Jamamina: A Green Nanostructured Lipid Carrier with NaDES and Curcumin for Redox Modulation and Inflammatory Disorders
Abstract
1. Introduction
2. Results
2.1. Cytokine Modulation and Anti-Oxidation Potential
2.2. Metalloprotease Activity
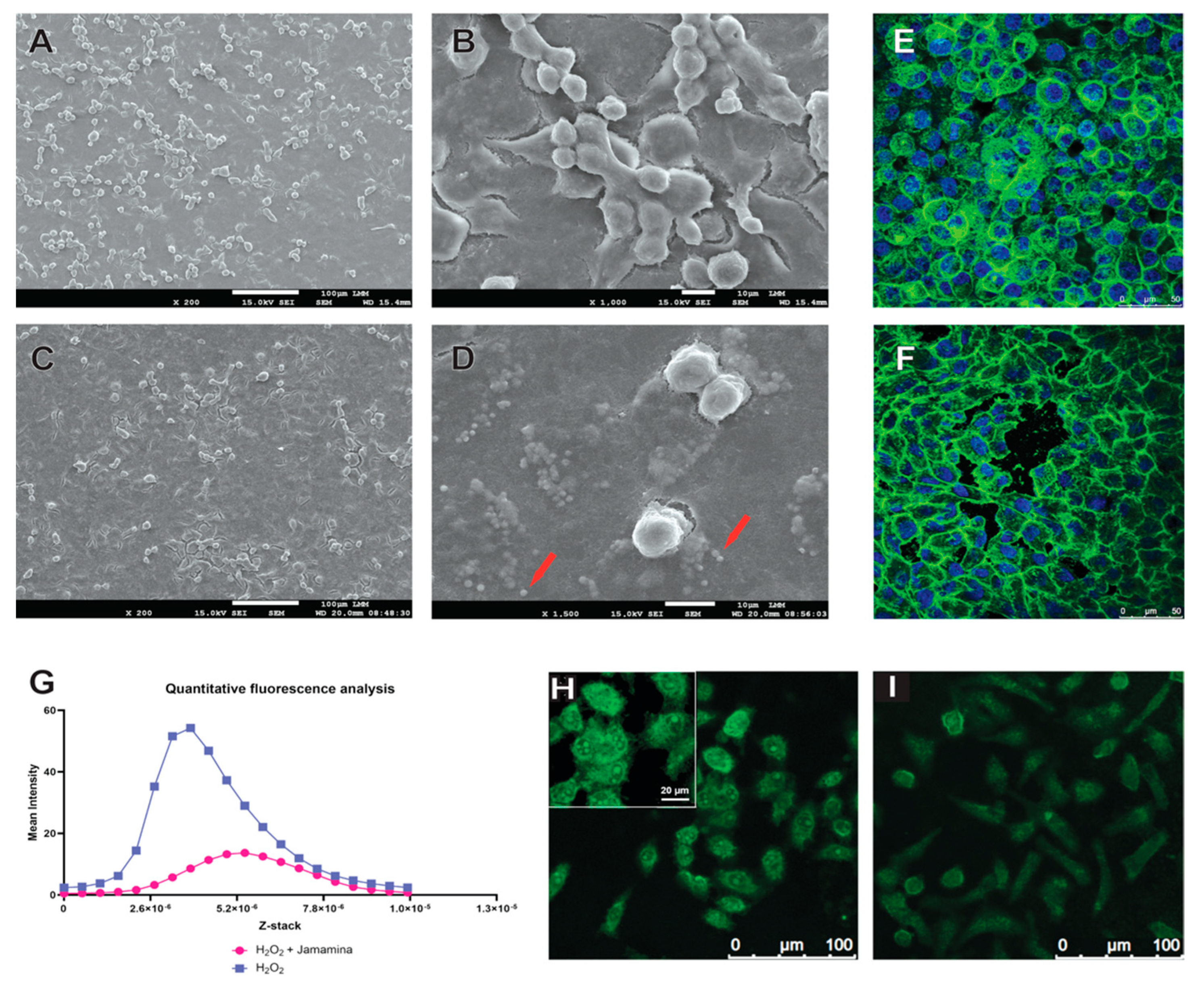
2.3. Fibroblast-like Cell Morphodynamics
3. Discussion
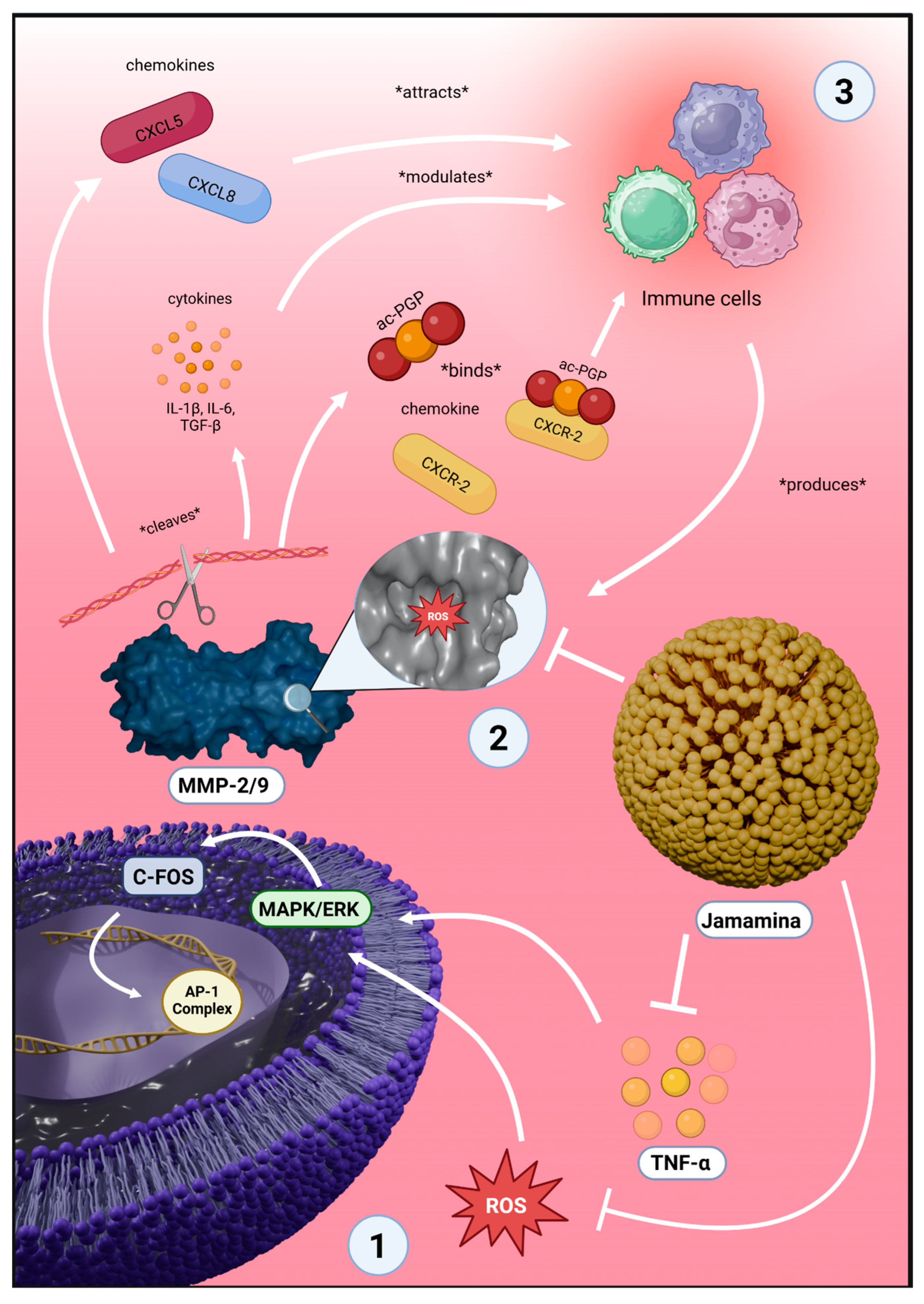
3.1. Cytokine Reprogramming and MMP Suppression by Jamamina NLCs
3.2. Morphodynamics and Cytoskeletal Protection Under Oxidative Stress
3.3. Redefining Therapeutics Through Circular Nanodesign
4. Materials and Methods
4.1. Jamamina Profile
4.2. Cell Maintenance
4.3. Morphology Study of the Cells Post-NLC Treatment
4.4. DPPH Radical Scavenging Assay
4.5. Reactive Oxygen Species (ROS) Production Assay
4.6. Immunofluorescence for the Localization of Actin Filaments
4.7. Immunoenzymatic Tests
4.8. Matrix Metalloprotease Tests
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NLC | Nanostructured Lipid Carrier |
| NaDES | Natural Deep Eutectic Solvent |
| NIB | Nova Indústria Brasil (New Industry Brazil) |
| SDGs | Sustainable Development Goals |
| DLS | Dynamic Light Scattering |
| ZP | Zeta Potential |
| EE | Encapsulation Efficiency |
| HD | Hydrodynamic Diameter |
| PDI | Polydispersity Index |
| MMP | Metalloproteinase |
| TEM | Transmission Electron Microscopy |
| SEM | Scanning Electron Microscope |
| XRD | X-ray Diffraction |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| ROS | Reactive Oxygen Species |
| IL | Interleukin |
| TNF | Tumor Necrosis Factor |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
References
- Devrimci-Ozguven, H.; Kundakci, T.N.; Kumbasar, H.; Boyvat, A. The depression, anxiety, life satisfaction and affective expression levels in psoriasis patients. J. Eur. Acad. Dermatol. Venereol. 2000, 14, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Wittkowski, A.; Richards, H.L.; Griffiths, C.E.; Main, C.J. The impact of psychological and clinical factors on quality of life in individuals with atopic dermatitis. J. Psychosom. Res. 2004, 57, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Ben-Gashir, M.A.; Seed, P.T.; Hay, R.J. Quality of life and disease severity are correlated in children with atopic dermatitis. Br. J. Dermatol. 2004, 150, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.L.; Balkrishnan, R.; Feldman, S.R.; Fleischer, A.B., Jr.; Manuel, J.C. The burden of atopic dermatitis: Impact on the patient, family, and society. Pediatr. Dermatol. 2005, 22, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Bhosle, M.J.; Kulkarni, A.; Feldman, S.R.; Balkrishnan, R. Quality of life in patients with psoriasis. Health Qual. Life Outcomes 2006, 4, 35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kurd, S.K.; Troxel, A.B.; Crits-Christoph, P.; Gelfand, J.M. The risk of depression, anxiety, and suicidality in patients with psoriasis: A population-based cohort study. Arch. Dermatol. 2010, 146, 891–895. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Richards, H.L.; Fortune, D.G.; Griffiths, C.E.; Main, C.J. The contribution of perceptions of stigmatisation to disability in patients with psoriasis. J. Psychosom. Res. 2001, 50, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Schuh, L.; Salgado, L.A.; Piau, T.B.; Silveira, A.P.; Leal, C.; Romera, L.F.; Radicchi, M.A.; Santos, M.-K.M.S.; Falcao, L.; Grisolia, C.K.; et al. Integrating Natural Deep Eutectic Solvents into Nanostructured Lipid Carriers: An Industrial Look. Pharmaceuticals 2024, 17, 855. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.I.; Choi, S.; Roh, W.S.; Lee, J.H.; Kim, T.-G. Cellular Senescence and Inflammaging in the Skin Microenvironment. Int. J. Mol. Sci. 2021, 22, 3849. [Google Scholar] [CrossRef]
- Gauthier, V.; Kyriazi, M.; Nefla, M.; Pucino, V.; Raza, K.; Buckley, C.D.; Alsaleh, G. Fibroblast heterogeneity: Keystone of tissue homeostasis and pathology in inflammation and ageing. Front. Immunol. 2023, 14, 1137659. [Google Scholar] [CrossRef]
- Bener, M.; Özyürek, M.; Güçlü, K.; Apak, R. Optimization of Microwave-Assisted Extraction of Curcumin From Curcuma longa L. (Turmeric) and Evaluation of Antioxidant Activity in Multi-Test Systems. Rec. Nat. Prod. 2016, 10, 542–554. [Google Scholar]
- Farhood, B.; Mortezaee, K.; Goradel, N.H.; Khanlarkhani, N.; Salehi, E.; Nashtaei, M.S.; Najafi, M.; Sahebkar, A. Curcumin as an anti-inflammatory agent: Implications to radiotherapy and chemotherapy. J. Cell. Physiol. 2018, 234, 5728–5740. [Google Scholar] [CrossRef]
- Naeini, M.B.; Momtazi, A.A.; Jaafari, M.R.; Johnston, T.P.; Barreto, G.; Banach, M.; Sahebkar, A. Antitumor effects of curcumin: A lipid perspective. J. Cell. Physiol. 2019, 234, 14743–14758. [Google Scholar] [CrossRef]
- Vollono, L.; Falconi, M.; Gaziano, R.; Iacovelli, F.; Dika, E.; Terracciano, C.; Bianchi, L.; Campione, E. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2169. [Google Scholar] [CrossRef]
- Ganassin, R.; da Silva, V.C.M.; Araujo, V.H.S.; Tavares, G.R.; da Silva, P.B.; Cáceres-Vélez, P.R.; Porcel, J.E.M.; Rodrigues, M.C.; Andreozzi, P.; Fernandes, R.P.; et al. Solid lipid nanoparticles loaded with curcumin: Development and In Vitro toxicity against Ct26 cells. Nanomedicine 2022, 17, 167–179. [Google Scholar] [CrossRef]
- Mello, V.C.; Araújo, V.H.S.; de Paiva, K.L.R.; Simões, M.M.; Marques, D.C.; da Silva Costa, N.R.; de Souza, I.F.; da Silva, P.B.; Santos, I.; Almeida, R.; et al. Development of New Natural Lipid-Based Nanoparticles Loaded with Aluminum-Phthalocyanine for Photodynamic Therapy against Melanoma. Nanomaterials 2022, 12, 3547. [Google Scholar] [CrossRef]
- Silva, C.N.S.; Fernandes, C.P.; Ferreira, J.C.V.A.M.; Florentino, R.A.C.A.C.; Bereau, J.-C.R.D. Development of Nanoemulsions with Tucumã (Astrocaryum vulgare) Fruit. Oil. J. Nanomed. Res. 2015, 2, 24. [Google Scholar] [CrossRef]
- Batista, L.L.; Koga, R.d.C.R.; Teixeira, A.V.T.d.L.; Teixeira, T.A.; de Melo, E.L.; Carvalho, J.C.T. Clinical Safety of a Pharmaceutical Formulation Containing an Extract of Acmella oleracea in Patients with Premature Ejaculation: A Pilot Study. Am. J. Men’s Health 2023, 17, 15579883231167819. [Google Scholar] [CrossRef]
- Rossato, A.; da Silva Silveira, L.; Oliveira, P.S.; de Souza Filho, W.P.; Wagner, R.; Klein, B.; de Souza, D.; Baldissera, M.D.; Sagrillo, M.R. Evaluation of anti-inflammatory and healing activity of a nano-structured lipid carrier containing tucuman butter oil and butter. Discip. Sci. Nat. Tecnol. 2020, 21, 99–108. [Google Scholar] [CrossRef]
- Confederação Nacional da Indústria. Nova Indústria Brasil Está em Sintonia com o Mapa Estratégico da Indústria 2023; Envolverde/ODS9: 2024. Available online: https://envolverde.com.br/tudo-sobre-ods/ods9/nova-industria-brasil-das-seis-missoes-a-mais-importante-e-a-transicao-energetica-avalia-a-cni/ (accessed on 27 August 2025).
- Governo Federal. Brasil Ganha Nova Política Industrial com Metas e Ações Para o Desenvolvimento Até 2033. Ministério do Desenvolvimento, Indústria, Comércio e Serviços. Available online: https://www.gov.br/mdic/pt-br/assuntos/noticias/2024/janeiro/brasil-ganha-nova-politica-industrial-com-metas-e-acoes-para-o-desenvolvimento-ate-2033 (accessed on 27 August 2025).
- Kumar, A.; Singh, R.; Pathak, R.; Singh, A.; Rai, S.; Singh, J.; Singh, H. Integrating Green Nanotechnology with Sustainable Development Goals: A Pathway to Sustainable Innovation. Discov. Sustain. 2024, 5, 364. [Google Scholar] [CrossRef]
- Nhani, G.B.B.; da Silva, D.G.; Leite, M.P.; dos Santos, P.A.; Chorilli, M.; Bauab, T.M.; Grespan, R. High-Tech Sustainable Beauty: Exploring Nanotechnology for the Development of Cosmetics Using Plant and Animal By-Products. Cosmetics 2024, 11, 112. [Google Scholar] [CrossRef]
- Peixoto Araujo, N.M.; Arruda, H.S.; Marques, D.R.P.; de Oliveira, W.Q.; Pereira, G.A.; Pastore, G.M. Functional and nutritional properties of selected Amazon fruits: A review. Food Res. Int. 2021, 147, 110520. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.O.; Garcia, L.C. Jambu (Spilanthes oleracea L.). In Hortaliças não-convencionais da Amazônia; ALICE; Embrapa Amazônia Ocidental: Manaus, Brasil, 1997. [Google Scholar]
- Dubey, S.; Maity, S.; Singh, M.; Saraf, S.A.; Saha, S. Phytochemistry, Pharmacology and Toxicology of Spilanthes acmella: A Review. Adv. Pharmacol. Sci. 2013, 2013, 423750. [Google Scholar] [CrossRef]
- Durand, E.; Villeneuve, P.; Bourlieu-lacanal, C.; Carrière, F. Chapter Six—Natural Deep Eutectic Solvents: Hypothesis for Their Possible Roles in Cellular Functions and Interaction with Membranes and Other Organized Biological Systems. In Advances in Botanical Research; Verpoorte, R., Witkamp, G.J., Choi, Y.H., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 97; pp. 133–158. [Google Scholar] [CrossRef]
- Schuh, L.; Reginato, M.; Florêncio, I.; Falcao, L.; Boron, L.; Gris, E.F.; Mello, V.; Báo, S.N. From Nature to Innovation: The Uncharted Potential of Natural Deep Eutectic Solvents. Molecules 2023, 28, 7653. [Google Scholar] [CrossRef]
- ANVISA. Instrução Normativa—IN n 211, de 1 de março de 2023. Available online: https://www.in.gov.br/en/web/dou/-/instrucao-normativa-in-n-211-de-1-de-marco-de-2023-468509746 (accessed on 27 August 2025).
- ANVISA. Consultas ANVISA—Medicamentos. Available online: https://consultas.anvisa.gov.br/#/medicamentos/q/?substancia=1176 (accessed on 17 June 2024).
- Mello, V.C.; de Brito, G.O.; Radicchi, M.A.; Florêncio, I.; Piau, T.B.; Ferreira, E.A.; de Azevedo Chang, L.F.; Silveira, A.P.; Simões, M.M.; de Paiva, K.L.R.; et al. Advanced Solubilization of Brazilian Cerrado Byproduct Extracts Using Green Nanostructured Lipid Carriers and NaDESs for Enhanced Antioxidant Potentials. Antioxidants 2025, 14, 290. [Google Scholar] [CrossRef]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A Panoramic Review of IL-6: Structure, Pathophysiological Roles and Inhibitors. Bioorganic Med. Chem. 2020, 28, 115327. [Google Scholar] [CrossRef]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and Therapeutic Potential of Interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Caldito, N. Role of Tumor Necrosis Factor-Alpha in the Central Nervous System: A Focus on Autoimmune Disorders. Front. Immunol. 2023, 14, 1213448. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, T.; Sawada, Y. Psoriasis and Systemic Inflammatory Disorders. Int. J. Mol. Sci. 2022, 23, 4457. [Google Scholar] [CrossRef]
- Liu, Y.-Z.; Wang, Y.-X.; Jiang, C.-L. Inflammation: The Common Pathway of Stress-Related Diseases. Front. Hum. Neurosci. 2017, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Chen, W.-W.; Zhang, X.; Huang, W.-J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef]
- Michalak, K.P.; Michalak, A.Z. Understanding chronic inflammation: Couplings between cytokines, ROS, NO, Cai2+, HIF-1α, Nrf2 and autophagy. Front. Immunol. 2025, 16, 1558263. [Google Scholar] [CrossRef]
- Corre, I.; Paris, F.; Huot, J. The p38 pathway, a major pleiotropic cascade that transduces stress and metastatic signals in endothelial cells. Oncotarget 2017, 8, 55684–55714. [Google Scholar] [CrossRef]
- Liu, X.; Ye, F.; Xiong, H.; Hu, D.; Limb, G.A.; Xie, T.; Peng, L.; Yang, W.; Sun, Y.; Zhou, M.; et al. Il-1 beta upregulates Il-8 production in human müller cells through activation of the P38 mapk and erk1/2 signaling pathways. Inflammation 2014, 37, 1486–1495. [Google Scholar] [CrossRef]
- Chen, J.; Liao, M.-Y.; Gao, X.-L.; Zhong, Q.; Tang, T.-T.; Yu, X.; Liao, Y.-H.; Cheng, X. Il-17a induces pro-inflammatory cytokines production in macrophages via mapkinases, nf-κb and ap-1. Cell. Physiol. Biochem. 2013, 32, 1265–1274. [Google Scholar] [CrossRef]
- Winston, B.W.; Chan, E.D.; Johnson, G.L.; Riches, D.W. Activation of P38mapk, mkk3, and mkk4 by tnf-alpha in mouse bone marrow-derived macrophages. J. Immunol. 1997, 159, 4491–4497. [Google Scholar] [CrossRef]
- Chen, N.-N.; Wei, F.; Wang, L.; Cui, S.; Wan, Y.; Liu, S. Tumor necrosis factor alpha induces neural stem cell apoptosis through activating P38 mapk pathway. Neurochem. Res. 2016, 41, 3052–3062. [Google Scholar] [CrossRef]
- Wang, X.-J.; Kong, K.-M.; Qi, W.-L.; Ye, W.-L.; Song, P.-S. Interleukin-1 beta induction of neuron apoptosis depends on p38 mitogen-activated protein kinase activity after spinal cord injury. Acta Pharmacol. Sin. 2005, 26, 934–942. [Google Scholar] [CrossRef]
- Jung, Y.D.; Fan, F.; McConkey, D.J.; E Jean, M.; Liu, W.; Reinmuth, N.; Stoeltzing, O.; A Ahmad, S.; A Parikh, A.; Mukaida, N.; et al. Role of P38 mapk, ap-1, and nf-κb in interleukin-1β-induced il-8 expression in human vascular smooth muscle cells. Cytokine 2002, 18, 206–213. [Google Scholar] [CrossRef]
- Suzuki, M.; Tetsuka, T.; Yoshida, S.; Watanabe, N.; Kobayashi, M.; Matsui, N.; Okamoto, T. The role of P38 mitogen-activated protein kinase in il-6 and il-8 production from the tnf-α- or il-1β-stimulated rheumatoid synovial fibroblasts. FEBS Lett. 2000, 465, 23–27. [Google Scholar] [CrossRef]
- Westra, J.; Doornbos-van-der-Meer, B.; de Boer, P.; van Leeuwen, M.A.; van Rijswijk, M.H.; Limburg, P.C. Strong inhibition of tnf-α Production and inhibition of il-8 and cox-2 mrna expression in monocyte-derived macrophages by rwj 67657, a P38 mitogen-activated protein kinase (Mapk) inhibitor. Arthritis Res. Ther. 2004, 6, R384. [Google Scholar] [CrossRef]
- Chen, K.-H.; Weng, M.-S.; Lin, J.-K. Tangeretin suppresses il-1β-induced cyclooxygenase (Cox)-2 expression through inhibition of P38 mapk, jnk, and akt activation in human lung carcinoma cells. Biochem. Pharmacol. 2006, 73, 215–227. [Google Scholar] [CrossRef]
- Beltrán, A.E.; Briones, A.M.; García-Redondo, A.B.; Rodríguez, C.; Miguel, M.; Álvarez, Y.; Alonso, M.J.; Martínez-González, J.; Salaices, M. P38 mapk contributes to angiotensin ii-induced cox-2 expression in aortic fibroblasts from normotensive and hypertensive rats. J. Hypertens. 2009, 27, 142–154. [Google Scholar] [CrossRef]
- Whitaker, R.H.; Cook, J.G. Stress relief techniques: P38 mapk determines the balance of cell cycle and apoptosis pathways. Biomolecules 2021, 11, 1444. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding mapk signaling pathways in apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Thornton, T.M.; Rincon, M. Non-classical P38 map kinase functions: Cell cycle checkpoints and survival. Int. J. Biol. Sci. 2009, 5, 44–51. [Google Scholar] [CrossRef]
- Lucas, R.M.; Luo, L.; Stow, J.L. Erk1/2 in immune signalling. Biochem. Soc. Trans. 2022, 50, 1341–1352. [Google Scholar] [CrossRef]
- Quément, C.L.; Guénon, I.; Gillon, J.-Y.; Lagente, V.; Boichot, E. mp-12 induces il-8/cxcl8 secretion through egfr and erk1/2 activation in epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2008, 294, L1076–L1084. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, X.; Zhang, J.; Yang, Y.; Chen, D.; Cao, J. Il-34 regulates il-6 and il-8 production in human lung fibroblasts via mapk, pi3k-akt, jak and nf-κb signaling pathways. Int. Immunopharmacol. 2018, 61, 119–125. [Google Scholar] [CrossRef]
- Shan, L.; Redhu, N.; Saleh, A.; Halayko, A.; Chakir, J.; Gounni, A. Thymic stromal lymphopoietin receptor-mediated il-6 and cc/cxc chemokines expression in human airway smooth muscle cells: Role of mapks (Erk1/2, P38, and jnk) and stat3 pathways. J. Immunol. 2010, 184, 7134–7143. [Google Scholar] [CrossRef]
- Chen, J.; Luo, X.; Liu, M.; Peng, L.; Zhao, Z.; He, C.; He, Y. Silencing long non-coding rna neat1 attenuates rheumatoid arthritis via the mapk/erk signalling pathway by downregulating microrna-129 and microrna-204. RNA Biol. 2021, 18, 657–668. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, L.; Yang, Q.; Xiao, X.; Ye, Z.; Li, R.; Guan, Y.; Wu, X. nlrp12 C.1382dup promotes the development of crohn’s disease through the erk/nlrp3/il-1β pathway. Gene 2024, 931, 148855. [Google Scholar] [CrossRef]
- Butturini, E.; Carcereri de Prati, A.; Mariotto, S. Redox Regulation of STAT1 and STAT3 Signaling. Int. J. Mol. Sci. 2020, 21, 7034. [Google Scholar] [CrossRef]
- Ma, J.-H.; Qin, L.; Li, X. Role of STAT3 signaling pathway in breast cancer. Cell Commun. Signal. 2020, 18, 33. [Google Scholar] [CrossRef]
- Mohassab, A.M.; Hassan, H.A.; Abdelhamid, D.; Abdel-Aziz, M. STAT3 transcription factor as target for anti-cancer therapy. Pharmacol. Rep. 2020, 72, 1101–1124. [Google Scholar] [CrossRef]
- Zou, S.; Tong, Q.; Liu, B.; Huang, W.; Tian, Y.; Fu, X. Targeting STAT3 in Cancer Immunotherapy. Mol. Cancer 2020, 19, 145. [Google Scholar] [CrossRef]
- El-Tanani, M.; Al Khatib, A.O.; Aladwan, S.M.; Abuelhana, A.; McCarron, P.A.; Tambuwala, M.M. Importance of STAT3 signalling in cancer, metastasis and therapeutic interventions. Cell. Signal. 2022, 92, 110275. [Google Scholar] [CrossRef]
- Hu, Y.; Dong, Z.; Liu, K. Unraveling the complexity of STAT3 in cancer: Molecular understanding and drug discovery. J. Exp. Clin. Cancer Res. 2024, 43, 23. [Google Scholar] [CrossRef]
- Zhang, G.; Hou, S.; Li, S.; Wang, Y.; Cui, W. Role of STAT3 in cancer cell epithelial-mesenchymal transition (Review). Int. J. Oncol. 2024, 64, 48. [Google Scholar] [CrossRef]
- Kalliolias, G.D.; Ivashkiv, L.B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 2016, 12, 49–62. [Google Scholar] [CrossRef]
- Varfolomeev, E.; Vucic, D. Intracellular regulation of TNF activity in health and disease. Cytokine 2018, 101, 26–32. [Google Scholar] [CrossRef]
- Huyghe, J.; Priem, D.; Bertrand, M.J. Cell death checkpoints in the TNF pathway. Trends Immunol. 2023, 44, 628–643. [Google Scholar] [CrossRef]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2022, 23, 289–303. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 inflammasome activation and cell death. Cell. Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, X.; Escames, G.; Lei, W.; Zhang, X.; Li, M.; Jing, T.; Yao, Y.; Qiu, Z.; Wang, Z.; et al. The NLRP3 inflammasome: Contributions to inflammation-related diseases. Cell. Mol. Biol. Lett. 2023, 28, 51. [Google Scholar] [CrossRef]
- Fu, J.; Wu, H. Structural Mechanisms of NLRP3 Inflammasome Assembly and Activation. Annu. Rev. Immunol. 2023, 41, 301–316. [Google Scholar] [CrossRef]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22: From Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Murray, P.J. Cytokine signaling modules in inflammatory responses. Immunity 2008, 28, 477–487. [Google Scholar] [CrossRef]
- Kulbe, H.; Chakravarty, P.; Leinster, D.A.; Charles, K.A.; Kwong, J.; Thompson, R.G.; Coward, J.I.; Schioppa, T.; Robinson, S.C.; Gallagher, W.M.; et al. A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Cancer Res. 2012, 72, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Kureshi, C.T.; Dougan, S.K. Cytokines in cancer. Cancer Cell 2025, 43, 15–35. [Google Scholar] [CrossRef]
- Burska, A.; Boissinot, M.; Ponchel, F. Cytokines as Biomarkers in Rheumatoid Arthritis. Mediat. Inflamm. 2014, 2014, 545493. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Liu, C.; Chu, D.; Kalantar-Zadeh, K.; George, J.; Young, H.A.; Liu, G. Cytokines: From Clinical Significance to Quantification. Adv. Sci. 2021, 8, e2004433. [Google Scholar] [CrossRef]
- Wei, K.; Nguyen, H.N.; Brenner, M.B. Fibroblast pathology in inflammatory diseases. J. Clin. Investig. 2021, 131, e149538. [Google Scholar] [CrossRef]
- Morgan, M.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457, Erratum in Nat. Immunol. 2017, 18, 1271. [Google Scholar] [CrossRef]
- Wu, S.; Cao, Z.; Lu, R.; Zhang, Z.; Sethi, G.; You, Y. Interleukin-6 (IL-6)-associated tumor microenvironment remodelling and cancer immunotherapy. Cytokine Growth Factor Rev. 2025, 16, 1359–6101. [Google Scholar] [CrossRef] [PubMed]
- Schumertl, T.; Lokau, J.; Garbers, C. IL-6 Signaling in Immunopathology: From Basic Biology to Selective Therapeutic Intervention. Immunotargets Ther. 2025, 14, 681–695. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Oliveira, G.L.S. Determinação da capacidade antioxidante de produtos naturais in vitro pelo método do DPPH. Rev. Bras. Pl. Med. 2015, 17, 36–44. [Google Scholar] [CrossRef]
- Buhrmann, C.; Brockmueller, A.; Mueller, A.L.; Shayan, P.; Shakibaei, M. Curcumin Attenuates Environment-Derived Osteoarthritis by Sox9/NF-kB Signaling Axis. Int. J. Mol. Sci. 2021, 22, 7645. [Google Scholar] [CrossRef]
- Dehzad, M.J.; Ghalandari, H.; Nouri, M.; Askarpour, M. Antioxidant and anti-inflammatory effects of curcumin/turmeric supplementation in adults: A GRADE-assessed systematic review and dose–response meta-analysis of randomized controlled trials. Cytokine 2023, 164, 156144. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Razi, B.; Aslani, S.; Abbasifard, M.; Imani, D.; Sathyapalan, T.; Sahebkar, A. Effect of curcumin on proinflammatory cytokines: A meta-analysis of randomized controlled trials. Cytokine 2021, 143, 155541. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Xu, X.; Yi, P.; Hao, Y. Curcumin reinforces MSC-derived exosomes in attenuating osteoarthritis via modulating the miR-124/NF-kB and miR-143/ROCK1/TLR9 signalling pathways. J. Cell. Mol. Med. 2020, 24, 10855–10865. [Google Scholar] [CrossRef] [PubMed]
- Thapa Magar, T.B.; Mallik, S.K.; Gurung, P.; Lim, J.; Kim, Y.-T.; Shrestha, R.; Kim, Y.-W. Chlorin E6-Curcumin-Mediated Photodynamic Therapy Promotes an Anti-Photoaging Effect in UVB-Irradiated Fibroblasts. Int. J. Mol. Sci. 2023, 24, 13468. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Tsuruta, D. What Are Reactive Oxygen Species, Free Radicals, and Oxidative Stress in Skin Diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef]
- Mondal, S.; Adhikari, N.; Banerjee, S.; Amin, S.A.; Jha, T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur. J. Med. Chem. 2020, 194, 112260, Erratum in Eur. J. Med. Chem. 2020, 205, 112642. [Google Scholar] [CrossRef]
- Ozkan, E.; Bakar-Ates, F. The Trinity of Matrix Metalloproteinases, Inflammation, and Cancer: A Literature Review of Recent Updates. Anti-Inflammatory Anti-Allergy Agents. Med. Chem. 2020, 19, 206–221. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, W.J. The Role of Matrix Metalloproteinase in Inflammation with a Focus on Infectious Diseases. Int. J. Mol. Sci. 2022, 23, 10546. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiao, L.; Qiang, C.; Chen, C.; Shen, Z.; Ding, F.; Lv, L.; Zhu, T.; Lu, Y.; Cui, X. The role of matrix metalloproteinase 9 in fibrosis diseases and its molecular mechanisms. Biomed. Pharmacother. 2024, 171, 116116. [Google Scholar] [CrossRef]
- Nissinen, L.; Kähäri, V.M. Matrix metalloproteinases in inflammation. Biochim. Biophys. Acta 2014, 1840, 2571–2580. [Google Scholar] [CrossRef] [PubMed]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Bassiouni, W.; Ali, M.A.M.; Schulz, R. Multifunctional intracellular matrix metalloproteinases: Implications in disease. FEBS J. 2021, 288, 7162–7182. [Google Scholar] [CrossRef]
- Kou, L.; Jiang, X.; Lin, X.; Huang, H.; Wang, J.; Yao, Q.; Chen, R. Matrix Metalloproteinase Inspired Therapeutic Strategies for Bone Diseases. Curr. Pharm. Biotechnol. 2021, 22, 451–467. [Google Scholar] [CrossRef]
- Feng, C.; Chen, X.; Yin, X.; Jiang, Y.; Zhao, C. Matrix Metalloproteinases on Skin Photoaging. J. Cosmet. Dermatol. 2024, 23, 3847–3862. [Google Scholar] [CrossRef]
- Punzo, A.; Porru, E.; Silla, A.; Simoni, P.; Galletti, P.; Roda, A.; Tagliavini, E.; Samorì, C.; Caliceti, C. Grape Pomace for Topical Application: Green NaDES Sustainable Extraction, Skin Permeation Studies, Antioxidant and Anti-Inflammatory Activities Characterization in 3D Human Keratinocytes. Biomolecules 2021, 11, 1181. [Google Scholar] [CrossRef]
- Villa, C.; Caviglia, D.; Robustelli della Cuna, F.S.; Zuccari, G.; Russo, E. NaDES Application in Cosmetic and Pharmaceutical Fields: An Overview. Gels 2024, 10, 107. [Google Scholar] [CrossRef]
- Deng, G.; Li, K.; Chen, S.; Chen, P.; Zheng, H.; Yu, B.; Zhang, K. Interleukin-10 promotes proliferation and migration, and inhibits tendon differentiation via the JAK/Stat3 pathway in tendon-derived stem cells in vitro. Mol. Med. Rep. 2018, 18, 5044–5052. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murphy, G.; Nagase, H. Progress in matrix metalloproteinase research. Mol. Asp. Med. 2008, 29, 290–308. [Google Scholar] [CrossRef] [PubMed]
- Birch, H.L. Extracellular Matrix and Ageing. Subcell. Biochem. 2018, 90, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggard, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Michen, B.; Geers, C.; Vanhecke, D.; Endes, C.; Rothen-Rutishauser, B.; Balog, S.; Petri-Fink, A. Avoiding drying-artifacts in transmission electron microscopy: Characterizing the size and colloidal state of nanoparticles. Sci. Rep. 2015, 5, 9793. [Google Scholar] [CrossRef]
- Lynch, I.; Dawson, K.A. Protein-nanoparticle interactions. Nano Today 2008, 3, 40–47. [Google Scholar] [CrossRef]
- Lima, T.; Bernfur, K.; Vilanova, M.; Cedervall, T. Understanding the Lipid and Protein Corona Formation on Different Sized Polymeric Nanoparticles. Sci. Rep. 2020, 10, 1129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Aberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Sun, Z.; Li, M.; Li, Z.; Bunyak, F.; Ersoy, I.; Trzeciakowski, J.P.; Staiculescu, M.C.; Jin, M.; Martinez-Lemus, L.; et al. Vasoactive agonists exert dynamic and coordinated effects on vascular smooth muscle cell elasticity, cytoskeletal remodelling and adhesion. J. Physiol. 2014, 592, 1249–1266. [Google Scholar] [CrossRef]
- Thelin, M.A.; Svensson, K.J.; Shi, X.; Bagher, M.; Axelsson, J.; Isinger-Ekstrand, A.; van Kuppevelt, T.H.; Johansson, J.; Nilbert, M.; Zaia, J.; et al. Dermatan Sulfate Is Involved in the Tumorigenic Properties of Esophagus Squamous Cell Carcinoma. Cancer Res. 2012, 72, 1943–1952. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kang, M.K.; Kim, Y.H.; Lee, E.J.; Oh, H.; Kim, S.I.; Oh, S.Y.; Kang, Y.H. Eucalyptol Ameliorates Dysfunction of Actin Cytoskeleton Formation and Focal Adhesion Assembly in Glucose-Loaded Podocytes and Diabetic Kidney. Mol. Nutr. Food Res. 2019, 63, e1900489. [Google Scholar] [CrossRef] [PubMed]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Katoh, K. FAK-Dependent Cell Motility and Cell Elongation. Cells 2020, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Prudent, J.; Popgeorgiev, N.; Gadet, R.; Deygas, M.; Rimokh, R.; Gillet, G. Mitochondrial Ca2+ uptake controls actin cytoskeleton dynamics during cell migration. Sci. Rep. 2016, 6, 36570. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.M.; Paiva, K.L.R.; de Souza, I.F.; Mello, V.C.; Martins da Silva, I.G.; Souza, P.E.N.; Muehlmann, L.A.; Báo, S.N. The Potential of Photodynamic Therapy Using Solid Lipid Nanoparticles with Aluminum Phthalocyanine Chloride as a Nanocarrier for Modulating Immunogenic Cell Death in Murine Melanoma In Vitro. Pharmaceutics 2024, 16, 941. [Google Scholar] [CrossRef]
- Datta, A.; Deng, S.; Gopal, V.; Yap, K.C.-H.; Halim, C.E.; Lye, M.L.; Ong, M.S.; Tan, T.Z.; Sethi, G.; Hooi, S.C.; et al. Cytoskeletal Dynamics in Epithelial-Mesenchymal Transition: Insights into Therapeutic Targets for Cancer Metastasis. Cancers 2021, 13, 1882. [Google Scholar] [CrossRef]
- Fink, A.; Doll, C.R.; Yagüe Relimpio, A.; Dreher, Y.; Spatz, J.P.; Göpfrich, K.; Cavalcanti-Adam, E.A. Extracellular Cues Govern Shape and Cytoskeletal Organization in Giant Unilamellar Lipid Vesicles. ACS Synth. Biol. 2023, 12, 369–374. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef]
- Cui, J.; Li, H.; Zhang, T.; Lin, F.; Chen, M.; Zhang, G.; Feng, Z. Research progress on the mechanism of curcumin anti-oxidative stress based on signaling pathway. Front. Pharmacol. 2025, 16, 1548073. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Ursini, F. Homeostatic control of redox status and health. IUBMB Life 2021, 74, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, J.; Kontny, E. Taurine and inflammatory diseases. Amino Acids 2012, 46, 7–20. [Google Scholar] [CrossRef]
- Vicente, G.; Moon, Y.J.K.; Rosa, D.W.; Lima, L.A.; Saleh, N.A.; da Rosa, J.S.; Creczynski-Pasa, T.B.; Biavatti, M.W.; Dalmarco, E.M.; Fröde, T.S. Anti-Inflammatory Profile of Jungia sellowii Less. by Downregulation of Proinflammatory Mediators and Inhibition of NF-κB and p38 Pathways. Mediators Inflamm. 2020, 38, 9078956. [Google Scholar] [CrossRef]
- Qiu, P.; Wheater, M.K.; Qiu, Y.; Sosne, G. Thymosin β4 inhibits TNF-α-induced NF-κB activation, IL-8 expression, and the sensitizing effects by its partners PINCH-1 and ILK. FASEB J. 2011, 25, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Burlec, A.F.; Hăncianu, M.; Ivănescu, B.; Macovei, I.; Corciovă, A. Exploring the Therapeutic Potential of Natural Compounds in Psoriasis and Their Inclusion in Nanotechnological Systems. Antioxidants 2024, 13, 912. [Google Scholar] [CrossRef]
- Zhao, P.; Sun, T.; Lyu, C.; Liang, K.; Du, Y. Cell mediated ECM-degradation as an emerging tool for anti-fibrotic strategy. Cell Regen. 2023, 12, 29. [Google Scholar] [CrossRef]
- Maitz, J.; Wang, Y.; Fathi, A.; Escobar, F.X.; Parungao, R.; Zuijlen, P.; Maitz, P.; Li, Z. The effects of cross-linking a collagen-elastin dermal template on scaffold bio-stability and degradation. J. Tissue Eng. Regen. Med. 2020, 14, 1189–1200. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, S.N.; Kim, K.; Joo, d.a.H.; Shin, S.; Lee, J.; Lee, H.K.; Kim, J.; Kwon, S.B.; Kim, M.J.; et al. Biological effects of rutin on skin aging. Int. J. Mol. Med. 2016, 38, 357–363. [Google Scholar] [CrossRef]
- Kang, J.H.; Jang, M.; Seo, S.J.; Choi, A.; Shin, D.; Seo, S.; Lee, S.H.; Kim, H.N. Mechanobiological Adaptation to Hyperosmolarity Enhances Barrier Function in Human Vascular Microphysiological System. Adv. Sci. 2023, 10, e2206384. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, J.; Chen, B.; Luo, M.; Cheng, W.; Wang, Y.; Liu, J.; Yang, H. Strain Stimulations with Different Intensities on Fibroblast Viability and Protein Expression. Open Life Sci. 2017, 12, 285–293. [Google Scholar] [CrossRef][Green Version]
- Mbese, Z.; Alven, S.; Aderibigbe, B.A.; Mbese, Z.; ALVEN, S.; Aderibigbe, B.A. Collagen-Based Nanofibers for Skin Regeneration and Wound Dressing Applications. Polymers 2021, 13, 4368. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.A.O.; Martins, D.C.M.; de Castro Cantuária, A.P.; de Andrade, R.V.; Lacorte, C.; de Almeida, J.A.; Aguiar, L.R.; Corrêa, J.R.; da Silva, I.G.M.; Franco, O.L.; et al. Host defense peptides combined with MTA extract increase the repair in dental pulp cells: In vitro and ex vivo study. Sci. Rep. 2023, 13, 9531. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, S.P.; Li, H.; Taniguchi, G.T. Zymography and reverse zymography for detecting MMPs and TIMPs. Methods Mol Biol. 2010, 622, 257–269. [Google Scholar] [CrossRef] [PubMed]

| Targeted Pathway or Mechanism | Molecular and Cellular Outcomes | Physiological Implications and Observed Effects |
|---|---|---|
| Oxidative Stress Reduction: neutralization of ROS (e.g., hydroxyl radicals); enhancement of endogenous antioxidant enzyme expression (e.g., SOD, catalase); reduction in NF-κB activation via ROS inhibition [127] | Stabilization of cellular redox balance; decreased oxidative damage to proteins, lipids, and DNA; reduced expression of NF-κB-regulated inflammatory cytokines (TNF-α, IL-6) [128] | Reduced inflammation; prevention of chronic inflammatory states; enhanced tissue integrity and cellular survival [129] |
| Inflammatory Pathway Modulation: Inhibition of ROS-induced MAPK signaling (p38, ERK, JNK); reduced activation of NF-κB pathway (through IκB stabilization); downregulation of pro-inflammatory cytokines (TNF-α, IL-6); upregulation of anti-inflammatory cytokine IL-10 [130] | Decreased leukocyte recruitment and transmigration; attenuation of inflammatory cytokine cascade; enhanced anti-inflammatory cytokine expression (IL-10) [131] | Alleviation of inflammatory skin diseases (e.g., psoriasis); regulation of cytokine-mediated tissue repair; reduced risk of chronic inflammation-associated pathologies [132] |
| Extracellular Matrix (ECM) Remodeling Regulation: inhibition of MMP-2 and MMP-9 enzymatic activities; decreased TNF-α mediated MMP-9 transcription (via MAPK/NF-κB pathway inhibition); reduced cleavage of collagen, elastin, and fibronectin [133] | Preservation of ECM structural proteins; enhanced structural stability and elasticity of dermal matrix; reduced generation of bioactive ECM fragments (ac-PGP) triggering further neutrophil recruitment [134] | Reduced skin aging signs (wrinkles, sagging); protection against fibrosis, scarring, and ECM degradation-associated diseases [135] |
| Cellular Microenvironment and Adhesion Modulation: osmotic modulation and membrane fluidity alteration via NaDES; integrin clustering and focal adhesion kinase (FAK) activation; cytoskeletal rearrangements (actin polymerization); enhanced STAT3-mediated cell proliferation (via IL-10) [136] | Increased cells adhesion and cell–cell interactions; enhanced cytoskeletal organization and mechanotransduction; stimulation of proliferation and improved cell viability through STAT3 pathway [137] | Improved skin regeneration and wound healing; enhanced dermal density and tissue regeneration potential [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romera, L.F.; Schuh, L.; Leal, C.; Chang, L.F.d.A.; Santos, B.M.d.; da Rocha, P.H.A.d.J.; Radicchi, M.A.; Gris, E.F.; Falcao, L.; Báo, S.N.; et al. Jamamina: A Green Nanostructured Lipid Carrier with NaDES and Curcumin for Redox Modulation and Inflammatory Disorders. Int. J. Mol. Sci. 2025, 26, 8373. https://doi.org/10.3390/ijms26178373
Romera LF, Schuh L, Leal C, Chang LFdA, Santos BMd, da Rocha PHAdJ, Radicchi MA, Gris EF, Falcao L, Báo SN, et al. Jamamina: A Green Nanostructured Lipid Carrier with NaDES and Curcumin for Redox Modulation and Inflammatory Disorders. International Journal of Molecular Sciences. 2025; 26(17):8373. https://doi.org/10.3390/ijms26178373
Chicago/Turabian StyleRomera, Luís Felipe, Luísa Schuh, Caio Leal, Leonardo Froes de Azevedo Chang, Brenda Martins dos Santos, Pedro Henrique Almeida de Jesus da Rocha, Marina Arantes Radicchi, Eliana Fortes Gris, Leila Falcao, Sônia Nair Báo, and et al. 2025. "Jamamina: A Green Nanostructured Lipid Carrier with NaDES and Curcumin for Redox Modulation and Inflammatory Disorders" International Journal of Molecular Sciences 26, no. 17: 8373. https://doi.org/10.3390/ijms26178373
APA StyleRomera, L. F., Schuh, L., Leal, C., Chang, L. F. d. A., Santos, B. M. d., da Rocha, P. H. A. d. J., Radicchi, M. A., Gris, E. F., Falcao, L., Báo, S. N., & Mello, V. C. (2025). Jamamina: A Green Nanostructured Lipid Carrier with NaDES and Curcumin for Redox Modulation and Inflammatory Disorders. International Journal of Molecular Sciences, 26(17), 8373. https://doi.org/10.3390/ijms26178373







