β-Caryophyllene Ameliorates Thioacetamide-Induced Liver Fibrosis in Rats: A Preventative Approach
Abstract
1. Introduction
2. Results
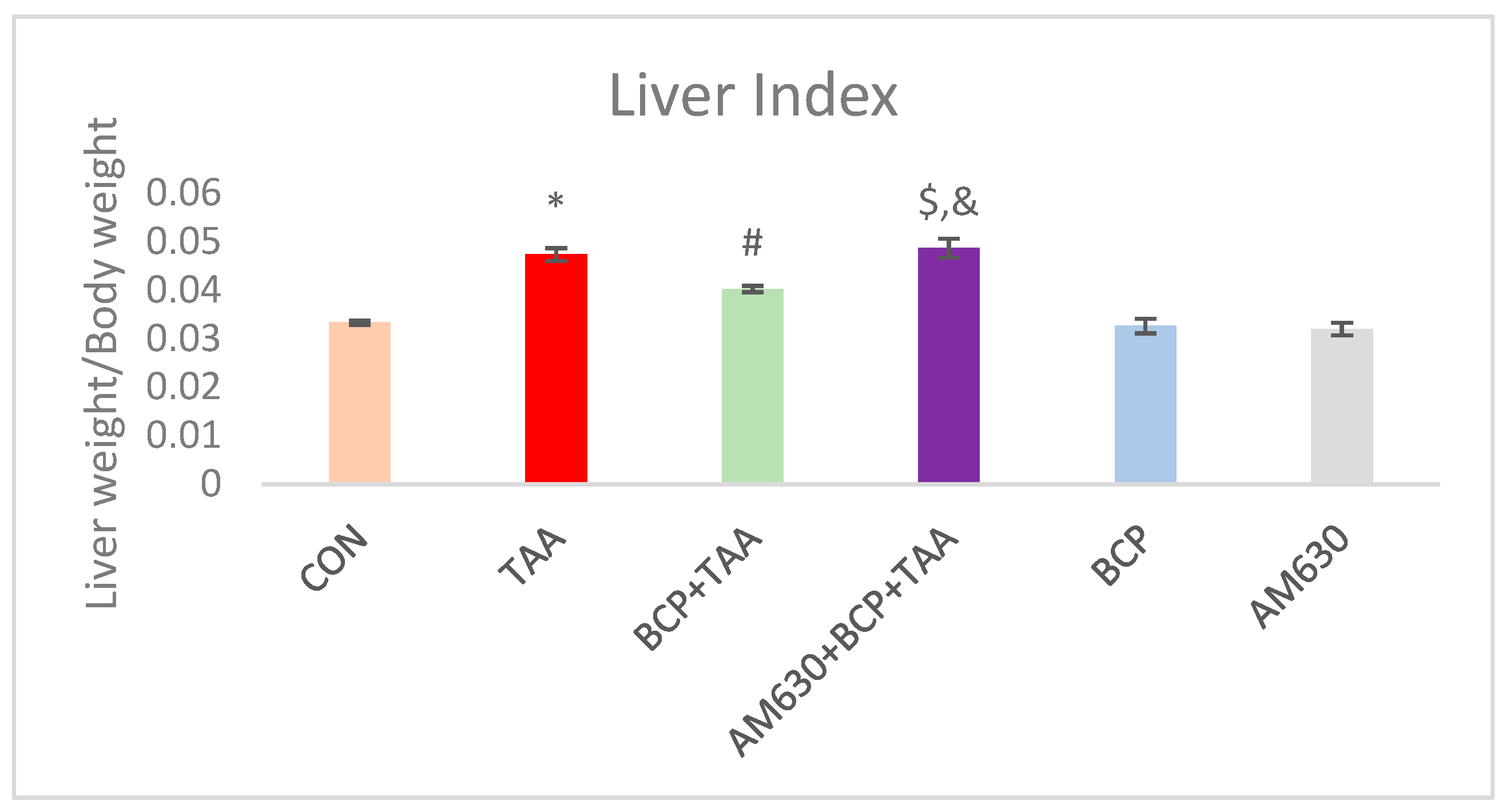
2.1. Liver Index
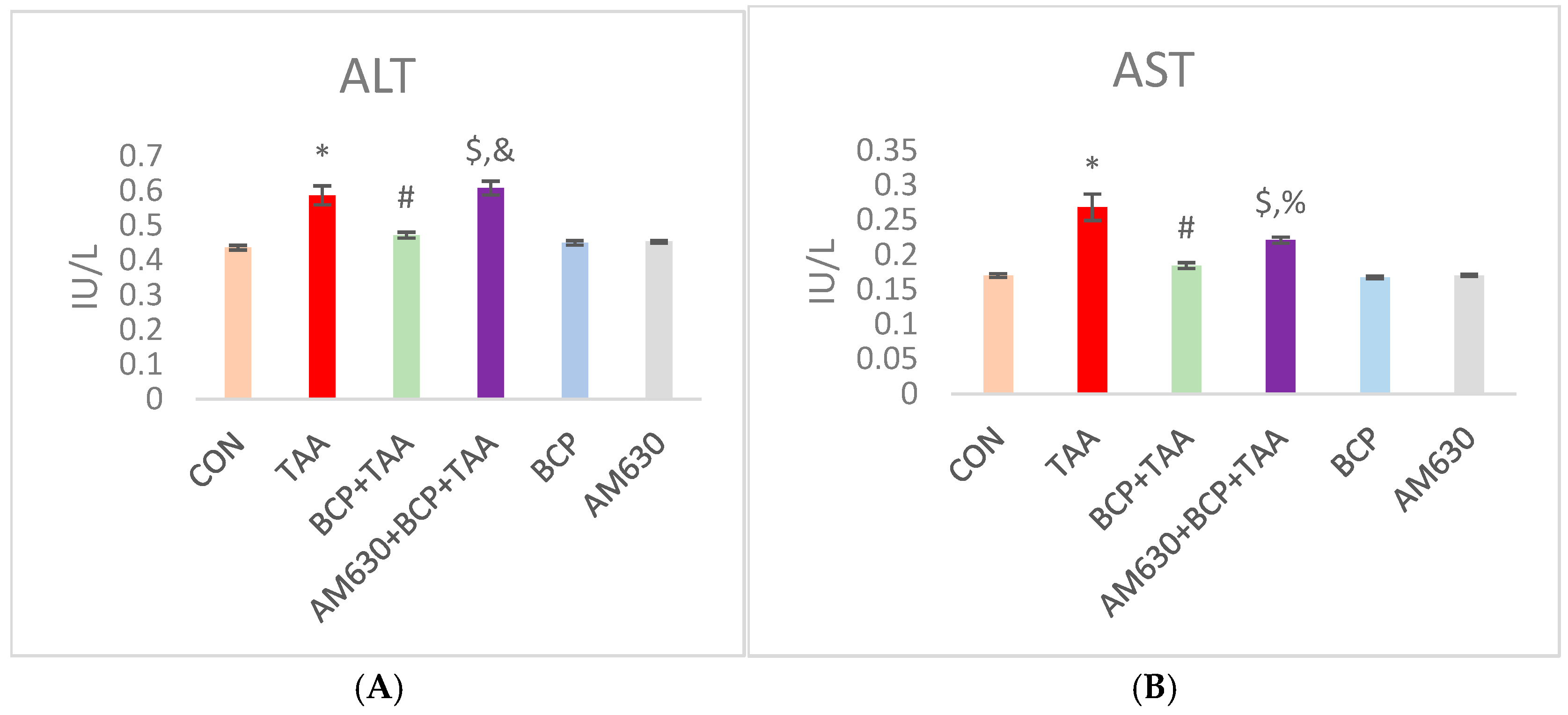
2.2. Effect of BCP on Serum Parameters
2.3. Effect of BCP on Oxidative Stress
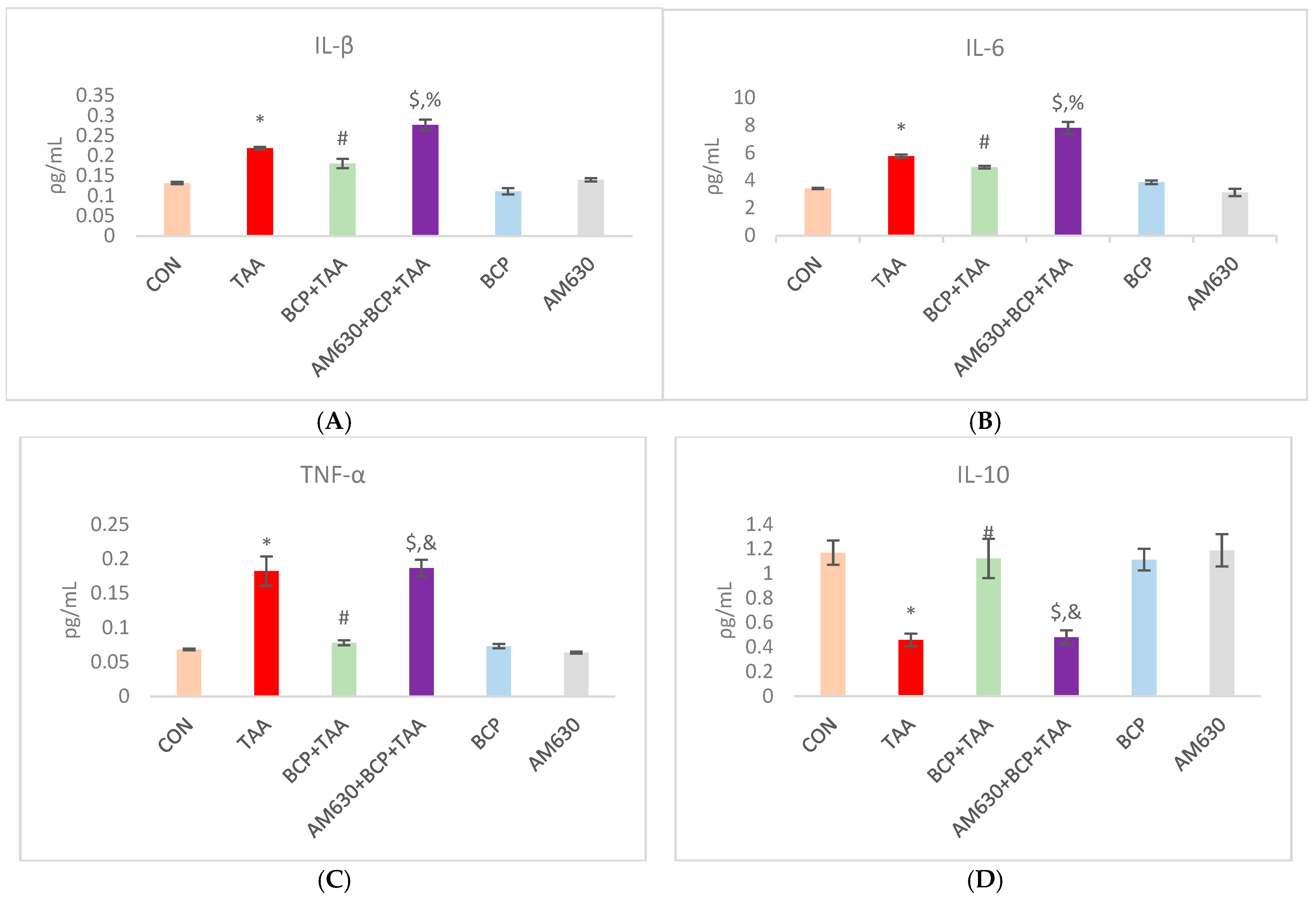
2.4. Effect of BCP on Inflammation
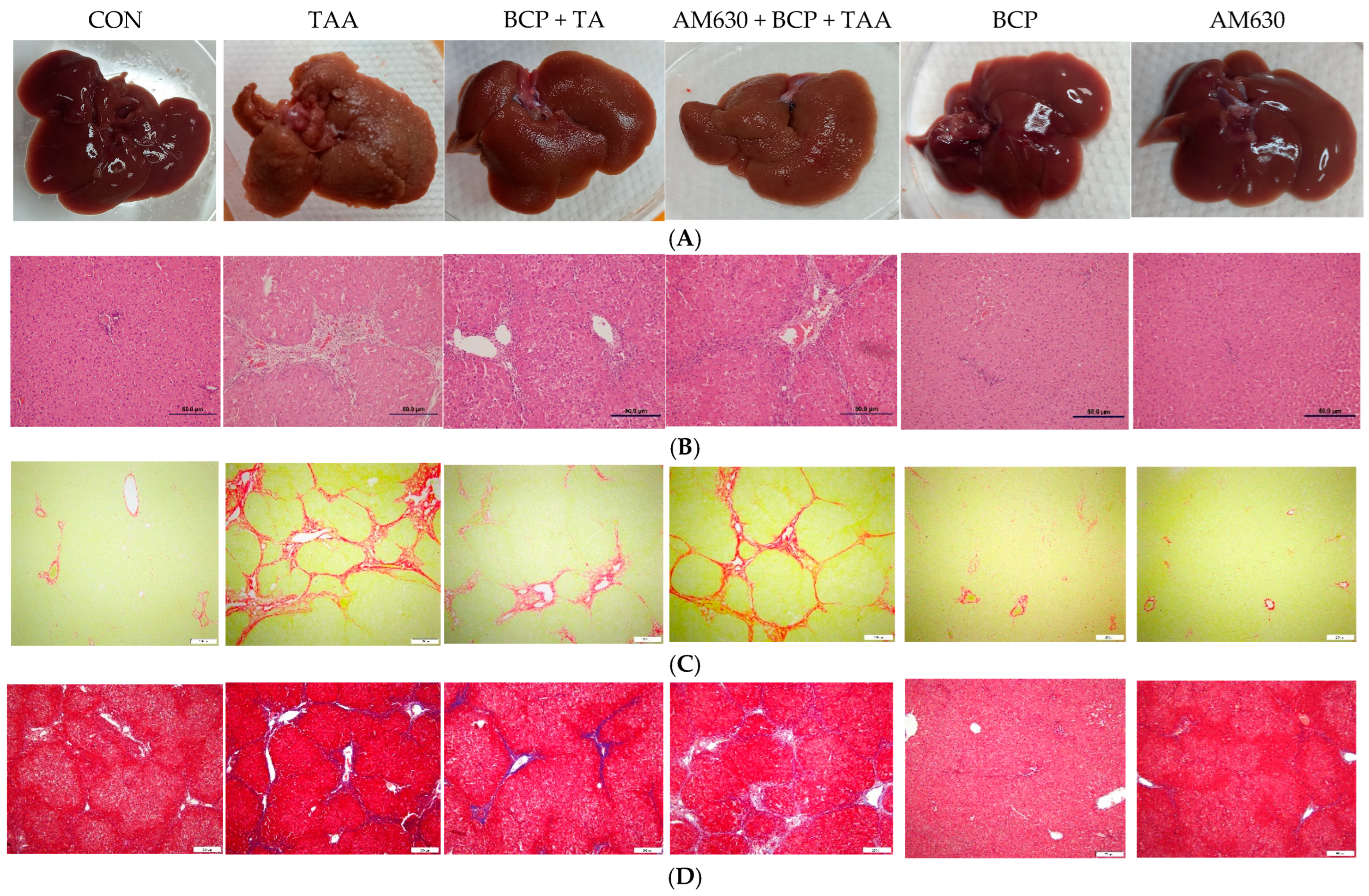
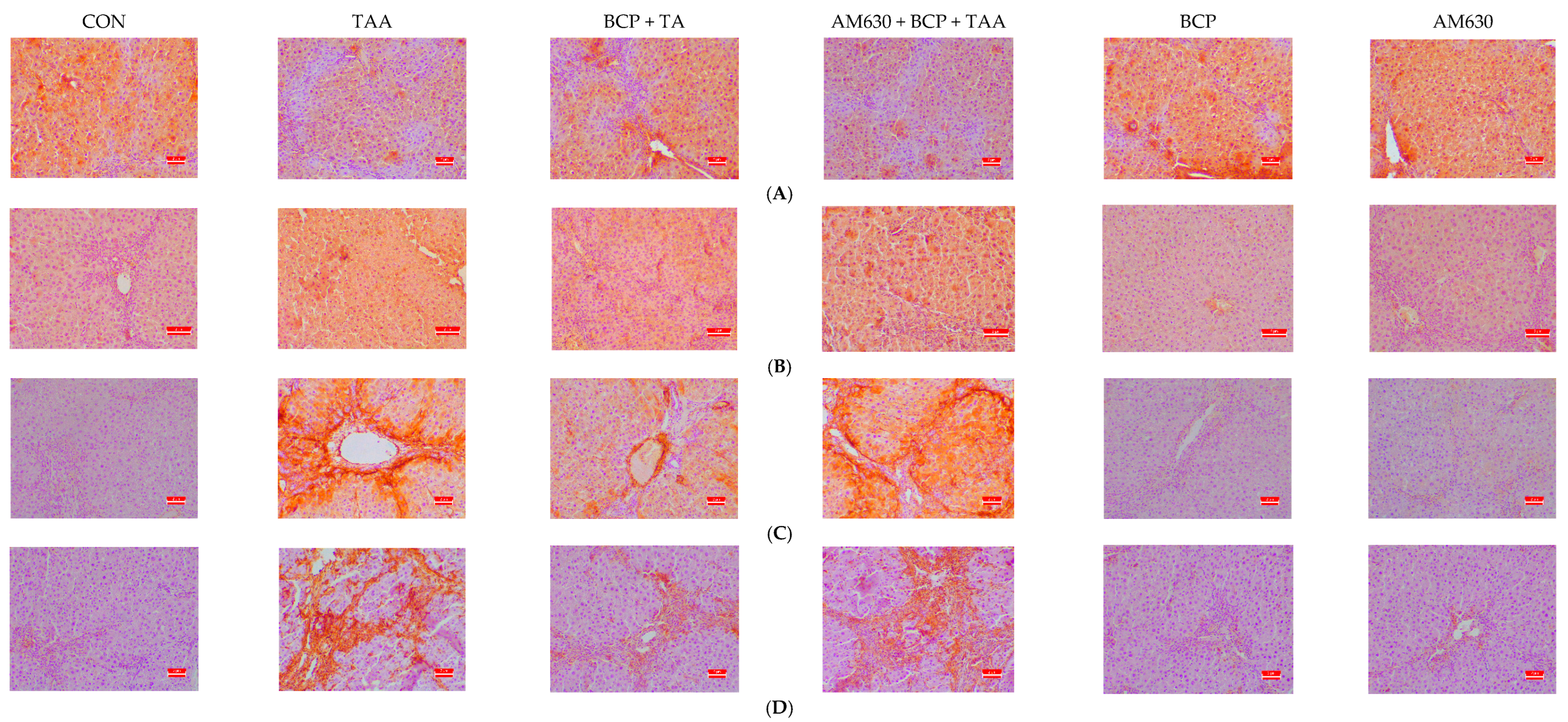
2.5. Histological Examination and Gross Morphology
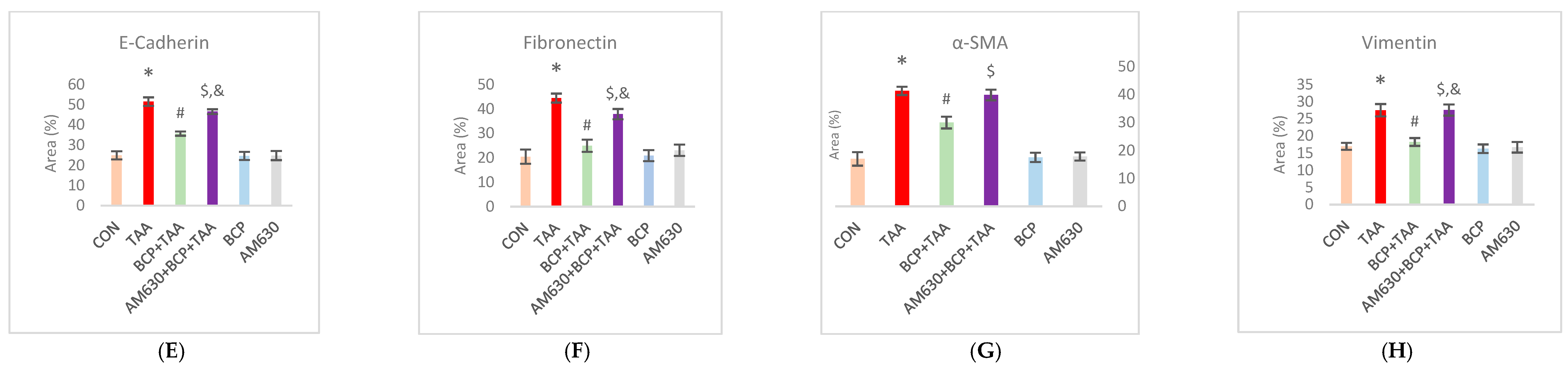
2.6. Immunohistochemical Staining of Liver Sections
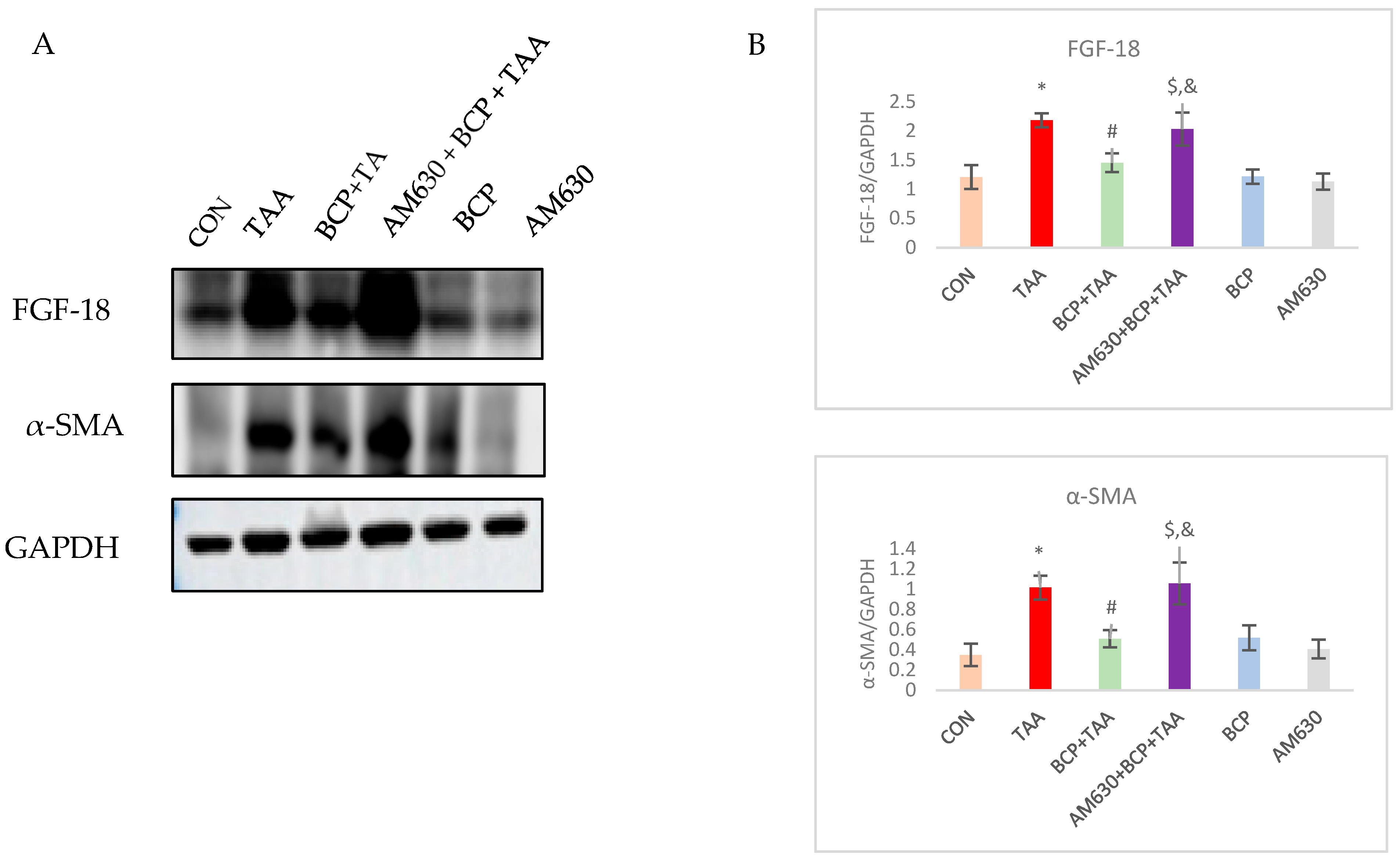
2.7. Effect of BCP on FGF-18 and α-SMA Expression
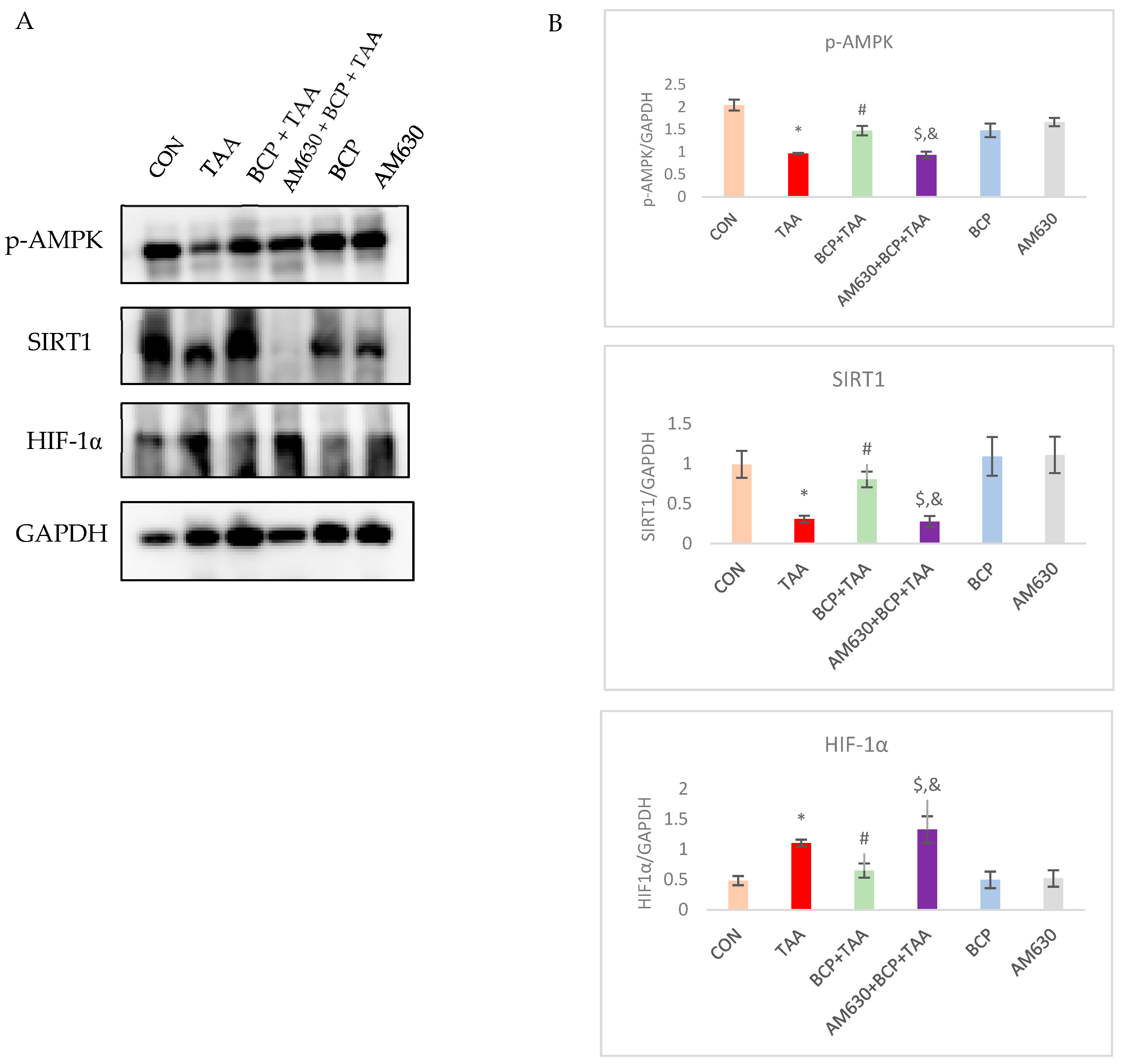
2.8. Effect of BCP on Expression of AMPK-SIRT1-HIF1
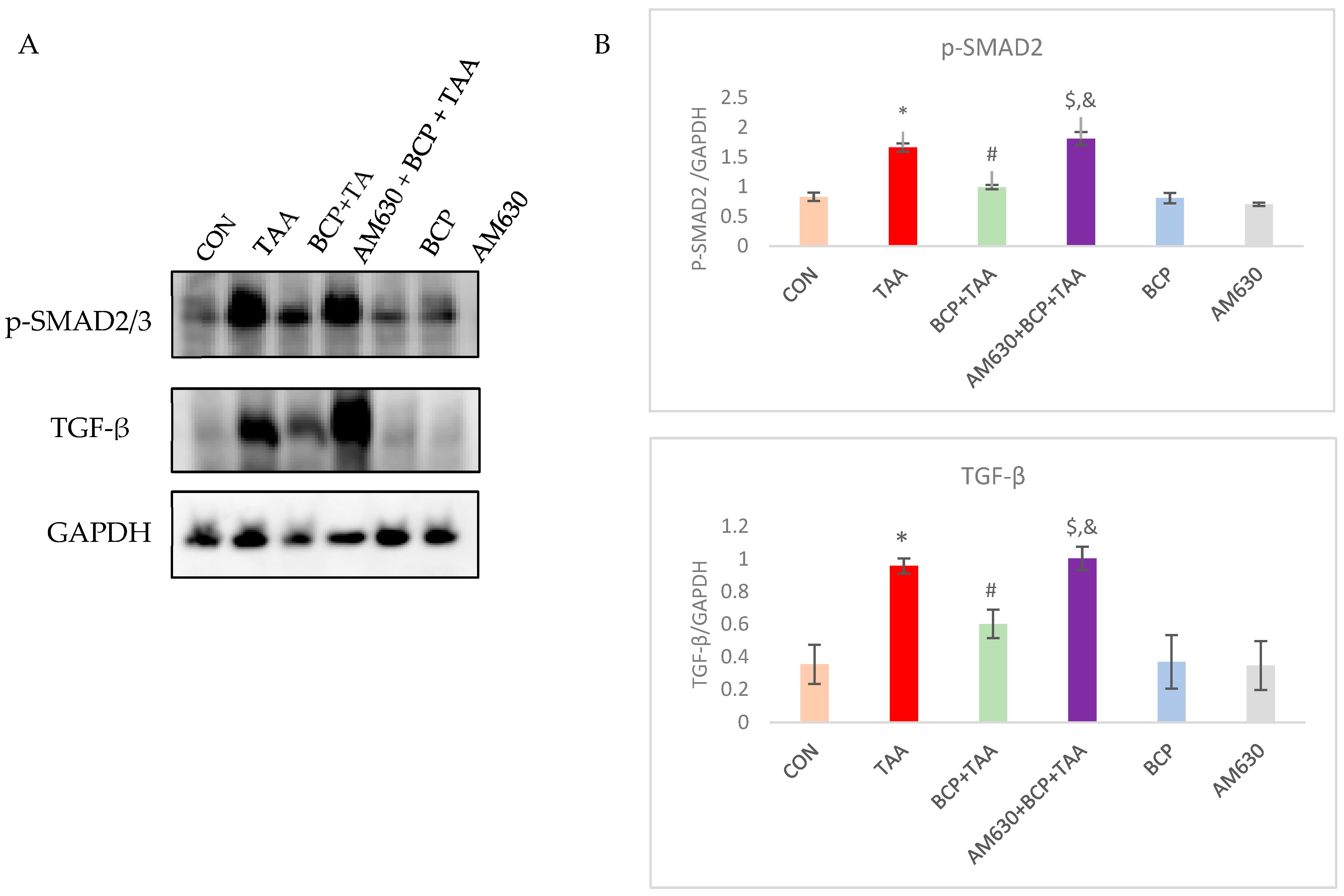
2.9. Effect of BCP on TGF-β Signaling Pathway Activation
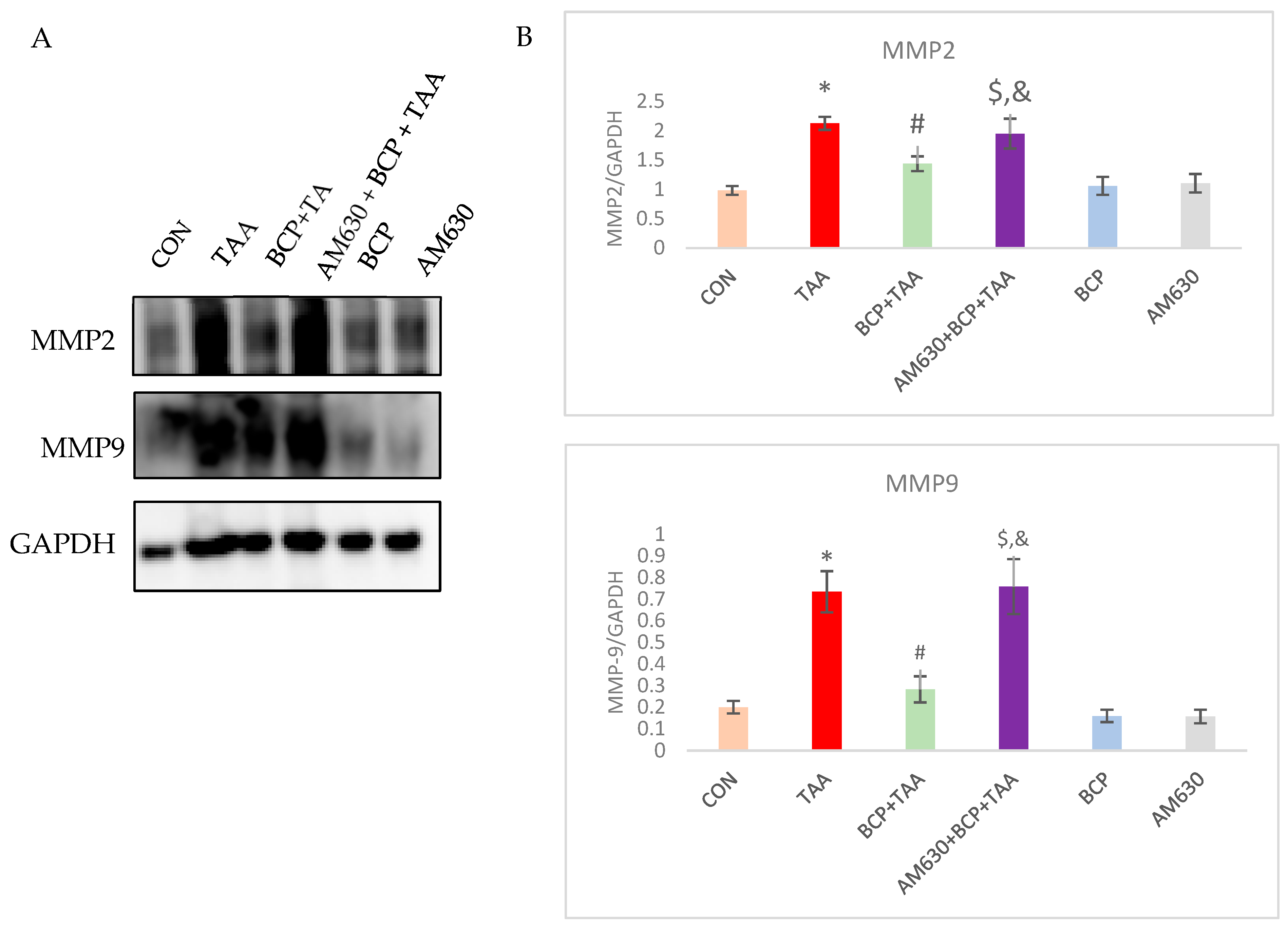
2.10. Effect of BCP on Matrix Metalloprotease Activation
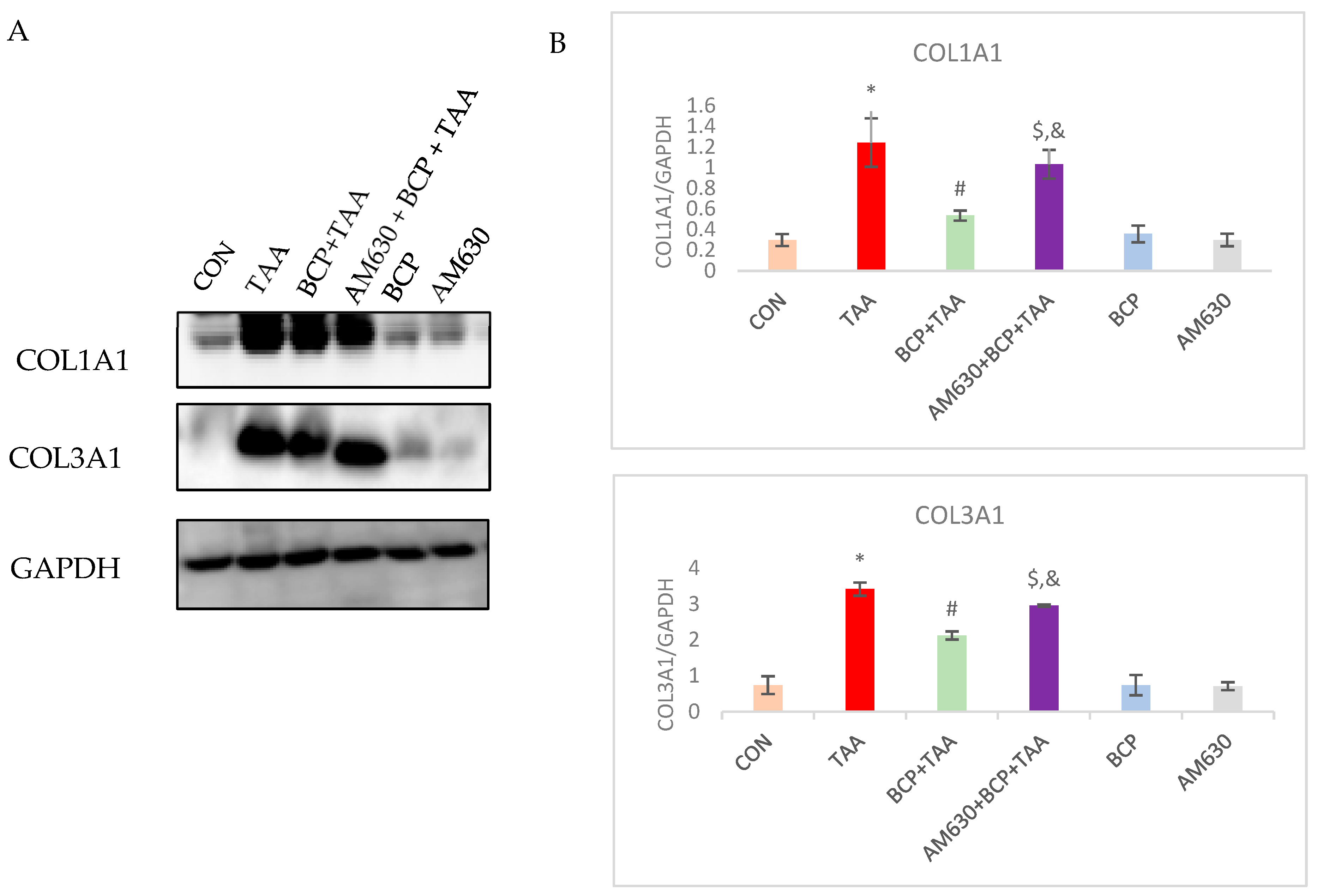
2.11. Effect of BCP on Collagen Expression
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Experimental Animals
4.3. Experimental Protocol
4.4. Tissue Collection
4.5. Tissues Homogenization and Sample Preparation
4.6. Liver Weight Index
4.7. Functional Markers Analysis
4.8. Malondialdehyde (MDA) Assay
4.9. Glutathione (GSH) Assay
4.10. Catalase Assay
4.11. Assessment of Pro- and Anti-Inflammatory Cytokines
4.12. Western Blotting Assay
4.13. Histological Examination
4.14. Immunohistochemical Staining
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine aminotransferase |
| AMPK | AMP-activated protein kinase |
| AST | Aspartate aminotransferase |
| BCP | β-Caryophyllene |
| CB1, CB2 | Cannabinoid receptors 1 and 2 |
| CON | Control |
| ECS | Endocannabinoid system |
| FGF-18 | Fibroblast Growth Factor |
| GSH | Glutathione |
| HIF-1α | Hypoxia inducible factor |
| IL-1β | Interleukin-1β |
| IL-10 | Interleukin-10 |
| IL-6 | Interleukin-6 |
| MDA | Malondialdehyde |
| MMP | Matrix Metalloproteases |
| p-SMAD2 | Phosphorylated-Caenorhabditis elegans SMA (“small” worm phenotype) and MAD family (“Mothers Against Decapentaplegic”) of genes in Drosophila |
| SIRT1 | Sirtuin 1 |
| TAA | Thioacetamide |
| TASO | Thioacetamide-S-oxide |
| TGF-β | Transforming growth factor-beta |
| TNF-α | Tumor Necrosis Factor alpha |
| α-SMA | Alpha Smooth Muscle Actin |
References
- Berumen, J.; Baglieri, J.; Kisseleva, T.; Mekeel, K. Liver fibrosis: Pathophysiology and clinical implications. WIREs Mech. Dis. 2021, 13, e1499. [Google Scholar] [CrossRef]
- Acharya, P.; Chouhan, K.; Weiskirchen, S.; Weiskirchen, R. Cellular Mechanisms of Liver Fibrosis. Front. Pharmacol. 2021, 12, 671640. [Google Scholar] [CrossRef]
- Cheemerla, S.; Balakrishnan, M. Global Epidemiology of Chronic Liver Disease. Clin. Liver Dis. 2021, 17, 365–370. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- Gan, C.; Yuan, Y.; Shen, H.; Gao, J.; Kong, X.; Che, Z.; Guo, Y.; Wang, H.; Dong, E.; Xiao, J. Liver diseases: Epidemiology, causes, trends and predictions. Signal Transduct. Target. Ther. 2025, 10, 33. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Costa, M.A.; Almada, M.; Correia-da-Silva, G.; Teixeira, N.A. Endogenous cannabinoids revisited: A biochemistry perspective. Prostaglandins Other Lipid Mediat. 2013, 102, 13–30. [Google Scholar] [CrossRef]
- Bie, B.; Wu, J.; Foss, J.F.; Naguib, M. An overview of the cannabinoid type 2 receptor system and its therapeutic potential. Curr. Opin. Anaesthesiol. 2018, 31, 407–414. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, S.; Wang, Q.; Hu, W.; Wang, D.; Li, X.; Su, T.; Qin, X.; Zhang, X.; Ma, K.; et al. Effects of cannabinoid receptor type 2 on endogenous myocardial regeneration by activating cardiac progenitor cells in mouse infarcted heart. Sci. China Life Sci. 2014, 57, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Wawryk-Gawda, E.; Chłapek, K.; Zarobkiewicz, M.K.; Lis-Sochocka, M.; Chylińska-Wrzos, P.; Boguszewska-Czubara, A.; Sławiński, M.A.; Franczak, A.; Jodłowska-Jędrych, B.; Biała, G. CB2R agonist prevents nicotine induced lung fibrosis. Exp. Lung Res. 2018, 44, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Ofek, O.; Karsak, M.; Leclerc, N.; Fogel, M.; Frenkel, B.; Wright, K.; Tam, J.; Attar-Namdar, M.; Kram, V.; Shohami, E.; et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc. Natl. Acad. Sci. USA 2006, 103, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Swanson, M.L.; Regner, K.R.; Moore, B.M., 2nd; Park, F. Cannabinoid Type 2 Receptor Activation Reduces the Progression of Kidney Fibrosis Using a Mouse Model of Unilateral Ureteral Obstruction. Cannabis Cannabinoid Res. 2022, 7, 790–803. [Google Scholar] [CrossRef]
- Julien, B.; Grenard, P.; Teixeira-Clerc, F.; Van Nhieu, J.T.; Li, L.; Karsak, M.; Zimmer, A.; Mallat, A.; Lotersztajn, S. Antifibrogenic role of the cannabinoid receptor CB2 in the liver. Gastroenterology 2005, 128, 742–755. [Google Scholar] [CrossRef]
- Walker, O.L.S.; Holloway, A.C.; Raha, S. The role of the endocannabinoid system in female reproductive tissues. J. Ovarian Res. 2019, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Mallat, A.; Teixeira-Clerc, F.; Deveaux, V.; Manin, S.; Lotersztajn, S. The endocannabinoid system as a key mediator during liver diseases: New insights and therapeutic openings. Br. J. Pharmacol. 2011, 163, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Cintosun, A.; Lara-Corrales, I.; Pope, E. Mechanisms of Cannabinoids and Potential Applicability to Skin Diseases. Clin. Drug Investig. 2020, 40, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, E.; Selvi, E.; Balistreri, E.; Lorenzini, S.; Maggio, R.; Natale, M.R.; Capecchi, P.L.; Lazzerini, P.E.; Bardelli, M.; Laghi-Pasini, F.; et al. Cannabinoids inhibit fibrogenesis in diffuse systemic sclerosis fibroblasts. Rheumatology 2009, 48, 1050–1056. [Google Scholar] [CrossRef]
- Defer, N.; Wan, J.; Souktani, R.; Escoubet, B.; Perier, M.; Caramelle, P.; Manin, S.; Deveaux, V.; Bourin, M.C.; Zimmer, A.; et al. The cannabinoid receptor type 2 promotes cardiac myocyte and fibroblast survival and protects against ischemia/reperfusion-induced cardiomyopathy. Faseb J. 2009, 23, 2120–2130. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef]
- Bastaki, M.; Api, A.M.; Aubanel, M.; Bauter, M.; Cachet, T.; Demyttenaere, J.C.R.; Diop, M.M.; Harman, C.L.; Hayashi, S.M.; Krammer, G.; et al. Dietary administration of β-caryophyllene and its epoxide to Sprague-Dawley rats for 90 days. Food Chem. Toxicol. 2020, 135, 110876. [Google Scholar] [CrossRef]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Calleja, M.A.; Vieites, J.M.; Montero-Meléndez, T.; Torres, M.I.; Faus, M.J.; Gil, A.; Suárez, A. The antioxidant effect of β-caryophyllene protects rat liver from carbon tetrachloride-induced fibrosis by inhibiting hepatic stellate cell activation. Br. J. Nutr. 2013, 109, 394–401. [Google Scholar] [CrossRef]
- Kamikubo, R.; Yoshida, H.; Fushimi, T.; Kamei, Y.; Akagawa, M. β-Caryophyllene, a dietary phytocannabinoid, alleviates high-fat diet-induced hepatic steatosis in mice via AMPK activation. Biosci. Biotechnol. Biochem. 2024, 88, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Arizuka, N.; Murakami, T.; Suzuki, K. The effect of β-caryophyllene on nonalcoholic steatohepatitis. J. Toxicol. Pathol. 2017, 30, 263–273. [Google Scholar] [CrossRef]
- Mazzantini, C.; El Bourji, Z.; Parisio, C.; Davolio, P.L.; Cocchi, A.; Pellegrini-Giampietro, D.E.; Landucci, E. Anti-Inflammatory Properties of Cannabidiol and Beta-Caryophyllene Alone or Combined in an In Vitro Inflammation Model. Pharmaceuticals 2024, 17, 467. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.F.; Swefy, S.E.; Hasan, R.A.; Ibrahim, A. Role of cannabinoid receptors in hepatic fibrosis and apoptosis associated with bile duct ligation in rats. Eur. J. Pharmacol. 2014, 742, 118–124. [Google Scholar] [CrossRef]
- Alshanwani, A.R.; Hagar, H.; Shaheen, S.; Alhusaini, A.M.; Arafah, M.M.; Faddah, L.M.; Alharbi, F.M.; Sharma, A.K.; Fayed, A.; Badr, A.M. A promising antifibrotic drug, pyridoxamine attenuates thioacetamide-induced liver fibrosis by combating oxidative stress, advanced glycation end products, and balancing matrix metalloproteinases. Eur. J. Pharmacol. 2022, 923, 174910. [Google Scholar] [CrossRef] [PubMed]
- Shaker, M.E.; Eisa, N.H.; Elgaml, A.; El-Mesery, A.; El-Shafey, M.; El-Dosoky, M.; El-Mowafy, M.; El-Mesery, M. Ingestion of mannose ameliorates thioacetamide-induced intrahepatic oxidative stress, inflammation and fibrosis in rats. Life Sci. 2021, 286, 120040. [Google Scholar] [CrossRef]
- Shin, M.R.; Lee, J.A.; Kim, M.; Lee, S.; Oh, M.; Moon, J.; Nam, J.W.; Choi, H.; Mun, Y.J.; Roh, S.S. Gardeniae Fructus Attenuates Thioacetamide-Induced Liver Fibrosis in Mice via Both AMPK/SIRT1/NF-κB Pathway and Nrf2 Signaling. Antioxidants 2021, 10, 1837. [Google Scholar] [CrossRef]
- Zhou, S.; Gu, J.; Liu, R.; Wei, S.; Wang, Q.; Shen, H.; Dai, Y.; Zhou, H.; Zhang, F.; Lu, L. Spermine Alleviates Acute Liver Injury by Inhibiting Liver-Resident Macrophage Pro-Inflammatory Response Through ATG5-Dependent Autophagy. Front. Immunol. 2018, 9, 948. [Google Scholar] [CrossRef]
- Parola, M.; Pinzani, M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol. Asp. Med. 2019, 65, 37–55. [Google Scholar] [CrossRef]
- Abdel-Rahman, R.F.; Fayed, H.M.; Asaad, G.F.; Ogaly, H.A.; Hessin, A.F.; Salama, A.A.A.; Abd El-Rahman, S.S.; Arbid, M.S.; Mohamed, M.A.E. The involvement of TGF-β1 /FAK/α-SMA pathway in the antifibrotic impact of rice bran oil on thioacetamide-induced liver fibrosis in rats. PLoS ONE 2021, 16, e0260130. [Google Scholar] [CrossRef]
- Tsai, M.-Y.; Yang, W.-C.; Lin, C.-F.; Wang, C.-M.; Liu, H.-Y.; Lin, C.-S.; Lin, J.-W.; Lin, W.-L.; Lin, T.-C.; Fan, P.-S.; et al. The Ameliorative Effects of Fucoidan in Thioacetaide-Induced Liver Injury in Mice. Molecules 2021, 26, 1937. [Google Scholar] [CrossRef]
- Santacroce, G.; Gentile, A.; Soriano, S.; Novelli, A.; Lenti, M.V.; Di Sabatino, A. Glutathione: Pharmacological aspects and implications for clinical use in non-alcoholic fatty liver disease. Front. Med. 2023, 10, 1124275. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Chen, Y.; Yang, M. β-Caryophyllene inhibits high glucose-induced oxidative stress, inflammation and extracellular matrix accumulation in mesangial cells. Int. Immunopharmacol. 2020, 84, 106556. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef]
- Scandiffio, R.; Geddo, F.; Cottone, E.; Querio, G.; Antoniotti, S.; Gallo, M.P.; Maffei, M.E.; Bovolin, P. Protective Effects of (E)-β-Caryophyllene (BCP) in Chronic Inflammation. Nutrients 2020, 12, 3273. [Google Scholar] [CrossRef]
- Carpino, G.; Morini, S.; Ginanni Corradini, S.; Franchitto, A.; Merli, M.; Siciliano, M.; Gentili, F.; Onetti Muda, A.; Berloco, P.; Rossi, M.; et al. Alpha-SMA expression in hepatic stellate cells and quantitative analysis of hepatic fibrosis in cirrhosis and in recurrent chronic hepatitis after liver transplantation. Dig. Liver Dis. 2005, 37, 349–356. [Google Scholar] [CrossRef]
- Cardoso-Lezama, I.; Ramos-Tovar, E.; Arellanes-Robledo, J.; Vargas-Pozada, E.E.; Vásquez-Garzón, V.R.; Villa-Treviño, S.; Muriel, P. Serum α-SMA is a potential noninvasive biomarker of liver fibrosis. Toxicol. Mech. Methods 2024, 34, 13–19. [Google Scholar] [CrossRef]
- Tennakoon, A.H.; Izawa, T.; Wijesundera, K.K.; Golbar, H.M.; Tanaka, M.; Ichikawa, C.; Kuwamura, M.; Yamate, J. Characterization of glial fibrillary acidic protein (GFAP)-expressing hepatic stellate cells and myofibroblasts in thioacetamide (TAA)-induced rat liver injury. Exp. Toxicol. Pathol. 2013, 65, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Foo, N.-P.; Lin, S.-H.; Lee, Y.-H.; Wu, M.-J.; Wang, Y.-J. α-Lipoic acid inhibits liver fibrosis through the attenuation of ROS-triggered signaling in hepatic stellate cells activated by PDGF and TGF-β. Toxicology 2011, 282, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Selim, N.M.; Melk, M.M.; Melek, F.R.; Saleh, D.O.; Sobeh, M.; El-Hawary, S.S. Phytochemical profiling and anti-fibrotic activities of Plumbago indica L. and Plumbago auriculata Lam. in thioacetamide-induced liver fibrosis in rats. Sci. Rep. 2022, 12, 9864. [Google Scholar] [CrossRef]
- Ibrahim, M.Y.; Alamri, Z.Z.; Juma, A.S.M.; Hamood, S.A.; Shareef, S.H.; Abdulla, M.A.; Jayash, S.N. Hepatoprotective Effects of Biochanin A on Thioacetamide-Induced Liver Cirrhosis in Experimental Rats. Molecules 2023, 28, 7608. [Google Scholar] [CrossRef] [PubMed]
- Roeb, E. Matrix metalloproteinases and liver fibrosis (translational aspects). Matrix Biol. 2018, 68, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; El-Tawil, O.S.; Al-Mokaddem, A.K.; Abd El-Rahman, S.S. Promoted inhibition of TLR4/miR-155/ NF(k)B p65 signaling by cannabinoid receptor 2 agonist (AM1241), aborts inflammation and progress of hepatic fibrosis induced by thioacetamide. Chem. Biol. Interact. 2021, 336, 109398. [Google Scholar] [CrossRef]
- Seitz, T.; Hellerbrand, C. Role of fibroblast growth factor signalling in hepatic fibrosis. Liver Int. 2021, 41, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Qiu, W.R.; Wang, Y.P.; Hill, D.; Ring, B.D.; Scully, S.; Bolon, B.; DeRose, M.; Luethy, R.; Simonet, W.S.; et al. FGF-18, a novel member of the fibroblast growth factor family, stimulates hepatic and intestinal proliferation. Mol. Cell Biol. 1998, 18, 6063–6074. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Seki, T.; Kobayashi, K.; Komazawa-Sakon, S.; Shichino, S.; Nishina, T.; Fukuhara, K.; Ikejima, K.; Nagai, H.; Igarashi, Y.; et al. Fibroblast growth factor 18 stimulates the proliferation of hepatic stellate cells, thereby inducing liver fibrosis. Nat. Commun. 2023, 14, 6304. [Google Scholar] [CrossRef]
- Xu, F.; Liu, C.; Zhou, D.; Zhang, L. TGF-β/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J. Histochem. Cytochem. 2016, 64, 157–167. [Google Scholar] [CrossRef]
- Tian, C.; Huang, R.; Xiang, M. SIRT1: Harnessing multiple pathways to hinder NAFLD. Pharmacol. Res. 2024, 203, 107155. [Google Scholar] [CrossRef]
- Yang, N.; Sun, R.; Zhang, X.; Wang, J.; Wang, L.; Zhu, H.; Yuan, M.; Xu, Y.; Ge, C.; He, J.; et al. Alternative pathway of bile acid biosynthesis contributes to ameliorate NASH after induction of NAMPT/NAD+/SIRT1 axis. Biomed. Pharmacother. 2023, 164, 114987. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, C.; Hu, K.; Greenberg, A.; Wu, D.; Ausman, L.M.; McBurney, M.W.; Wang, X.-D. Ablation of systemic SIRT1 activity promotes nonalcoholic fatty liver disease by affecting liver-mesenteric adipose tissue fatty acid mobilization. Biochim. E Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 2783–2790. [Google Scholar] [CrossRef]
- ElBaset, M.A.; Salem, R.S.; Ayman, F.; Ayman, N.; Shaban, N.; Afifi, S.M.; Esatbeyoglu, T.; Abdelaziz, M.; Elalfy, Z.S. Effect of Empagliflozin on Thioacetamide-Induced Liver Injury in Rats: Role of AMPK/SIRT-1/HIF-1α Pathway in Halting Liver Fibrosis. Antioxidants 2022, 11, 2152. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between Oxidative Stress and SIRT1: Impact on the Aging Process. Int. J. Mol. Sci. 2013, 14, 3834–3859. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.R.; Lee, S.H.; Roh, S.S. The Potential Hepatoprotective Effect of Paeoniae Radix Alba in Thioacetamide-Induced Acute Liver Injury in Rats. Evid. Based Complement. Altern. Med. 2022, 2022, 7904845. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Sun, T.; Wang, X. Activation of type 2 cannabinoid receptors (CB2R) promotes fatty acid oxidation through the SIRT1/PGC-1α pathway. Biochem. Biophys. Res. Commun. 2013, 436, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Al-Hashem, F.; Al-Humayed, S.; Amin, S.N.; Kamar, S.S.; Mansy, S.S.; Hassan, S.; Abdel-Salam, L.O.; Ellatif, M.A.; Alfaifi, M.; Haidara, M.A.; et al. Metformin inhibits mTOR–HIF-1α axis and profibrogenic and inflammatory biomarkers in thioacetamide-induced hepatic tissue alterations. J. Cell. Physiol. 2019, 234, 9328–9337. [Google Scholar] [CrossRef]
- Mooli, R.G.; Mukhi, D.; Watt, M.; Nagati, V.; Reed, S.M.; Gandhi, N.K.; Oertel, M.; Ramakrishnan, S.K. Hypoxia-Inducible Factor-2α Promotes Liver Fibrosis by Inducing Hepatocellular Death. Int. J. Mol. Sci. 2024, 25, 13114. [Google Scholar] [CrossRef]
- Roth, K.J.; Copple, B.L. Role of Hypoxia-Inducible Factors in the Development of Liver Fibrosis. Cell Mol. Gastroenterol. Hepatol. 2015, 1, 589–597. [Google Scholar] [CrossRef]
- Li, S.; Qian, Q.; Ying, N.; Lai, J.; Feng, L.; Zheng, S.; Jiang, F.; Song, Q.; Chai, H.; Dou, X. Activation of the AMPK-SIRT1 pathway contributes to protective effects of Salvianolic acid A against lipotoxicity in hepatocytes and NAFLD in mice. Front. Pharmacol. 2020, 11, 560905. [Google Scholar] [CrossRef]
- Yang, J.D.; Abdelmalek, M.F.; Pang, H.; Guy, C.D.; Smith, A.D.; Diehl, A.M.; Suzuki, A. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology 2014, 59, 1406–1414. [Google Scholar] [CrossRef]
- Della Torre, S. Beyond the X Factor: Relevance of Sex Hormones in NAFLD Pathophysiology. Cells 2021, 10, 2502. [Google Scholar] [CrossRef]
- Cooper, K.M.; Delk, M.; Devuni, D.; Sarkar, M. Sex differences in chronic liver disease and benign liver lesions. JHEP Rep. 2023, 5, 100870. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, M.; Patel, P.; Dunn-Valadez, S.; Dao, C.; Khan, V.; Ali, H.; El-Serag, L.; Hernaez, R.; Sisson, A.; Thrift, A.P.; et al. Women Have a Lower Risk of Nonalcoholic Fatty Liver Disease but a Higher Risk of Progression vs Men: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 19, 61–71.e15. [Google Scholar] [CrossRef] [PubMed]
- Garate-Carrillo, A.; Gonzalez, J.; Ceballos, G.; Ramirez-Sanchez, I.; Villarreal, F. Sex related differences in the pathogenesis of organ fibrosis. Transl. Res. 2020, 222, 41–55. [Google Scholar] [CrossRef]
- Li, L.; Hu, Z.; Li, W.; Hu, M.; Ran, J.; Chen, P.; Sun, Q. Establishment of a standardized liver fibrosis model with different pathological stages in rats. Gastroenterol. Res. Pract. 2012, 2012, 560345. [Google Scholar] [CrossRef]
- Yang, X.M.; Wu, Z.; Wang, X.; Zhou, Y.; Zhu, L.; Li, D.; Nie, H.Z.; Wang, Y.H.; Li, J.; Ma, X. Disulfiram inhibits liver fibrosis in rats by suppressing hepatic stellate cell activation and viability. BMC Pharmacol. Toxicol. 2022, 23, 54. [Google Scholar] [CrossRef]
- Xu, J.W.; Gong, J.; Chang, X.M.; Luo, J.Y.; Dong, L.; Hao, Z.M.; Jia, A.; Xu, G.P. Estrogen reduces CCL4-induced liver fibrosis in rats. World J. Gastroenterol. 2002, 8, 883–887. [Google Scholar] [CrossRef]
- Eisa, M.A.; Mansour, A.M.; Salama, S.A.; Elsadek, B.E.M.; Ashour, A.A.; Abdelghany, T.M. Estrogen/estrogen receptor activation protects against DEN-induced liver fibrosis in female rats via modulating TLR-4/NF-kβ signaling. Eur. J. Pharmacol. 2023, 960, 176165. [Google Scholar] [CrossRef]
- Lee, Y.H.; Son, J.Y.; Kim, K.S.; Park, Y.J.; Kim, H.R.; Park, J.H.; Kim, K.-B.; Lee, K.Y.; Kang, K.W.; Kim, I.S.; et al. Estrogen Deficiency Potentiates Thioacetamide-Induced Hepatic Fibrosis in Sprague-Dawley Rats. Int. J. Mol. Sci. 2019, 20, 3709. [Google Scholar] [CrossRef] [PubMed]

| Antibody | Dilution Ratio | Company and Catalog Number |
|---|---|---|
| COL3A1 | 1:1000 | Santa Cruz Biotechnology (Dallas, TA, USA) (sc-271249) |
| COL1A1 | 1:1000 | Santa Cruz Biotechnology (sc-293182) |
| SIRT1 | 1:500 | Santa Cruz Biotechnology (sc-74465) |
| FGF-18 | 1:1000 | Santa Cruz Biotechnology (sc-393471) |
| HIF-1α | 1:500 | Santa Cruz Biotechnology (sc-13515) |
| p-AMPK | 1:500 | Cell Signaling Technology (Danvers, MA, USA) (2535s) |
| p-SMAD | 1:500 | Cell Signaling Technology (8828s) |
| TGF-β | 1:1000 | Cell Signaling Technology (3711s) |
| α-SMA | 1:1000 | Cell Signaling Technology (14968s) |
| MMP-2 | 1:500 | Abcam (Waltham, MA, USA) (ab97779) |
| MMP-9 | 1:500 | Abcam (ab658803) |
| GAPDH | 1:1000 | Santa Cruz Biotechnology (sc-365062) |
| Antibody | Dilution Ratio | Company and Catalog Number |
|---|---|---|
| Fibronectin | 1:50 | Santa Cruz Biotechnology (sc-8422) |
| E-cadherin | 1:50 | Santa Cruz Biotechnology (sc-52751) |
| α-SMA | 1:50 | Cell Signaling Technology (14968s) |
| Vimentin | 1:50 | Cell Signaling Technology (5741s) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bader Eddin, L.; Mahgoub, A.; Almarzooqi, S.; Adeghate, E.; Subramanya, S.B.; Ojha, S. β-Caryophyllene Ameliorates Thioacetamide-Induced Liver Fibrosis in Rats: A Preventative Approach. Int. J. Mol. Sci. 2025, 26, 8493. https://doi.org/10.3390/ijms26178493
Bader Eddin L, Mahgoub A, Almarzooqi S, Adeghate E, Subramanya SB, Ojha S. β-Caryophyllene Ameliorates Thioacetamide-Induced Liver Fibrosis in Rats: A Preventative Approach. International Journal of Molecular Sciences. 2025; 26(17):8493. https://doi.org/10.3390/ijms26178493
Chicago/Turabian StyleBader Eddin, Lujain, Amar Mahgoub, Saeeda Almarzooqi, Ernest Adeghate, Sandeep B. Subramanya, and Shreesh Ojha. 2025. "β-Caryophyllene Ameliorates Thioacetamide-Induced Liver Fibrosis in Rats: A Preventative Approach" International Journal of Molecular Sciences 26, no. 17: 8493. https://doi.org/10.3390/ijms26178493
APA StyleBader Eddin, L., Mahgoub, A., Almarzooqi, S., Adeghate, E., Subramanya, S. B., & Ojha, S. (2025). β-Caryophyllene Ameliorates Thioacetamide-Induced Liver Fibrosis in Rats: A Preventative Approach. International Journal of Molecular Sciences, 26(17), 8493. https://doi.org/10.3390/ijms26178493








