye Patients Under Desiccating Stress. Am. J. Ophthalmol. 2016, 161, 133–141.e2. [Google Scholar] [CrossRef]
- Lam, H.; Bleiden, L.; de Paiva, C.S.; Farley, W.; Stern, M.E.; Pflugfelder, S.C. Tear cytokine profiles in dysfunctional tear syndrome. Am. J. Ophthalmol. 2009, 147, 198–205.e1. [Google Scholar] [CrossRef]
- El Annan, J.; Goyal, S.; Zhang, Q.; Freeman, G.J.; Sharpe, A.H.; Dana, R. Regulation of T-cell chemotaxis by programmed death-ligand 1 (PD-L1) in dry eye-associated corneal inflammation. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3418–3423. [Google Scholar] [CrossRef] [PubMed]
- Nagar, S.; Ajouz, L.; Nichols, K.K.; Kumar, S.; Zhao, C.; Naidoo, K.K.; Robinson, M.R.; Borchman, D. Relationship Between Human Meibum Lipid Composition and the Severity of Meibomian Gland Dysfunction: A Spectroscopic Analysis. Investig. Ophthalmol. Vis. Sci. 2023, 64, 22. [Google Scholar] [CrossRef] [PubMed]
- Asiedu, K. Candidate Molecular Compounds as Potential Indicators for Meibomian Gland Dysfunction. Front. Med. 2022, 9, 873538. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, S.N.; Shao, Y.; Xiao, D.; Tang, L.Y.; Cheng, Z.; Peng, J. Lipidomics Profiles Revealed Alterations in Patients with Meibomian Gland Dysfunction After Exposure to Intense Pulsed Light. Front. Neurol. 2022, 13, 827544. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.M.; Tong, L.; Yong, S.S.; Li, B.; Chaurasia, S.S.; Shui, G.; Wenk, M.R. Meibum lipid composition in Asians with dry eye disease. PLoS ONE 2011, 6, e24339. [Google Scholar] [CrossRef]
- Nguyen, A.; Naidoo, K.K.; Ajouz, L.; Xu, X.; Zhao, C.; Robinson, M.R.; Borchman, D. Changes in Human Meibum Lipid Composition Related to the Presence and Severity of Meibomian Gland Dysfunction. J. Ocul. Pharmacol. Ther. 2024, 40, 562–570. [Google Scholar] [CrossRef]
- Garcia-Queiruga, J.; Pena-Verdeal, H.; Sabucedo-Villamarin, B.; Paz-Tarrio, M.; Guitian-Fernandez, E.; Garcia-Resua, C.; Yebra-Pimentel, E.; Giraldez, M.J. Meibum Lipidomic Analysis in Evaporative Dry Eye Subjects. Int. J. Mol. Sci. 2024, 25, 4782. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, J.D.; Nichols, K.K. Dry Eye Disease Associated with Meibomian Gland Dysfunction: Focus on Tear Film Characteristics and the Therapeutic Landscape. Ophthalmol. Ther. 2023, 12, 1397–1418. [Google Scholar] [CrossRef]
- Mondal, K.; Mandal, N. Role of Bioactive Sphingolipids in Inflammation and Eye Diseases. Adv. Exp. Med. Biol. 2019, 1161, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Paranjpe, V.; Galor, A.; Grambergs, R.; Mandal, N. The role of sphingolipids in meibomian gland dysfunction and ocular surface inflammation. Ocul. Surf. 2022, 26, 100–110. [Google Scholar] [CrossRef]
- Ham, B.M.; Cole, R.B.; Jacob, J.T. Identification and comparison of the polar phospholipids in normal and dry eye rabbit tears by MALDI-TOF mass spectrometry. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3330–3338. [Google Scholar] [CrossRef] [PubMed]
- Borchman, D. Lipid conformational order and the etiology of cataract and dry eye. J. Lipid Res. 2021, 62, 100039. [Google Scholar] [CrossRef]
- Miyamoto, M.; Sassa, T.; Sawai, M.; Kihara, A. Lipid polarity gradient formed by omega-hydroxy lipids in tear film prevents dry eye disease. Elife 2020, 9, e53582. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, Y.; Zhang, K.; Mou, Y.; Jin, X.; Huang, X. The alterations of ocular surface metabolism and the related immunity inflammation in dry eye. Adv. Ophthalmol. Pract. Res. 2025, 5, 1–12. [Google Scholar] [CrossRef]
- Jantti, J.; Viitaja, T.; Sevon, J.; Lajunen, T.; Raitanen, J.E.; Schlegel, C.; Viljanen, M.; Paananen, R.O.; Moilanen, J.; Ruponen, M.; et al. Early-Stage Development of an Anti-Evaporative Liposomal Formulation for the Potential Treatment of Dry Eyes. ACS Pharmacol. Transl. Sci. 2023, 6, 1518–1530. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki-Dantas, M.C.; de Freitas, D.; Fornazari, D.; Dos Santos, M.S.; Wakamatsu, T.H.; Barquilha, C.N.; Ferrer, M.T.; Holzhausen, H.C.N.; Alves, M. Phospholipid Nanoemulsion-Based Ocular Lubricant for the Treatment of Dry Eye Subtypes: A Multicenter and Prospective Study. Ophthalmol. Ther. 2024, 13, 3203–3213. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Hammer, C.M.; Paulsen, F. Urea and ocular surface: Synthesis, secretion and its role in tear film homeostasis. Ocul. Surf. 2023, 27, 41–47. [Google Scholar] [CrossRef]
- Chai, P.; Zhao, F.; Jia, R.; Zhou, X.; Fan, X. Lactate/lactylation in ocular development and diseases. Trends Mol. Med. 2024. [Google Scholar] [CrossRef]
- Mondal, H.; Kim, H.J.; Mohanto, N.; Jee, J.P. A Review on Dry Eye Disease Treatment: Recent Progress, Diagnostics, and Future Perspectives. Pharmaceutics 2023, 15, 990. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, C.; Peng, F.; Wu, L.; Zhuo, D.; Wang, L.; Zhang, M.; Li, Z.; Tian, L.; Jie, Y.; et al. Identification of glutamine as a potential therapeutic target in dry eye disease. Signal Transduct. Target. Ther. 2025, 10, 27. [Google Scholar] [CrossRef]
- Galbis-Estrada, C.; Pinazo-Duran, M.D.; Martinez-Castillo, S.; Morales, J.M.; Monleon, D.; Zanon-Moreno, V. A metabolomic approach to dry eye disorders. The role of oral supplements with antioxidants and omega 3 fatty acids. Mol. Vis. 2015, 21, 555–567. [Google Scholar]
- Jiang, Y.; Yang, C.; Zheng, Y.; Liu, Y.; Chen, Y. A Set of Global Metabolomic Biomarker Candidates to Predict the Risk of Dry Eye Disease. Front. Cell Dev. Biol. 2020, 8, 344. [Google Scholar] [CrossRef]
- Yazdani, M.; Elgstoen, K.B.P.; Rootwelt, H.; Shahdadfar, A.; Utheim, O.A.; Utheim, T.P. Tear Metabolomics in Dry Eye Disease: A Review. Int. J. Mol. Sci. 2019, 20, 3755. [Google Scholar] [CrossRef]
- Seen, S.; Tong, L. Dry eye disease and oxidative stress. Acta Ophthalmol. 2018, 96, e412–e420. [Google Scholar] [CrossRef] [PubMed]
- Dogru, M.; Kojima, T.; Simsek, C.; Tsubota, K. Potential Role of Oxidative Stress in Ocular Surface Inflammation and Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES163–DES168. [Google Scholar] [CrossRef]
- Bu, J.; Liu, Y.; Zhang, R.; Lin, S.; Zhuang, J.; Sun, L.; Zhang, L.; He, H.; Zong, R.; Wu, Y.; et al. Potential New Target for Dry Eye Disease-Oxidative Stress. Antioxidants 2024, 13, 422. [Google Scholar] [CrossRef]
- Ouyang, W.; Yan, D.; Hu, J.; Liu, Z. Multifaceted mitochondrial as a novel therapeutic target in dry eye: Insights and interventions. Cell Death Discov. 2024, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zheng, L.Y.; Zhang, P.; Yu, C.Q. miR-146a and miR-155 expression in PBMCs from patients with Sjogren’s syndrome. J. Oral Pathol. Med. 2014, 43, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Zilahi, E.; Tarr, T.; Papp, G.; Griger, Z.; Sipka, S.; Zeher, M. Increased microRNA-146a/b, TRAF6 gene and decreased IRAK1 gene expressions in the peripheral mononuclear cells of patients with Sjogren’s syndrome. Immunol. Lett. 2012, 141, 165–168. [Google Scholar] [CrossRef]
- Sun, H.Y.; Lv, A.K.; Yao, H. Relationship of miRNA-146a to primary Sjogren’s syndrome and to systemic lupus erythematosus: A meta-analysis. Rheumatol. Int. 2017, 37, 1311–1316. [Google Scholar] [CrossRef]
- Wei, Y.; Li, N.; Zhao, L.; Yang, C.; Ma, B.; Li, X.; Wei, R.; Nian, H. MicroRNAs and Autoimmune-Mediated Eye Diseases. Front. Cell Dev. Biol. 2020, 8, 818. [Google Scholar] [CrossRef] [PubMed]
- Benavides-Aguilar, J.A.; Morales-Rodriguez, J.I.; Ambriz-Gonzalez, H.; Ruiz-Manriquez, L.M.; Banerjee, A.; Pathak, S.; Duttaroy, A.K.; Paul, S. The regulatory role of microRNAs in common eye diseases: A brief review. Front. Genet. 2023, 14, 1152110. [Google Scholar] [CrossRef]
- Rassi, D.M.; De Paiva, C.S.; Dias, L.C.; Modulo, C.M.; Adriano, L.; Fantucci, M.Z.; Rocha, E.M. Review: MicroRNAS in ocular surface and dry eye diseases. Ocul. Surf. 2017, 15, 660–669. [Google Scholar] [CrossRef]
- Kessal, K.; Liang, H.; Rabut, G.; Daull, P.; Garrigue, J.S.; Docquier, M.; Melik Parsadaniantz, S.; Baudouin, C.; Brignole-Baudouin, F. Conjunctival Inflammatory Gene Expression Profiling in Dry Eye Disease: Correlations with HLA-DRA and HLA-DRB1. Front. Immunol. 2018, 9, 2271. [Google Scholar] [CrossRef]
- Brignole-Baudouin, F.; Riancho, L.; Ismail, D.; Deniaud, M.; Amrane, M.; Baudouin, C. Correlation Between the Inflammatory Marker HLA-DR and Signs and Symptoms in Moderate to Severe Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2438–2448. [Google Scholar] [CrossRef] [PubMed]
- Paik, B.; Tong, L. Polymorphisms in Lymphotoxin-Alpha as the “Missing Link” in Prognosticating Favourable Response to Omega-3 Supplementation for Dry Eye Disease: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 4236. [Google Scholar] [CrossRef]
- Roshandel, D.; Semnani, F.; Rayati Damavandi, A.; Masoudi, A.; Baradaran-Rafii, A.; Watson, S.L.; Morgan, W.H.; McLenachan, S. Genetic predisposition to ocular surface disorders and opportunities for gene-based therapies. Ocul. Surf. 2023, 29, 150–165. [Google Scholar] [CrossRef]
- Kim, W.; Woo, I.H.; Eom, Y.; Song, J.S. Short-term changes in tear osmolarity after instillation of different osmolarity eye drops in patients with dry eye. Sci. Rep. 2023, 13, 11012. [Google Scholar] [CrossRef]
- Baudouin, C.; Aragona, P.; Messmer, E.M.; Tomlinson, A.; Calonge, M.; Boboridis, K.G.; Akova, Y.A.; Geerling, G.; Labetoulle, M.; Rolando, M. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: Proceedings of the OCEAN group meeting. Ocul. Surf. 2013, 11, 246–258. [Google Scholar] [CrossRef]
- Lemp, M.A.; Bron, A.J.; Baudouin, C.; Benitez Del Castillo, J.M.; Geffen, D.; Tauber, J.; Foulks, G.N.; Pepose, J.S.; Sullivan, B.D. Tear osmolarity in the diagnosis and management of dry eye disease. Am. J. Ophthalmol. 2011, 151, 792–798.e1. [Google Scholar] [CrossRef]
- Tashbayev, B.; Utheim, T.P.; Utheim, O.A.; Raeder, S.; Jensen, J.L.; Yazdani, M.; Lagali, N.; Vitelli, V.; Dartt, D.A.; Chen, X. Utility of Tear Osmolarity Measurement in Diagnosis of Dry Eye Disease. Sci. Rep. 2020, 10, 5542. [Google Scholar] [CrossRef]
- Tomlinson, A.; Khanal, S.; Ramaesh, K.; Diaper, C.; McFadyen, A. Tear film osmolarity: Determination of a referent for dry eye diagnosis. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4309–4315. [Google Scholar] [CrossRef]
- Suzuki, M.; Massingale, M.L.; Ye, F.; Godbold, J.; Elfassy, T.; Vallabhajosyula, M.; Asbell, P.A. Tear osmolarity as a biomarker for dry eye disease severity. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4557–4561. [Google Scholar] [CrossRef] [PubMed]
- Galor, A.; Lighthizer, N. Corneal Sensitivity Testing Procedure for Ophthalmologic and Optometric Patients. J. Vis. Exp. 2024, 210, e66597. [Google Scholar] [CrossRef] [PubMed]
- Merayo-Lloves, J.; Gomez Martin, C.; Lozano-Sanroma, J.; Renedo Laguna, C. Assessment and safety of the new esthesiometer BRILL: Comparison with the Cochet-Bonnet Esthesiometer. Eur. J. Ophthalmol. 2024, 34, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, R.E.; Quiroga-Garza, M.E.; Ramos-DÁVila, E.M.; PantaleÓN-GarcÍA, J.; Khodor, A.L.I.; Komai, S.; Rodriguez-Gutierrez, L.A.; Ma, S.; Mousa, H.M.; Mattes, R.; et al. Comparative Evaluation of the Corneal Sensitivity Thresholds between the Novel Non-Contact and Cochet-Bonnet Esthesiometers. Am. J. Ophthalmol. 2025, 271, 407–416. [Google Scholar] [CrossRef]
- Vazquez, A.; Blanco-Vazquez, M.; Martinez-Plaza, E.; Sobas, E.M.; Gonzalez-Garcia, M.J.; Lopez-Miguel, A.; Ortega, E.; Enriquez-de-Salamanca, A.; Calonge, M. Corneal Sensory Changes and Nerve Plexus Abnormalities in Chronic Neuropathic Ocular Pain and Dry Eye Postrefractive Surgery. Am. J. Ophthalmol. 2025, 276, 170–185. [Google Scholar] [CrossRef]
- Vidas Pauk, S.; Petricek, I.; Jukic, T.; Popovic-Suic, S.; Tomic, M.; Kalauz, M.; Jandrokovic, S.; Masnec, S. Noninvasive Tear Film Break-up Time Assessment Using Handheld Lipid Layer Examination Instrument. Acta Clin. Croat. 2019, 58, 63–71. [Google Scholar] [CrossRef]
- Tsubota, K. Short Tear Film Breakup Time-Type Dry Eye. Investig. Ophthalmol. Vis. Sci. 2018, 59, DES64–DES70. [Google Scholar] [CrossRef] [PubMed]
- Correa-Sandoval, D.C.; Quintanilla-Treviño, P.M.; Amparo, F.; Garza-Leon, M.A. Noninvasive tear breakup time evaluation with multifunctional topography supports the diagnosis of evaporative dry eye disease. Pan-Am. J. Ophthalmol. 2024, 6, 80. [Google Scholar] [CrossRef]
- Yazdani, M.; Fiskadal, J.; Chen, X.; Utheim, O.A.; Raeder, S.; Vitelli, V.; Utheim, T.P. Tear Film Break-Up Time and Dry Eye Disease Severity in a Large Norwegian Cohort. J. Clin. Med. 2021, 10, 884. [Google Scholar] [CrossRef]
- El Barche, F.Z.; Benyoussef, A.A.; El Habib Daho, M.; Lamard, A.; Quellec, G.; Cochener, B.; Lamard, M. Automated tear film break-up time measurement for dry eye diagnosis using deep learning. Sci. Rep. 2024, 14, 11723. [Google Scholar] [CrossRef]
- Pondelis, N.; Dieckmann, G.M.; Jamali, A.; Kataguiri, P.; Senchyna, M.; Hamrah, P. Infrared meibography allows detection of dimensional changes in meibomian glands following intranasal neurostimulation. Ocul. Surf. 2020, 18, 511–516. [Google Scholar] [CrossRef]
- Palamar, M.; Kiyat, P.; Ertam, I.; Yagci, A. Evaluation of dry eye and meibomian gland dysfunction with meibography in vitiligo. Eye 2017, 31, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.J.; Sobel, R.K.; Allen, R.C. Meibography: A review of techniques and technologies. Saudi J. Ophthalmol. 2012, 26, 349–356. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Yu, C.; Li, Q.; Chang, P.; Wang, D.; Li, Z.; Zhao, Y.; Zhang, H.; Tang, N.; et al. Unsupervised Learning Based on Meibography Enables Subtyping of Dry Eye Disease and Reveals Ocular Surface Features. Investig. Ophthalmol. Vis. Sci. 2023, 64, 43. [Google Scholar] [CrossRef]
- Alhatem, A.; Cavalcanti, B.; Hamrah, P. In vivo confocal microscopy in dry eye disease and related conditions. Semin. Ophthalmol. 2012, 27, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Kheirkhah, A.; Cavalcanti, B.M.; Cruzat, A.; Jamali, A.; Hamrah, P. Correlation of corneal immune cell changes with clinical severity in dry eye disease: An in vivo confocal microscopy study. Ocul. Surf. 2021, 19, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Dua, H.S.; Tong, L.; Kundu, G.; Khamar, P.; Gorimanipalli, B.; D’Souza, S. Role of in vivo confocal microscopy in dry eye disease and eye pain. Indian J. Ophthalmol. 2023, 71, 1099–1104. [Google Scholar] [CrossRef]
- Sim, R.; Yong, K.; Liu, Y.C.; Tong, L. In Vivo Confocal Microscopy in Different Types of Dry Eye and Meibomian Gland Dysfunction. J. Clin. Med. 2022, 11, 2349. [Google Scholar] [CrossRef]
- He, J.; Ogawa, Y.; Mukai, S.; Saijo-Ban, Y.; Kamoi, M.; Uchino, M.; Yamane, M.; Ozawa, N.; Fukui, M.; Mori, T.; et al. In Vivo Confocal Microscopy Evaluation of Ocular Surface with Graft-Versus-Host Disease-Related Dry Eye Disease. Sci. Rep. 2017, 7, 10720. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, P.; Liang, H.; Reboussin, E.; Rabut, G.; Warcoin, E.; Brignole-Baudouin, F.; Melik-Parsadaniantz, S.; Baudouin, C.; Labbe, A.; Reaux-Le Goazigo, A. Proinflammatory Markers, Chemokines, and Enkephalin in Patients Suffering from Dry Eye Disease. Int. J. Mol. Sci. 2018, 19, 1221. [Google Scholar] [CrossRef]
- Walter, S.D.; Gronert, K.; McClellan, A.L.; Levitt, R.C.; Sarantopoulos, K.D.; Galor, A. omega-3 Tear Film Lipids Correlate with Clinical Measures of Dry Eye. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2472–2478. [Google Scholar] [CrossRef]
- Huang, S.C.; Lei, Y.P.; Hsiao, M.C.; Hsieh, Y.K.; Tang, Q.P.; Chen, C.; Hsu, M.Y. Multicomponent Dietary Supplementation: Impact on Tear Secretion and Ocular Surface Inflammation in Dry Eye Syndrome Patients. Antioxidants 2025, 14, 103. [Google Scholar] [CrossRef]
- Hussain, M.; Shtein, R.M.; Pistilli, M.; Maguire, M.G.; Oydanich, M.; Asbell, P.A.; Group, D.S.R. The Dry Eye Assessment and Management (DREAM) extension study—A randomized clinical trial of withdrawal of supplementation with omega-3 fatty acid in patients with dry eye disease. Ocul. Surf. 2020, 18, 47–55. [Google Scholar] [CrossRef]
- Khanal, S.; Ngo, W.; Nichols, K.K.; Wilson, L.; Barnes, S.; Nichols, J.J. Human meibum and tear film derived (O-acyl)-omega-hydroxy fatty acids in meibomian gland dysfunction. Ocul. Surf. 2021, 21, 118–128. [Google Scholar] [CrossRef]
- Bland, H.C.; Moilanen, J.A.; Ekholm, F.S.; Paananen, R.O. Investigating the Role of Specific Tear Film Lipids Connected to Dry Eye Syndrome: A Study on O-Acyl-omega-hydroxy Fatty Acids and Diesters. Langmuir 2019, 35, 3545–3552. [Google Scholar] [CrossRef] [PubMed]
- Viitaja, T.; Raitanen, J.E.; Moilanen, J.; Paananen, R.O.; Ekholm, F.S. The Properties and Role of O-Acyl-omega-hydroxy Fatty Acids and Type I-St and Type II Diesters in the Tear Film Lipid Layer Revealed by a Combined Chemistry and Biophysics Approach. J. Org. Chem. 2021, 86, 4965–4976. [Google Scholar] [CrossRef] [PubMed]
- Pucker, A.D.; Ngo, W.; Postnikoff, C.K.; Fortinberry, H.; Nichols, J.J. Tear Film miRNAs and Their Association with Human Dry Eye Disease. Curr. Eye Res. 2022, 47, 1479–1487. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Benítez-Del-Castillo, J.; Loya-Garcia, D.; Inomata, T.; Iyar, G.; Liang, L.; Pult, H.; Sabater, A.L.; Starr, C.E.; Vehof, J.; et al. TFOS DEWS III Diagnostic Methodology. Am. J. Ophthalmol. 2025, 1–101. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, B.G.; Kim, J.S.; Hwang, J.H. Matrix Metalloproteinase 9 Point-of-Care Immunoassay Result Predicts Response to Topical Cyclosporine Treatment in Dry Eye Disease. Transl. Vision. Sci. Technol. 2018, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Schargus, M.; Ivanova, S.; Kakkassery, V.; Dick, H.B.; Joachim, S. Correlation of Tear Film Osmolarity and 2 Different MMP-9 Tests with Common Dry Eye Tests in a Cohort of Non-Dry Eye Patients. Cornea 2015, 34, 739–744. [Google Scholar] [CrossRef]
- Sambursky, R.; Davitt, W.F., 3rd; Latkany, R.; Tauber, S.; Starr, C.; Friedberg, M.; Dirks, M.S.; McDonald, M. Sensitivity and specificity of a point-of-care matrix metalloproteinase 9 immunoassay for diagnosing inflammation related to dry eye. JAMA Ophthalmol. 2013, 131, 24–28. [Google Scholar] [CrossRef]
- Bron, A.J.; Willshire, C. Tear Osmolarity in the Diagnosis of Systemic Dehydration and Dry Eye Disease. Diagnostics 2021, 11, 387. [Google Scholar] [CrossRef]
- Park, J.; Choi, Y.; Han, G.; Shin, E.; Han, J.; Chung, T.Y.; Lim, D.H. Evaluation of tear osmolarity measured by I-Pen osmolarity system in patients with dry eye. Sci. Rep. 2021, 11, 7726. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.V.; Ying, G.S.; Pistilli, M.; Maguire, M.G.; Asbell, P.A.; Dry Eye, A.; Management Study Research, G. Association of Tear Osmolarity with Signs and Symptoms of Dry Eye Disease in the Dry Eye Assessment and Management (DREAM) Study. Investig. Ophthalmol. Vis. Sci. 2023, 64, 5. [Google Scholar] [CrossRef]
- Harrell, C.R.; Feulner, L.; Djonov, V.; Pavlovic, D.; Volarevic, V. The Molecular Mechanisms Responsible for Tear Hyperosmolarity-Induced Pathological Changes in the Eyes of Dry Eye Disease Patients. Cells 2023, 12, 2755. [Google Scholar] [CrossRef]
- Pflugfelder, S.; Nakhleh, L.; Kikukawa, Y.; Tanaka, S.; Kosugi, T. Non-Invasive Tear Break-Up Detection with the Kowa DR-1alpha and Its Relationship to Dry Eye Clinical Severity. Int. J. Mol. Sci. 2022, 23, 14774. [Google Scholar] [CrossRef] [PubMed]
- Aldeyra Therapeutics, Inc. Reproxalap: Our Novel Small Molecule Drug Candidate for Dry Eye; Aldeyra Therapeutics, Inc.: Lexington, MA, USA, 2024. [Google Scholar]
- Aldeyra Therapeutics, Inc. Aldeyra Therapeutics achieves primary endpoint in phase 3 dry eye disease clinical trial of reproxalap. Business Wire, 8 August 2024. [Google Scholar]
- Hu, L.; Zhang, T.; Ma, H.; Pan, Y.; Wang, S.; Liu, X.; Dai, X.; Zheng, Y.; Lee, L.P.; Liu, F. Discovering the Secret of Diseases by Incorporated Tear Exosomes Analysis via Rapid-Isolation System: iTEARS. ACS Nano 2022, 16, 11720–11732. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, J.; Frenia, K.; Gelman, J.; Beatty, M.; Zhou, M.; Ma, L.; Pieramici, S.; Eger, N.; Dhaliwal, D.; Labriola, L.T.; et al. Translating Biomarker Discovery: From Bench to Bedside in Dry Eye Disease. Int. J. Mol. Sci. 2025, 26, 8556. https://doi.org/10.3390/ijms26178556
Jones J, Frenia K, Gelman J, Beatty M, Zhou M, Ma L, Pieramici S, Eger N, Dhaliwal D, Labriola LT, et al. Translating Biomarker Discovery: From Bench to Bedside in Dry Eye Disease. International Journal of Molecular Sciences. 2025; 26(17):8556. https://doi.org/10.3390/ijms26178556
Chicago/Turabian StyleJones, Jeremy, Kyla Frenia, Julia Gelman, Maria Beatty, Melody Zhou, Levin Ma, Sean Pieramici, Noah Eger, Deepinder Dhaliwal, Leanne T. Labriola, and et al. 2025. "Translating Biomarker Discovery: From Bench to Bedside in Dry Eye Disease" International Journal of Molecular Sciences 26, no. 17: 8556. https://doi.org/10.3390/ijms26178556
APA StyleJones, J., Frenia, K., Gelman, J., Beatty, M., Zhou, M., Ma, L., Pieramici, S., Eger, N., Dhaliwal, D., Labriola, L. T., & Xiao, K. (2025). Translating Biomarker Discovery: From Bench to Bedside in Dry Eye Disease. International Journal of Molecular Sciences, 26(17), 8556. https://doi.org/10.3390/ijms26178556







