Generation of Active Neurons from Mouse Embryonic Stem Cells Using Retinoic Acid and Purmorphamine
Abstract
1. Introduction
2. Results
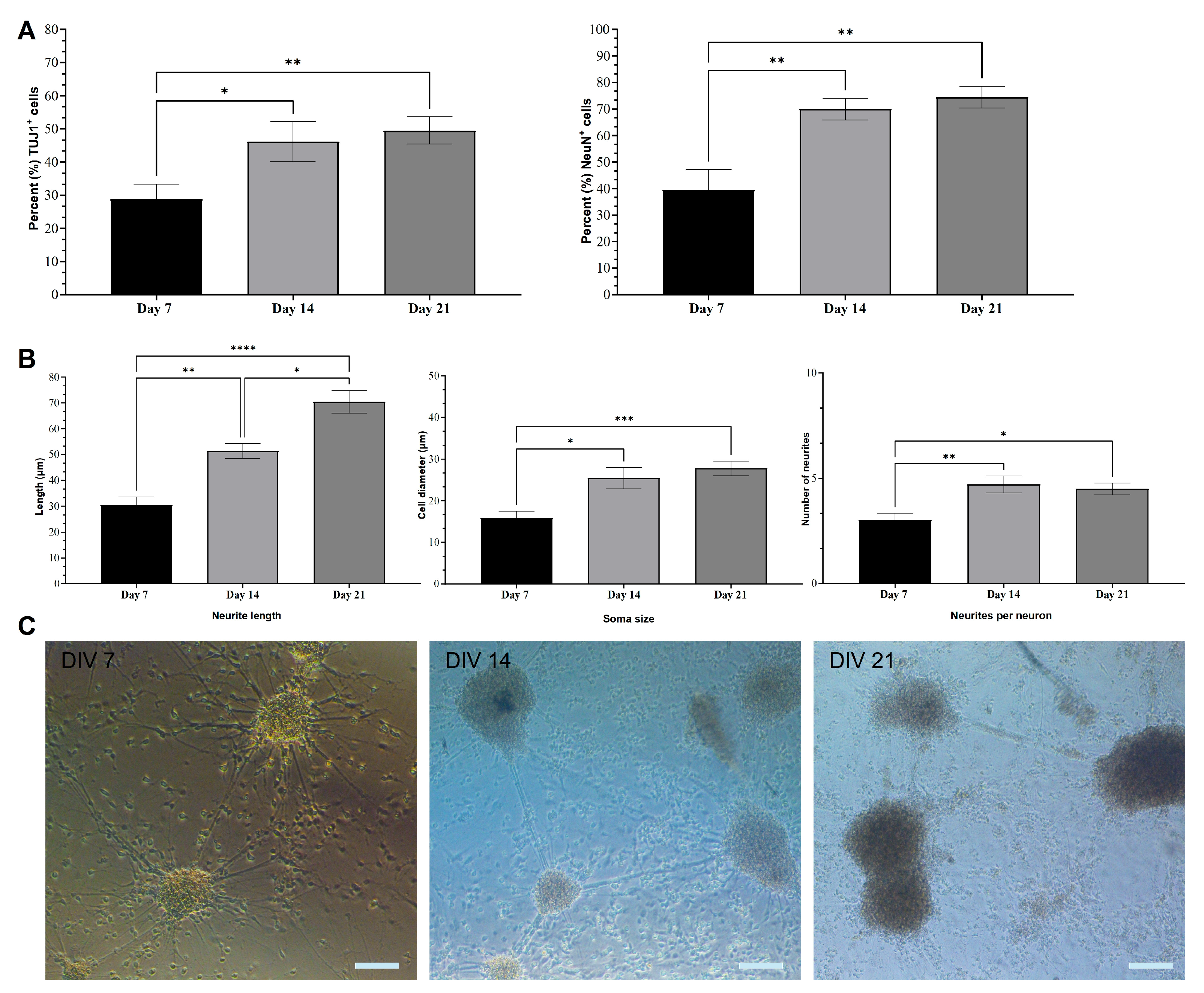
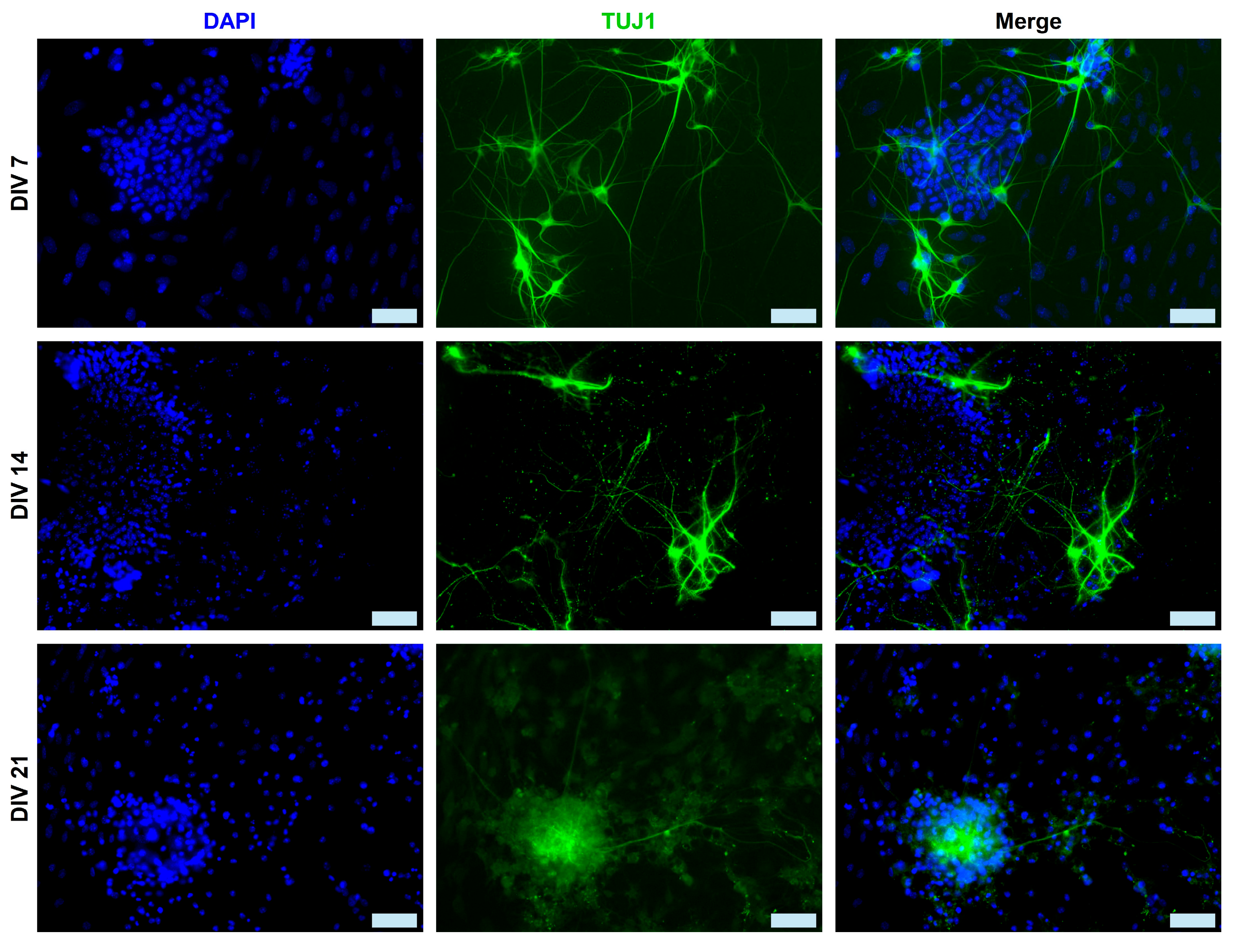
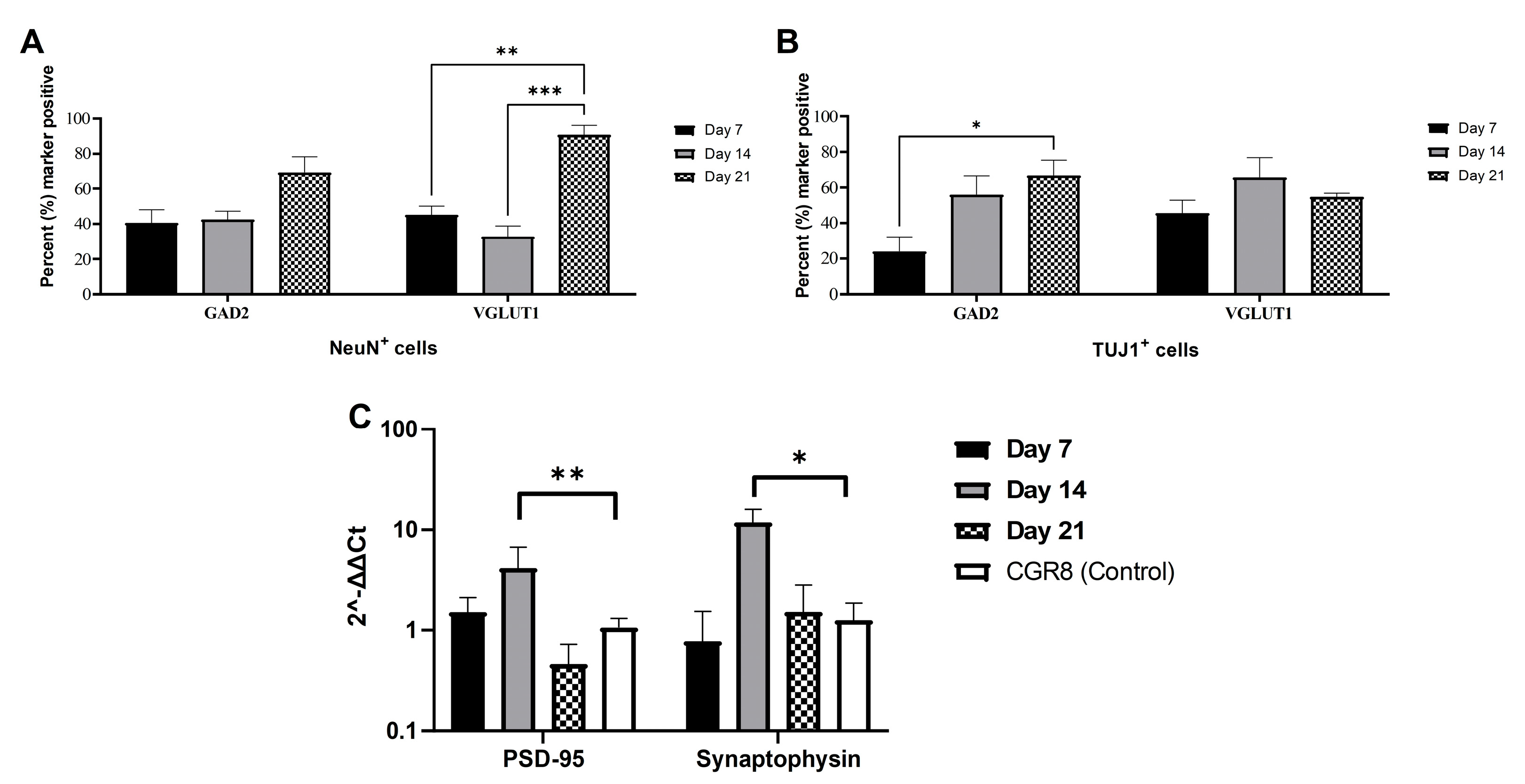
2.1. Immunocytochemical Analysis of mESC-Derived Neurons
2.2. Morphological Characterization of mESC-Derived Neurons
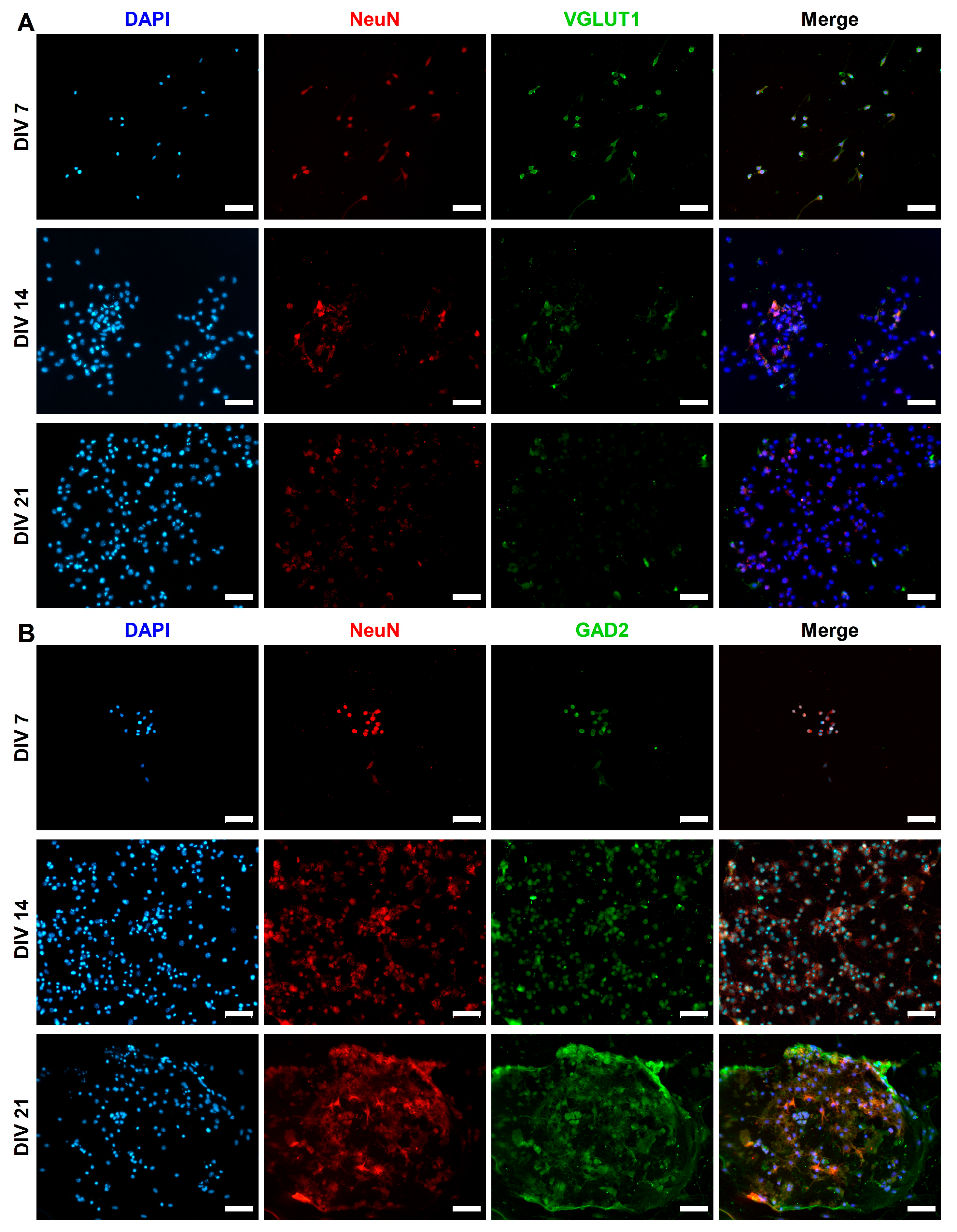
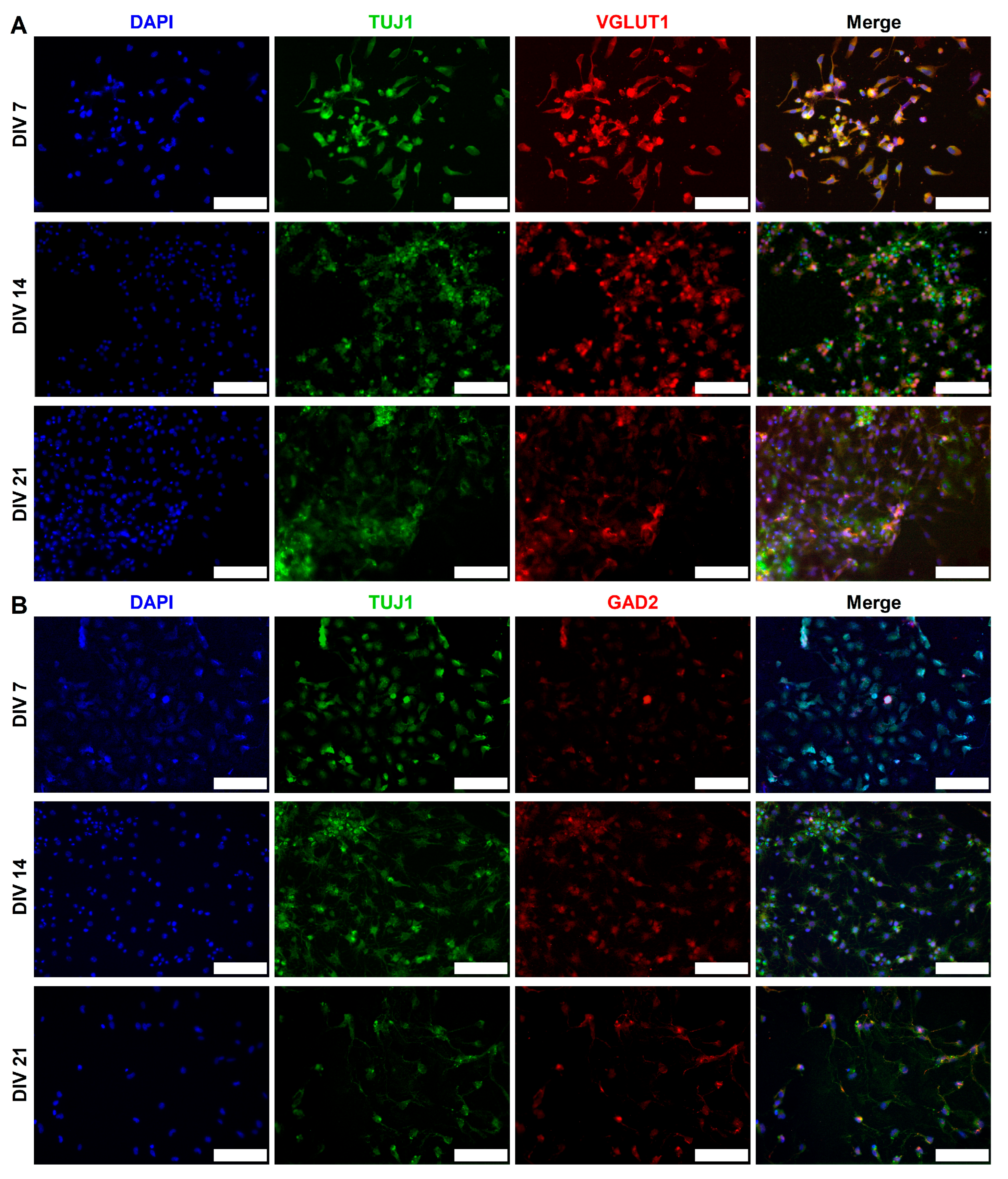
2.3. Expression of Synaptic Markers in CGR8 Cell-Derived Neurons
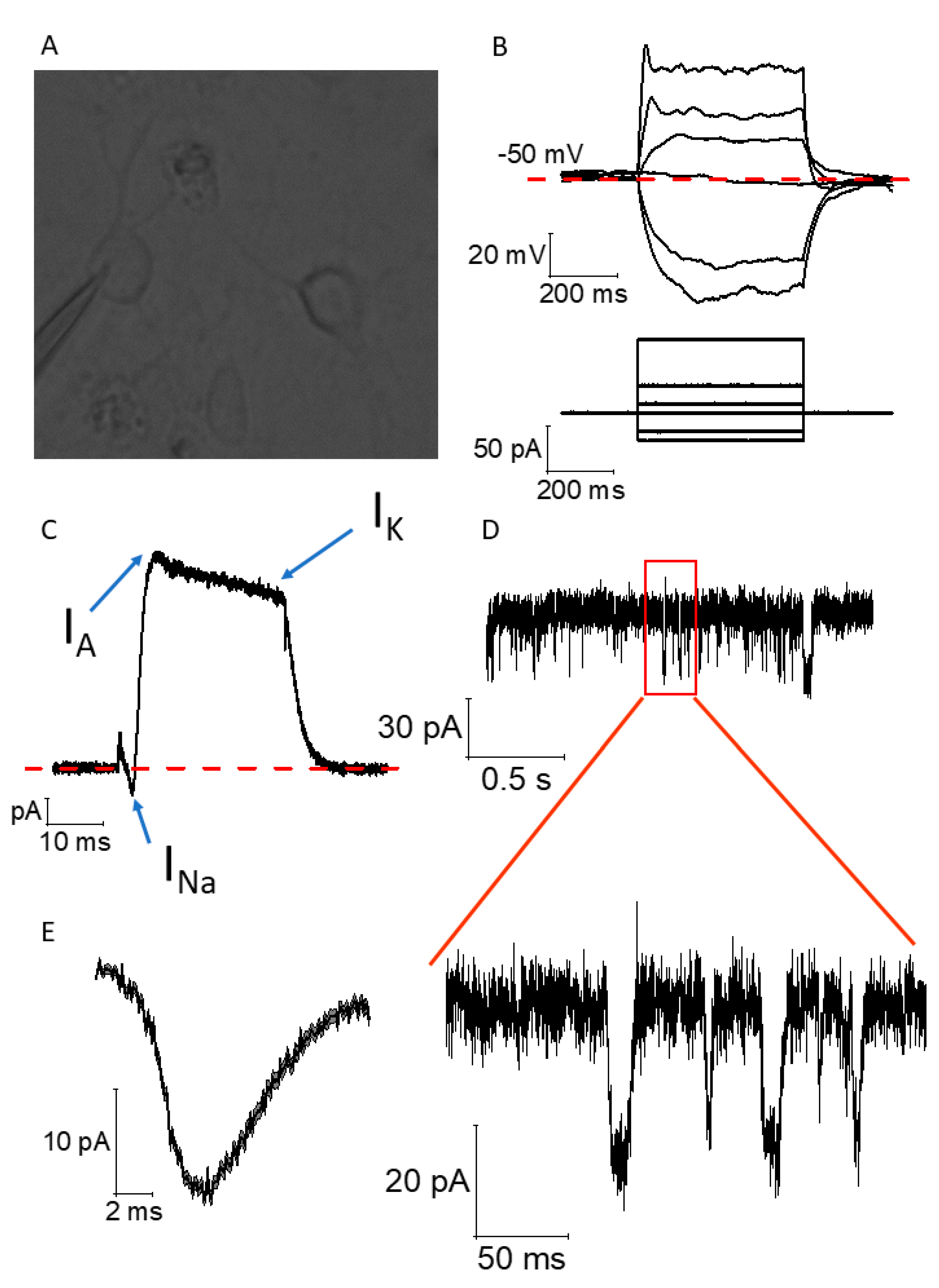
2.4. Functional Maturation of CGR8 Cell-Derived Neurons and Formation of Local Networks
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Immunohistochemistry
4.3. Image Analysis
4.4. RNA Extraction and cDNA Synthesis
4.5. Real-Time PCR
4.6. Electrophysiology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LIF | Leukemia Inhibitory Factor |
| mESC | Mouse embryonic stem cell |
| RA | Retinoic acid |
| RMP | Resting membrane potential |
References
- Merkle, F.T.; Maroof, A.; Wataya, T.; Sasai, Y.; Studer, L.; Eggan, K.; Schier, A.F. Generation of neuropeptidergic hypothalamic neurons from human pluripotent stem cells. Development 2015, 142, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Corti, S.; Bonjean, R.; Legier, T.; Rattier, D.; Melon, C.; Salin, P.; Toso, E.A.; Kyba, M.; Goff, L.K.-L.; Maina, F.; et al. Enhanced differentiation of human induced pluripotent stem cells toward the midbrain dopaminergic neuron lineage through GLYPICAN-4 downregulation. Stem Cells Transl. Med. 2021, 10, 725–742. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, K.; Lekholm, E.; Ahemaiti, A.; Fredriksson, R. Differentiation of Human Embryonic Stem Cells into Neuron, Cholinergic, and Glial Cells. Stem Cells Int. 2020, 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Birey, F.; Andersen, J.; Makinson, C.D.; Islam, S.; Wei, W.; Huber, N.; Fan, H.C.; Metzler, K.R.C.; Panagiotakos, G.; Thom, N.; et al. Assembly of functionally integrated human forebrain spheroids. Nature 2017, 545, 54. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.; Li, C.; Lan, T.; Wei, Y.; Tang, C.; Zhou, X.; Zhou, R.; Rosa, A.; Zheng, X.; et al. Inducible motor neuron differentiation of human induced pluripotent stem cells in vivo. Cell Prolif. 2022, 55, e13319. [Google Scholar] [CrossRef]
- Konstantinides, N.; Desplan, C. Neuronal differentiation strategies: Insights from single-cell sequencing and machine learning. Development 2020, 147, dev193631. [Google Scholar] [CrossRef]
- Saxena, S.; Choudhury, S.; Mohan, K.N. Reproducible differentiation and characterization of neurons from mouse embryonic stem cells. MethodsX 2020, 7, 101073. [Google Scholar] [CrossRef]
- Wongpaiboonwattana, W.; Stavridis, M.P. Neural Differentiation of Mouse Embryonic Stem Cells in Serum-free Monolayer Culture. J. Vis. Exp. 2015, 2015, 52823. [Google Scholar] [CrossRef]
- Yuan, P.; Ding, L.; Chen, H.; Wang, Y.; Li, C.; Zhao, S.; Yang, X.; Ma, Y.; Zhu, J.; Qi, X.; et al. Neural Stem Cell-Derived Exosomes Regulate Neural Stem Cell Differentiation Through miR-9-Hes1 Axis. Front. Cell Dev. Biol. 2021, 9, 601600. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; He, H.; Qin, J.; Cheng, X.; Zhao, H.; Tian, M.; Zhalng, X.; Jin, G. Expression and function of Ndel1 during the differentiation of neural stem cells induced by hippocampal exosomesticle. Stem Cell Res. Ther. 2021, 12, 51. [Google Scholar] [CrossRef]
- D’Aiuto, L.; Zhi, Y.; Kumar Das, D.; Wilcox, M.R.; Johnson, J.W.; McClain, L.; MacDonald, M.L.; Di Maio, R.; E Schurdak, M.; Piazza, P.; et al. Large-scale generation of human iPSC-derived neural stem cells/early neural progenitor cells and their neuronal differentiation. Organogenesis 2014, 10, 365. [Google Scholar] [CrossRef]
- de Leeuw, V.C.; van Oostrom, C.T.M.; Westerink, R.H.S.; Piersma, A.H.; Heusinkveld, H.J.; Hessel, E.V.S. An efficient neuron-astrocyte differentiation protocol from human embryonic stem cell-derived neural progenitors to assess chemical-induced developmental neurotoxicity. Reprod. Toxicol. 2020, 98, 107–116. [Google Scholar] [CrossRef]
- Bianchi, F.; Malboubi, M.; Li, Y.; George, J.H.; Jerusalem, A.; Szele, F.; Thompson, M.S.; Ye, H. Rapid and efficient differentiation of functional motor neurons from human iPSC for neural injury modelling. Stem Cell Res. 2018, 32, 126–134. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, X.J.; Renier, N.; Wu, Z.; Atkin, T.; Sun, Z.; Ozair, M.Z.; Tchieu, J.; Zimmer, B.; Fattahi, F.; et al. Combined small-molecule inhibition accelerates the derivation of functional, early-born, cortical neurons from human pluripotent stem cells. Nat. Biotechnol. 2017, 35, 154. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Karki, P.; Lei, L.; Wang, H.; Fliegel, L. Na+/H+ exchanger isoform 1 facilitates cardiomyocyte embryonic stem cell differentiation. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H159–H170. [Google Scholar] [CrossRef] [PubMed]
- Hannes, T.; Wolff, M.; Doss, M.X.; Pfannkuche, K.; Haustein, M.; Müller-Ehmsen, J.; Sachinidis, A.; Hescheler, J.; Khalil, M.; Halbach, M. Electrophysiological Characteristics of Embryonic Stem Cell-Derived Cardiomyocytes are Cell Line-Dependent. Cell. Physiol. Biochem. 2015, 35, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Juneja, D.S.; Nasuto, S.; Delivopoulos, E. Fast and Efficient Differentiation of Mouse Embryonic Stem Cells Into ATP-Responsive Astrocytes. Front. Cell. Neurosci. 2020, 13, 579. [Google Scholar] [CrossRef]
- Hannig, M.; Figulla, H.R.; Sauer, H.; Wartenberg, M. Control of leucocyte differentiation from embryonic stem cells upon vasculogenesis and confrontation with tumour tissue. J. Cell. Mol. Med. 2010, 14, 303. [Google Scholar] [CrossRef]
- Schaedlich, K.; Knelangen, J.M.; Santos, A.N.; Fischer, B.; Santos, A.N. A simple method to sort ESC-derived adipocytes. Cytom. Part A 2010, 77A, 990–995. [Google Scholar] [CrossRef]
- Delivopoulos, E.; Shakesheff, K.M.; Peto, H. Neuralization of mouse embryonic stem cells in alginate hydrogels under retinoic acid and SAG treatment. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Milan, Italy, 25–29 August 2015; Volume 2015. [Google Scholar] [CrossRef]
- Wessler, I.; Michel-Schmidt, R.; Brochhausen, C.; Kirkpatrick, C.J. Subcellular distribution of choline acetyltransferase by immunogold electron microscopy in non-neuronal cells: Placenta, airways and murine embryonic stem cells. Life Sci. 2012, 91, 977–980. [Google Scholar] [CrossRef]
- Davis, D.A.; Vajaria, R.; Delivopoulos, E.; Vasudevan, N. Localisation of oestrogen receptors in stem cells and in stem cell-derived neurons of the mouse. J. Neuroendocr. 2023, 35, e13220. [Google Scholar] [CrossRef]
- Delivopoulos, E.; Ouberai, M.M.; Coffey, P.D.; Swann, M.J.; Shakesheff, K.M.; Welland, M.E. Serum protein layers on parylene-C and silicon oxide: Effect on cell adhesion. Colloids Surf. B Biointerfaces 2015, 126, 169–177. [Google Scholar] [CrossRef]
- Finkensieper, A.; Bekhite, M.M.; Fischer, H.; Nitza, S.; Figulla, H.R.; Müller, J.P.; Sauer, H.; Wartenberg, M. Antibacterial Capacity of Differentiated Murine Embryonic Stem Cells During Defined In Vitro Inflammatory Conditions. Stem Cells Dev. 2013, 22, 1977–1990. [Google Scholar] [CrossRef]
- Gissel, C.; Voolstra, C.; Doss, M.X.; Koehler, C.I.; Winkler, J.; Hescheler, J.; Sachinidis, A. An optimized embryonic stem cell model for consistent gene expression and developmental studies: A fundamental study. Thromb. Haemost. 2005, 94, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Fannon, O.M.; Bithell, A.; Whalley, B.J.; Delivopoulos, E. A Fiber Alginate Co-culture Platform for the Differentiation of mESC and Modeling of the Neural Tube. Front. Neurosci. 2021, 14, 1386. [Google Scholar] [CrossRef] [PubMed]
- de Amores Sousa, M.C.; Rodrigues, C.A.; Ferreira, I.A.; Diogo, M.M.; Linhardt, R.J.; Cabral, J.M.S.; Ferreira, F.C. Functionalization of Electrospun Nanofibers and Fiber Alignment Enhance Neural Stem Cell Proliferation and Neuronal Differentiation. Front. Bioeng. Biotechnol. 2020, 8, 580135. [Google Scholar] [CrossRef] [PubMed]
- Kheradmand, H.; Babaloo, H.; Vojgani, Y.; Mirzakhanlouei, S.; Bayat, N. PCL/gelatin scaffolds and beta-boswellic acid synergistically increase the efficiency of CGR8 stem cells differentiation into dopaminergic neuron: A new paradigm of Parkinson’s disease cell therapy. J. Biomed. Mater. Res. A 2021, 109, 562–571. [Google Scholar] [CrossRef]
- Murray, A.F.; Delivopoulos, E. Adhesion and Growth of Neuralized Mouse Embryonic Stem Cells on Parylene-C/SiO2 Substrates. Materials 2021, 14, 3174. [Google Scholar] [CrossRef]
- Hu, B.-Y.; Zhang, S.-C. Differentiation of spinal motor neurons from pluripotent human stem cells. Nat. Protoc. 2009, 4, 1295. [Google Scholar] [CrossRef]
- Qu, Q.; Li, D.; Louis, K.R.; Li, X.; Yang, H.; Sun, Q.; Crandall, S.R.; Tsang, S.; Zhou, J.; Cox, C.L.; et al. High-efficiency motor neuron differentiation from human pluripotent stem cells and the function of Islet-1. Nat. Commun. 2014, 5, 3449. [Google Scholar] [CrossRef]
- Wichterle, H.; Peljto, M. Differentiation of Mouse Embryonic Stem Cells to Spinal Motor Neurons. Curr. Protoc. Stem Cell Biol. 2008, 5, 1H.1.1–1H.1.9. [Google Scholar] [CrossRef] [PubMed]
- Lek, M.; Dias, J.M.; Marklund, U.; Uhde, C.W.; Kurdija, S.; Lei, Q.; Sussel, L.; Rubenstein, J.L.; Matise, M.P.; Arnold, H.-H.; et al. A homeodomain feedback circuit underlies step-function interpretation of a Shh morphogen gradient during ventral neural patterning. Development 2010, 137, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, J.; Ericson, J. Specification of neuronal fates in the ventral neural tube. Curr. Opin. Neurobiol. 2001, 11, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Shimazaki, T.; Sobue, G.; Okano, H. Retinoic-acid-concentration-dependent acquisition of neural cell identity during in vitro differentiation of mouse embryonic stem cells. Dev. Biol. 2004, 275, 124–142. [Google Scholar] [CrossRef]
- Wu, C.Y.; Whye, D.; Mason, R.W.; Wang, W. Efficient Differentiation of Mouse Embryonic Stem Cells into Motor Neurons. J. Vis. Exp. 2012, 64, 3813. [Google Scholar] [CrossRef]
- Li, Y.; Mao, X.; Zhou, X.; Su, Y.; Zhou, X.; Shi, K.; Zhao, S. An optimized method for neuronal differentiation of embryonic stem cells in vitro. J. Neurosci. Methods 2020, 330, 108486. [Google Scholar] [CrossRef]
- Mao, X.; Zhao, S. Neuronal Differentiation from Mouse Embryonic Stem Cells In vitro. J. Vis. Exp. 2020, 2020, e61190. [Google Scholar] [CrossRef]
- Wessler, I.; Michel-Schmidt, R.; Schmidt, H.; Kaltwasser, S.; Unger, R.; Kirkpatrick, C.J. Upregulated acetylcholine synthesis during early differentiation in the embryonic stem cell line CGR8. Neurosci. Lett. 2013, 547, 32–36. [Google Scholar] [CrossRef]
- Peljto, M.; Dasen, J.S.; Mazzoni, E.O.; Jessell, T.M.; Wichterle, H. Functional Diversity of ESC-Derived Motor Neuron Subtypes Revealed through Intraspinal Transplantation. Cell Stem Cell 2010, 7, 355–366. [Google Scholar] [CrossRef]
- Hanafiah, A.; Geng, Z.; Wang, Q.; Gao, Z. Differentiation and Characterization of Neural Progenitors and Neurons from Mouse Embryonic Stem Cells. J. Vis. Exp. 2020, 2020, e61446. [Google Scholar] [CrossRef]
- Briggs, J.A.; Li, V.C.; Lee, S.; Woolf, C.J.; Klein, A.; Kirschner, M.W. Mouse embryonic stem cells can differentiate via multiple paths to the same state. Elife 2017, 6, e26945. [Google Scholar] [CrossRef]
- Sarnat, H.B. Immunocytochemical markers of neuronal maturation in human diagnostic neuropathology. Cell Tissue Res. 2015, 359, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.B.; Das, S.; Miller, K.E. Subcellular localization of neuronal nuclei (NeuN) antigen in size and calcitonin gene-related peptide (CGRP) populations of dorsal root ganglion (DRG) neurons during acute peripheral inflammation. Neurosci. Lett. 2021, 760, 135974. [Google Scholar] [CrossRef] [PubMed]
- Nassauw, L.V.; Wu, M.; De Jonge, F.; Adriaensen, D.; Timmermans, J.P. Cytoplasmic, but not nuclear, expression of the neuronal nuclei (NeuN) antibody is an exclusive feature of Dogiel type II neurons in the guinea-pig gastrointestinal tract. Histochem. Cell Biol. 2005, 124, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Zink, A.; Conrad, J.; Telugu, N.S.; Diecke, S.; Heinz, A.; Wanker, E.; Priller, J.; Prigione, A. Assessment of Ethanol-Induced Toxicity on iPSC-Derived Human Neurons Using a Novel High-Throughput Mitochondrial Neuronal Health (MNH) Assay. Front. Cell Dev. Biol. 2020, 8, 590540. [Google Scholar] [CrossRef]
- Ohara, Y.; Koganezawa, N.; Yamazaki, H.; Roppongi, R.T.; Sato, K.; Sekino, Y.; Shirao, T. Early-stage development of human induced pluripotent stem cell-derived neurons. J. Neurosci. Res. 2015, 93, 1804–1813. [Google Scholar] [CrossRef]
- Pawlowski, M.; Ortmann, D.; Bertero, A.; Tavares, J.M.; Pedersen, R.A.; Vallier, L.; Kotter, M.R. Inducible and Deterministic Forward Programming of Human Pluripotent Stem Cells into Neurons, Skeletal Myocytes, and Oligodendrocytes. Stem Cell Rep. 2017, 8, 803. [Google Scholar] [CrossRef]
- Oh, Y.; Jang, J. Directed Differentiation of Pluripotent Stem Cells by Transcription Factors. Mol. Cells 2019, 42, 200–209. [Google Scholar] [CrossRef]
- Mehrban, N.; Zhu, B.; Tamagnini, F.; Young, F.I.; Wasmuth, A.; Hudson, K.L.; Thomson, A.R.; Birchall, M.A.; Randall, A.D.; Song, B.; et al. Functionalized α-Helical Peptide Hydrogels for Neural Tissue Engineering. ACS Biomater. Sci. Eng. 2015, 1, 431–439. [Google Scholar] [CrossRef]
- Nistor, P.A.; May, P.W.; Tamagnini, F.; Randall, A.D.; Caldwell, M.A. Long-term culture of pluripotent stem-cell-derived human neurons on diamond—A substrate for neurodegeneration research and therapy. Biomaterials 2015, 61, 139–149. [Google Scholar] [CrossRef]
- Hergenreder, E.; Minotti, A.P.; Zorina, Y.; Oberst, P.; Zhao, Z.; Munguba, H.; Calder, E.L.; Baggiolini, A.; Walsh, R.M.; Liston, C.; et al. Combined small-molecule treatment accelerates maturation of human pluripotent stem cell-derived neurons. Nat. Biotechnol. 2024, 42, 1515–1525. [Google Scholar] [CrossRef]
- van Horn, M.R.; Benfey, N.J.; Shikany, C.; Severs, L.J.; Deemyad, T. Neuron-astrocyte networking: Astrocytes orchestrate and respond to changes in neuronal network activity across brain states and behaviors. J. Neurophysiol. 2021, 126, 627–636. [Google Scholar] [CrossRef]
- Oliveira, J.F.; Araque, A. Astrocyte regulation of neural circuit activity and network states. Glia 2022, 70, 1455. [Google Scholar] [CrossRef] [PubMed]
- Pirttimaki, T.M.; Parri, H.R. Astrocyte Plasticity. Neuroscientist 2013, 19, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.S.; Chung, W.S. Astrocytes regulate neuronal network activity by mediating synapse remodeling. Neurosci. Res. 2023, 187, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Ciceri, G.; Baggiolini, A.; Cho, H.S.; Kshirsagar, M.; Benito-Kwiecinski, S.; Walsh, R.M.; Aromolaran, K.A.; Gonzalez-Hernandez, A.J.; Munguba, H.; Koo, S.Y.; et al. An epigenetic barrier sets the timing of human neuronal maturation. Nature 2024, 626, 881–890. [Google Scholar] [CrossRef]
- Brännvall, K.; Korhonen, L.; Lindholm, D. Estrogen-receptor-dependent regulation of neural stem cell proliferation and differentiation. Mol. Cell. Neurosci. 2002, 21, 512–520. [Google Scholar] [CrossRef]
- López-González, R.; Camacho-Arroyo, I.; Velasco, I. Progesterone and 17β-estradiol increase differentiation of mouse embryonic stem cells to motor neurons. IUBMB Life 2011, 63, 930–939. [Google Scholar] [CrossRef]
- Lovén, J.; Zinin, N.; Wahlström, T.; Müller, I.; Brodin, P.; Fredlund, E.; Ribacke, U.; Pivarcsi, A.; Påhlman, S.; Henriksson, M. MYCN-regulated microRNAs repress estrogen receptor-alpha (ESR1) expression and neuronal differentiation in human neuroblastoma. Proc. Natl. Acad. Sci. USA 2010, 107, 1553–1558. [Google Scholar] [CrossRef]
- Piiper, A.; Dikic, I.; Lutz, M.P.; Leser, J.; Kronenberger, B.; Elez, R.; Cramer, H.; Müller-Esterl, W.; Zeuzem, S. Cyclic AMP induces transactivation of the receptors for epidermal growth factor and nerve growth factor, thereby modulating activation of MAP kinase, Akt, and neurite outgrowth in PC12 cells. J. Biol. Chem. 2002, 277, 43623–43630. [Google Scholar] [CrossRef]
- Tzeng, H.H.; Hsu, C.H.; Chung, T.H.; Lee, W.C.; Lin, C.H.; Wang, W.C.; Hsiao, C.Y.; Leu, Y.W.; Wang, T.H.; Kango-Singh, M. Cell Signaling and Differential Protein Expression in Neuronal Differentiation of Bone Marrow Mesenchymal Stem Cells with Hypermethylated Salvador/Warts/Hippo (SWH) Pathway Genes. PLoS ONE 2015, 10, e0145542. [Google Scholar] [CrossRef]
- Tan, B.T.; Wang, L.; Li, S.; Long, Z.Y.; Wu, Y.M.; Liu, Y. Retinoic acid induced the differentiation of neural stem cells from embryonic spinal cord into functional neurons in vitro. Int. J. Clin. Exp. Pathol. 2015, 8, 8129–8135. [Google Scholar]


| Target | Company/Cat No. | Host | Dilution |
|---|---|---|---|
| Β-Tubulin III (TUJ1) | Abcam, Cambridge, UK (ab 18207) | Mouse | 1:300 |
| VGLUT1 | 2BScientific Kidlington, UK (135-316-SY) | Chicken | 1:300 |
| GAD2 | St Johns (STJ23739) | Rabbit | 1:500 |
| Rabbit | ThermoFisher, UK (A-11011) | Goat | 1:300 |
| Chicken | ThermoFisher, UK (A-21449) | Goat | 1:300 |
| Mouse | ThermoFisher, UK (A-11001) | Goat | 1:300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vajaria, R.; Davis, D.; Tamagnini, F.; McMillan, D.G.G.; Vasudevan, N.; Delivopoulos, E. Generation of Active Neurons from Mouse Embryonic Stem Cells Using Retinoic Acid and Purmorphamine. Int. J. Mol. Sci. 2025, 26, 8372. https://doi.org/10.3390/ijms26178372
Vajaria R, Davis D, Tamagnini F, McMillan DGG, Vasudevan N, Delivopoulos E. Generation of Active Neurons from Mouse Embryonic Stem Cells Using Retinoic Acid and Purmorphamine. International Journal of Molecular Sciences. 2025; 26(17):8372. https://doi.org/10.3390/ijms26178372
Chicago/Turabian StyleVajaria, Ruby, DeAsia Davis, Francesco Tamagnini, Duncan G. G. McMillan, Nandini Vasudevan, and Evangelos Delivopoulos. 2025. "Generation of Active Neurons from Mouse Embryonic Stem Cells Using Retinoic Acid and Purmorphamine" International Journal of Molecular Sciences 26, no. 17: 8372. https://doi.org/10.3390/ijms26178372
APA StyleVajaria, R., Davis, D., Tamagnini, F., McMillan, D. G. G., Vasudevan, N., & Delivopoulos, E. (2025). Generation of Active Neurons from Mouse Embryonic Stem Cells Using Retinoic Acid and Purmorphamine. International Journal of Molecular Sciences, 26(17), 8372. https://doi.org/10.3390/ijms26178372






