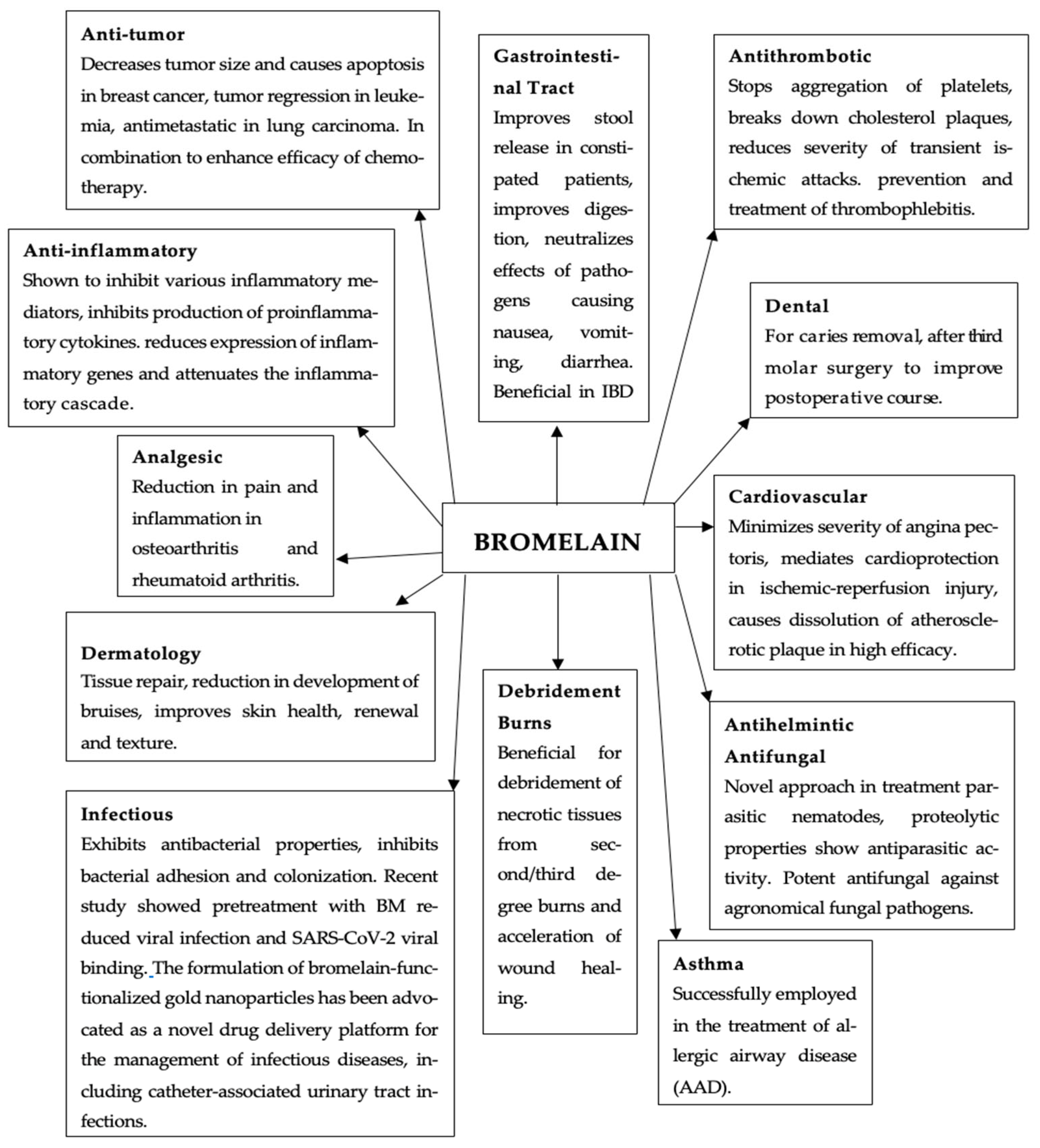
Bromelain in Obesity Therapy: A Review of Anti-Inflammatory and Metabolic Mechanisms
Abstract
1. Introduction
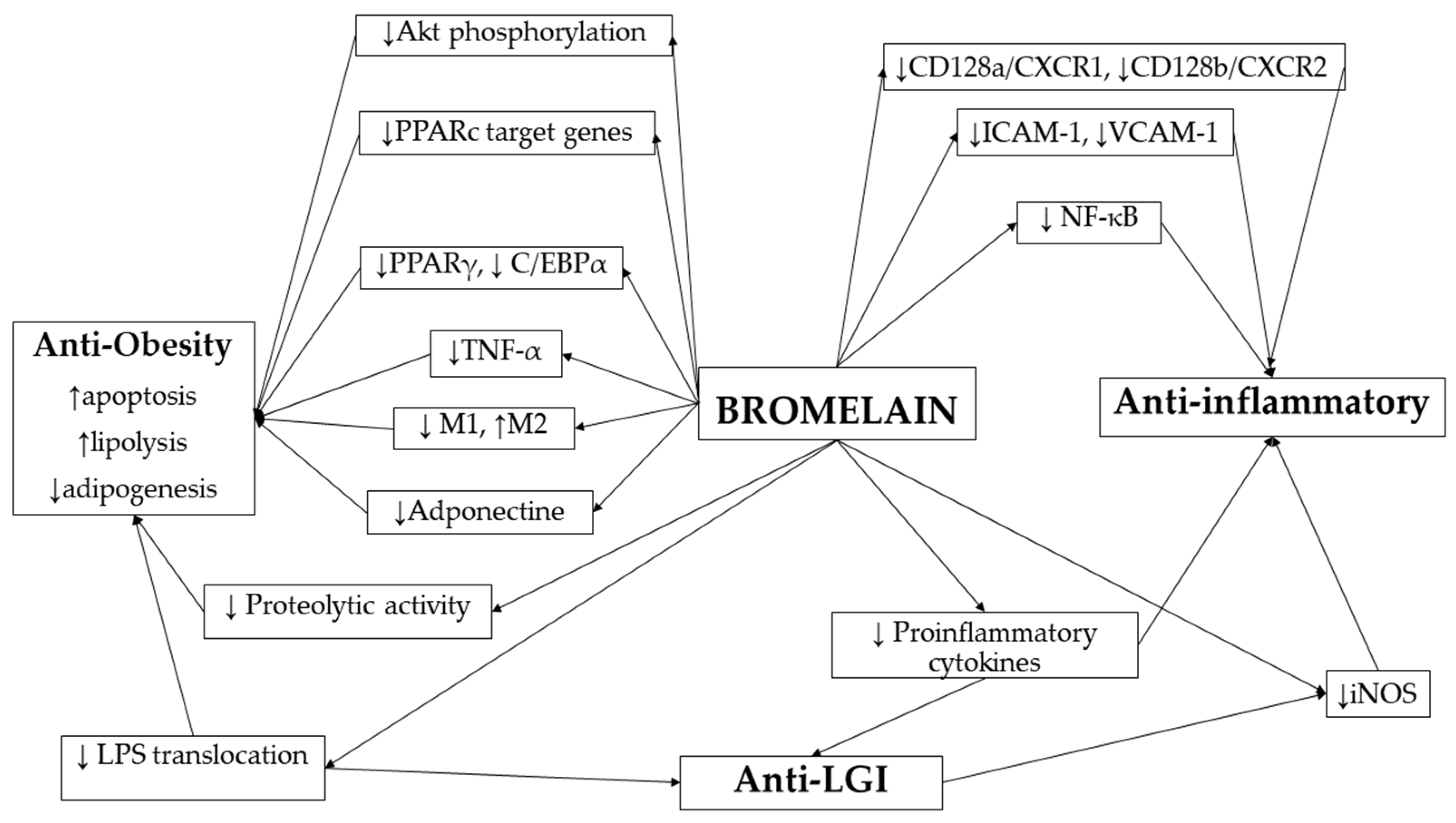
2. Mechanisms of Bromelain Action in Obesity and Inflammation
2.1. Bromelain as a Regulator of Adipocyte Function and Metabolic Signaling
2.2. The Effect of Bromelain on the Immune System and Inflammatory Pathways
2.3. The Role of Bromelain in Obesity-Related Low-Grade Inflammation
2.4. Potential Effect of Bromelain on Circadian Regulation of Adipose Tissue
3. Evidence from In Vitro (Cell) and In Vivo (Human, Animal) Studies Related to the Functions of Bromelain—Immunomodulation, Fibrinolysis, Treatment of Obesity, Inflammation, Cardiovascular Diseases, NAFLD, and Diabetes
3.1. Obesity-Related Models
3.2. Anti-Inflammation and Immunomodulation
3.3. Cardiovascular Diseases, Dyslipidemia, and Hypertension
3.4. Blood Coagulation and Fibrinolysis
3.5. Nonalcoholic Fatty Liver Disease and Diabetes
4. Pharmacological Properties, Bioavailability, and Aspects of Use
4.1. Pharmacological Properties
4.2. Clinical Applications and Therapeutic Use
4.3. Dosage, Pharmacokinetics, and Safety
4.4. Contraindications and Drug Interactions
4.5. Limitations of Recent Studies and Strength of Current Evidence
5. Materials and Methods
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3T3-L1 | Preadipocyte cell line derived from mouse embryonic fibroblasts |
| ACC | Acetyl-CoA carboxylase |
| AGEs | Advanced glycation end-products |
| Akt | Protein kinase B (PKB), also known as Akt |
| AP-1 | Activator protein 1 (transcription factor complex including c-Jun and c-Fos) |
| Ap2 | Adipocyte protein 2 (also known as FABP4—fatty acid binding protein 4) |
| Apoe−/− mice | Apolipoprotein E-deficient mice (model of atherosclerosis) |
| APTT | Activated partial thromboplastin time |
| BAT | Brown adipose tissue |
| BMI | Body mass index |
| C/EBP | CCAAT/enhancer-binding protein |
| CCI | Chronic Constriction Injury |
| CD | Cluster of differentiation |
| CD (np. CD7, CD8α, CD14 itd.) | Cluster of differentiation |
| CD1 mice | Nice-type CD1 |
| CD128a/CXCR1 | CXC chemokine receptor 1 |
| CD128b/CXCR2 | CXC chemokine receptor 2 |
| CKD | Chronic kidney disease |
| COX-2 | Cycloxygenase |
| CPT-1 | Carnitine palmitoyltransferase 1 |
| CRP | C-reactive protein |
| CTLA-4 | Cytotoxic T lymphocyte antigen 4 |
| CU/mL | Casein units per milliliter |
| CVD | Cardiovascular disease |
| EC | Enzyme Commission Number |
| EP | Leptin gene expression |
| ERK | Extracellular signal-regulated kinase |
| ERK, JNK, MAPK, p38 | Protein kinases involved in cellular signaling pathways |
| ESRD | End-stage renal disease |
| FAS | Fatty acid synthase |
| FD 4 | Fluorescein isothiocyanate-dextran 4 |
| FFAs | Free fatty acids |
| FIB | Fibrinogen |
| FIP unit | Federation Internationale Pharmaceutique unit |
| Gia1 | Inhibitory G protein alpha subunit 1 |
| GLUT-2 | Glucose transporter type 2 |
| GLUT4 | Glucose transporter type 4 |
| HC | Hip circumference |
| HDL-C | High-density lipoprotein cholesterol |
| hDPCs | Human dental pulp cells |
| HFD | High-fat diet |
| Hgb1c | Glycated hemoglobin |
| HGFs | Human gingival fibroblasts |
| HIPEs | High internal phase emulsions |
| HOMA-IR | Homeostasis Model Assessment of Insulin Resistance |
| HSL | Hormone-sensitive lipase |
| IBD | Inflammatory bowel disease |
| ICAM-1 | Intercellular adhesion molecule 1 |
| IFN-γ | Interferon gamma |
| IL-1β | Interleukin 1 beta |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| IL-10 | Interleukin 10 |
| iNOS | Inducible nitric oxide synthase |
| IR | Insulin resistance |
| IRS-1 | Insulin receptor substrate 1 |
| JNK | C-Jun N-terminal kinase |
| LDL-C | Low-density lipoprotein cholesterol |
| LPL | Lipoprotein lipase |
| M1 | Classically activated (pro-inflammatory) macrophages |
| M2 | Alternatively activated (anti-inflammatory) macrophages |
| MAPK | Mitogen-activated protein kinase |
| MCL | Mitotic clonal expansion |
| MCP-1 | Monocyte chemoattractant protein 1 |
| M-CSF | Macrophage colony-stimulating factor |
| MIP-1α | Macrophage inflammatory protein 1 alpha |
| MIP-1β | Macrophage inflammatory protein 1 beta |
| MLCK | Myosin light chain kinase |
| MMP-8, MMP-1, MMP-3, MMP-13, MMP-9 | Matrix metalloproteinases |
| mRNA | Messenger ribonucleic acid |
| mTORC1 | Mechanistic target of rapamycin complex 1 |
| NAFLD | Non-alcoholic fatty liver disease |
| NF-κB | Nuclear factor kappa B |
| NK | Natural killer cells |
| NO | Nitric oxide |
| OA | Osteoarthritis |
| OPG | Osteoprotegerin |
| p38 | p38 MAP kinase (a subfamily of MAPK) |
| PBMC | Peripheral blood mononuclear cell |
| PCOS | Polycystic ovary syndrome |
| PDE3B | Phosphodiesterase 3B |
| PGE2 | Prostaglandin E2 |
| PPARγ (PPARc) | Peroxisome proliferator-activated receptor gamma |
| PT | Prothrombin time |
| RANKL | Receptor activator of nuclear factor κ B ligand |
| RAAS | Renin–angiotensin–aldosterone system |
| SBM | Stem bromelain |
| SRBC | Sheep red blood cells |
| SREBP-1c | Sterol regulatory element-binding protein 1c |
| T cells | T lymphocytes |
| T2DM | Type 2 diabetes mellitus |
| TC | Total cholesterol |
| TER | Transepithelial electrical resistance |
| TG | Triglyceride |
| TLR4 | Toll-like receptor 4 |
| TNF-α | Tumor necrosis factor alpha |
| TSC2 | Tuberous sclerosis complex 2 |
| UC/mL | Enzymatic activity unit concentration |
| VCAM-1 | Vascular cell adhesion molecule 1 |
| ICAM-1 | Intercellular cell adhesion molecule 1 |
| WAT | White adipose tissue |
| WC | Waist circumference |
| WHR | Waist-to-hip ratio |
References
- Caballero, B. Humans Against Obesity: Who Will Win? Adv. Nutr. Int. Rev. J. 2019, 10, S4–S9. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 23 April 2025).
- The World Obesity Federation. World Obesity Atlas 2023. 2023. Available online: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2023 (accessed on 23 April 2025).
- Karri, S.; Sharma, S.; Hatware, K.; Patil, K. Natural anti-obesity agents and their therapeutic role in management of obesity: A future trend perspective. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 110, 224–238. [Google Scholar] [CrossRef]
- Hikisz, P.; Bernasinska-Slomczewska, J. Beneficial Properties of Bromelain. Nutrients 2021, 13, 4313. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Nikhade, P.; Patel, A.; Mankar, N.; Sedani, S. Bromelain: A Potent Phytomedicine. Cureus 2022, 14, e27876. [Google Scholar] [CrossRef] [PubMed]
- Castell, J.V.; Friedrich, G.; Kuhn, C.S.; Poppe, G.E. Intestinal absorption of undegraded proteins in men: Presence of bromelain in plasma after oral intake. Am. J. Physiol. 1997, 273 Pt 1, G139–G146. [Google Scholar] [CrossRef]
- Manzoor, Z.; Nawaz, A.; Mukhtar, H.; Haq, I. Bromelain: Methods of extraction, purification and therapeutic applications. Braz. Arch. Biol. Technol. 2016, 59, e16150010. [Google Scholar] [CrossRef]
- Báez, R.; Lopes, M.T.; Salas, C.E.; Hernández, M. In vivo antitumoral activity of stem pineapple (Ananas comosus) bromelain. Planta Medica 2007, 73, 1377–1383. [Google Scholar] [CrossRef]
- Pavan, R.; Jain, S.; Shraddha Kumar, A. Properties and therapeutic application of bromelain: A review. Biotechnol. Res. Int. 2012, 2012, 976203. [Google Scholar] [CrossRef]
- Taussig, S.J.; Szekerczes, J.; Batkin, S. Inhibition of Tumour Growth In Vitro by Bromelain, an Extract of the Pineapple Plant (Ananas comosus). Planta Medica 1985, 51, 538–539. [Google Scholar] [CrossRef]
- Ley, C.M.; Tsiami, A.; Ni, Q.; Robinson, N. A review of the use of bromelain in cardiovascular diseases. Zhong Xi Yi Jie He Xue Bao J. Chin. Integr. Med. 2011, 9, 702–710. [Google Scholar] [CrossRef]
- Secor, E.R.; Szczepanek, S.M., Jr.; Castater, C.A.; Adami, A.J.; Matson, A.P.; Rafti, E.T.; Guernsey, L.; Natarajan, P.; McNamara, J.T.; Schramm, C.M.; et al. Bromelain Inhibits Allergic Sensitization and Murine Asthma via Modulation of Dendritic Cells. Evid. Based Complement. Altern. Med. 2013, 2013, 702196. [Google Scholar] [CrossRef] [PubMed]
- Wasso, S.; Maina, N.; Kagira, J. Toxicity and anthelmintic efficacy of chitosan encapsulated bromelain against gastrointestinal strongyles in Small East African goats in Kenya. Vet. World 2020, 13, 177–183. [Google Scholar] [CrossRef] [PubMed]
- López-García, B.; Hernández, M.; Segundo, B.S. Bromelain, a cysteine protease from pineapple (Ananas comosus) stem, is an inhibitor of fungal plant pathogens. Lett. Appl. Microbiol. 2012, 55, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Akhter, J.; Queromes, G.; Pillai, K.; Kepenekian, V.; Badar, S.; Mekkawy, A.H.; Frobert, E.; Valle, S.J.; Morris, D.L. The Combination of Bromelain and Acetylcysteine (BromAc) Synergistically Inactivates SARS-CoV-2. Viruses 2021, 13, 425. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kang, J.H.; Lee, D.Y.; Jeong, J.W.; Kim, J.H.; Moon, S.S.; Hur, S.J. Methods for improving meat protein digestibility in older adults. J. Anim. Sci. Technol. 2023, 65, 32–56. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. AMS 2017, 13, 851–863. [Google Scholar] [CrossRef]
- Pi-Sunyer, F.X. The obesity epidemic: Pathophysiology and consequences of obesity. Obes. Res. 2002, 10, 97S–104S. [Google Scholar] [CrossRef]
- Aloysius, T.A.; Carvajal, A.K.; Slizyte, R.; Skorve, J.; Berge, R.K.; Bjørndal, B. Chicken Protein Hydrolysates Have Anti-Inflammatory Effects on High-Fat Diet Induced Obesity in Mice. Medicines 2018, 6, 5. [Google Scholar] [CrossRef]
- Piché, M.E.; Tchernof, A.; Després, J.-P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500, Erratum in Circ. Res. 2020, 127, e107. [Google Scholar] [CrossRef]
- Li, M.; Cui, M.; Li, G.; Liu, Y.; Xu, Y.; Eftekhar, S.P.; Ala, M. The Pathophysiological Associations Between Obesity, NAFLD, and Atherosclerotic Cardiovascular Diseases. Horm. Metab. Res. 2024, 10, 683–696. [Google Scholar] [CrossRef]
- Cho, Y.; Hong, N.; Kim, K.-W.; Cho, S.J.; Lee, M.; Lee, Y.-H.; Lee, Y.-H.; Kang, E.S.; Cha, B.-S.; Lee, B.-W. The effectiveness of intermittent fasting to reduce body mass index and glucose metabolism: A systematic Review and meta-analysis. J. Clin. Med. 2019, 8, 1645. [Google Scholar] [CrossRef] [PubMed]
- Ye, J. Mechanisms of insulin resistance in obesity. Front. Med. 2013, 7, 14–24. [Google Scholar] [CrossRef]
- Welsh, A.; Hammad, M.; Piña, I.L.; Kulinski, J. Obesity and cardiovascular health. Eur. J. Prev. Cardiol. 2024, 8, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, L.; Aronne, L.J.; Beilin, L.J.; Burke, V.; Igel, L.I.; Lloyd-Jones, D.; Sowers, J. Obesity-related hypertension: Pathogenesis, cardiovascular risk, and treatment: A position paper of The Obesity Society and the American Society of Hypertension. J. Clin. Hypertens. 2013, 15, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Rocchini, A.P.; Key, J.; Bondie, D.; Chico, R.; Moorehead, C.; Katch, V.; Martin, M. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N. Engl. J. Med. 1989, 321, 580–585. [Google Scholar] [CrossRef]
- Haley, M.J.; Lawrence, C.B. Obesity and stroke: Can we translate from rodents to patients? J. Cereb. Blood Flow. Metab. 2016, 36, 2007–2021. [Google Scholar] [CrossRef]
- Andersen, K.K.; Olsen, T.S. The obesity paradox in stroke: Lower mortality and lower risk of readmission for recurrent stroke in obese stroke patients. Int. J. Stroke 2015, 10, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, S.; Wakabayashi, H.; Yoshida, T.; Mori, N.; Watanabe, R.; Nishioka, E. Obese Japanese Patients with Stroke Have Higher Functional Recovery in Convalescent Rehabilitation Wards: A Retrospective Cohort Study. J. Stroke Cerebrovasc. Dis. 2016, 25, 26–33. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Furth, S.L.; Zoccali, C.; World Kidney Day Steering Committee. Obesity and kidney disease: Hidden consequences of the epidemic. J. Nephrol. 2017, 30, 1–10. [Google Scholar] [CrossRef]
- Blüher, M. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr. Opin. Lipidol. 2010, 21, 38–43. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, Y.; Zhao, X.; Cui, H.; Han, M.; Ren, X.; Gang, X.; Wang, G. Obesity and chronic kidney disease. Am. J. Physiol. Endocrinol. Metab. 2023, 324, E24–E41. [Google Scholar] [CrossRef]
- Reynolds, K.; Gu, D.; Muntner, P.; Kusek, J.W.; Chen, J.; Wu, X.; Duan, X.; Chen, C.S.; Klag, M.J.; Whelton, P.K.; et al. A population-based, prospective study of blood pressure and risk for end-stage renal disease in China. J. Am. Soc. Nephrol. JASN 2021, 18, 1928–1935. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Electronic address: Asrm@asrm.org; Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: A committee opinion. Fertil. Steril. 2021, 116, 1266–1285. [Google Scholar] [CrossRef]
- Rich-Edwards, J.W.; Goldman, M.B.; Willett, W.C.; Hunter, D.J.; Stampfer, M.J.; Colditz, G.A.; Manson, J.E. Adolescent body mass index and infertility caused by ovulatory disorder. Am. J. Obstet. Gynecol. 1994, 171, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef]
- Brown, K.A.; Scherer, P.E. Update on Adipose Tissue and Cancer. Endocr. Rev. 2023, 44, 961–974. [Google Scholar] [CrossRef]
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Rakib, A.; Al Mamun, A.; Mandal, M.; Sinha, P.; Singh, U.P. Obesity–cancer axis crosstalk: Molecular insights and therapeutic approaches. Acta Pharm. Sin. B 2025, 15, 2930–2944. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. CCAAT/Enhancer-Binding Protein Beta Is Required for Mitotic Clonal Expansion During Adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 850–855. [Google Scholar] [CrossRef]
- Alessi, M.C.; Lijnen, H.R.; Bastelica, D.; Juhan-Vague, I. Adipose Tissue and Atherothrombosis. Pathophysiol. Haemost. Thromb. 2003, 33, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Dave, S.; Kaur, N.J.; Nanduri, R.; Dkhar, H.K.; Kumar, A.; Gupta, P. Inhibition of Adipogenesis and Induction of Apoptosis and Lipolysis by Stem Bromelain in 3T3-L1 Adipocytes. PLoS ONE 2012, 7, e30831. [Google Scholar] [CrossRef]
- Zhang, H.H.; Huang, J.; Düvel, K.; Boback, B.; Wu, S.; Squillace, R.M.; Wu, C.-L.; Manning, B.D.; Blagosklonny, M.V. Insulin Stimulates Adipogenesis Through the Akt-TSC2-mTORC1 Pathway. PLoS ONE 2009, 4, e6189. [Google Scholar] [CrossRef]
- Tamai, M.; Shimada, T.; Hiramatsu, N.; Hayakawa, K.; Okamura, M.; Tagawa, Y.; Takahashi, S.; Nakajima, S.; Yao, J.; Kitamura, M. Selective Deletion of Adipocytes, but Not Preadipocytes, by TNF-Alpha Through C/EBP and PPARγ-Mediated Suppression of NF-κB. Lab. Investig. 2010, 90, 1385–1395, Erratum in Lab. Investig. 2017, 97, 228. [Google Scholar] [CrossRef][Green Version]
- Prins, J.B.; Niesler, C.U.; Winterford, C.M.; Bright, N.A.; Siddle, K.; O’Rahilly, S.; Walker, N.I.; Cameron, D.P. Tumor Necrosis Factor-Alpha Induces Apoptosis of Human Adipose Cells. Diabetes 1997, 46, 1939–1944. [Google Scholar] [CrossRef]
- Kumar, V.; Mangla, B.; Javed, S.; Ahsan, W.; Kumar, P.; Garg, V.; Dureja, H. Bromelain: A Review of Its Mechanisms, Pharmacological Effects and Potential Applications. Food Funct. 2023, 14, 8101–8128. [Google Scholar] [CrossRef] [PubMed]
- Cancello, R.; Henegar, C.; Viguerie, N.; Taleb, S.; Poitou, C.; Rouault, C.; Coupaye, M.; Pelloux, V.; Hugol, D.; Bouillot, J.L.; et al. Reduction of Macrophage Infiltration and Chemoattractant Gene Expression Changes in White Adipose Tissue of Morbidly Obese Subjects After Surgery-Induced Weight Loss. Diabetes 2005, 54, 2277–2286. [Google Scholar] [CrossRef]
- El-Shazly, S.A.; Ahmed, M.M.; Al-Harbi, M.S.; Alkafafy, M.E.; El-Sawy, H.B.; Amer, S.A.M. Physiological and Molecular Study on the Anti-Obesity Effects of Pineapple (Ananas comosus) Juice in Male Wistar Rat. Food Sci. Biotechnol. 2018, 27, 1429–1438. [Google Scholar] [CrossRef]
- Hasoon, D.A.A.-W.; Kadhim, K.A.; Rahmah, A.M. Effect of Bromelain in Obese Diabetic Patients in Iraq. Rev. Latinoam. Hipertens. 2022, 17, 5. [Google Scholar]
- Vatanen, T.; Kostic, A.D.; d’Hennezel, E.; Siljander, H.; Franzosa, E.A.; Yassour, M.; Kolde, R.; Vlamakis, H.; Arthur, T.D.; Hämä Läinen, A.M.; et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell 2016, 165, 1551. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Kansakar, U.; Trimarco, V.; Manzi, M.V.; Cervi, E.; Mone, P.; Santulli, G. Exploring the Therapeutic Potential of Bromelain: Applications, Benefits, and Mechanisms. Nutrients 2024, 16, 2060. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Hsia, C.-C.; Hu, P.-A.; Yeh, C.-H.; Chen, C.-T.; Peng, C.-L.; Wang, C.-H.; Lee, T.-S. Bromelain Ameliorates Atherosclerosis by Activating the TFEB-Mediated Autophagy and Antioxidant Pathways. Antioxidants 2023, 12, 72. [Google Scholar] [CrossRef]
- Singh, V.; Kaur, R.; Kumari, P.; Pasricha, C.; Singh, R. ICAM-1 and VCAM-1: Gatekeepers in Various Inflammatory and Cardiovascular Disorders. Clin. Chim. Acta 2023, 548, 117487. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Kim, M.R.; Lee, B.N.; Och, W.M.; Min, K.S.; Im, Y.G.; Hwang, Y.C. Anti-Inflammatory and Mineralization Effects of Bromelain on Lipopolysaccharide-Induced Inflammation of Human Dental Pulp Cells. Medicina 2021, 57, 591. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J.-B.; Lee, J.-T.; Park, H.-R.; Kim, J.-B. Medicinal Effects of Bromelain (Ananas comosus) Targeting Oral Environment as an Anti-Oxidant and Anti-Inflammatory Agent. J. Food Nutr. Res. 2018, 6, 773–784. [Google Scholar] [CrossRef]
- Thanos, D.; Georgopoulos, K.; Greenberg, M.E.; Leder, P. c-Jun Dimerizes with Itself and with c-Fos, Forming Complexes of Different DNA Binding Affinities. Cell 1988, 55, 917–924. [Google Scholar] [CrossRef]
- Onken, J.E.; Greer, P.K.; Calingaert, B.; Hale, L.P. Bromelain Treatment Decreases Secretion of Pro-Inflammatory Cytokines and Chemokines by Colon Biopsies In Vitro. Clin. Immunol. 2008, 126, 345–352. [Google Scholar] [CrossRef]
- Hou, R.C.-W.; Chen, Y.-S.; Huang, J.-R.; Jeng, K.-C.G. Cross-Linked Bromelain Inhibits Lipopolysaccharide-Induced Cytokine Production Involving Cellular Signaling Suppression in Rats. J. Agric. Food Chem. 2006, 54, 2193–2198. [Google Scholar] [CrossRef]
- Bottega, R.; Persico, I.; De Seta, F.; Romano, F.; Di Lorenzo, G. Anti-Inflammatory Properties of a Proprietary Bromelain Extract (Bromeyal™) after In Vitro Simulated Gastrointestinal Digestion. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211034686. [Google Scholar] [CrossRef]
- Habashi, S.A.; Sabouni, F.; Moghimi, A.; Majd, S.A. Modulation of Lipopolysaccharide Stimulated Nuclear Factor Kappa B-Mediated iNOS/NO Production by Bromelain in Rat Primary Microglial Cells. Iran. Biomed. J. 2015, 20, 33–40. [Google Scholar]
- Kienzler, A.-K.; Rizzi, M.; Goldacker, S.; Warnatz, K.; Salzer, U.; Schlesier, M.; Peter, H.H.; Eibel, H. Bromelain Treatment Decreases Neutrophil Migration to Sites of Inflammation. Clin. Immunol. 2008, 128, 66–74. [Google Scholar] [CrossRef]
- Hale, L.P.; Greer, P.K.; Sempowski, G.D. Bromelain Treatment Alters Leukocyte Expression of Cell Surface Molecules Involved in Cellular Adhesion and Activation. Clin. Immunol. 2002, 104, 183–190. [Google Scholar] [CrossRef]
- Lu, H.C.; Ng, M.Y.; Liao, Y.W.; Maekawa, S.; Lin, T.; Yu, C.C. Bromelain Inhibits the Inflammation and Senescence Effect in Diabetic Periodontitis: A Preliminary In Vitro Study. J. Dent. Sci. 2023, 18, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.R.; Wu, C.C.; Hou, R.C.W.; Jeng, K.C. Bromelain Inhibits Lipopolysaccharide-Induced Cytokine Production in Human THP-1 Monocytes via the Removal of CD14. Immunol. Investig. 2008, 37, 263–277. [Google Scholar] [CrossRef]
- Man, A.W.C.; Xia, N.; Li, H. Circadian Rhythm in Adipose Tissue: Novel Antioxidant Target for Metabolic and Cardiovascular Diseases. Antioxidants 2020, 9, 968. [Google Scholar] [CrossRef] [PubMed]
- Maury, E.; Navez, B.; Brichard, S.M. Circadian Clock Dysfunction in Human Omental Fat Links Obesity to Metabolic Inflammation. Nat. Commun. 2021, 12, 2388. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Forstermann, U. Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr. Opin. Pharm. 2013, 13, 161–167. [Google Scholar] [CrossRef]
- Chang, L.; Garcia-Barrio, M.T.; Chen, Y.E. Perivascular Adipose Tissue Regulates Vascular Function by Targeting Vascular Smooth Muscle Cells. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1094–1109. [Google Scholar] [CrossRef]
- Victorio, J.A.; Fontes, M.T.; Rossoni, L.V.; Davel, A.P. Different Anti-Contractile Function and Nitric Oxide Production of Thoracic and Abdominal Perivascular Adipose Tissues. Front. Physiol. 2016, 7, 295. [Google Scholar] [CrossRef]
- Omar, A.; Chatterjee, T.K.; Tang, Y.; Hui, D.Y.; Weintraub, N.L. Proinflammatory phenotype of perivascular adipocytes. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1631–1636. [Google Scholar] [CrossRef]
- Bailey-Downs, L.C.; Tucsek, Z.; Toth, P.; Sosnowska, D.; Gautam, T.; Sonntag, W.E.; Csiszar, A.; Ungvari, Z. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: A paracrine mechanism contributing to vascular redox dysregulation and inflammation. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 780–792. [Google Scholar] [CrossRef]
- Ley, C.M.; Ni, Q.; Liao, X.; Gao, H.-L.; Robinson, N. Bromelain and Cardiovascular Risk Factors in Diabetes: An Exploratory Randomized, Placebo Controlled, Double Blind Clinical Trial. Chin. J. Integr. Med. 2016, 22, 728–737. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Cheng, S.; Zhai, M.; Ma, F.; Nian, Y.; Ding, L.; Hu, B. Interfacial Protein Fibril Polymorphisms Regulate In Vivo Adipose Expansion for Control of Obesity. ACS Nano 2024, 18, 17969–17986. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.A.; Chen, C.H.; Guo, B.C.; Kou, Y.R.; Lee, T.S. Bromelain Confers Protection Against the Non-Alcoholic Fatty Liver Disease in Male C57BL/6 Mice. Nutrients 2020, 12, 1458. [Google Scholar] [CrossRef]
- Ahounou Aïkpe, J.F.; Hamadou, A.; Bonoy, L.; Kotinan, A.T.; Gbenou, J.D.; Dansou, P.H. Preventive Effects of Ananas comosus Juice on Obesity Risk Factors in Female Wistar Rats. Open J. Appl. Sci. 2020, 10, 534–542. [Google Scholar] [CrossRef]
- Pereira, I.C.; Sátiro Vieira, E.E.; de Oliveira Torres, L.R.; da Carneiro Silva, F.C.; de Castro e Sousa, J.M.; Torres-Leal, F.L. Bromelain supplementation and inflammatory markers: A systematic review of clinical trials. Clin. Nutr. Espen 2023, 55, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Barth, H.; Guseo, A.; Klein, R. In vitro study on the immunological effect of bromelain and trypsin on mononuclear cells from humans. Eur. J. Med. Res. 2005, 10, 325–331. [Google Scholar]
- Rose, B.; Herder, C.; Loffler, H.; Meierhoff, G.; Schloot, N.C.; Walz, M.; Martin, S. Dose-dependent induction of IL-6 by plant-derived proteases in vitro. Clin. Exp. Immunol. 2006, 143, 85–92. [Google Scholar] [CrossRef]
- Engwerda, C.R.; Andrew, D.; Ladhams, A.; Mynott, T.L. Bromelain Modulates T Cell and B Cell Immune Responses In Vitro and In Vivo. Cell. Immunol. 2001, 210, 66–75. [Google Scholar] [CrossRef]
- Madkhali, J.Y.; Hussein, R.H.; Alnahdi, H.S. Therapeutic Effect of Bromelain and Papain on Intestinal Injury Induced by Indomethacin in Male Rats. Int. J. Health Sci. 2023, 17, 23–30. [Google Scholar]
- Zhou, Z.; Wang, L.; Feng, P.; Yin, L.; Wang, C.; Zhi, S.; Dong, J.; Wang, J.; Lin, Y.; Chen, D.; et al. Inhibition of Epithelial TNF-α Receptors by Purified Fruit Bromelain Ameliorates Intestinal Inflammation and Barrier Dysfunction in Colitis. Front. Immunol. 2017, 8, 1468. [Google Scholar] [CrossRef]
- Maleki, M.S.M.; Kiasari, R.E.; Mousavi, S.J.S.; Hashemi-Moghaddam, H.; Shabani, A.A.; Madanchi, H.; Sardari, S. Bromelain-Loaded Nanocomposites Decrease Inflammatory and Cytotoxicity Effects of Gliadin on Caco-2 Cells and Peripheral Blood Mononuclear Cells of Celiac Patients. Sci. Rep. 2023, 13, 21180. [Google Scholar] [CrossRef] [PubMed]
- Paksoy, T.; Ustaoğlu, G.; Şehirli, A.Ö.; Ünsal, R.B.K.; Sayıner, S.; Orhan, K.; Aycı, N.B.; Çetinel, Ş.; Aksoy, U.; Öğünç, A.V. Effect of Bromelain on Periodontal Destruction and Alveolar Bone in Rats with Experimental Periodontitis. Int. Immunopharmacol. 2023, 121, 110446. [Google Scholar] [CrossRef] [PubMed]
- Bakare, A.O.; Owoyele, B.V. Bromelain Reduced Pro-Inflammatory Mediators as a Common Pathway That Mediate Antinociceptive and Anti-Anxiety Effects in Sciatic Nerve Ligated Wistar Rats. Sci. Rep. 2021, 11, 289. [Google Scholar] [CrossRef]
- Sulumer, A.N. Bromelain Ameliorates Inflammation and Hyperlipidemia by Modulating Oxidative Stress and Lipid Metabolism in Hyperlipidemic Rats. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Kasemsuk, T.; Vivithanaporn, P.; Unchern, S. Anti-Inflammatory Effects of Bromelain in LPS-Induced Human U937 Macrophages. Chiang Mai J. Sci. 2018, 45, 299–307. [Google Scholar]
- Pothacharoen, P.; Chaiwongsa, R.; Chanmee, T.; Insuan, O.; Wongwichai, T.; Janchai, P.; Vaithanomsat, P. Bromelain Extract Exerts Antiarthritic Effects via Chondroprotection and the Suppression of TNF-α–Induced NF-κB and MAPK Signaling. Plants 2021, 10, 2273. [Google Scholar] [CrossRef]
- Insuan, O.; Janchai, P.; Thongchuai, B.; Chaiwongsa, R.; Khamchun, S.; Saoin, S.; Insuan, W.; Pothacharoen, P.; Apiwatanapiwat, W.; Boondaeng, A.; et al. Anti-Inflammatory Effect of Pineapple Rhizome Bromelain Through Downregulation of the NF-κB- and MAPKs-Signaling Pathways in Lipopolysaccharide (LPS)-Stimulated RAW264.7 Cells. Curr. Issues Mol. Biol. 2021, 43, 93–106. [Google Scholar] [CrossRef]
- Desser, L.; Rehberger, A.; Paukovits, W. Proteolytic Enzymes and Amylase Induce Cytokine Production in Human Peripheral Blood Mononuclear Cells In Vitro. Cancer Biother. 1994, 9, 253–263. [Google Scholar] [CrossRef]
- Ghensi, P.D.; Cucchi, A.D.; Bonaccorso, A.M.; Ferroni, L.; Gardin, C.; Mortellaro, C.M.; Zavan, B. In Vitro Effect of Bromelain on the Regenerative Properties of Mesenchymal Stem Cells. J. Craniofac. Surg. 2019, 30, 1064–1067. [Google Scholar] [CrossRef]
- Quarta, S.; Santarpino, G.; Carluccio, M.A.; Calabriso, N.; Scoditti, E.; Siculella, L.; Damiano, F.; Maffia, M.; Verri, T.; De Caterina, R.; et al. Analysis of the Anti-Inflammatory and Anti-Osteoarthritic Potential of Flonat Fast®, a Combination of Harpagophytum Procumbens DC. ex Meisn., Boswellia serrata Roxb., Curcuma longa L., Bromelain and Escin (Aesculus hippocastanum), Evaluated in In Vitro Models of Inflammation Relevant to Osteoarthritis. Pharmaceuticals 2022, 15, 1263. [Google Scholar] [CrossRef]
- Brochard, S.; Pontin, J.; Bernay, B.; Boumediene, K.; Conrozier, T.; Baugé, C. The Benefit of Combining Curcumin, Bromelain and Harpagophytum to Reduce Inflammation in Osteoarthritic Synovial Cells. BMC Complement. Med. Ther. 2021, 21, 261. [Google Scholar] [CrossRef] [PubMed]
- Secor, E.R., Jr.; Singh, A.; Guernsey, L.A.; McNamara, J.T.; Zhan, L.; Maulik, N.; Thrall, R.S. Bromelain Treatment Reduces CD25 Expression on Activated CD4+ T Cells In Vitro. Int. Immunopharmacol. 2009, 9, 340–346. [Google Scholar] [CrossRef]
- Gaspani, L.; Limiroli, E.; Ferrario, P.; Bianchi, M. In Vivo and In Vitro Effects of Bromelain on PGE2 and SP Concentrations in the Inflammatory Exudate in Rats. Pharmacology 2002, 65, 83–86. [Google Scholar] [CrossRef]
- Wen, S.; Huang, T.H.W.; Li, G.Q.; Yamahara, J.; Roufogalis, B.D.; Li, Y. Bromelain Improves Decrease in Defecation in Postoperative Rats: Modulation of Colonic Gene Expression of Inducible Nitric Oxide Synthase. Life Sci. 2006, 78, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Errasti, M.E.; Prospitti, A.; Viana, C.A.; Gonzalez, M.M.; Ramos, M.V.; Rotelli, A.E.; Caffini, N.O. Effects on Fibrinogen, Fibrin, and Blood Coagulation of Proteolytic Extracts from Fruits of Pseudananas macrodontes, Bromelia balansae, and B. hieronymi (Bromeliaceae) in Comparison with Bromelain. Blood Coagul. Fibrinolysis 2016, 27, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Azarkan, M.; González, M.M.; Calvo Esposito, R.; Errasti, M.E. Stem Bromelain Proteolytic Machinery: Study of the Effects of Its Components on Fibrin(ogen) and Blood Coagulation. Protein Pept. Lett. 2020, 27, 1159–1170. [Google Scholar] [CrossRef]
- Kaur, H.; Corscadden, K.; Lott, C.; Elbatarny, H.S.; Othman, M. Bromelain Has Paradoxical Effects on Blood Coagulability: A Study Using Thromboelastography. Blood Coagul. Fibrinolysis 2016, 27, 745–752. [Google Scholar] [CrossRef]
- Eckert, K.; Grabowska, E.; Stange, R.; Schneider, U.; Eschmann, K.; Maurer, H.R. Effects of Oral Bromelain Administration on the Impaired Immunocytotoxicity of Mononuclear Cells from Mammary Tumor Patients. Oncol. Rep. 1999, 6, 1191–1199. [Google Scholar] [CrossRef]
- Lotz-Winter, H. On the Pharmacology of Bromelain: An Update with Special Regard to Animal Studies on Dose-Dependent Effects. Planta Med. 1990, 56, 249–253. [Google Scholar] [CrossRef]
- Livio, M.; Bertoni, M.P.; De Gaetano, G.; Donati, M.B. Effects of Bromelain on Fibrinogen Level, Prothrombin Complex and Platelet Aggregation in the Rat—A Preliminary Report. Drugs Exp. Clin. Res. 1978, 4, 49–53. [Google Scholar]
- Juhasz, B. Bromelain Induces Cardioprotection Against Ischemia-Reperfusion Injury Through Akt/FOXO Pathway in Rat Myocardium. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1365–H1370. [Google Scholar] [CrossRef]
- Metzig, C.; Grabowska, E.; Eckert, K.; Rehse, K.; Maurer, H.R. Bromelain Proteases Reduce Human Platelet Aggregation In Vitro, Adhesion to Bovine Endothelial Cells and Thrombus Formation in Rat Vessels In Vivo. In Vivo 1999, 13, 7–12. [Google Scholar] [PubMed]
- Pirotta, F.; de Giuli-Morghen, C. Bromelain: Anti-inflammatory and serum fibronolytic activity after oral administration in the rat. Drugs Exp. Clin. Res. 1978, 4, 1–20. [Google Scholar]
- Hu, P.A.; Wang, S.H.; Chen, C.H.; Guo, B.C.; Huang, J.W.; Lee, T.S. New Mechanisms of Bromelain in Alleviating Non-Alcoholic Fatty Liver Disease-Induced Deregulation of Blood Coagulation. Nutrients 2022, 14, 2329. [Google Scholar] [CrossRef]
- Tarantino, G.; Balsano, C.; Santini, S.J.; Brienza, G.; Clemente, I.; Cosimini, B.; Sinatti, G. It Is High Time Physicians Thought of Natural Products for Alleviating NAFLD. Is There Sufficient Evidence to Use Them? Int. J. Mol. Sci. 2021, 22, 13424. [Google Scholar] [CrossRef]
- Fiore, G.; Pascuzzi, M.C.; Di Profio, E.; Corsello, A.; Agostinelli, M.; La Mendola, A.; Milanta, C.; Campoy, C.; Calcaterra, V.; Zuccotti, G.; et al. Bioactive Compounds in Childhood Obesity and Associated Metabolic Complications: Current Evidence, Controversies and Perspectives. Pharmacol. Res. 2023, 187, 106599. [Google Scholar] [CrossRef] [PubMed]
- Tassman, G.C.; Zafran, J.N.; Zayon, G.M. A Double-Blind Crossover Study of a Plant Proteolytic Enzyme in Oral Surgery. J. Dent. Med. 1965, 20, 51–54. [Google Scholar] [PubMed]
- Maurer, H.R. Bromelain: Biochemistry, Pharmacology and Medical Use. CMLS Cell. Mol. Life Sci. 2001, 58, 1234–1245. [Google Scholar] [CrossRef]
- Taussig, S.J.; Yokoyama, M.M.; Chinen, A. Bromelain: A Proteolytic Enzyme and Its Clinical Application: A Review. Hiroshima J. Med. Sci. 1975, 24, 185–193. [Google Scholar]
- Frazier, I.N.; Martin, G.J. Bromelain—The Pharmacology of the Enzyme. Arch. Int. Pharmacodyn. Ther. 1963, 145, 166–189. [Google Scholar]
- Donath, F.; Mai, I.; Maurer, A.; Brockmoeller, J.; Kuhn, C.S.; Friedrich, G.; Roots, I. Dose-Related Bioavailability of Bromelain and Trypsin After Repeated Oral Administration. Clin. Pharmacol. Ther. 1997, 61, 157–162. [Google Scholar] [CrossRef]
- Chakraborty, A.J.; Mitra, S.; Tallei, T.E.; Tareq, A.M.; Nainu, F.; Cicia, D.; Dhama, K.; Emran, T.B.; Simal-Gandara, J.; Capasso, R. Bromelain: A Potential Bioactive Compound—A Comprehensive Overview from a Pharmacological Perspective. Life 2021, 11, 317. [Google Scholar] [CrossRef] [PubMed]
- Colletti, A.; Li, S.; Marengo, M.; Adinolfi, S.; Cravotto, G. Recent Advances and Insights into Bromelain Processing, Pharmacokinetics and Therapeutic Uses. Appl. Sci. 2021, 11, 8428. [Google Scholar] [CrossRef]
| Associated Diseases | Ref. | Findings Correlating with Obesity |
|---|---|---|
| Insulin resistance (IR) | [22,23,24] | Insulin resistance serves as a pivotal link between development of NAFLD and consequent progression of atherosclerotic cardiovascular diseases in obese patients with increased levels of leptin, lipotoxicity, and mitochondrial dysfunction and decreased levels of adiponectin being the underlying mechanisms. Insulin resistance along with oxidative stress, inflammation, gut dysbiosis, renin–angiotensin–aldosterone system (RAAS) overactivity, and endothelial dysfunction have been stated as different mechanisms that collectively associate the pathophysiology between NAFLD and CVD with obesity being its primary driver. |
| Type 2 Diabetes Mellitus (T2DM) | [25] | Abdominal obesity increases the risk of glucose intolerance and insulin resistance, which are key factors in the development of type 2 diabetes. Studies have demonstrated a steep increase in the relative risk of developing type 2 diabetes with increasing body mass index (BMI). The higher levels of free fatty acids (FFAs) in obesity impair glucose use and increase hepatic glucose output, contributing to the development of T2DM. |
| Cardiovascular Disease (CVD) | [19,21,25] | Obesity is widely recognized as an independent risk factor for CVD, including coronary heart disease and heart failure. Mechanisms linking obesity to CVD include increased vascular volume, sympathetic nervous system activation, and changes in Na+/H+-ATPase activity, all potentially influenced by hyperinsulinemia. Elevated BMI values were responsible for 4 million deaths in 2015, with two-thirds of this number attributed to cardiovascular disease. Numerous studies have identified obesity as an important risk factor for the development of heart failure. |
| Nonalcoholic Fatty Liver Disease (NAFLD) | [19,22] | Increased FFAs in obesity contribute to lipid accumulation in the liver, leading to inflammation and liver damage. Studies have shown that weight loss can improve liver function and reduce fat accumulation in individuals with NAFLD. |
| Hypertension | [22,26,27] | Multiple studies state the activation of RAAS due to obesity by upregulation of angiotensinogen, angiotensin 1, and angiotensin-converting enzyme to be the cause of developing hypertension. Obesity predisposes renal sodium reabsorption thereby necessitating an increase in arterial pressure to maintain sodium balance. Other potential mechanisms include a decrease in natriuretic peptides with subsequent impairment in salt excretion. |
| Stroke | [28,29,30] | Obesity-related chronic inflammation plays an essential role in the progressive elevation of stroke risk, as increased plasma levels of interleukin-6 (IL-6) and C-reactive protein (CRP) are well-established inflammatory biomarkers implicated in the pathogenesis of cerebrovascular events. Although the obesity paradox implies obesity to have a protective stroke outcome in patients, such as reduced risk of recurrent stroke and improved functional recovery, its existence has not been confirmed by all studies. In spite of this, clinical evidence in an experimental study of obesity in rat models showed increased ischemic brain damage in the obese rodents. |
| Chronic Kidney Disease (CKD) and Other Renal Complications | [31,32,33,34] | Various population-based studies have shown direct link between high BMI in obese patients and progressive loss in GFR over time and incidence of end-stage renal disease (ESRD). Cardiovascular and metabolic effects such as high blood pressure and diabetes mellitus due to obesity aid in the disruption of kidney function. With visceral adipose tissue being the primary source of cytokine release affecting renal hemodynamics, persistent inflammation also contributes to fibrosis in the kidney. Furthermore, a cohort analysis found that participants with stage 2 hypertension had a 3.4-fold-higher chance of acquiring glomerulonephritis-related ESRD and a 2.2-fold-higher risk of developing non-glomerulonephritis-related ESRD than those with normal blood pressure. |
| Fertility | [35,36] | Ovulatory dysfunction has been noted to be more common in women with obesity; in addition, greater BMI at the age of 18 years predicted anovulatory infertility with or without polycystic ovary syndrome (PCOS), which is a common symptom of obesity. Recent evidence states sperm epigenetics are altered in men with obesity. Moreover, increased abdominal adiposity in men of subfertile couples has been associated with reduced sperm count, concentration, and motility. |
| Cancers | [37,38,39,40] | Systemically, metabolic syndrome, including dyslipidemia and insulin resistance, occurs in the setting of adipose inflammation and operates in concert with local mechanisms to sustain the inflamed microenvironment and promote tumor growth. Recent investigations have established a significant association between overweight or obesity, defined as a BMI of 25 or greater, and the increased risk of developing at least thirteen distinct malignancies. These include cancers of the esophagus, stomach, colon and rectum, liver, gallbladder, pancreas, postmenopausal breast, uterus, ovary, kidney, meningioma, thyroid, and multiple myeloma. Dysregulation of adipocytes and infiltrating immune cells in the adipose tissue results in chronic inflammation, which plays a crucial role in the progression of obesity-associated cancers. |
| Fields of Study | Ref. | Dosage | Subjects | Outcomes |
|---|---|---|---|---|
| Human | [50] | 1000 mg/d (2 × 500 mg) for 8 weeks, p.o.; | obese diabetic patients | ↓ BMI, waist circumference (WC), waist-to-hip ratio (WHR), ↓ serum leptin, |
| [74] | 1050 mg/d (3 × 350 mg) for 12 weeks, p.o. | diabetic patients with normal weight | n.c. body mass, hip circumference (HC), and WC | |
| Animal | [53] | 20 mg/kg/day for 4 weeks, p.o. | Apoe−/− mice with hyperlipidemia (apolipoprotein E-deficient mouse model of atherosclerosis) | ↓ body mass, WAT weight, ↑ BAT weight, ↓ size of droplet of BAT and WAT |
| [75] | 100 µL high-internal-phase oil-in-water emulsions (HIPEs) with added bromelain fibrils for 10 weeks, p.o. | mice +HFD | ↑ excretion of fats in the feces | |
| [76] | 20 mg/kg/day for 12 weeks, p.o. | C57BL/6 mice + HFD | ↓ body weight by ~30%, ↓ organ weight by ~20% in liver weight and ~40% in white adipose tissue weight | |
| Cell | [43] | Enzyme Commission Number (EC) 3.4.22.32, 10–100 µg/mL | preadipose 3T3 clonal cell line | ↓ adipogenesis, ↑ apoptosis, ↑ lipolysis |
| Fields of Study | Ref. | Dosage | Subjects | Outcomes |
|---|---|---|---|---|
| Human | [79] | 1000 mg/d (2 × 500 mg) for 8 weeks, p.o. | obese diabetic patients | ↓ leptin, IL-6, TNF-α |
| Animal | [82] | EC 3.4.22.32, 35–200 µg, i.v. | BALB/c + sheep red blood cells (SRBCs) mice | ↑ B cell ↓ mRNA IL-2 |
| [83] | EC 3.4.22.32, 1000 mg/kg/day for 3 weeks, p.o. | Wistar albino rats | ↓ TNF-α, IL-10, MCP-1, PGE2, ↓ NF-κB, CRP, MPO | |
| [84] | EC 3.4.22.33,10, 80 mg/kg/day for 14 days, p.o. | Sprague-Dawley rats + 2,4,6-trinitrobenzene sulfonic acid (TNBS) | ↑ occluding, ↓ TNFRs, NF-κB, MLCK, FD-4, | |
| [86] | EC 3.4.22.32, 5 mg/kg/d, or 10 mg/kg/d, p.o. for 14 days | Wistar albino rats | ↓ TNF-α, IL-6, M-CSF, MMP-8, RANKL, ↑ OPG | |
| [87] | EC 3.4.22.32, 30 mg/kg and 50 mg/kg, p.o. | Wistar albino rats | ↓ IL-1β, IL-6, TNF-α, PGE2, NF-κB, iNOS, NO3-/NO2-, glutamine | |
| [88] | 250 mg/kg/day, p.o.; | rats | ↓ IL-1β, IL-6, TNF-α | |
| [97] | 10 and 20 mg/kg, p.o. | rats | ↓ P, PGE2 | |
| Cells | [56] | 6.25–5000 µg/mL | RAW264.7—mouse macrophages +LPS | ↓ iNOS, COX-2 (dose-dependent), ↓ MAPK (p-ERK, p-JNK, p-p38), ↓ c-Fos expression and c-Jun phosphorylation, ↓ NO (dose-dependent) |
| [55] | EC 3.4.22.32, 2.5, 5, 10, 20 µg/mL | human dental pulp cells (hDPCs) + LPS | ↓ IL-1β, IL-6, IL-8 (mRNA + protein), ↓ ICAM-1, VCAM-1 ↓ ERK, p38 MAPK, NF-κB (p65) n.c. JNK | |
| [59] | EC 3.4.22.32, 1 mg/mL | colonic mucosa biopsies from patients with Crohn’s disease or ulcerative colitis | ↓ G-CSF, GM-CSF, IFN-γ, CCL4, MIP-1β, TNF-α | |
| [61] | 35 mg/L (0.035 mg/mL) | stomach, intestinal, and chondrocyte human cellular models (AGS, Caco-2, and SW1353) | ↓ IL-8, COX-2, iNOS | |
| [64] | EC 3.4.22.32, from 1 µg/mL to >1000 µg/mL | human leukocytes in whole blood, PBMCs | ↓ CD7, CD8α, CD14, CD16, CD21, CD41, CD42a, CD44, CD45RA, CD48, CD57, CD62L, CD128a, CD128b, CD4, CD40, CD56, CD61, CD79, CD132. ↑ CD5, CD11b, CD11c, CD13, CD15, CD18, CD53 | |
| [65] | EC 3.4.22.32, 2.5, 5, 10, 20, 40 µg/mL | human gingival fibroblasts (HGFs) + AGEs | ↓ IL-6, IL-8, p16, ↓ NF-kB, MAPK/ERK | |
| [67] | E.C. 3.4.22.32, 50–100 μg/mL | PBMCs and monocytic leukemia THP-1 cells + LPS | ↓ TNF-α, IL-1β, IL-6 | |
| [80] | 10–1000 µg/mL | PBMCs obtained from subjects with encephalomyelitis disseminate and healthy control group | ↑IL-6, GM-CSF, TNF-α, IFN-γ | |
| [81] | 5.4 FIP-E./mg | modified mixed lymphocyte culture (MMLC) | ↑IL-6, | |
| [82] | EC 3.4.22.32, 0–200 µg/mL | CD41 T cells, CD81 T cells, B cells from mouse splenocytes | ↑ proliferation (in splenocytes), ↓ IL-2 ↑costimulation by B cells | |
| [84] | EC 3.4.22.33 <80 µg/mL | intestinal epithelial cells IEC-6 (rat) and Caco-2 (human) + LPS | ↓ TNFR2, NF-κB, MLCK ↑ TER | |
| [85] | EC 3.4.22.32, 10 mg/mL in nanocomposites and 500 µg/mL free bromelain | Caco-2 (intestinal line), PBMCs (blood mononuclear lymphocytes) celiac and healthy + gliadin | ↓ CCR5, CXCR3 ↓ IL-1β, IL-6, TNF-α, IFN-γ ↑ IL-10, cytotoxic T lymphocyte antigen 4 (CTLA-4) | |
| [89] | EC 3.4.22.32, 100 µg/mL | human monocytic cell line U937 + LPS | ↓ IL-1β, IL-6, IL-8, COX-2, MIP-1α/β, MCP-1 | |
| [90] | EC 3.4.22.3210–40 µg/mL | SW982 synovial fibroblasts + TNF-α | ↓ TNF-α, IL-1β, IL-6, IL-8, ↓ phosphorylation NF-κB p65, IκB-α, ↓ activation MAPK: p38, JNK n.c. ERK | |
| [91] | crude (CBM) and purified rhizome bromelain (PBM) (CBM: 20, 40 and 80 µg/mL) (PBM: 10, 20 and 40 µg/mL) | RAW 264.7 macrophage cells + LPS | CBM: ↓ IL-6, NO (only with 80 µg/mL), p38, iNOS and COX-2 n.c. TNF-α, ERK1/2, JNK, p65, IκB-α. PBM: ↓ IL-6, TNF-α, NO, ERK1/2, JNK, p38, p65, IκB-α., iNOS and COX-2 | |
| [92] | EC 3.4.22.3215 100 mg | PBMCs obtained from healthy subjects | ↓ TNF-α, IL-1β, IL-6 | |
| [93] | EC 3.4.22.32, 20 ng/mL, after 3 and 7 days | mesenchymal stem cells (MSCs) | ↑ IL-10 ↓ IL-1 n.c. IL-2, IL-6 and IL-8 | |
| [94] | EC 3.4.22.32, 50–100 µg/mL | human monocytoid THP-1 cells, (Human Microvascular Endothelial Cells-1 (HMEC-1) | ↓ IL-6, IL-8, MMP-9, n.c. COX-2 | |
| [95] | EC 3.4.22.32, 14.7 µg/mL | human synovium from OA patients + LPS | n.c. IL-6, NGF, MMP-1, MMP-3, MMP-13, PGE2, MMPs | |
| [96] | EC 3.4.22.32, 25–100 µg/mL | CD4+ CD25 cells isolated from mouse spleens | ↓ CD25 and sCD25 | |
| [98] | EC 3.4.22.32, 10–100 µg/mL | RAW264.7—mouse macrophages (ATCC) | ↓ NF-κB, NO |
| Fields of Study | Ref. | Dosage | Subjects | Outcomes |
|---|---|---|---|---|
| Humans | [75] | 1050 mg/d (3×350 mg) for 12 weeks, p.o. | diabetic patients with normal weight | n.c. blood pressure; n.c. total cholesterol, TG, high-density lipoprotein cholesterol (HDL-C); ↓ LDL |
| Animals | [54] | 20 mg/kg/day, for 4 weeks, p.o. | Apoe−/− mice with hyperlipidemia | n.c. mean arterial pressure; ↓ TC, TG, no-HDL-C; ↓ inflammation of the aorta, formation of atherosclerosis |
| [77] | 20 mg/kg/day for 12 weeks, p.o. | C57BL/6 mice + HFD | ↓ TC ~15% ↓ TG ~25% | |
| [88] | 250 mg/kg/day, p.o. | rats | ↓ TC, TG, LDL-C |
| Fields of Study | Ref. | Dosage | Subjects | Outcomes |
|---|---|---|---|---|
| Human | [102] | 3000 Federation Internationale Pharmaceutique units (F.I.P units) for 10 days, p.o. | breast cancer patients and healthy subjects | ↑ activated partial thromboplastin time was increased from 38 to 46 s n.c. leaving prothrombin time and plasminogen |
| Animal | [103] | minimum 5 mg/kg/day, p.o. | rabbits | ↑ antiprothrombin time and serum plasmin |
| [104] | 1, 5, 10, 20, and 30 mg/kg, i.v. | rats | ↓prothrombin time, prothrombin levels, FactorX (Stuart-Factor), fibrinogen levels | |
| [105] | 10 mg/kg twice a day for 15 days, i.p. | Sprague-Dawley rats | ↓ infarction spread ↑aortic flow, ↑left ventricular functional recovery throughout reperfusion | |
| [106] | (a) 60 mg/kg, p.o.; (b) 30 mg/kg, i.v. | rats | (a) ↓ thrombus formation in 11% of arterioles and 6% of venules (b) ↓ thrombus formation in arterioles (13%) and venules (5%) | |
| [107] | 25 and 100 mg/kg, p.o. | rats | ↑ serum fibrinolytic activity ↓absorption time of hematoma ↓ pentobarbital-induced sleeping time | |
| [108] | 20 mg/kg/day for 12 weeks, p.o. | C57BL/6 mice + HFD | ↓ PT, APTT, and FIB (fibrinogen) | |
| [101] | 1.5 mg/kg, i.p. | CD1 mice (mice type CD1) | ↑ hypercoagulation | |
| Cell | [99] | EC 3.4.22.32, (B4882), from 0.003 casein units per milliliter (CU/mL) to 10 CU/mL | (a) fibrinogen; (b) platelet-poor plasma of healthy donors | (a) ↑ fibrinolytic effect (b)↑ PT, APTT |
| [100] | EC 3.4.22.32, 0.8 μL at a concentration of 1.0 UC/mL | (a) fibrinogen; (b) pool of platelet-poor plasma | (a)↑ fibrinolytic effect (b)↑ PT, APTT | |
| [101] | 0.4 U/mL | whole blood samples taken from healthy individuals and hypercoagulable | ↑ PT by 47% (healthy) and 22% (hypercoagulable) ↑ APTT by 20% (healthy) and 10% (hypercoagulable) ↓ platelet aggregation | |
| [106] | 10 mg/mL | platelets with thrombin (0.2 U/mL). | ↓ platelet aggregation |
| Fields of Study | Ref. | Dosage | Subjects | Outcomes |
|---|---|---|---|---|
| Human | [50] | 1000 mg/d (2 × 500 mg) for 8 weeks, p.o. | obese diabetic patients | ↓Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) n.c. fasting glucose |
| [75] | 1050 mg/d (3 × 350 mg) for 12 weeks, p.o. | diabetic patients with normal weight | ↓post prandial blood glucose ↓ fasting glucose, n.c. Hgb1c | |
| Animal | [77] | 20 mg/kg/day for 12 weeks, p.o. | C57BL/6 mice + HFD | ↓ lipid accumulation in the liver |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sethia, Y.; Polak-Szczybyło, E.; Tabarkiewicz, J. Bromelain in Obesity Therapy: A Review of Anti-Inflammatory and Metabolic Mechanisms. Int. J. Mol. Sci. 2025, 26, 8347. https://doi.org/10.3390/ijms26178347
Sethia Y, Polak-Szczybyło E, Tabarkiewicz J. Bromelain in Obesity Therapy: A Review of Anti-Inflammatory and Metabolic Mechanisms. International Journal of Molecular Sciences. 2025; 26(17):8347. https://doi.org/10.3390/ijms26178347
Chicago/Turabian StyleSethia, Yashvi, Ewelina Polak-Szczybyło, and Jacek Tabarkiewicz. 2025. "Bromelain in Obesity Therapy: A Review of Anti-Inflammatory and Metabolic Mechanisms" International Journal of Molecular Sciences 26, no. 17: 8347. https://doi.org/10.3390/ijms26178347
APA StyleSethia, Y., Polak-Szczybyło, E., & Tabarkiewicz, J. (2025). Bromelain in Obesity Therapy: A Review of Anti-Inflammatory and Metabolic Mechanisms. International Journal of Molecular Sciences, 26(17), 8347. https://doi.org/10.3390/ijms26178347