Probiotics: A Little Help for Enteral Nutritional Therapy in Critically Ill Adults
Abstract
1. Introduction
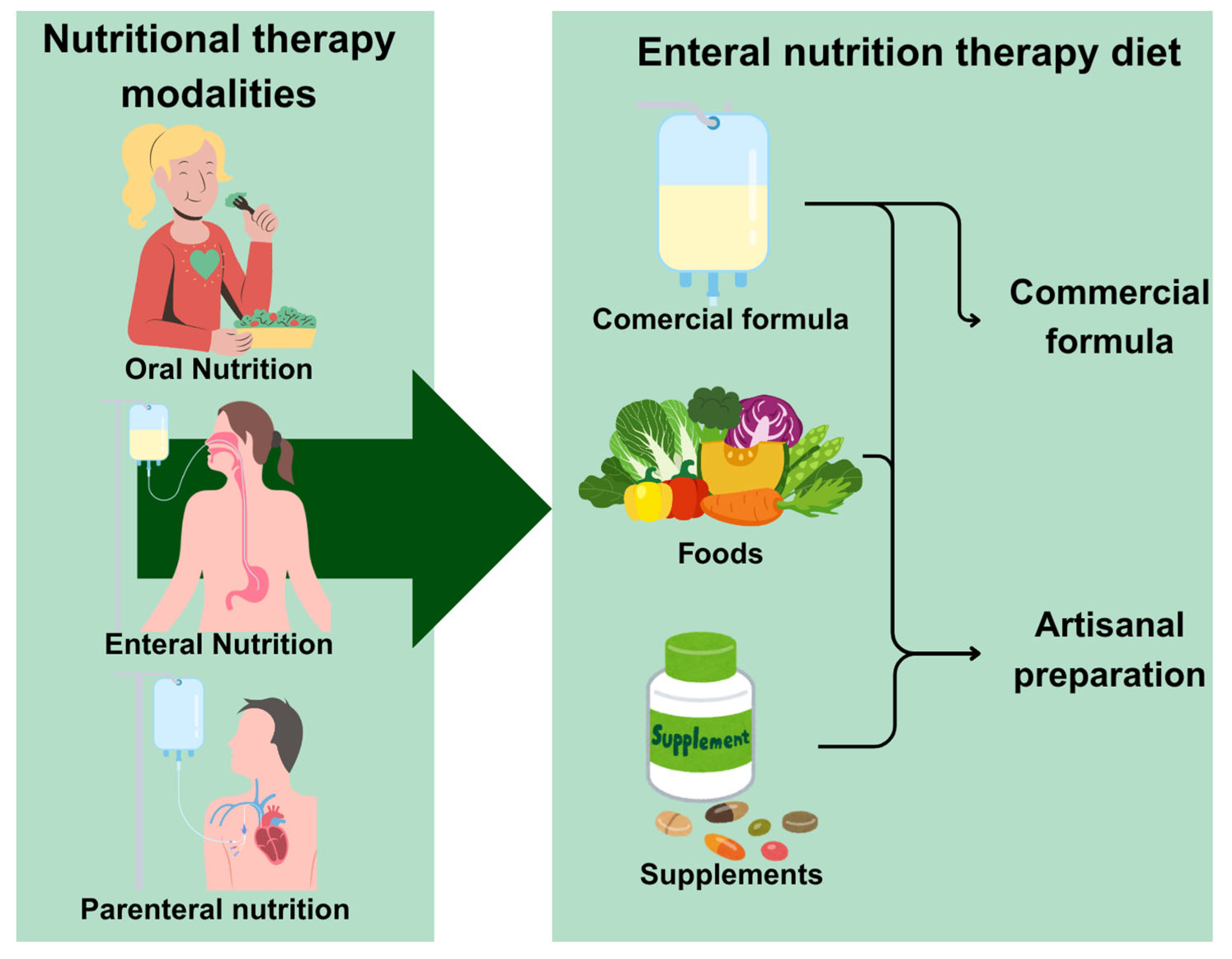
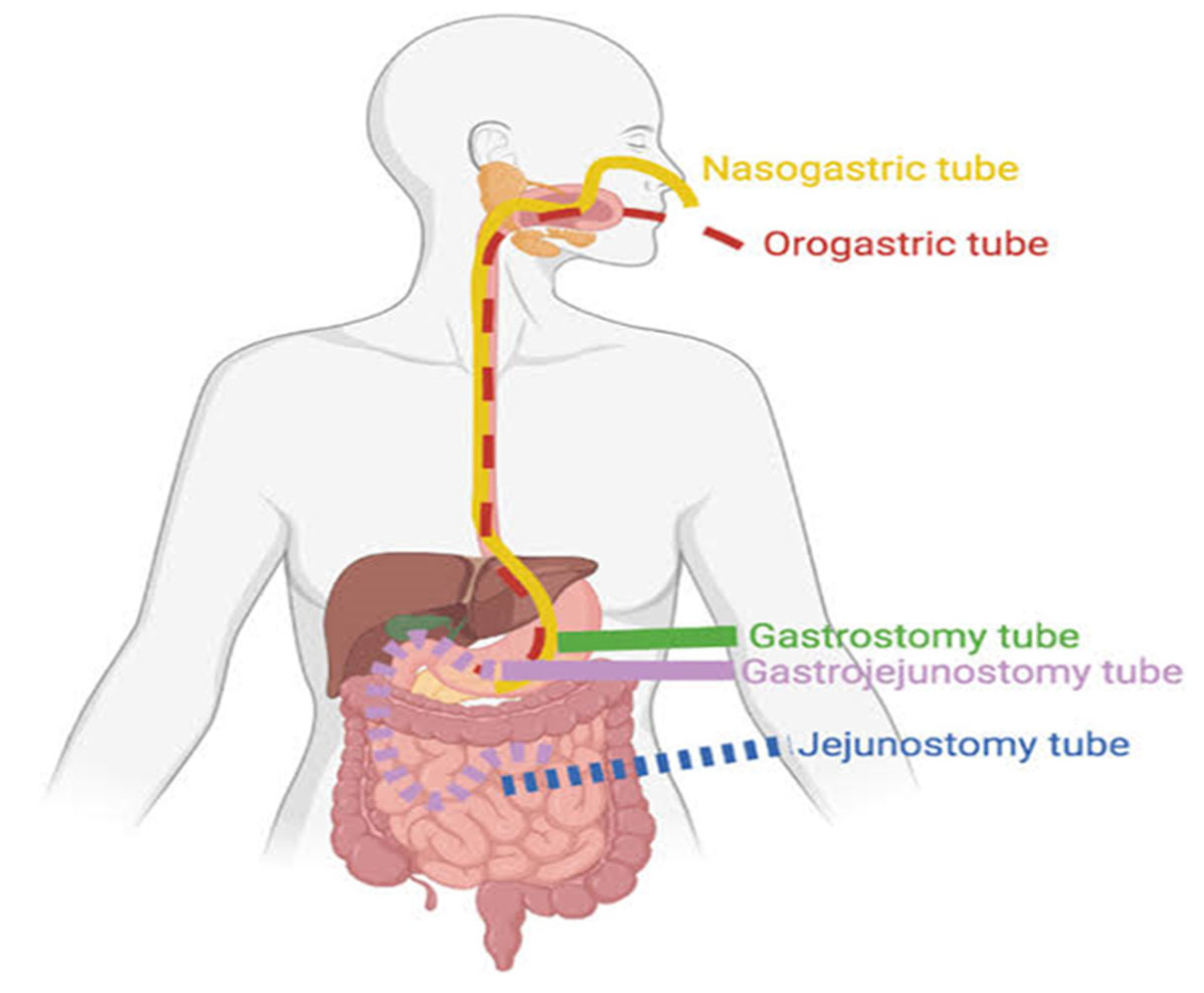
2. Enteral Nutritional Therapy (ENT)
3. Probiotics: Definition, Characteristics, and Potential Health Effects
4. Probiotics as a Supplement in ENT: The Adult Experience
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waitzberg, D.L.; Plopper, C.; Terra, R.M. Access routes for nutritional therapy. World J. Surg. 2000, 24, 1468–1476. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Austin, P.; Boeykens, K.; Chourdakis, M.; Cuerda, C.; Jonkers-Schuitema, C.; Lichota, M.; Nyulasi, I.; Schneider, S.M.; Stanga, Z. ESPEN guideline on home enteral nutrition. Clin. Nutr. 2020, 39, 5–22. [Google Scholar] [CrossRef]
- Santos, D.C.d.; Ataide, C.D.G.; Mota da Costa, N.; Oliveira Junior, V.P.d.; Egea, M.B. Blenderized formulations in home enteral nutrition: A narrative review about challenges in nutritional security and food safety. Nutr. Rev. 2022, 80, 1580–1598. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Zommiti, M.; Feuilloley, M.G.J.; Connil, N. Update of Probiotics in Human World: A Nonstop Source of Benefactions till the End of Time. Microorganisms 2020, 8, 1907. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Ding, B.; Feng, C.; Yin, S.; Zhang, T.; Qi, X.; Lv, H.; Guo, X.; Dong, K.; Zhu, Y. Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J. Affect. Disord. 2017, 207, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Mundi, M.S.; Patel, J.; McClave, S.A.; Hurt, R.T. Current perspective for tube feeding in the elderly: From identifying malnutrition to providing of enteral nutrition. Clin. Interv. Aging 2018, 13, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Reber, E.; Gomes, F.; Vasiloglou, M.F.; Schuetz, P.; Stanga, Z. Nutritional risk screening and assessment. J. Clin. Med. 2019, 8, 1065. [Google Scholar] [CrossRef]
- Norton, C. Oxford Handbook of Gastrointestinal Nursing; Oxford University Press: New York, NY, USA, 2008. [Google Scholar]
- Toledo, D.; Castro, M. Assessment of the current outlook of nutritional therapy within the intensive care unit. BRASPEN J. 2017, 32, 297–301. [Google Scholar]
- Fugazza, A.; Capogreco, A.; Cappello, A.; Nicoletti, R.; Da Rio, L.; Galtieri, P.A.; Maselli, R.; Carrara, S.; Pellegatta, G.; Spadaccini, M.; et al. Percutaneous endoscopic gastrostomy and jejunostomy: Indications and techniques. World J. Gastrointest. Endosc. 2022, 14, 250–266. [Google Scholar] [CrossRef]
- Borghi, R.; Araujo, T.; Vieira, R.; Souza, T.d.; Waitzberg, D. ILSI Task Force on enteral nutrition; estimated composition and costs of blenderized diets. Nutr. Hosp. 2013, 28, 2033–2038. [Google Scholar]
- White, S.; Brereton, L. Examining the role of patient values in decisions about long-term enteral feeding: A qualitative study. Clin. Nutr. 2018, 37, 1046–1052. [Google Scholar] [CrossRef]
- Mezzomo, T.; Fiori, L.; de Oliveira Reis, L.; Schieferdecker, M. Nutritional composition and cost of home-prepared enteral tube feeding. Clin. Nutr. ESPEN 2021, 42, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Afonso, M.G.; Arroyo, L.H.; Gastaldi, A.A.; Assalin, A.C.B.; Yamamura, M.; Girão, F.B. Teaching and learning strategies in Home Enteral Nutritional Therapy: Knowledge gains perceived by caregivers. Rev. Lat.-Am. Enferm. 2023, 31, e3888. [Google Scholar]
- Mezzomo, T.; Lemos, K.; Zaparolli, M.; Fiori, L.; Schieferdeker, M. Glycemic index and glycemic load of standard enteral homemade diets for domiciliary use. CABI Databases 2018, 38, 168–173. [Google Scholar]
- Orlandoni, P.; Jukic Peladic, N.; Amoruso, A.; Pane, M.; Di Rosa, M.; Vedruccio, J.; Santini, F. Safety and efficacy of probiotic supplementation in reducing the incidence of infections and modulating inflammation in the elderly with feeding tubes: A pilot, double-blind, placebo-controlled study, “IntegPRO”. Nutrients 2021, 13, 391. [Google Scholar] [CrossRef]
- Lim, M.; Yong, B.; Mar, M.; Ang, S.; Chan, M.; Lam, M.; Chong, N.; Lopez, V. Caring for patients on home enteral nutrition: Reported complications by home carers and perspectives of community nurses. J. Clin. Nurs. 2018, 27, 2825–2835. [Google Scholar] [CrossRef]
- Sabaté, E. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Sharifi-Rad, J.; Rodrigues, C.F.; Stojanović-Radić, Z.; Dimitrijević, M.; Aleksić, A.; Neffe-Skocińska, K.; Zielińska, D.; Kołożyn-Krajewska, D.; Salehi, B.; Milton Prabu, S. Probiotics: Versatile bioactive components in promoting human health. Medicina 2020, 56, 433. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Szychowiak, P.; Villageois-Tran, K.; Patrier, J.; Timsit, J.-F.; Ruppé, É. The role of the microbiota in the management of intensive care patients. Ann. Intensive Care 2022, 12, 3. [Google Scholar] [CrossRef]
- Nakov, R.; Segal, J.P.; Settanni, C.R.; Bibbò, S.; Gasbarrini, A.; Cammarota, G.; Ianiro, G. Microbiome: What intensivists should know. Minerva Anestesiol. 2020, 86, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Hussein, L.A. Novel prebiotics and next-generation probiotics: Opportunities and challenges. In Functional Foods and Nutraceuticals in Metabolic and Non-Communicable Diseases; Singh, R.B., Watanabe, S., Isaza, A.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 431–457. [Google Scholar]
- Bock, P.M.; Martins, A.F.; Schaan, B.D. Understanding how pre-and probiotics affect the gut microbiome and metabolic health. Am. J. Physiol.-Endocrinol. Metab. 2024, 327, E89–E102. [Google Scholar] [CrossRef] [PubMed]
- McAleer, J.P.; Kolls, J.K. Contributions of the intestinal microbiome in lung immunity. Eur. J. Immunol. 2018, 48, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, A.R.; Kullberg, R.F.; Wiersinga, W.J. Probiotics in the intensive care unit. Antibiotics 2022, 11, 217. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarpellini, E.; Paravati, M.R.; Scarlata, G.G.M.; Boccuto, L.; Tilocca, B.; Roncada, P.; Luzza, F. Gut microbiota and critically ill patients: Immunity and its modulation via probiotics and immunonutrition. Nutrients 2023, 15, 3569. [Google Scholar] [CrossRef]
- Andersen, S.; Banks, M.; Bauer, J. Nutrition support and the gastrointestinal microbiota: A systematic review. J. Acad. Nutr. Diet. 2020, 120, 1498–1516. [Google Scholar] [CrossRef]
- Tian, X.; Xia, G.; Zhang, M.; Tan, C.; Yang, M.; Wang, H.; Song, X.; Chai, S.; Yin, J.; Song, W. Effect of enteral nutrition on the intestinal microbiome and risk of death in ischemic stroke patients. J. Parenter. Enter. Nutr. 2022, 46, 1847–1858. [Google Scholar] [CrossRef]
- Xu, W.; Zhong, M.; Pan, T.; Qu, H.; Chen, E. Gut microbiota and enteral nutrition tolerance in non-abdominal infection septic ICU patients: An observational study. Nutrients 2022, 14, 5342. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.; Mazmanian, S.K. The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Garcez, F.B.; Garcia de Alencar, J.C.; Fernandez, S.S.M.; Avelino-Silva, V.I.; Sabino, E.C.; Martins, R.C.R.; Franco, L.A.M.; Lima Ribeiro, S.M.; Possolo de Souza, H.; Avelino-Silva, T.J. Association between gut microbiota and delirium in acutely ill older adults. J. Gerontol. Ser. A 2023, 78, 1320–1327. [Google Scholar] [CrossRef]
- Gungabissoon, U.; Hacquoil, K.; Bains, C.; Irizarry, M.; Dukes, G.; Williamson, R.; Deane, A.M.; Heyland, D.K. Prevalence, risk factors, clinical consequences, and treatment of enteral feed intolerance during critical illness. J. Parenter. Enter. Nutr. 2015, 39, 441–448. [Google Scholar] [CrossRef]
- Heyland, D.K.; Ortiz, A.; Stoppe, C.; Patel, J.J.; Yeh, D.D.; Dukes, G.; Chen, Y.J.; Almansa, C.; Day, A.G. Incidence, risk factors, and clinical consequence of enteral feeding intolerance in the mechanically ventilated critically ill: An analysis of a multicenter, multiyear database. Crit. Care Med. 2021, 49, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Zanza, C.; Romenskaya, T.; Thangathurai, D.; Ojetti, V.; Saviano, A.; Abenavoli, L.; Robba, C.; Cammarota, G.; Franceschi, F.; Piccioni, A. Microbiome in critical care: An unconventional and unknown ally. Curr. Med. Chem. 2022, 29, 3179–3188. [Google Scholar] [CrossRef]
- Ma, L.; Tu, H.; Chen, T. Postbiotics in human health: A narrative review. Nutrients 2023, 15, 291. [Google Scholar] [CrossRef]
- Rafique, N.; Jan, S.Y.; Dar, A.H.; Dash, K.K.; Sarkar, A.; Shams, R.; Pandey, V.K.; Khan, S.A.; Amin, Q.A.; Hussain, S.Z. Promising bioactivities of postbiotics: A comprehensive review. J. Agric. Food Res. 2023, 14, 100708. [Google Scholar] [CrossRef]
- Martín, R.; Langella, P. Emerging health concepts in the probiotics field: Streamlining the definitions. Front. Microbiol. 2019, 10, 1047. [Google Scholar] [CrossRef] [PubMed]
- Katkowska, M.; Garbacz, K.; Kusiak, A. Probiotics: Should all patients take them? Microorganisms 2021, 9, 2620. [Google Scholar] [CrossRef]
- Shimizu, K.; Ojima, M.; Ogura, H. Gut microbiota and probiotics/synbiotics for modulation of immunity in critically ill patients. Nutrients 2021, 13, 2439. [Google Scholar] [CrossRef]
- Cook, D.J.; Johnstone, J.; Marshall, J.C.; Lauzier, F.; Thabane, L.; Mehta, S.; Dodek, P.M.; McIntyre, L.; Pagliarello, J.; Henderson, W. Probiotics: Prevention of severe pneumonia and endotracheal colonization trial—PROSPECT: A pilot trial. Trials 2016, 17, 377. [Google Scholar] [CrossRef]
- Johnstone, J.; Meade, M.; Lauzier, F.; Marshall, J.; Duan, E.; Dionne, J.; Arabi, Y.M.; Heels-Ansdell, D.; Thabane, L.; Lamarche, D. Effect of probiotics on incident ventilator-associated pneumonia in critically ill patients: A randomized clinical trial. JAMA 2021, 326, 1024–1033. [Google Scholar] [CrossRef]
- Litton, E.; Anstey, M.; Broadhurst, D.; Chapman, A.; Currie, A.; Ferrier, J.; Gummer, J.; Higgins, A.; Lim, J.; Manning, L. Early and sustained Lactobacillus plantarum probiotic therapy in critical illness: The randomised, placebo-controlled, restoration of gut microflora in critical illness trial (ROCIT). Intensive Care Med. 2021, 47, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodpoor, A.; Hamishehkar, H.; Asghari, R.; Abri, R.; Shadvar, K.; Sanaie, S. Effect of a probiotic preparation on ventilator-associated pneumonia in critically ill patients admitted to the intensive care unit: A prospective double-blind randomized controlled trial. Nutr. Clin. Pract. 2019, 34, 156–162. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Folwarski, M.; Skonieczna-Żydecka, K.; Ruszkowski, J.; Makarewicz, W. The use of Lactobacillus plantarum 299v (DSM 9843) in cancer patients receiving home enteral nutrition–study protocol for a randomized, double-blind, and placebo-controlled trial. Nutr. J. 2020, 19, 98. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Siedlecka, K.; Folwarski, M.; Ruszkowski, J.; Skonieczna-Żydecka, K.; Szafrański, W.; Makarewicz, W. Effects of 4 weeks of Lactobacillus plantarum 299v supplementation on nutritional status, enteral nutrition tolerance, and quality of life in cancer patients receiving home enteral nutrition–a double-blind, randomized, and placebo-controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9684–9694. [Google Scholar] [PubMed]
- Alberda, C.; Marcushamer, S.; Hewer, T.; Journault, N.; Kutsogiannis, D. Feasibility of a Lactobacillus casei drink in the intensive care unit for prevention of antibiotic associated diarrhea and Clostridium difficile. Nutrients 2018, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Rongrungruang, Y.; Krajangwittaya, D.; Pholtawornkulchai, K.; Tiengrim, S.; Thamlikitkul, V. Randomized controlled study of probiotics containing Lactobacillus casei (Shirota strain) for prevention of ventilator-associated pneumonia. J. Med. Assoc. Thai 2015, 98, 253–259. [Google Scholar]
- Wang, J.; Ke, H.; Liu, K.-X.; Qu, J.-M. Effects of exogenous probiotics on the gut microbiota and clinical outcomes in critically ill patients: A randomized controlled trial. Ann. Palliat. Med. 2021, 10, 1180190–1181190. [Google Scholar] [CrossRef]
- de Castro Soares, G.G.; Marinho, C.H.; Pitol, R.; Andretta, C.; Oliveira, E.; Martins, C.; Riella, M.C. Sporulated Bacillus as alternative treatment for diarrhea of hospitalized adult patients under enteral nutrition: A pilot randomized controlled study. Clin. Nutr. ESPEN 2017, 22, 13–18. [Google Scholar] [CrossRef]
- Yang, L.; Liang, Y.; Li, R.; Chen, Y.; Zhang, X. Efficacy of probiotics combined with enteral nutrition therapy on intestinal flora, digestive tract symptoms and endogenous environment in patients with gastric cancer undergoing chemotherapy. Pak. J. Med. Sci. 2024, 40, 2344. [Google Scholar] [CrossRef]
- Malik, A.A.; Rajandram, R.; Tah, P.C.; Hakumat-Rai, V.-R.; Chin, K.-F. Microbial cell preparation in enteral feeding in critically ill patients: A randomized, double-blind, placebo-controlled clinical trial. J. Crit. Care 2016, 32, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.; Abedi, N. Association of using enteral nutrition containing probiotics and dietary inflammatory index with inflammatory factors serum levels and gastrointestinal complications in infected patients with COVID-19. Nutr. Food Sci. 2024, 54, 1219–1233. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Y.; Huang, Y.; Cui, Y.; Xia, L.; Rao, Z.; Zhou, Y.; Wu, X. Effects of fiber and probiotics on diarrhea associated with enteral nutrition in gastric cancer patients: A prospective randomized and controlled trial. Medicine 2017, 96, e8418. [Google Scholar] [CrossRef]
- Pocidoni, J.B.N.; Cruz, M.R.R.d.; Morimoto, I.M.I.; Mendonça, L.; Engelhardt, C.W.; Fujimura, J.N. Supplementation for diarrhea control in hospitalized geriatric patients on enteral nutrition. Geriatr. Gerontol. Aging 2019, 13, 28–35. [Google Scholar] [CrossRef]
- Seifi, N.; Rezvani, R.; Sedaghat, A.; Nematy, M.; Khadem-Rezaiyan, M.; Safarian, M. The effects of synbiotic supplementation on enteral feeding tolerance, protein homeostasis, and muscle wasting of critically ill adult patients: A randomized controlled trial. Trials 2022, 23, 846. [Google Scholar] [CrossRef]
- Tzikos, G.; Tsalkatidou, D.; Stavrou, G.; Thoma, G.; Chorti, A.; Tsilika, M.; Michalopoulos, A.; Papavramidis, T.; Giamarellos-Bourboulis, E.J.; Kotzampassi, K. A four-probiotic regime to reduce surgical site infections in multi-trauma patients. Nutrients 2022, 14, 2620. [Google Scholar] [CrossRef] [PubMed]
- Tsilika, M.; Thoma, G.; Aidoni, Z.; Tsaousi, G.; Fotiadis, K.; Stavrou, G.; Malliou, P.; Chorti, A.; Massa, H.; Antypa, E. A four-probiotic preparation for ventilator-associated pneumonia in multi-trauma patients: Results of a randomized clinical trial. Int. J. Antimicrob. Agents 2022, 59, 106471. [Google Scholar] [CrossRef]
- Wan, G.; Wang, L.; Zhang, G.; Zhang, J.; Lu, Y.; Li, J.; Yi, X. Effects of probiotics combined with early enteral nutrition on endothelin-1 and C-reactive protein levels and prognosis in patients with severe traumatic brain injury. J. Int. Med. Res. 2020, 48, 0300060519888112. [Google Scholar] [CrossRef]
- Xie, H.; Cai, M.; Zhang, Y. Influence of early enteral nutrition plus probiotics on intestinal function of senile patients with sepsis. Am. J. Transl. Res. 2023, 15, 445. [Google Scholar] [PubMed]
- D’Onofrio, V.; Del Chierico, F.; Belci, P.; Vernocchi, P.; Quagliariello, A.; Reddel, S.; Conta, G.; Mancino, M.V.; Fadda, M.; Scigliano, M.C. Effects of a synbiotic formula on functional bowel disorders and gut microbiota profile during long-term home enteral nutrition (LTHEN): A pilot study. Nutrients 2020, 13, 87. [Google Scholar] [CrossRef]
- de Oliveira Junior, V.P.; dos Santos, D.C.; Fernandes, S.S.; Egea, M.B. Prescription and Delivery of Enteral Nutrition for ICU Patients: A Case Study of a Hospital in the Interior of Brazil. Food Nutr. Sci. 2023, 14, 880–896. [Google Scholar] [CrossRef]
- Lavori, P.W. Placebo control groups in randomized treatment trials: A statistician’s perspective. Biol. Psychiatry 2000, 47, 717–723. [Google Scholar] [CrossRef]
- Battaglini, D.; Torres, A. Gut microbiota and its impact on critical illness. Curr. Opin. Crit. Care 2025, 31, 189–197. [Google Scholar] [CrossRef]
- Klassert, T.E.; Zubiria-Barrera, C.; Denkel, L.; Neubert, R.; Schneegans, A.; Kulle, A.; Vester, A.; Bloos, F.; Schulze, C.; Epstude, J. Skin dysbiosis and loss of microbiome site specificity in critically ill patients. Microbiol. Spectr. 2024, 12, e03078-23. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Cui, S.; Huang, N.; Jin, G.; Chen, C.; Fan, Y.; Zhang, C.; Li, J. Efficacy of probiotics or synbiotics in critically ill patients: A systematic review and meta-analysis. Clin. Nutr. ESPEN 2024, 59, 48–62. [Google Scholar] [CrossRef]
- Lan, S.-H.; Hung, S.-H.; Chang, S.-P.; Lu, L.-C.; Lai, C.-C.; Lin, W.-T. Pro-, pre-and synbiotics for the prevention of incidental ventilator-associated pneumonia among critically ill patients: A systematic review and meta-analysis of randomized controlled trials. Expert Rev. Anti-Infect. Ther. 2022, 20, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Wieërs, G.; Belkhir, L.; Enaud, R.; Leclercq, S.; Philippart de Foy, J.-M.; Dequenne, I.; de Timary, P.; Cani, P.D. How probiotics affect the microbiota. Front. Cell. Infect. Microbiol. 2020, 9, 454. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Invernici, M.M.; Furlaneto, F.A.; Messora, M.R. Effectiveness of multi-strain versus single-strain probiotics: Current status and recommendations for the future. J. Clin. Gastroenterol. 2018, 52, S35–S40. [Google Scholar] [CrossRef]
- Chapman, C.M.C.; Gibson, G.R.; Rowland, I. Health benefits of probiotics: Are mixtures more effective than single strains? Eur. J. Nutr. 2011, 50, 1–17. [Google Scholar] [CrossRef]
- Chapman, C.; Gibson, G.R.; Rowland, I. In vitro evaluation of single-and multi-strain probiotics: Inter-species inhibition between probiotic strains, and inhibition of pathogens. Anaerobe 2012, 18, 405–413. [Google Scholar] [CrossRef]
- Sanders, M.E.; Benson, A.; Lebeer, S.; Merenstein, D.J.; Klaenhammer, T.R. Shared mechanisms among probiotic taxa: Implications for general probiotic claims. Curr. Opin. Biotechnol. 2018, 49, 207–216. [Google Scholar] [CrossRef]
- Leser, T.; Baker, A. Molecular mechanisms of Lacticaseibacillus rhamnosus, LGG® probiotic function. Microorganisms 2024, 12, 794. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.; Wan, Y.; Hu, T.; Liu, L.; Yang, S.; Gong, Z.; Zeng, Q.; Wei, Y.; Yang, W. A novel postbiotic from Lactobacillus rhamnosus GG with a beneficial effect on intestinal barrier function. Front. Microbiol. 2019, 10, 477. [Google Scholar] [CrossRef]
- Capurso, L. Thirty years of Lactobacillus rhamnosus GG: A review. J. Clin. Gastroenterol. 2019, 53, S1–S41. [Google Scholar] [CrossRef]
- Lebeer, S.; Bron, P.A.; Marco, M.L.; Van Pijkeren, J.-P.; Motherway, M.O.C.; Hill, C.; Pot, B.; Roos, S.; Klaenhammer, T. Identification of probiotic effector molecules: Present state and future perspectives. Curr. Opin. Biotechnol. 2018, 49, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Fidanza, M.; Panigrahi, P.; Kollmann, T.R. Lactiplantibacillus plantarum–nomad and ideal probiotic. Front. Microbiol. 2021, 12, 712236. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, G.; Fang, J. Progress on the mechanisms of Lactobacillus plantarum to improve intestinal barrier function in ulcerative colitis. J. Nutr. Biochem. 2024, 124, 109505. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, C.; Wa, Y.; Qu, H.; Gu, R.; Chen, D.; Song, Z.; Chen, X. Correlation between exopolysaccharide biosynthesis and gastrointestinal tolerance of Lactiplantibacillus plantarum. J. Appl. Microbiol. 2022, 132, 584–591. [Google Scholar] [CrossRef]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 2021, 13, 1907272. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y. Analysis and identification of the main antimicrobial metabolites of Lactobacillus plantarum LPZN19. Electron. J. Biotechnol. 2024, 71, 74–88. [Google Scholar] [CrossRef]
- Banna, G.L.; Torino, F.; Marletta, F.; Santagati, M.; Salemi, R.; Cannarozzo, E.; Falzone, L.; Ferraù, F.; Libra, M. Lactobacillus rhamnosus GG: An overview to explore the rationale of its use in cancer. Front. Pharmacol. 2017, 8, 603. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, V.; Salvatore, F. The role of the gut microbiome in the healthy adult status. Clin. Chim. Acta 2015, 451, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, T.C.; Brandão, L.R.; de Oliveira, M.P.; da Costa, W.K.A.; Magnani, M. Health benefits and technological effects of Lacticaseibacillus casei-01: An overview of the scientific literature. Trends Food Sci. Technol. 2021, 114, 722–737. [Google Scholar] [CrossRef]
- Shen, N.T.; Maw, A.; Tmanova, L.L.; Pino, A.; Ancy, K.; Crawford, C.V.; Simon, M.S.; Evans, A.T. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: A systematic review with meta-regression analysis. Gastroenterology 2017, 152, 1889–1900.e9. [Google Scholar] [CrossRef]
- Lee, N.-K.; Son, S.-H.; Jeon, E.B.; Jung, G.H.; Lee, J.-Y.; Paik, H.-D. The prophylactic effect of probiotic Bacillus polyfermenticus KU3 against cancer cells. J. Funct. Foods 2015, 14, 513–518. [Google Scholar] [CrossRef]
- Lee, N.-K.; Kim, W.-S.; Paik, H.-D. Bacillus strains as human probiotics: Characterization, safety, microbiome, and probiotic carrier. Food Sci. Biotechnol. 2019, 28, 1297–1305. [Google Scholar] [CrossRef]
- Stenfors Arnesen, L.P.; Fagerlund, A.; Granum, P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Ho, C.L. Recent development of probiotic bifidobacteria for treating human diseases. Front. Bioeng. Biotechnol. 2021, 9, 770248. [Google Scholar] [CrossRef]
- Bocchio, F.; Mancabelli, L.; Milani, C.; Lugli, G.A.; Tarracchini, C.; Longhi, G.; De Conto, F.; Turroni, F.; Ventura, M. Compendium of Bifidobacterium-based probiotics: Characteristics and therapeutic impact on human diseases. Microbiome Res. Rep. 2024, 4, 2. [Google Scholar] [CrossRef]
- Warda, A.K.; Clooney, A.G.; Ryan, F.; de Almeida Bettio, P.H.; Di Benedetto, G.; Ross, R.P.; Hill, C. A postbiotic consisting of heat-treated lactobacilli has a bifidogenic effect in pure culture and in human fermented fecal communities. Appl. Environ. Microbiol. 2021, 87, e02459-20. [Google Scholar] [CrossRef] [PubMed]
- Toscano, M.; De Grandi, R.; Stronati, L.; De Vecchi, E.; Drago, L. Effect of Lactobacillus rhamnosus HN001 and Bifidobacterium longum BB536 on the healthy gut microbiota composition at phyla and species level: A preliminary study. World J. Gastroenterol. 2017, 23, 2696. [Google Scholar] [CrossRef] [PubMed]
- Tojo, R.; Suárez, A.; Clemente, M.G.; de los Reyes-Gavilán, C.G.; Margolles, A.; Gueimonde, M.; Ruas-Madiedo, P. Intestinal microbiota in health and disease: Role of bifidobacteria in gut homeostasis. World J. Gastroenterol. WJG 2014, 20, 15163. [Google Scholar] [CrossRef]
- Čitar, M.; Hacin, B.; Tompa, G.; Štempelj, M.; Rogelj, I.; Dolinšek, J.; Narat, M.; Matijašić, B.B. Human intestinal mucosa-associated Lactobacillus and Bifidobacterium strains with probiotic properties modulate IL-10, IL-6 and IL-12 gene expression in THP-1 cells. Benef. Microbes 2015, 6, 325–336. [Google Scholar] [CrossRef]
- Boonma, P.; Spinler, J.K.; Venable, S.F.; Versalovic, J.; Tumwasorn, S. Lactobacillus rhamnosus L34 and Lactobacillus casei L39 suppress Clostridium difficile-induced IL-8 production by colonic epithelial cells. BMC Microbiol. 2014, 14, 177. [Google Scholar] [CrossRef]
- Gunaratnam, S.; Diarra, C.; Paquette, P.D.; Ship, N.; Millette, M.; Lacroix, M. The acid-dependent and independent effects of Lactobacillus acidophilus CL1285, Lacticaseibacillus casei LBC80R, and Lacticaseibacillus rhamnosus CLR2 on Clostridioides difficile R20291. Probiotics Antimicrob. Proteins 2021, 13, 949–956. [Google Scholar] [CrossRef]
- Zaman, M.K.; Chin, K.-F.; Rai, V.; Majid, H.A. Fiber and prebiotic supplementation in enteral nutrition: A systematic review and meta-analysis. World J. Gastroenterol. WJG 2015, 21, 5372. [Google Scholar] [CrossRef]
- Reis, A.M.d.; Fruchtenicht, A.V.; Loss, S.H.; Moreira, L.F. Use of dietary fibers in enteral nutrition of critically ill patients: A systematic review. Rev. Bras. Ter. Intensiv. 2018, 30, 358–365. [Google Scholar] [CrossRef]
- Kato, Y.; Nakao, M.; Iwasa, M.; Hasegawa, S.; Yamada, K. Soluble fiber improves management of diarrhea in elderly patients receiving enteral nutrition. Food Nutr. Sci. 2012, 3, 24495. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Ren, F. A role of exopolysaccharide produced by Streptococcus thermophilus in the intestinal inflammation and mucosal barrier in Caco-2 monolayer and dextran sulphate sodium-induced experimental murine colitis. Molecules 2019, 24, 513. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Lu, Y.-H.; Liu, X.-T.; Wu, W.-T.; Li, W.-Q.; Lai, S.-Q.; Aadil, R.M.; Riaz Rajoka, M.S.; Wang, L.-H.; Zeng, X.-A. Metabolic properties, functional characteristics, and practical application of Streptococcus thermophilus. Food Rev. Int. 2024, 40, 792–813. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, Z.; Liang, J.; Dou, J.; Guo, F.; Xu, Z.; Wang, T. Advances in genetic tools and their application in Streptococcus thermophilus. Foods 2023, 12, 3119. [Google Scholar] [CrossRef]
- Baliza, D.D.M.d.S.; Silva, J.F.M.d.; Pimenta, R.S. Evaluation of the applicability of a probiotic strain of Saccharomyces cerevisiae in cereal bars. Braz. J. Food Technol. 2018, 21, e2017148. [Google Scholar]
- Shruthi, B.; Deepa, N.; Somashekaraiah, R.; Adithi, G.; Divyashree, S.; Sreenivasa, M. Exploring biotechnological and functional characteristics of probiotic yeasts: A review. Biotechnol. Rep. 2022, 34, e00716. [Google Scholar] [CrossRef]
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces boulardii: What makes it tick as successful probiotic? J. Fungi 2020, 6, 78. [Google Scholar] [CrossRef]
- Hanchi, H.; Mottawea, W.; Sebei, K.; Hammami, R. The genus Enterococcus: Between probiotic potential and safety concerns—An update. Front. Microbiol. 2018, 9, 1791. [Google Scholar] [CrossRef]
- Lauková, A.; Kandričáková, A.; Buňková, L.; Pleva, P.; Ščerbová, J. Sensitivity to enterocins of biogenic amine-producing faecal Enterococci from ostriches and pheasants. Probiotics Antimicrob. Proteins 2017, 9, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Franz, C.M.; Huch, M.; Abriouel, H.; Holzapfel, W.; Gálvez, A. Enterococci as probiotics and their implications in food safety. Int. J. Food Microbiol. 2011, 151, 125–140. [Google Scholar] [CrossRef]
- Besseling-Van Der Vaart, I.; Heath, M.; Guagnini, F.; Kramer, M. In vitro evidence for efficacy in food intolerance for the multispecies probiotic formulation Ecologic® Tolerance (Syngut™). Benef. Microbes 2016, 7, 111–118. [Google Scholar] [CrossRef]
- Murphy, K. Imunobiologia de Janeway-8; Artmed Editora: Porto Alegre-RS, Brazil, 2014. [Google Scholar]
- Nakamura, S.; Morimoto, Y.V.; Kudo, S. A lactose fermentation product produced by Lactococcus lactis subsp. lactis, acetate, inhibits the motility of flagellated pathogenic bacteria. Microbiology 2015, 161, 701–707. [Google Scholar] [CrossRef]
- Rivai, M.I.; Lusikooy, R.E.; Putra, A.E.; Elliyanti, A. Effects of Lactococcus lactis on colorectal cancer in various terms: A narrative review. Ann. Med. Surg. 2024, 86, 3503–3507. [Google Scholar] [CrossRef] [PubMed]
- Carasi, P.; Malamud, M.; Serradell, M. Potentiality of food-isolated Lentilactobacillus kefiri strains as probiotics: State-of-art and perspectives. Curr. Microbiol. 2022, 79, 21. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.M.; Mattarelli, P.; O’toole, P.W.; Pot, B.; Vandamme, P.; Walter, J. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Butera, A.; Folini, E.; Cosola, S.; Russo, G.; Scribante, A.; Gallo, S.; Stablum, G.; Menchini Fabris, G.B.; Covani, U.; Genovesi, A. Evaluation of the Efficacy of Probiotics Domiciliary Protocols for the Management of Periodontal Disease, in Adjunction of Non-Surgical Periodontal Therapy (NSPT): A Systematic Literature Review. Appl. Sci. 2023, 13, 663. [Google Scholar] [CrossRef]


| Reference | Study Design | Studied Group | Study Duration | No PTP | No Male PTP | No Female PTP | Age (INT) | Age (PLA or CON) |
|---|---|---|---|---|---|---|---|---|
| [44] | Prospective, randomized, double-blind, controlled clinical trial | ICU patients on mechanical ventilation ≥ 72 h | stay in the ICU or until 60 d | 150 | 88 | 62 | 58.8 ± 17.0 | 61.2 ± 15.4 |
| [45] | Prospective, randomized, double-blind clinical trial | ICU patients on mechanical ventilation ≥ 72 h | 60 d | 2.650 | 1.587 | 1.063 | 60.1 ± 16.2 | 59.6 ± 16.8 |
| [46] | Randomized double-blind controlled clinical trial | Patients with >48 h of ICU admission | 60 d | 208 | 134 | 74 | 62.1 ± 15.7 | 62.6 ± 14.5 |
| [47] | Randomized controlled trial | Critically ill patients undergoing mechanical ventilation for >48 h in the ICU | 14 d | 102 | 55 | 45 | 59.1 ± 12.9 | 57.5 ± 14.5 |
| [48,49] | Randomized, double-blind, and placebo-controlled | Cancer patients receiving home enteral nutrition | 12 w | 35 | 27 | 8 | 60.0 ± 10.9 | 61.1 ± 8.9 |
| [50] | Parallel group randomized trial | Patients hospitalized in the ICU | 7 d | 32 | 25 | 7 | 59.9 ± 15.6 | 57.5 ± 15.0 |
| [51] | Prospective, randomized, controlled study | Patients on mechanical ventilation from medical wards | 28 d | 150 | 62 | 88 | 68.9 ± 18.4 | 73.1 ± 13.2 |
| [52] | A single-blind, randomized controlled trial | Patients admitted to the respiratory intensive care unit (RICU) | until death or discharge, and evaluated after 15 days | 61 | 34 | 27 | 81 (61–95) | 81 (70–96) |
| [53] | Randomized, prospective, double-blind study | Patients hospitalized in the ICU | 5 d | 58 | ND | ND | 65.0 ± 20.7 | 70.8 ± 18.1 |
| [54] | Retrospective study | Patients with gastric cancer undergoing chemotherapy | 14 d | 80 | 43 | 37 | 58.3 ± 8.3 | 57.6 ± 8.3 |
| [55] | Randomized, double-blind study | Critically ill patients on mechanical ventilation in the ICU | 7 d | 60 | 40 | 20 | 60 | 55 |
| [56] | Cross-sectional study | Patients with COVID-19 who were admitted to the ICU tolerated approximately 80% of ENT | 7 d | 100 | ND | ND | 45.63 | ND |
| [57] | Prospective randomized-controlled trial | Postoperative patients with gastric cancer (stage II or III tumors) | 7 d | 120 | 62 | 58 | 66.5 ± 7.1 | 63.5 ± 8.5 |
| [58] | Retrospective, analytical, and longitudinal study | Patients admitted to the hospital | While the diarrhea lasted | 75 | 32 | 43 | 71.8 ± 7.9 | |
| [59] | Randomized controlled triple-blind clinical trial | Patients hospitalized in the ICU | 14 d | 38 | 23 | 15 | 38.5 ± 17.94 | 47.61 ± 22.51 |
| [60] | Multi-center, randomized, double-blind, placebo-controlled trial | Surgical site infections in multiple-trauma patients on mechanical ventilation in the ICU | 15 d | 103 | 90 | 13 | 38.4 ± 16.9 | 44.1 ± 13.9 |
| [61] | Randomized, controlled study | Multi-trauma patients on mechanical ventilation in the ICU | 15 d | 112 | 94 | 18 | 38.1 ± 17.2 | 43.8 ± 14.4 |
| [62] | Prospective study | Patients with severe traumatic brain injury are being transferred from the hospital | 15 d | 76 | 36 | 40 | 35.9 ± 13.1 | 38.6 ± 11.3 |
| [63] | Retrospective study | Patients with senile sepsis | 2 w | 108 | 61 | 47 | 71.3 ± 7.7 | |
| [64] | Randomized open-label intervention study | Patients on home enteral nutrition with neurological disorders | 4 m | 20 | ND | ND | 75.2 ± 4.3 | |
| [18] | Pilot, Double-Blind, Placebo-Controlled Study | Geriatric patients on home enteral nutrition with different comorbidities | 60 d | 32 | 8 | 24 | 80.0 ± 10.2 | 79.3 ± 10.0 |
| References | Main Results | Intervention |
|---|---|---|
| [44] | No comparison between groups was needed to analyze feasibility outcomes. The rate of VAP was 19%; bloodstream infection (19.3%); urinary tract infections (12.7%); skin and soft tissue infections (4.0%); and Bristol stool type 6 or 7 occurred in 133 (88.7%) of patients. The median stay in the ICU was 12 days, and in the hospital, it was 26 days. | 1 capsule of Culturelle® content 1 × 1010 CFU of Lacticaseibacillus rhamnosus GG (former Lactobacillus rhamnosus) was administered twice/day until discharge or death vs. placebo during the patient’s stay in the ICU |
| [45] | = diarrhea, antimicrobial use, length of hospital stay, and VAP. | 1 × 1010 UFC de L. rhamnosus GG (i-Health Inc®) |
| [46] | = Days alive and out of the hospital to Day 60; nosocomial infection; mortality in the ICU and hospital; and overall quality of life at day 60, as assessed by median EQ-5D-5L VAS scores. No participant had more than one nosocomial infection. | 1 capsule containing 2 × 1010 CFU of L. plantarum 299 (Lp299 DSM 6595) once daily |
| [48,49] | ↑ Serum albumin concentration compared with control, serum albumin after week 4 compared with baseline, total protein after 4 weeks compared with baseline, and self-assessment of quality of life compared with baseline. ↓ Frequency of vomiting and flatulence compared to baseline. = vomiting and flatulence between groups, total lymphocyte count, body mass, BMI, fat mass content, muscle mass, and total body weight in both groups after 4 weeks of treatment | Two capsules contain 1010 UFC of L. plantarum 299v (Sanprobi IBS®) twice a day |
| [50] | ↓ Antibiotic-associated diarrhea and infectious episodes. = Adverse events probiotic group and the control group. | 93 mL of drink (Danactive®) with L. casei sp. paracasei CNCM I1518 (formally DN-114 001) with 10 billion bolused twice daily |
| [51] | ↓ VAP within the intervention group and the incidence of diarrhea. = Time on mechanical ventilation, ICU stay, and hospitalization. | 80 mL of a fermented milk product containing 8 × 109 CFU L. casei (Shirota strain) (Yakult®) administered enterally once daily. The exact amount of L. casei was used for oral hygiene once daily |
| [52] | ↓ Duration of fever, incidence of constipation, bactericides, Escherichia coli and Enterococcus, Bacteroides, and serum LPS level. = mortality; length of hospital stay; gastrointestinal adverse effects; and Bifidobacterium and Limosilactobacillus; content of DAO (intestinal barrier); and IL-10 and TNF-α. | 1 tablet of MIYA-BM® with 106 UFC Clostridium butyricum 3 times/day |
| [53] | = Diarrhea cessation; serum albumin; Subjective Global Assessment (SGA) score; food osmolality; and antibiotic use. B. cereus was associated with a ↓ diarrhea period (2.5 versus 3.7 days). | 4 vials with 5 mL of 106 of Bacillus cereus A 05 (Biovicerin®) each 6 h or 10 g of soluble fiber (Fiber mais®) every 8 h |
| [54] | ↑ Serum levels of total protein, prealbumin, albumin, and transferrin *. ↓ Levels of Staphylococcus, Escherichia coli, and Enterococcus; IgA, IgM, and IgG levels; levels of endotoxin and D-lactic acid; and incidence of gastrointestinal symptoms (compared with the start and with the control) | Probiotic capsules (Bifidobacterium) were taken orally, one tablet at a time, tid, or given via tube feeding after being fully dissolved in warm boiled water |
| [55] | ↑ Bowel function. ↓ Duration of ventilation by 40% and length of stay in the ICU by 31%. = inflammatory markers (leukocyte count and CRP levels) in both groups. | Lactobacillus acidophilus, Lacticaseibacillus casei (former Lactobacillus casei), Lactobacillus lactis, Bifidobacterium bifidum, Bifidobacterium longum, and Bifidobacterium infantis (30 bilhões de UFC) administered twice daily |
| [56] | ↓ Dietary inflammatory index; h-CRP; serum erythrocyte sedimentation rate level; incidence of diarrhea, abdominal pain, and vomiting compared to control. | 300 g/day probiotic (L. acidophilus La-5 and Bifidobacterium lactis Bb-12) yogurt vs. 300 g/day yogurt |
| [57] | ↓ cases of diarrhea compared with FE; intestinal disorders compared with FF; and length of hospital stay compared with FF. = length of hospital stays between FEB and FE; and total lymphocyte count, albumin, prealbumin, and transferrin levels between groups. | Three groups being fiber-free nutrition formula (FF), fiber-enriched nutrition formula (FE), and fiber- and probiotic-enriched nutrition formula (FEB) (live bifidobacteria and lactobacilli) in tablets |
| [58] | Time of diarrhea in patient (d = days): T5 = T3 (2 d) > T5 (1.4 d) > T3 (1.2 d, 2 g) > T3 (0.6 d, 3 g > T7 (0.5 d) > T2 (0.33) ↑ (3) diarrhea control (55.8%) | Several groups: (T1) fiber supplement (partially hydrolyzed guar gum and inulin); (T2) partially hydrolyzed guar gum + inulin + Lactobacillus reuteri culture) (10 g); (T3) L. acidophilus, L. rhamnosus, L. paracasei and Bifidobacterium lactis (1 g, 2 g, and 3 g); (T4) L-glutamine supplement; (T5) synbiotic (1) + probiotic (3) (15 g + 3 g or 10 + 2 g); (T6) synbiotic (1) + L-glutamine (4) (10 + 20 g); (T7) probiotic (3) + L-glutamine (4) (1 + 10 g); (8) fiber (1) + synbiotic of L. paracasei, L. rhamnosus, L. acidophilus, Bifidobacterium lactis, and fructooligosaccharides. |
| [59] | ↑ Enteral feed volume, calories, and protein; Intake compared with the first day. = Duration of ENT and days of ENT per day of ICU stay; average energy deficit; enteral feeding intolerance; protein intake; nitrogen balance; and mid-arm circumference measurements. | Symbiotic capsule containing L. casei (1.5 × 109 CFU), L. acidophilus (1.5 × 1010 CFU), L. rhamnosus (3.5 × 109 CFU), Lactobacillus bulgaricus (2.5 × 108 CFU), Bifidobacterium breve (1 × 1010 CFU), Bifidobacterium longum (5 × 108 CFU), S. thermophilus (1.5 × 108 CFU), and fructooligosaccharides |
| [47] | ↓ Gastric residue (57.4% control vs. 30% probiotics); incidence of VAP; length of ICU stays; diarrhea, gastric, and oropharyngeal colonization; and incidence of multidrug-resistant pathogens. = Kaplan–Meier survival curves for time to the first episode of VAP. | 2 capsules of probiotic-containing Limosilactobacillus (former Lactobacillus), Bifidobacterium, and Streptococcus thermophilus with 1010 CFU |
| [60] | ↑ Staphylococcus aureus isolated from the surgical traumas. ↓ Incidence of Surgical Site Infections: Most captured were related to osteosynthesis, followed by facial fractures. | Two sachets of L. acidophilus LA-5 (1.75 × 109 CFU), Lactiplantibacillus plantarum (former Lactobacillus plantarum) UBLP-40 (0.5 × 109 CFU), Bifidobacterium animalis subsp. lactis BB-12 (1.75 × 109 CFU) and Saccharomyces boulardii Unique-28 (1.5 × 109 CFU) twice/day (one through the nasogastric tube and one spread on the oropharynx) |
| [61] | ↓ incidence rate of VAP and sepsis, and length of stay in the ICU compared to the placebo group. | Two sachets of L. acidophilus LA-5 (1.75 × 109 CFU), L. plantarum UBLP-40 (0.5 × 109 CFU), Bifidobacterium animalis subsp. lactis BB-12 (1.75 × 109 CFU) and S. boulardii Unique-28 (1.5 × 109 CFU) twice/day (one through the nasogastric tube and one spread on the oropharynx) |
| [62] | ↓ Compared with baseline, serum levels of inflammatory factors (IL-6, IL-10, TNF-α, ET-1, and CRP). ↓ ET-1 in 15 days and the concentrations of IL-6, IL-10, and TNF-α in 7 and 15 days; duration of hospitalization and incidence of pulmonary infection; and Glasgow Coma Scale (GCS) in 15 days compared with the control. = 1-month mortality rates, rates of intracranial, incisional, or bloodstream infection, sepsis, septic shock, or systemic inflammatory response syndrome, and Sepsis-related Organ Assessment (SOFA) or Acute Physiology and Chronic Health Evaluation II (APACHE II) scores | 6 tablets (210 mg) containing Bifidobacterium longum, Lactobacillus bulgaricus, and Enterococcus faecalis (1.0 × 107 CFU) twice a day by tube or orally |
| [63] | ↑ Overall response rate and albumin and prealbumin levels compared to the placebo group. ↓ Intestinal fatty acid binding protein, diamine oxidase, D-lactate, and 28-day mortality compared to a placebo group. | 3 tablets containing Bifidobacterium longum, L. acidophilus, and Enterococcus (Biid-triple Viable Enteric-coated Capsules) three times a day |
| [64] | ↑ stool consistency (BCS value) and Shannon index compared with baseline; increased microbiota biodiversity in half of the intervention patients; propionic and butanoic acids and ketones, such as 2-octanone and 2-pentadecanone, Volatile Metabolome Profile compared with baseline. ↓ “Constipation Scoring System” (CSS) questionnaire compared to the beginning = nutritional measures and hematochemical values; Beta diversity indices and Lactococcus spp., Limosilactobacillus spp., and Bifidobacterium spp. Maintained the same relative abundance compared to the beginning | One sachet Syngut (109 CFU of L. acidophilus W22, 3.33 × 106 CFU of Bifidobacterium lactis W51, 3,33 × 102 CFU de L. plantarum W21, 3.33 × 106 CFU of Lactococcus lactis W21, and 0.375 g of Inulin |
| [18] | = Clinical manifestations of infections; incidence of bacterial infections; CRP levels, intestinal function, and nutritional status between the two groups. | Proxian® probiotic supplement, with L. plantarum LP01 (LMG P-21021) ≥ 1 billion live cells/dose, Lentilactobacillus buchneri (20 mg) Lb26 (DSM 16341), Bifidobacterium animalis subsp. lactis BS01 (LMG P-21384) ≥ 1 billion live cells/dose and enriched with zinc (Zn) and selenium (Se) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, G.M.d.; Egea, M.B. Probiotics: A Little Help for Enteral Nutritional Therapy in Critically Ill Adults. Int. J. Mol. Sci. 2025, 26, 8458. https://doi.org/10.3390/ijms26178458
Almeida GMd, Egea MB. Probiotics: A Little Help for Enteral Nutritional Therapy in Critically Ill Adults. International Journal of Molecular Sciences. 2025; 26(17):8458. https://doi.org/10.3390/ijms26178458
Chicago/Turabian StyleAlmeida, Graciele Magda de, and Mariana Buranelo Egea. 2025. "Probiotics: A Little Help for Enteral Nutritional Therapy in Critically Ill Adults" International Journal of Molecular Sciences 26, no. 17: 8458. https://doi.org/10.3390/ijms26178458
APA StyleAlmeida, G. M. d., & Egea, M. B. (2025). Probiotics: A Little Help for Enteral Nutritional Therapy in Critically Ill Adults. International Journal of Molecular Sciences, 26(17), 8458. https://doi.org/10.3390/ijms26178458







