Neuromedin U Receptor NMUR3 Regulates Autophagy, Thereby Enhancing Thermal Tolerance in C. elegans
Abstract
1. Introduction
2. Results
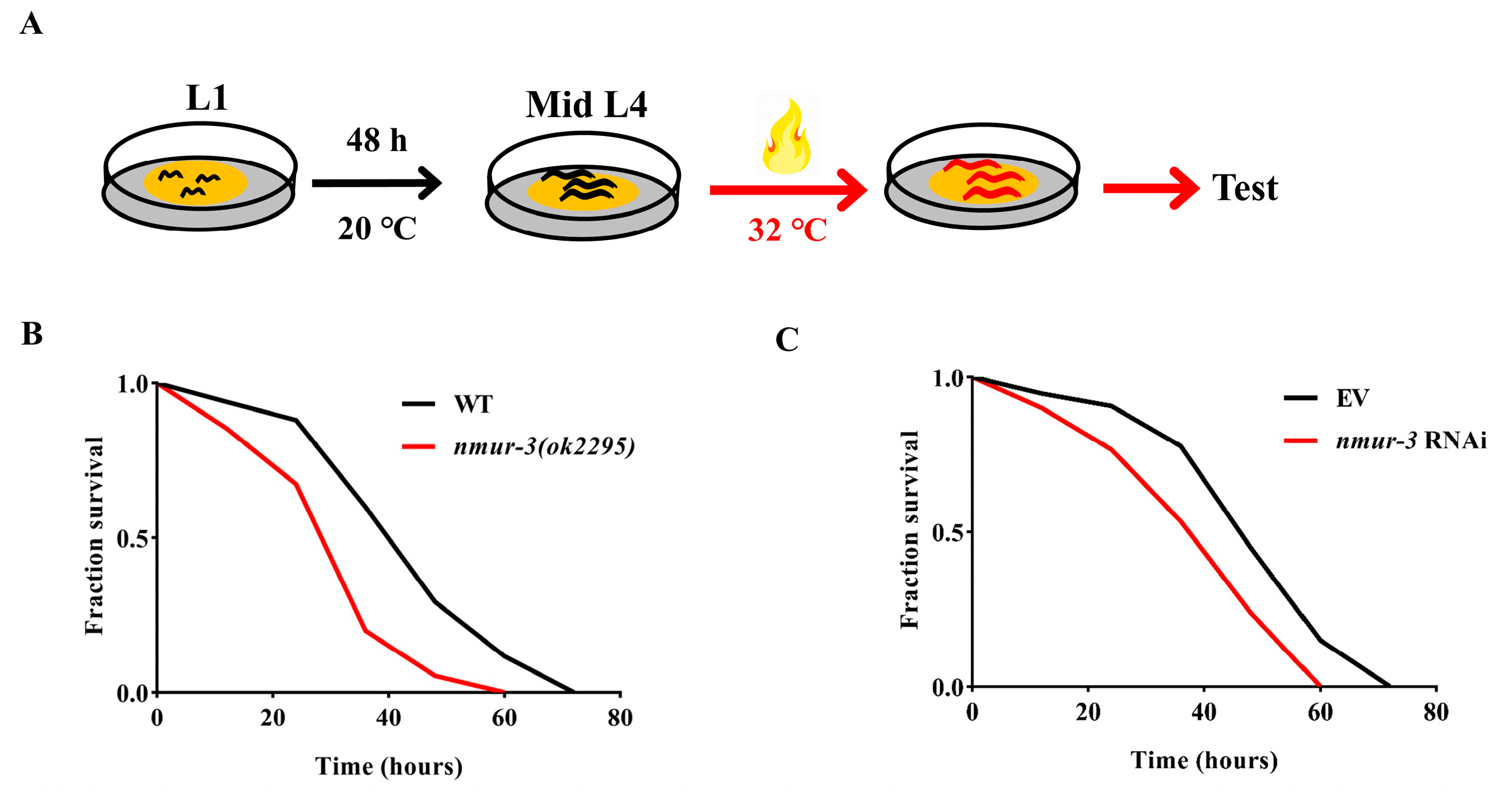
2.1. NMUR-3 Mediates Resistance to Heat Shock in Worms
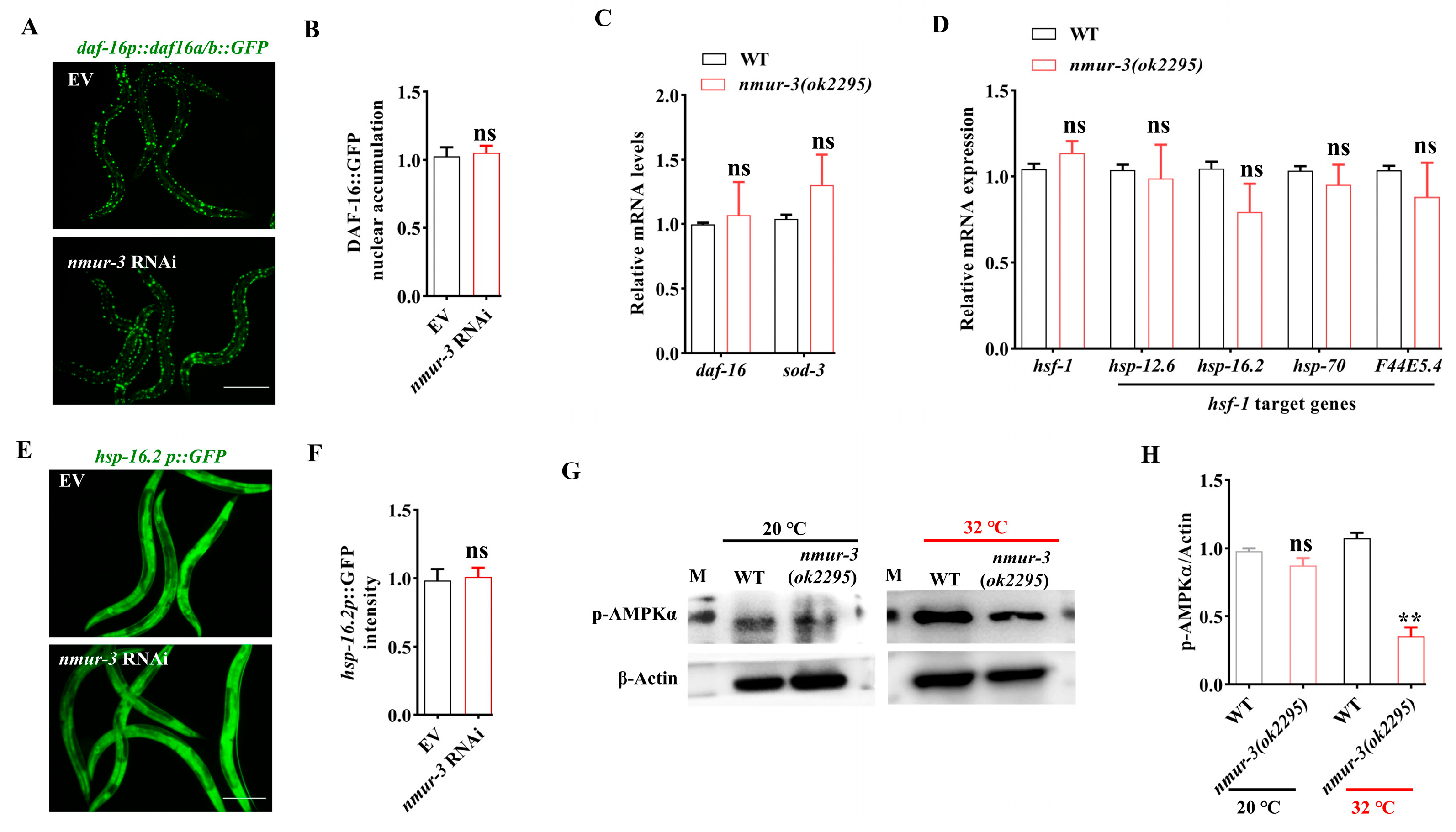
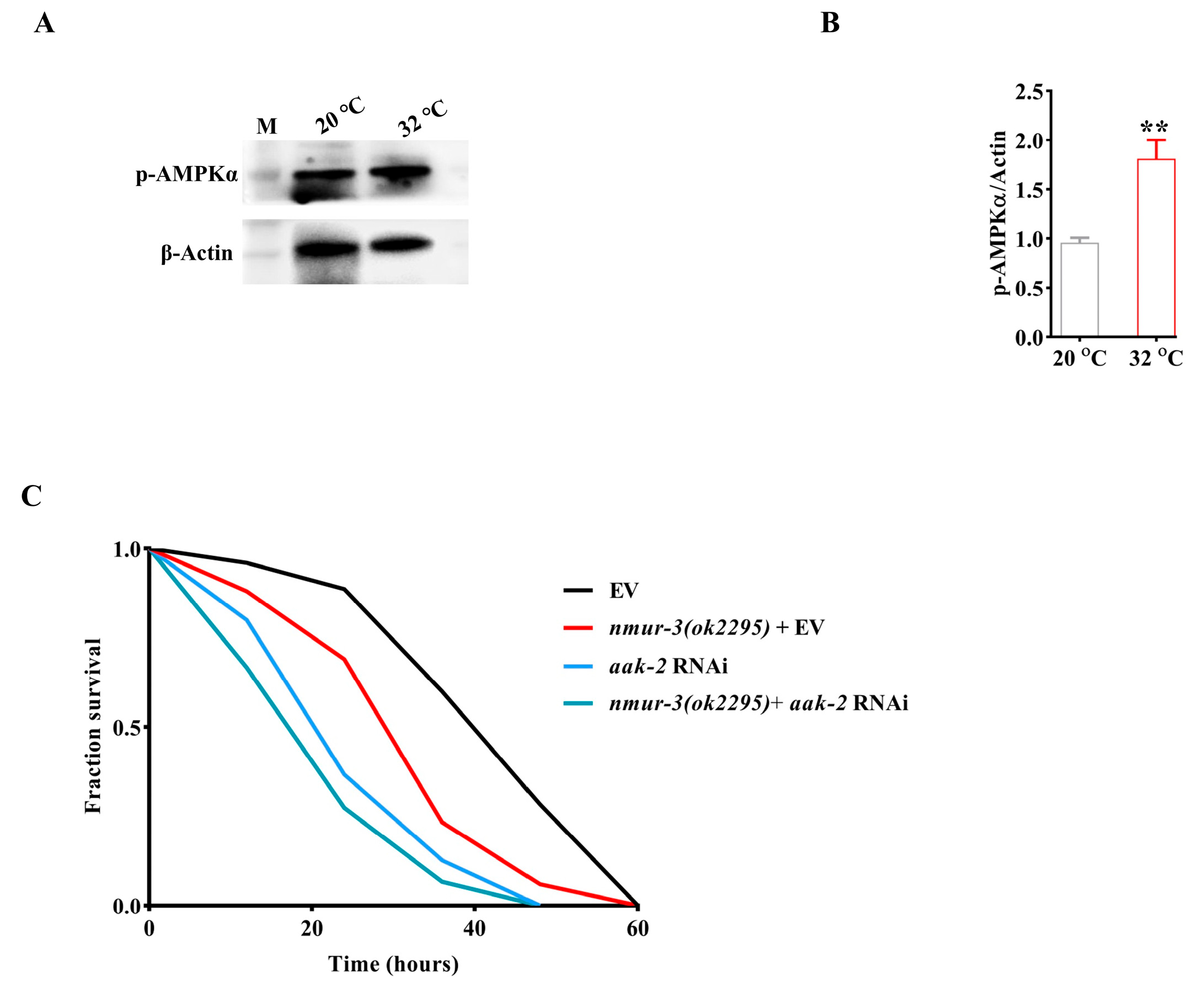
2.2. NMUR-3-Mediated Thermal Tolerance Is Dependent on AMPK in Worms
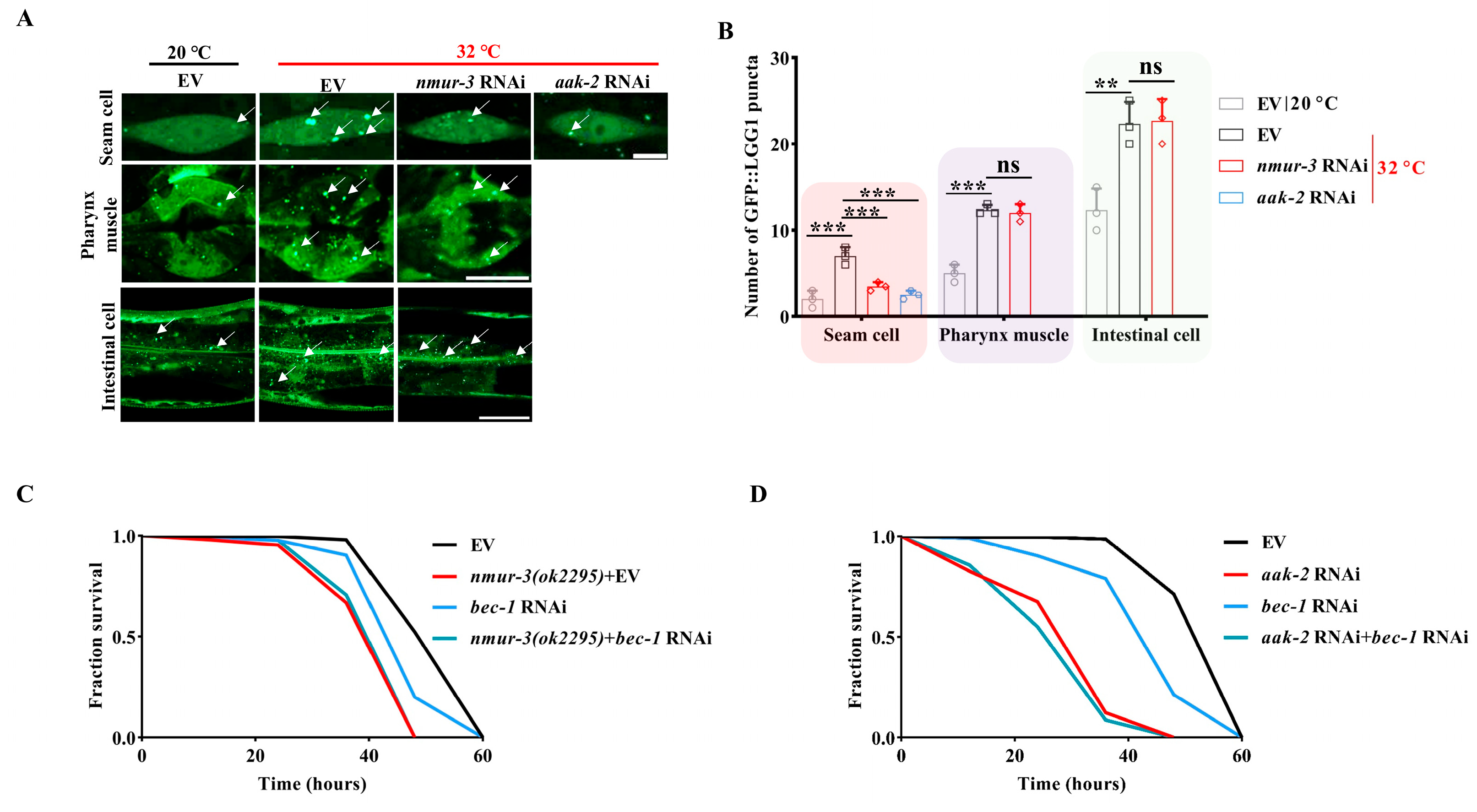
2.3. NMUR-3 Increases Heat Resistance by Inducing Autophagy via AMPK
3. Discussion
4. Materials and Methods
4.1. Nematode Strains and Bacteria
4.2. Synchronization Methods
4.3. Oil Red O (ORO) Staining
4.4. RNAi in C. elegans
4.5. Lifespan Analysis
4.6. C. elegans Infection Experiment
4.7. C. elegans Stress Response Experiment Under Heat Shock and High-Salt Conditions
4.8. RNA Extraction, cDNA Synthesis and RT-qPCR Analysis
4.9. Microscopy
4.10. Western Blot
4.11. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blackwell, T.K.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88 Pt B, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, U.T.T.; Youn, E.; Le, T.A.N.; Ha, N.M.; Tran, S.H.; Lee, S.; Cha, J.W.; Park, J.S.; Kwon, H.C.; Kang, K. Photodynamic treatment increases the lifespan and oxidative stress resistance of Caenorhabditis elegans. Free Radic. Biol. Med. 2024, 221, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Feinbaum, R.; Alloing, G.; Emerson, F.E.; Garsin, D.A.; Inoue, H.; Tanaka-Hino, M.; Hisamoto, N.; Matsumoto, K.; Tan, M.W.; et al. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 2002, 297, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.J.; Cheesman, H.K.; Liu, P.; Peterson, N.D.; Anderson, S.M.; Pukkila-Worley, R. Innate Immunity in the C. elegans Intestine Is Programmed by a Neuronal Regulator of AWC Olfactory Neuron Development. Cell Rep. 2020, 31, 107478. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Blackwell, T.K. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Gene Dev. 2003, 17, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Wu, J.; Tan, Y.; Shi, Y.; Yang, W.; Tu, H.; Tan, W. β-asarone protects against age-related motor decline via activation of SKN-1/Nrf2 and subsequent induction of GST-4. Pharmacol. Res. 2024, 209, 107450. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.T.; McCarroll, S.A.; Bargmann, C.I.; Fraser, A.; Kamath, R.S.; Ahringer, J.; Li, H.; Kenyon, C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 2003, 424, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Erkut, C.; Penkov, S.; Khesbak, H.; Vorkel, D.; Verbavatz, J.M.; Fahmy, K.; Kurzchalia, T.V. Trehalose renders the dauer larva of Caenorhabditis elegans resistant to extreme desiccation. Curr. Biol. 2011, 21, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.D.; Chen, X.; Yu, L.; Hu, M.L.; Pan, Y.R.; Qin, D.L.; Wu, J.M.; Li, L.; Law, B.Y.; Wong, V.K.; et al. Targeting autophagy to discover the Piper wallichii petroleum ether fraction exhibiting antiaging and anti-Alzheimer’s disease effects in Caenorhabditis elegans. Phytomedicine 2023, 117, 154916. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.G.; Ma, Y.C.; Dai, L.L.; Zhang, K.Q. Autophagy protects C. elegans against necrosis during Pseudomonas aeruginosa infection. Proc. Natl. Acad. Sci. USA 2014, 111, 12480–12485. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Howell, S.H. Heat Stress Responses and Thermotolerance in Maize. Int. J. Mol. Sci. 2021, 22, 948. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, R.I. Regulation of the heat shock transcriptional response: Cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998, 12, 3788–3796. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.; Chang, J.; Gensch, E.; Rudner, A.; Tabtiang, R. A C. elegans mutant that lives twice as long as wild type. Nature 1993, 366, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Barrett, L.N.; Westerheide, S.D. The CBP-1/p300 Lysine Acetyltransferase Regulates the Heat Shock Response in C. elegans. Front. Aging 2022, 3, 861761. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; He, B.; Deng, J.; Pang, S.; Tang, H. Histone acetylation promotes long-lasting defense responses and longevity following early life heat stress. PLoS Genet. 2019, 15, e1008122. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Niu, M.F.; Su, J.; Ma, Z.Y.; Jin, M.M.; Qiao, W.N.; Zhang, Y.; Feng, Y.Y.; An, N.; Hou, Y.L.; et al. Cloning and expression patterns of neuromedin U and its receptors in pigs. Neuropeptides 2017, 64, 47–60. [Google Scholar] [CrossRef]
- Bargmann, C.I. Beyond the connectome: How neuromodulators shape neural circuits. Bioessays 2012, 34, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Ensho, T.; Maruyama, K.; Qattali, A.W.; Yasuda, M.; Uemura, R.; Murakami, N.; Nakahara, K. Comparison of glucose tolerance between wild-type mice and mice with double knockout of neuromedin U and neuromedin S. J. Vet. Med. Sci. 2019, 81, 1305–1312. [Google Scholar] [CrossRef]
- Mirabeau, O.; Joly, J.S. Molecular evolution of peptidergic signaling systems in bilaterians. Proc. Natl. Acad. Sci. USA 2013, 110, E2028–E2037. [Google Scholar] [CrossRef] [PubMed]
- Maier, W.; Adilov, B.; Regenass, M.; Alcedo, J. A neuromedin U receptor acts with the sensory system to modulate food type-dependent effects on C. elegans lifespan. PLoS Biol. 2010, 8, e1000376. [Google Scholar] [CrossRef] [PubMed]
- Watteyne, J.; Peymen, K.; Van der Auwera, P.; Borghgraef, C.; Vandewyer, E.; Van Damme, S.; Rutten, I.; Lammertyn, J.; Jelier, R.; Schoofs, L.; et al. Neuromedin U signaling regulates retrieval of learned salt avoidance in a C. elegans gustatory circuit. Nat. Commun. 2020, 11, 2076. [Google Scholar] [CrossRef] [PubMed]
- Wibisono, P.; Wibisono, S.; Watteyne, J.; Chen, C.H.; Sellegounder, D.; Beets, I.; Liu, Y.; Sun, J. Neuronal GPCR NMUR-1 regulates distinct immune responses to different pathogens. Cell Rep. 2022, 38, 110321. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.; Curran, S.P. Adaptive capacity to bacterial diet modulates aging in C. elegans. Cell Metab. 2014, 19, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, T.; Sakamoto, K. FoxO/Daf-16 restored thrashing movement reduced by heat stress in Caenorhabditis elegans. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 170, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.W.; Mukhopadhyay, A.; Dixit, B.L.; Raha, T.; Green, M.R.; Tissenbaum, H.A. Identification of direct DAF-16 targets controlling longevity, metabolism and diapause by chromatin immunoprecipitation. Nat. Genet. 2006, 38, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Tataridas-Pallas, N.; Aman, Y.; Williams, R.; Chapman, H.; Cheng, K.J.H.; Gomez-Paredes, C.; Bates, G.P.; Labbadia, J. Mitochondrial clearance and increased HSF-1 activity are coupled to promote longevity in fasted Caenorhabditis elegans. Iscience 2024, 27, 109834. [Google Scholar] [CrossRef] [PubMed]
- Rea, S.L.; Wu, D.; Cypser, J.R.; Vaupel, J.W.; Johnson, T.E. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat. Genet. 2005, 37, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, R.; von Gromoff, E.D.; Drepper, F.; Knapp, B.; Warscheid, B.; Baumeister, R.; Qi, W. Reprogramming of the transcriptome after heat stress mediates heat hormesis in Caenorhabditis elegans. Nat. Commun. 2023, 14, 4176. [Google Scholar] [CrossRef] [PubMed]
- Corton, J.M.; Gillespie, J.G.; Hardie, D.G. Role of the AMP-activated protein kinase in the cellular stress response. Curr. Biol. 1994, 4, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Oakhill, J.S.; Chen, Z.-P.; Scott, J.W.; Steel, R.; Castelli, L.A.; Ling, N.; Macaulay, S.L.; Kemp, B.E. β-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK). Proc. Natl. Acad. Sci.USA 2010, 107, 19237–19241. [Google Scholar] [CrossRef] [PubMed]
- Kumsta, C.; Chang, J.T.; Schmalz, J.; Hansen, M. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nat. Commun. 2017, 8, 14337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xie, H.; Pan, P.; Wang, Q.; Yang, B.; Li, Y.; Wei, Y.; Sun, Y.; Wei, Y.; Jiang, Q.; et al. EGCG alleviates heat-stress-induced fat deposition by targeting HSP70 through activation of AMPK-SIRT1-PGC-1α in porcine subcutaneous preadipocytes. Biochem. Pharmacol. 2024, 225, 116250. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Chen, S.; Li, K.; Zhang, C.; Tan, Y.; Deng, Q.; Chai, Y.; Wang, X.; Chen, G.; Feng, K.; et al. Targeting autophagy enhances heat stress-induced apoptosis via the ATP-AMPK-mTOR axis for hepatocellular carcinoma. Int. J. Hyperther. 2019, 36, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xie, H.; Pan, P.; Qu, Q.; Xia, Q.; Gao, X.; Zhang, S.; Jiang, Q. Heat stress promotes lipid accumulation by inhibiting the AMPK-PGC-1α signaling pathway in 3T3-L1 preadipocytes. Cell Stress Chaperones 2021, 26, 563–574. [Google Scholar] [CrossRef]
- Sulston, J.E.; Brenner, S. The DNA of Caenorhabditis elegans. Genetics 1974, 77, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Xiao, Y.; Ji, X.L.; Zhang, K.Q.; Zou, C.G. The cAMP-PKA pathway-mediated fat mobilization is required for cold tolerance in C. elegans. Sci. Rep. 2017, 7, 638. [Google Scholar] [CrossRef] [PubMed]
- Kamath, R.S.; Ahringer, J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods 2003, 30, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Marquina-Solis, J.; Feng, L.; Vandewyer, E.; Beets, I.; Hawk, J.; Colón-Ramos, D.A.; Yu, J.; Fox, B.W.; Schroeder, F.C.; Bargmann, C.I. Antagonism between neuropeptides and monoamines in a distributed circuit for pathogen avoidance. Cell Rep. 2024, 43, 114042. [Google Scholar] [CrossRef] [PubMed]
- Garsin, D.A.; Venkatesh, S.R.; Siddiqui, R.; Sandhu, A.; Ramani, M.; Houston, I.R.; Watts, J.L.; Singh, V. Homeostatic control of stearoyl desaturase expression via patched-like receptor PTR-23 ensures the survival of C. elegans during heat stress. PLoS Genet. 2023, 19, e1011067. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Yang, J.; Wang, S.; Ma, Y.; Zou, C. Neuromedin U Receptor NMUR3 Regulates Autophagy, Thereby Enhancing Thermal Tolerance in C. elegans. Int. J. Mol. Sci. 2025, 26, 8471. https://doi.org/10.3390/ijms26178471
He L, Yang J, Wang S, Ma Y, Zou C. Neuromedin U Receptor NMUR3 Regulates Autophagy, Thereby Enhancing Thermal Tolerance in C. elegans. International Journal of Molecular Sciences. 2025; 26(17):8471. https://doi.org/10.3390/ijms26178471
Chicago/Turabian StyleHe, Limei, Jianqi Yang, Shufang Wang, Yicheng Ma, and Chenggang Zou. 2025. "Neuromedin U Receptor NMUR3 Regulates Autophagy, Thereby Enhancing Thermal Tolerance in C. elegans" International Journal of Molecular Sciences 26, no. 17: 8471. https://doi.org/10.3390/ijms26178471
APA StyleHe, L., Yang, J., Wang, S., Ma, Y., & Zou, C. (2025). Neuromedin U Receptor NMUR3 Regulates Autophagy, Thereby Enhancing Thermal Tolerance in C. elegans. International Journal of Molecular Sciences, 26(17), 8471. https://doi.org/10.3390/ijms26178471





