From Pathophysiology to Innovative Therapies in Eye Diseases: A Brief Overview
Abstract
1. Introduction
2. Anatomy of the Eye
3. Pathophysiology of Eye Diseases at the Molecular Level—Introduction
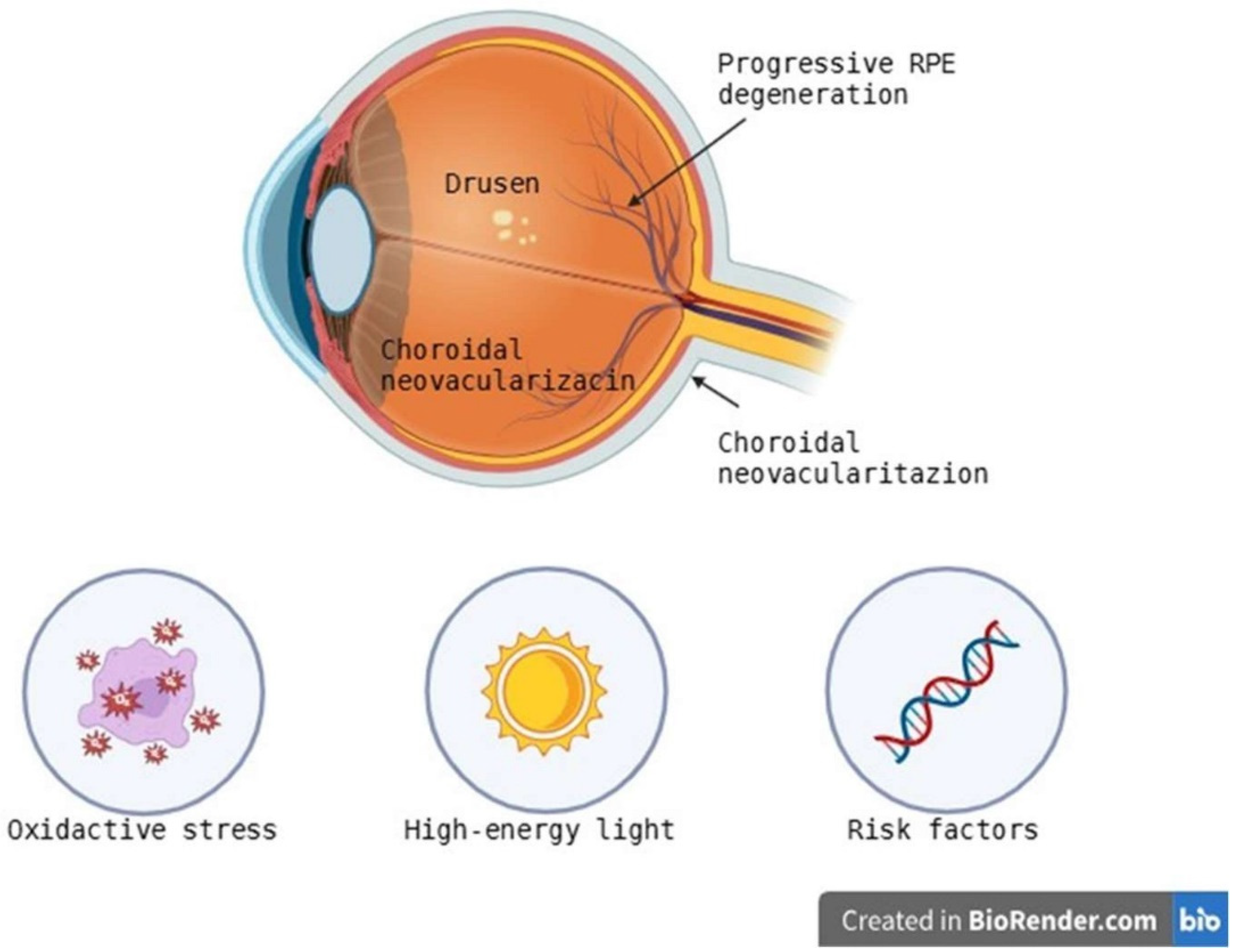
3.1. AMD—Age-Related Macular Degeneration
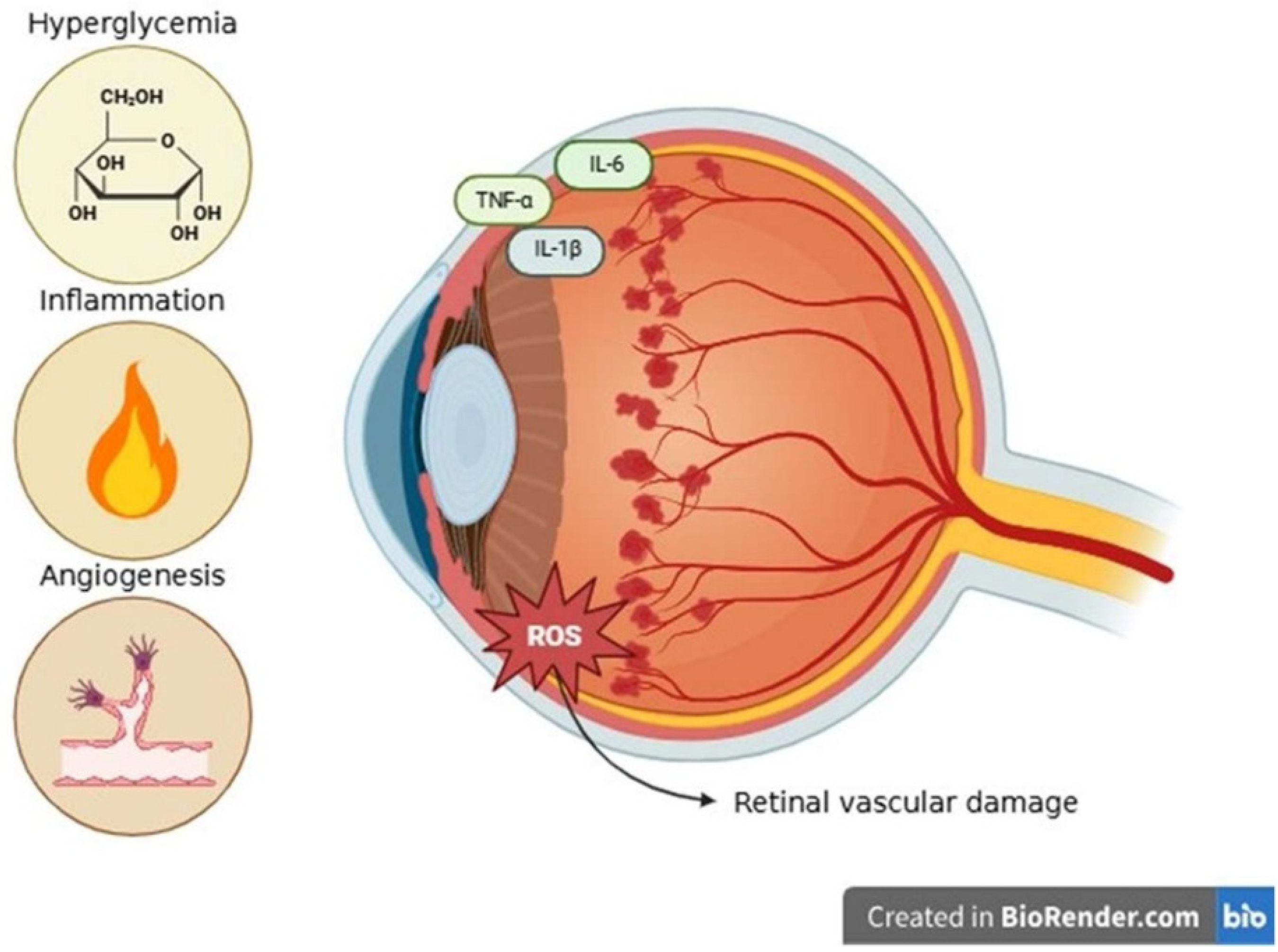
3.2. Diabetic Retinopathy
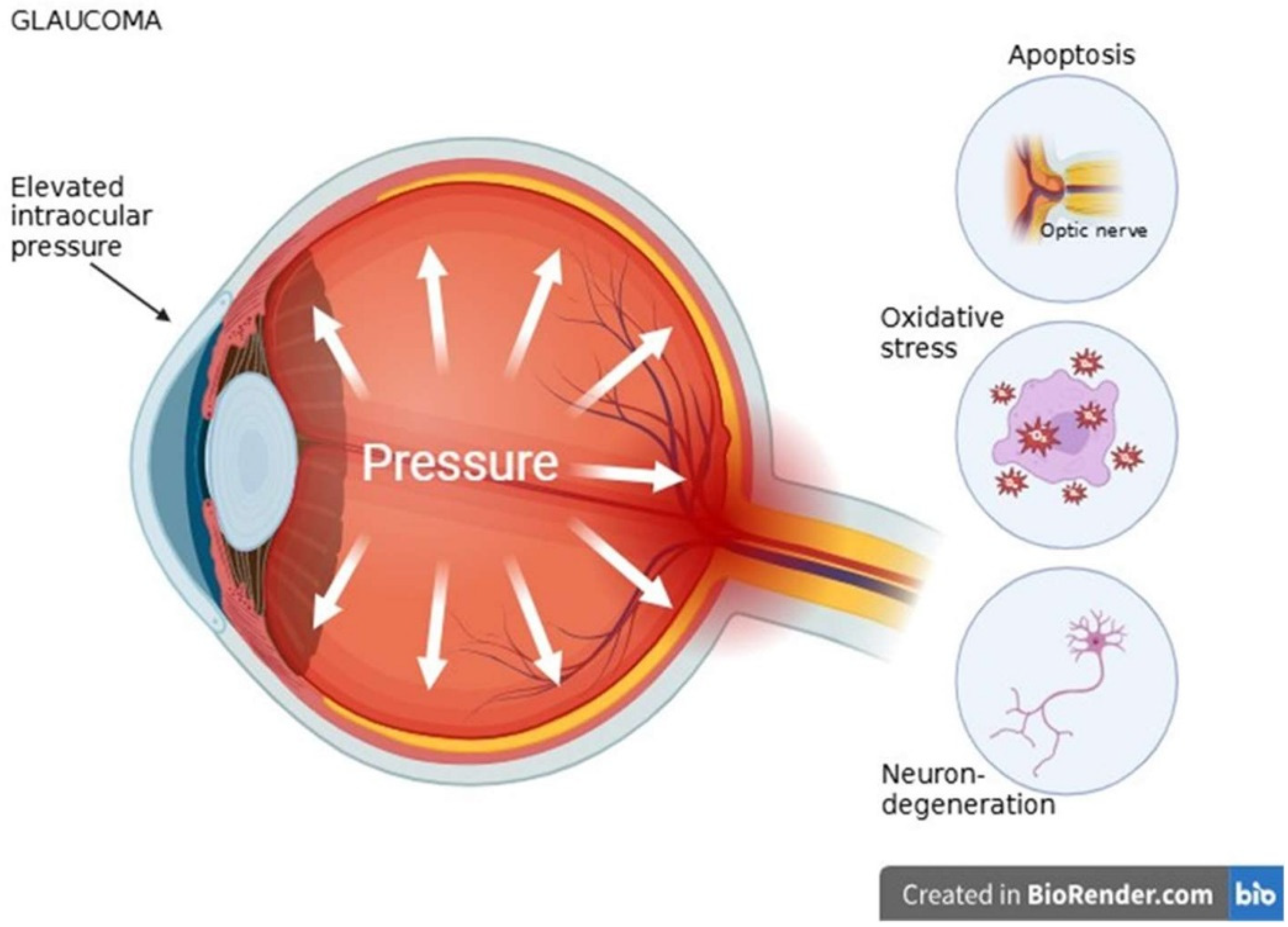
3.3. Glaucoma
3.4. Cataract
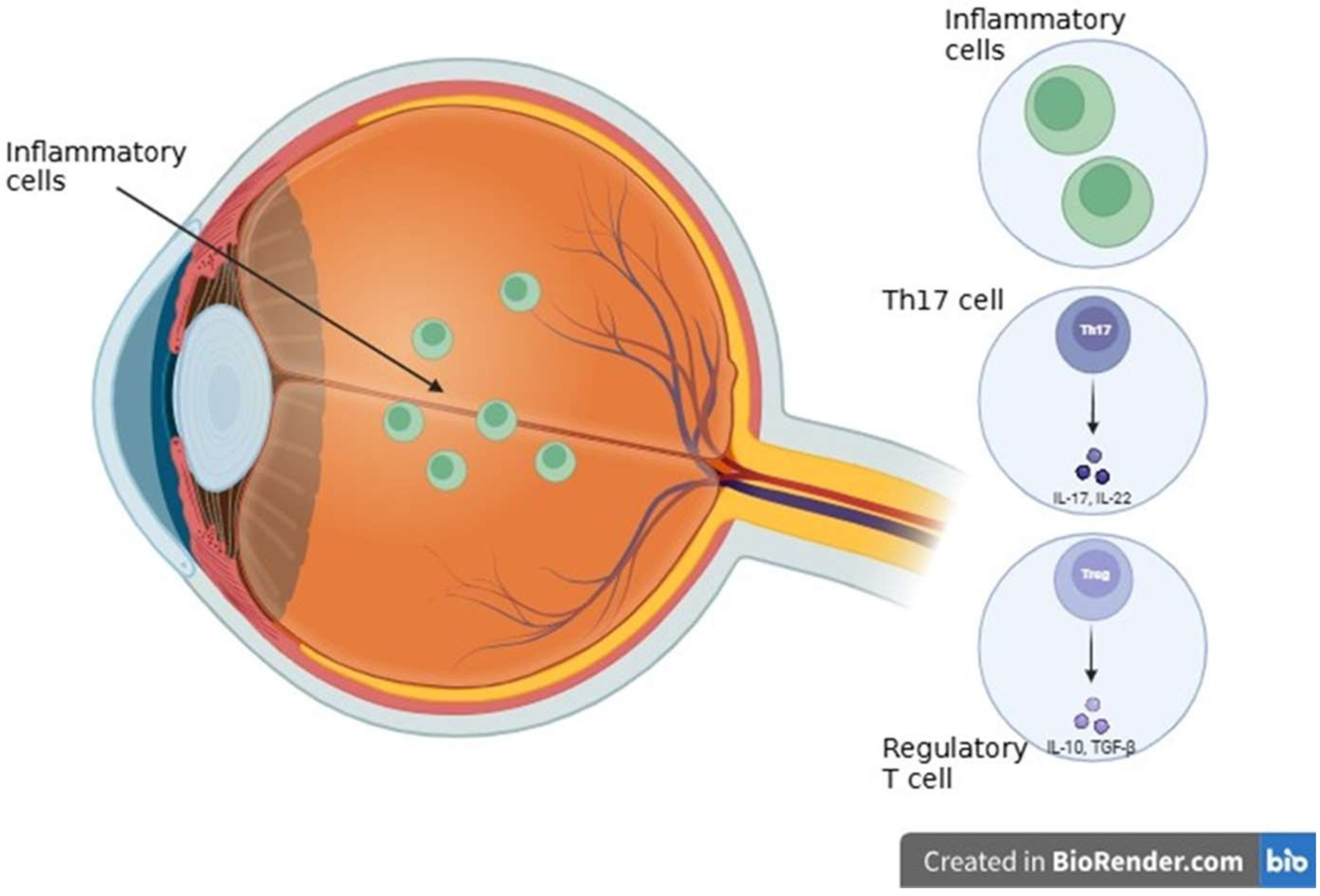
3.5. Inflammatory and Autoimmune Diseases
4. Modern Diagnostic Methods and Molecular Technologies
4.1. Genomics and Transcriptomics in Diagnostics
4.2. Proteomics and Metabolomics of the Eye
4.3. Molecular Imaging and Biomarkers
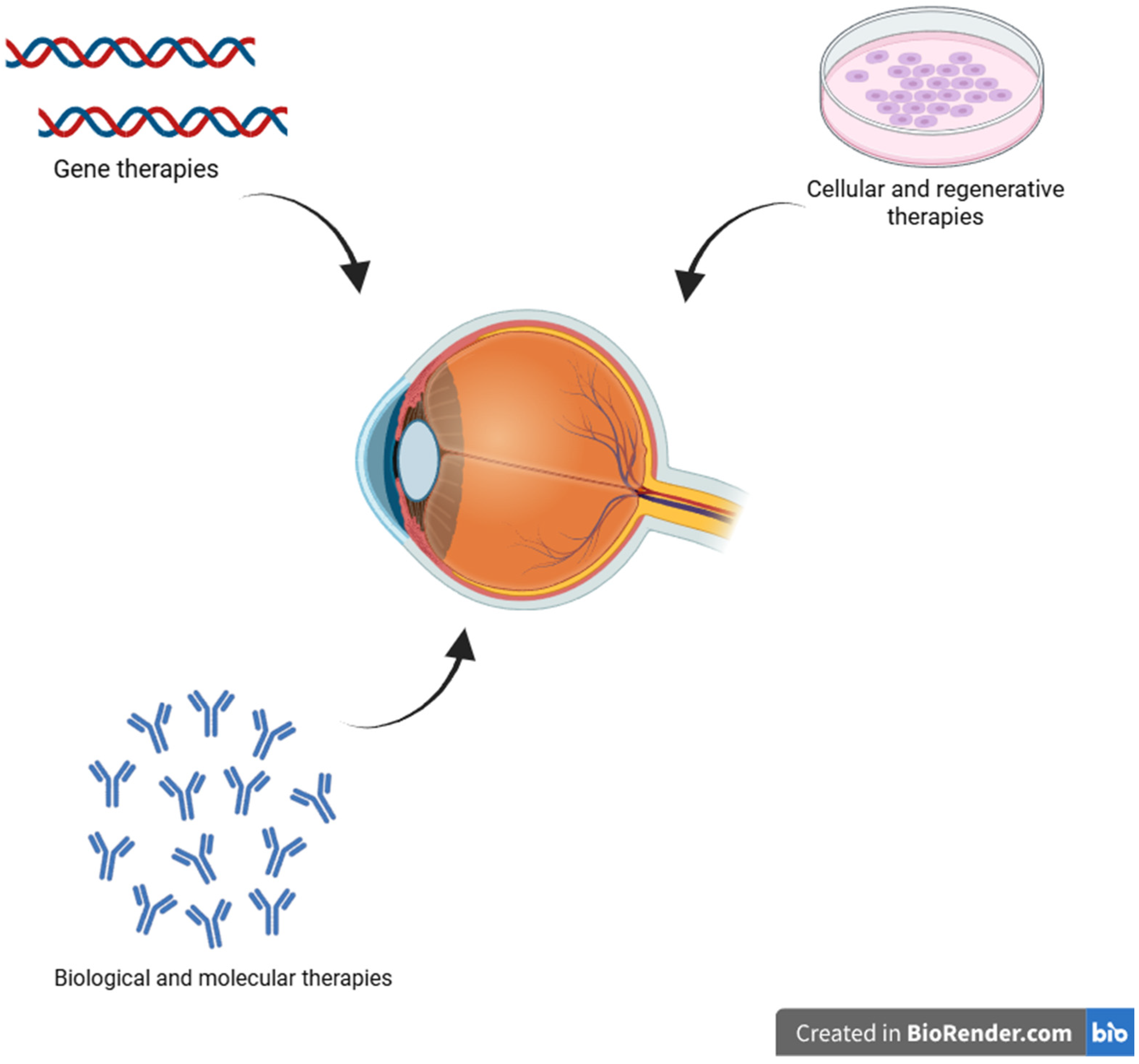
5. Innovative Therapeutic Approaches
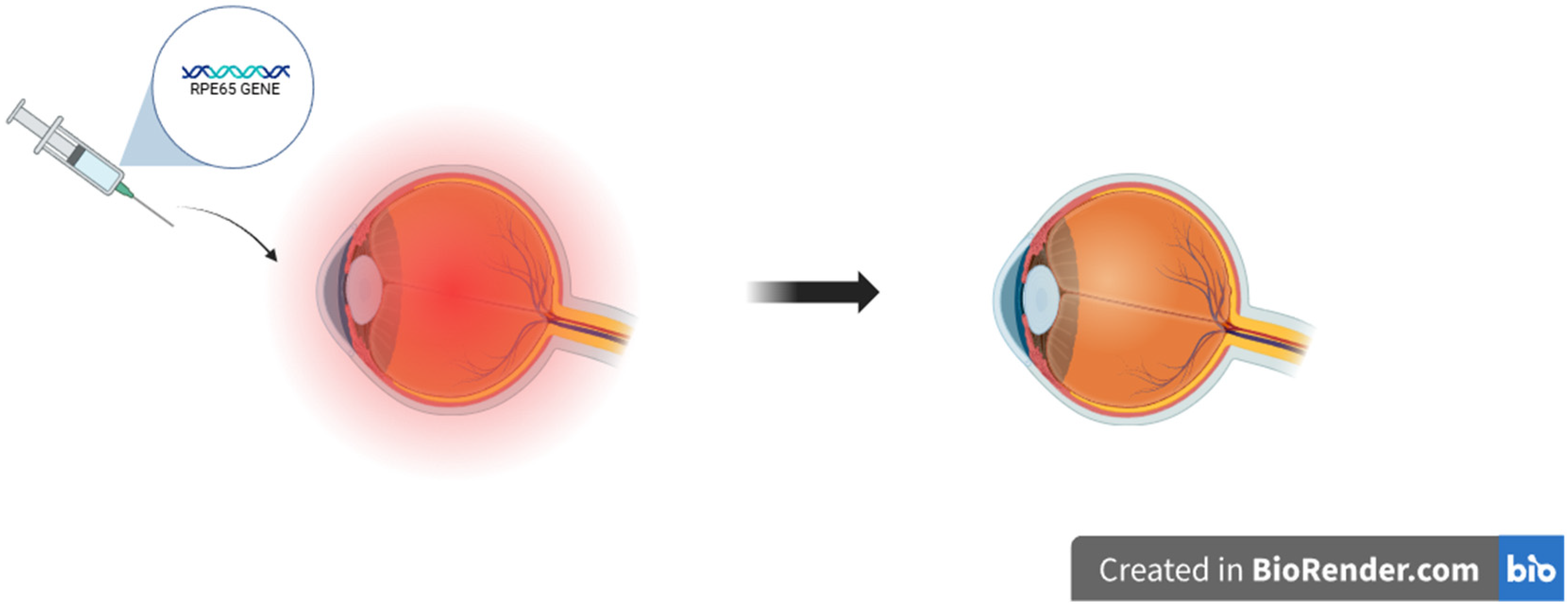
5.1. Gene Therapies
5.2. Cellular and Regenerative Therapies
5.3. Biological and Molecular Therapies
5.4. Targeted Therapies
6. Future Research Directions and Clinical Perspectives: Toward Personalized Ophthalmic Therapies
7. Current Limitations and Challenges
8. Synergistic Interactions and Multimodal Therapeutic Strategies
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAV2 | approved adeno-associated virus 2 |
| ACAID | Anterior Chamber-Associated Immune Deviation |
| AGE | advanced glycation and end-products |
| AI | artificial intelligence |
| AMD | age-related macular degeneration |
| ANGPT2 | angiopoietin-2 |
| BM | Bruch’s membrane |
| BRB | blood-retina barrier |
| CAT | catalase |
| cfRNA | cell-free RNA |
| CLET | Limbal epithelial cell transplantation |
| CNV | choroidal neovascularization |
| CRISPR | clustered regularly interspaced short palindromic repeats |
| CRVO | central retinal vein occlusion |
| CSSCs | corneal stromal stem cells |
| ctDNA | circulating tumor DNA |
| DAF | decay-accelerating factor |
| DCs | dendritic cells |
| DED | dry eye disease |
| DME | diabetic macular edema |
| DR | diabetic retinopathy |
| FA | fluorescein angiography |
| FAF | fundus autofluorescence |
| GCIPL | ganglion cell-inner plexiform layer |
| GPX | glutathione peroxidase |
| GWAS | genome-wide association study |
| HIF-1α | transcription factor 1 alpha |
| HLA-DR/IFNG | Human Leukocyte Antigen—DR isotype/Interferon gamma |
| hESCs | human embryonic stem cells |
| HSK | herpes simplex keratitis |
| HRMS | high-resolution mass spectrometry |
| IL-1beta/IL1B | Interleukin 1beta |
| IL-6 | Interleukin 6 |
| IOP | intraocular pressure |
| iPSC | induced pluripotent stem cell |
| iPSCs | induced pluripotent stem cells |
| IRD | inherited retinal dystrophies |
| IRDs | inherited retinal diseases |
| LC-MS | liquid chromatography-mass spectrometry |
| LCA | Leber congenital amaurosis |
| LESCs | limbal epithelial stem cells |
| MAC | membrane attack complex |
| MALDI-MSI | matrix-assisted laser desorption/ionization imaging mass spectrometry |
| MCP | membrane cofactor protein |
| MMP-9 | Matrix metalloproteinase-9 |
| MOMP | membrane permeabilization |
| MRI | Magnetic resonance imaging |
| MS | mass spectrometry |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NGS | next-generation sequencing |
| NMR | nuclear magnetic resonance spectroscopy |
| O-GlcNAcylation | O-linked β-N-acetylglucosaminylation |
| OCT | Optical Coherence Tomography |
| OCTA | OCT angiography |
| PDR | proliferative diabetic retinopathy |
| PEA | proximity extension assays |
| PET | Positron Emission Tomography |
| PKC | protein kinase C |
| PRDXs | peroxiredoxins |
| PRRs | pattern recognition receptors |
| RGC | retinal ganglion cell |
| RGCs | retinal ganglion cells |
| RNA | ribonucleic acid |
| RNA-seq | RNA sequencing |
| ROP | retinopathy of prematurity |
| RP | retinitis pigmentosa |
| RPE | retinal pigment epithelium |
| scRNA-seq | single-cell RNA sequencing |
| SOD | superoxide dismutase |
| TM | trabecular meshwork |
| UV | ultraviolet |
| VEGF | vascular endothelial growth factor |
| VEGF-A | vascular endothelial growth factor A |
| WES | whole-exome sequencing |
| WGS | whole-genome sequencing |
| WHO | World Health Organization |
| XAI | Explainable AI |
References
- World Health Organization. World Report on Vision; WHO: Geneva, Switzerland, 2019. Available online: https://www.who.int/publications/i/item/9789241516570 (accessed on 9 July 2025).
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef]
- Daiger, S.P.; Sullivan, L.S.; Bowne, S.J. Genes and mutations causing retinitis pigmentosa. Clin. Genet. 2013, 84, 132–141. [Google Scholar] [CrossRef]
- Peng, P.; Yu, Y.; Ma, W.; Lyu, S.; Ma, L.; Liu, T.; Dong, Y.; Wei, C. Proteomic characterization of aqueous humor in corneal endothelial decompensation after penetrating keratoplasty. Exp. Eye Res. 2023, 230, 109457. [Google Scholar] [CrossRef]
- Drexler, W.; Fujimoto, J.G. Optical Coherence Tomography: Technology and Applications, 2nd ed.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Gillam, W.; Godbole, N.; Sangam, S.; DeTommaso, A.; Foreman, M.; Lucke-Wold, B. Neurologic Injury-Related Predisposing Factors of Post-Traumatic Stress Disorder: A Critical Examination. Biomedicines 2023, 11, 2732. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Hu, J.; Zhe, J.; Ramasamy, S.; Ahmed, U.; Paulus, Y.M. Advanced nanomaterials for imaging of eye diseases. ADMET DMPK 2024, 12, 269–298. [Google Scholar] [CrossRef] [PubMed]
- Ba, J.; Peng, R.S.; Xu, D.; Li, Y.H.; Shi, H.; Wang, Q.; Yu, J. Intravitreal anti-VEGF injections for treating wet age-related macular degeneration: A systematic review and meta-analysis. Drug Des. Devel. Ther. 2015, 9, 5397–5405. [Google Scholar] [CrossRef] [PubMed]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Belliveau, A.P.; Somani, A.N.; Dossani, R.H. Pupillary Light Reflex. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Koenig, K.M.; Gross, J.M. Evolution and development of complex eyes: A celebration of diversity. Development 2020, 147, dev182923. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, T.; Mehra, D.; Le, P.H. Histology, Eye. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Van Zyl, T.; Yan, W.; McAdams, A.M.; Monavarfeshani, A.; Hageman, G.S.; Sanes, J.R. Cell atlas of the human ocular anterior segment: Tissue-specific and shared cell types. Proc. Natl. Acad. Sci. USA 2022, 119, e2200914119. [Google Scholar] [CrossRef]
- Ludwig, P.E.; Lopez, M.J.; Sevensma, K.E. Anatomy, Head and Neck, Eye Cornea. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Machiele, R.; Motlagh, M.; Zeppieri, M.; Patel, B.C. Intraocular Pressure. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Viet, J.; Reboutier, D.; Hardy, S.; Lachke, S.A.; Paillard, L.; Gautier-Courteille, C. Modeling ocular lens disease in Xenopus. Dev. Dyn. 2019, 249, 610–621. [Google Scholar] [CrossRef]
- Mahabadi, N.; Al Khalili, Y. Neuroanatomy, Retina. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Fague, L.; Liu, Y.A.; Marsh-Armstrong, N. The basic science of optic nerve regeneration. Ann. Transl. Med. 2021, 9, 1276. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, J.; Li, D. Functions and Diseases of the Retinal Pigment Epithelium. Front. Pharmacol. 2021, 12, 727870. [Google Scholar] [CrossRef]
- Simó, R.; Villarroel, M.; Corraliza, L.; Hernández, C.; Garcia-Ramírez, M. The retinal pigment epithelium: Something more than a constituent of the blood-retinal barrier—Implications for the pathogenesis of diabetic retinopathy. J. Biomed. Biotechnol. 2010, 2010, 190724. [Google Scholar] [CrossRef]
- Somasundaran, S.; Constable, I.J.; Mellough, C.B.; Carvalho, L.S. Retinal pigment epithelium and age-related macular degeneration: A review of major disease mechanisms. Clin. Exp. Ophthalmol. 2020, 48, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; McHarg, S.; Tilakaratna, V.; Brace, N.; Bishop, P.N. Bruch’s Membrane Compartmentalizes Complement Regulation in the Eye with Implications for Therapeutic Design in Age-Related Macular Degeneration. Front. Immunol. 2017, 8, 1778. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef]
- Koksaldi, S.; Kayabasi, M.; Karti, O.; Saatci, A.O. Clinical anatomy of the macula. Med. Hypothesis Discov. Innov. Ophthalmol. 2025, 14, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Vyawahare, H.; Shinde, P. Age-Related Macular Degeneration: Epidemiology, Pathophysiology, Diagnosis, and Treatment. Cureus 2022, 14, e29583. [Google Scholar] [CrossRef]
- Ptito, M.; Bleau, M.; Bouskila, J. The Retina: A Window into the Brain. Cells 2021, 10, 3269. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M. Integrin-mediated electric axon guidance underlying optic nerve formation in the embryonic chick retina. Commun. Biol. 2023, 6, 680. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.J.; Mehta, S.N. Nanotechnology in Retinal Disease: Current Concepts and Future Directions. J. Ocul. Pharmacol. Ther. 2024, 40, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Schumann, U.; Liu, L.; Aggio-Bruce, R.; Cioanca, A.V.; Shariev, A.; Madigan, M.C.; Valter, K.; Wen, J.; Natoli, R. Spatial transcriptomics reveals regionally altered gene expression that drives retinal degeneration. Commun. Biol. 2025, 8, 629, Erratum in Commun. Biol. 2025, 8, 1013. https://doi.org/10.1038/s42003-025-08451-8. [Google Scholar] [CrossRef]
- Joyal, J.-S.; Gantner, M.L.; Smith, L.E. Retinal energy demands control vascular supply of the retina in development and disease: The role of neuronal lipid and glucose metabolism. Prog. Retin. Eye Res. 2018, 64, 131–156. [Google Scholar] [CrossRef]
- Barabino, S.; Chen, Y.; Chauhan, S.; Dana, R. Ocular surface immunity: Homeostatic mechanisms and their disruption in dry eye disease. Prog. Retin. Eye Res. 2012, 31, 271–285. [Google Scholar] [CrossRef]
- MacDonald, I.M.; Tran, M.; Musarella, M.A. Ocular genetics: Current understanding. Surv. Ophthalmol. 2004, 49, 159–196. [Google Scholar] [CrossRef]
- Scioli, M.G.; Storti, G.; D’Amico, F.; Rodríguez Guzmán, R.; Centofanti, F.; Doldo, E.; Céspedes Miranda, E.M.; Orlandi, A. Oxidative stress and new pathogenetic mechanisms in endothelial dysfunction: Potential diagnostic biomarkers and therapeutic targets. J. Clin. Med. 2020, 9, 1995. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog. Retin. Eye Res. 2015, 49, 67–81. [Google Scholar] [CrossRef]
- Xu, X.; Pang, Y.; Fan, X. Mitochondria in oxidative stress, inflammation and aging: From mechanisms to therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of cellular senescence: Cell cycle arrest and senescence associated secretory phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Tuo, J.; Bojanowski, C.M.; Chan, C.C. Genetic factors of age-related macular degeneration. Prog. Retin. Eye Res. 2004, 23, 229–249. [Google Scholar] [CrossRef]
- Lee, C.; Kim, M.-J.; Kumar, A.; Lee, H.-W.; Yang, Y.; Kim, Y. Vascular endothelial growth factor signaling in health and disease: From molecular mechanisms to therapeutic perspectives. Signal Transduct. Target. Ther. 2025, 10, 1–40. [Google Scholar] [CrossRef]
- Nashine, S. Potential Therapeutic Candidates for Age-Related Macular Degeneration (AMD). Cells 2021, 10, 2483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Owen, L.A.; Lillvis, J.H.; Zhang, S.X.; Kim, I.K.; DeAngelis, M.M. AMD Genomics: Non-Coding RNAs as Biomarkers and Therapeutic Targets. J. Clin. Med. 2022, 11, 1484. [Google Scholar] [CrossRef]
- García-Layana, A.; Cabrera-López, F.; García-Arumí, J.; Arias-Barquet, L.; Ruiz-Moreno, J.M. Early and intermediate age-related macular degeneration: Update and clinical review. Clin. Interv. Aging 2017, 12, 1579–1587. [Google Scholar] [CrossRef]
- Cruz-González, F.; Cieza-Borrella, C.; López Valverde, G.; Lorenzo-Pérez, R.; Hernández-Galilea, E.; González-Sarmiento, R. CFH (rs1410996), HTRA1 (rs112000638) and ARMS2 (rs10490923) gene polymorphisms are associated with AMD risk in Spanish patients. Ophthalmic Genet. 2014, 35, 68–73. [Google Scholar] [CrossRef]
- Deng, Y.; Qiao, L.; Du, M.; Qu, C.; Wan, L.; Li, J.; Huang, L. Age-related macular degeneration: Epidemiology, genetics, patho-physiology, diagnosis, and targeted therapy. Genes. Dis. 2021, 9, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Schaumberg, D.A.; Hankinson, S.E.; Guo, Q.; Rimm, E.; Hunter, D.J. A prospective study of 2 major age-related macular degeneration susceptibility alleles and interactions with modifiable risk factors. Arch. Ophthalmol. 2007, 125, 55–62. [Google Scholar] [CrossRef]
- Geerlings, M.J.; de Jong, E.K.; den Hollander, A.I. The complement system in age-related macular degeneration: A review of rare genetic variants and implications for personalized treatment. Mol. Immunol. 2017, 84, 65–76. [Google Scholar] [CrossRef]
- Raychaudhuri, S.; Iartchouk, O.; Chin, K.; Tan, P.L.; Tai, A.K.; Ripke, S.; Gowrisankar, S.; Vemuri, S.; Montgomery, K.; Yu, Y.; et al. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nat. Genet. 2011, 43, 1232–1236. [Google Scholar] [CrossRef]
- Hughes, A.E.; Orr, N.; Esfandiary, H.; Diaz-Torres, M.; Goodship, T.; Chakravarthy, U. A common CFH haplotype, with deletion of CFHR1 and CFHR3, is associated with lower risk of age-related macular degeneration. Nat. Genet. 2006, 38, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.B.; Cooke Bailey, J.N.; Hoffman, J.D.; Pericak-Vance, M.A.; Scott, W.K.; Kovach, J.L.; Schwartz, S.G.; Agarwal, A.; Brantley, M.A., Jr.; Haines, J.L.; et al. Estimating cumulative pathway effects on risk for age-related macular degeneration using mixed linear models. BMC Bioinform. 2015, 16, 329. [Google Scholar] [CrossRef] [PubMed]
- Armento, A.; Ueffing, M.; Clark, S.J. The complement system in age-related macular degeneration. Cell. Mol. Life Sci. 2021, 78, 4487–4505. [Google Scholar] [CrossRef]
- Buonfiglio, F.; Korb, C.A.; Stoffelns, B.; Pfeiffer, N.; Gericke, A. Recent Advances in Our Understanding of Age-Related Macular Degeneration: Mitochondrial Dysfunction, Redox Signaling, and the Complement System. Aging Dis. 2024, 16, 1535–1575. [Google Scholar] [CrossRef]
- Fan, W.; Huang, W.; Chen, J.; Li, N.; Mao, L.; Hou, S. Retinal microglia: Functions and diseases. Immunology 2022, 166, 268–286. [Google Scholar] [CrossRef]
- Domènech, B.; Marfany, G. The Relevance of Oxidative Stress in the Pathogenesis and Therapy of Retinal Dystrophies. Antioxidants 2020, 9, 347. [Google Scholar] [CrossRef]
- Böhm, E.W.; Buonfiglio, F.; Voigt, A.M.; Bachmann, P.; Safi, T.; Pfeiffer, N.; Gericke, A. Oxidative stress in the eye and its role in the pathophysiology of ocular diseases. Redox Biol. 2023, 68, 102967. [Google Scholar] [CrossRef]
- Ochoa Hernández, M.E.; Lewis-Luján, L.M.; Burboa Zazueta, M.G.; Del Castillo Castro, T.; De La Re Vega, E.; Gálvez-Ruiz, J.C.; Trujillo-López, S.; López Torres, M.A.; Iloki-Assanga, S.B. Role of Oxidative Stress and Inflammation in Age Related Macular Degeneration: Insights into the Retinal Pigment Epithelium (RPE). Int. J. Mol. Sci. 2025, 26, 3463. [Google Scholar] [CrossRef]
- Chakravarthy, H.; Georgyev, V.; Wagen, C.; Hosseini, A.; Matsubara, J. Blue light-induced phototoxicity in retinal cells: Implications in age-related macular degeneration. Front. Aging Neurosci. 2024, 16, 1509434. [Google Scholar] [CrossRef]
- Mammadzada, P.; Corredoira, P.M.; André, H. The role of hypoxia-inducible factors in neovascular age-related macular degeneration: A gene therapy perspective. Cell. Mol. Life Sci. 2020, 77, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Penn, J.S.; Madan, A.; Caldwell, R.B.; Bartoli, M.; Caldwell, R.W.; Hartnett, M.E. Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 2008, 27, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zhang, S.; Huang, M.; Liu, Y.; Zou, X.; Chen, X.; Zhang, Z. Treatment of neovascular age-related macular degeneration with anti-vascular endothelial growth factor drugs: Progress from mechanisms to clinical applications. Front. Med. 2024, 11, 1411278. [Google Scholar] [CrossRef]
- Emerson, M.V.; Lauer, A.K. Current and emerging therapies for the treatment of age-related macular degeneration. Clin. Ophthalmol. 2008, 2, 377–388. [Google Scholar] [CrossRef]
- Kropp, M.; Golubnitschaja, O.; Mazurakova, A.; Koklesova, L.; Sargheini, N.; Vo, T.K.S.; de Clerck, E.; Polivka, J., Jr.; Potuznik, P.; Polivka, J.; et al. Diabetic retinopathy as the leading cause of blindness and early predictor of cascading complications—Risks and mitigation. EPMA J. 2023, 14, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Sun, X.; Fan, C.; Li, R.; Zhou, S.; Yu, H. The pathophysiological mechanisms underlying diabetic retinopathy. Front. Cell Dev. Biol. 2022, 10, 963615. [Google Scholar] [CrossRef]
- Dolar-Szczasny, J.; Drab, A.; Rejdak, R. Biochemical Changes in Anterior Chamber of the Eye in Diabetic Patients—A Review. J. Clin. Med. 2024, 13, 2581. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Wu, M.Y.; Yiang, G.T.; Lai, T.T.; Li, C.J. The Oxidative Stress and Mitochondrial Dysfunction during the Pathogenesis of Diabetic Retinopathy. Oxid. Med. Cell Longev. 2018, 2018, 3420187. [Google Scholar] [CrossRef]
- Safi, S.Z.; Qvist, R.; Kumar, S.; Batumalaie, K.; Ismail, I.S. Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. Biomed. Res. Int. 2014, 2014, 801269. [Google Scholar] [CrossRef] [PubMed]
- Paneque, A.; Fortus, H.; Zheng, J.; Werlen, G.; Jacinto, E. The Hexosamine Biosynthesis Pathway: Regulation and Function. Genes 2023, 14, 933. [Google Scholar] [CrossRef]
- Pan, D.; Xu, L.; Guo, M. The role of protein kinase C in diabetic microvascular complications. Front. Endocrinol 2022, 13, 973058. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Majsterek, I. Relationship between Biochemical Pathways and Non-Coding RNAs Involved in the Progression of Diabetic Retinopathy. J. Clin. Med. 2024, 13, 292. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Tang, P.; Lv, H. Targeting oxidative stress in diabetic retinopathy: Mechanisms, pathology, and novel treatment approaches. Front. Immunol. 2025, 16, 1571576. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Gui, F.; You, Z.; Fu, S.; Wu, H.; Zhang, Y. Endothelial Dysfunction in Diabetic Retinopathy. Front. Endocrinol 2020, 11, 591. [Google Scholar] [CrossRef]
- Song, S.; Yu, X.; Zhang, P.; Dai, H. Increased levels of cytokines in the aqueous humor correlate with the severity of diabetic retinopathy. J. Diabetes Complicat. 2020, 34, 107641. [Google Scholar] [CrossRef]
- Al-Kharashi, A.S. Role of oxidative stress, inflammation, hypoxia and angiogenesis in the development of diabetic retinopathy. Saudi J. Ophthalmol. 2018, 32, 318–323. [Google Scholar] [CrossRef]
- Al-Shabrawey, M.; Elsherbiny, M.; Nussbaum, J.; Othman, A.; Megyerdi, S.; Tawfik, A. Targeting Neovascularization in Ischemic Retinopathy: Recent Advances. Expert. Rev. Ophthalmol. 2013, 8, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.; Srishti, R.; Khanum, A.; Thirumalesh, M.B.; Dave, V.; Arora, A.; Bansal, R.; Surve, A.; Azad, S.; Kumar, V. Vitreous hemorrhage—Causes, diagnosis, and management. Indian. J. Ophthalmol. 2023, 71, 28–38. [Google Scholar] [CrossRef]
- Jayaram, H.; Kolko, M.; Friedman, D.S.; Gazzard, G. Glaucoma: Now and beyond. Lancet 2023, 402, 1788–1801. [Google Scholar] [CrossRef]
- Wang, L.H.; Huang, C.H.; Lin, I.C. Advances in Neuroprotection in Glaucoma: Pharmacological Strategies and Emerging Technologies. Pharmaceuticals 2024, 17, 1261. [Google Scholar] [CrossRef]
- D’Souza, S.; Lang, R.A. Retinal ganglion cell interactions shape the developing mammalian visual system. Development 2020, 147, dev196535. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, D. Apoptosis in glaucoma: A new direction for the treatment of glaucoma (Review). Mol. Med. Rep. 2024, 29, 82. [Google Scholar] [CrossRef]
- Glover, H.L.; Schreiner, A.; Dewson, G.; Tait, S.W.G. Mitochondria and cell death. Nat. Cell Biol. 2024, 26, 1434–1446. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; van Delft, M.F.; Dewson, G. Too much death can kill you: Inhibiting intrinsic apoptosis to treat disease. EMBO J. 2021, 40, e107341. [Google Scholar] [CrossRef]
- Palioura, S.; Vavvas, D.G. Neuroprotection in Glaucoma; IntechOpen: London, UK, 2013. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Jiang, S.; Musayeva, A.; Gericke, A. Oxidative Stress and Vascular Dysfunction in the Retina: Therapeutic Strategies. Antioxidants 2020, 9, 761. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar] [CrossRef]
- Lee, H.P.; Tsung, T.H.; Tsai, Y.C.; Chen, Y.H.; Lu, D.W. Glaucoma: Current and New Therapeutic Approaches. Biomedicines 2024, 12, 2000. [Google Scholar] [CrossRef]
- Gholami, M.; Ghelichkhani, Z.; Aghakhani, R.; Klionsky, D.J.; Motaghinejad, O.; Motaghinejad, M.; Koohi, M.K.; Hassan, J. Minocycline acts as a neuroprotective agent against tramadol-induced neurodegeneration: Behavioral and molecular evidence. Int. J. Prev. Med. 2024, 15, 47. [Google Scholar] [CrossRef]
- Teister, J.; Anders, F.; Beck, S.; Funke, S.; von Pein, H.; Prokosch, V.; Pfeiffer, N.; Grus, F. Decelerated neurodegeneration after intravitreal injection of α-synuclein antibodies in a glaucoma animal model. Sci. Rep. 2017, 7, 6260. [Google Scholar] [CrossRef] [PubMed]
- Pietrucha-Dutczak, M.; Amadio, M.; Govoni, S.; Lewin-Kowalik, J.; Smedowski, A. The role of endogenous neuroprotective mechanisms in the prevention of retinal ganglion cells degeneration. Front. Neurosci. 2018, 12, 834. [Google Scholar] [CrossRef]
- Sarkis, S.; Chamard, C.; Johansen, B.; Daien, V.; Michon, F. Challenging glaucoma with emerging therapies: An overview of advancements against the silent thief of sight. Front. Med. 2025, 12, 1527319. [Google Scholar] [CrossRef]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative stress and inflammation in the pathogenesis of neurological disorders: Mechanisms and implications. Acta Pharm. Sin. B 2025, 15, 15–34. [Google Scholar] [CrossRef]
- Shu, D.Y.; Chaudhary, S.; Cho, K.S.; Lennikov, A.; Miller, W.P.; Thorn, D.C.; Yang, M.; McKay, T.B. Role of oxidative stress in ocular diseases: A balancing act. Metabolites 2023, 13, 187. [Google Scholar] [CrossRef]
- Lou, M.F.; Augusteyn, R.C. Oxidation-induced mixed disulfide and cataract formation: A review. Antioxidants 2025, 14, 425. [Google Scholar] [CrossRef]
- Roskamp, K.W.; Paulson, C.N.; Brubaker, W.D.; Martin, R.W. Function and aggregation in structural eye lens crystallins. Acc. Chem. Res. 2020, 53, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.K.; Santhoshkumar, P. Lens aging: Effects of crystallins. Biochim. Biophys. Acta 2009, 1790, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Cvekl, A.; Vijg, J. Aging of the eye: Lessons from cataracts and age-related macular degeneration. Ageing Res. Rev. 2024, 99, 102407. [Google Scholar] [CrossRef]
- Ray, N.J. Biophysical chemistry of the ageing eye lens. Biophys. Rev. 2015, 7, 353–368. [Google Scholar] [CrossRef]
- Nordström, M.; Zetterberg, M.; Torén, K.; Schiöler, L.; Holm, M. The more smoking the more cataract: A study on smoking, snus use and cataract in a Swedish population. Acta Ophthalmol. 2025, 103, 77–84. [Google Scholar] [CrossRef]
- MacFarlane, E.R.; Donaldson, P.J.; Grey, A.C. UV light and the ocular lens: A review of exposure models and resulting biomolecular changes. Front. Ophthalmol. 2024, 4, 1414483. [Google Scholar] [CrossRef]
- Zarei-Ghanavati, S.; Hadi, Y.; Habibi, A.; Ashraf Khorasani, M.; Yoo, S.H. Cataract and diabetes: Review of the literature. J. Cataract. Refract. Surg. 2024, 50, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Nizami, A.A.; Gulani, A.C. Cataract. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Kulbay, M.; Wu, K.Y.; Nirwal, G.K.; Bélanger, P.; Tran, S.D. Oxidative stress and cataract formation: Evaluating the efficacy of antioxidant therapies. Biomolecules 2024, 14, 1055. [Google Scholar] [CrossRef] [PubMed]
- Truscott, R.J. Age-related nuclear cataract—Oxidation is the key. Exp. Eye Res. 2005, 80, 709–725. [Google Scholar] [CrossRef]
- Ramkumar, S.; Fan, X.; Wang, B.; Yang, S.; Monnier, V.M. Reactive cysteine residues in the oxidative dimerization and Cu2+ induced aggregation of human γD-crystallin: Implications for age-related cataract. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3595–3604. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.A.; Lee, W.; Giblin, F.J.; David, L.L.; Kantorow, M. Methionine sulfoxide reductase A (MsrA) restores alpha-crystallin chaperone activity lost upon methionine oxidation. Biochim. Biophys. Acta 2009, 1790, 1665–1672. [Google Scholar] [CrossRef][Green Version]
- Álvarez-Barrios, A.; Álvarez, L.; García, M.; Artime, E.; Pereiro, R.; González-Iglesias, H. Antioxidant defenses in the human eye: A focus on metallothioneins. Antioxidants 2021, 10, 89. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef]
- Korovila, I.; Hugo, M.; Castro, J.P.; Weber, D.; Höhn, A.; Grune, T.; Jung, T. Proteostasis, oxidative stress and aging. Redox Biol. 2017, 13, 550–567. [Google Scholar] [CrossRef]
- Karakosta, C.; Samiotaki, M.; Panayotou, G.; Papaconstantinou, D.S.; Moschos, M.M. Lens cytoskeleton: An update on the etiopathogenesis of human cataracts. Cureus 2024, 16, e56793. [Google Scholar] [CrossRef]
- Wei, Z.; Hao, C.; Huangfu, J.; Srinivasagan, R.; Zhang, X.; Fan, X. Aging lens epithelium is susceptible to ferroptosis. Free Radic. Biol. Med. 2021, 167, 94–108. [Google Scholar] [CrossRef]
- Kłodnicka, K.; Januszewski, J.; Forma, A.; Pająk, W.; Teresińska, B.; Baj, J. Iron in multiple sclerosis—From pathophysiology to disease progression—A narrative literature review. Acta Neurobiol. Exp. (Wars) 2025, 85, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Pal, P.; Gupta, S.K.; Potdar, M.B.; Belgamwar, A.V. Microglial-mediated immune mechanisms in autoimmune uveitis: Elucidating pathogenic pathways and targeted therapeutics. J. Neuroimmunol. 2024, 395, 578433. [Google Scholar] [CrossRef]
- Wang, X.; Wang, T.; Lam, E.; Alvarez, D.; Sun, Y. Ocular vascular diseases: From retinal immune privilege to inflammation. Int. J. Mol. Sci. 2023, 24, 12090. [Google Scholar] [CrossRef]
- Cunha-Vaz, J. The blood-retinal barrier in the management of retinal disease: EURETINA award lecture. Ophthalmologica 2017, 237, 1–10. [Google Scholar] [CrossRef]
- Taylor, A.W. Ocular immune privilege. Eye 2009, 23, 1885–1889. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chun, J.; Ahn, M.; Jung, K.; Moon, C.; Shin, T. Blood-retina barrier dysfunction in experimental autoimmune uveitis: The pathogenesis and therapeutic targets. Anat. Cell Biol. 2022, 55, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Gadina, M.; Gazaniga, N.; Vian, L.; Furumoto, Y. Small molecules to the rescue: Inhibition of cytokine signaling in immune-mediated diseases. J. Autoimmun. 2017, 85, 20–31. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Guo, K.; Zhang, X. Cytokines that modulate the differentiation of Th17 cells in autoimmune uveitis. J. Immunol. Res. 2021, 2021, 6693542. [Google Scholar] [CrossRef]
- Manicassamy, S.; Pulendran, B. Dendritic cell control of tolerogenic responses. Immunol. Rev. 2011, 241, 206–227. [Google Scholar] [CrossRef]
- Griffin, G.K.; Newton, G.; Tarrio, M.L.; Bu, D.X.; Maganto-Garcia, E.; Azcutia, V.; Alcaide, P.; Grabie, N.; Luscinskas, F.W.; Croce, K.J.; et al. IL-17 and TNF-α sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J. Immunol. 2012, 188, 6287–6299. [Google Scholar] [CrossRef]
- Valenzuela, R.A.; Vega-Tapia, F.; Elizalde, N.; Flores, I.; Rojas, F.M.; Goecke, A.; Cuitino, L.; Urzua, C.A. IL-10 and IL-6/IL-10 as predictive biomarkers for treatment response in non-infectious uveitis. Front. Immunol. 2025, 16, 1584905. [Google Scholar] [CrossRef] [PubMed]
- Ratnapriya, R.; Swaroop, A. Genetic architecture of retinal and macular degenerative diseases: The promise and challenges of next-generation sequencing. Genome Med. 2013, 5, 98. [Google Scholar] [CrossRef][Green Version]
- Dias, M.F.; Joo, K.; Kemp, J.A.; Fialho, S.L.; Cunha, A.D.S.; Woo, S.J.; Kwon, Y.J. Molecular genetics and emerging therapies for retinitis pigmentosa: Basic research and clinical perspectives. Prog. Retin. Eye Res. 2018, 63, 107–131, Erratum in Prog. Retin. Eye Res. 2018, 66, 220–221. https://doi.org/10.1016/j.preteyeres.2018.08.001. [Google Scholar] [CrossRef] [PubMed]
- Stone, E.M.; Andorf, J.L.; Whitmore, S.S.; DeLuca, A.P.; Giacalone, J.C.; Streb, L.M.; Braun, T.A.; Mullins, R.F.; Scheetz, T.E.; Sheffield, V.C.; et al. Clinically focused molecular investigation of 1000 consecutive families with inherited retinal disease. Ophthalmology 2017, 124, 1314–1331. [Google Scholar] [CrossRef]
- Boycott, K.M.; Rath, A.; Chong, J.X.; Hartley, T.; Alkuraya, F.S.; Baynam, G.; Brookes, A.J.; Brudno, M.; Carracedo, A.; Dunnen, J.T.D.; et al. International cooperation to enable the diagnosis of all rare genetic diseases. Am. J. Hum. Genet. 2017, 100, 695–705. [Google Scholar] [CrossRef]
- Gupta, P.R.; Kheir, W.; Peng, B.; Duan, J.; Chiang, J.P.; Iannaccone, A. Identification of numerous novel disease-causing variants in patients with inherited retinal diseases, combining careful clinical-functional phenotyping with systematic, broad NGS panel-based genotyping. Mol. Vis. 2022, 28, 203–219. [Google Scholar]
- Jose, P.F.-S.; Corton, M.; Blanco-Kelly, F.; Avila-Fernandez, A.; Lopez-Martinez, M.A.; Sanchez-Navarro, I.; Sanchez-Alcudia, R.; Perez-Carro, R.; Zurita, O.; Sanchez-Bolivar, N.; et al. Targeted Next-Generation Sequencing Improves the Diagnosis of Autosomal Dominant Retinitis Pigmentosa in Spanish Patients. Investig. Opthalmol. Vis. Sci. 2015, 56, 2173–2182. [Google Scholar] [CrossRef]
- Shah, M.; Shanks, M.; Packham, E.; Williams, J.; Haysmoore, J.; MacLaren, R.E.; Németh, A.H.; Clouston, P.; Downes, S.M. Next generation sequencing using phenotype-based panels for genetic testing in inherited retinal diseases. Ophthalmic Genet. 2020, 41, 331–337. [Google Scholar] [CrossRef]
- Jin, E.; Burnier, J.V. Liquid biopsy in uveal melanoma: Are we there yet? Ocul. Oncol. Pathol. 2021, 7, 1–16. [Google Scholar] [CrossRef]
- Lalman, C.; Yang, Y.; Walker, J.L. Artificial Intelligence in Ocular Transcriptomics: Applications of Unsupervised and Supervised Learning. Cells 2025, 14, 1315. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, H.; Xiao, L.; Zhang, M.; Yan, N. Single-cell RNA sequencing in the study of human retinal organoids. Exp. Eye Res. 2025, 256, 110417. [Google Scholar] [CrossRef]
- Van der Pol, Y.; Mouliere, F. Toward the early detection of cancer by decoding the epigenetic and environmental fingerprints of cell-free DNA. Cancer Cell 2019, 36, 350–368. [Google Scholar] [CrossRef] [PubMed]
- Collin, R.W.J.; Garanto, A. Applications of long-read sequencing technologies in inherited eye diseases. Mol. Genet. Genomic Med. 2023, 11, e2067. [Google Scholar] [CrossRef]
- Ballesta-Alcaraz, L.; Bernal, M.; Vilchez, J.R.; Palacios, J.A.; Jiménez, P.; Garrido, P.; Gutiérrez-Bautista, J.F.; Ruiz-Cabello, F. Application of Optical Genome Mapping for the Diagnosis and Risk Stratification of Myeloid and Lymphoid Malignancies. Int. J. Mol. Sci. 2025, 26, 5763. [Google Scholar] [CrossRef] [PubMed]
- Theodorakis, N.; Feretzakis, G.; Tzelves, L.; Paxinou, E.; Hitas, C.; Vamvakou, G.; Verykios, V.S.; Nikolaou, M. Integrating Machine Learning with Multi-Omics Technologies in Geroscience: Towards Personalized Medicine. J. Pers. Med. 2024, 14, 931. [Google Scholar] [CrossRef]
- Jenkins, A.J.; Joglekar, M.V.; Hardikar, A.A.; Keech, A.C.; O’NEal, D.N.; Januszewski, A.S. Biomarkers in Diabetic Retinopathy. Rev. Diabet. Stud. 2015, 12, 159–195. [Google Scholar] [CrossRef]
- Brown, C.N.; Green, B.D.; Thompson, R.B.; Hollander, A.I.D.; Lengyel, I. On behalf of the EYE-RISK consortium Metabolomics and Age-Related Macular Degeneration. Metabolites 2018, 9, 4. [Google Scholar] [CrossRef]
- Voigt, A.P.; Mullin, N.K.; Stone, E.M.; Tucker, B.A.; Scheetz, T.E.; Mullins, R.F. Single-cell RNA sequencing in vision research: Insights into human retinal health and disease. Prog. Retin. Eye Res. 2021, 83, 100934. [Google Scholar] [CrossRef]
- Moqri, M.; Herzog, C.; Poganik, J.R.; Ying, K.; Justice, J.N.; Belsky, D.W.; Higgins-Chen, A.T.; Chen, B.H.; Cohen, A.A.; Fuellen, G.; et al. Validation of biomarkers of aging. Nat. Med. 2024, 30, 360–372. [Google Scholar] [CrossRef]
- Xue, M.; Ke, Y.; Ren, X.; Zhou, L.; Liu, J.; Zhang, X.; Shao, X.; Li, X. Proteomic analysis of aqueous humor in patients with pathologic myopia. J. Proteom. 2021, 234, 104088. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zheng, S.G.; Zhou, H. Metabolomics of ocular immune diseases. Metabolomics 2025, 21, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, Y.; Yu, B.; Deng, Y.; Wang, Y.; Fang, S.; Song, X.; Fan, X.; Zhou, H. Multi-Omics Approaches to Discover Biomarkers of Thyroid Eye Disease: A Systematic Review. Int. J. Biol. Sci. 2024, 20, 6038–6055. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.; Das, S.; Shetty, R.; Zhou, L.; Ghosh, A.; Deshpande, V. Tear proteomics in dry eye disease. Indian J. Ophthalmol. 2023, 71, 1203–1214. [Google Scholar] [CrossRef]
- Chen, S.; Bai, W. Artificial intelligence technology in ophthalmology public health: Current applications and future directions. Front. Cell Dev. Biol. 2025, 13, 1576465. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Z.; Zhang, Y.; Wu, K.; Li, X.; Zhou, S. Tear metabolomics reveals novel potential biomarkers in epithelial herpes simplex keratitis. BMC Ophthalmol. 2025, 25, 1–9. [Google Scholar] [CrossRef]
- Rosa, C.; Marsch, L.A.; Winstanley, E.L.; Brunner, M.; Campbell, A.N. Using digital technologies in clinical trials: Current and future applications. Contemp. Clin. Trials 2020, 100, 106219. [Google Scholar] [CrossRef]
- Kimoto, K.; Kubota, T. Anti-VEGF Agents for Ocular Angiogenesis and Vascular Permeability. J. Ophthalmol. 2011, 2012, 852183. [Google Scholar] [CrossRef]
- Sahel, D.K.; Giriprasad, G.; Jatyan, R.; Guha, S.; Korde, A.; Mittal, A.; Bhand, S.; Chitkara, D. Next-generation CRISPR/Cas-based ultrasensitive diagnostic tools: Current progress and prospects. RSC Adv. 2024, 14, 32411–32435. [Google Scholar] [CrossRef] [PubMed]
- Kellner, M.J.; Koob, J.G.; Gootenberg, J.S.; Abudayyeh, O.O.; Zhang, F. SHERLOCK: Nucleic acid detection with CRISPR nucleases. Nat. Protoc. 2019, 14, 2986–3012, Erratum in Nat Protoc. 2020, 15, 1311. https://doi.org/10.1038/s41596-020-0302-z. [Google Scholar] [CrossRef]
- Schumacher-Schuh, A.; Bieger, A.; Borelli, W.V.; Portley, M.K.; Awad, P.S.; Bandres-Ciga, S. Advances in Proteomic and Metabolomic Profiling of Neurodegenerative Diseases. Front. Neurol. 2022, 12, 792227. [Google Scholar] [CrossRef]
- Dalamaga, M. Clinical metabolomics: Useful insights, perspectives and challenges. Metabol. Open 2024, 22, 100290. [Google Scholar] [CrossRef]
- Dmuchowska, D.A.; Bartolo Khodjayeva, M.; Ciborowski, M. Editorial: Metabolomics in human and animal ophthalmic research. Front. Mol. Biosci. 2025, 12, 1594647. [Google Scholar] [CrossRef]
- Jones, G.; Altman, J.; Ahmed, S.; Lee, T.J.; Zhi, W.; Sharma, S.; Sharma, A. Unraveling the Intraday Variations in the Tear Fluid Proteome. Invest. Ophthalmol. Vis. Sci. 2024, 65, 2. [Google Scholar] [CrossRef] [PubMed]
- Explainable AI Models in Ophthalmic Diagnostics. arXiv 2024, arXiv:2402.06322. Available online: https://arxiv.org/abs/2402.06322 (accessed on 9 July 2025).
- Sheng, B.; Chen, X.; Li, T.; Ma, T.; Yang, Y.; Bi, L.; Zhang, X. An overview of artificial intelligence in diabetic retinopathy and other ocular diseases. Front. Public. Health 2022, 10, 971943. [Google Scholar] [CrossRef]
- El-Ateif, S.; Idri, A. Multimodality Fusion Strategies in Eye Disease Diagnosis. J. Imaging Inform. Med. 2024, 37, 2524–2558. [Google Scholar] [CrossRef]
- Jain, S.; Eadon, M.T. Spatial transcriptomics in health and disease. Nat. Rev. Nephrol. 2024, 20, 659–671. [Google Scholar] [CrossRef]
- Wei, L.; Niraula, D.; Gates, E.D.H.; Fu, J.; Luo, Y.; Nyflot, M.J.; Bowen, S.R.; El Naqa, I.M.; Cui, S. Artificial intelligence (AI) and machine learning (ML) in precision oncology: A review on enhancing discoverability through multiomics integration. Br. J. Radiol. 2023, 96, 20230211. [Google Scholar] [CrossRef]
- Hu, M.L.; Edwards, T.L.; O’Hare, F.; Hickey, D.G.; Wang, J.H.; Liu, Z.; Ayton, L.N. Gene therapy for inherited retinal diseases: Progress and possibilities. Clin. Exp. Optom. 2021, 104, 444–454. [Google Scholar] [CrossRef]
- Boon, N.; Wijnholds, J.; Pellissier, L.P. Research models and gene augmentation therapy for CRB1 retinal dystrophies. Front. Neurosci. 2020, 14, 860. [Google Scholar] [CrossRef]
- Mendell, J.R.; Al-Zaidy, S.A.; Rodino-Klapac, L.R.; Goodspeed, K.; Gray, S.J.; Kay, C.N.; Boye, S.L.; Boye, S.E.; George, L.A.; Salabarria, S.; et al. Current clinical applications of in vivo gene therapy with AAVs. Mol. Ther. 2021, 29, 464–488. [Google Scholar] [CrossRef] [PubMed]
- Banou, L.; Sarrafpour, S.; Teng, C.C.; Liu, J. Ocular gene therapy: An overview of viral vectors, immune responses, and future directions. Yale J. Biol. Med. 2024, 97, 491–503. [Google Scholar] [CrossRef]
- Dhurandhar, D.; Sahoo, N.K.; Mariappan, I.; Narayanan, R. Gene therapy in retinal diseases: A review. Indian J. Ophthalmol. 2021, 69, 2257–2265. [Google Scholar] [CrossRef]
- Drag, S.; Dotiwala, F.; Upadhyay, A.K. Gene therapy for retinal degenerative diseases: Progress, challenges, and future directions. Invest. Ophthalmol. Vis. Sci. 2023, 64, 39. [Google Scholar] [CrossRef]
- Henderson, J.; O’Callaghan, J.; Campbell, M. Gene therapy for glaucoma: Targeting key mechanisms. Vision. Res. 2024, 225, 108502. [Google Scholar] [CrossRef]
- Hakim, A.; Guido, B.; Narsineni, L.; Chen, D.W.; Foldvari, M. Gene therapy strategies for glaucoma from IOP reduction to retinal neuroprotection: Progress towards non-viral systems. Adv. Drug Deliv. Rev. 2023, 196, 114781. [Google Scholar] [CrossRef]
- Anton, N.; Geamănu, A.; Iancu, R.; Pîrvulescu, R.A.; Popa-Cherecheanu, A.; Barac, R.I.; Bandol, G.; Bogdănici, C.M. A mini-review on gene therapy in glaucoma and future directions. Int. J. Mol. Sci. 2024, 25, 11019. [Google Scholar] [CrossRef]
- Camacho, D.K.; Go, C.C.; Chaqour, B.; Shindler, K.S.; Ross, A.G. Emerging gene therapy technologies for retinal ganglion cell neuroprotection. J. Neuro-Ophthalmol. 2023, 43, 330–340. [Google Scholar] [CrossRef]
- Mohan, R.R.; Martin, L.M.; Sinha, N.R. Novel insights into gene therapy in the cornea. Exp. Eye Res. 2021, 202, 108361. [Google Scholar] [CrossRef]
- Amador, C.; Shah, R.; Ghiam, S.; Kramerov, A.A.; Ljubimov, A.V. Gene therapy in the anterior eye segment. Curr. Gene Ther. 2022, 22, 104–131. [Google Scholar] [CrossRef]
- Utine, C.A.; Güven, S. Tissue engineering and ophthalmology. Turk. J. Ophthalmol. 2024, 54, 159–169. [Google Scholar] [CrossRef]
- Voisin, A.; Pénaguin, A.; Gaillard, A.; Leveziel, N. Stem cell therapy in retinal diseases. Neural Regen. Res. 2023, 18, 1478–1485. [Google Scholar] [CrossRef]
- Maeda, T.; Mandai, M.; Sugita, S.; Kime, C.; Takahashi, M. Strategies of pluripotent stem cell-based therapy for retinal degen- eration: Update and challenges. Trends Mol. Med. 2022, 28, 388–404. [Google Scholar] [CrossRef]
- Maeda, A.; Mandai, M.; Takahashi, M. Gene and induced pluripotent stem cell therapy for retinal diseases. Annu. Rev. Genom. Hum. Genet. 2019, 20, 201–216. [Google Scholar] [CrossRef]
- Nurković, J.S.; Vojinović, R.; Dolićanin, Z. Corneal stem cells as a source of regenerative cell-based therapy. Stem Cells Int. 2020, 2020, 8813447. [Google Scholar] [CrossRef]
- Shukla, S.; Shanbhag, S.S.; Tavakkoli, F.; Varma, S.; Singh, V.; Basu, S. Limbal epithelial and mesenchymal stem cell therapy for corneal regeneration. Curr. Eye Res. 2020, 45, 265–277. [Google Scholar] [CrossRef]
- Ting, D.S.J.; Peh, G.S.L.; Adnan, K.; Mehta, J.S. Translational and regulatory challenges of corneal endothelial cell therapy: A global perspective. Tissue Eng. Part. B Rev. 2022, 28, 52–62. [Google Scholar] [CrossRef]
- Takayanagi, H.; Hayashi, R. Status and prospects for the development of regenerative therapies for corneal and ocular diseases. Regen. Ther. 2024, 26, 819–825. [Google Scholar] [CrossRef]
- Doss, M.X.; Sachinidis, A. Current challenges of iPSC-based disease modeling and therapeutic implications. Cells 2019, 8, 403. [Google Scholar] [CrossRef]
- Li, T.; Xing, H.M.; Qian, H.D.; Gao, Q.; Xu, S.L.; Ma, H.; Chi, Z.L. Small extracellular vesicles derived from human induced pluripotent stem cell-differentiated neural progenitor cells mitigate retinal ganglion cell degeneration in a mouse model of optic nerve injury. Neural Regen. Res. 2025, 20, 587–597. [Google Scholar] [CrossRef]
- Tomczak, W.; Winkler-Lach, W.; Tomczyk-Socha, M.; Misiuk-Hojło, M. Advancements in ocular regenerative therapies. Biology 2023, 12, 737. [Google Scholar] [CrossRef]
- Li, J.Y.; Cortina, M.S.; Greiner, M.A.; Kuo, A.N.; Miller, D.D.; Shtein, R.M.; Veldman, P.B.; Yin, J.; Kim, S.J.; Shen, J.F. Outcomes and Complications of Limbal Stem Cell Allograft Transplantation: A Report by the American Academy of Ophthalmology. Ophthalmology 2024, 131, 1121–1131. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Q.; Temple, S. Stem cell therapies for retinal diseases: Recapitulating development to replace degenerated cells. Development 2017, 144, 1368–1381. [Google Scholar] [CrossRef]
- Lin, Y.T.; Wang, C.K.; Yang, S.C.; Hsu, S.C.; Lin, H.; Chang, F.P.; Kuo, T.C.; Shen, C.N.; Chiang, P.M.; Hsiao, M.; et al. Elimination of undifferentiated human embryonic stem cells by cardiac glycosides. Sci. Rep. 2017, 7, 5289. [Google Scholar] [CrossRef]
- Movahed, A.Y.; Bagheri, R.; Savatier, P.; Šarić, T.; Moradi, S. Elimination of tumorigenic pluripotent stem cells from their differentiated cell therapy products: An important step toward ensuring safe cell therapy. Stem Cell Rep. 2025, 20, 102543. [Google Scholar] [CrossRef]
- Wuputra, K.; Ku, C.C.; Wu, D.C.; Lin, Y.C.; Saito, S.; Yokoyama, K.K. Prevention of tumor risk associated with the reprogramming of human pluripotent stem cells. J. Exp. Clin. Cancer Res. 2020, 39, 100. [Google Scholar] [CrossRef]
- Bedel, A.; Beliveau, F.; Lamrissi-Garcia, I.; Rousseau, B.; Moranvillier, I.; Rucheton, B.; Guyonnet-Dupérat, V.; Cardinaud, B.; de Verneuil, H.; Moreau-Gaudry, F.; et al. Preventing Pluripotent Cell Teratoma in Regenerative Medicine Applied to Hematology Disorders. Stem Cells Transl. Med. 2017, 6, 382–393. [Google Scholar] [CrossRef]
- Mostafa, M.; Al Fatease, A.; Alany, R.G.; Abdelkader, H. Recent advances of ocular drug delivery systems: Prominence of ocular implants for chronic eye diseases. Pharmaceutics 2023, 15, 1746. [Google Scholar] [CrossRef]
- Choe, S.; Heo, J.W.; Oh, B.L. Quiescence and subsequent anterior chamber inflammation in adalimumab-treated pediatric noninfectious uveitis. Korean J. Ophthalmol. 2020, 34, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, Z.; Tao, T.; Duan, R.; Wang, X.; Su, W. TNF-α in uveitis: From bench to clinic. Front. Pharmacol. 2021, 12, 740057, Erratum in Front Pharmacol. 2022, 13, 817235. https://doi.org/10.3389/fphar.2022.817235. [Google Scholar] [CrossRef]
- Ricci, F.; Bandello, F.; Navarra, P.; Staurenghi, G.; Stumpp, M.; Zarbin, M. Neovascular age-related macular degeneration: Therapeutic management and new-upcoming approaches. Int. J. Mol. Sci. 2020, 21, 8242. [Google Scholar] [CrossRef]
- Liberski, S.; Wichrowska, M.; Kocięcki, J. Aflibercept versus faricimab in the treatment of neovascular age-related macular degeneration and diabetic macular edema: A review. Int. J. Mol. Sci. 2022, 23, 9424. [Google Scholar] [CrossRef]
- Gross, J.G.; Glassman, A.R.; Liu, D.; Sun, J.K.; Antoszyk, A.N.; Baker, C.W.; Bressler, N.M.; Elman, M.J.; Ferris, F.L., 3rd; Gardner, T.W.; et al. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized clinical trial. JAMA Ophthalmol. 2018, 136, 1138–1148, Erratum in JAMA Ophthalmol. 2019, 137, 467. https://doi.org/10.1001/jamaophthalmol.2019.0032. [Google Scholar] [CrossRef]
- Shughoury, A.; Bhatwadekar, A.; Jusufbegovic, D.; Hajrasouliha, A.; Ciulla, T.A. The evolving therapeutic landscape of diabetic retinopathy. Expert Opin. Biol. Ther. 2023, 23, 969–985. [Google Scholar] [CrossRef]
- Uludag, G.; Hassan, M.; Matsumiya, W.; Pham, B.H.; Chea, S.; Than, N.T.T.; Doan, H.L.; Akhavanrezayat, A.; Halim, M.S.; Do, D.V.; et al. Efficacy and safety of intravitreal anti-VEGF therapy in diabetic retinopathy: What we have learned and what should we learn further? Expert Opin. Biol. Ther. 2022, 22, 1275–1291. [Google Scholar] [CrossRef]
- Bancalari, A.; Schade, R. Update in the treatment of retinopathy of prematurity. Am. J. Perinatol. 2022, 39, 22–30. [Google Scholar] [CrossRef]
- Stahl, A.; Sukgen, E.A.; Wu, W.C.; Lepore, D.; Nakanishi, H.; Mazela, J.; Moshfeghi, D.M.; Vitti, R.; Athanikar, A.; Chu, K.; et al. Effect of intravitreal aflibercept vs laser photocoagulation on treatment success of retinopathy of prematurity: The FIREFLEYE randomized clinical trial. JAMA 2022, 328, 348–359. [Google Scholar] [CrossRef]
- Chang, E.; Josan, A.S.; Purohit, R.; Patel, C.K.; Xue, K. A network meta-analysis of retreatment rates following bevacizumab, ranibizumab, aflibercept, and laser for retinopathy of prematurity. Ophthalmology 2022, 129, 1389–1401. [Google Scholar] [CrossRef]
- Amoaku, W.M.; Chakravarthy, U.; Gale, R.; Gavin, M.; Ghanchi, F.; Gibson, J.; Harding, S.; Johnston, R.L.; Kelly, S.P.; Lotery, A.; et al. Defining response to anti-VEGF therapies in neovascular. AMD Eye 2015, 29, 721–731, Erratum in Eye 2015, 29, 1397–1398. https://doi.org/10.1038/eye.2015.159. [Google Scholar] [CrossRef]
- Moraru, A.D.; Danielescu, C.; Iorga, R.E.; Moraru, R.L.; Zemba, M.; Branisteanu, D.C. Review of Guideline Recommendations for Optimal Anti-VEGF Therapy in Age-Related Macular Degeneration. Life 2024, 14, 1220. [Google Scholar] [CrossRef]
- Wallsh, J.O.; Gallemore, R.P. Anti-VEGF-Resistant Retinal Diseases: A Review of the Latest Treatment Options. Cells 2021, 10, 1049. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Z.; Webster, K.A.; Paulus, Y.M. Treatment Strategies for Anti-VEGF Resistance in Neovascular Age-Related Macular Degeneration by Targeting Arteriolar Choroidal Neovascularization. Biomolecules 2024, 14, 252. [Google Scholar] [CrossRef]
- Borchert, G.A.; Kiire, C.A.; Stone, N.M.; Akil, H.; Gkika, T.; Fischer, M.D.; Xue, K.; Cehajic-Kapetanovic, J.; MacLaren, R.E.; Charbel Issa, P.; et al. Real-world six-month outcomes in patients switched to faricimab following partial response to anti-VEGF therapy for neovascular age-related macular degeneration and diabetic macular oedema. Eye 2024, 38, 3569–3577. [Google Scholar] [CrossRef]
- Tsai, A.S.; Chou, H.D.; Ling, X.C.; Al-Khaled, T.; Valikodath, N.; Cole, E.; Yap, V.L.; Chiang, M.F.; Chan, R.V.P.; Wu, W.C. Assessment and management of retinopathy of prematurity in the era of anti-vascular endothelial growth factor (VEGF). Prog. Retin. Eye Res. 2022, 88, 101018. [Google Scholar] [CrossRef]
- Korobelnik, J.F.; Loewenstein, A.; Eldem, B.; Joussen, A.M.; Koh, A.; Lambrou, G.N.; Lanzetta, P.; Li, X.; Lövestam-Adrian, M.; Navarro, R.; et al. Guidance for anti-VEGF intravitreal injections during the COVID-19 pandemic. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1149–1156. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Wang, S.; Ding, M.; Zhang, M.; Tang, J.; Deng, A. Chorioretinal venous anastomosis for non-ischemic retinal vein occlusion. Front. Ophthalmol. 2022, 2, 869843. [Google Scholar] [CrossRef]
- Hiyama, T.; Harada, Y.; Doi, T.; Kiuchi, Y. Early administration of adalimumab for paediatric uveitis due to Behçet’s disease. Pediatr. Rheumatol. 2019, 17, 29. [Google Scholar] [CrossRef]
- Jabs, D.A. Immunosuppression for the uveitides. Ophthalmology 2018, 125, 193–202. [Google Scholar] [CrossRef]
- Moustardas, P.; Aberdam, D.; Lagali, N. MAPK pathways in ocular pathophysiology: Potential therapeutic drugs and challenges. Cells 2023, 12, 617. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cain-Hom, C.; Choy, L.; Hagenbeek, T.J.; de Leon, G.P.; Chen, Y.; Finkle, D.; Venook, R.; Wu, X.; Ridgway, J.; et al. Therapeutic antibody targeting of individual Notch receptors. Nature 2010, 464, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.; Brahmbhatt, A.; Deng, S.X.; Zheng, J.J. Wnt signaling activation: Targets and therapeutic opportunities for stem cell therapy and regenerative medicine. RSC Chem. Biol. 2021, 2, 1144–1157. [Google Scholar] [CrossRef]
- Lin, F.; Su, Y.; Zhao, C.; Akter, F.; Yao, S.; Huang, S.; Shao, X.; Yao, Y. Tackling visual impairment: Emerging avenues in ophthalmology. Front. Med. 2025, 12, 1567159. [Google Scholar] [CrossRef]
- Wood, E.H.; Tang, P.H.; De la Huerta, I.; Korot, E.; Muscat, S.; Palanker, D.A.; Williams, G.A. Stem cell therapies, gene-based therapies, optogenetics, and retinal prosthetics: Current state and implications for the future. Retina 2019, 39, 820–835. [Google Scholar] [CrossRef]
- Molla Desta, G.; Birhanu, A.G. Advancements in single-cell RNA sequencing and spatial transcriptomics: Transforming biomedical research. Acta Biochim. Pol. 2025, 72, 13922. [Google Scholar] [CrossRef]
- Liu, J.; Liao, X.; Li, N.; Xu, Z.; Yang, W.; Zhou, H.; Liu, Y.; Zhang, Z.; Wang, G.; Hou, S. Single-cell RNA sequencing reveals inflammatory retinal microglia in experimental autoimmune uveitis. MedComm 2024, 5, e534. [Google Scholar] [CrossRef]
- Dougan, M.; Luoma, A.M.; Dougan, S.K.; Wucherpfennig, K.W. Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell 2021, 184, 1575–1588. [Google Scholar] [CrossRef]
- Yang, Y.; Dunbar, H. Clinical perspectives and trends: Microperimetry as a trial endpoint in retinal disease. Ophthalmologica 2021, 244, 418–450. [Google Scholar] [CrossRef]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860, Erratum in Lancet 2017, 390, 848. https://doi.org/10.1016/S0140-6736(17)32235-3. [Google Scholar] [CrossRef]
- Khaparde, A.; Mathias, G.P.; Poornachandra, B.; Thirumalesh, M.B.; Shetty, R.; Ghosh, A. Gene therapy for retinal diseases: From genetics to treatment. Indian J. Ophthalmol. 2024, 72, 1091–1101. [Google Scholar] [CrossRef]
- Bi, Z.; Li, J.; Liu, Q.; Fang, Z. Deep learning-based optical coherence tomography and retinal images for detection of diabetic retinopathy: A systematic and meta analysis. Front. Endocrinol. 2025, 16, 1485311. [Google Scholar] [CrossRef]
- Leandro, I.; Lorenzo, B.; Aleksandar, M.; Dario, M.; Rosa, G.; Agostino, A.; Daniele, T. OCT-based deep-learning models for the identification of retinal key signs. Sci. Rep. 2023, 13, 14628. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Gao, A.; Giunta, M.; Tran, S.D. What’s new in ocular drug delivery: Advances in suprachoroidal injection since 2023. Pharmaceuticals 2024, 17, 1007. [Google Scholar] [CrossRef]
- Thenuwara, G.; Curtin, J.; Tian, F. Advances in diagnostic tools and therapeutic approaches for gliomas: A comprehensive review. Sensors 2023, 23, 9842. [Google Scholar] [CrossRef]
- Henderson, M.L.; Zieba, J.K.; Li, X.; Campbell, D.B.; Williams, M.R.; Vogt, D.L.; Bupp, C.P.; Edgerly, Y.M.; Rajasekaran, S.; Hartog, N.L.; et al. Gene therapy for genetic syndromes: Understanding the current state to guide future care. BioTech 2024, 13, 1. [Google Scholar] [CrossRef]
- Olawade, D.B.; Weerasinghe, K.; Mathugamage, M.D.D.E.; Odetayo, A.; Aderinto, N.; Teke, J.; Boussios, S. Enhancing ophthalmic diagnosis and treatment with artificial intelligence. Medicina 2025, 61, 433. [Google Scholar] [CrossRef]
- Salih, S.; Elliyanti, A.; Alkatheeri, A.; AlYafei, F.; Almarri, B.; Khan, H. The role of molecular imaging in personalized medicine. J. Pers. Med. 2023, 13, 369. [Google Scholar] [CrossRef] [PubMed]
- Najjar, R. Redefining radiology: A review of artificial intelligence integration in medical imaging. Diagnostics 2023, 13, 2760. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs-Krapels, S.; Ditewig, B.; Boulding, H.; Chalkidou, A.; Erskine, J.; Shokraneh, F. Purchasing high-cost medical devices and equipment in hospitals: A systematic review. BMJ Open 2022, 12, e057516. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Acevedo, A.; Davidoff, E.J.; Timmins, L.M.; Marrero-Berrios, I.; Patel, M.; White, C.; Lowe, C.; Sherba, J.J.; Hartmanshenn, C.; et al. The growing role of precision and personalized medicine for cancer treatment. Technol. (Singap. World Sci.) 2018, 6, 79–100. [Google Scholar] [CrossRef]
- Marques, L.; Costa, B.; Pereira, M.; Silva, A.; Santos, J.; Saldanha, L.; Silva, I.; Magalhães, P.; Schmidt, S.; Vale, N. Advancing precision medicine: A review of innovative in silico approaches for drug development, clinical pharmacology and personalized healthcare. Pharmaceutics 2024, 16, 332. [Google Scholar] [CrossRef]
- Garg, P.; Malhotra, J.; Kulkarni, P.; Horne, D.; Salgia, R.; Singhal, S.S. Emerging therapeutic strategies to overcome drug resistance in cancer cells. Cancers 2024, 16, 2478. [Google Scholar] [CrossRef]
- Shankar, L.K.; Schöder, H.; Sharon, E.; Wolchok, J.; Knopp, M.V.; Wahl, R.L.; Ellingson, B.M.; Hall, N.C.; Yaffe, M.J.; Towbin, A.J.; et al. Harnessing imaging tools to guide immunotherapy trials: Summary from the National Cancer Institute Cancer Imaging Steering Committee workshop. Lancet Oncol. 2023, 24, e133–e143. [Google Scholar] [CrossRef] [PubMed]


| Technology | Description | Key Features | Applications in Ophthalmology |
|---|---|---|---|
| Whole Genome Sequencing (WGS) [126,129] | Sequencing of the entire genome, coding and non-coding regions | Comprehensive, detects all variants | Rare Mendelian disorders, polygenic diseases |
| Whole Exome Sequencing (WES) [126,128,129,131] | Sequencing protein-coding regions (~1–2% of genome) | Focused on exons, cost-effective | Diagnosing inherited retinal diseases (IRDs) |
| Next-Generation Sequencing (NGS) [126,127,131] | High-throughput sequencing of multiple DNA fragments simultaneously | Affordable, automated, scalable | Rare genetic disorders, oncology, targeted gene panels |
| Targeted NGS Panels [4,128,131] | Sequencing specific gene sets | Higher diagnostic yield, faster results | IRDs like LCA, RP, Stargardt disease |
| Liquid Biopsy [4,126,128,133] | Detects circulating tumor DNA (ctDNA) or cell-free RNA (cfRNA) in fluids | Non-invasive, dynamic monitoring | Uveal melanoma, systemic ocular diseases |
| RNA Sequencing (RNA-seq) [133,134,135,138,139] | Sequencing of transcriptome (all RNA molecules) | Bulk and single-cell options, functional insight | Transcriptional dysregulation in retinal diseases |
| Single-Cell RNA-seq (scRNA-seq) [134,135,138,139,140,141] | Gene expression at the individual cell level | High resolution, detects cell heterogeneity | Diabetic retinopathy, autoimmune uveitis |
| Long-Read Sequencing [127,138] | Sequencing long DNA fragments | Detects structural variants, splice mutations | Usher syndrome, mitochondrial disorders |
| Optical Genome Map-ping [138] | Visualizes structural variants in the genome | High precision, structural variant detection | Complex inherited retinal diseases |
| Spatial Transcriptomics [138,150] | Maps gene expression spatially within tissue | Tissue-specific transcriptional profiling | Retinal microenvironment heterogeneity |
| AI-Powered Bioinformatics [139,140,141,144,145,146,147] | Machine learning for multi-omics data integration | Enhances classification, biomarker discovery | Glaucoma, AMD, retinal degeneration |
| CRISPR-based Diagnostics [151,152,153] | Nucleic acid detection with CRISPR nucleases | Ultra-sensitive mutation detection | Emerging genomic diagnostic tool |
| Ophthalmic Disease | Key Molecular Findings | Technologies Used |
|---|---|---|
| Glaucoma [130,144,145,150] | Dysregulated proteins: ALD1A1, CRYAA, clusterin; metabolites: citrate, lactate, pyruvate | LC-MS, HRMS, LC-MS/MS |
| Diabetic Retinopathy (DR) [140,145] | Markers of polyol pathway dysregulation, glycolytic imbalance, lipid peroxidation | Metabolomic profiling of aqueous/vitreous humor |
| Age-Related Macular Degeneration (AMD) [141,144,152] | Alterations in lipid metabolism, mitochondrial dysfunction, and complement cascade proteins | Plasma and aqueous humor proteomics/metabolomics |
| Autoimmune/Tumor Diseases [145,146] | Proteomic signatures linked to inflammation and tissue remodeling | Circulating proteomics (e.g., Olink PEA) |
| Dry Eye Disease (DED) [145,147] | Elevated IL-1β, IL-6, MMP-9, lipid mediators, osmolarity metabolites | Tear proteomics and metabolomics |
| Herpes simplex Keratitis (HSK) [149] | Viral-immune interaction markers | Tear metabolomics |
| Imaging Technology | Ophthalmic Applications | Key Biomarkers/Findings | Notes and Emerging Uses |
|---|---|---|---|
| Optical Coherence Tomography (OCT) [6,7,139,151,158] | Retinal layer imaging in AMD, DR, glaucoma | Retinal thickness, macular edema, GCIPL thinning | Standard for disease monitoring and therapy response |
| Fundus Autofluorescence (FAF) [6,128,152] | RPE dysfunction, Stargardt disease, retinitis pigmentosa | Lipofuscin (A2E) accumulation, RPE stress | Detects early degeneration, toxicity markers |
| Fluorescein Angiography (FA) [6,152] | Retinal vascular leakage in diabetic macular edema, CRVO | Vascular leakage, capillary nonperfusion, neovascularization | Guides anti-angiogenic therapy |
| Magnetic Resonance Imaging (MRI) [7,146] | Optic nerve pathology, orbital tumors, inflammatory diseases | Visualization beyond retina, optic neuritis, thyroid eye disease | Systemic correlation and orbital imaging |
| Positron Emission Tomography (PET) [8,133] | Ocular melanoma, autoimmune thyroid orbitopathy | Glucose metabolism, immune activation | Expanding use in ocular oncology and inflammatory orbitopathies |
| MALDI Imaging Mass Spectrometry (MALDI-MSI) [133,141,144,152] | AMD, ROP, choroidal melanoma | Oxidized phospholipids, complement peptides, lipid dysregulation | Label-free, spatially resolved molecular detection |
| Spatial Transcriptomics [134,150] | Diabetic retinopathy, non-infectious uveitis | Localized VEGF-A, ANGPT2, IL1B, immune clusters | Maps gene expression within retinal architecture |
| OCT Angiography (OCTA) [6,7,140] | Proliferative diabetic retinopathy, neovascular AMD | Capillary perfusion, neovascularization | Non-invasive vascular imaging |
| AI-Driven Imaging Analysis [10,139,151,158] | AMD, diabetic macular edema, glaucoma | Automated detection and classification of structural changes | Integration with omics for composite digital biomarkers |
| Theranostic Molecular Imaging [7,8,151] | Uveitis, AMD | Targeted nanobody-fluorophores, inflammatory and angiogenic markers | Concurrent diagnosis and therapy delivery |
| Disease | Main Pathophysiological Mechanisms | Imaging Techniques | Therapies |
|---|---|---|---|
| AMD | Lipid peroxidation, mitochondrial dysfunction, complement activation | OCT, FAF, MALDI-MSI | Anti-VEGF therapy, iPSC-derived cells, gene therapy |
| Glaucoma | Oxidative stress, apoptosis of RGCs, neurodegeneration | OCT, OCTA, GCIPL analysis | Neuroprotection, IOP-lowering drugs, gene therapy |
| Diabetic Retinopathy (DR) | Polyol pathway dysregulation, angiogenesis, advanced glycation end-products | OCT, FA, OCTA | Anti-VEGF therapy, targeted molecular therapy, gene therapy |
| DME | Vascular leakage, inflammation, cytokine upregulation | OCT, OCTA | Anti-VEGF agents, corticosteroids |
| Keratitis | Immune-mediated inflammation, corneal neovascularization | OCT, molecular imaging | Biologic therapies, tear-based diagnostics, gene therapy |
| Dry Eye Disease (DED) | Hyperosmolarity, inflammatory cytokines, immune cell infiltration | Tear proteomics, anterior segment OCT | Omics-based diagnostics, epithelial regeneration |
| Retinopathy of Prematurity | Pathological angiogenesis due to oxygen fluctuations | FA, OCTA | Anti-VEGF agents |
| Technology/Platform | Clinical Stage | Target Diseases | Data Type |
|---|---|---|---|
| OCT/OCTA | Established in clinical routine | AMD, glaucoma, DR, DME | Structural imaging |
| FA/FAF | Widely used in clinical ophthalmology | DR, AMD, Stargardt disease | Fluorescence-based imaging |
| MALDI-MSI | Preclinical/early clinical research | AMD, uveal melanoma | Spatial proteomics |
| Spatial transcriptomics | Early-stage studies | DR, uveitis | Spatial gene expression |
| Proteomics | Translational/early clinical | Glaucoma, DED, autoimmune diseases | Protein biomarkers |
| Metabolomics | Translationa | AMD, DR, HSK, DED | Small-molecule metabolites |
| AI in Imaging | Partially deployed in clinics | AMD, DR, glaucoma | Imaging diagnostics |
| Theranostics | Preclinical/Phase I | Uveitis, AMD | Imaging + therapy |
| Gene therapie | Approved (RPE65), others in trials | IRDs, glaucoma | Genetic correction |
| Cell therapies | Partially approved (e.g., Holoclar) | AMD, RP, corneal disorders | Regenerative medicine |
| Biologic therapies (e.g., anti-VEGF, anti-TNF) | Approved | AMD, DR, PDR, uveitis | Targeted molecular therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kłodnicka, K.; Januszewski, J.; Tyc, H.; Michalska, A.; Forma, A.; Teresińska, B.; Rejdak, R.; Baj, J.; Dolar-Szczasny, J. From Pathophysiology to Innovative Therapies in Eye Diseases: A Brief Overview. Int. J. Mol. Sci. 2025, 26, 8496. https://doi.org/10.3390/ijms26178496
Kłodnicka K, Januszewski J, Tyc H, Michalska A, Forma A, Teresińska B, Rejdak R, Baj J, Dolar-Szczasny J. From Pathophysiology to Innovative Therapies in Eye Diseases: A Brief Overview. International Journal of Molecular Sciences. 2025; 26(17):8496. https://doi.org/10.3390/ijms26178496
Chicago/Turabian StyleKłodnicka, Karolina, Jacek Januszewski, Hanna Tyc, Aleksandra Michalska, Alicja Forma, Barbara Teresińska, Robert Rejdak, Jacek Baj, and Joanna Dolar-Szczasny. 2025. "From Pathophysiology to Innovative Therapies in Eye Diseases: A Brief Overview" International Journal of Molecular Sciences 26, no. 17: 8496. https://doi.org/10.3390/ijms26178496
APA StyleKłodnicka, K., Januszewski, J., Tyc, H., Michalska, A., Forma, A., Teresińska, B., Rejdak, R., Baj, J., & Dolar-Szczasny, J. (2025). From Pathophysiology to Innovative Therapies in Eye Diseases: A Brief Overview. International Journal of Molecular Sciences, 26(17), 8496. https://doi.org/10.3390/ijms26178496







