Modulation of INa, Ih, and IK(erg) by Extracellular or Intracellular QX-314 (N-(2,6-dimethylphenylcarbamoylmethyl) triethylammonium bromide) in Pituitary Tumor Cells
Abstract
1. Introduction
2. Results
2.1. Inhibitory Effect of QX-314 on the Amplitude of Voltage-Gated Na+ Currents (INa) Measured from GH3 Cells
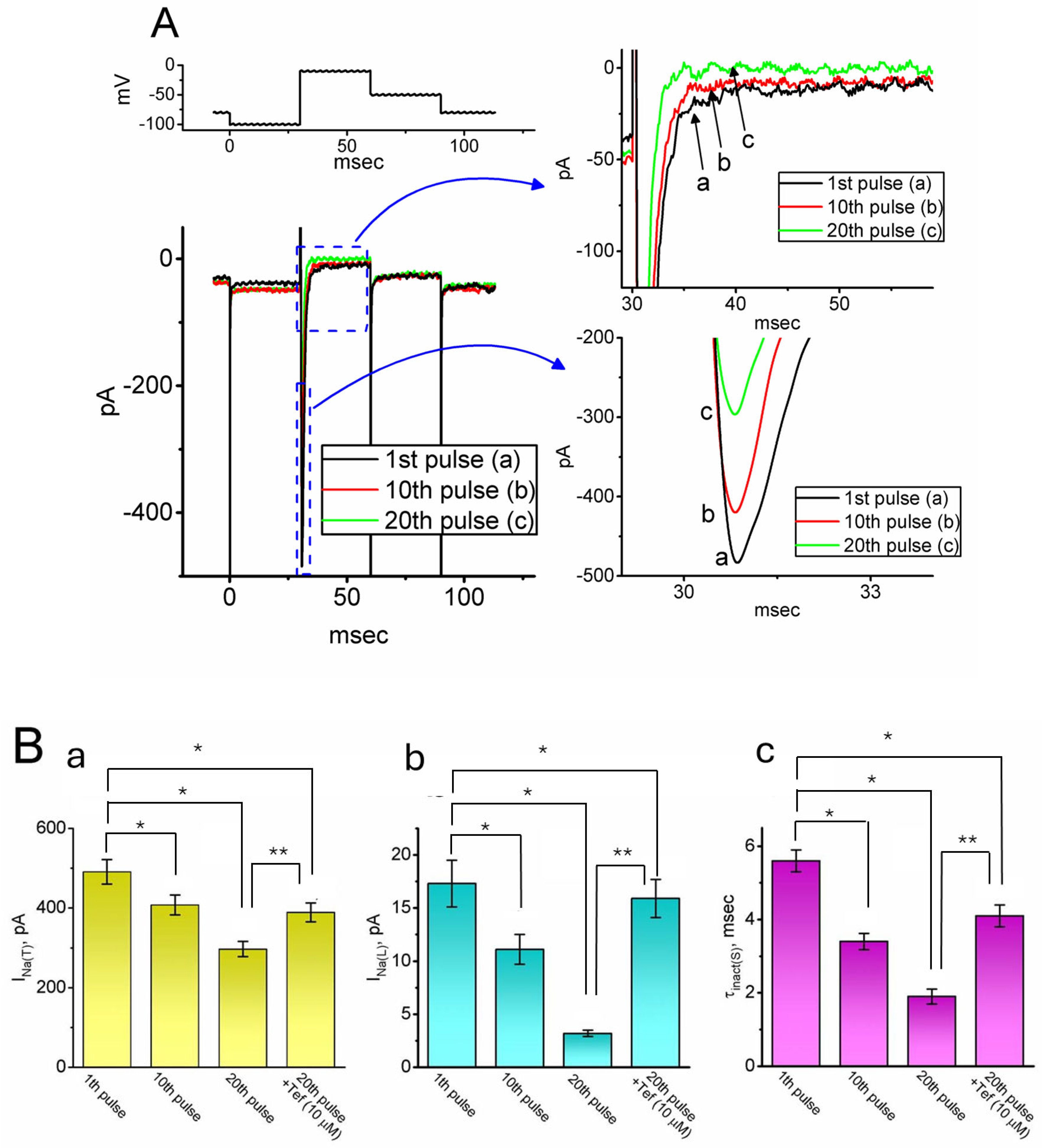
2.2. Effect of Intracellular Dialysis with QX-314 on the Amplitude of INa
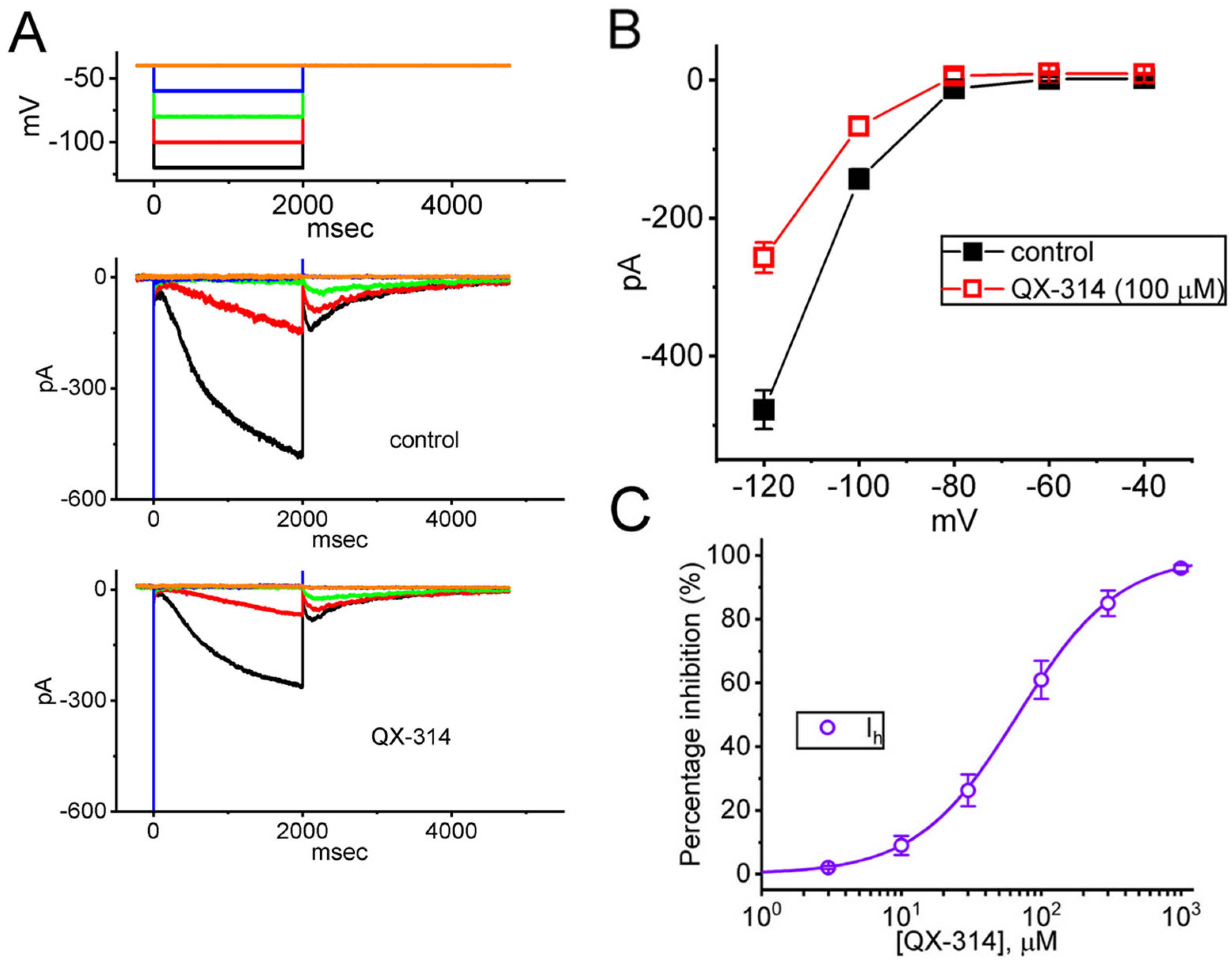
2.3. Inhibitory Effect of QX-314 on the Current Versus Voltage (I-V) Relationship of Hyperpolarization-Activated Cation Currents (Ih) in GH3 Cells
2.4. Impact of Intracellular Dialysis with QX-314 on the Ih Amplitude
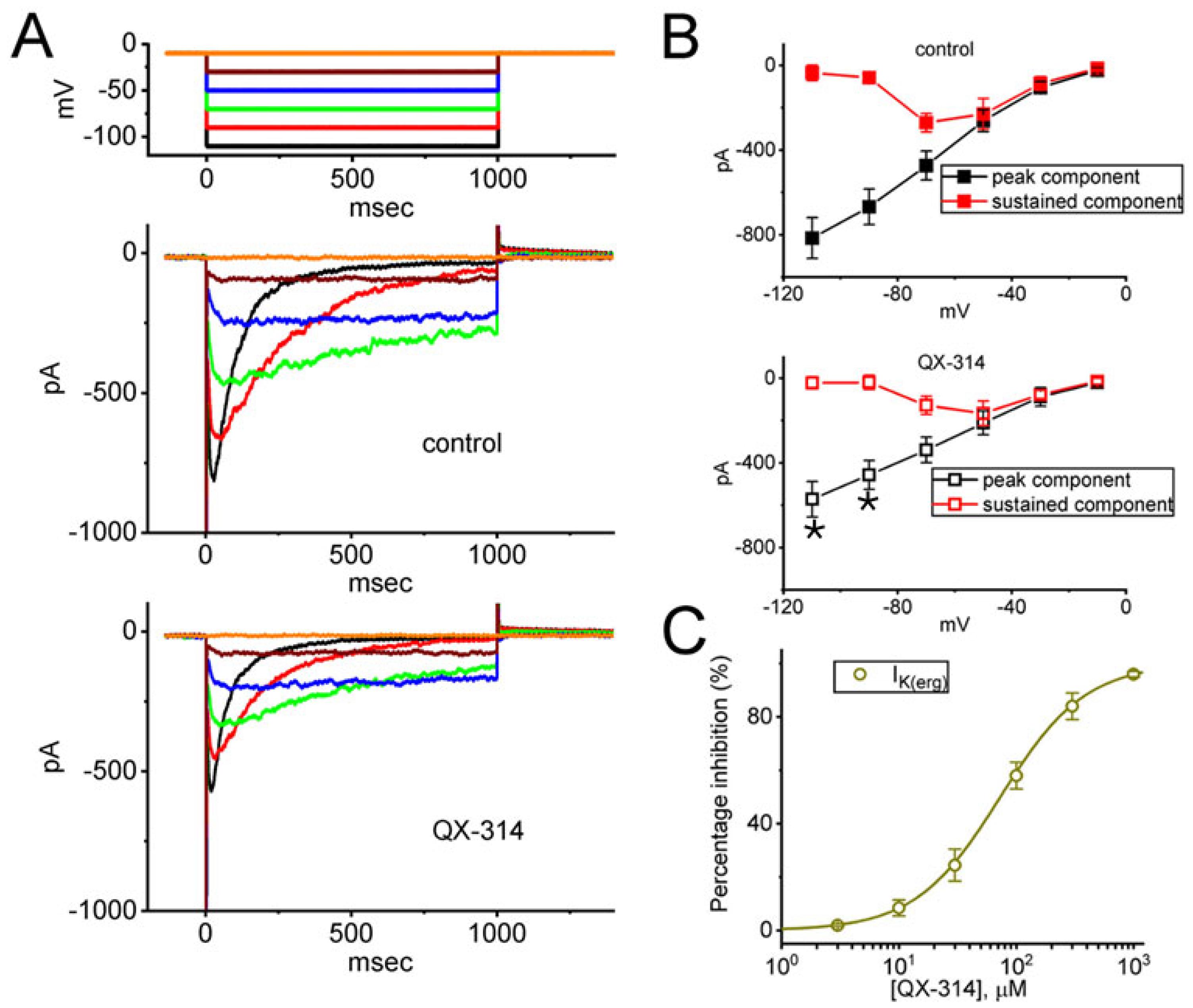
2.5. Suppressive Effect of QX-314 on the Amplitude of erg-Mediated K+ Current (IK(erg)) in GH3 Cells
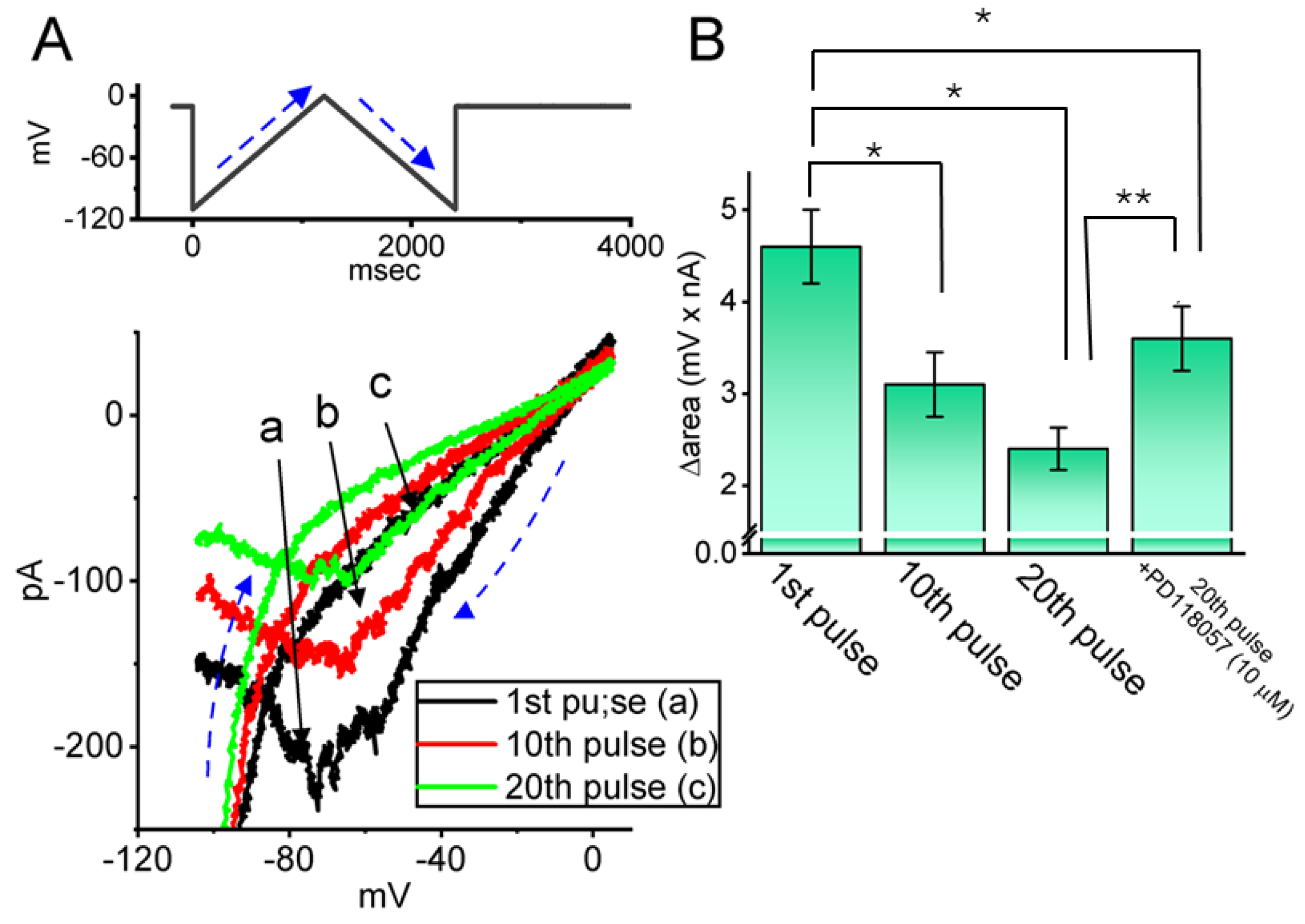
2.6. Impact of Intracellular Dialysis with QX-314 on Hys(V) of IK(erg) Activated by the Upright Isosceles-Triangular Ramp Voltage (Vramp)
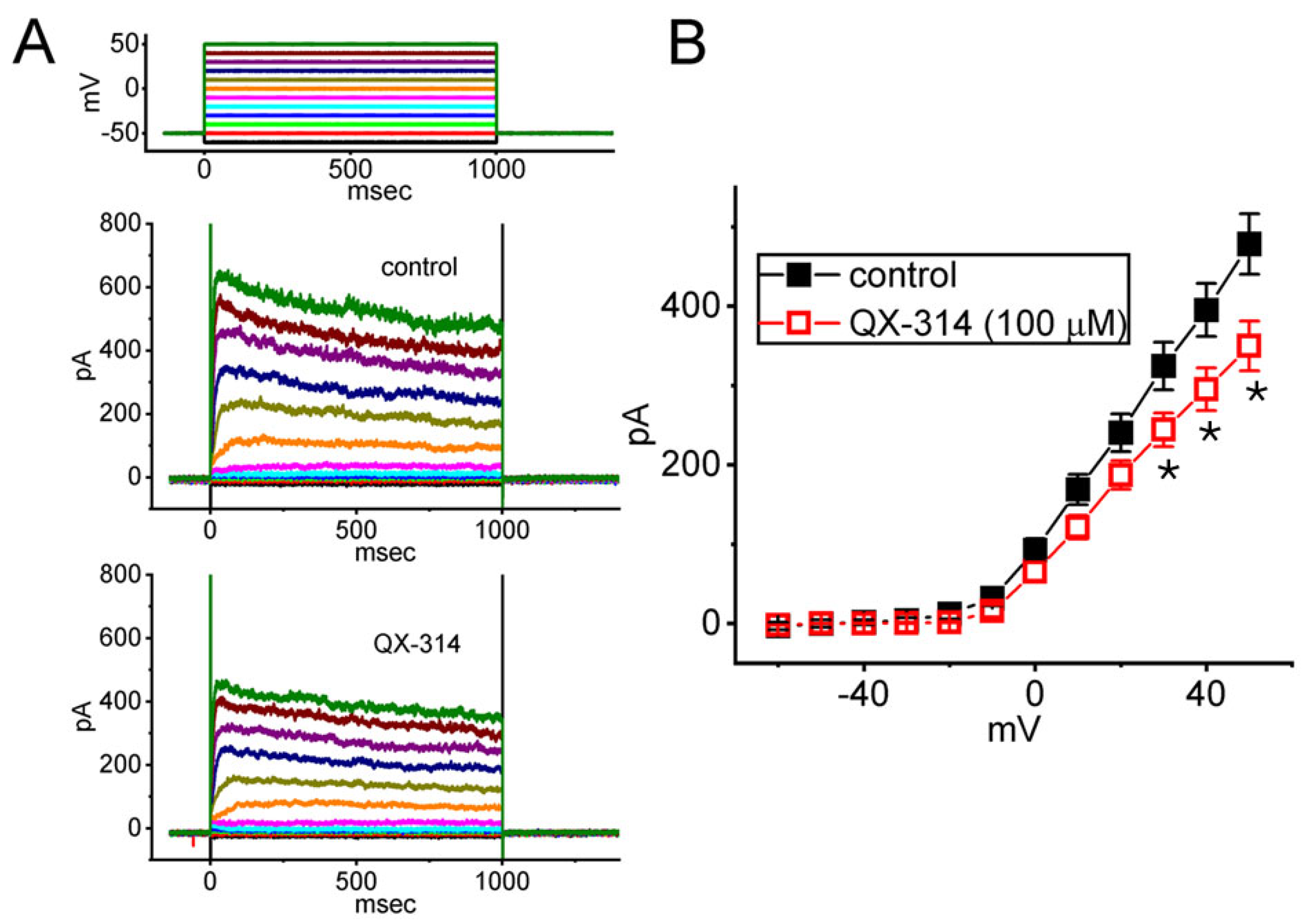
2.7. Mild Inhibitory Effect of QX-314 on Averaged I-V Relationship of Delayed Rectifier K+ Current (IK(DR)) Recorded from GH3 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals, Drugs, Reagents, and Solutions Used in This Study
4.2. Cell Preparation
4.3. Electrophysiological Assessment via Patch-Clamp Current Recording
4.4. Whole-Cell Current Recordings
4.5. Data Analyses
4.6. Curve-Fitting Procedures and Statistical Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
References
- Agnew, W.S.; Cooper, E.C.; Shenkel, S.; Correa, A.M.; James, W.M.; Ukomadu, C.; Tomiko, S.A. Voltage-sensitive sodium channels: Agents that perturb inactivation gating. Ann. N. Y. Acad. Sci. 1991, 625, 200–223. [Google Scholar] [CrossRef]
- Barravecchia, I.; Demontis, G.C. Hcn1 channels: A versatile tool for signal processing by primary sensory neurons. Prog. Biophys. Mol. Biol. 2021, 166, 133–146. [Google Scholar] [CrossRef]
- Puopolo, M.; Binshtok, A.M.; Yao, G.L.; Oh, S.B.; Woolf, C.J.; Bean, B.P. Permeation and block of trpv1 channels by the cationic lidocaine derivative qx-314. J. Neurophysiol. 2013, 109, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Blake, K.J.; Baral, P.; Voisin, T.; Lubkin, A.; Pinho-Ribeiro, F.A.; Adams, K.L.; Roberson, D.P.; Ma, Y.C.; Otto, M.; Woolf, C.J.; et al. Staphylococcus aureus produces pain through pore-forming toxins and neuronal trpv1 that is silenced by qx-314. Nat. Commun. 2018, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Ongun, S.; Sarkisian, A.; McKemy, D.D. Selective cold pain inhibition by targeted block of trpm8-expressing neurons with quaternary lidocaine derivative qx-314. Commun. Biol. 2018, 1, 53. [Google Scholar] [CrossRef] [PubMed]
- Tochitsky, I.; Jo, S.; Andrews, N.; Kotoda, M.; Doyle, B.; Shim, J.; Talbot, S.; Roberson, D.; Lee, J.; Haste, L.; et al. Inhibition of inflammatory pain and cough by a novel charged sodium channel blocker. Br. J. Pharmacol. 2021, 178, 3905–3923. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Liu, J.; Zhang, W. Quaternary lidocaine derivatives: Past, present, and future. Drug Des. Dev. Ther. 2021, 15, 195–207. [Google Scholar] [CrossRef]
- Klasfauseweh, T.; Israel, M.R.; Ragnarsson, L.; Cox, J.J.; Durek, T.; Carter, D.A.; Leffler, A.; Vetter, I.; Deuis, J.R. Low potency inhibition of na(v)1.7 by externally applied qx-314 via a depolarizing shift in the voltage-dependence of activation. Eur. J. Pharmacol. 2022, 925, 175013. [Google Scholar] [CrossRef]
- Rocchetti, M.; Armato, A.; Cavalieri, B.; Micheletti, M.; Zaza, A. Lidocaine inhibition of the hyperpolarization-activated current (i(f)) in sinoatrial myocytes. J. Cardiovasc. Pharmacol. 1999, 34, 434–439. [Google Scholar] [CrossRef]
- Bischoff, U.; Bräu, M.E.; Vogel, W.; Hempelmann, G.; Olschewski, A. Local anaesthetics block hyperpolarization-activated inward current in rat small dorsal root ganglion neurones. Br. J. Pharmacol. 2003, 139, 1273–1280. [Google Scholar] [CrossRef]
- Putrenko, I.; Schwarz, S.K. Lidocaine blocks the hyperpolarization-activated mixed cation current, i(h), in rat thalamocortical neurons. Anesthesiology 2011, 115, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Putrenko, I.; Yip, R.; Schwarz, S.K.W.; Accili, E.A. Cation and voltage dependence of lidocaine inhibition of the hyperpolarization-activated cyclic nucleotide-gated hcn1 channel. Sci. Rep. 2017, 7, 1281. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.K.; Macleod, B.A.; Ries, C.R.; Schwarz, S.K. The quaternary lidocaine derivative, qx-314, produces long-lasting local anesthesia in animal models in vivo. Anesthesiology 2007, 107, 305–311. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Yin, Q.; Yang, L.; Liu, J.; Zhang, W. Qx-oh, a qx-314 derivative agent, produces long-acting local anesthesia in rats. Eur. J. Pharm. Sci. 2017, 105, 212–218. [Google Scholar] [CrossRef]
- Yoon, J.H.; Son, J.Y.; Kim, M.J.; Kang, S.H.; Ju, J.S.; Bae, Y.C.; Ahn, D.K. Preemptive application of qx-314 attenuates trigeminal neuropathic mechanical allodynia in rats. Korean J. Physiol. Pharmacol. 2018, 22, 331–341. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, X.; Yu, S. Qx-314 inhibits acid-induced activation of esophageal nociceptive c fiber neurons. Neurogastroenterol. Motil. 2019, 31, e13543. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A. Forty years of sodium channels: Structure, function, pharmacology, and epilepsy. Neurochem. Res. 2017, 42, 2495–2504. [Google Scholar] [CrossRef]
- Guérineau, N.C.; Monteil, A.; Lory, P. Sodium background currents in endocrine/neuroendocrine cells: Towards unraveling channel identity and contribution in hormone secretion. Front. Neuroendocrinol. 2021, 63, 100947. [Google Scholar] [CrossRef]
- Peters, C.H.; Liu, P.W.; Morotti, S.; Gantz, S.C.; Grandi, E.; Bean, B.P.; Proenza, C. Bidirectional flow of the funny current (i(f)) during the pacemaking cycle in murine sinoatrial node myocytes. Proc. Natl. Acad. Sci. USA 2021, 118, e2104668118. [Google Scholar] [CrossRef]
- Wu, S.N.; Chen, B.S.; Hsu, T.I.; Peng, H.; Wu, Y.H.; Lo, Y.C. Analytical studies of rapidly inactivating and noninactivating sodium currents in differentiated ng108-15 neuronal cells. J. Theor. Biol. 2009, 259, 828–836. [Google Scholar] [CrossRef]
- Stueber, T.; Eberhardt, M.J.; Hadamitzky, C.; Jangra, A.; Schenk, S.; Dick, F.; Stoetzer, C.; Kistner, K.; Reeh, P.W.; Binshtok, A.M.; et al. Quaternary lidocaine derivative qx-314 activates and permeates human trpv1 and trpa1 to produce inhibition of sodium channels and cytotoxicity. Anesthesiology 2016, 124, 1153–1165. [Google Scholar] [CrossRef]
- Hwang, S.M.; Lee, K.; Im, S.T.; Go, E.J.; Kim, Y.H.; Park, C.K. Co-application of eugenol and qx-314 elicits the prolonged blockade of voltage-gated sodium channels in nociceptive trigeminal ganglion neurons. Biomolecules 2020, 10, 1513. [Google Scholar] [CrossRef] [PubMed]
- Kretschmannova, K.; Gonzalez-Iglesias, A.E.; Tomić, M.; Stojilkovic, S.S. Dependence of hyperpolarisation-activated cyclic nucleotide-gated channel activity on basal cyclic adenosine monophosphate production in spontaneously firing gh3 cells. J. Neuroendocrinol. 2006, 18, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Stojilkovic, S.S.; Tabak, J.; Bertram, R. Ion channels and signaling in the pituitary gland. Endocr. Rev. 2010, 31, 845–915. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Wang, S.J.; Cui, J.; He, W.J.; Ruan, H.Z. The role of hcn channels within the periaqueductal gray in neuropathic pain. Brain Res. 2013, 1500, 36–44. [Google Scholar] [CrossRef]
- Depuydt, A.S.; Peigneur, S.; Tytgat, J. Review: Hcn channels in the heart. Curr. Cardiol. Rev. 2022, 18, e040222200836. [Google Scholar] [CrossRef]
- Toyoda, H.; Won, J.; Kim, W.; Kim, H.; Davy, O.; Saito, M.; Kim, D.; Tanaka, T.; Kang, Y.; Oh, S.B. The nature of noradrenergic volume transmission from locus coeruleus to brainstem mesencephalic trigeminal sensory neurons. Front. Cell. Neurosci. 2022, 16, 841239. [Google Scholar] [CrossRef]
- Zaborska, K.E.; Jordan, K.L.; Thorson, A.S.; Dadi, P.K.; Schaub, C.M.; Nakhe, A.Y.; Dickerson, M.T.; Lynch, J.C.; Weiss, A.J.; Dobson, J.R.; et al. Liraglutide increases islet ca(2+) oscillation frequency and insulin secretion by activating hyperpolarization-activated cyclic nucleotide-gated channels. Diabetes Obes. Metab. 2022, 24, 1741–1752. [Google Scholar] [CrossRef]
- Chang, W.T.; Ragazzi, E.; Liu, P.Y.; Wu, S.N. Effective block by pirfenidone, an antifibrotic pyridone compound (5-methyl-1-phenylpyridin-2[h-1]-one), on hyperpolarization-activated cation current: An additional but distinctive target. Eur. J. Pharmacol. 2020, 882, 173237. [Google Scholar] [CrossRef]
- Calejo, A.I.; Jorgačevski, J.; Rituper, B.; Guček, A.; Pereira, P.M.; Santos, M.A.; Potokar, M.; Vardjan, N.; Kreft, M.; Gonçalves, P.P.; et al. Hyperpolarization-activated cyclic nucleotide-gated channels and camp-dependent modulation of exocytosis in cultured rat lactotrophs. J. Neurosci. 2014, 34, 15638–15647. [Google Scholar] [CrossRef]
- Spinelli, V.; Sartiani, L.; Mugelli, A.; Romanelli, M.N.; Cerbai, E. Hyperpolarization-activated cyclic-nucleotide-gated channels: Pathophysiological, developmental, and pharmacological insights into their function in cellular excitability. Can. J. Physiol. Pharmacol. 2018, 96, 977–984. [Google Scholar] [CrossRef]
- Chang, W.T.; Gao, Z.H.; Li, S.W.; Liu, P.Y.; Lo, Y.C.; Wu, S.N. Characterization in dual activation by oxaliplatin, a platinum-based chemotherapeutic agent of hyperpolarization-activated cation and electroporation-induced currents. Int. J. Mol. Sci. 2020, 21, 396. [Google Scholar] [CrossRef]
- Chuang, C.W.; Chang, K.P.; Cho, H.Y.; Chuang, T.H.; Yu, M.C.; Wu, C.L.; Wu, S.N. Characterization of inhibitory capability on hyperpolarization-activated cation current caused by lutein (β,ε-carotene-3,3′-diol), a dietary xanthophyll carotenoid. Int. J. Mol. Sci. 2022, 23, 7186. [Google Scholar] [CrossRef] [PubMed]
- Oniani, T.; Vinnenberg, L.; Chaudhary, R.; Schreiber, J.A.; Riske, K.; Williams, B.; Pape, H.C.; White, J.A.; Junker, A.; Seebohm, G.; et al. Effects of axonal demyelination, inflammatory cytokines and divalent cation chelators on thalamic hcn channels and oscillatory bursting. Int. J. Mol. Sci. 2022, 23, 6285. [Google Scholar] [CrossRef]
- Yavuz, M.; Aydın, B.; Çarçak, N.; Onat, F. Decreased hyperpolarization-activated cyclic nucleotide-gated channel 2 activity in a rat model of absence epilepsy and the effect of zd7288, an ih inhibitor, on the spike-and-wave discharges. Pharmacology 2022, 107, 227–234. [Google Scholar] [CrossRef]
- Deng, W.S.; Jiang, Y.X.; Han, X.H.; Xue, Y.; Wang, H.; Sun, P.; Chen, L. Hcn channels modulate the activity of the subthalamic nucleus in vivo. J. Mol. Neurosci. 2015, 55, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Chiappe, F.; Frenkel, L.; Colque, C.C.; Ricciuti, A.; Hahm, B.; Cerredo, K.; Muraro, N.I.; Ceriani, M.F. High-frequency neuronal bursting is essential for circadian and sleep behaviors in drosophila. J. Neurosci. 2021, 41, 689–710. [Google Scholar] [CrossRef]
- Liu, J.J.; Eyring, K.W.; König, G.M.; Kostenis, E.; Tsien, R.W. Oxytocin-modulated ion channel ensemble controls depolarization, integration and burst firing in ca2 pyramidal neurons. J. Neurosci. 2022, 42, 7707–7720. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Liu, N.; Lv, M.; Ma, L.; Peng, H.; Peng, S.; Liu, T. Lidocaine inhibits hcn currents in rat spinal substantia gelatinosa neurons. Anesth. Analg. 2016, 122, 1048–1059. [Google Scholar] [CrossRef]
- Zhao, W.; Liang, P.; Liu, J.; Li, H.; Liao, D.; Chen, X.; Li, Q.; Zhou, C. Capsazepine prolongation of the duration of lidocaine block of sensory transmission in mice may be mediated by modulation of hcn channel currents. PeerJ 2019, 7, e7111. [Google Scholar] [CrossRef]
- Perkins, K.L.; Wong, R.K. Intracellular qx-314 blocks the hyperpolarization-activated inward current iq in hippocampal ca1 pyramidal cells. J. Neurophysiol. 1995, 73, 911–915. [Google Scholar] [CrossRef]
- Kilb, W.; Luhmann, H.J. Characterization of a hyperpolarization-activated inward current in cajal-retzius cells in rat neonatal neocortex. J. Neurophysiol. 2000, 84, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Martinson, A.S.; van Rossum, D.B.; Diatta, F.H.; Layden, M.J.; Rhodes, S.A.; Martindale, M.Q.; Jegla, T. Functional evolution of erg potassium channel gating reveals an ancient origin for ikr. Proc. Natl. Acad. Sci. USA 2014, 111, 5712–5717. [Google Scholar] [CrossRef]
- Cho, H.Y.; Chuang, T.H.; Wu, S.N. Effective perturbations on the amplitude and hysteresis of erg-mediated potassium current caused by 1-octylnonyl 8-[(2-hydroxyethyl)[6-oxo-6(undecyloxy)hexyl]amino]-octanoate (sm-102), a cationic lipid. Biomedicines 2021, 9, 1367. [Google Scholar] [CrossRef]
- Chen, L.; Cho, H.Y.; Chuang, T.H.; Ke, T.L.; Wu, S.N. The effectiveness of isoplumbagin and plumbagin in regulating amplitude, gating kinetics, and voltage-dependent hysteresis of erg-mediated k(+) currents. Biomedicines 2022, 10, 780. [Google Scholar] [CrossRef]
- Cui, E.D.; Estright, A.W.; Pressler, R.T.; Strowbridge, B.W. Spike afterhyperpolarizations govern persistent firing dynamics in rat neocortical and hippocampal pyramidal cells. J. Neurosci. 2022, 42, 7690–7706. [Google Scholar] [CrossRef]
- Hasan, S.; Delicata, F.; Guasti, L.; Duranti, C.; Haidar, F.M.; Arcangeli, A.; Imbrici, P.; Pessia, M.; Valentino, M.; D’Adamo, M.C. Locus coeruleus neurons’ firing pattern is regulated by erg voltage-gated k(+) channels. Int. J. Mol. Sci. 2022, 23, 15334. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Lin, J.F.; Yu, M.C.; Wu, S.N.; Wu, C.L.; Cho, H.Y. Characterization in potent modulation on voltage-gated na(+) current exerted by deltamethrin, a pyrethroid insecticide. Int. J. Mol. Sci. 2022, 23, 14733. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Soderlund, D.M. Actions of tefluthrin on rat na(v)1.7 voltage-gated sodium channels expressed in xenopus oocytes. Pestic. Biochem. Physiol. 2011, 101, 21–26. [Google Scholar] [CrossRef]
- Wu, S.N.; Wu, C.L.; Cho, H.Y.; Chiang, C.W. Effective perturbations by small-molecule modulators on voltage-dependent hysteresis of transmembrane ionic currents. Int. J. Mol. Sci. 2022, 23, 9453. [Google Scholar] [CrossRef]
- Yeung, S.Y.; Greenwood, I.A. Pharmacological and biophysical isolation of k+ currents encoded by ether-à-go-go-related genes in murine hepatic portal vein smooth muscle cells. Am. J. Physiol. Cell Physiol. 2007, 292, C468–C476. [Google Scholar] [CrossRef] [PubMed]
- Olschewski, A.; Bräu, M.E.; Olschewski, H.; Hempelmann, G.; Vogel, W. Atp-dependent potassium channel in rat cardiomyocytes is blocked by lidocaine. Possible impact on the antiarrhythmic action of lidocaine. Circulation 1996, 93, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Trellakis, S.; Benzenberg, D.; Urban, B.W.; Friederich, P. Differential lidocaine sensitivity of human voltage-gated potassium channels relevant to the auditory system. Otol. Neurotol. 2006, 27, 117–123. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.C.-F.; Hsiao, H.-T.; Wu, S.-N. Modulation of INa, Ih, and IK(erg) by Extracellular or Intracellular QX-314 (N-(2,6-dimethylphenylcarbamoylmethyl) triethylammonium bromide) in Pituitary Tumor Cells. Int. J. Mol. Sci. 2025, 26, 8469. https://doi.org/10.3390/ijms26178469
Wang JC-F, Hsiao H-T, Wu S-N. Modulation of INa, Ih, and IK(erg) by Extracellular or Intracellular QX-314 (N-(2,6-dimethylphenylcarbamoylmethyl) triethylammonium bromide) in Pituitary Tumor Cells. International Journal of Molecular Sciences. 2025; 26(17):8469. https://doi.org/10.3390/ijms26178469
Chicago/Turabian StyleWang, Jeffrey Chi-Fei, Hung-Tsung Hsiao, and Sheng-Nan Wu. 2025. "Modulation of INa, Ih, and IK(erg) by Extracellular or Intracellular QX-314 (N-(2,6-dimethylphenylcarbamoylmethyl) triethylammonium bromide) in Pituitary Tumor Cells" International Journal of Molecular Sciences 26, no. 17: 8469. https://doi.org/10.3390/ijms26178469
APA StyleWang, J. C.-F., Hsiao, H.-T., & Wu, S.-N. (2025). Modulation of INa, Ih, and IK(erg) by Extracellular or Intracellular QX-314 (N-(2,6-dimethylphenylcarbamoylmethyl) triethylammonium bromide) in Pituitary Tumor Cells. International Journal of Molecular Sciences, 26(17), 8469. https://doi.org/10.3390/ijms26178469




