The Identification of Gyrophoric Acid, a Phytochemical Derived from Lichen, as a Potent Inhibitor for Aggregation of Amyloid Beta Peptide: In Silico and Biochemical Evaluation
Abstract
1. Introduction
2. Results
2.1. XP Molecular Docking and MM–GBSA Calculation
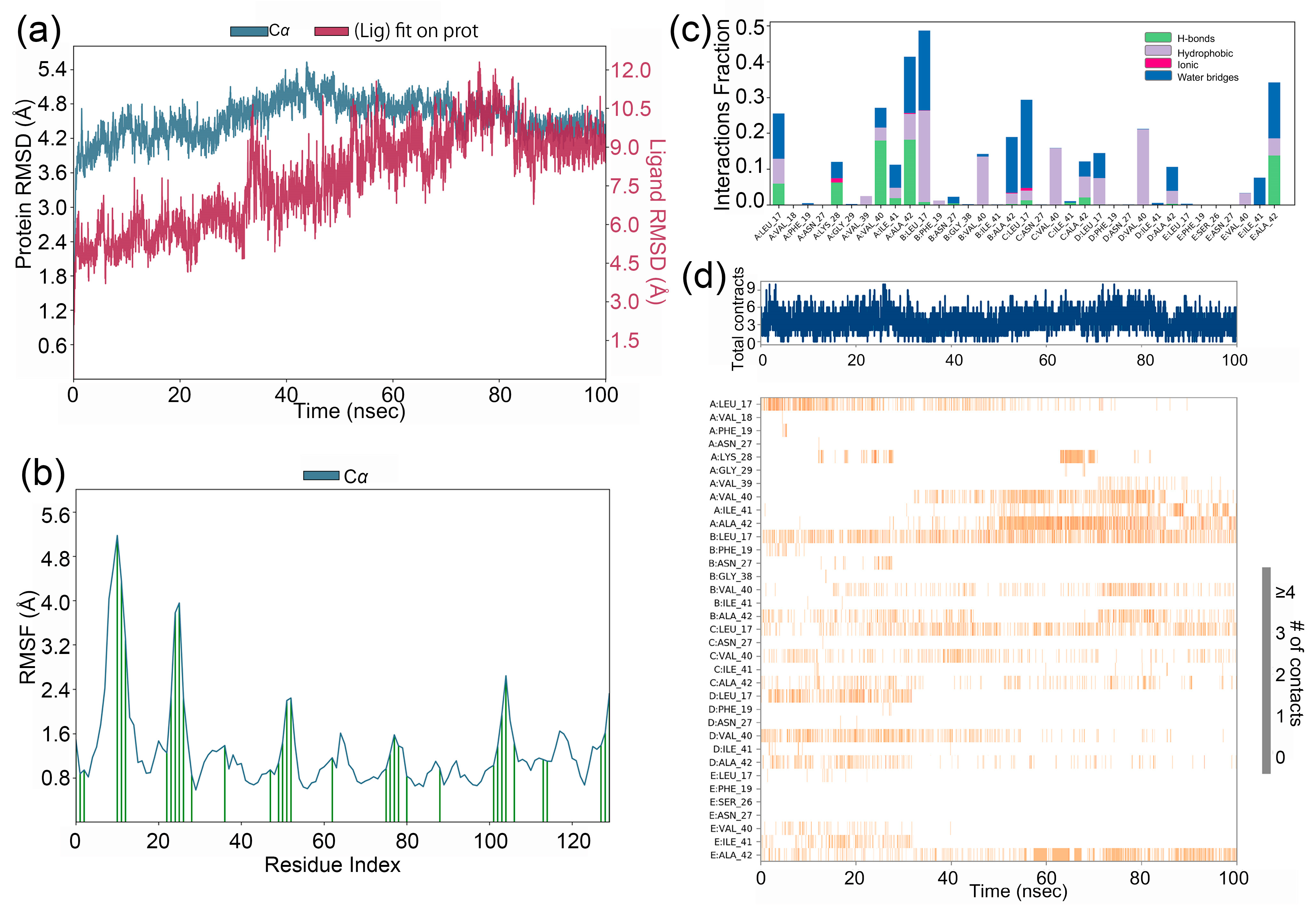
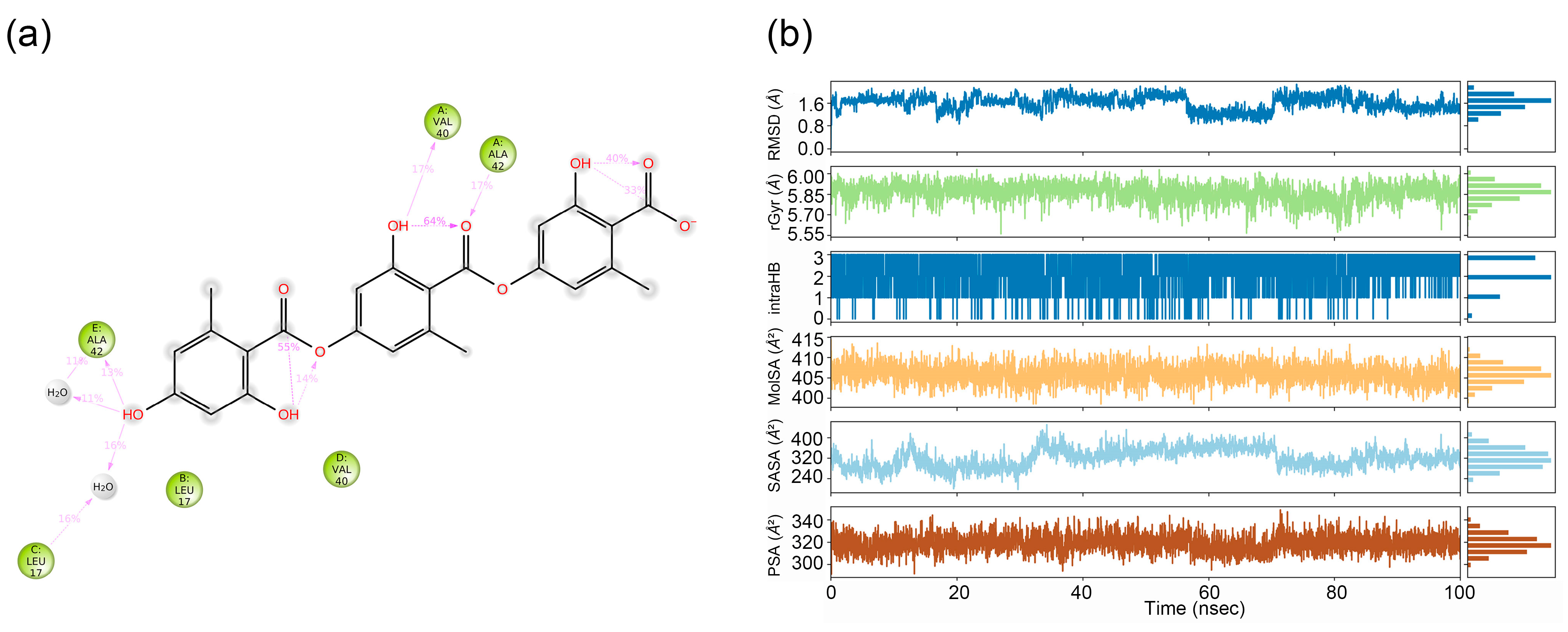
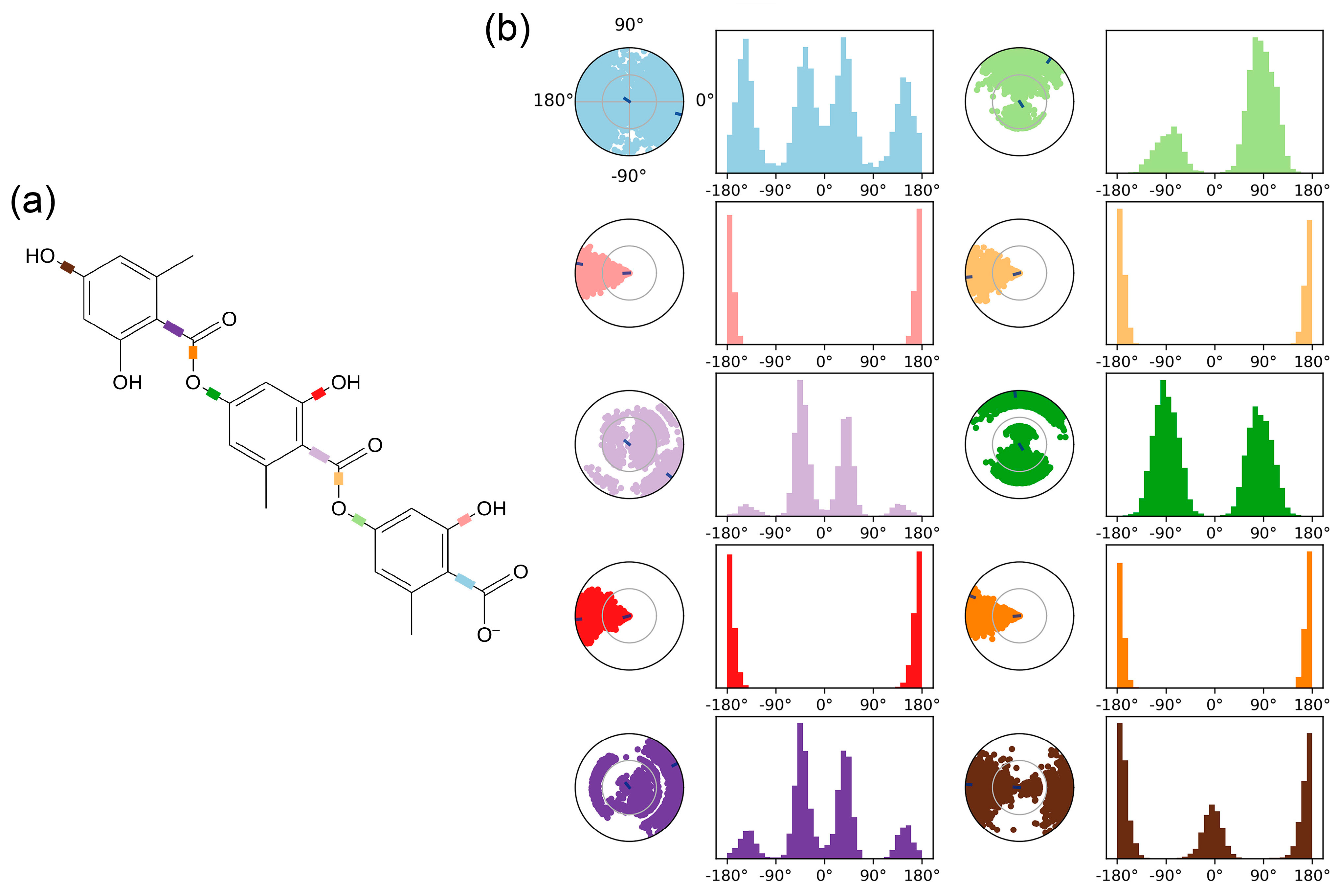
2.2. Molecular Dynamic (MD) Simulation
2.3. ADMET Properties and Druglikeness Prediction
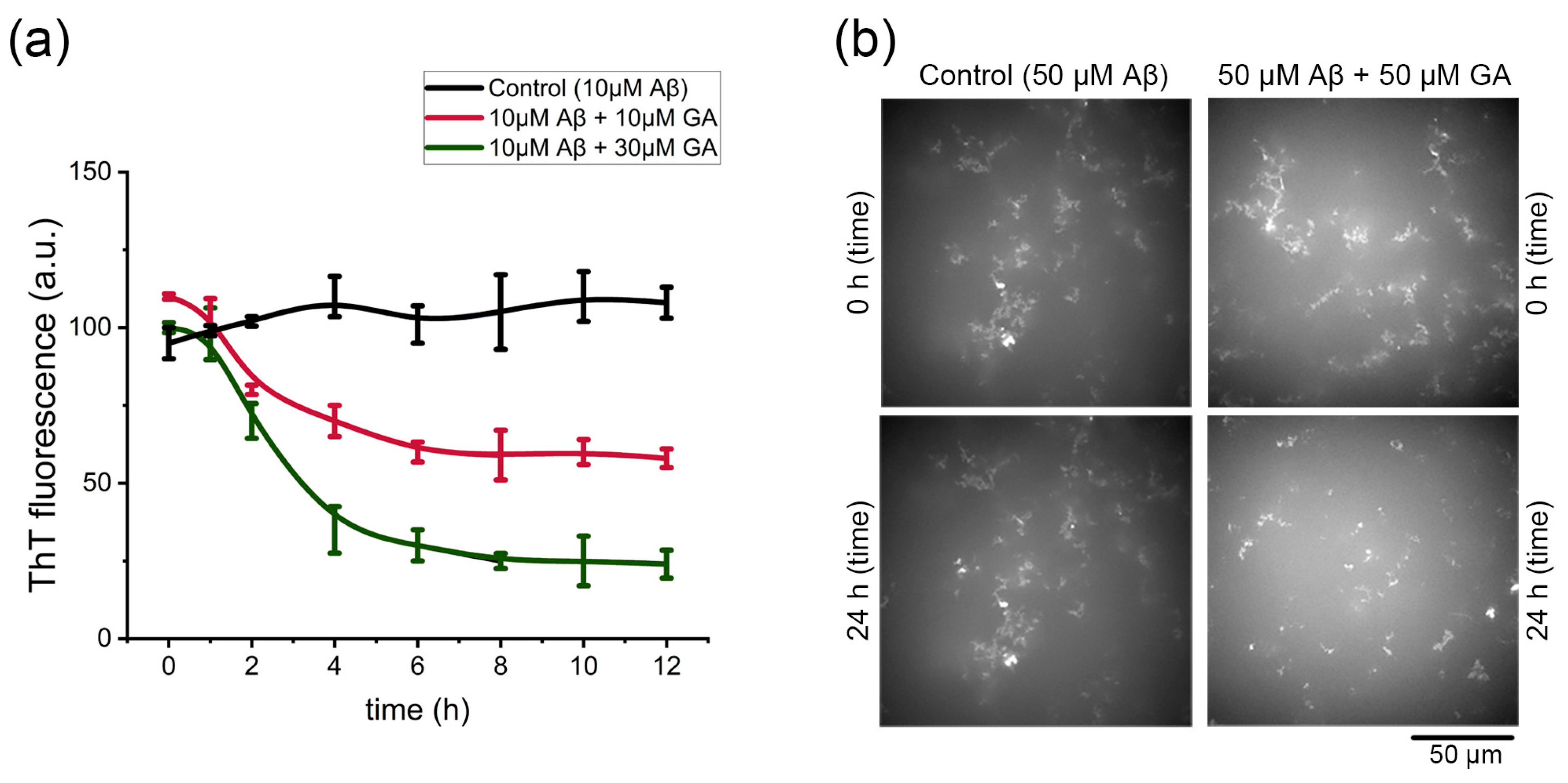
2.4. Gyrophoric Acid Disassembles Aβ42 Fibrils
3. Discussion
4. Materials and Methods
4.1. Preparation of Aβ Fibrils
4.2. Thioflavin T (ThT) Fluorescence Assay
4.3. Confocal Microscopy Real-Time Measurement
4.4. In Silico Analysis
4.5. MM–GBSA Binding Free Energy Calculations
4.6. MD Simulation
4.7. ADMET and Bioavailability Radar Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Aβ | amyloid beta |
| ThT | thioflavin T |
| MD | molecular dynamics |
| ADMET | absorption, distribution, metabolism, excretion, toxicity |
| AChE | acetylcholinesterase |
| MM–GBSA | molecular mechanics–generalized Born surface area |
| RMSD | root-mean-square deviation |
References
- Blass, J.P. Alzheimer’s disease and Alzheimer’s dementia: Distinct but overlapping entities. Neurobiol. Aging 2002, 23, 1077–1084. [Google Scholar] [CrossRef]
- Sohrabi, H.R.; Weinborn, M. Cognitive impairments in Alzheimer’s disease and other neurodegenerative diseases. In Neurodegeneration and Alzheimer’s Disease; Wiley: New York, NY, USA, 2019; pp. 267–290. [Google Scholar] [CrossRef]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef]
- Cummings, J.; Feldman, H.H.; Scheltens, P. The “rights” of precision drug development for Alzheimer’s disease. Alz Res. Ther. 2019, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Gheidari, D.; Bayat, M. Current treads of targeted nanoparticulate carriers for the treatment of Alzheimer’s disease. In Nanomedical Drug Delivery for Neurodegenerative Diseases; Elsevier: Amsterdam, The Netherlands, 2022; pp. 17–39. [Google Scholar] [CrossRef]
- Korczyn, A.D.; Grinberg, L.T. Is Alzheimer disease a disease? Nat. Rev. Neurol. 2024, 20, 245–251. [Google Scholar] [CrossRef]
- Song, K.; Li, Y.; Zhang, H.; An, N.; Wei, Y.; Wang, L.; Tian, C.; Yuan, M.; Sun, Y.; Xing, Y.; et al. Oxidative stress-mediated blood-brain barrier (BBB) disruption in neurological diseases. Oxid. Med. Cell. Longev. 2020, 2020, 4356386. [Google Scholar] [CrossRef]
- Lamontagne-Kam, D.; Ulfat, A.K.; Hervé, V.; Vu, T.-M.; Brouillette, J. Implication of tau propagation on neurodegeneration in Alzheimer’s disease. Front. Neuro. Sci. 2023, 17, 1219299. [Google Scholar] [CrossRef]
- Tolstova, A.P.; Adzhubei, A.A.; Strelkova, M.A.; Makarov, A.A.; Mitkevich, V.A. Survey of the Aβ-peptide structural diversity: Molecular dynamics approaches. Biophys. Rev. 2024, 16, 701–722. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef]
- Iqubal, A.; Rahman, S.O.; Ahmed, M.; Bansal, P.; Haider, M.R.; Iqubal, M.K.; Najmi, A.K.; Pottoo, F.H.; Haque, S.E. Current quest in natural bioactive compounds for Alzheimer’s disease: Multi-targeted-designed-ligand based approach with Preclinical and Clinical Based Evidence. Curr. Drug Targets 2021, 22, 685–720. [Google Scholar] [CrossRef]
- Mohammadi, M.; Bagheri, L.; Badreldin, A.; Fatehi, P.; Pakzad, L.; Suntres, Z.; van Wijnen, A.J. Biological effects of gyrophoric acid and other lichen derived metabolites, on cell proliferation, apoptosis and cell signaling pathways. Chem.-Biol. Interact. 2022, 351, 109768. [Google Scholar] [CrossRef]
- Goga, M.; Kello, M.; Vilkova, M.; Petrova, K.; Backor, M.; Adlassnig, W.; Lang, I. Oxidative stress mediated by gyrophoric acid from the lichen Umbilicaria hirsuta affected apoptosis and stress/survival pathways in HeLa cells. BMC Complement Altern Med. 2019, 19, 221. [Google Scholar] [CrossRef] [PubMed]
- Urbanska, N.; Karasova, M.; Jendzelovska, Z.; Majerník, M.; Kolesarova, M.; Kecsey, D.; Jendzelovsky, R.; Bohus, P.; Kiskova, T. Gyrophoric Acid, a secondary metabolite of lichens, exhibits antidepressant and anxiolytic activity in vivo in wistar rats. Int. J. Mol. Sci. 2024, 25, 11840. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yan, C.; Ospondpant, D.; Wang, L.; Lin, S.; Tang, W.L.; Dong, T.T.; Tong, P.; Xu, Q.; Tsim, K.W.K. Unveiling the therapeutic potential of Lobaria extract and its depsides/depsidones in combatting Aβ42 peptides aggregation and neurotoxicity in Alzheimer’s disease. Front. Pharmacol. 2024, 15, 1426569. [Google Scholar] [CrossRef]
- Tran, L.; Ha-Duong, T. Exploring the Alzheimer amyloid-β peptide conformational ensemble: A review of molecular dynamics approaches. Peptides 2015, 69, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Sucharitha, P.; Ramesh Reddy, K.; Satyanarayana, S.V.; Garg, T. Absorption, distribution, metabolism, excretion, and toxicity assessment of drugs using computational tools. In Computational Approaches for Novel Therapeutic and Diagnostic Designing to Mitigate SARS-CoV-2 Infection; Elsevier: Amsterdam, The Netherlands, 2022; pp. 335–355. [Google Scholar] [CrossRef]
- Ferreira, L.L.G.; Andricopulo, A.D. ADMET modeling approaches in drug discovery. Drug Discov. Today 2019, 24, 1157–1165. [Google Scholar] [CrossRef]
- Saini, R.K.; Shuaib, S.; Goyal, B. Molecular insights into Aβ42 protofibril destabilization with a fluorinated compound D744: A molecular dynamics simulation study. J. Mol. Recognit. 2017, 30, e2656. [Google Scholar] [CrossRef]
- Colvin, M.T.; Silvers, R.; Ni, Q.Z.; Can, T.V.; Sergeyev, I.; Rosay, M.; Donovan, K.J.; Michael, B.; Wall, J.; Linse, S.; et al. Atomic resolution structure of monomorphic Aβ42 amyloid fibrils. J. Am. Chem. Soc. 2016, 138, 9663–9674. [Google Scholar] [CrossRef]
- Xiao, Y.; Ma, B.; McElheny, D.; Parthasarathy, S.; Long, F.; Hoshi, M.; Nussinov, R.; Ishii, Y. Aβ(1–42) fibril structure illuminates self-recognition and replication of amyloid in Alzheimer’s disease. Nat. Struct. Mol. Biol. 2015, 22, 499–505. [Google Scholar] [CrossRef]
- Kundaikar, H.S.; Degani, M.S. Insights into the interaction mechanism of ligands with Aβ42 based on molecular dynamics simulations and mechanics: Implications of role of common binding site in drug design for Alzheimer’s disease. Chem. Biol. Drug Des. 2015, 86, 805–812. [Google Scholar] [CrossRef]
- Lu, J.X.; Qiang, W.; Yau, W.M.; Schwieters, C.D.; Meredith, S.C.; Tycko, R. Molecular structure of β-amyloid fibrils in Alzheimer’s disease brain tissue. Cell 2013, 154, 1257–1268. [Google Scholar] [CrossRef]
- Halder, A.K.; Mishra, P.; Basak, S.; Roy, D.; Das, A.; Karmakar, S.; Mondal, R.; Banerjee, S.; De, P.; Chatterjee, A.; et al. Structural insights into the interactions of repositioning and known drugs for Alzheimer’s disease with hen egg white lysozyme by MM-GBSA. J. Biomol. Struct. Dyn. 2025, 43, 4629–4647. [Google Scholar] [CrossRef]
- Raskatov, J.A. Conformational selection as the driving force of amyloid β chiral inactivation. ChemBioChem. 2020, 21, 2945–2949. [Google Scholar] [CrossRef]
- Mahmoodi, N.; Bayat, M.; Gheidari, D.; Sadeghian, Z. In silico evaluation of cis-dihydroxy-indeno [1,2-d]imidazolones as inhibitors of glycogen synthase kinase-3: Synthesis, molecular docking, physicochemical data, ADMET, MD simulation, and DFT calculations. J. Saudi Chem. Soc. 2024, 28, 101894. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Srivastava, R. Theoretical studies on the molecular properties, toxicity, and biological efficacy of 21 new chemical entities. ACS Omega 2021, 6, 24891–24901. [Google Scholar] [CrossRef] [PubMed]
- Capuzzi, S.J.; Muratov, E.N.; Tropsha, A. Phantom PAINS: Problems with the utility of alerts for pan-assay Iinterference compounds. J. Chem. Inf. Model. 2017, 57, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.I.A.; Linse, S.; Luheshi, L.M.; Hellstrand, E.; White, D.A.; Rajah, L.; Otzen, D.E.; Vendruscolo, M.; Dobson, C.M.; Knowles, T.P.J. Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 9758–9763. [Google Scholar] [CrossRef]
- Thakur, M.; Bhardwaj, S.; Kumar, V.; Rodrigo-Comino, J. Lichens as effective bioindicators for monitoring environmental changes: A comprehensive review. Total Environ. Adv. 2023, 9, 200085. [Google Scholar] [CrossRef]
- Urena-Vacas, I.; Gonzalez-Burgos, E.; Divakar, P.K.; Gomez-Serranillos, M.P. Lichen depsidones with biological interest. Planta Medica. 2021, 88, 855–880. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem. 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]

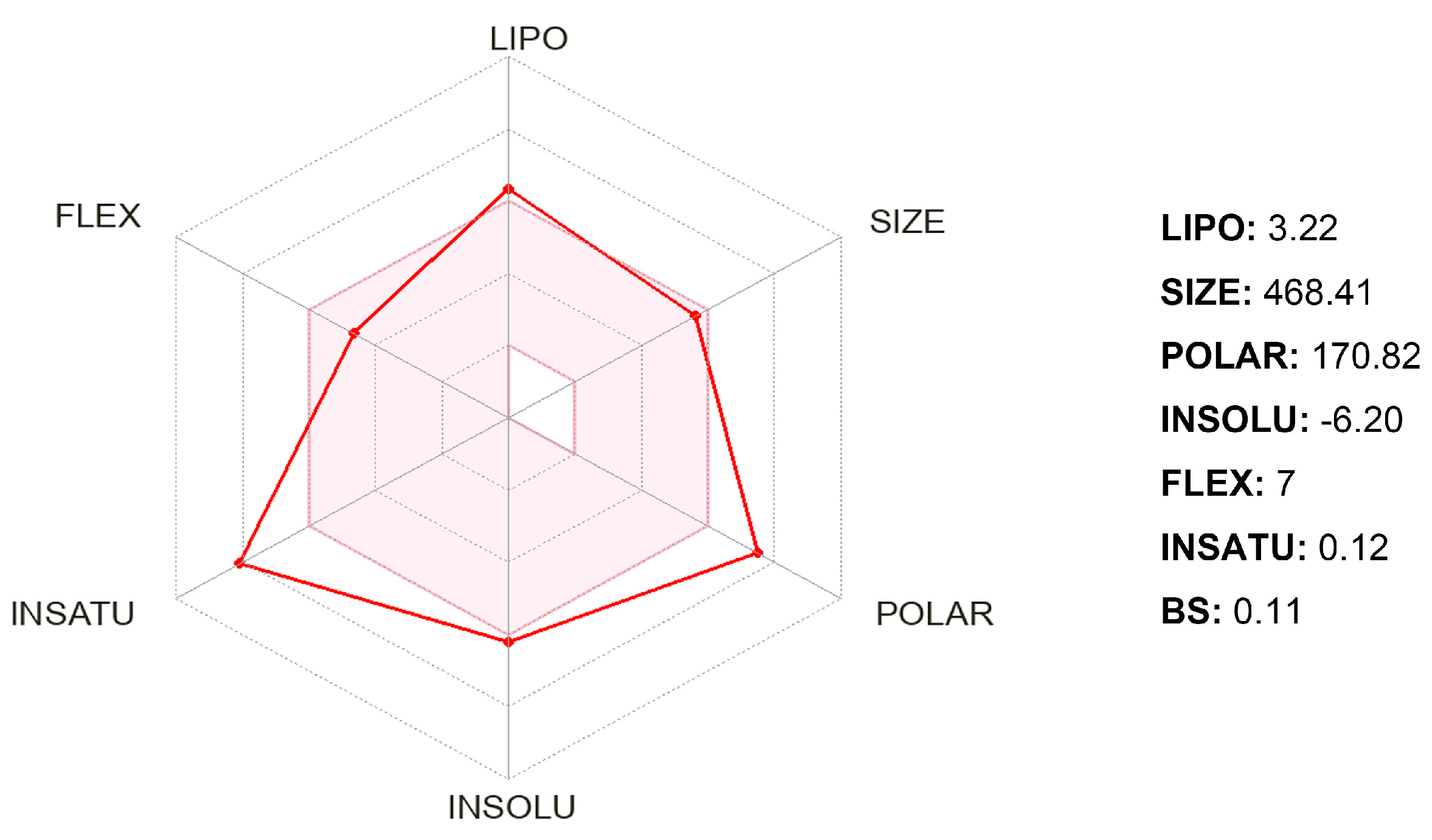
| Property or Descriptor with Range or Recommended Values | Gyrophoric Acid | Property or Descriptor with Range or Recommended Values | Gyrophoric Acid |
|---|---|---|---|
| Mol. Wt (130–725) | 468.416 | QPlogPo/w (−2.0–6.5) | 3.256 |
| SASA (300–1000) | 763.078 | QPlogS (−6.5–0.5) | −6.041 |
| FOSA (0–750) | 208.919 | CIQPlogS (−6.5–0.5) | −6.959 |
| FISA (7–330) | 329.438 | QPlogHERG (concern below −5) | −4.367 |
| PISA (0–450) | 224.721 | QPlogBB (−3.0–1.2) | −3.595 |
| Volume (500–2000) | 1363.561 | QPlogKp (−8.0–−1.0) | −5.935 |
| donorHB (0.0–6.0) | 2.000 | IP(eV) (7.9–10.5) | 9.524 |
| accptHB (2.0–20.0) | 7.000 | EA(eV) (−0.9–1.7) | 0.621 |
| dip^2/V (0.0–0.13) | 0.130 | #metab (1–8) | 7 |
| ACxDN^.5/SA (0.0–0.05) | 0.013 | QPlogKhsa (−1.5–1.5) | 0.354 |
| glob (0.75–0.95) | 0.780 | HumanOralAbsorption | 1 |
| QPpolrz (13.0–70.0) | 44.783 | PHOA (<25% is poor, >80% is high) | 50.942 |
| QPlogPC16 (4.0–18.0) | 15.378 | QPlogKhsa (−1.5 to 1.5) | 0.354 |
| QPlogPoct (8.0–35.0) | 23.996 | RuleOfFive (maximum is 4) | 0 |
| QPlogPw (4.0–45.0) | 13.164 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Hu, H.; Gao, J.; Lai, Q.W.S.; Eshboev, F.; Leung, K.W.; Dong, T.T.; Xu, Q.; Tsim, K.W.K. The Identification of Gyrophoric Acid, a Phytochemical Derived from Lichen, as a Potent Inhibitor for Aggregation of Amyloid Beta Peptide: In Silico and Biochemical Evaluation. Int. J. Mol. Sci. 2025, 26, 8500. https://doi.org/10.3390/ijms26178500
Yang M, Hu H, Gao J, Lai QWS, Eshboev F, Leung KW, Dong TT, Xu Q, Tsim KWK. The Identification of Gyrophoric Acid, a Phytochemical Derived from Lichen, as a Potent Inhibitor for Aggregation of Amyloid Beta Peptide: In Silico and Biochemical Evaluation. International Journal of Molecular Sciences. 2025; 26(17):8500. https://doi.org/10.3390/ijms26178500
Chicago/Turabian StyleYang, Meixia, Haitao Hu, Jin Gao, Queenie Wing Sze Lai, Farkhod Eshboev, Ka Wing Leung, Tina Tingxia Dong, Qin Xu, and Karl Wah Keung Tsim. 2025. "The Identification of Gyrophoric Acid, a Phytochemical Derived from Lichen, as a Potent Inhibitor for Aggregation of Amyloid Beta Peptide: In Silico and Biochemical Evaluation" International Journal of Molecular Sciences 26, no. 17: 8500. https://doi.org/10.3390/ijms26178500
APA StyleYang, M., Hu, H., Gao, J., Lai, Q. W. S., Eshboev, F., Leung, K. W., Dong, T. T., Xu, Q., & Tsim, K. W. K. (2025). The Identification of Gyrophoric Acid, a Phytochemical Derived from Lichen, as a Potent Inhibitor for Aggregation of Amyloid Beta Peptide: In Silico and Biochemical Evaluation. International Journal of Molecular Sciences, 26(17), 8500. https://doi.org/10.3390/ijms26178500








