Ganglioside Profiling Uncovers Distinct Patterns in High-Risk Neuroblastoma
Abstract
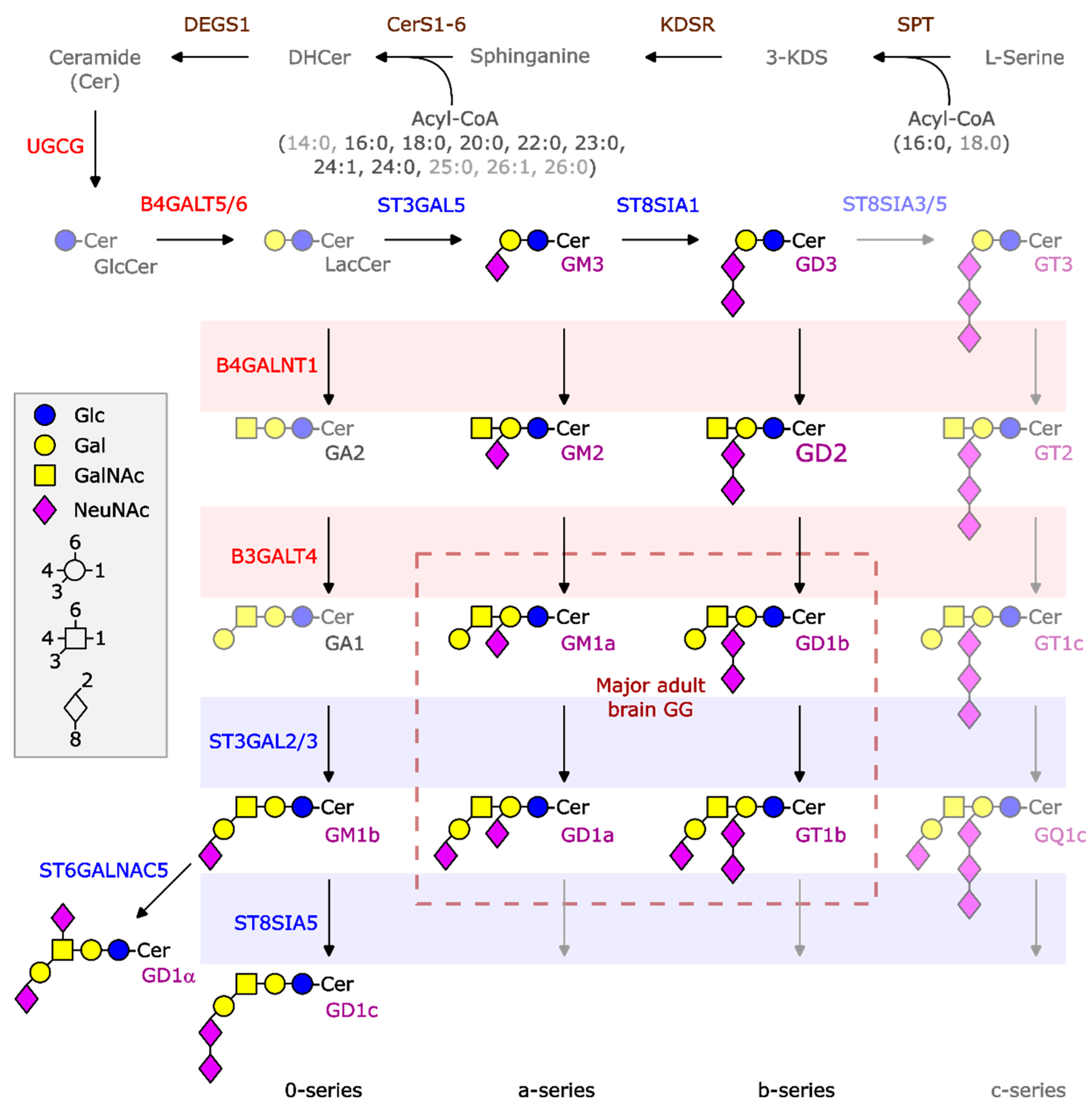
1. Introduction
2. Results
2.1. Patient and Tumor Samples
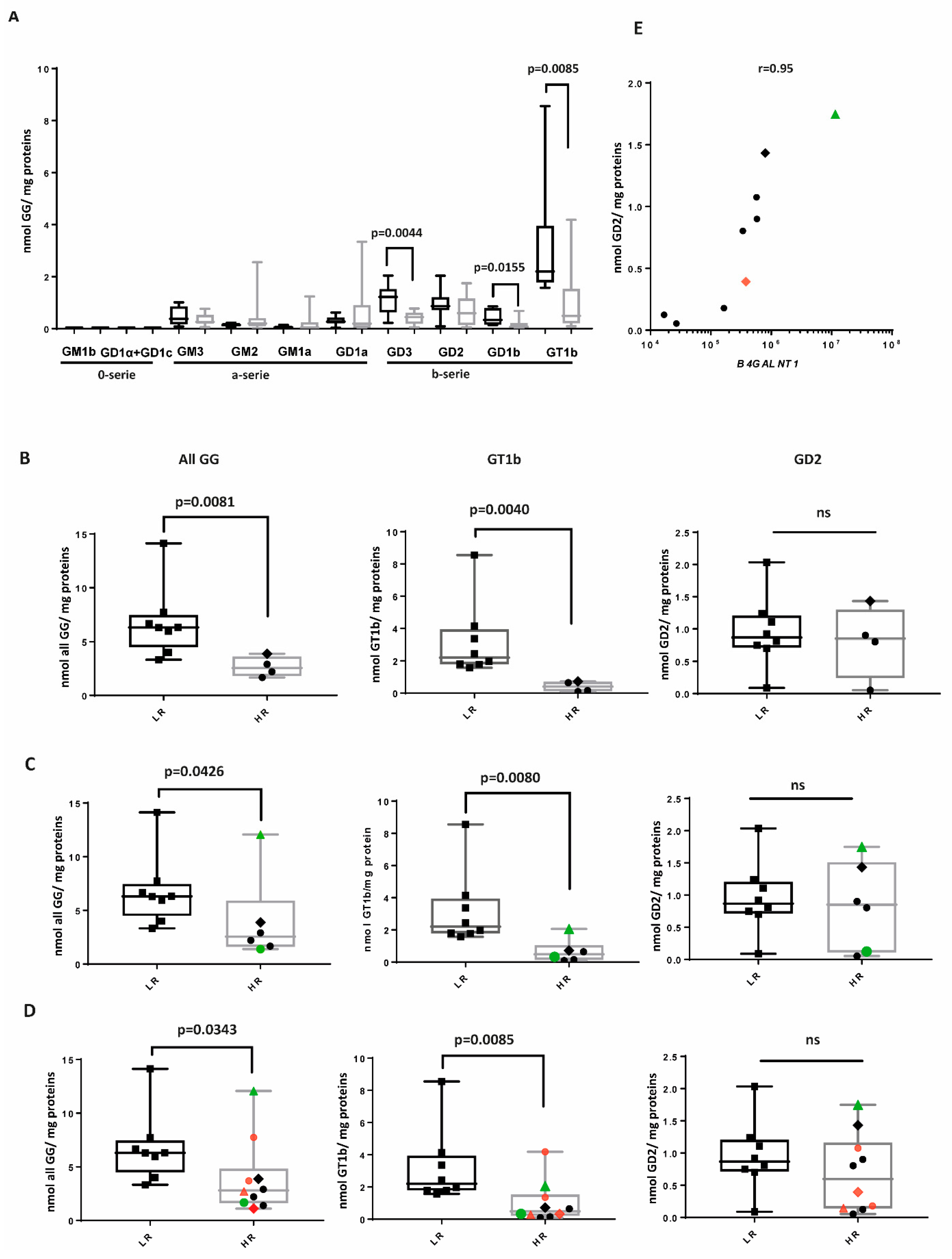
2.2. The b-Series of GGs Is Associated with LR NBL
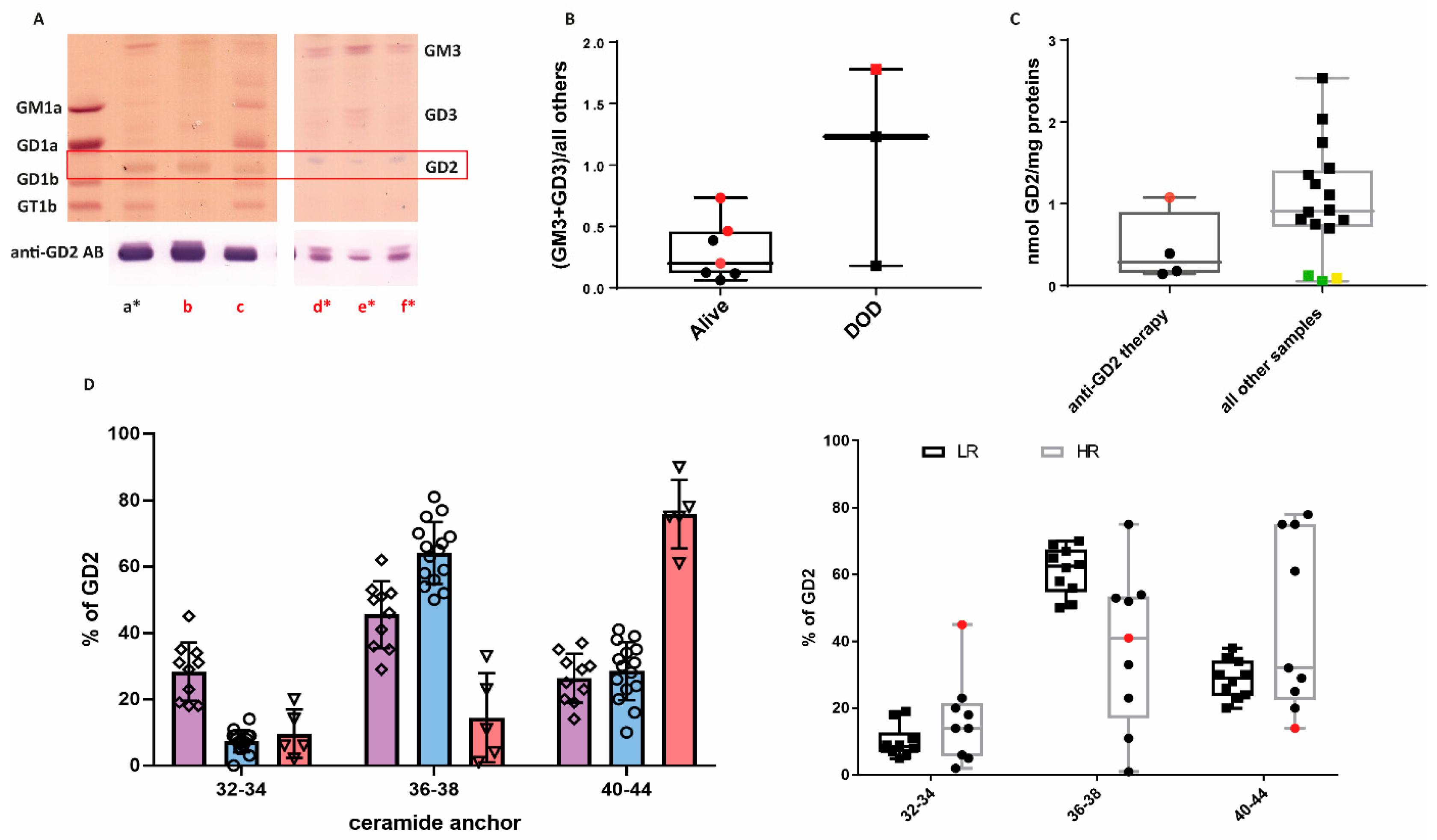
2.3. GG Profiles of HR Samples
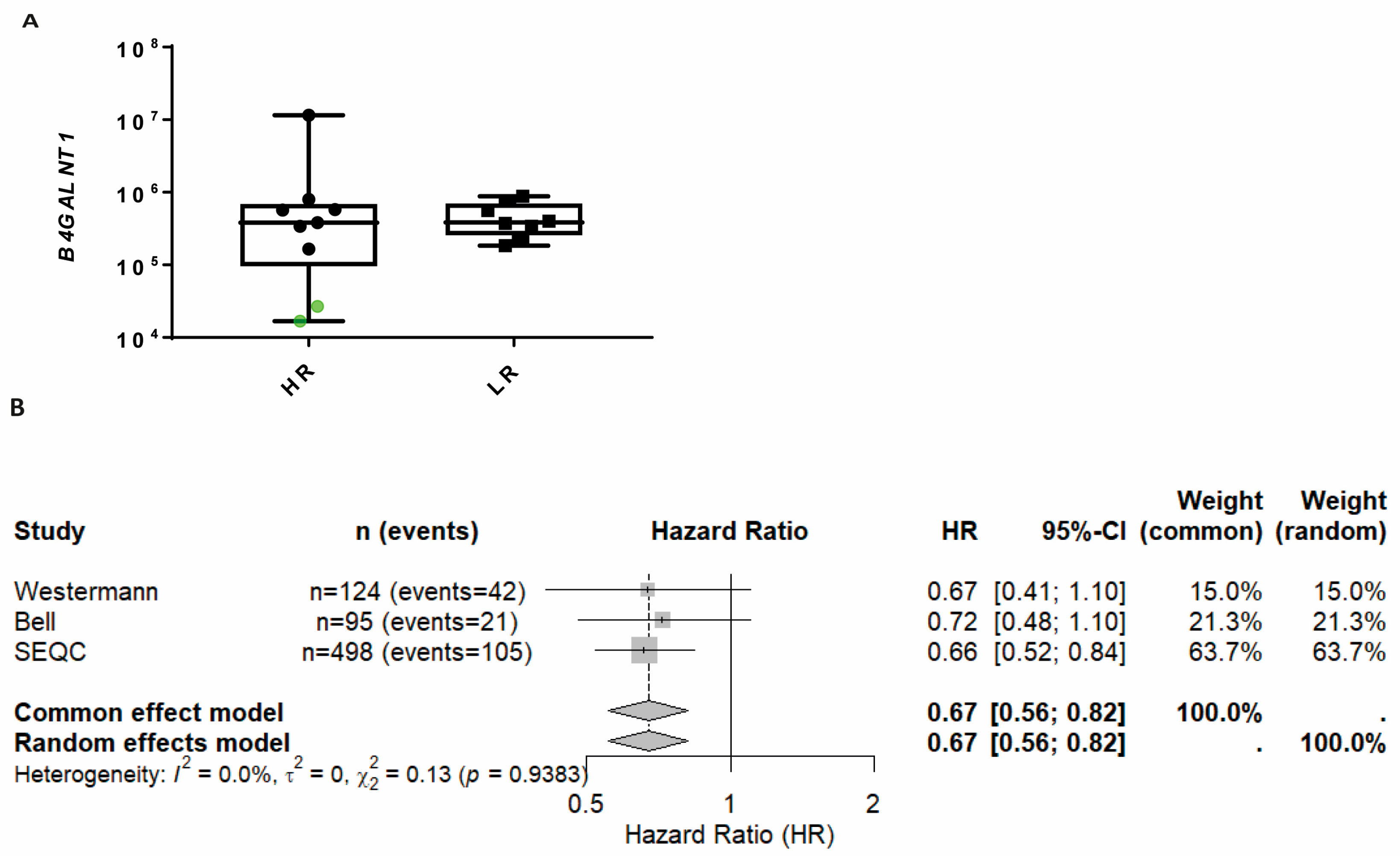
2.4. B4GALNT1 Downregulation as a Predictive Marker of Poor Outcome
2.5. Neuroblastoma Cell Lines Preferentially Express the a-Series of Gangliosides
3. Discussion
4. Materials and Methods
4.1. Patients and Tissues
4.2. Cell Cultures
4.3. Lipid Extraction
4.4. Thin-Layer Chromatography and Immune Overlay
4.5. LC-MS2 Analysis of Gangliosides
4.6. Gene Expression Analysis of Tumor Samples
4.7. GG Transcriptome Profiles of NBL Cell Lines
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATRA | All-trans retinoic acid |
| CER | Ceramide |
| CERS | Ceramide synthase |
| GG | Ganglioside |
| GSL | Glycosphingolipid |
| HR | High-risk |
| LR | Low-risk |
| MHC-1 | Major histocompatibility complex I |
| NBL | Neuroblastoma |
| NK | Natural killer cell |
| TrkA | Tyrosin kinase receptor A |
References
- Berthold, F.; Faldum, A.; Ernst, A.; Boos, J.; Dilloo, D.; Eggert, A.; Fischer, M.; Fruhwald, M.; Henze, G.; Klingebiel, T.; et al. Extended induction chemotherapy does not improve the outcome for high-risk neuroblastoma patients: Results of the randomized open-label GPOH trial NB2004-HR. Ann. Oncol. 2020, 31, 422–429. [Google Scholar] [CrossRef]
- Hero, B.; Simon, T.; Spitz, R.; Ernestus, K.; Gnekow, A.K.; Scheel-Walter, H.G.; Schwabe, D.; Schilling, F.H.; Benz-Bohm, G.; Berthold, F. Localized infant neuroblastomas often show spontaneous regression: Results of the prospective trials NB95-S and NB97. J. Clin. Oncol. 2008, 26, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Streby, K.A.; Parisi, M.T.; Shulkin, B.L.; LaBarre, B.; Bagatell, R.; Diller, L.; Grupp, S.A.; Matthay, K.K.; Voss, S.D.; Yu, A.L.; et al. Impact of diagnostic and end-of-induction Curie scores with tandem high-dose chemotherapy and autologous transplants for metastatic high-risk neuroblastoma: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2023, 70, e30418. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Hero, B.; Schulte, J.H.; Deubzer, H.; Hundsdoerfer, P.; von Schweinitz, D.; Fuchs, J.; Schmidt, M.; Prasad, V.; Krug, B.; et al. 2017 GPOH Guidelines for Diagnosis and Treatment of Patients with Neuroblastic Tumors. Klin. Padiatr. 2017, 229, 147–167. [Google Scholar] [CrossRef]
- Matthay, K.K.; Maris, J.M.; Schleiermacher, G.; Nakagawara, A.; Mackall, C.L.; Diller, L.; Weiss, W.A. Neuroblastoma. Nat. Rev. Dis. Primers 2016, 2, 16078. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, V.P.; Matthay, K.K. Neuroblastoma: Clinical and biological approach to risk stratification and treatment. Cell Tissue Res. 2018, 372, 195–209. [Google Scholar] [CrossRef]
- Heipertz, A.E.; Pajtler, K.W.; Pfaff, E.; Schramm, K.; Blattner-Johnson, M.; Milde, T.; Jones, B.C.; Zuliani, C.; Hutter, C.; Lohi, O.; et al. Outcome of Children and Adolescents with Relapsed/Refractory/Progressive Malignancies Treated with Molecularly Informed Targeted Drugs in the Pediatric Precision Oncology Registry INFORM. JCO Precis Oncol. 2023, 7, e2300015, Erratum in JCO Precis. Oncol. 2023, 7, e2300341. [Google Scholar] [CrossRef]
- Wienke, J.; Dierselhuis, M.P.; Tytgat, G.A.M.; Kunkele, A.; Nierkens, S.; Molenaar, J.J. The immune landscape of neuroblastoma: Challenges and opportunities for novel therapeutic strategies in pediatric oncology. Eur. J. Cancer 2021, 144, 123–150. [Google Scholar] [CrossRef]
- Wolfl, M.; Jungbluth, A.A.; Garrido, F.; Cabrera, T.; Meyen-Southard, S.; Spitz, R.; Ernestus, K.; Berthold, F. Expression of MHC class I, MHC class II, and cancer germline antigens in neuroblastoma. Cancer Immunol. Immunother. 2005, 54, 400–406. [Google Scholar] [CrossRef]
- DuBois, S.G.; Macy, M.E.; Henderson, T.O. High-Risk and Relapsed Neuroblastoma: Toward More Cures and Better Outcomes; American Society of Clinical Oncology Educational Book: Alexandria, VA, USA, 2022; Volume 42, pp. 1–13. [Google Scholar] [CrossRef]
- Schengrund, C.L.; Shochat, S.J. Gangliosides in neuroblastomas. Neurochem. Pathol. 1988, 8, 189–202. [Google Scholar] [CrossRef]
- D’Angelo, G.; Capasso, S.; Sticco, L.; Russo, D. Glycosphingolipids: Synthesis and functions. FEBS J. 2013, 280, 6338–6353. [Google Scholar] [CrossRef]
- Cheung, I.Y.; Mauguen, A.; Modak, S.; Ragupathi, G.; Basu, E.M.; Roberts, S.S.; Kushner, B.H.; Cheung, N.K. Effect of Oral beta-Glucan on Antibody Response to Ganglioside Vaccine in Patients with High-Risk Neuroblastoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2023, 9, 242–250. [Google Scholar] [CrossRef]
- Del Bufalo, F.; De Angelis, B.; Caruana, I.; Del Baldo, G.; De Ioris, M.A.; Serra, A.; Mastronuzzi, A.; Cefalo, M.G.; Pagliara, D.; Amicucci, M.; et al. GD2-CART01 for Relapsed or Refractory High-Risk Neuroblastoma. N. Engl. J. Med. 2023, 388, 1284–1295. [Google Scholar] [CrossRef]
- Wu, Z.L.; Schwartz, E.; Seeger, R.; Ladisch, S. Expression of GD2 ganglioside by untreated primary human neuroblastomas. Cancer Res. 1986, 46, 440–443. [Google Scholar]
- Tang, X.X.; Shimada, H.; Ikegaki, N. Clinical Relevance of CD4 Cytotoxic T Cells in High-Risk Neuroblastoma. Front. Immunol. 2021, 12, 650427. [Google Scholar] [CrossRef] [PubMed]
- Uemura, S.; Ishida, T.; Thwin, K.K.M.; Yamamoto, N.; Tamura, A.; Kishimoto, K.; Hasegawa, D.; Kosaka, Y.; Nino, N.; Lin, K.S.; et al. Dynamics of Minimal Residual Disease in Neuroblastoma Patients. Front. Oncol. 2019, 9, 455. [Google Scholar] [CrossRef]
- Paret, C.; Ustjanzew, A.; Ersali, S.; Seidmann, L.; Jennemann, R.; Ziegler, N.; Malki, K.E.; Russo, A.; Wingerter, A.; Ortmuller, F.; et al. GD2 Expression in Medulloblastoma and Neuroblastoma for Personalized Immunotherapy: A Matter of Subtype. Cancers 2022, 14, 6051. [Google Scholar] [CrossRef]
- Ustjanzew, A.; Nedwed, A.S.; Sandhoff, R.; Faber, J.; Marini, F.; Paret, C. Unraveling the glycosphingolipid metabolism by leveraging transcriptome-weighted network analysis on neuroblastic tumors. Cancer Metab. 2024, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Massa, P.T. Specific suppression of major histocompatibility complex class I and class II genes in astrocytes by brain-enriched gangliosides. J. Exp. Med. 1993, 178, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- van der Haar Avila, I.; Windhouwer, B.; van Vliet, S.J. Current state-of-the-art on ganglioside-mediated immune modulation in the tumor microenvironment. Cancer Metastasis Rev. 2023, 42, 941–958. [Google Scholar] [CrossRef]
- Ladisch, S.; Wu, Z.L.; Feig, S.; Ulsh, L.; Schwartz, E.; Floutsis, G.; Wiley, F.; Lenarsky, C.; Seeger, R. Shedding of GD2 ganglioside by human neuroblastoma. Int. J. Cancer 1987, 39, 73–76. [Google Scholar] [CrossRef]
- Li, R.X.; Ladisch, S. Shedding of human neuroblastoma gangliosides. Biochim. Biophys. Acta 1991, 1083, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Morini, M.; Barco, S.; Ardito, M.; Cafaro, A.; Pigliasco, F.; Rossi, L.; Fragola, M.; Segalerba, D.; Conte, M.; Garaventa, A.; et al. Detection of plasma circulating GD2 ganglioside in patients with neuroblastoma and age-matched healthy children. Diagnostic and prognostic evaluation. Oncologist 2025, 30, oyaf008. [Google Scholar] [CrossRef]
- Sandhoff, R.; Sandhoff, K. Emerging concepts of ganglioside metabolism. FEBS Lett. 2018, 592, 3835–3864. [Google Scholar] [CrossRef]
- Brodeur, G.M.; Seeger, R.C.; Barrett, A.; Berthold, F.; Castleberry, R.P.; D’Angio, G.; De Bernardi, B.; Evans, A.E.; Favrot, M.; Freeman, A.I.; et al. International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J. Clin. Oncol. 1988, 6, 1874–1881. [Google Scholar] [CrossRef]
- van Groningen, T.; Koster, J.; Valentijn, L.J.; Zwijnenburg, D.A.; Akogul, N.; Hasselt, N.E.; Broekmans, M.; Haneveld, F.; Nowakowska, N.E.; Bras, J.; et al. Neuroblastoma is composed of two super-enhancer-associated differentiation states. Nat. Genet. 2017, 49, 1261–1266. [Google Scholar] [CrossRef]
- Cohn, S.L.; Salwen, H.; Quasney, M.W.; Ikegaki, N.; Cowan, J.M.; Herst, C.V.; Kennett, R.H.; Rosen, S.T.; DiGiuseppe, J.A.; Brodeur, G.M. Prolonged N-myc protein half-life in a neuroblastoma cell line lacking N-myc amplification. Oncogene 1990, 5, 1821–1827. [Google Scholar]
- Kaufman, M.E.; Vayani, O.R.; Moore, K.; Chlenski, A.; Wu, T.; Lee, S.M.; Desai, A.V.; He, C.; Cohn, S.L.; Applebaum, M.A. Characterizing Relationships between T-cell Inflammation and Outcomes in Patients with High-Risk Neuroblastoma According to Mesenchymal and Adrenergic Signatures. Cancer Res. Commun. 2024, 4, 2255–2266. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, G.; Schilbach-Stuckle, K.; Handgretinger, R.; Kaiser, P.; Hameister, H. Cytogenetic and molecular characterization of a newly established neuroblastoma cell line LS. Hum. Genet. 1991, 86, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Kendsersky, N.M.; Odrobina, M.; Mabe, N.W.; Farrel, A.; Grossmann, L.; Tsang, M.; Groff, D.; Wolpaw, A.J.; Narch, A.; Zammarchi, F.; et al. Lineage dependence of the neuroblastoma surfaceome defines tumor cell state-dependent and -independent immunotherapeutic targets. Neuro Oncol. 2025, 27, 1372–1384. [Google Scholar] [CrossRef]
- Schlesinger, H.R.; Gerson, J.M.; Moorhead, P.S.; Maguire, H.; Hummeler, K. Establishment and characterization of human neuroblastoma cell lines. Cancer Res. 1976, 36, 3094–3100. [Google Scholar]
- Jalava, A.M.; Heikkila, J.; Akerlind, G.; Pettit, G.R.; Akerman, K.E. Effects of bryostatins 1 and 2 on morphological and functional differentiation of SH-SY5Y human neuroblastoma cells. Cancer Res. 1990, 50, 3422–3428. [Google Scholar]
- George, R.E.; Sanda, T.; Hanna, M.; Frohling, S.; Luther, W., II; Zhang, J.; Ahn, Y.; Zhou, W.; London, W.B.; McGrady, P.; et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature 2008, 455, 975–978. [Google Scholar] [CrossRef]
- El-Badry, O.M.; Romanus, J.A.; Helman, L.J.; Cooper, M.J.; Rechler, M.M.; Israel, M.A. Autonomous growth of a human neuroblastoma cell line is mediated by insulin-like growth factor II. J. Clin. Investig. 1989, 84, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Biedler, J.L.; Spengler, B.A. A novel chromosome abnormality in human neuroblastoma and antifolate-resistant Chinese hamster cell lives in culture. J. Natl. Cancer Inst. 1976, 57, 683–695. [Google Scholar] [CrossRef]
- Melodia, A.; Cornara, L.; Bertelli, R.; Canepa, M.; Gimelli, G.; Repetto, G.; Cornaglia-Ferraris, P. New line of human neuroblastoma derived from bone marrow. Pathologica 1986, 78, 371–384. [Google Scholar]
- Berlak, M.; Tucker, E.; Dorel, M.; Winkler, A.; McGearey, A.; Rodriguez-Fos, E.; da Costa, B.M.; Barker, K.; Fyle, E.; Calton, E.; et al. Mutations in ALK signaling pathways conferring resistance to ALK inhibitor treatment lead to collateral vulnerabilities in neuroblastoma cells. Mol. Cancer 2022, 21, 126. [Google Scholar] [CrossRef] [PubMed]
- Gross, N.; Favre, S.; Beck, D.; Meyer, M. Differentiation-related expression of adhesion molecules and receptors on human neuroblastoma tissues, cell lines and variants. Int. J. Cancer 1992, 52, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Tumilowicz, J.J.; Nichols, W.W.; Cholon, J.J.; Greene, A.E. Definition of a continuous human cell line derived from neuroblastoma. Cancer Res. 1970, 30, 2110–2118. [Google Scholar]
- Lode, H.N.; Xiang, R.; Varki, N.M.; Dolman, C.S.; Gillies, S.D.; Reisfeld, R.A. Targeted interleukin-2 therapy for spontaneous neuroblastoma metastases to bone marrow. J. Natl. Cancer Inst. 1997, 89, 1586–1594. [Google Scholar] [CrossRef]
- Ratner, N.; Brodeur, G.M.; Dale, R.C.; Schor, N.F. The “neuro” of neuroblastoma: Neuroblastoma as a neurodevelopmental disorder. Ann. Neurol. 2016, 80, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.K.; Tsai, Y.T.; Ariga, T. Functional roles of gangliosides in neurodevelopment: An overview of recent advances. Neurochem. Res. 2012, 37, 1230–1244. [Google Scholar] [CrossRef]
- Schengrund, C.L. Gangliosides and Neuroblastomas. Int. J. Mol. Sci. 2020, 21, 5313. [Google Scholar] [CrossRef]
- Hettmer, S.; Malott, C.; Woods, W.; Ladisch, S.; Kaucic, K. Biological stratification of human neuroblastoma by complex “B” pathway ganglioside expression. Cancer Res. 2003, 63, 7270–7276. [Google Scholar]
- Laviad, E.L.; Albee, L.; Pankova-Kholmyansky, I.; Epstein, S.; Park, H.; Merrill, A.H., Jr.; Futerman, A.H. Characterization of ceramide synthase 2: Tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J. Biol. Chem. 2008, 283, 5677–5684. [Google Scholar] [CrossRef]
- Novgorodov, S.A.; Chudakova, D.A.; Wheeler, B.W.; Bielawski, J.; Kindy, M.S.; Obeid, L.M.; Gudz, T.I. Developmentally regulated ceramide synthase 6 increases mitochondrial Ca2+ loading capacity and promotes apoptosis. J. Biol. Chem. 2011, 286, 4644–4658. [Google Scholar] [CrossRef]
- Becker, I.; Wang-Eckhardt, L.; Yaghootfam, A.; Gieselmann, V.; Eckhardt, M. Differential expression of (dihydro)ceramide synthases in mouse brain: Oligodendrocyte-specific expression of CerS2/Lass2. Histochem. Cell Biol. 2008, 129, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Mabe, N.W.; Huang, M.; Dalton, G.N.; Alexe, G.; Schaefer, D.A.; Geraghty, A.C.; Robichaud, A.L.; Conway, A.S.; Khalid, D.; Mader, M.M.; et al. Transition to a mesenchymal state in neuroblastoma confers resistance to anti-GD2 antibody via reduced expression of ST8SIA1. Nat. Cancer 2022, 3, 976–993. [Google Scholar] [CrossRef]
- Matthay, K.K.; Reynolds, C.P.; Seeger, R.C.; Shimada, H.; Adkins, E.S.; Haas-Kogan, D.; Gerbing, R.B.; London, W.B.; Villablanca, J.G. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A children’s oncology group study. J. Clin. Oncol. 2009, 27, 1007–1013, Erratum in J. Clin. Oncol. 2014, 32, 1862–1863. [Google Scholar] [CrossRef]
- Leskawa, K.C.; Hogan, E.L. Quantitation of the in vitro neuroblastoma response to exogenous, purified gangliosides. J. Neurosci. Res. 1985, 13, 539–550. [Google Scholar] [CrossRef]
- Hettmer, S.; McCarter, R.; Ladisch, S.; Kaucic, K. Alterations in neuroblastoma ganglioside synthesis by induction of GD1b synthase by retinoic acid. Br. J. Cancer 2004, 91, 389–397. [Google Scholar] [CrossRef]
- Li, R.; Ladisch, S. Alteration of neuroblastoma ganglioside metabolism by retinoic acid. J. Neurochem. 1992, 59, 2297–2303. [Google Scholar] [CrossRef]
- Osanai, T.; Watanabe, Y.; Sanai, Y. Glycolipid sialyltransferases are enhanced during neural differentiation of mouse embryonic carcinoma cells, P19. Biochem. Biophys. Res. Commun. 1997, 241, 327–333. [Google Scholar] [CrossRef]
- Chiricozzi, E.; Biase, E.D.; Maggioni, M.; Lunghi, G.; Fazzari, M.; Pome, D.Y.; Casellato, R.; Loberto, N.; Mauri, L.; Sonnino, S. GM1 promotes TrkA-mediated neuroblastoma cell differentiation by occupying a plasma membrane domain different from TrkA. J. Neurochem. 2019, 149, 231–241. [Google Scholar] [CrossRef]
- Li, R.; Ladisch, S. Inhibition of endogenous ganglioside synthesis does not block neurite formation by retinoic acid-treated neuroblastoma cells. J. Biol. Chem. 1997, 272, 1349–1354. [Google Scholar] [CrossRef]
- Kracun, I.; Rosner, H.; Drnovsek, V.; Vukelic, Z.; Cosovic, C.; Trbojevic-Cepe, M.; Kubat, M. Gangliosides in the human brain development and aging. Neurochem. Int. 1992, 20, 421–431. [Google Scholar] [CrossRef]
- Dalton, G.; Kim, W.-J.; Mabe, N.; Huang, M.; Rotiroti, M.; Tousley, A.; Stegmaier, K.; Majzner, R. 244 Targeting gangliosides in pediatric cancer. J. Immunother. Cancer 2023, 11, 283. [Google Scholar]
- Terzic, T.; Cordeau, M.; Herblot, S.; Teira, P.; Cournoyer, S.; Beaunoyer, M.; Peuchmaur, M.; Duval, M.; Sartelet, H. Expression of Disialoganglioside (GD2) in Neuroblastic Tumors: A Prognostic Value for Patients Treated with Anti-GD2 Immunotherapy. Pediatr. Dev. Pathol. 2018, 21, 355–362. [Google Scholar] [CrossRef]
- Schumacher-Kuckelkorn, R.; Volland, R.; Gradehandt, A.; Hero, B.; Simon, T.; Berthold, F. Lack of immunocytological GD2 expression on neuroblastoma cells in bone marrow at diagnosis, during treatment, and at recurrence. Pediatr. Blood Cancer 2017, 64, 46–56. [Google Scholar] [CrossRef]
- Ladisch, S. Biological Significance of Tumor Gangliosides: Shedding, Transfer, and Immunosuppression. In Frontiers in Bioactive Lipids; Vanderhoek, J.Y., Ed.; Springer: Boston, MA, USA, 1996; pp. 215–221. [Google Scholar] [CrossRef]
- Ladisch, S.; Li, R.; Olson, E. Ceramide structure predicts tumor ganglioside immunosuppressive activity. Proc. Natl. Acad. Sci. USA 1994, 91, 1974–1978. [Google Scholar] [CrossRef]
- Bergelson, L.D.; Dyatlovitskaya, E.V.; Klyuchareva, T.E.; Kryukova, E.V.; Lemenovskaya, A.F.; Matveeva, V.A.; Sinitsyna, E.V. The role of glycosphingolipids in natural immunity. Gangliosides modulate the cytotoxicity of natural killer cells. Eur. J. Immunol. 1989, 19, 1979–1983. [Google Scholar] [CrossRef]
- Yamaji, T.; Teranishi, T.; Alphey, M.S.; Crocker, P.R.; Hashimoto, Y. A small region of the natural killer cell receptor, Siglec-7, is responsible for its preferred binding to alpha 2,8-disialyl and branched alpha 2,6-sialyl residues. A comparison with Siglec-9. J. Biol. Chem. 2002, 277, 6324–6332. [Google Scholar] [CrossRef]
- Falco, M.; Biassoni, R.; Bottino, C.; Vitale, M.; Sivori, S.; Augugliaro, R.; Moretta, L.; Moretta, A. Identification and molecular cloning of p75/AIRM1, a novel member of the sialoadhesin family that functions as an inhibitory receptor in human natural killer cells. J. Exp. Med. 1999, 190, 793–802. [Google Scholar] [CrossRef]
- Ibarlucea-Benitez, I.; Weitzenfeld, P.; Smith, P.; Ravetch, J.V. Siglecs-7/9 function as inhibitory immune checkpoints in vivo and can be targeted to enhance therapeutic antitumor immunity. Proc. Natl. Acad. Sci. USA 2021, 118, e2107424118. [Google Scholar] [CrossRef]
- Theruvath, J.; Menard, M.; Smith, B.A.H.; Linde, M.H.; Coles, G.L.; Dalton, G.N.; Wu, W.; Kiru, L.; Delaidelli, A.; Sotillo, E.; et al. Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication. Nat. Med. 2022, 28, 333–344. [Google Scholar] [CrossRef]
- Rapoport, E.; Mikhalyov, I.; Zhang, J.; Crocker, P.; Bovin, N. Ganglioside binding pattern of CD33-related siglecs. Bioorg. Med. Chem. Lett. 2003, 13, 675–678. [Google Scholar] [CrossRef]
- Vuchkovska, A.; Glanville, D.G.; Scurti, G.M.; Nishimura, M.I.; White, P.; Ulijasz, A.T.; Iwashima, M. Siglec-5 is an inhibitory immune checkpoint molecule for human T cells. Immunology 2022, 166, 238–248. [Google Scholar] [CrossRef]
- Balis, F.M.; Busch, C.M.; Desai, A.V.; Hibbitts, E.; Naranjo, A.; Bagatell, R.; Irwin, M.; Fox, E. The ganglioside G(D2) as a circulating tumor biomarker for neuroblastoma. Pediatr. Blood Cancer 2020, 67, e28031. [Google Scholar] [CrossRef]
- Karlsson, G.; Mansson, J.E.; Wikstrand, C.; Bigner, D.; Svennerholm, L. Characterization of the binding epitope of the monoclonal antibody DMAb-1 to ganglioside GM2. Biochim. Biophys. Acta 1990, 1043, 267–272. [Google Scholar] [CrossRef]
- Hettmer, S.; Ladisch, S.; Kaucic, K. Low complex ganglioside expression characterizes human neuroblastoma cell lines. Cancer Lett. 2005, 225, 141–149. [Google Scholar] [CrossRef][Green Version]
- Kaneko, T.; Okita, H.; Nakajima, H.; Iijima, K.; Ogasawara, N.; Miyagawa, Y.; Katagiri, Y.U.; Nakagawa, A.; Kiyokawa, N.; Sato, T.; et al. Neuroblastoma cells can be classified according to glycosphingolipid expression profiles identified by liquid chromatography-tandem mass spectrometry. Int. J. Oncol. 2010, 37, 1279–1288. [Google Scholar] [CrossRef]
- Rebhan, M.; Vacun, G.; Bayreuther, K.; Rosner, H. Altered ganglioside expression by SH-SY5Y cells upon retinoic acid-induced neuronal differentiation. Neuroreport 1994, 5, 941–944. [Google Scholar] [CrossRef]
- Harenza, J.L.; Diamond, M.A.; Adams, R.N.; Song, M.M.; Davidson, H.L.; Hart, L.S.; Dent, M.H.; Fortina, P.; Reynolds, C.P.; Maris, J.M. Transcriptomic profiling of 39 commonly-used neuroblastoma cell lines. Sci. Data 2017, 4, 170033, Erratum in Sci. Data 2017, 4, 170183. [Google Scholar] [CrossRef]
- Julien, S.; Bobowski, M.; Steenackers, A.; Le Bourhis, X.; Delannoy, P. How Do Gangliosides Regulate RTKs Signaling? Cells 2013, 2, 751–767. [Google Scholar] [CrossRef]
- Sasaki, H.; Momoi, T.; Yamanaka, C.; Yorifuji, T.; Kaji, M.; Mikawa, H. Changes in the ganglioside composition of human neuroblastoma cells under different growth conditions. Int. J. Cancer 1991, 47, 742–745. [Google Scholar] [CrossRef]
- Molenaar, J.J.; Koster, J.; Zwijnenburg, D.A.; van Sluis, P.; Valentijn, L.J.; van der Ploeg, I.; Hamdi, M.; van Nes, J.; Westerman, B.A.; van Arkel, J.; et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature 2012, 483, 589–593. [Google Scholar] [CrossRef]
- Hartlieb, S.A.; Sieverling, L.; Nadler-Holly, M.; Ziehm, M.; Toprak, U.H.; Herrmann, C.; Ishaque, N.; Okonechnikov, K.; Gartlgruber, M.; Park, Y.G.; et al. Alternative lengthening of telomeres in childhood neuroblastoma from genome to proteome. Nat. Commun. 2021, 12, 1269. [Google Scholar] [CrossRef]
- Hagemann, S.; Misiak, D.; Bell, J.L.; Fuchs, T.; Lederer, M.I.; Bley, N.; Hammerle, M.; Ghazy, E.; Sippl, W.; Schulte, J.H.; et al. IGF2BP1 induces neuroblastoma via a druggable feedforward loop with MYCN promoting 17q oncogene expression. Mol. Cancer 2023, 22, 88. [Google Scholar] [CrossRef]
- Consortium, S.M.-I. A comprehensive assessment of RNA-seq accuracy, reproducibility and information content by the Sequencing Quality Control Consortium. Nat. Biotechnol. 2014, 32, 903–914. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rucker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]

| Patient ID | Risk | Last Therapy Before Sample Collection | Age at Diagnosis | Sex | Histology | Sample Localization | Disease Status at Sample Collection | MYCN | GG Profile | Ceramide |
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | LR (4S) | none | 8 m | m | NBL, pd | Metastatic (skin, lumbar) | Primary disease | NA | A | normal |
| #2 | LR (4S) | none | 2 m | m | NBL, pd | Primary tumor (adrenal gland) | Primary disease | NA | A | normal |
| #3 | LR | none | 5 m | f | NBL, pd | Primary tumor (adrenal gland) | Primary disease | NA | A | normal |
| #4 | LR | none | 1 m | f | NBL, pd | Primary tumor (adrenal gland) | Primary disease | NA | A | normal |
| #5 | LR | none | 4 m | f | NBL, pd | Primary tumor (adrenal gland) | Primary disease | NA | A | normal |
| #6 | LR | none | 4 y | f | NBL, dif/mature GN | Primary tumor (adrenal gland) | Primary disease | NA | A | normal |
| #7 | LR | none | 5 m | m | NBL, pd | Primary tumor (adrenal gland) | Primary disease | NA | A | normal |
| #8 | LR | none | 9 m | m | NBL, pd | Primary tumor (retroperitoneal) | Primary disease | NA | A | normal |
| #9 | HR | none | 1 y | m | NBL, pd | Metastatic (lymph node, supraclavicular) | Primary disease | A | B | normal |
| #10 | HR | none | 2 y | m | NBL, pd | Primary tumor (adrenal gland) | Primary disease | A | C | long |
| #11 | HR | none | 8 y | m | NBL, pd | Metastatic (lymph node, cervical) | Primary disease | NA | B | long |
| #12 | HR | none | 1 y | m | NBL, pd | Primary tumor (retroperitoneal) | Primary disease | A | B | short |
| #13 | HR | chemotherapy | 4 y | f | NBL, pd | Metastatic (orbital) | Primary refractory, PD | NA | C | long |
| #14 | HR | chemotherapy | 8 y | m | NBL, dif | Primary tumor (retroperitoneal) | Primary disease | NA | D | normal |
| #15 | HR | anti-GD2 mAb | 4 y | f | NBL, pd | Metastatic (jaw) | 1st Relapse, PD | NA | A | short |
| #16 | HR | anti-GD2 mAb | 1 y | m | GNB | Metastatic (soft tissue, axilla) | Primary refractory, SD | A | D | normal |
| #17 | HR | anti-GD2 mAb | 8 y | m | NBL, pd | Metastatic (cns) | 1st relapse (initial) | NA | B | long |
| #18 | HR | anti-GD2 mAb | 8 y | m | NBL, pd | Metastatic (lung) | 1st relapse, PD | NA | E | n. d. |
| Profile | Prevalent GGs | Risk Status |
|---|---|---|
| A | b-series, particularly GT1b | All LR and some HR |
| B | GD2 | HR |
| C | GM3 and or GD3 | HR |
| D | GT1b and GD1a | HR |
| E | GD1a and GM2 | HR |
| Cell Line | Origin | Species | Subtype | Age | Sex | MYCN | ALK | GG Profile |
|---|---|---|---|---|---|---|---|---|
| NBL-S [28] | Adrenal tumor (primary) | Human | Adr [29] | 36 m | male | No | No | E |
| LS [30] | Abdominal tumor | Human | Mes [31] | 16 m | female | Yes | No | (E) |
| CHP-134 [32] | Left adrenal area | Human | Adr [27] | 13 m | male | Yes | No | B |
| SH-SY5Y [33] | Bone marrow (subclon) | Human | Adr [27] | 4 y | female | No | F1174L [34] | E |
| SK-N-AS [35] | Bone marrow (metastasis) | Human | Mes [27] | 6 y | female | No | No | C |
| SK-N-BE(2)-C [36] | Bone marrow (subclon) | Human | Adr [27] | 26 m | male | Yes | No | E |
| GI-ME-N [37] | Bone marrow (metastasis) | Human | Mes [27] | 42 m | female | No | No | C |
| LA-N-5 [38] | Bone marrow (metastasis) | Human | Adr [27] | 4 m | male | Yes | R1275Q [38] | (A) |
| ACN [39] | Bone marrow (metastasis) | Human | Mes [27] | 36 m | male | No | No | C |
| IMR-32 [40] | Bone marrow (metastasis) | Human | Adr [27] | 13 m | male | Yes | No | (E) |
| NXS2 [41] | Murine | - | - | - | unknown | unknown | E |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paret, C.; Wingerter, A.; Seidmann, L.; Ustjanzew, A.; Sathyamurthy, S.; Ludwig, J.; Schwickerath, P.; Brignole, C.; Pastorino, F.; Wagner, S.; et al. Ganglioside Profiling Uncovers Distinct Patterns in High-Risk Neuroblastoma. Int. J. Mol. Sci. 2025, 26, 8431. https://doi.org/10.3390/ijms26178431
Paret C, Wingerter A, Seidmann L, Ustjanzew A, Sathyamurthy S, Ludwig J, Schwickerath P, Brignole C, Pastorino F, Wagner S, et al. Ganglioside Profiling Uncovers Distinct Patterns in High-Risk Neuroblastoma. International Journal of Molecular Sciences. 2025; 26(17):8431. https://doi.org/10.3390/ijms26178431
Chicago/Turabian StyleParet, Claudia, Arthur Wingerter, Larissa Seidmann, Arsenij Ustjanzew, Shobha Sathyamurthy, Jannis Ludwig, Philipp Schwickerath, Chiara Brignole, Fabio Pastorino, Saskia Wagner, and et al. 2025. "Ganglioside Profiling Uncovers Distinct Patterns in High-Risk Neuroblastoma" International Journal of Molecular Sciences 26, no. 17: 8431. https://doi.org/10.3390/ijms26178431
APA StyleParet, C., Wingerter, A., Seidmann, L., Ustjanzew, A., Sathyamurthy, S., Ludwig, J., Schwickerath, P., Brignole, C., Pastorino, F., Wagner, S., El Malki, K., Roth, W., Sandhoff, R., & Faber, J. (2025). Ganglioside Profiling Uncovers Distinct Patterns in High-Risk Neuroblastoma. International Journal of Molecular Sciences, 26(17), 8431. https://doi.org/10.3390/ijms26178431







