The Human Amniotic Membrane: A Rediscovered Tool to Improve Wound Healing in Oral Surgery
Abstract
1. Introduction
2. Human Amniotic Membrane’s Structural Complexity
3. The hAM in Tissue Repair
Stem Cell Characteristics of Amnion-Derived Cells
4. hAM’s Multifaceted Properties
4.1. Biomechanical Properties
4.2. Epithelialization
4.3. Inhibition of Inflammation
4.4. Angiogenesis
4.5. Inhibition of Scarring
4.6. Lack of Immunogenicity
4.7. Antimicrobial/Antiviral Properties
4.8. Aesthetic Properties
4.9. Reducing Pain at the Site of Application
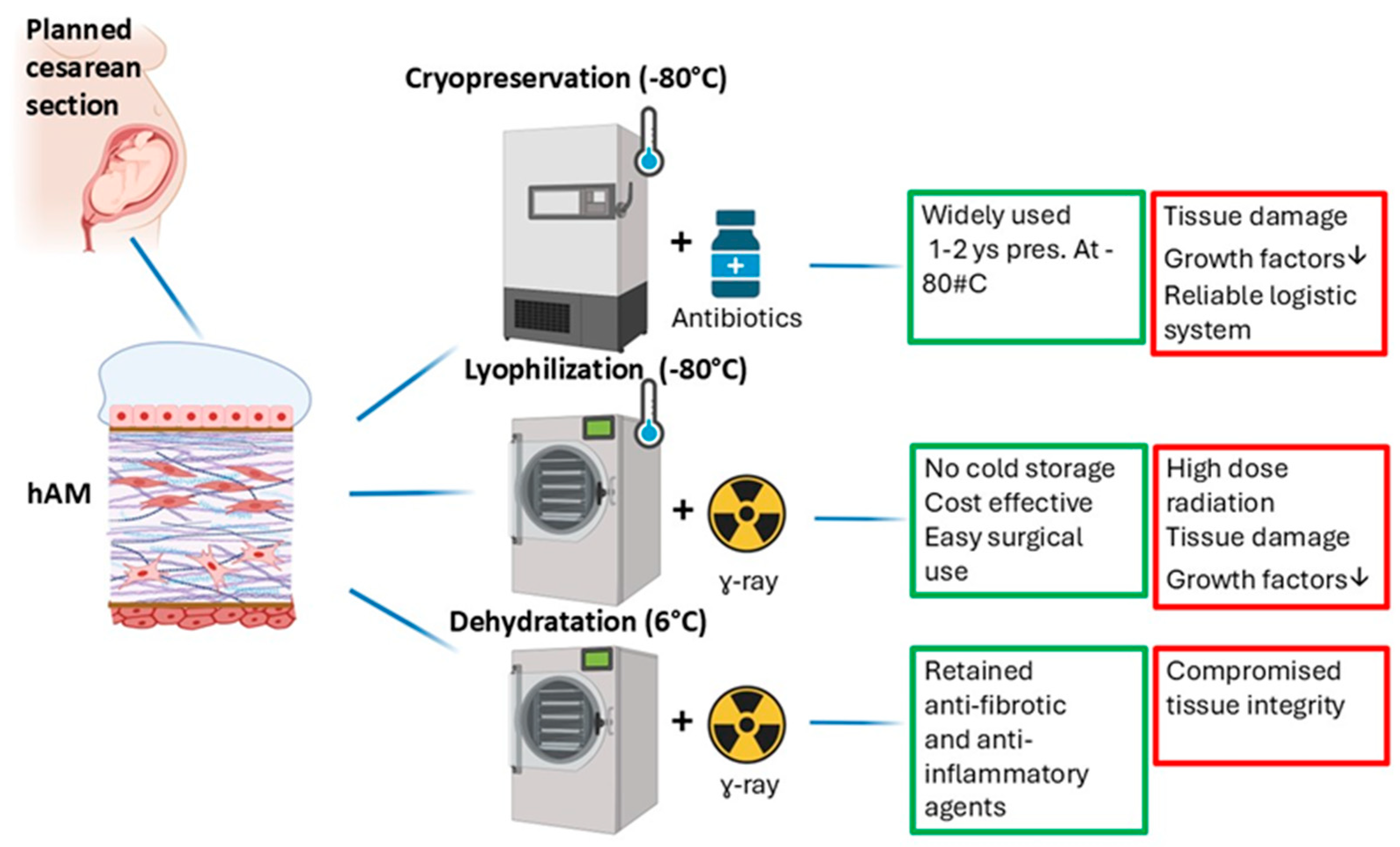
5. Preparation and Utilization of hAM
5.1. Cryopreservation
5.2. Lyophilization
5.3. Dehydration
6. The hAM in Oral Surgery
7. Conclusions
Unresolved Questions and Future Research Agenda
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mohan, R.; Bajaj, A.; Gundappa, M. Human Amnion Membrane: Potential Applications in Oral and Periodontal Field. J. Int. Soc. Prev. Community Dent. 2017, 7, 15–21. [Google Scholar] [CrossRef]
- Okuyama, K.; Yanamoto, S. Saliva in Balancing Oral and Systemic Health, Oral Cancer, and Beyond: A Narrative Review. Cancers 2024, 16, 4276. [Google Scholar] [CrossRef]
- Waasdorp, M.; Krom, B.P.; Bikker, F.J.; van Zuijlen, P.P.; Niessen, F.B.; Gibbs, S. The Bigger Picture: Why Oral Mucosa Heals Better Than Skin. Biomolecules 2021, 11, 1165. [Google Scholar] [CrossRef]
- Shang, L.; Deng, D.; Buskermolen, J.K.; Janus, M.M.; Krom, B.P.; Roffel, S.; Waaijman, T.; van Loveren, C.; Crielaard, W.; Gibbs, S. Multi-species oral biofilm promotes reconstructed human gingiva epithelial barrier function. Sci. Rep. 2018, 8, 16061. [Google Scholar] [CrossRef] [PubMed]
- Laheij, A.M.; de Soet, J.J.; Veerman, E.C.; Bolscher, J.G.; van Loveren, C. The influence of oral bacteria on epithelial cell migration in vitro. Mediat. Inflamm. 2013, 2013, 154532. [Google Scholar] [CrossRef]
- Karin, M.; Clevers, H. Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef]
- Gibbs, S.; Ponec, M. Intrinsic regulation of differentiation markers in human epidermis, hard palate and buccal mucosa. Arch. Oral Biol. 2000, 45, 149–158. [Google Scholar] [CrossRef]
- Qin, R.; Steel, A.; Fazel, N. Oral mucosa biology and salivary biomarkers. Clin. Dermatol. 2017, 35, 477–483. [Google Scholar] [CrossRef]
- Turabelidze, A.; Guo, S.; Chung, A.Y.; Chen, L.; Dai, Y.; Marucha, P.T.; DiPietro, L.A. Intrinsic differences between oral and skin keratinocytes. PLoS ONE 2014, 9, e101480. [Google Scholar] [CrossRef]
- Semlali, A.; Chakir, J.; Goulet, J.P.; Chmielewski, W.; Rouabhia, M. Whole cigarette smoke promotes human gingival epithelial cell apoptosis and inhibits cell repair processes. J. Periodontal Res. 2011, 46, 533–541. [Google Scholar] [CrossRef]
- Shah, R.; Domah, F.; Shah, N.; Domah, J. Surgical Wound Healing in the Oral Cavity: A Review. Dent. Update 2020, 47, 135–143. [Google Scholar] [CrossRef]
- Politis, C.; Schoenaers, J.; Jacobs, R.; Agbaje, J.O. Wound Healing Problems in the Mouth. Front. Physiol. 2016, 7, 507. [Google Scholar] [CrossRef]
- Toma, A.I.; Fuller, J.M.; Willett, N.J.; Goudy, S.L. Oral wound healing models and emerging regenerative therapies. Transl. Res. 2021, 236, 17–34. [Google Scholar] [CrossRef]
- Gulameabasse, S.; Gindraux, F.; Catros, S.; Fricain, J.C.; Fenelon, M. Chorion and amnion/chorion membranes in oral and periodontal surgery: A systematic review. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1216–1229. [Google Scholar] [CrossRef]
- Wolf, H.F.; Rateitschak, E.M.; Rateitschak, K.H. Parodontologie, 3rd ed.; Elsevier Masson: Paris, France, 2005. [Google Scholar]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.M.; Kowolik, M.J.; Janowski, G.M. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.A.; Amudha, D. Guided tissue regeneration: A review. J. Dent. Health Oral Disord. Ther. 2017, 6, 67–73. [Google Scholar] [CrossRef]
- Malek, A.; Bersinger, N.A. Human placental stem cells: Biomedical potential and clinical relevance. J. Stem. Cells. 2011, 6, 75–92. [Google Scholar] [PubMed]
- Caruso, M.; Evangelista, M.; Parolini, O. Human term placental cells: Phenotype, properties and new avenues in regenerative medicine. Int. J. Mol. Cell. Med. 2012, 1, 64–74. [Google Scholar]
- Gupta, I.; Gupta, R.; Gokhale, S.T.; Sharma, A. Placental tissues: Fixing smiles. Int. J. Innov. Sci. Res. 2014, 7, 57–62. [Google Scholar]
- Bryant-Greenwood, G.D. The extracellular matrix of the human fetal membranes: Structure and function. Placenta 1998, 19, 1–11. [Google Scholar] [CrossRef]
- Kesting, M.R.; Wolff, K.D.; Nobis, C.P.; Rohleder, N.H. Amniotic membrane in oral and maxillofacial surgery. Oral Maxillofac. Surg. 2014, 18, 153–164. [Google Scholar] [CrossRef]
- Sacco, R.; Akintola, O.; Sacco, N.; Acocella, A.; Calasans-Maia, M.D.; Maranzano, M.; Olate, S. The Use of Human Amniotic Membrane (hAM) as a Treatment Strategy of Medication-Related Osteonecrosis of the Jaw (MRONJ): A Systematic Review and Meta-Analysis of the Literature. Medicina 2023, 59, 968. [Google Scholar] [CrossRef] [PubMed]
- Sadler, T.W. Langman’s Medical Embryology; Slock: London, UK, 2000. [Google Scholar]
- Baradaran-Rafii, A.; Arjmand, B.; Javadi, M. Amniotic membrane transplantation. Iran J. Ophthal. Res. 2007, 2, 58–75. [Google Scholar]
- Mamede, A.C.; Carvalho, M.J.; Abrantes, A.M.; Laranjo, M.; Maia, C.J.; Botelho, M.F. Amniotic membrane: From structure and functions to clinical applications. Cell Tissue Res. 2012, 349, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Benirschke, K. Pathology of the Human Placenta; Springer: New York, NY, USA, 2000. [Google Scholar]
- Toda, A.; Okabe, M.; Yoshida, T.; Nikaido, T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J. Pharmacol. Sci. 2007, 105, 215–228. [Google Scholar] [CrossRef]
- Dawiec, G.; Niemczyk, W.; Wiench, R.; Niemczyk, S.; Skaba, D. Introduction to Amniotic Membranes in Maxillofacial Surgery—A Scoping Review. Medicina 2024, 60, 663. [Google Scholar] [CrossRef]
- Nouri, M.; Ebrahimi, M.; Bagheri, T.; Fatemi, M.J.; Najafbeygi, A.; Araghi, S.; Molaee, M. Healing effects of dried and acellular human amniotic membrane and mepitelas for coverage of skin graft donor areas; A randomized clinical trial. Bull. Emerg. Trauma 2018, 6, 195–200. [Google Scholar] [CrossRef]
- Wolf, H.; Schmidt, W.; Drenckhahn, D. Immunocytochemical analysis of the cytoskeleton of the human amniotic epithelium. Cell Tissue Res. 1991, 266, 385–389. [Google Scholar] [CrossRef]
- Takashima, S.; Yasuo, M.; Sanzen, N.; Sekiguchi, K.; Okabe, M.; Yoshida, T.; Toda, A.; Nikaido, T. Characterization of laminin isoforms in human amnion. Tissue Cell 2008, 40, 75–81. [Google Scholar] [CrossRef]
- Hu, Z.; Luo, Y.; Ni, R.; Hu, Y.; Yang, F.; Du, T.; Zhu, Y. Biological importance of human amniotic membrane in tissue engineering and regenerative medicine. Mater. Today Bio 2023, 22, 100790. [Google Scholar] [CrossRef]
- Tseng, S.C. Evolution of amniotic membrane transplantation. Clin. Exp. Ophthalmol. 2007, 35, 109–110. [Google Scholar] [CrossRef]
- Manuelpillai, U.; Moodley, Y.; Borlongan, C.V.; Parolini, O. Amniotic membrane and amniotic cells: Potential therapeutic tools to combat tissue inflammation and fibrosis? Placenta 2011, 32 (Suppl. 4), S320–S325. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.A.; Johari, H.G.; Eskandari, S.; Rajabnejad, A. The application of amniotic membrane in ophthalmic surgery. J. Ophthalmic Vis. Res. 2008, 3, 84–93. [Google Scholar]
- Niknejad, H.; Peirovi, H.; Jorjani, M.; Ahmadiani, A.; Ghanavi, J.; Seifalian, A.M. Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cells Mater. 2008, 15, 88–99. [Google Scholar] [CrossRef]
- Mamun, A.A.; Shao, C.; Geng, P.; Wang, S.; Xiao, J. Recent advances in molecular mechanisms of skin wound healing and its treatments. Front. Immunol. 2024, 15, 1395479. [Google Scholar] [CrossRef] [PubMed]
- Duan-Arnold, Y.; Gyurdieva, A.; Johnson, A.; Uveges, T.E.; Jacobstein, D.A.; Danilkovitch, A. Retention of endogenous viable cells enhances the anti-inflammatory activity of cryopreserved amnion. Adv. Wound Care 2015, 4, 523–533. [Google Scholar] [CrossRef]
- Elkhenany, H.; El-Derby, A.; Abd Elkodous, M.; Salah, R.A.; Lotfy, A.; El-Badri, N. Applications of the amniotic membrane in tissue engineering and regeneration: The hundred-year challenge. Stem Cell Res. Ther. 2022, 13, 8. [Google Scholar] [CrossRef]
- Cai, J.; Li, W.; Su, H.; Qin, D.; Yang, J.; Zhu, F.; Xu, J.; He, W.; Guo, X.; Labuda, K.; et al. Generation of human induced pluripotent stem cells from umbilical cord matrix and amniotic membrane mesenchymal cells. J. Biol. Chem. 2010, 285, 11227–11234. [Google Scholar] [CrossRef]
- Evangelista, M.; Soncini, M.; Parolini, O. Placenta-derived stem cells: New hope for cell therapy? Cytotechnology 2008, 58, 33–42. [Google Scholar] [CrossRef]
- Parolini, O.; Alviano, F.; Bagnara, G.P.; Bilic, G.; Bühring, H.J.; Evangelista, M.; Hennerbichler, S.; Liu, B.; Magatti, M.; Mao, N.; et al. Concise review: Isolation and characterization of cells from human term placenta: Outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 2008, 26, 300–311. [Google Scholar] [CrossRef]
- Sakuragawa, N.; Kakinuma, K.; Kikuchi, A.; Okano, H.; Uchida, S.; Kamo, I.; Kobayashi, M.; Yokoyama, Y. Human amnion mesenchyme cells express phenotypes of neuroglial progenitor cells. J. Neurosci. Res. 2004, 78, 208–214. [Google Scholar] [CrossRef]
- Wassmer, C.H.; Berishvili, E. Immunomodulatory properties of amniotic membrane derivatives and their potential in regenerative medicine. Curr. Diabetes Rep. 2020, 20, 31. [Google Scholar] [CrossRef]
- Pratama, G.; Vaghjiani, V.; Tee, J.Y.; Liu, Y.H.; Chan, J.; Tan, C.; Murthi, P.; Gargett, C.; Manuelpillai, U. Changes in culture expanded human amniotic epithelial cells: Implications for potential therapeutic applications. PLoS ONE 2011, 6, e26136. [Google Scholar] [CrossRef]
- Zhang, Q.; Lai, D. Application of human amniotic epithelial cells in regenerative medicine: A systematic review. Stem Cell Res. Ther. 2020, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Lehmann, T.; Cai, H.; Stolz, D.; Strom, S. Stem cell characteristics of amniotic epithelial cells. Stem Cells 2005, 23, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, T.; Kawa, S.; Aizawa, T.; Ota, M.; Akaike, T.; Kato, K.; Konishi, I.; Nikaido, T. Human amnion-isolated cells normalize blood glucose in streptozotocin-induced diabetic mice. Cell Transplant. 2003, 12, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Takashima, S.; Ise, H.; Zhao, P.; Akaike, T.; Nikaido, T. Human amniotic epithelial cells possess hepatocyte-like characteristics and functions. Cell Struct. Funct. 2004, 29, 73–84. [Google Scholar] [CrossRef]
- Jafari, A.; Mirzaei, Y.; Mer, A.H.; Rezaei-Tavirani, M.; Jafari, Z.; Niknejad, H. Comparison of the effects of preservation methods on structural, biological, and mechanical properties of the human amniotic membrane for medical applications. Cell Tissue Bank. 2024, 25, 305–323. [Google Scholar] [CrossRef]
- Papait, A.; Ragni, E.; Cargnoni, A.; Vertua, E.; Romele, P.; Masserdotti, A.; Perucca Orfei, C.; Signoroni, P.B.; Magatti, M.; Silini, A.R.; et al. Comparison of EV-free fraction, EVs, and total secretome of amniotic mesenchymal stromal cells for their immunomodulatory potential: A translational perspective. Front. Immunol. 2022, 13, 960909. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Samadikuchaksaraei, A.; Seifalian, A.M.; Urbanska, A.M.; Ghanbarian, H.; Hardy, J.G.; Omrani, M.D.; Mozafari, M.; Reis, R.L.; Kundu, S.C. Silk fibroin/amniotic membrane 3D bi-layered artificial skin. Biomed. Mater. 2018, 13, 035003. [Google Scholar] [CrossRef]
- Tseng, S.C.; Prabhasawat, P.; Lee, S.H. Amniotic membrane transplantation for conjunctival surface reconstruction. Am. J. Ophthalmol. 1997, 124, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, J.; Shinozaki, N.; Tsubota, K. Transplantation of amniotic membrane and limbal autograft for patients with recurrent pterygium associated with symblepharon. Br. J. Ophthalmol. 1998, 82, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Mermet, I.; Pottier, N.; Sainthillier, J.M.; Malugani, C.; Cairey-Remonnay, S.; Maddens, S.; Riethmuller, D.; Tiberghien, P.; Humbert, P.; Aubin, F. Use of amniotic membrane transplantation in the treatment of venous leg ulcers. Wound Repair Regen. 2007, 15, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Solomon, A.; Rosenblatt, M.; Monroy, D.; Ji, Z.; Pflugfelder, S.C.; Tseng, S.C. Suppression of interleukin 1alpha and interleukin 1beta in human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br. J. Ophthalmol. 2001, 85, 444–449. [Google Scholar] [CrossRef]
- Byrne, H.M.; Chaplain, M.A.J.; Evans, D.L.; Hopkinson, I. Mathematical modelling of angiogenesis in wound healing comparison of theory and experiment. Comput. Math. Methods Med. 2000, 2, 175–197. [Google Scholar] [CrossRef]
- Kim, S.W.; Zhang, H.Z.; Kim, C.E.; An, H.S.; Kim, J.M.; Kim, M.H. Amniotic mesenchymal stem cells have robust angiogenic properties and are effective in treating hindlimb ischaemia. Cardiovasc. Res. 2012, 93, 525–534. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Baum, C.L.; Arpey, C.J. Normal cutaneous wound healing: Clinical correlation with cellular and molecular events. Dermatol. Surg. 2005, 31, 674–686. [Google Scholar] [CrossRef]
- Law, E.J.; Taib, H.; Berahim, Z. Amniotic Membrane: An Approach to Periodontal Regeneration. Cureus 2022, 14, e27832. [Google Scholar] [CrossRef]
- Solomon, A.; Wajngarten, M.; Alviano, F.; Anteby, I.; Elchalal, U.; Pe’Er, J.; Levi-Schaffer, F. Suppression of inflammatory and fibrotic responses in allergic inflammation by the amniotic membrane stromal matrix. Clin. Exp. Allergy 2005, 35, 941–948. [Google Scholar] [CrossRef]
- Tseng, S.C.; Espana, E.M.; Kawakita, T.; Di Pascuale, M.A.; Li, W.; He, H.; Liu, T.S.; Cho, T.H.; Gao, Y.Y.; Yeh, L.K.; et al. How does amniotic membrane work? Ocul. Surf. 2004, 2, 177–187. [Google Scholar] [CrossRef]
- Morelli, S.S.; Mandal, M.; Goldsmith, L.T.; Kashani, B.N.; Ponzio, N.M. The maternal immune system during pregnancy and its influence on fetal development. Res. Rep. Biol. 2015, 6, 171–189. [Google Scholar] [CrossRef]
- Tersigni, C.; Meli, F.; Neri, C.; Iacoangeli, A.; Franco, R.; Lanzone, A.; Scambia, G.; Di Simone, N. Role of Human Leukocyte Antigens at the Feto-Maternal Interface in Normal and Pathological Pregnancy: An Update. Int. J. Mol. Sci. 2020, 21, 4756. [Google Scholar] [CrossRef] [PubMed]
- Banas, R.A.; Trumpower, C.; Bentlejewski, C.; Marshall, V.; Sing, G.; Zeevi, A. Immunogenicity and immunomodulatory effects of amnion-derived multipotent progenitor cells. Hum. Immunol. 2008, 69, 321–328. [Google Scholar] [CrossRef] [PubMed]
- van ‘t Hof, L.J.; Schotvanger, N.; Haasnoot, G.W.; van der Keur, C.; Roelen, D.L.; Lashley, L.E.; Claas, F.H.; Eikmans, M.; van der Hoorn, M.L.P. Maternal-Fetal HLA Compatibility in Uncomplicated and Preeclamptic Naturally Conceived Pregnancies. Front. Immunol. 2021, 12, 673131. [Google Scholar] [CrossRef] [PubMed]
- Zhanzak, Z.; Cina, D.; Johnson, A.C.; Larsen, C.P. Implications of MHC-restricted immunopeptidome in transplantation. Front. Immunol. 2024, 15, 1436233. [Google Scholar] [CrossRef]
- Migliario, M.; Yerra, P.; Gino, S.; Sabbatini, M.; Renò, F. Laser Biostimulation Induces Wound Healing-Promoter β2-Defensin Expression in Human Keratinocytes via Oxidative Stress. Antioxidants 2023, 12, 1550. [Google Scholar] [CrossRef]
- Hao, Y.; Ma, D.H.; Hwang, D.G.; Kim, W.S.; Zhang, F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea 2008, 19, 348–352. [Google Scholar] [CrossRef]
- Li, W.; He, H.; Kawakita, T.; Espana, E.M.; Tseng, S.C. Amniotic membrane induces apoptosis of interferon-γ activated macrophages in vitro. Exp. Eye Res. 2006, 82, 282–292. [Google Scholar] [CrossRef]
- Kanyshkova, T.G.; Buneva, V.N.; Nevinsky, G.A. Lactoferrin and its biological functions. Biochemistry 2001, 66, 1–7. [Google Scholar] [CrossRef]
- Mao, Y.; Hoffman, T.; Singh-Varma, A.; Duan-Arnold, Y.; Moorman, M.; Danilkovitch, A.; Kohn, J. Antimicrobial peptides secreted from human cryopreserved viable amniotic membrane contribute to its antibacterial activity. Sci. Rep. 2017, 7, 13722. [Google Scholar] [CrossRef]
- Papait, A.; Cargnoni, A.; Sheleg, M.; Silini, A.R.; Kunis, G.; Ofir, R.; Parolini, O. Perinatal cells: A promising COVID-19 therapy? Front. Bioeng. Biotechnol. 2020, 8, 619980. [Google Scholar] [CrossRef]
- Brijacak, N.; Dekaris, I.; Gagro, A.; Gabrić, N. Therapeutic effect of amniotic membrane in persistent epithelial defects and corneal ulcers in herpetic keratitis. Coll. Antropol. 2008, 32 (Suppl. 2), 21–25. [Google Scholar]
- Buday, M.C.; Ozturk, M. Evaluation of folded amniotic membrane and injectable amniotic membrane pieces as soft tissue filler materials. Auris Nasus Larynx 2019, 46, 451–456. [Google Scholar] [CrossRef]
- Davis, A.; Augenstein, A. Amniotic allograft implantation for midface aging correction: A retrospective comparative study with platelet-rich plasma. Aesth. Plast. Surg. 2019, 43, 1345–1352. [Google Scholar] [CrossRef]
- Ley-Chavez, E.; Martinez-Pardo, M.E.; Roman, R.; Oliveros-Lozano Fde, J.; Canchola-Martinez, E. Application of biological dressings from radiosterilized amnios with cobalt 60 and serologic studies on the handling of burns in pediatric patients. Ann. Transplant. 2003, 8, 46–49. [Google Scholar]
- Ramakrishnan, K.M.; Jayaraman, V. Management of partial thickness burn wounds by amniotic membrane: A cost-effective treatment in developing countries. Burns 1997, 23 (Suppl. 1), S33–S36. [Google Scholar] [CrossRef] [PubMed]
- Elahi, A.; Taib, H.; Berahim, Z.; Ahmad, A.; Ab Hamid, S.S.; Mocktar, N.A. Amniotic membrane as a scaffold for periodontal tissue engineering. J. Health Sci. Med. Res. 2020, 39, 169–180. [Google Scholar] [CrossRef]
- Fénelon, M.; Catros, S.; Meyer, C.; Fricain, J.C.; Obert, L.; Auber, F.; Louvrier, A.; Gindraux, F. Applications of Human Amniotic Membrane for Tissue Engineering. Membranes 2021, 11, 387. [Google Scholar] [CrossRef] [PubMed]
- Sanders, F.W.; Huang, J.; Alió Del Barrio, J.L.; Hamada, S.; McAlinden, C. Amniotic membrane transplantation: Structural and biological properties, tissue preparation, application and clinical indications. Eye 2024, 38, 668–679. [Google Scholar] [CrossRef]
- Walkden, A. Amniotic membrane transplantation in ophthalmology: An updated perspective. Clin. Ophthalmol. 2020, 14, 2057–2072. [Google Scholar] [CrossRef]
- Adds, P.J.; Hunt, C.J.; Dart, J.K. Amniotic membrane grafts,”fresh”or frozen? A clinical and in vitro comparison. Br. J. Ophthalmol. 2001, 85, 905–907. [Google Scholar] [CrossRef] [PubMed]
- Paolin, A.; Trojan, D.; Leonardi, A.; Mellone, S.; Volpe, A.; Orlandi, A.; Cogliati, E. Cytokine expression and ultrastructural alterations in fresh-frozen, freeze-dried and γ-irradiated human amniotic membranes. Cell Tissue Bank. 2016, 17, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.P. Cell membrane oxidative damage induced by gamma-radiation and apoptotic sensitivity. J. Environ. Pathol. Toxicol. Oncol. 2004, 23, 6. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sekiyama, E.; Takaoka, M.; Bentley, A.J.; Yokoi, N.; Fullwood, N.J.; Kinoshita, S. The use of trehalose-treated freeze-dried amniotic membrane for ocular surface reconstruction. Biomaterials 2008, 29, 3729–3737. [Google Scholar] [CrossRef]
- Laranjo, M. Preservation of amniotic membrane. In Amniotic Membrane; Mamede, A., Botelho, M., Eds.; Springer: Dordrech, The Netherlands, 2015. [Google Scholar]
- Maral, T.; Borman, H.; Arslan, H.; Demirhan, B.; Akinbingol, G.; Haberal, M. Effectiveness of human amnion preserved long-term in glycerol as a temporary biological dressing. Burns 1999, 25, 625–635. [Google Scholar] [CrossRef]
- Ingraldi, A.L.; Audet, R.G.; Tabor, A.J. The Preparation and Clinical Efficacy of Amnion-Derived Membranes: A Review. J. Funct. Biomater. 2023, 14, 531. [Google Scholar] [CrossRef]
- Debelian, G.J.; Olsen, I.; Tronstad, L. Anaerobic bacteremia and fungemia in patients undergoing endodontic therapy: An overview. Ann. Periodontol. 1998, 3, 281–287. [Google Scholar] [CrossRef]
- Li, X.; Kolltveit, K.M.; Tronstad, L.; Olsen, I. Systemic diseases caused by oral infection. Clin. Microbiol. Rev. 2000, 13, 547–558. [Google Scholar] [CrossRef]
- Cohen, S.R.; Corrigan, M.; Wilmot, J.; Trotman, C.A. Cumulative operative procedures in patients aged 14 years and older with unilateral or bilateral cleft lip and palate. Plast. Reconstr. Surg. 1995, 96, 267–271. [Google Scholar] [CrossRef]
- Kido, D.; Mizutani, K.; Takeda, K.; Mikami, R.; Matsuura, T.; Iwasaki, K.; Izumi, Y. Impact of diabetes on gingival wound healing via oxidative stress. PLoS ONE 2017, 12, e0189601. [Google Scholar] [CrossRef]
- Evans, E.W. Treating Scars on the Oral Mucosa. Facial Plast. Surg. Clin. N. Am. 2017, 25, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Sattar, M.; Sayed, O.M.; Lane, M.E. Oral transmucosal drug delivery current status and future prospects. Int. J. Pharm. 2014, 471, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Naumova, E.A.; Dierkes, T.; Sprang, J.; Arnold, W.H. The oral mucosal surface and blood vessels. Head Face Med. 2013, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Lawson, V.G. Oral cavity reconstruction using pectoralis major muscle and amnion. Arch. Otolaryngol. 1985, 111, 230–233. [Google Scholar] [CrossRef]
- Anamika, S.; Komal, Y. Amniotic membrane—A Novel material for the root coverage: A case series. J. Indian Soc. Periodontol. 2015, 19, 444–448. [Google Scholar]
- Rehan, M.; Khatri, M.; Bansal, M.; Puri, K.; Kumar, A. Comparative Evaluation of Coronally Advanced Flap Using Amniotic Membrane and Platelet-rich Fibrin Membrane in Gingival Recession: An 18-Month Clinical Study. Contemp. Clin. Dent. 2018, 9, 188–194. [Google Scholar] [CrossRef]
- Kaur, J.; Bathla, S.C. Regenerative potential of autologous platelet-rich fibrin with and without amnion membrane in the treatment of Grade-II furcation defects: A clinicoradiographic study. J. Indian Soc. Periodontol. 2018, 22, 235–242. [Google Scholar]
- Temraz, A.; Ghallab, N.A.; Hamdy, R.; El-Dahab, O.A. Clinical and radiographic evaluation of amnion chorion membrane and demineralized bone matrix putty allograft for management of periodontal intrabony defects: A randomized clinical trial. Cell Tissue Bank. 2019, 20, 117–128. [Google Scholar] [CrossRef]
- Kadkhoda, Z.; Tavakoli, A.; Rafiei, S.C.; Zolfaghari, F.; Akbari, S. Effect of Amniotic Membrane Dressing on Pain and Healing of Palatal Donor Site: A Randomized Controlled Trial. Int. J. Organ Transplant. Med. 2020, 11, 55–62. [Google Scholar]
- Kumar, S.; Hirani, T.; Shah, S.; Mehta, R.; Bhakkand, S.R.; Shishoo, D. Treating Public Health Dilemma of Gingival Recession by the Dehydrated Amnion Allograft: A 5-Year Longitudinal Study. Front. Oral Health 2020, 1, 540211. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, N.; Lavu, V.; Balaji, S.K. Clinical efficacy of amniotic membrane with biphasic calcium phosphate in guided tissue regeneration of intrabony defects- a randomized controlled clinical trial. Biomater. Res. 2021, 25, 15. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Bhattacharya, H.S.; Rastogi, P.; Agarwal, M.C.; Agarwal, A. Evaluation of association between potential stress markers and periodontal health in medical and dental students: A questionnaire-based study. Natl. J. Maxillofac. Surg. 2022, 13, 90–94. [Google Scholar] [CrossRef]
- Nath, J.; Changmai, A.; Bhattacharjee, K.; Phukan, A.H.; Chakraborty, D.; Das, U. Management of Gingival Recession by Coronally Advanced Flap with and Without Amniotic Membrane: A Clinical Study. J. Pharm. Bioallied Sci. 2022, 14 (Suppl. 1), S486–S489. [Google Scholar] [CrossRef]
- Akhlaghi, F.; Hesami, N.; Rad, M.R.; Nazeman, P.; Fahimipour, F.; Khojasteh, A. Improved bone regeneration through amniotic membrane loaded with buccal fat pad-derived MSCs as an adjuvant in maxillomandibular reconstruction. J. Cranio-Maxillofac. Surg. 2019, 47, 1266–1273. [Google Scholar] [CrossRef]
- Hazarika, K.; Malik, K.; Adhyapok, A.K.; Debnath, S.C. Lyophilised Amniotic Membrane in Intraoral Surgical Defects: A Prospective Clinical Study. Ann. Maxillofac. Surg. 2022, 12, 5–10. [Google Scholar] [CrossRef]
- Faraj, S.A.; Kutkut, A.; Taylor, R.C.; Villasante-Tezanos, A.; Huja, S.S.; Dawson, D.R.; Almehmadi, N.; Al-Sabbagh, M. Comparison of Dehydrated Human Amnion-Chorion and Type 1 Bovine Collagen Membranes in Alveolar Ridge Preservation: A Clinical and Histological Study. J. Oral Implantol. 2021, 47, 385–393. [Google Scholar] [CrossRef]
- Babaki, D.; Khoshsimaybargard, M.; Yaghoubi, S.; Gholami, M. Comparison of Vestibular Depth Relapse and Wound Healing After Reconstructive Preprosthetic Surgery Using Cryopreserved Amniotic Membrane and Acellular Dermal Matrix-A Comparative Study. Ann. Maxillofac. Surg. 2021, 11, 12–16. [Google Scholar]
- Gajul, M.; Bhate, K.; Awate, S.; Kakodkar, P.; Shah, S. Comparative evaluation of the efficacy of wound healing with and without dehydrated human amniotic/chorionic membrane in alveoloplasty: A pilot study. J. Korean Assoc. Oral Maxillofac. Surg. 2021, 47, 279–285. [Google Scholar] [CrossRef]
- Çanakçi, F.G.; Er, N.; Duygu, G.; Varol, G.F. Surgical management of stage-2 medication-related osteonecrosis of the jaw with transplantation of human amniotic membrane: Preliminary results. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e28–e31. [Google Scholar] [CrossRef]
- Ragazzo, M.; Val, M.; Montagner, G.; Trojan, D.; Fusetti, S.; Guarda Nardini, L. Human amniotic membrane: An improvement in the treatment of Medication-related osteonecrosis of the jaw (MRONJ)? A case–control study. Cell Tissue Bank. 2022, 23, 129–141. [Google Scholar] [CrossRef]
- Odet, S.; Meyer, C.; Gaudet, C.; Weber, E.; Quenot, J.; Derruau, S.; Laurence, S.; Bompy, L.; Girodon, M.; Chatelain, B.; et al. Tips and Tricks and Clinical Outcome of Cryopreserved Human Amniotic Membrane Application for the Management of Medication-Related Osteonecrosis of the Jaw (MRONJ): A Pilot Study. Front. Bioeng. Biotechnol. 2022, 10, 936074, Erratum in Front. Bioeng. Biotechnol. 2022, 10, 1058241. [Google Scholar] [CrossRef] [PubMed]
- Pakkala, T.; Virtanen, L.; Oksanen, J.; Jones, J.C.; Hormia, M. Function of Laminins and Laminin-Binding Integrins in Gingival Epithelial Cell Adhesion. J. Periodontol. 2002, 40, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Kothiwale, S.V.; Anuroopa, P.; Gajiwala, A.L. A clinical and radiological evaluation of DFDBA with amniotic membrane vs bovine derived xenograft with amniotic membrane in human periodontal grade II furcation defects. Cell Tissue Bank. 2009, 10, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Mallapragda, S.; Gupta, R.; Gupta, S.; Sharma, H.; Srivastava, S.; Raj, A. Evaluation of Regenerative Efficacy of Amnion and Chorion Membrane in Treatment of Mandibular Molar Furcation Defects: A Clinico-radiographic Study. J. Contemp. Dent. Pract. 2024, 25, 160–167. [Google Scholar]
- Arai, N.; Tsuno, H.; Okabe, M.; Yoshida, T.; Koike, C.; Noguchi, M.; Nikaido, T. Clinical application of a hyperdry amniotic membrane on surgical defects of the oral mucosa. J. Oral Maxillofac. Surg. 2012, 70, 2221–2228. [Google Scholar] [CrossRef]
- Kesting, M.R.; Loeffelbein, D.J.; Classen, M.; Slotta-Huspenina, J.; Hasler, R.J.; Jacobsen, F.; Kreutzer, K.; Al-Benna, S.; Wolff, K.D.; Steinstraesser, L. Repair of oronasal fistulas with human amniotic membrane in minipigs. Br. J. Oral Maxillofac. Surg. 2010, 48, 131–135. [Google Scholar] [CrossRef]
- Sharma, S.; Mehra, H.; Gupta, H.; Agarwal, R.; Gangwar, A.; Kumar, A. Comparison of the Efficacy of Amniotic Membrane Versus Buccal Fat Pad in Treatment of Oral Submucous Fibrosis. J. Maxillofac. Oral Surg. 2023, 22, 525–532. [Google Scholar] [CrossRef]
- Chen, E.; Tofe, A. A literature review of the safety and biocompatibility of amnion tissue. J. Implant. Adv. Clin. Dent. 2009, 2, 67–75. [Google Scholar]
- Bagde, H.; Pawar, S.K.; Vasisth, D.; Vadvadgi, V.H.; Laddha, R.B.; Wagh, P.P. Comparison of Amnion Membrane and Hyaluronic Acid in Gingival Recession Coverage and Gain in Clinical Attachment Level following Coronally Advanced Flap Procedure-A Clinical Study. J. Pharm. Bioallied Sci. 2023, 15 (Suppl. 2), S1104–S1107. [Google Scholar] [CrossRef]
- Sundar, T.A.; Shetty, P.; Hegde, P.; Shreya, S. Hyaluronic acid versus amniotic membrane in wound healing and bone regeneration in extraction sockets-A randomized controlled trial. J. Oral Biol. Craniofac. Res. 2025, 15, 305–309. [Google Scholar] [CrossRef]
- Abdel-Fatah, R.; Saleh, W. Efficacy of amniotic membrane with coronally advanced flap in the treatment of gingival recession: An updated systematic review and meta-analysis. BMC Oral Health 2024, 24, 133. [Google Scholar] [CrossRef]


| Year | Author | Patients | Indications | Treatment | Assessment Methods | Results |
|---|---|---|---|---|---|---|
| 2018 | Rehan et al. [101] | 10 | Gingival recession | Coronally advanced flap + platelet-rich fibrin (PRF) Coronally advanced flap + hAM | Plaque index; Gingival index; Bleeding on probing; Clinical attachment level; Depth of recession; Width of recession; Width of attached gingiva. | The hAM showed the better percentage of root coverage as compared to PRF. |
| 2018 | Kaur and Bathla [102] | 15 | Periodontal furcation defect | PRF + hAM hAM alone | Measurement of dental plaque index; Measurement of gingival index; Measurement of gingival recession depth; Measurement of pocket depth; Measurement of clinical attachment level. | All clinical and radiographic parameters showed statistically significant improvement at the sites treated with PRF and the amnion membrane compared to those with PRF alone. |
| 2019 | Temraz et al. [103] | 22 | Periodontal pockets | Open flap debridement + hAM Open flap debridement + demineralized bone matrix | Measurement of dental plaque index; Measurement of gingival index; Measurement of pocket depth; Measurement of clinical attachment level; Radiographic measurement. | Both the hAM barrier and demineralized bone matrix putty allograft provided significant improvement in clinical and radiographic outcomes after 6 months, yet no significant differences were noticed between them. |
| 2020 | Kadkh-oda et al. [104] | 27 | Healing of palatal donor site after free gingival graft surgery | hAM Only suture | Clinical assessment; Pain score. | Mean color match scores were higher in the hAM group than in the control group. |
| 2020 | Kumar et al. [105] | 51 | Gingival recession | Coronally advanced flap + hAM Coronally advanced flap alone | Measurement of clinical attachment level; Measurement of pocket depth; Measurement of recession width; Measurement of keratinized tissue width; Measurement of thickness of keratinized gingiva (TKG). | Intergroup comparison showed a non-significant difference in all settings except the TKG. The hAM was proven to help improve the TKG. |
| 2021 | Venkat-esan et al. [106] | 50 | Periodontal pockets | hAM + Biphasic calcium phosphate Collagen membrane + Biphasic calcium phosphate | Measurement of clinical attachment level; Measurement of pocket depth. | The hAM can be used as a barrier membrane, in conjunction with Biphasic calcium phosphate, and provides comparable results to a collagen membrane with Biphasic calcium phosphate. |
| 2022 | Agraw-al et al. [107] | 20 | Periodontal pockets | Open flap debridement + demineralized freeze-dried bone allograft + hAM Open flap debridement + demineralized freeze-dried bone allograft + collagen membrane | Measurement of dental plaque index; Measurement of gingival index; Measurement of pocket depth; Measurement of clinical attachment level; Radiographic measurement. | For all the clinical and radiographic parameters, no statistically significant difference was noted between both the groups. |
| 2022 | Nath et al. [108] | 18 | Gingival recession | Coronally advanced flap + hAM Coronally advanced flap alone | Measurement of width of attached gingiva; Measurement of clinical attachment level; Measurement of pocket depth; Measurement of width of keratinized gingiva; Measurement of length of gingival recession; Measurement of width of gingival recession. | Combined, a coronally advanced flap and the hAM have additional advantage in the outcome of periodontal therapy in the management of gingival recession. |
| Year | Author | Patients | Indications | Treatment | Assessment Methods | Results |
|---|---|---|---|---|---|---|
| 2019 | Akhlagi et al. [109] | 9 | Maxillomandibular bone defects following tumor surgery | Iliac crest bone graft + hAM Iliac crest bone graft + hAM + buccal fat pad-derived stem cells | Computed tomography image assessment | The mean increase in bone width was found to be significantly greater in the hAM + buccal fat pad-derived stem cell group |
| 2022 | Hazari-ka et al. [110] | 15 | Mucosal defect after excision of precancerous lesions | hAM | Clinical assessment of operability; Hemostatic status; Pain; Feeding situation; Epithelialization; Change in mouth opening; Mucosal suppleness and safety. | The hAM is a cost-effective material for immediate coverage of intraoral surgical defects. |
| Year | Author | Patients | Indications | Treatment | Assessment Methods | Results |
|---|---|---|---|---|---|---|
| 2021 | Faraj et al. [111] | 21 | Alveolar ridge preservation | hAM Collagen membrane | Clinical assessment of ridge dimensions | Human amnion–chorion membrane or type 1 bovine collagen as the open barrier did not change healing. |
| 2021 | Babaki et al. [112] | 28 | Mandibular vestibuloplasty | hAM Acellular dermal matrix | Clinical assessment of relapses and healing | An acellular dermal matrix accelerates wound healing compared to the hAM. |
| 2021 | Gajul et al. [113] | 10 | Alveoloplasty | hAM Control | Assessment by Landry, Turnbull, and Howley Index | The hAM group showed an improved healing index for tissue color, bleeding on palpation, granulation tissue, suppuration, and overall healing. |
| Year | Author | Patients | Indications | Treatment | Assessment Methods | Results |
|---|---|---|---|---|---|---|
| 2022 | Canakci et al. [114] | 5 | MRONJ (zoledronic acid) | hAM | Clinical assessment | There was complete mucosal coverage in 4 patients |
| 2022 | Ragazzo et al. [115] | 49 | MRONJ (zoledronic acid, clodronic acid, ibandronic acid) | hAM | Clinical assessment | hAM seems to stimulate soft tissue healing and reducing pain perception in the postoperative period. |
| 2022 | Odet et al. [116] | 8 | MRONJ (bisphosphates, denosumab) | hAM | Clinical assessment | Of the lesions, 80% had complete or partial wound healing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabbatini, M.; Boffano, P.; Ferrillo, M.; Migliario, M.; Renò, F. The Human Amniotic Membrane: A Rediscovered Tool to Improve Wound Healing in Oral Surgery. Int. J. Mol. Sci. 2025, 26, 8470. https://doi.org/10.3390/ijms26178470
Sabbatini M, Boffano P, Ferrillo M, Migliario M, Renò F. The Human Amniotic Membrane: A Rediscovered Tool to Improve Wound Healing in Oral Surgery. International Journal of Molecular Sciences. 2025; 26(17):8470. https://doi.org/10.3390/ijms26178470
Chicago/Turabian StyleSabbatini, Maurizio, Paolo Boffano, Martina Ferrillo, Mario Migliario, and Filippo Renò. 2025. "The Human Amniotic Membrane: A Rediscovered Tool to Improve Wound Healing in Oral Surgery" International Journal of Molecular Sciences 26, no. 17: 8470. https://doi.org/10.3390/ijms26178470
APA StyleSabbatini, M., Boffano, P., Ferrillo, M., Migliario, M., & Renò, F. (2025). The Human Amniotic Membrane: A Rediscovered Tool to Improve Wound Healing in Oral Surgery. International Journal of Molecular Sciences, 26(17), 8470. https://doi.org/10.3390/ijms26178470










