Boosted Hydrogen Evolution Catalysis Using Biomass-Derived Mesoporous Carbon Nanosponges
Abstract
1. Introduction
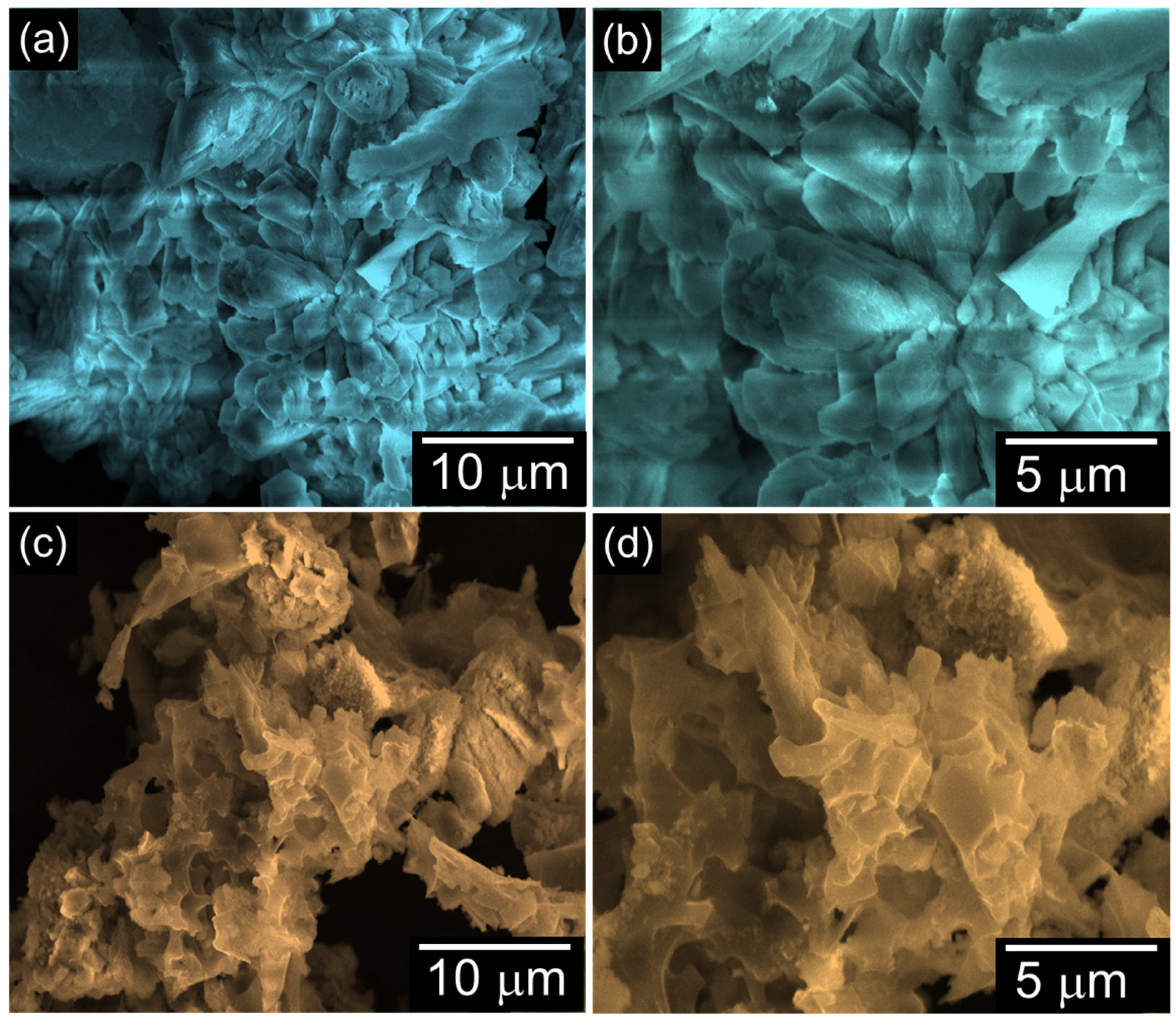
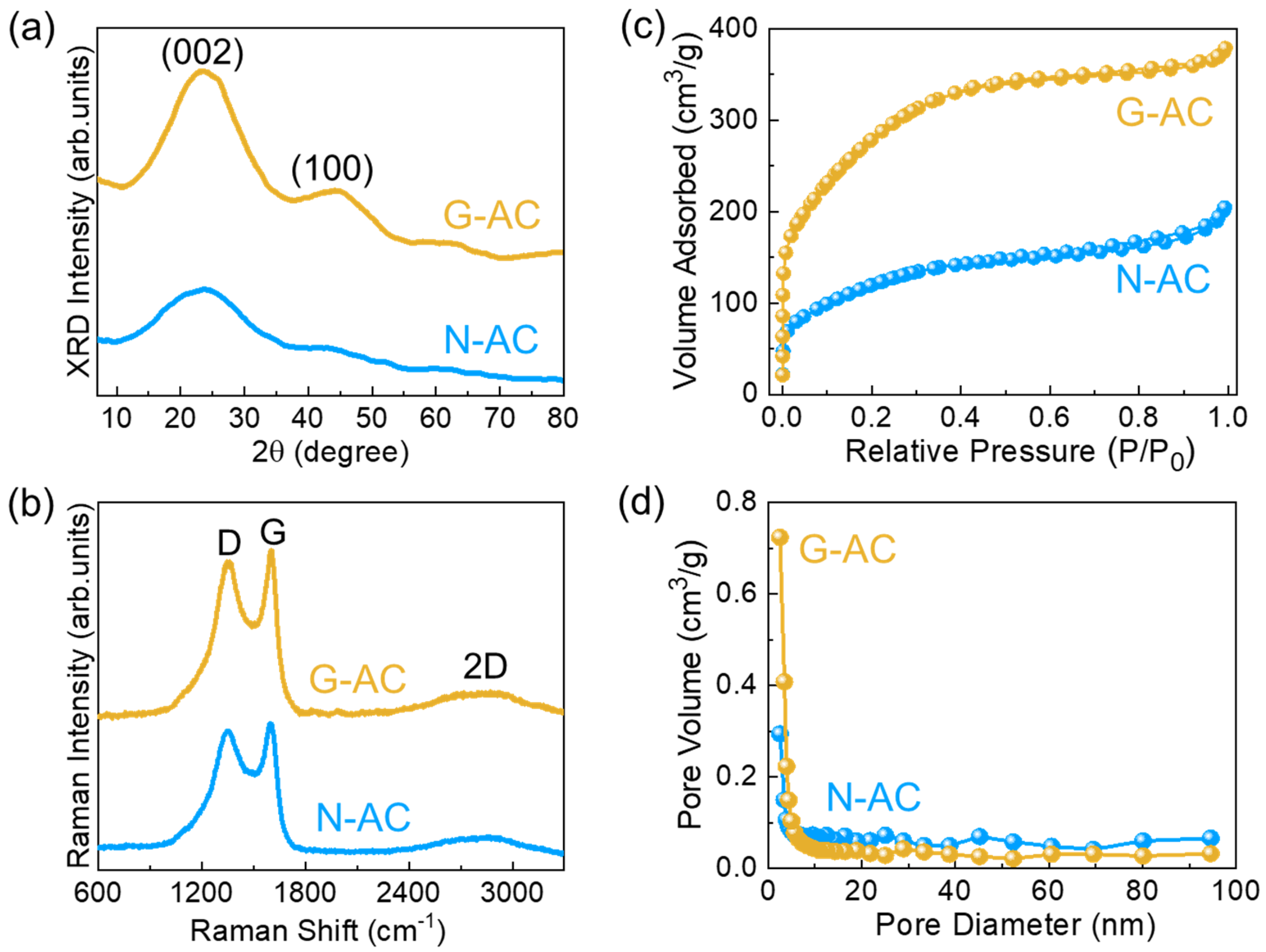
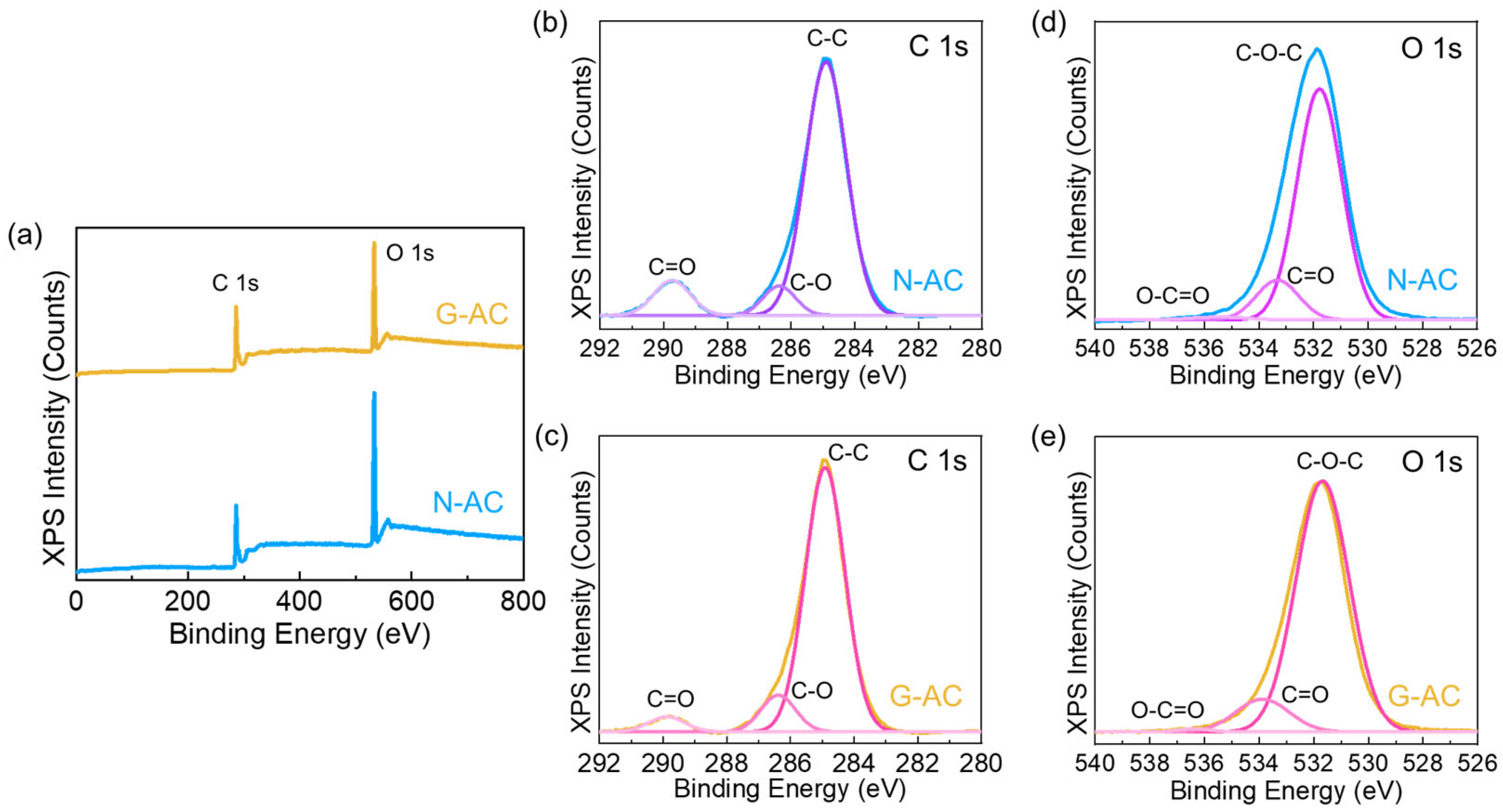
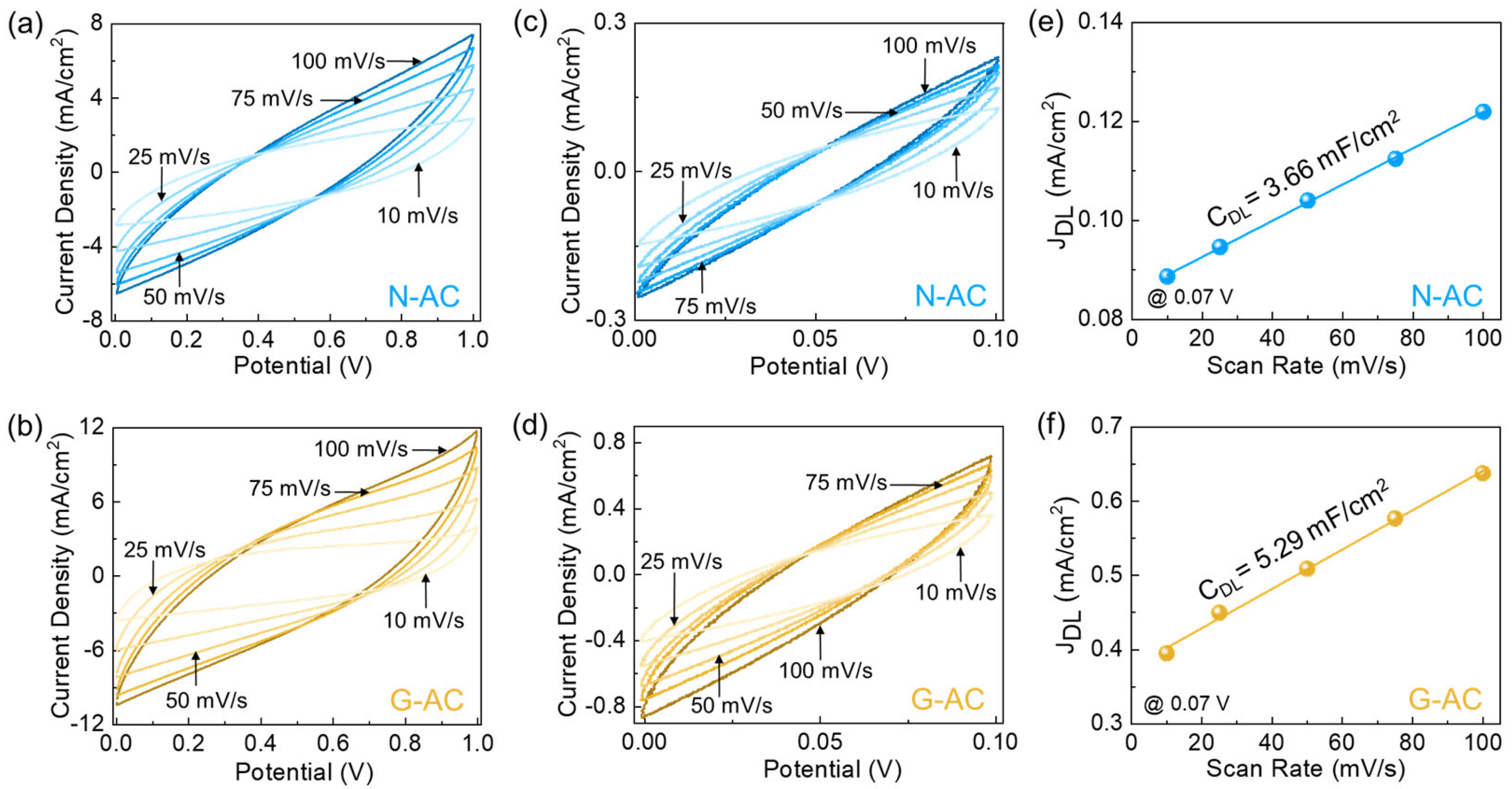
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Preparation of Activated Carbon Nanostructures
3.3. Material Characterizations
3.4. Electrocatalytic HER Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Wang, J.; Yao, Z.; Xia, Q.; Xu, H.; Guo, W.; Wu, X. Enhanced oxygen activation in antibiotic degradation using N-doped carbon skeleton encapsulated high-entropy alloy catalysts. Chem. Eng. J. 2025, 518, 164832. [Google Scholar] [CrossRef]
- Li, H.; Zhang, G.; Jia, X.; Pan, Y.; Yang, Z.; Li, G.; Guan, T.; Wang, K. Chemical vapor deposition-assisted activation for tailoring mesoporous carbon from polar components of coal tar pitch for zinc-ion hybrid capacitors. Chem. Eng. J. 2025, 519, 164974. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Wu, M.; Feng, X.; Redfern, S.A.T.; Shang, Y.; Yong, X.; Feng, T.; Wu, K.; Liu, Z.; et al. Carbon-Quantum-Dots-Loaded Ruthenium Nanoparticles as an Efficient Electrocatalyst for Hydrogen Production in Alkaline Media. Adv. Mater. 2018, 30, 1800676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Guan, J.; Tu, Y.; Chen, S.; Wang, Y.; Wang, S.; Yu, L.; Ma, C.; Deng, D.; Bao, X. Highly efficient H2 production from H2S via a robust graphene-encapsulated metal catalyst. Energy Environ. Sci. 2020, 13, 119–126. [Google Scholar] [CrossRef]
- Jamesh, M.-I.; Hu, D.; Wang, J.; Naz, F.; Feng, J.; Yu, L.; Cai, Z.; Colmenares, J.C.; Lee, D.-J.; Chu, P.K.; et al. Recent advances in noble metal-free electrocatalysts to achieve efficient alkaline water splitting. J. Mater. Chem. A 2024, 12, 11771–11820. [Google Scholar] [CrossRef]
- Sekar, S.; Lee, E.; Yun, J.; Arumugasamy, S.K.; Choi, M.-J.; Lee, Y.; Lee, S. High-performance electrocatalyst of activated carbon-decorated molybdenum trioxide nanocomposites for effective production of H2 and H2O2. Sep. Purif. Technol. 2025, 361, 131614. [Google Scholar] [CrossRef]
- Sekar, S.; Sadhasivam, S.; Lee, Y.; Lee, S. Enhanced bifunctional water electrolysis activities of activated carbon-decorated trimetallic Ni-Al-La nanocomposites. Appl. Surf. Sci. 2025, 698, 163098. [Google Scholar] [CrossRef]
- Sekar, S.; Ilanchezhiyan, P.; Ahmed, A.T.A.; Lee, Y.; Lee, S. Substantial Electrocatalytic Oxygen Evolution Performances of Activated Carbon-Decorated Vanadium Pentoxide Nanocomposites. Int. J. Energy Res. 2024, 2024, 9953038. [Google Scholar] [CrossRef]
- Sadeq, A.M.; Homod, R.Z.; Hussein, A.K.; Togun, H.; Mahmoodi, A.; Isleem, H.F.; Patil, A.R.; Moghaddam, A.H. Hydrogen energy systems: Technologies, trends, and future prospects. Sci. Total Environ. 2024, 939, 173622. [Google Scholar] [CrossRef]
- Hossain Bhuiyan, M.M.; Siddique, Z. Hydrogen as an alternative fuel: A comprehensive review of challenges and opportunities in production, storage, and transportation. Int. J. Hydrogen Energy 2025, 102, 1026–1044. [Google Scholar] [CrossRef]
- Segovia-Hernández, J.G.; Hernández, S.; Cossío-Vargas, E.; Juarez-García, M.; Sánchez-Ramírez, E. Green hydrogen production for sustainable development: A critical examination of barriers and strategic opportunities. RSC Sustain. 2025, 3, 134–157. [Google Scholar] [CrossRef]
- Abdullah, A.; Tariq, F.; Kulkarni, M.A.; Thaalbi, H.; Din, H.U.; Kang, S.H.; Ryu, S.-W. High-efficiency electrocatalytic hydrogen generation under harsh acidic condition by commercially viable Pt nanocluster-decorated non-polar faceted GaN nanowires. Int. J. Hydrogen Energy 2024, 94, 1257–1265. [Google Scholar] [CrossRef]
- Sekar, S.; Shanmugam, A.; Senthilkumar, G.; Thangasami, K.; Jung, H.; Lee, Y.; Lee, S. Enhanced Hydrogen Evolution Reaction Using Biomass-Activated Carbon Nanosheets Derived from Eucalyptus Leaves. Materials 2025, 18, 670. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, Y.; Wang, Y.; Zhao, L.; Zhao, X.; Du, J.; Wu, H.; Chen, A. Next-Generation Green Hydrogen: Progress and Perspective from Electricity, Catalyst to Electrolyte in Electrocatalytic Water Splitting. Nano-Micro Lett. 2024, 16, 237. [Google Scholar] [CrossRef]
- Sekar, S.; Shanmugam, A.; Lee, Y.; Lee, S. Highly Efficient Electrocatalyst of 2D–2D gC3N4–MoS2 Composites for Enhanced Overall Water Electrolysis. Materials 2025, 18, 3775. [Google Scholar] [CrossRef]
- Sekar, S.; Sim, D.H.; Lee, S. Excellent Electrocatalytic Hydrogen Evolution Reaction Performances of Partially Graphitized Activated-Carbon Nanobundles Derived from Biomass Human Hair Wastes. Nanomaterials 2022, 12, 531. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Yu, J.; Liu, X.; Zhang, X.; Liu, H.; Zhou, W. Water Splitting: From Electrode to Green Energy System. Nano-Micro Lett. 2020, 12, 131. [Google Scholar] [CrossRef]
- Chatenet, M.; Pollet, B.G.; Dekel, D.R.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R.D.; Bazant, M.Z.; Eikerling, M.; Staffell, I.; et al. Water electrolysis: From textbook knowledge to the latest scientific strategies and industrial developments. Chem. Soc. Rev. 2022, 51, 4583–4762. [Google Scholar] [CrossRef]
- Sekar, S.; Aqueel Ahmed, A.T.; Sim, D.H.; Lee, S. Extraordinarily high hydrogen-evolution-reaction activity of corrugated graphene nanosheets derived from biomass rice husks. Int. J. Hydrogen Energy 2022, 47, 40317–40326. [Google Scholar] [CrossRef]
- Veeramani, K.; Janani, G.; Kim, J.; Surendran, S.; Lim, J.; Jesudass, S.C.; Mahadik, S.; Lee, H.; Kim, T.-H.; Kim, J.K.; et al. Hydrogen and value-added products yield from hybrid water electrolysis: A critical review on recent developments. Renew. Sustain. Energy Rev. 2023, 177, 113227. [Google Scholar] [CrossRef]
- Wan, Z.; Yang, K.; Li, P.; Yang, S.; Wang, X.; Gao, R.; Xie, X.; Deng, G.; Yang, M.; Wang, Z. Atomically dispersed Au single sites and nanoengineered structural defects enable a high electrocatalytic activity and durability for hydrogen evolution reaction and overall urea electrolysis. Electrochim. Acta 2024, 499, 144685. [Google Scholar] [CrossRef]
- Li, P.; Luo, S.; Xiong, Z.; Xiao, H.; Wang, X.; Peng, K.; Xie, X.; Zhang, Z.; Deng, G.; Yang, M.; et al. RuxMoS2 interfacial heterojunctions achieve efficient overall water splitting and stability in both alkaline and acidic media under large current density exceeding 100 mA cm−2. Mol. Catal. 2025, 570, 114710. [Google Scholar] [CrossRef]
- Hussain, M.B.; Ahmad, M.; Cheng, X.; Mehmood, R.; Ajmal, Z.; Hussain, S.; Tayyab, M.; Gitis, V. ZrO2 supported Pt nanoparticles for robust electrocatalytic hydrogen evolution reactions. Int. J. Hydrogen Energy 2025, 106, 825–833. [Google Scholar] [CrossRef]
- Sekar, S.; Sadhasivam, S.; Nangai, E.K.; Saravanan, S.; Kim, D.Y.; Lee, S. Enhanced Hydrogen Evolution Reaction Performances of Ultrathin CuBi2O4 Nanoflakes. Int. J. Energy Res. 2023, 2023, 5038466. [Google Scholar] [CrossRef]
- Ahmed, K.; Hameed, S.; Patchigolla, K.; Dawood, N.; Ghouri, Z.K. Carbon-based electrocatalysts for hydrogen evolution reaction. Energy Convers. Manage. X 2025, 26, 100892. [Google Scholar] [CrossRef]
- Li, W.; Xu, Y.; Wang, G.; Xu, T.; Wang, K.; Zhai, S.; Si, C. Sustainable Carbon-Based Catalyst Materials Derived From Lignocellulosic Biomass for Energy Storage and Conversion: Atomic Modulation and Properties Improvement. Carbon Energy 2025, 7, e708. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Graphene-based electrocatalysts: Hydrogen evolution reactions and overall water splitting. Int. J. Hydrogen Energy 2021, 46, 21401–21418. [Google Scholar] [CrossRef]
- Cui, W.; Liu, Q.; Cheng, N.; Asiri, A.M.; Sun, X. Activated carbon nanotubes: A highly-active metal-free electrocatalyst for hydrogen evolution reaction. Chem. Commun. 2014, 50, 9340–9342. [Google Scholar] [CrossRef]
- Liu, T.; Yabu, H. Biomass-Derived Electrocatalysts: Low-Cost, Robust Materials for Sustainable Electrochemical Energy Conversion. Adv. Energy Sustain. Res. 2024, 5, 2300168. [Google Scholar] [CrossRef]
- Feng, Y.; Jiang, J.; Xu, Y.; Wang, S.; An, W.; Chai, Q.; Prova, U.H.; Wang, C.; Huang, G. Biomass derived diverse carbon nanostructure for electrocatalysis, energy conversion and storage. Carbon 2023, 211, 118105. [Google Scholar] [CrossRef]
- Gao, J.; Chu, X.; Lu, H.; Wang, H.; Li, X.; Yin, Z.; Tan, X. Efficient carbon-based electrocatalyst derived from biomass for hydrogen peroxide generation. Mater. Today Commun. 2021, 26, 102051. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, Y.; Zheng, R.; Wang, Q.; Li, X.; Wei, H.; Wang, L.; Li, Z.; Wang, F.; Han, N. Biomass-derived carbon material as efficient electrocatalysts for the oxygen reduction reaction. Biomass Bioenergy 2023, 168, 106676. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Bystrzejewski, M.; De Adhikari, A.; Huczko, A.; Wang, N. Methods for the conversion of biomass waste into value-added carbon nanomaterials: Recent progress and applications. Prog. Energy Combust. Sci. 2022, 92, 101023. [Google Scholar] [CrossRef]
- Fahad Halim, A.F.M.; Poinern, G.E.J.; Fawcett, D.; Sharma, R.; Surendran, S.; Rajeshkannan, R. Biomass-Derived Carbon Nanomaterials: Synthesis and Applications in Textile Wastewater Treatment, Sensors, Energy Storage, and Conversion Technologies. CleanMat 2025, 2, 4–58. [Google Scholar] [CrossRef]
- Han, G.; Hu, M.; Liu, Y.; Gao, J.; Han, L.; Lu, S.; Cao, H.; Wu, X.; Li, B. Efficient carbon-based catalyst derived from natural cattail fiber for hydrogen evolution reaction. J. Solid State Chem. 2019, 274, 207–214. [Google Scholar] [CrossRef]
- Prabu, N.; Saravanan, R.S.A.; Kesavan, T.; Maduraiveeran, G.; Sasidharan, M. An efficient palm waste derived hierarchical porous carbon for electrocatalytic hydrogen evolution reaction. Carbon 2019, 152, 188–197. [Google Scholar] [CrossRef]
- Cao, X.; Li, Z.; Chen, H.; Zhang, C.; Zhang, Y.; Gu, C.; Xu, X.; Li, Q. Synthesis of biomass porous carbon materials from bean sprouts for hydrogen evolution reaction electrocatalysis and supercapacitor electrode. Int. J. Hydrogen Energy 2021, 46, 18887–18897. [Google Scholar] [CrossRef]
- Prabu, N.; Kesavan, T.; Maduraiveeran, G.; Sasidharan, M. Bio-derived nanoporous activated carbon sheets as electrocatalyst for enhanced electrochemical water splitting. Int. J. Hydrogen Energy 2019, 44, 19995–20006. [Google Scholar] [CrossRef]
- Arul Saravanan, K.R.; Prabu, N.; Sasidharan, M.; Maduraiveeran, G. Nitrogen-self doped activated carbon nanosheets derived from peanut shells for enhanced hydrogen evolution reaction. Appl. Surf. Sci. 2019, 489, 725–733. [Google Scholar] [CrossRef]
- Hoang, V.C.; Gomes, V.G.; Dinh, K.N. Ni- and P-doped carbon from waste biomass: A sustainable multifunctional electrode for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Electrochim. Acta 2019, 314, 49–60. [Google Scholar] [CrossRef]
- Wang, Q.; Fei, Z.; Shen, D.; Cheng, C.; Dyson, P.J. Ginkgo Leaf-Derived Carbon Supports for the Immobilization of Iron/Iron Phosphide Nanospheres for Electrocatalytic Hydrogen Evolution. Small 2024, 20, 2309830. [Google Scholar] [CrossRef]
- Sundriyal, S.; Shrivastav, V.; Kaur, A.; Mansi; Deep, A.; Dhakate, S.R. Surface and diffusion charge contribution study of neem leaves derived porous carbon electrode for supercapacitor applications using acidic, basic, and neutral electrolytes. J. Energy Storage 2021, 41, 103000. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, X.; Li, L.; Li, X.; Wu, H.; Zheng, J.; Yao, J.; Li, G. Porous and high specific surface area carbon material derived from ginkgo leaves for high-performance symmetric supercapacitors. Biomass Bioenergy 2024, 191, 107481. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, S.; Xu, K.; Zhang, Y.; Zhang, L.; Lou, G.; Wu, Y.; Zhu, E.; Chen, H.; Shen, Z.; et al. Sustainable activated carbons from dead ginkgo leaves for supercapacitor electrode active materials. Chem. Eng. Sci. 2018, 181, 36–45. [Google Scholar] [CrossRef]
- Sekar, S.; Aqueel Ahmed, A.T.; Pawar, S.M.; Lee, Y.; Im, H.; Kim, D.Y.; Lee, S. Enhanced water splitting performance of biomass activated carbon-anchored WO3 nanoflakes. Appl. Surf. Sci. 2020, 508, 145127. [Google Scholar] [CrossRef]
- Sekar, S.; Lee, S.; Vijayarengan, P.; Kalirajan, K.M.; Santhakumar, T.; Sekar, S.; Sadhasivam, S. Upcycling of Wastewater via Effective Photocatalytic Hydrogen Production Using MnO2 Nanoparticles—Decorated Activated Carbon Nanoflakes. Nanomaterials 2020, 10, 1610. [Google Scholar] [CrossRef]
- Manasa, P.; Lei, Z.J.; Ran, F. Biomass Waste Derived Low Cost Activated Carbon from Carchorus Olitorius (Jute Fiber) as Sustainable and Novel Electrode Material. J. Energy Storage 2020, 30, 101494. [Google Scholar] [CrossRef]
- Sankar, S.; Saravanan, S.; Ahmed, A.T.A.; Inamdar, A.I.; Im, H.; Lee, S.; Kim, D.Y. Spherical activated-carbon nanoparticles derived from biomass green tea wastes for anode material of lithium-ion battery. Mater. Lett. 2019, 240, 189–192. [Google Scholar] [CrossRef]
- Xiao, P.-W.; Meng, Q.; Zhao, L.; Li, J.-J.; Wei, Z.; Han, B.-H. Biomass-derived flexible porous carbon materials and their applications in supercapacitor and gas adsorption. Mater. Des. 2017, 129, 164–172. [Google Scholar] [CrossRef]
- Qian, W.; Sun, F.; Xu, Y.; Qiu, L.; Liu, C.; Wang, S.; Yan, F. Human hair-derived carbon flakes for electrochemical supercapacitors. Energy Environ. Sci. 2014, 7, 379–386. [Google Scholar] [CrossRef]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]
- Fu, P.; Zhou, L.; Sun, L.; Huang, B.; Yuan, Y. Nitrogen-doped porous activated carbon derived from cocoon silk as a highly efficient metal-free electrocatalyst for the oxygen reduction reaction. RSC Adv. 2017, 7, 13383–13389. [Google Scholar] [CrossRef]
- Watcharamaisakul, S.; Janphuang, N.; Chueangam, W.; Srisom, K.; Rueangwittayanon, A.; Rittihong, U.; Tunmee, S.; Chanlek, N.; Pornsetmetakul, P.; Wirojsirasak, W.; et al. Synthesis of Turbostratic Graphene Derived from Biomass Waste Using Long Pulse Joule Heating Technique. Nanomaterials 2025, 15, 468. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nano. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Homayounfard, A.M.; Maleki, M.; Ghanbari, H.; Kahnamouei, M.H.; Safaei, B. Growth of few-layer flower-like MoS2 on heteroatom-doped activated carbon as a hydrogen evolution reaction electrode. Int. J. Hydrogen Energy 2024, 55, 1360–1370. [Google Scholar] [CrossRef]
- Rajasekaran, S.J.; Raghavan, V. Facile synthesis of activated carbon derived from Eucalyptus globulus seed as efficient electrode material for supercapacitors. Diam. Relat. Mater 2020, 109, 108038. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Bharathi Sankar, A.; Sri Rohita, D.; Nanaji, K.; Narasinga Rao, T.; Karthik, M. Achieving High Voltage and Excellent Rate Capability Supercapacitor Electrodes Derived From Bio-renewable and Sustainable Resource. ChemistrySelect 2020, 5, 8759–8772. [Google Scholar] [CrossRef]
- Lin, P.; Xia, Y.; Liu, Z. Influence of Different Activators on the Structure and Properties of Activated Carbon Based on Bamboo Fiber. Polymers 2022, 14, 5500. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, L.-y.; Liang, Y.; Chen, Q.; Li, Z.-s.; Li, C.-h.; Wang, Z.-h.; Li, W. Coconut-based activated carbon fibers for efficient adsorption of various organic dyes. RSC Adv. 2018, 8, 42280–42291. [Google Scholar] [CrossRef]
- Shrestha, D.; Maensiri, S.; Wongpratat, U.; Lee, S.W.; Nyachhyon, A.R. Shorea robusta derived activated carbon decorated with manganese dioxide hybrid composite for improved capacitive behaviors. J. Environ. Chem. Eng. 2019, 7, 103227. [Google Scholar] [CrossRef]
- Bejjanki, D.; Banothu, P.; Kumar, V.B.; Kumar, P.S. Biomass-Derived N-Doped Activated Carbon from Eucalyptus Leaves as an Efficient Supercapacitor Electrode Material. C 2023, 9, 24. [Google Scholar] [CrossRef]
- Sukhbaatar, B.; Qing, W.; Seo, J.; Yoon, S.; Yoo, B. Uniformly dispersed ruthenium nanoparticles on porous carbon from coffee waste outperform platinum for hydrogen evolution reaction in alkaline media. Sci. Rep. 2024, 14, 5850. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Yao, Y.; Zhang, J.-W.; Pan, L.; Zhang, X.; Zou, J.-J. Electrocatalysts for Hydrogen Evolution in Alkaline Electrolytes: Mechanisms, Challenges, and Prospective Solutions. Adv. Sci. 2018, 5, 1700464. [Google Scholar] [CrossRef]
- Sukhbaatar, B.; Yoon, S.; Yoo, B. Simple synthesis of a CoO nanoparticle-decorated nitrogen-doped carbon catalyst from spent coffee grounds for alkaline hydrogen evolution. J. Mater. Sci. 2022, 57, 18075–18088. [Google Scholar] [CrossRef]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801, Correction in Sci. Rep. 2020, 10, 1–1. [Google Scholar] [CrossRef]
- Yan, X.; Jia, Y.; Odedairo, T.; Zhao, X.; Jin, Z.; Zhu, Z.; Yao, X. Activated carbon becomes active for oxygen reduction and hydrogen evolution reactions. Chem. Commun. 2016, 52, 8156–8159. [Google Scholar] [CrossRef]
- Chinnadurai, D.; Karuppiah, P.; Chen, S.-M.; Kim, H.-j.; Prabakar, K. Metal-free multiporous carbon for electrochemical energy storage and electrocatalysis applications. New J. Chem. 2019, 43, 11653–11659. [Google Scholar] [CrossRef]
- Sathiskumar, C.; Ramakrishnan, S.; Vinothkannan, M.; Rhan Kim, A.; Karthikeyan, S.; Yoo, D.J. Nitrogen-Doped Porous Carbon Derived from Biomass Used as Trifunctional Electrocatalyst toward Oxygen Reduction, Oxygen Evolution and Hydrogen Evolution Reactions. Nanomaterials 2020, 10, 76. [Google Scholar] [CrossRef]
- Xia, C.; Surendran, S.; Ji, S.; Kim, D.; Chae, Y.; Kim, J.; Je, M.; Han, M.-K.; Choe, W.-S.; Choi, C.H.; et al. A sulfur self-doped multifunctional biochar catalyst for overall water splitting and a supercapacitor from Camellia japonica flowers. Carbon Energy 2022, 4, 491–505. [Google Scholar] [CrossRef]
- Thomas, A.; Padmanabhan, N.T.; Jelmy, E.J.; Gopinath, P.; John, H. Multifunctional carbonaceous materials derived from tea waste: Towards sustainable solutions for wastewater treatment and hydrogen evolution. Clean Waste Syst. 2025, 10, 100233. [Google Scholar] [CrossRef]
- Hoang, V.C.; Dinh, K.N.; Gomes, V.G. Hybrid Ni/NiO composite with N-doped activated carbon from waste cauliflower leaves: A sustainable bifunctional electrocatalyst for efficient water splitting. Carbon 2020, 157, 515–524. [Google Scholar] [CrossRef]
- Sekar, S.; Kim, D.Y.; Lee, S. Excellent Oxygen Evolution Reaction of Activated Carbon-Anchored NiO Nanotablets Prepared by Green Routes. Nanomaterials 2020, 10, 1382. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.; Ahmed, A.T.A.; Inamdar, A.I.; Im, H.; Im, Y.B.; Lee, Y.; Kim, D.Y.; Lee, S. Biomass-derived ultrathin mesoporous graphitic carbon nanoflakes as stable electrode material for high-performance supercapacitors. Mater. Des. 2019, 169, 107688. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Sekar, S.; Sadhasivam, S.; Shanmugam, A.; Saravanan, S.; Pugazhendi, I.; Lee, Y.; Kim, D.Y.; Manikandan, R.; Chang, S.-C.; Lee, S. Enhanced bifunctional water electrolysis performance of spherical ZnMn2O4 nanoparticles. Int. J. Hydrogen Energy 2025, 141, 721–728. [Google Scholar] [CrossRef]
- Ahmed, A.T.A.; Ansari, A.S.; Sree, V.G.; Jana, A.; Meena, A.; Sekar, S.; Cho, S.; Kim, H.; Im, H. Nitrogen-Doped CuO@CuS Core–Shell Structure for Highly Efficient Catalytic OER Application. Nanomaterials 2023, 13, 3160. [Google Scholar] [CrossRef]
- Singh, D.K.; Jenjeti, R.N.; Sampath, S.; Eswaramoorthy, M. Two in one: N-doped tubular carbon nanostructure as an efficient metal-free dual electrocatalyst for hydrogen evolution and oxygen reduction reactions. J. Mater. Chem. A 2017, 5, 6025–6031. [Google Scholar] [CrossRef]
- Kumar, M.; Nagaiah, T.C. A NiCu–MoS2 electrocatalyst for pH-universal hydrogen evolution reaction and Zn–air batteries driven self-power water splitting. J. Mater. Chem. A 2023, 11, 18336–18348. [Google Scholar] [CrossRef]
- Chen, H.; Luo, X.; Huang, S.; Yu, F.; Li, D.; Chen, Y. Phosphorus-doped activated carbon as a platinum-based catalyst support for electrocatalytic hydrogen evolution reaction. J. Electroanal. Chem. 2023, 948, 117820. [Google Scholar] [CrossRef]
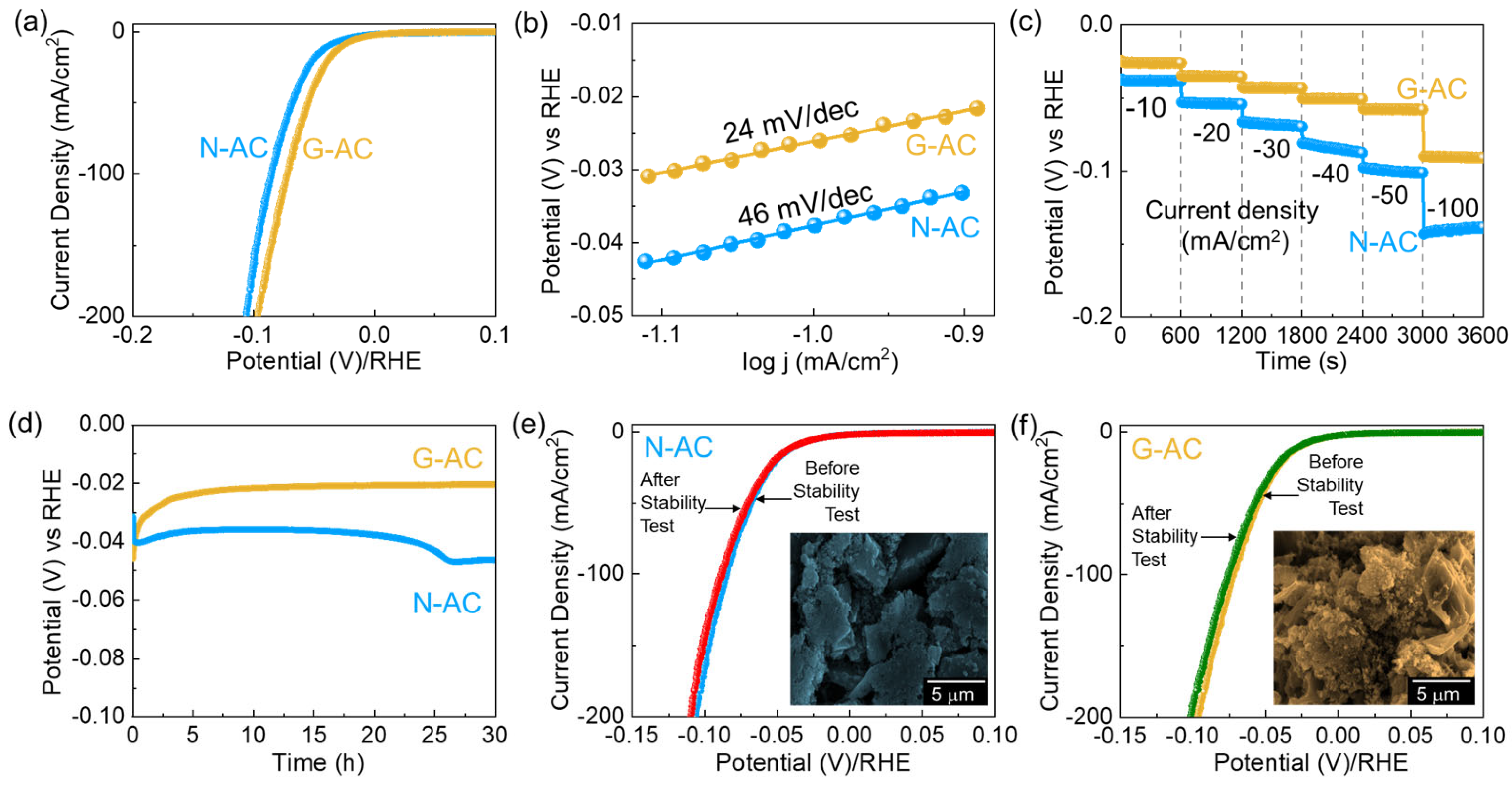
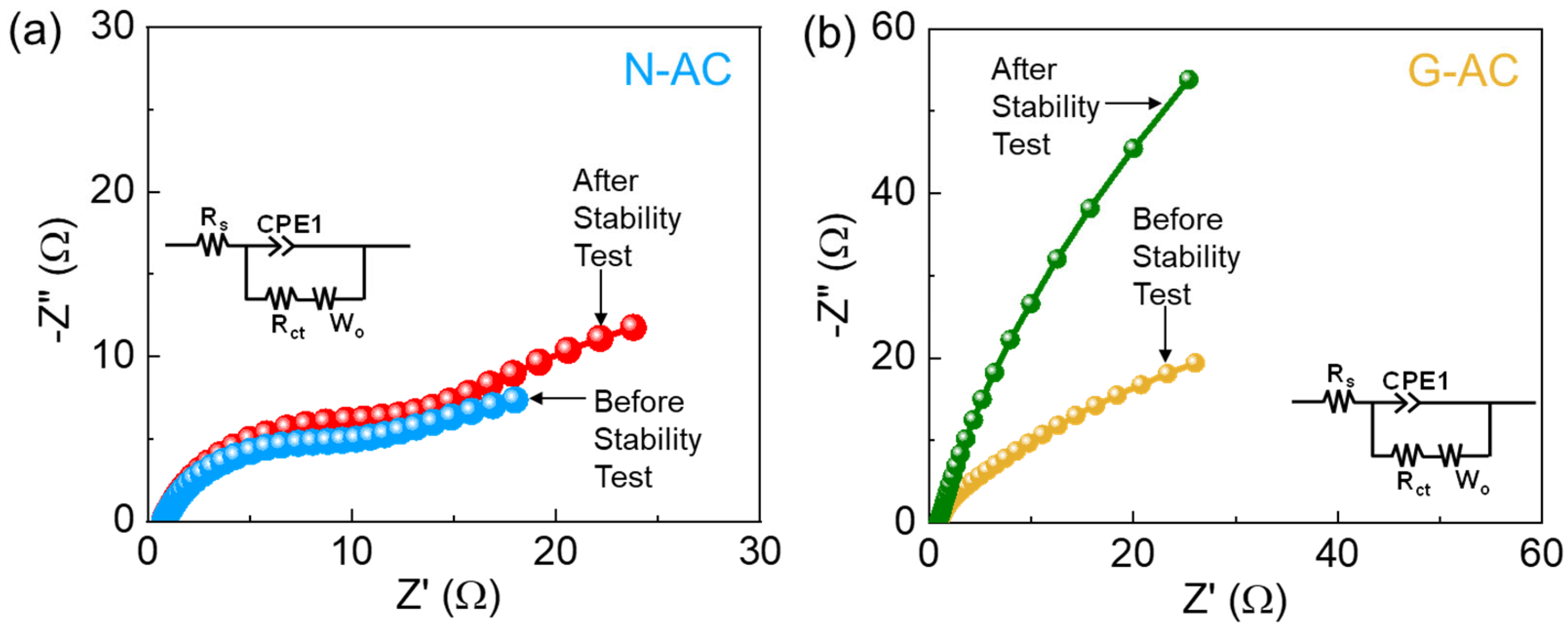

| Biomass Resources | Catalyst | Activation Source | η (mV) | ST (mV/dec) | Electrolyte | Ref. |
|---|---|---|---|---|---|---|
| Ginkgo Leaves | G-AC | KOH | 26 | 24 | 0.5 M H2SO4 | This Work |
| Neem Leaves | N-AC | KOH | 40 | 46 | 0.5 M H2SO4 | This Work |
| Bean Sprouts | BS-800 | HF Etching | 413 | 98 | 0.5 M H2SO4 | [37] |
| Carrots | PC | Thermal Annealing | 939 | 273 | 0.1 M KOH | [40] |
| Ooty Varkey | NACS | KOH | 380 | 85 | 0.5 M H2SO4 | [38] |
| Cattail Fiber | NPCF | KOH | 244 | 135 | 0.5 M H2SO4 | [35] |
| Eucalyptus Leaves | ELC-700 | KOH | 39 | 36 | 0.5 M H2SO4 | [13] |
| Palm Waste | HPNS | KOH | 330 | 63 | 0.5 M H2SO4 | [36] |
| Human Hair | HH-AC-700 | KOH | 16 | 51 | 0.5 M H2SO4 | [16] |
| Commercial AC | D-AC | NH3 | 334 | 66 | 0.5 M H2SO4 | [66] |
| Broccoli Stems | NA9 | KOH | 184 | 164 | 1 M H2SO4 | [67] |
| Peanut Shells | PSAC | KOH | 80 | 75 | 0.5 M H2SO4 | [39] |
| Golden Shower Pods | N-PC | Urea | 179 | 98 | 1 M KOH | [68] |
| Rice Husk | RH-CG-600 | KOH | 33 | 67 | 0.5 M H2SO4 | [19] |
| Camellia Japonica Flower | SA-Came | KOH | 154 | 89 | 1 M KOH | [69] |
| Tea Waste | G-ACOH | KOH | 349 | 128 | 0.5 M H2SO4 | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekar, S.; Sadhasivam, S.; Shanmugam, A.; Sekar, S.; Lee, Y.; Lee, S. Boosted Hydrogen Evolution Catalysis Using Biomass-Derived Mesoporous Carbon Nanosponges. Int. J. Mol. Sci. 2025, 26, 8502. https://doi.org/10.3390/ijms26178502
Sekar S, Sadhasivam S, Shanmugam A, Sekar S, Lee Y, Lee S. Boosted Hydrogen Evolution Catalysis Using Biomass-Derived Mesoporous Carbon Nanosponges. International Journal of Molecular Sciences. 2025; 26(17):8502. https://doi.org/10.3390/ijms26178502
Chicago/Turabian StyleSekar, Sankar, Sutha Sadhasivam, Atsaya Shanmugam, Saravanan Sekar, Youngmin Lee, and Sejoon Lee. 2025. "Boosted Hydrogen Evolution Catalysis Using Biomass-Derived Mesoporous Carbon Nanosponges" International Journal of Molecular Sciences 26, no. 17: 8502. https://doi.org/10.3390/ijms26178502
APA StyleSekar, S., Sadhasivam, S., Shanmugam, A., Sekar, S., Lee, Y., & Lee, S. (2025). Boosted Hydrogen Evolution Catalysis Using Biomass-Derived Mesoporous Carbon Nanosponges. International Journal of Molecular Sciences, 26(17), 8502. https://doi.org/10.3390/ijms26178502







