Proteomic Insights into Childhood Obesity: A Systematic Review of Protein Biomarkers and Advances
Abstract
1. Introduction
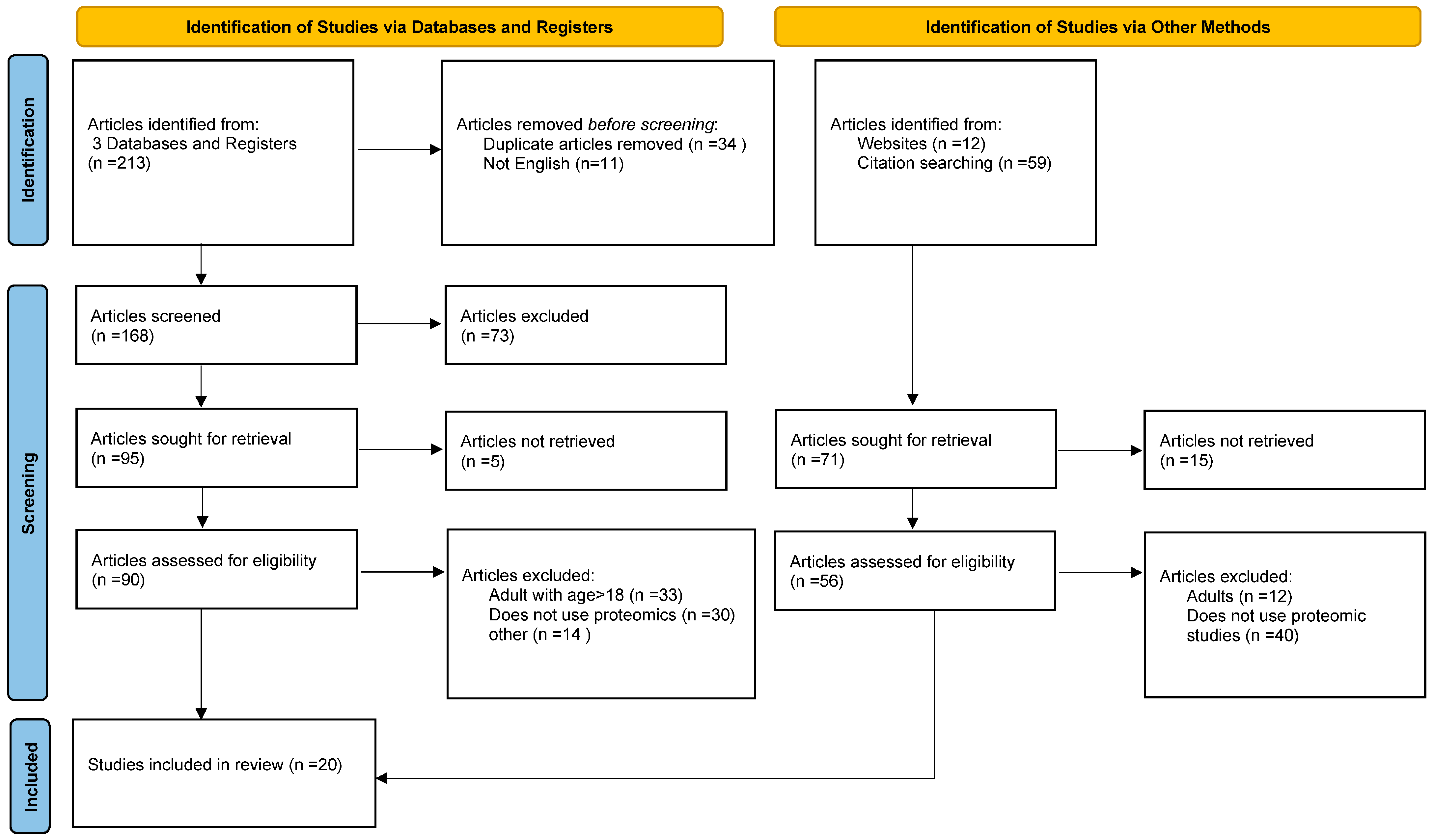
2. Materials and Methods
3. Results
- -
- APOA1 (Apolipoprotein-A1): Studies have shown a decrease in various isoforms of APOA1 in obese children compared to lean controls. This reduction is more pronounced in insulin-resistant individuals and may be partially reversed with weight loss. APOA1 particles can increase cholesterol efflux from cells and risk for cardiovascular disease [18,20,21,27,28,40,41,46,47,48].
- -
- CLU (Clusterin; Apolipoprotein J): This is involved in lipid transport, apoptosis regulation, and protection against oxidative stress. Reduced levels of clusterin have been observed in obese pediatric populations, suggesting a compromised capacity to manage cellular stress and lipid transport. This protein is known to accumulate in the artery wall during the development of atherosclerosis and has been detected in the infarcted heart during myocardial infarction. Given its multifaceted role, alterations in clusterin levels may contribute to the pathophysiology of obesity and its related complications [30,31,39,49,50,51].
- -
- APOE (Apolipoprotein E): This is involved in lipid transport and clearance [28].
- -
- HP (Haptoglobin): An increase in low-molecular-weight isoforms of haptoglobin (as well as a decrease in high isoforms) has been observed in obese children, especially those with insulin resistance. These elevated levels correlate positively with inflammatory markers such as interleukin-6 and NAMPT/visfatin. It is also an acute-phase/inflammation marker [6,18,28,33,39,49,52,53,54].
- -
- CFB (Complement Factor B), CFH (Complement Factor H), and CFI (Complement Factor I): These proteins are integral components of the immune system’s complement pathway. Elevated levels have been associated with an increased BMI, suggesting a link between obesity and immune system activation [8,9,20,22,28,31,32,33,39,40,43,55,56,57].
- -
- VDBP (Vitamin D Binding Protein): This protein transports vitamin D metabolites in circulation. Lower concentrations in obese children may reflect alterations in vitamin D metabolism associated with obesity. Upregulation was paralleled by the adiponectin-interactive protein DsbA-L, suggesting that the VD regulation of adiponectin involves post-transcriptional events. Some pediatric studies link higher VDBP levels with higher HOMA-IR or impaired glucose metabolism, suggesting that VDBP may be involved in the vitamin D–insulin axis. Higher or altered VDBP levels in obese children compared to lean ones are often correlated with insulin resistance or inflammatory markers. Using a proteomic approach, multimeric adiponectin has been identified as a key plasma protein that links VDD with pediatric obesity [8,32,36,43,49,58].
- -
- ADIPOQ (Adiponectin): This is a clinically relevant protein biomarker that reflects metabolic health in children with obesity. Its HMW form is especially significant in insulin sensitivity and is often reduced in obese, insulin-resistant, or vitamin D-deficient children [32].
- -
- -
- -
- -
- PON1 (Paraoxonase 1): This plays a key antioxidant, anti-inflammatory, and detoxifying role. Alterations in its activity—often due to genetic polymorphisms—are associated with a broad spectrum of diseases, particularly those involving oxidative stress, inflammation, lipid dysregulation, and toxin exposure [5,58,64,65,66,67,68].
- -
- ALDH (Aldehyde dehydrogenase): This is a family of enzymes that detoxify aldehydes (through lipid peroxidation, alcohol metabolism, etc.), contributing to oxidative stress control. ALDH isoforms are often upregulated in obese children, particularly in those with metabolic syndrome or fatty liver disease. This suggests a compensatory response to oxidative stress and lipid peroxidation associated with excess adiposity. It has been identified in plasma and liver tissue proteomics in obesity-related NAFLD and may serve as a marker of oxidative stress load or liver dysfunction in early obesity-related organ damage [27,28,69,70].
- -
- ALB (Albumin): This is a major plasma protein; it maintains oncotic pressure and transports hormones, fatty acids, and drugs. In pediatric obesity, total albumin levels may remain normal, but post-translational modifications (e.g., glycation; oxidation) increase. Lower “functional” albumin may reflect oxidative stress or systemic inflammation. It is identified as differentially abundant or modified in obese vs. lean children in serum proteomic panels. Changes in albumin may reflect subclinical inflammation, oxidative damage, or early renal impairment in obesity [20,71,72].
- -
- CD38 (Cluster of differentiation 38): This is a transmembrane enzyme that breaks down NAD+, a key cofactor in cellular metabolism. CD38 is heavily involved in diet-induced obesity and is the protein that shows the most consistent association with cardiometabolic and brain health. Its receptor is involved in immune cell activation, calcium signaling, and NAD+ metabolism. In pediatric obesity, CD38 levels have been found to be lower in metabolically healthy obese (MHO) children compared to metabolically unhealthy obese (MUO) ones. This suggests a potential protective role: lower CD38 may reflect reduced inflammation and a better metabolic profile. It is also linked to brain–immune signaling and blood–brain barrier integrity [40,73].
- -
- LAIR2 (Leukocyte-Associated Immunoglobulin-Like Receptor 2) collagen-binding protein: This is a soluble immune modulator that inhibits immune cell overactivation by interfering with LAIR1. In pediatric obesity, it is lower in MHO children than in MUO children. It may reflect reduced chronic low-grade inflammation, making it a potential marker of a healthier immune–inflammatory status in obesity [40].
- -
- MANF (Mesencephalic Astrocyte-derived Neurotrophic Factor): This is a neurotrophic factor that supports neuron survival and maintains endoplasmic reticulum (ER) function and cellular stress responses. It is reduced in metabolically healthy obese compared to non-metabolically healthy obese children. Its decrease in healthier obese children might suggest lower ER stress or better neural and metabolic balance, highlighting links between metabolic health and brain function [40].
- -
- NRP2 (Neuropilin-2): This is a co-receptor for VEGF isoforms and plays a role in neurological diseases in obese children. It is involved in angiogenesis, neurodevelopment, and immune cell migration. In pediatric obesity, it is lower in MHO children. This suggests that there is improved vascular and neuroimmune regulation in metabolically healthier obesity phenotypes [40].
- -
- HSP90AA1 (Heat Shock Protein beta 1 A, PCYOX1, and HSP90AA1): These are small heat shock proteins involved in cell protection, cytoskeletal stability, and anti-apoptotic functions. They may be upregulated as a cellular defense mechanism against metabolic or oxidative stress. Elevated levels may correlate with insulin resistance or inflammatory stress. They have been detected to be upregulated in obese children with insulin resistance in targeted proteomics studies. They represent a potential early marker of cellular stress and insulin resistance development, predictive for liver steatosis [20,44].
- -
- PDIA3 (Protein Disulfide-isomerase A3): This is an ER-resident protein that assists in protein folding and redox regulation and is often upregulated in obese individuals’ children, indicating ER stress and misfolded protein response, which are common in insulin resistance and obesity. It has been identified in both the liver and plasma proteomes of obese pediatric patients, often in those with NAFLD. It may reflect ER dysfunction and metabolic inflammation, possibly preceding overt comorbidities [20,28,48,58,72].
- -
- Plasma lyso-PEs, especially 16:0 and 22:6 species: Lyso-PEs are bioactive lipid intermediates, formed via the partial hydrolysis of phosphatidylethanolamines, typically by phospholipase A2. They influence membrane fluidity, cell signaling, and inflammation. A pattern of Lyso-PE 16:0 (palmitoyl-LysoPE) ↓ decreasing and Lyso-PE 22:6 (docosahexaenoyl-LysoPE) ↑ increasing was observed in overweight vs. normal-weight children even before classical lipid profile abnormalities. These changes may reflect early alterations in lipid metabolism, cellular stress, or low-grade inflammation and may indicate subclinical shifts in lipid signaling or mitochondrial lipid remodeling [20,41,61,62,74,75].
- -
- IGFBP1 (Insulin-Like Growth Factor Binding Protein 1): This binds to IGF1, regulating its availability for tissues. It is inversely regulated by insulin—a high level of insulin suppresses IGFBP1 production. IGFBP1 is consistently found to be reduced in obese and insulin-resistant children. This reflects hyperinsulinemia, even before glucose tolerance declines. IGFBP1 (and IGFBP2) are often measured in proteomic studies via multiplex immunoassays, SOMAscan, or targeted mass spectrometry. These proteins are among the most reliable early indicators of metabolic dysfunction, especially when combined with inflammatory and lipid-related markers [55,76,77,78,79].
- -
- -
- -
- S100 Proteins (S100-A8 and S100-A9): These calcium-binding proteins form the calprotectin complex, which is involved in inflammatory processes. Their elevated expression in obese individuals further highlights the inflammatory component of obesity [80].
- -
- -
- NEBL (Nebulette): This may reflect subclinical cardiovascular remodeling or cardiac stress in pediatric obesity [43].
- -
- Galectin 3BP (Galectin-3 binding protein): This is a novel marker of obesity and metabolic syndrome [35].
- -
- SEM4A, PSB3, DDC, C1RL1, T132A (uncharacterized), pyruvate carboxylase, and C1-esterase inhibitor: These proteins implicate inflammation, metabolic stress, proteostasis dysregulation, and immune activation in the early pathogenesis of diabetic kidney disease. They could be used as markers in early risk stratification and targeted interventions in obese youth-onset T2D [39].
- -
- RAS-GTPase: This is involved in insulin signaling, activating the PI3K/Akt and MAPK pathways. Its dysregulation leads to insulin resistance. eIF4E Eukaryotic translation initiation factor 4E leads to translation initiation and metabolic stress [29].
- -
- -
- SSP1 (Osteopontin): This is a known bone remodeling and inflammatory marker. It indicates that in obese adolescents, inflammatory/integrity pathways play a key role in bone development and density changes [45].
- -
- MFAP5 (Microfibrillar-associated protein 5): This is an extracellular matrix protein implicated in bone strength, used to identify youth at risk for poor bone accrual. It is particularly important in the context of excess adiposity [45].
- -
- p38 MAPK (Mitogen-activated protein kinase): This is involved in inflammatory signaling (e.g., TNF-α; IL-6), insulin signaling modulation, adipocyte differentiation and lipolysis, and muscle and liver glucose homeostasis [29].
- -
- AHSG (Fetuin A): Children with obesity or overweight consistently show elevated serum and salivary fetuin-A levels. Increased fetuin-A correlates with increased BMI, waist circumference, HOMA-IR (insulin resistance index), and triglyceride levels. Weight loss or physical activity programs can lower fetuin-A levels, reflecting improved insulin sensitivity and metabolic health [63].
- -
- MSR1 (Macrophage scavenger receptor type I): Membrane glycoproteins are implicated in the pathological deposition of cholesterol in the arterial wall. This protein plays a role in clearing infectious agents and toxic molecules in pro- and anti-inflammatory responses and cardiometabolic and brain health [80].
- -
- STAT3: Proteomic/phosphoproteomic studies in children are still emerging. However, STAT3′s downstream involvement in energy homeostasis, inflammation, and hypothalamic regulation suggest that it is an important therapeutic and mechanistic target in pediatric metabolic research [41].
- -
- PDK1 (Phosphoinositide-Dependent Kinase 1): PDK1 is a central kinase downstream of PI3K, responsible for the phosphorylation and activation of Akt/PKB and other AGC kinases—key players in insulin, growth factor, and metabolic signaling. Though direct evidence in pediatric obesity is currently limited, PDK1′s role in insulin pathway functionality is well established. Future proteomic or phosphoproteomic studies may reveal its regulation in relation to adiposity, insulin resistance, or growth hormone signaling in youth [41].
- -
- OLFM1: This is a brain-enriched protein that shows decreased levels in children with obesity [40].
- -
- ANPEP (Aminopeptidase N): This points to alterations in the gut–metabolic axis or digestive enzyme regulation in obese children [43].
- -
- FHL3 (Four and a Half LIM Domains 3): This may reflect impaired muscle development, lower lean mass, or altered muscle signaling in obesity [43].
- -
- UMOD (Uromodulin): This suggests a kidney–metabolism connection; lower levels may indicate systemic inflammation or early renal stress [43].
- -
- KIM1 (Kidney Injury Molecule-1): Elevated plasma levels correlate with decreased vagal heart rate variability (HRV), a marker of poor cardiovascular autonomic health. KIM1 is one of the three proteins (with IDUA and BOC) most consistently associated with at least three heart rate parameters, suggesting early cardiovascular stress in children with obesity [32].
- -
- -
- IDUA (Alpha-L-iduronidase): Plasma IDUA levels are positively correlated with HRV—a higher IDUA means a better vagal tone. It is ranked among the key proteins associated with ≥3 HRV metrics, implying a potentially protective cardiovascular effect [42].
- -
- -
- MYH10: This points to improved muscle–adipocyte physiology and insulin signaling [41].
- -
- CNDP1: Its changes correlate with BMI, but its role in obesity requires further investigation [41].
- -
- CDH2 (Cadherin 2): A cell–cell adhesion molecule (N-cadherin), may reflect hepatic or adipose tissue remodeling, showing significant correlation with the severity of steatosis [45].
- -
- -
- LILRA5 (Leukocyte Immunoglobulin-Like Receptor A5): This is an immune receptor on leukocytes; it is possibly linked to inflammation-driven liver pathology, showing a significant correlation with the severity of steatosis [27].
- -
- ALPL (Alkaline Phosphatase): This is significantly upregulated in obese subjects. Elevated ALPL levels are correlated with cardiovascular risk factors, including systolic/diastolic blood pressure, mean arterial pressure, carotid intima–media thickness, and a trend toward higher fasting insulin, a consistent and novel neutrophil activation marker in obesity [34,84].
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D-DIGE | 2-dimensional Differential Gel Electrophoresis |
| BMI | Body Mass Index |
| DIA | Data-Independent Acquisition |
| ELISA | Enzyme-Linked ImmunoSorbent Assay |
| LC-MS/MS | Liquid Chromatography–Tandem Mass Spectrometry |
| LSG | Laparoscopic Sleeve Gastrectomy |
| MALDI-TOF | Matrix-Assisted Laser Desorption/Ionization Time-Of-Flight |
| MHO | Metabolically Healthy Obese |
| MRM | Multiple Reaction Monitoring |
| MS | Mass Spectrometry |
| MUO | Metabolically Unhealthy Obese |
| SRM | Selected Reaction Monitoring |
| T2D | Type 2 Diabetes |
| TMT | Tandem Mass Tagging |
| PEA | Proximity Extension Assay |
| PPI | Protein–Protein Interaction |
| RCT | Randomized Controlled Trial |
References
- WHO European Childhood Obesity Surveillance Initiative (COSI). Available online: https://www.who.int/europe/initiatives/who-european-childhood-obesity-surveillance-initiative-(cosi) (accessed on 30 June 2022).
- Vasile, C.M.; Padovani, P.; Rujinski, S.D.; Nicolosu, D.; Toma, C.; Turcu, A.A.; Cioboata, R. The Increase in Childhood Obesity and Its Association with Hypertension during Pandemics. J. Clin. Med. 2023, 12, 5909. [Google Scholar] [CrossRef] [PubMed]
- Lifshitz, F. Obesity in Children. J. Clin. Res. Pediatr. Endocrinol. 2011, 1, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Weihe, P.; Weihrauch-Blüher, S. Metabolic Syndrome in Children and Adolescents: Diagnostic Criteria, Therapeutic Options and Perspectives. Curr. Obes. Rep. 2019, 8, 472–479. [Google Scholar] [CrossRef]
- Hawton, K.; Shirodkar, D.; Siese, T.; Hamilton-Shield, J.P.; Giri, D. A recent update on childhood obesity: Aetiology, treatment and complications. J. Pediatr. Endocrinol. Metab. 2025, 38, 429–441. [Google Scholar] [CrossRef]
- Chung, S. Childhood Obesity and Cardiovascular Disease Risk. Curr. Atheroscler. Rep. 2023, 25, 405–415. [Google Scholar] [CrossRef]
- Ding, W.; Mak, R.H. Early markers of obesity-related renal injury in childhood. Pediatr. Nephrol. 2015, 30, 1–4. [Google Scholar] [CrossRef]
- Lim, C.Y.S.; Foo, Y.W.; Tok, C.L.X.; Lim, Y.Y.; Loke, K.Y.; Lee, Y.S.; Ng, N.B.H. Screening for metabolic complications of childhood and adolescent obesity: A scoping review of national and international guidelines. Obes. Rev. 2022, 23, e13513. [Google Scholar] [CrossRef]
- Pacheco, L.S.; Blanco, E.; Burrows, R.; Reyes, M.; Lozoff, B.; Gahagan, S. Early Onset Obesity and Risk of Metabolic Syndrome Among Chilean Adolescents. Prev. Chronic. Dis. 2017, 14, 170132. [Google Scholar] [CrossRef]
- Malhotra, S.; Sivasubramanian, R.; Singhal, V. Adult obesity and its complications: A pediatric disease? Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Li, R.; Yao, P.; Yu, H.; Pan, A.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Liu, G. Proteomic signatures of healthy dietary patterns are associated with lower risks of major chronic diseases and mortality. Nat. Food 2024, 6, 47–57. [Google Scholar] [CrossRef]
- Thrush, A.B.; Antoun, G.; Nikpay, M.; A Patten, D.; DeVlugt, C.; Mauger, J.-F.; Beauchamp, B.L.; Lau, P.; Reshke, R.; Doucet, É.; et al. Diet-resistant obesity is characterized by a distinct plasma proteomic signature and impaired muscle fiber metabolism. Int. J. Obes. 2018, 42, 353–362. [Google Scholar] [CrossRef]
- O’rEilly, M.; Dillon, E.; Guo, W.; Finucane, O.; McMorrow, A.; Murphy, A.; Lyons, C.; Jones, D.; Ryan, M.; Gibney, M.; et al. High-density lipoprotein proteomic composition, and not efflux capacity, reflects differential modulation of reverse cholesterol transport by saturated and monounsaturated fat diets. Circulation 2016, 133, 13. [Google Scholar] [CrossRef]
- Calabrese, F.M.; Porrelli, A.; Vacca, M.; Comte, B.; Nimptsch, K.; Pinart, M.; Pischon, T.; Pujos-Guillot, E.; De Angelis, M. Metaproteomics Approach and Pathway Modulation in Obesity and Diabetes: A Narrative Review. Nutrients 2021, 14, 47. [Google Scholar] [CrossRef]
- Figarska, S.M.; Rigdon, J.; Ganna, A.; Elmståhl, S.; Lind, L.; Gardner, C.D.; Ingelsson, E. Proteomic profiles before and during weight loss: Results from randomized trial of dietary intervention. Sci. Rep. 2020, 10, 7913. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, L.; Garlantézec, R.; Audouze, K.; Bustamante, M.; Carracedo, Á.; Chatzi, L.; González, J.R.; Gražulevičienė, R.; Keun, H.; E Lau, C.-H.; et al. Childhood exposure to non-persistent endocrine disrupting chemicals and multi-omic profiles: A panel study. Environ. Int. 2023, 173, 107856. [Google Scholar] [CrossRef]
- Stratakis, N.; Anguita-Ruiz, A.; Fabbri, L.; Maitre, L.; González, J.R.; Andrusaityte, S.; Basagaña, X.; Borràs, E.; Keun, H.C.; Chatzi, L.; et al. Multi-omics architecture of childhood obesity and metabolic dysfunction uncovers biological pathways and prenatal determinants. Nat. Commun. 2025, 16, 654. [Google Scholar] [CrossRef] [PubMed]
- Martos-Moreno, G.Á.; Sackmann-Sala, L.; Barrios, V.; E Berrymann, D.; Okada, S.; Argente, J.; Kopchick, J.J. Proteomic analysis allows for early detection of potential markers of metabolic impairment in very young obese children. Int. J. Pediatr. Endocrinol. 2014, 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, X.; Heianza, Y.; Manson, J.E.; Qi, L. Proteomic Signature of BMI and Risk of Cardiovascular Disease. Clin. Chem. 2024, 70, 1474–1484. [Google Scholar] [CrossRef]
- Rodriguez-Muñoz, A.; Motahari-Rad, H.; Martin-Chaves, L.; Benitez-Porres, J.; Rodriguez-Capitan, J.; Gonzalez-Jimenez, A.; Insenser, M.; Tinahones, F.J.; Murri, M. A Systematic Review of Proteomics in Obesity: Unpacking the Molecular Puzzle. Curr. Obes. Rep. 2024, 13, 403–438, Correction in Curr. Obes. Rep. 2024, 13, 439. https://doi.org/10.1007/s13679-024-00575-y. [Google Scholar] [CrossRef]
- Medeiros, I.; Aguiar, A.J.F.C.; Fortunato, W.M.S.; Teixeira, A.F.G.S.; e Silva, E.G.S.O.; Bezerra, I.W.L.; Maia, J.K.d.S.; Piuvezam, G.; Morais, A.H.d.A. In silico structure-based design of peptides or proteins as therapeutic tools for obesity or diabetes mellitus: A protocol for systematic review and meta analysis. Medicine 2023, 102, e33514. [Google Scholar] [CrossRef]
- Castro, A.P.P.; Hermsdorff, H.H.M.; Milagres, L.C.; de Albuquerque, F.M.; Filgueiras, M.d.S.; Rocha, N.P.; de Novaes, J.F. Increased ApoB/ApoA1 ratio is associated with excess weight, body adiposity, and altered lipid profile in children. J. Pediatr. 2019, 95, 238–246. [Google Scholar] [CrossRef]
- Conroy, R.; Espinal, Y.; Fennoy, I.; Accacha, S.; Boucher-Berry, C.; Carey, D.E.; Close, S.; DeSantis, D.; Gupta, R.; Hassoun, A.A.; et al. Retinol binding protein 4 is associated with adiposity-related co-morbidity risk factors in children. J. Pediatr. Endocrinol. Metab. 2011, 24, 913–919. [Google Scholar] [CrossRef][Green Version]
- Doumatey, A.P.; Zhou, J.; Zhou, M.; Prieto, D.; Rotimi, C.N.; Adeyemo, A. Proinflammatory and lipid biomarkers mediate metabolically healthy obesity: A proteomics study. Obesity 2016, 24, 1257–1265. [Google Scholar] [CrossRef]
- Huen, K.; Harley, K.; Beckman, K.; Eskenazi, B.; Holland, N. Associations of PON1 and Genetic Ancestry with Obesity in Early Childhood. PLoS ONE 2013, 8, e62565. [Google Scholar] [CrossRef]
- Liao, J.; Goodrich, J.A.; Chen, W.; Qiu, C.; Chen, J.C.; Costello, E.; Alderete, T.L.; Chatzi, L.; Gilliland, F.; Chen, Z. Cardiometabolic profiles and proteomics associated with obesity phenotypes in a longitudinal cohort of young adults. Sci. Rep. 2024, 14, 7384. [Google Scholar] [CrossRef] [PubMed]
- Giraudi, P.J.; Pascut, D.; Banfi, C.; Ghilardi, S.; Tiribelli, C.; Bondesan, A.; Caroli, D.; Minocci, A.; Sartorio, A. Serum proteome signatures associated with liver steatosis in adolescents with obesity. J. Endocrinol. Investig. 2024, 48, 213–225. [Google Scholar] [CrossRef]
- Galata, Z.; Moschonis, G.; Makridakis, M.; Dimitraki, P.; Nicolaides, N.C.; Manios, Y.; Bartzeliotou, A.; Chrousos, G.P.; Charmandari, E. Plasma proteomic analysis in obese and overweight prepubertal children. Eur. J. Clin. Investig. 2011, 41, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, S.W.; Poth, M.; McIver, H.; Ayika, C.; Eidelman, O.; Jozwik, C.; Pollard, H.B. Plasma Proteomic Signature in Overweight Girls Closely Correlates with Homeostasis Model Assessment (HOMA), an Objective Measure of Insulin Resistance. Hum. Genom. 2011, 2011, 323629. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gozal, D.; Jortani, S.; Snow, A.B.; Kheirandish-Gozal, L.; Bhattacharjee, R.; Kim, J.; Capdevila, O.S. Two-Dimensional Differential In-Gel Electrophoresis Proteomic Approaches Reveal Urine Candidate Biomarkers in Pediatric Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2009, 180, 1253–1261. [Google Scholar] [CrossRef]
- Oberbach, A.; Bergen, M.V.; Blüher, S.; Lehmann, S.; Till, H. Combined Serum Proteomic and Metabonomic Profiling After Laparoscopic Sleeve Gastrectomy in Children and Adolescents. J. Laparoendosc. Adv. Surg. Tech. 2012, 22, 184–188. [Google Scholar] [CrossRef]
- Walker, G.E.; Marzullo, P.; Prodam, F.; Bona, G.; Di Blasio, A.M. Obesity modifies expression profiles of metabolic markers in superficial and deep subcutaneous abdominal adipose tissue depots. Endocrine 2014, 46, 99–106. [Google Scholar] [CrossRef]
- López-Villar, E.; Martos-Moreno, G.Á.; Chowen, J.A.; Okada, S.; Kopchick, J.J.; Argente, J. A proteomic approach to obesity and type 2 diabetes. J. Cell. Mol. Med. 2015, 19, 1455–1470. [Google Scholar] [CrossRef]
- Pan, Y.; Choi, J.-H.; Shi, H.; Zhang, L.; Su, S.; Wang, X. Discovery and Validation of a Novel Neutrophil Activation Marker Associated with Obesity. Sci. Rep. 2019, 9, 3433. [Google Scholar] [CrossRef]
- Zhen, S.; Ma, Y.; Han, Y.; Zhao, Z.; Yang, X.; Wen, D. Serum galectin-3BP as a novel marker of obesity and metabolic syndrome in Chinese adolescents. BMJ Open Diabetes Res. Care 2021, 9, e001894. [Google Scholar] [CrossRef]
- Ramírez-Vélez, R.; García-Hermoso, A.; Correa-Rodríguez, M.; Fernández-Irigoyen, J.; Palomino-Echeverría, S.; Santamaría, E.; Correa-Bautista, J.E.; González-Ruíz, K.; Izquierdo, M. Effects of Different Doses of Exercise on Inflammation Markers Among Adolescents With Overweight/Obesity: HEPAFIT Study. J. Clin. Endocrinol. Metab. 2022, 107, e2619–e2627. [Google Scholar] [CrossRef]
- Selvaraju, V.; Babu, J.R.; Geetha, T. Multiplexed measurements of salivary fetuin-A, insulin, and adiponectin as potential non-invasive biomarkers in childhood obesity. Cytokine 2022, 153, 155843. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ayllon, M.; Plaza-Florido, A.; Mendez-Gutierrez, A.; Altmäe, S.; Solis-Urra, P.; Aguilera, C.M.; Catena, A.; Ortega, F.B.; Esteban-Cornejo, I. The effects of a 20-week exercise program on blood-circulating biomarkers related to brain health in overweight or obese children: The ActiveBrains project. J. Sport Health Sci. 2023, 12, 175–185. [Google Scholar] [CrossRef]
- Pyle, L.; Choi, Y.J.; Narongkiatikhun, P.; Sharma, K.; Waikar, S.; Layton, A.; Tommerdahl, K.L.; de Boer, I.; Vigers, T.; Nelson, R.G.; et al. Proteomic Analysis Uncovers Multiprotein Signatures Associated with Early Diabetic Kidney Disease in Youth with Type 2 Diabetes Mellitus. Clin. J. Am. Soc. Nephrol. 2024, 19, 1603–1612. [Google Scholar] [CrossRef]
- Olvera-Rojas, M.; Plaza-Florido, A.; Solis-Urra, P.; Osuna-Prieto, F.J.; Ortega, F.B. Neurological-related proteomic profiling in plasma of children with metabolic healthy and unhealthy overweight/obesity. Pediatr. Obes. 2024, 19, e13155. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, H.; Zhu, L. Profound perturbations are found in the proteome and metabolome in children with obesity after weight loss intervention. Heliyon 2024, 10, e31917. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Florido, A.; Olvera-Rojas, M.; Alcantara, J.M.A.; Radom-Aizik, S.; Ortega, F.B. Targeted proteomics involved in cardiovascular health and heart rate variability in children with overweight/obesity. Am. J. Hum. Biol. 2024, 36, e24113. [Google Scholar] [CrossRef]
- Niu, L.; Stinson, S.E.; Holm, L.A.; Lund, M.A.V.; Fonvig, C.E.; Cobuccio, L.; Meisner, J.; Juel, H.B.; Fadista, J.; Thiele, M.; et al. Plasma proteome variation and its genetic determinants in children and adolescents. Nat. Genet. 2025, 57, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.; Quesada-López, T.; Villarroya, F.; Casano, P.; López-Bermejo, A.; de Zegher, F.; Ibáñez, L. The Proteome of Exosomes at Birth Predicts Insulin Resistance, Adrenarche and Liver Fat in Childhood. Int. J. Mol. Sci. 2025, 26, 1721. [Google Scholar] [CrossRef]
- Beglarian, E.; Chen, J.C.; Li, Z.; Costello, E.; Wang, H.; Hampson, H.; Alderete, T.L.; Chen, Z.; Valvi, D.; Rock, S.; et al. Proteins and pathways involved in inflammation are longitudinally associated with total body bone mineral density among primarily Hispanic overweight/obese adolescents and young adults. J. Bone Miner. Res. 2025, 40, 372–381. [Google Scholar] [CrossRef]
- Gayret, Ö.B.; Taşdemir, M.; Erol, M.; Nacaroğlu, H.T.; Zengi, O.; Yiğit, Ö. Are there any new reliable markers to detect renal injury in obese children? Ren. Fail. 2018, 40, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Liang, Y.; Li, H.; Wang, M.; Wang, B.; Chen, X.; Zhou, N.; Cao, D.; Wu, J. Plasma metabolic fingerprinting of childhood obesity by GC/MS in conjunction with multivariate statistical analysis. J. Pharm. Biomed. Anal. 2010, 52, 265–272. [Google Scholar] [CrossRef]
- Alshahrani, A.; Aljada, A.; Masood, A.; Mujammami, M.; Alfadda, A.A.; Musambil, M.; Alanazi, I.O.; Al Dubayee, M.; Rahman, A.M.A.; Benabdelkamel, H. Proteomic Profiling Identifies Distinct Regulation of Proteins in Obese Diabetic Patients Treated with Metformin. Pharmaceuticals 2023, 16, 1345. [Google Scholar] [CrossRef]
- Ravnsborg, T.; Svaneklink, S.; Andersen, L.L.T.; Larsen, M.R.; Jensen, D.M.; Overgaard, M. First-trimester proteomic profiling identifies novel predictors of gestational diabetes mellitus. PLoS ONE 2019, 14, e0214457. [Google Scholar] [CrossRef]
- Chirico, V.; Lacquaniti, A.; Manti, S.; Cuppari, C.; D’Angelo, G.; Lanzafame, A.; Filippelli, M.; Munafó, C.; Salpietro, C.; Arrigo, T. New available biomarkers to face a worldwide emergency: The childhood obesity. J. Pediatr. Biochem. 2014, 4, 139–143. [Google Scholar] [CrossRef]
- Arnold, T.; Brandlhofer, S.; Vrtikapa, K.; Stangl, H.; Hermann, M.; Zwiauer, K.; Mangge, H.; Karwautz, A.; Huemer, J.; Koller, D.; et al. Effect of Obesity on Plasma Clusterin: A Proposed Modulator of Leptin Action. Pediatr. Res. 2011, 69, 237–242, Erratum in Pediatr. Res. 2011, 69, 358. https://doi.org/10.1203/PDR.0b013e3182172574. [Google Scholar] [CrossRef] [PubMed]
- Chiellini, C.; Bertacca, A.; Novelli, S.E.; Görgün, C.Z.; Ciccarone, A.; Giordano, A.; Xu, H.; Soukas, A.; Costa, M.; Gandini, D.; et al. Obesity modulates the expression of haptoglobin in the white adipose tissue via TNFα. J. Cell. Physiol. 2002, 190, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Manti, L.; D’Angelo, C.; Lanzafame, F.; Munafò, S.; Arrigo, C. New available biomarkers to face a world-wide emergency: The childhood obesity. J. Pediatr. Biochem. 2016. [Google Scholar]
- Huang, Y.; Nagamani, M.; Anderson, K.E.; Kurosky, A.; Haag, A.M.; Grady, J.J.; Lu, L.-J.W. A strong association between body fat mass and protein profiles in nipple aspirate fluid of healthy premenopausal non-lactating women. Breast Cancer Res. Treat. 2007, 104, 57–66. [Google Scholar] [CrossRef][Green Version]
- Rahman, A.; Hammad, M.M.; Al Khairi, I.; Cherian, P.; Al-Sabah, R.; Al-Mulla, F.; Abu-Farha, M.; Abubaker, J. Profiling of Insulin-Like Growth Factor Binding Proteins (IGFBPs) in Obesity and Their Association With Ox-LDL and Hs-CRP in Adolescents. Front. Endocrinol. 2021, 12, 727004. [Google Scholar] [CrossRef]
- Kosteria, I.; Kanaka-Gantenbein, C.; Anagnostopoulos, A.K.; Chrousos, G.P.; Tsangaris, G.T. Pediatric endocrine and metabolic diseases and proteomics. J. Proteom. 2018, 188, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Martínez-Barricarte, R.; Catalán, V.; Sabater, M.; Gómez-Ambrosi, J.; Ortega, F.J.; Ricart, W.; Blüher, M.; Frühbeck, G.; de Cordoba, S.R.; et al. Complement Factor H Is Expressed in Adipose Tissue in Association With Insulin Resistance. Diabetes 2010, 59, 200–209. [Google Scholar] [CrossRef]
- Newton, D.A.; Baatz, J.E.; Kindy, M.S.; Gattoni-Celli, S.; Shary, J.R.; Hollis, B.W.; Wagner, C.L. Insights image for vitamin D binding protein polymorphisms significantly impact vitamin D status in children. Pediatr. Res. 2019, 86, 674. [Google Scholar] [CrossRef]
- Higgins, V.; Omidi, A.; Tahmasebi, H.; Asgari, S.; Gordanifar, K.; Nieuwesteeg, M.; Adeli, K. Marked Influence of Adiposity on Laboratory Biomarkers in a Healthy Cohort of Children and Adolescents. J. Clin. Endocrinol. Metab. 2020, 105, e1781–e1797. [Google Scholar] [CrossRef]
- Cominetti, O.; Galindo, A.N.; Corthésy, J.; Valsesia, A.; Irincheeva, I.; Kussmann, M.; Saris, W.H.M.; Astrup, A.; McPherson, R.; Harper, M.-E.; et al. Obesity shows preserved plasma proteome in large independent clinical cohorts. Sci. Rep. 2018, 8, 16981. [Google Scholar] [CrossRef] [PubMed]
- Sylvetsky, A.C.; Issa, N.T.; Chandran, A.; Brown, R.J.; Alamri, H.J.; Aitcheson, G.; Walter, M.; Rother, K.I. Pigment Epithelium-Derived Factor Declines in Response to an Oral Glucose Load and Is Correlated with Vitamin D and BMI but Not Diabetes Status in Children and Young Adults. Horm. Res. Paediatr. 2017, 87, 301–306. [Google Scholar] [CrossRef]
- Oberbach, A.; Blüher, M.; Wirth, H.; Till, H.; Kovacs, P.; Kullnick, Y.; Schlichting, N.; Tomm, J.M.; Rolle-Kampczyk, U.; Murugaiyan, J.; et al. Combined Proteomic and Metabolomic Profiling of Serum Reveals Association of the Complement System with Obesity and Identifies Novel Markers of Body Fat Mass Changes. J. Proteome Res. 2011, 10, 4769–4788. [Google Scholar] [CrossRef]
- Reinehr, T.; Stoffel-Wagner, B.; Roth, C.L. Retinol-Binding Protein 4 and Its Relation to Insulin Resistance in Obese Children before and after Weight Loss. J. Clin. Endocrinol. Metab. 2008, 93, 2287–2293. [Google Scholar] [CrossRef]
- Saraswathi, V.; Ai, W.; Kumar, V.; Sharma, K.; Gopal, T.; Kumar, N.; Malhi, H.; Sehrawat, T.; Desouza, C.V. A Pilot Study on the Proteomics Profile of Serum Exosome-Enriched Extracellular Vesicles from Normal versus Individuals with Obesity-Related Insulin Resistance. Biomedicines 2024, 12, 799. [Google Scholar] [CrossRef] [PubMed]
- Copenhaver, M.M.; Yu, C.-Y.; Zhou, D.; Hoffman, R.P. Relationships of complement components C3 and C4 and their genetics to cardiometabolic risk in healthy, non-Hispanic white adolescents. Pediatr. Res. 2020, 87, 88–94. [Google Scholar] [CrossRef]
- Tryggestad, J.B.; Wang, J.J.; Zhang, S.X.; Thompson, D.M.; Short, K.R. Elevated plasma pigment epithelium-derived factor in children with type 2 diabetes mellitus is attributable to obesity: PEDF in youth with T2DM. Pediatr. Diabetes 2015, 16, 600–605. [Google Scholar] [CrossRef]
- Ege, S.; Akduman, H.; Aşır, A.; Korak, T. Excessive Weight Gain During Pregnancy Increased Ponoxarase 1 Level in Neonatal Cord Blood. Antioxidants 2025, 14, 105. [Google Scholar] [CrossRef]
- Seres, I.; Bajnok, L.; Harangi, M.; Sztanek, F.; Koncsos, P.; Paragh, G. Alteration of PON1 Activity in Adult and Childhood Obesity and Its Relation to Adipokine Levels. Paraoxonases Inflamm. Infect. Toxicol. 2010, 660, 129–142. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.; Xu, M.; Yue, T.; Ling, P.; Fang, T.; Luo, S.; Xu, S.; Weng, J. Comparative Proteomic Analysis of Liver Tissues and Serum in db/db Mice. Int. J. Mol. Sci. 2022, 23, 9687. [Google Scholar] [CrossRef]
- Pascut, D.; Giraudi, P.J.; Banfi, C.; Ghilardi, S.; Tiribelli, C.; Bondesan, A.; Caroli, D.; Minocci, A.; Grugni, G.; Sartorio, A. Proteome profiling identifies circulating biomarkers associated with hepatic steatosis in subjects with Prader-Willi syndrome. Front. Endocrinol. 2023, 14, 1254778. [Google Scholar] [CrossRef] [PubMed]
- Atabek, M.E.; Keskin, M.; Yazici, C.; Kendirci, M.; Hatipoglu, N.; Koklu, E.; Kurtoglu, S. Protein oxidation in obesity and insulin resistance. Eur. J. Pediatr. 2006, 165, 753–756. [Google Scholar] [CrossRef]
- Nishimura, R.; Kanda, A.; Sano, H.; Matsudaira, T.; Miyashita, Y.; Morimoto, A.; Shirasawa, T.; Kawaguchi, T.; Tajima, N. Glycated albumin is low in obese, non-diabetic children. Diabetes Res. Clin. Pract. 2006, 71, 334–338. [Google Scholar] [CrossRef]
- Vukovic, R.; Santos, T.J.D.; Ybarra, M.; Atar, M. Children With Metabolically Healthy Obesity: A Review. Front. Endocrinol. 2019, 10, 865. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N.; Gowda, S.G.B.; Gowda, D.; Sakurai, T.; Ikeda-Araki, A.; Bamai, Y.A.; Ketema, R.M.; Kishi, R.; Chiba, H.; Hui, S.-P. Determination of plasma lysophosphatidylethanolamines (lyso-PE) by LC-MS/MS revealed a possible relation between obesity and lyso-PE in Japanese preadolescent children: The Hokkaido study. Ann. Clin. Biochem. Int. J. Lab. Med. 2025, 62, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Anjos, S.; Feiteira, E.; Cerveira, F.; Melo, T.; Reboredo, A.; Colombo, S.; Dantas, R.; Costa, E.; Moreira, A.; Santos, S.; et al. Lipidomics Reveals Similar Changes in Serum Phospholipid Signatures of Overweight and Obese Pediatric Subjects. J. Proteome Res. 2019, 18, 3174–3183. [Google Scholar] [CrossRef]
- Bhangoo, A.; Gupta, R.; Shelov, S.P.; Carey, D.E.; Accacha, S.; Fennoy, I.; Altshuler, L.; Lowell, B.; Rapaport, R.; Rosenfeld, W.; et al. Fasting Serum IGFBP-1 as a Marker of Insulin Resistance in Diverse School Age Groups. Front. Endocrinol. 2022, 13, 840361. [Google Scholar] [CrossRef]
- Goudswaard, L.J.; Smith, M.L.; Hughes, D.A.; Taylor, R.; Lean, M.; Sattar, N.; Welsh, P.; McConnachie, A.; Blazeby, J.M.; Rogers, C.A.; et al. Using trials of caloric restriction and bariatric surgery to explore the effects of body mass index on the circulating proteome. Sci. Rep. 2023, 13, 21077. [Google Scholar] [CrossRef]
- Lewitt, M.S.; Boyd, G.W. Insulin-like Growth Factor-Binding Protein-1 (IGFBP-1) as a Biomarker of Cardiovascular Disease. Biomolecules 2024, 14, 1475. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.E.L.; A Gralewski, K.; Abrams, P.; Brar, P.C.; Gallagher, P.R.; Lipman, T.H.; Brooks, L.J.; Koren, D. Insulin-like growth factor-I and insulin-like growth factor binding protein-1 are related to cardiovascular disease biomarkers in obese adolescents: The IGF axis and adolescent obesity. Pediatr. Diabetes 2016, 17, 77–86. [Google Scholar] [CrossRef]
- Handakas, E.; Lau, C.H.; Alfano, R.; Chatzi, V.L.; Plusquin, M.; Vineis, P.; Robinson, O. A systematic review of metabolomic studies of childhood obesity: State of the evidence for metabolic determinants and consequences. Obes. Rev. 2022, 23, e13384. [Google Scholar] [CrossRef]
- Zaki, M.; El-Bassyouni, H.; Kamal, S.; El-Gammal, M.; Youness, E. Association of serum paraoxonase enzyme activity and oxidative stress markers with dyslipidemia in obese adolescents. Indian J. Endocrinol. Metab. 2014, 18, 340. [Google Scholar] [CrossRef]
- Uysal, M.; Mete, B.; Kara, E.; Demirhindi, H.; Haytoglu, Z.; Yuksel, B.; Turan, I.; Daglioglu, G.; Dogus, Y. Plasma pentraxin-3 levels and its role in childhood obesity—Is it anti-inflammatory? A matched group study. Clin. Endocrinol. 2024, 101, 13–22. [Google Scholar] [CrossRef]
- Luo, Y.; Luo, D.; Li, M.; Tang, B. Insulin Resistance in Pediatric Obesity: From Mechanisms to Treatment Strategies. Pediatr. Diabetes 2024, 2024, 2298306. [Google Scholar] [CrossRef]
- Pan, Y.; Choi, J.; Shi, H.; Su, S.; Wang, X. ALPL, A Novel Marker of Neutrophil Activation in Response to Obesity. FASEB J. 2018, 32, lb477. [Google Scholar] [CrossRef]
- Romano, A.; Del Vescovo, E.; Rivetti, S.; Triarico, S.; Attinà, G.; Mastrangelo, S.; Maurizi, P.; Ruggiero, A. Biomarkers Predictive of Metabolic Syndrome and Cardiovascular Disease in Childhood Cancer Survivors. J. Pers. Med. 2022, 12, 880. [Google Scholar] [CrossRef]
- Masood, A.; Benabdelkamel, H.; Alfadda, A.A. Obesity Proteomics: An Update on the Strategies and Tools Employed in the Study of Human Obesity. High-Throughput 2018, 7, 27. [Google Scholar] [CrossRef]
- Giuliani, E.; Schon, S.B.; Yang, K.; Burns, G.W.; Neff, L.M.; Remmer, H.A.; Teixeira, J.M.; Marsh, E.E. Obesity-induced follicular phase endometrial proteome dysregulation in a well-phenotyped population. FS Sci. 2022, 3, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Foratori-Junior, G.A.; Ventura, T.M.O.; Grizzo, L.T.; Carpenter, G.H.; Buzalaf, M.A.R.; Sales-Peres, S.H.D.C. Label-Free Quantitative Proteomic Analysis Reveals Inflammatory Pattern Associated with Obesity and Periodontitis in Pregnant Women. Metabolites 2022, 12, 1091. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, V.; De Amici, M.; Leonard, M.M.; De Silvestri, A.; Pelizzo, G.; Buttari, N.; Michev, A.; Leggio, M.; Larizza, D.; Cena, H. Serum Calprotectin Level in Children: Marker of Obesity and its Metabolic Complications. Ann. Nutr. Metab. 2018, 73, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, O.I.; Arzumanian, V.A.; Poverennaya, E.V.; Pyatnitskiy, M.A.; Ilgisonis, E.V.; Zgoda, V.G.; Plotnikova, O.A.; Sharafetdinov, K.K.; Lisitsa, A.V.; Tutelyan, V.A.; et al. Does Proteomic Mirror Reflect Clinical Characteristics of Obesity? J. Pers. Med. 2021, 11, 64. [Google Scholar] [CrossRef]
- Ahmed, H.; Fernandes, M.F.; Abbas, K.; Synowsky, S.A.; Shirran, S.L.; Ajjan, R.A.; Stewart, A.J. Quantitative proteomics identifies plasma protein alterations that associate with metabolic and thrombotic profile changes after bariatric surgery. Diabetes Obes. Metab. 2025, 27, 2647–2657. [Google Scholar] [CrossRef]
- Lind, L.; Figarska, S.; Sundström, J.; Fall, T.; Ärnlöv, J.; Ingelsson, E. Changes in Proteomic Profiles are Related to Changes in BMI and Fat Distribution During 10 Years of Aging. Obesity 2020, 28, 178–186. [Google Scholar] [CrossRef]
- Sharma, P.; Roy, A.; Dhamija, R.K.; Bhushan, S.; Baswal, K.; Kulandaisamy, R.; Yadav, S.; Kumar, S.; Inampudi, K.K. A comprehensive proteomic profiling of urinary exosomes and the identification of early non-invasive biomarker in patients with coronary artery disease. J. Proteom. 2024, 293, 105059. [Google Scholar] [CrossRef]
- Goknar, N.; Oktem, F.; Ozgen, I.T.; Torun, E.; Kuçukkoc, M.; Demir, A.D.; Cesur, Y. Determination of early urinary renal injury markers in obese children. Pediatr. Nephrol. 2015, 30, 139–144. [Google Scholar] [CrossRef]
- Xia, Z.; Shen, S.; Wang, L.; Sun, B.; Yin, J.; Huo, J.; Guo, X. Advances in biomarkers of transcriptomics, proteomics and metabolomics and childhood obesity. Chin. J. Sch. Health 2024, 45, 1364–1368. [Google Scholar] [CrossRef]
- Shi, X.; Liu, Y.; Chen, Y.; Jiang, Z.; Ye, M.; Wang, J. The correlation of apolipoprotein B and apolipoprotein A1 with metabolic dysfunction-associated steatotic liver disease in children and adolescents with obesity. Pediatr. Obes. 2025, 20, e70017. [Google Scholar] [CrossRef] [PubMed]
- Schauermann, M.; Wang, R.; Pons-Kuehnemann, J.; Hartmann, M.; Remer, T.; Hua, Y.; Bereket, A.; Wasniewska, M.; Shmoish, M.; Hochberg, Z.; et al. Targeted quantitative analysis of urinary bile acids by liquid chromatography-tandem mass spectrometry: Method development and application to healthy and obese children. J. Steroid Biochem. Mol. Biol. 2025, 249, 106712. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, J.K.; Piras, I.S.; Wu, X.; Sharma, R.; Garcia-Mansfield, K.; Willey, M.; Lovell, B.; Pirrotte, P.; Olson, M.L.; Shaibi, G.Q. Changes in proteomic cargo of circulating extracellular vesicles in response to lifestyle intervention in adolescents with hepatic steatosis. Clin. Nutr. ESPEN 2024, 60, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, S.; Herzog, R.I.; Caprio, S.; Santoro, N.; Tricò, D. Glutamate–Serine–Glycine Index: A Novel Potential Biomarker in Pediatric Non-Alcoholic Fatty Liver Disease. Children 2020, 7, 270. [Google Scholar] [CrossRef]
- Wu, Y.; Li, M.; Zhang, K.; Ma, J.; Gozal, D.; Zhu, Y.; Xu, Z. Quantitative Proteomics Analysis of Serum and Urine with DIA Mass Spectrometry Reveals Biomarkers for Pediatric Obstructive Sleep Apnea. Arch. Bronconeumol. 2025, 61, 67–75. [Google Scholar] [CrossRef]
- Carullo, N.; Zicarelli, M.; Michael, A.; Faga, T.; Battaglia, Y.; Pisani, A.; Perticone, M.; Costa, D.; Ielapi, N.; Coppolino, G.; et al. Childhood Obesity: Insight into Kidney Involvement. Int. J. Mol. Sci. 2023, 24, 17400. [Google Scholar] [CrossRef]
- Bălănescu, A.; Stan, I.; Codreanu, I.; Comănici, V.; Bălănescu, E.; Bălănescu, I.P. Circulating Hsp90 Isoform Levels in Over-weight and Obese Children and the Relation to Nonalcoholic Fatty Liver Disease: Results from a Cross-Sectional Study. Dis. Markers 2019, 2019, 9560247. [Google Scholar] [CrossRef]
- Małecki, P.; Tracz, J.; Łuczak, M.; Figlerowicz, M.; Mazur-Melewska, K.; Służewski, W.; Mania, A. Serum proteome assessment in nonalcoholic fatty liver disease in children: A preliminary study. Expert Rev. Proteomics 2020, 17, 623–632. [Google Scholar] [CrossRef]
- Manell, H.; Tsolakis, N.; Janson, C.; Malinovschi, A.; Alving, K. Multiarray screening identifies plasma proteins associated with Th17 cell differentiation and viral defense in coincident asthma and obesity. Pediatr. Allergy Immunol. 2024, 35, e14187. [Google Scholar] [CrossRef]
- Sztolsztener, K.; Żywno, H.; Hodun, K.; Konończuk, K.; Muszyńska-Rosłan, K.; Latoch, E. Apolipoproteins—New Biomarkers of Overweight and Obesity among Childhood Acute Lymphoblastic Leukemia Survivors? Int. J. Mol. Sci. 2022, 23, 10634. [Google Scholar] [CrossRef]
- Sunderland, K.L.; Tryggestad, J.B.; Wang, J.J.; Teague, A.M.; Pratt, L.V.; Zhang, S.X.; Thompson, D.M.; Short, K.R. Pigment epithelium-Derived Factor (PEDF) Varies with Body Composition and Insulin Resistance in Healthy Young People. J. Clin. Endocrinol. Metab. 2012, 97, E2114–E2118. [Google Scholar] [CrossRef]
- Hu, Z.; Han, L.; Liu, J.; Fowke, J.H.; Han, J.C.; Kakhniashvili, D.; LeWinn, K.Z.; Bush, N.R.; Mason, W.A.; Zhao, Q. Prenatal metabolomic profiles mediate the effect of maternal obesity on early childhood growth trajectories and obesity risk: The Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) Study. Am. J. Clin. Nutr. 2022, 116, 1343–1353. [Google Scholar] [CrossRef]
- Szczerbinski, L.; Wojciechowska, G.; Olichwier, A.; Taylor, M.A.; Puchta, U.; Konopka, P.; Paszko, A.; Citko, A.; Goscik, J.; Fiehn, O.; et al. Untargeted Metabolomics Analysis of the Serum Metabolic Signature of Childhood Obesity. Nutrients 2022, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Hellmuth, C.; Kirchberg, F.F.; Brandt, S.; Moß, A.; Walter, V.; Rothenbacher, D.; Brenner, H.; Grote, V.; Gruszfeld, D.; Socha, P.; et al. An individual participant data meta-analysis on metabolomics profiles for obesity and insulin resistance in European children. Sci. Rep. 2019, 9, 5053. [Google Scholar] [CrossRef]
- Concepcion, J.; Chen, K.; Saito, R.; Gangoiti, J.; Mendez, E.; Nikita, M.E.; Barshop, B.A.; Natarajan, L.; Sharma, K.; Kim, J.J.; et al. Identification of pathognomonic purine synthesis biomarkers by metabolomic profiling of adolescents with obesity and type 2 diabetes. PLoS ONE 2020, 15, e0234970. [Google Scholar] [CrossRef] [PubMed]
- Balikcioglu, P.G.; Trub, C.J.; Balikcioglu, M.; Ilkayeva, O.; White, P.J.; Muehlbauer, M.; Bain, J.R.; Armstrong, S.; Freemark, M. Branched-chain α-keto acids and glutamate/glutamine: Biomarkers of insulin resistance in childhood obesity, Endocrinol. Diabetes Metab. 2023, 6, e388. [Google Scholar] [CrossRef] [PubMed]
| Study | Cohort | Method | Key Findings | Protein |
|---|---|---|---|---|
| Giraudi et al. (2011) [27] | 59 obese adolescents (11–18 yo) | Proximity extension assay (PEA) | Focused on liver steatosis | CDH2, CTSO, and LILRA5 |
| Galata et al. (2011) [28] | Prepubertal boys (10 normal, 10 overweight, 10 obese) | 2-DE + MS | Changes in apolipoproteins highlight early dyslipidemia | APOA1, APOE, APOA4, CFB, CFH, CFI, HP, and CD5L |
| Rothwell et al. (2011) [29] | 5 girls (13–17 yo), but the control group comprised adult females | Clontech antibody microarrays | Plasma proteomics can reflect metabolic dysfunction in obese youth | Identified plasma protein signatures (groups of proteins) that correlate with insulin resistance (e.g., hILP/XIAP, Nup888, Hap-1, Ki-67, TNFa, and ARPK1) |
| Gozal et al. (2011) [30] | 120 children with overweight or obesity | 2D-DIGE + mass spectrometry + Western blotting | Pediatric obstructive sleep apnea (OSA) biomarker discovery | UMOD, UCN3, ORM1, KLK1, and AZGP1 |
| Oberbach et al. (2012) [31] | 6 morbidly obese children and adolescents | Likely LC-MS/MS (not specified) | Shifts in lipid metabolism, insulin sensitivity, and oxidative stress after bariatric procedures | CLU, PEDF, RBP4, and PON1 |
| Walker et al. (2014) [32] | 42 children and adolescents aged between 5 and 18 yo | Two-dimensional electrophoresis (2-DE) and then mass spectrometry (MS) and immunoblotting | The proteomic approach highlights multimeric adiponectin as a potential molecular link between pediatric obesity and vitamin D deficiency. Vitamin D deficiency was associated with the downregulation of high-molecular-weight adiponectin, a protein linked to insulin sensitivity | Adiponectin |
| Martos-Moreno et al. (2014) [18] | 22 obese prepubescents and 21 healthy prepubescents; 20 were obese before intervention and after weight reduction | Two-dimensional gel electrophoresis (2DE) + mass spectrometry (MS) | The study identified differential expression of proteins (231) suggesting early metabolic alterations in obesity | APOA1, Apo-J-clusterin, vitamin D binding, transthyretin, and haptoglobin |
| López-Villar et al. (2015) [33] | Review article on proteomic research tools and their applications in obesity and type 2 diabetes | Mass spectroscopy SRM (Selected Reaction Monitoring) or MRM (Multiple Reaction Monitoring) proteomic profiling of inflammatory pathways/bead-based targeted proteomics (e.g., Luminex, quantifying ~49 metabolic/inflammatory proteins) in serum | Obesity and diabetes linked to inflammatory protein expression | Complement C3, C5–C7, and lipid transport proteins |
| Pan et al. (2019) [34] | 12 obese vs. 12 lean African American male adolescents (aged 14–20) | Label-free quantitative proteomics (likely LC–MS/MS) | Novel protein associated with obesity inflammation ALPL | ALPL |
| Zhen et al. (2021) [35] | 60 (13–18 yo) Chinese normal weight/overweight/obese adolescents | TMT (Tandem Mass Tag) | Galectin-3BP associated with obesity and metabolic syndrome | Galectin3bp |
| Ramirez-Velez et al. (2022) [36] | 95 adolescents aged 11–17 yo | A multiplex proteomic assay | IGFBP1 is a sensitive marker of obesity-related metabolic risk | BLC, Eotaxin, MCP-4, FGF-6, and PARC |
| Selvaraju et al. (2022) [37] | 76 children (6–10 yo) | Multiplexed measurements | Saliva-based assays can detect key metabolic biomarkers | Fetuin-A, insulin, and adiponectin identified as non-invasive biomarkers |
| Rodriguez-Ayllon et al. (2023) [38] | 81 overweight/obese children ~10 yo | Olink 92 protein panel (PEA) | Novel biomarkers influenced by lifestyle interventions. While the exercise program did not affect the selected candidate biomarkers, the identification of MSR1 as a responsive protein suggests potential avenues for future research into its role in health and disease | msr1 |
| Pyle et al. (2024) [39] | Youth-onset T2D 374 baseline plasma samples; >90% had obesity | Multiprotein signature via mass spectrometry | Biomarkers identified for early diabetic kidney disease in obese children | SEM4A, DDC, C1RL1, and pyruvate carboxylase are linked to kidney risk |
| Olvera-Rojas et al. (2024) [40] | 84 10-yo with overweight/obesity | Olink 92 protein panel | Neurological/metabolic proteins such as CD38 and LAIR2 distinguish metabolic health within obesity | CD38, CPM, EDA2R, IL12, JAMB, KYNU, LAYN, MSR1, SMOC2, and LAIR2 |
| Liu et al. (2024) [41] | 6 children (10–14 yo) | TMT and LC-MS | The study identified 43 differentially expressed proteins and 165 metabolites, highlighting pathways involved in lipid metabolism and inflammation | LCAT, GSTA1, PRCP, MYH10, and CNDP1 |
| Plaza-Florido et al. (2024) [42] | 44 (10-yo) | Olink 92 protein panel | Potential novel biomarkers linking cardiovascular-related plasma proteins to autonomic nervous system function in children with overweight or obesity | Eight proteins—KIM1, IgG Fc receptor II-b, IDUA, BOC, IL1RL2, TNFRSF11A, VSIG2, and transferrin (TF) |
| Niu et al. (2025) [43] | 2147 children/adolescents (12-yo) | MS-DIA, high-throughput methods | Identified genetic determinants of proteome variation, BMI-related protein variation, pathway references | CRP, ACPS, SAA1, C3, CFH, CFI, ORM1, LBP, and PRG4 |
| Díaz et al. (2025) [44] | Cord blood-derived exosomes collected at birth from 20 AGA (appropriate-for-gestational-age) and 20 SGA (small-for-gestational-age) infants | Label-free MS followed by bioinformatic pathway and protein–protein interaction (PPI) analyses | Exosome proteomics at birth can reveal protein signatures that predict metabolic outcomes such as insulin resistance and liver fat accumulation later in childhood | PCYOX1 and HSP90AA1 are predictive for liver fat at age 7 |
| Beglarian et al. (2025) [45] | 304 (8–13 yo) Hispanic children and 169 (17–22 yo) Hispanic adolescents | Olink 650 protein panel | 44 inflammation-related proteins, e.g., early biomarkers for impaired bone health | PI3K-Akt pathways |
| Biomarker | Source | Associated Complication | Proteomic Method | Notes |
|---|---|---|---|---|
| APOA1 (Apolipoprotein-A1) | Plasma, serum | Insulin resistance, lipid metabolism | 2D-DIGE, LC-MS/MS | Reduced in obese and insulin-resistant children; reversible with weight loss |
| CLU (Apolipoprotein J, Clusterin) | Plasma, serum | Lipid transport, oxidative stress | 2D electrophoresis, MS | Reduced in obese children |
| HP (Haptoglobin) | Plasma, serum | Inflammation, insulin resistance | 2D-DIGE, MS | Low MW isoforms increased; correlates with IL-6, visfatin |
| CFB, CFH, CFI (Complement Factors B,H,I) | Plasma, serum | Immune activation, obesity | MALDI-TOF/MS analysis of 2-DE | Elevated in obese children |
| VDBP (Vitamin D Binding Protein) | Plasma | Vitamin D metabolism | LC-MS/MS | Reduced in obesity; linked with adiponectin pathway |
| HMW (Adiponectin) | Plasma | Vitamin D deficiency in obesity | 2D-DIGE + MS + Western blot | ↓ in vitamin D-deficient obese children; ↑ after 1 year of vitamin D supplementation |
| TTR (Transthyretin) | Plasma, serum | Thyroid hormone and retinol transport | LC-MS/MS | Decreased in obesity |
| PEDF | Adipose tissue | Insulin resistance, inflammation | Proteomic profiling | Induces insulin resistance and inflammation |
| RBP4 | Plasma | Insulin resistance, endothelial dysfunction | 2D-DIGE, MS | Elevated in obese children |
| PON1 | Serum | Oxidative stress, lipid metabolism | Activity assays, MS | Altered activity in obesity |
| ALDH (Aldehyde dehydrogenase), ALB (Albumin), HSPB1, PDIA3 | Plasma | Stress response, detoxification | LC-MS/MS | Altered expression in obesity |
| Lyso-PEs (16:0, 22:6) | Plasma | Lipid signaling | Lipidomics | Elevated in obese children |
| IGFBP1 | Plasma | Growth factor regulation | Immunoassays, MS | Reduced in obesity |
| CRP | Plasma | Chronic inflammation | Immunoassays | Correlates with BMI in obesity |
| S100-A8, S100-A9, calprotectin | Plasma | Inflammation | Proteomics | Overexpressed in obesity |
| PTX3 | Plasma | Cardiovascular inflammation | TMT-labeled LC-MS/MS and ELISA | Early marker for CV risk |
| Galectin 3BP | Serum | Obesity, metabolic syndrome | TMT-labeled LC-MS/MS and ELISA | Marker for metabolic syndrome risk |
| PCYOX1, HSP90AA1 | Plasma | Liver fat prediction | Targeted proteomics | Predictive for liver steatosis |
| RAS | Plasma, Cellular | Insulin signaling | Antibody microarrays: Clontech | Altered in obesity |
| PKC-η | Plasma, Cellular | Inflammation | Clontech | Upregulated in inflammation |
| PDK1 | Plasma, Cellular | Insulin/PI3K pathway | Antibody microarrays | Altered expression |
| STAT3 | Plasma, Cellular | Inflammation, metabolism | Antibody microarrays | Activated in obesity |
| p38 MAPK | Plasma, Cellular | Stress response | Antibody microarrays | Increased in metabolic stress |
| AHSG (Fetuin-A) | Plasma, saliva | Linked to insulin resistance | 2D-MS differential spots | Correlates with insulin resistance and adiposity |
| CD38 | Plasma | Regulation of cell growth, insulin secretion | LC-MS/MS | Altered expression in cardiovascular and neurological diseases and aging |
| MSR1 | Plasma | Immune response, lipid transport, inflammation metabolism | LC-MS/MS, Olink | Atherosclerosis—positively associated with TG levels and in clearing infectious agents |
| MANF | Plasma | Neuron projection development | LC-MS/MS, Olink | Neurological disease |
| LAIR2 | Plasma | Collagen binding | LC-MS/MS, Olink | Immunological response in obese |
| OLFM1 | Plasma | Neurological system | Olink | Decreased in obese children; may be involved in neurodevelopment and synaptic plasticity |
| ANPEP | Plasma | Digestive system and immune | Olink | Regulates peptide digestion and immune signaling |
| FHL3 | Plasma | Skeletal system | Olink | Muscle development, differentiation, and function |
| UMOD | Plasma | Urological system | Olink | Anti-inflammatory; protects renal function |
| KIM | Plasma, urine | Urological system | Olink | Elevation in obese youth indicates subclinical kidney stress |
| BOC | Plasma, serum | Cardiometabolic system | Olink | Early signs of metabolic and organ-specific stress |
| IDUA | Serum | Cardiovascular system | Olink | Implying a potentially protective cardiovascular effect |
| CDH2 | Serum | Cardiometabolic system | Olink PEA | Liver steatosis |
| CTSO | Serum | Cardiometabolic system | Olink PEA | Liver steatosis |
| LILRA5 | Serum | Cardiometabolic system | Olink PEA | Liver steatosis |
| SEM4A, PSB3, DDC, C1RL1, T132A | Plasma | Kidney disease | SomaScan v.7K | Multiprotein plasma signatures that strongly predict early DKD outcomes in youth-onset T2D—far outperforming clinical markers alone |
| ALPL | Purified neutrophils | Cardiovascular system | Quantitative, label-free mass spectrometry proteomics performed on purified neutrophils | ALPL is consistently elevated in obese neutrophils and correlates with CVD risk markers |
| NRP2 | Plasma | Metabolic health status | Olink | Decrease in metabolic health in children with obesity versus children with metabolic syndrome and obesity |
| SSP1 (Osteopontin) | Plasma | Bone mineral density, cardiovascular system | Olink | Increase in bone mineral density gains in obese children |
| MFAP5 | Plasma | BMD dynamics | Olink (inflammatory and cardiometabolic panel) | Biphasic: ↑ at baseline, ↓ longitudinally |
| GSTA1 | Serum | BMI and oxidative stress after weight loss | TMT-labeled LC-MS/MS | Decrease after weight loss |
| MYH10 | Serum | Obesity-related adipogenesis | TMT-labeled LC-MS/MS | Decrease after weight loss, regulates adipocyte GLUT4 activity |
| CNDP1 | Serum | Obesity and metabolic response | TMT-labeled LC-MS/MS | Increase after weight loss |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krakowczyk, D.; Szeliga, K.; Chyra, M.; Pietrowska, M.; Koszutski, T.; Gawlik-Starzyk, A.; Hyla-Klekot, L. Proteomic Insights into Childhood Obesity: A Systematic Review of Protein Biomarkers and Advances. Int. J. Mol. Sci. 2025, 26, 8522. https://doi.org/10.3390/ijms26178522
Krakowczyk D, Szeliga K, Chyra M, Pietrowska M, Koszutski T, Gawlik-Starzyk A, Hyla-Klekot L. Proteomic Insights into Childhood Obesity: A Systematic Review of Protein Biomarkers and Advances. International Journal of Molecular Sciences. 2025; 26(17):8522. https://doi.org/10.3390/ijms26178522
Chicago/Turabian StyleKrakowczyk, Dominika, Kamila Szeliga, Marcin Chyra, Monika Pietrowska, Tomasz Koszutski, Aneta Gawlik-Starzyk, and Lidia Hyla-Klekot. 2025. "Proteomic Insights into Childhood Obesity: A Systematic Review of Protein Biomarkers and Advances" International Journal of Molecular Sciences 26, no. 17: 8522. https://doi.org/10.3390/ijms26178522
APA StyleKrakowczyk, D., Szeliga, K., Chyra, M., Pietrowska, M., Koszutski, T., Gawlik-Starzyk, A., & Hyla-Klekot, L. (2025). Proteomic Insights into Childhood Obesity: A Systematic Review of Protein Biomarkers and Advances. International Journal of Molecular Sciences, 26(17), 8522. https://doi.org/10.3390/ijms26178522






