Nephroprotective Effect of Sansevieria trifasciata
Abstract
1. Introduction
2. Predisposing Factors for Kidney Diseases
3. Conventional Treatment in Renal Affections
4. Nephroprotective Effect of Sansevieria trifasciata
4.1. Flavonoids
4.2. Phenolic Acids
4.3. Terpenes: TP1 (Citronellol)
5. Prediction of the Pharmacokinetic Potential of Hydroxylated Aromatic Compounds from S. trifasciata
6. Materials and Methods
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication—Worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019, 96, 1048–1050. [Google Scholar] [CrossRef]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, V.A.; Tonelli, M.; Stanifer, J.W. The global burden of kidney disease and the sustainable development goals. Bull. World Health Organ. 2018, 96, 414–422C. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Simental-Mendía, L.E.; Butler, A.E.; Sahebkar, A. Protective effects of plant-derived natural products on renal complications. J. Cell. Physiol. 2019, 234, 12161–12172. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Chen, H.; Zhao, Y.Y. Traditional uses, phytochemistry, pharmacology, toxicology and quality control of Alisma orientale (Sam.) Juzep: A review. J. Ethnopharmacol. 2014, 158, 373–387. [Google Scholar] [CrossRef]
- Khan, M.A.; Kassianos, A.J.; Hoy, W.E.; Alam, A.K.; Healy, H.G.; Gobe, G.C. Promoting Plant-Based Therapies for Chronic Kidney Disease. J. Evidence-Based Integr. Med. 2022, 27, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Maroyi, A. Sansevieria Hyacinthoides (L.) Druce: A Review of Its Botany, Medicinal Uses, Phytochemistry, and Biological Activities. Asian J. Pharm. Clin. Res. 2019, 12, 21–26. [Google Scholar] [CrossRef]
- Palomo-Piñón, S.; Aguilar-Alonso, J.A.; Chávez-Iñiguez, J.S.; Hernández-Arellanes, F.E.; Mariano-Murga, J.A.; Flores-Rodríguez, J.C.; Pérez-López, M.J.; Pazos-Pérez, F.; Treviño-Becerra, A.; Guillen-Graf, A.E.; et al. Strategies to address diabetic kidney disease burden in Mexico: A narrative review by the Mexican College of Nephrologists. Front. Med. 2024, 11, 1–14. [Google Scholar] [CrossRef]
- Bakar, F.; Bahadir Acikara, Ö.; Ergene, B.; Nebioğlu, S.; Saltan Çitoğlu, G. Antioxidant activity and phytochemical screening of some asteraceae plants. Turkish J. Pharm. Sci. 2015, 12, 123–132. [Google Scholar] [CrossRef]
- Kasmawati, H.; Ruslin, R.; Arfan, A.; Sida, N.A.; Wibowo, A.; Hanafiah, M.A.K.M.; Yahya, M.F.M. In-depth insights into the antidiabetic activity of Dracaena trifasciata (Prain) Mabb. fractions and subfractions: In vitro and computational studies. J. Pharm. Pharmacogn. Res. 2025, 13, S13–S26. [Google Scholar] [CrossRef]
- Zhou, D.; Wu, Y.; Yan, H.; Shen, T.; Li, S.; Gong, J.; Li, G.; Mai, H.; Wang, D.; Tan, X. Gallic acid ameliorates calcium oxalate crystal-induced renal injury via upregulation of Nrf2/HO-1 in the mouse model of stone formation. Phytomedicine 2022, 106, 154429. [Google Scholar] [CrossRef]
- Sarmah, D.; Sengupta, R. A Review on the Role of Phytoconstituents Chrysin on the Protective Effect on Liver and Kidney. Curr. Drug Discov. Technol. 2023, 21, 60–74. [Google Scholar] [CrossRef]
- Kumari, S.; Kamboj, A.; Wanjari, M.; Sharma, A.K. Nephroprotective effect of Vanillic acid in STZ-induced diabetic rats. J. Diabetes Metab. Disord. 2021, 20, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Chen, H.Y.; Tang, Q.Q.; Li, Y.F.; Liu, X.S.; Lu, F.H.; Gu, Y.Y. Protective effect of quercetin on kidney diseases: From chemistry to herbal medicines. Front. Pharmacol. 2022, 13, 968226. [Google Scholar] [CrossRef]
- Burnier, M.; Damianaki, A. Hypertension as Cardiovascular Risk Factor in Chronic Kidney Disease. Circ. Res. 2023, 132, 1050–1063. [Google Scholar] [CrossRef]
- Maya-Quinta, R.; Rodriguez-Gomez, G.P.; Del Toro-Mijares, R.; Henriquez-Santos, F.; Martagon, A.J. Chronic kidney disease acquired knowledge in a diabetic and hypertensive population using a translated and validated questionnaire. Ren. Fail. 2023, 45, 2222836. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.M.; Zoungas, S.; Rossing, P.; Groop, P.H.; Cooper, M.E. Diabetic kidney disease. Nat. Rev. Dis. Prim. 2015, 1, 15018, Correction in Nat. Rev. Dis. Prim. 2015, 1, 15070. [Google Scholar] [CrossRef]
- Huang, Y.; Dou, X.; He, M.; Su, Y.; Lin, H.; Yang, Y. The G-allele of rs10830963 in MTNR1B Exerts Stage-Specific Effects Across the Trajectory of Type 2 Diabetes: A Multi-State Analysis. Int. J. Mol. Sci. 2025, 26, 7855. [Google Scholar] [CrossRef]
- Mallamaci, F.; Tripepi, G. Risk Factors of Chronic Kidney Disease Progression: Between Old and New Concepts. J. Clin. Med. 2024, 13, 678. [Google Scholar] [CrossRef]
- Gupta, A.K.; Versteeg, S.G.; Shear, N.H. Onychomycosis in the 21st Century: An Update on Diagnosis, Epidemiology, and Treatment. J. Cutan. Med. Surg. 2017, 21, 525–539. [Google Scholar] [CrossRef]
- Juan, D.R.; Flores, C. Enfermedad Renal Crónica: Epidemiología Y Factores De Riesgo Chronic Kidney Disease: Epidemiology and Risk Factors. Rev. Med. Clin. Condes 2010, 21, 502–507. [Google Scholar]
- Kazancioǧlu, R. Risk factors for chronic kidney disease: An update. Kidney Int. Suppl. 2013, 3, 368–371. [Google Scholar] [CrossRef]
- Ruggenenti, P.; Bettinaglio, P.; Pinares, F.; Remuzzi, G. Angiotensin converting enzyme insertion/deletion polymorphism and renoprotection in diabetic and nondiabetic nephropathies. Clin. J. Am. Soc. Nephrol. 2008, 3, 1511–1525. [Google Scholar] [CrossRef]
- Misra, S.; Stevermer, J.J. ACE inhibitors and ARBs: One or the other-not both-for high-risk patients. J. Fam. Pract. 2009, 58, 24–28. [Google Scholar]
- Kamal, A.; Fain, C.; Park, A.; Wang, P.; Gonzalez-Velez, E.; Leffler, D.A.; Hutfless, S.M. Angiotensin II receptor blockers and gastrointestinal adverse events of resembling sprue-like enteropathy: A systematic review. Gastroenterol. Rep. 2019, 7, 162–167. [Google Scholar] [CrossRef]
- Buitrago, A.; Sánchez, C. Mecanismos de acción de los inhibidores de cotransportador de sodio y glucosa tipo 2–SGLT2: Más allá del control de la glicemia. Rev. Colomb. Cardiol. 2020, 27, 22–25. [Google Scholar]
- Zaccardi, F.; Webb, D.R.; Htike, Z.Z.; Youssef, D.; Khunti, K.; Davies, M.J. Efficacy and safety of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes mellitus: Systematic review and network meta-analysis. Diabetes Obes. Metab. 2016, 18, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Sida, N.A.; Kasmawati, H.; Ruslin, R. Sansevieria trifasciata Prain: A Review on Its Phytochemicals and Pharmacological Potential. Indones. J. Pharm. Sci. Technol. 2024, 6, 26–36. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Taxonomy Summary for Taxonomy 39534, Sansevieria Trifas-Ciata (Snake Plant). Available online: https://pubchem.ncbi.nlm.nih.gov/taxonomy/Sansevieria-trifasciata (accessed on 21 July 2025).
- Mohany, M.; Ahmed, M.M.; Al-Rejaie, S.S. Molecular mechanistic pathways targeted by natural antioxidants in the prevention and treatment of chronic kidney disease. Antioxidants 2022, 11, 15. [Google Scholar] [CrossRef]
- Raslan, M.A.; Abdel-Rahman, R.F.; Fayed, H.M.; Ogaly, H.A.; Tahere, R.F. Metabolomic Profiling of Sansevieria trifasciata hort ex. Prain Leaves and Roots by HPLC-PAD-ESI/MS and its Hepatoprotective Effect via Activation of the NRF2/ARE Signaling Pathway in an Experimentally Induced Liver Fibrosis Rat Model. Egypt. J. Chem. 2021, 64, 6647–6671. [Google Scholar] [CrossRef]
- Kamiyama, M.; Iijima, K.; Okuzawa, R.; Kawata, R.; Kimura, A.; Shinohara, Y.; Shimada, A.; Yamanaka, M.; Youda, A.; Iwamoto, T. Augmented Intrarenal and Urinary Angiotensinogen in Diabetic Nephropathy: The Role of Isoflavones. Int. J. Mol. Sci. 2025, 26, 1443. [Google Scholar] [CrossRef]
- Pessoa, E.d.A.; Convento, M.B.; Castino, B.; Leme, A.M.; de Oliveira, A.S.; Aragão, A.; Fernandes, S.M.; Carbonel, A.; Dezoti, C.; Vattimo, M.d.F.; et al. Beneficial effects of isoflavones in the kidney of obese rats are mediated by ppar-gamma expression. Nutrients 2020, 12, 1624. [Google Scholar] [CrossRef]
- El-Din El-Hawary, S.S.; El-Mahdy El-Tantawy, M.; Rabeh, M.A.; Ali, Z.Y.; Albohy, A.; Fawaz, N.E. Sansevieria: An evaluation of potential cytotoxic activity in reference to metabolomic and molecular docking studies. Egypt. J. Chem. 2021, 64, 835–849. [Google Scholar] [CrossRef]
- Nirumand, M.C.; Hajialyani, M.; Rahimi, R.; Farzaei, M.H.; Zingue, S.; Nabavi, S.M.; Bishayee, A. Dietary plants for the prevention and management of kidney stones: Preclinical and clinical evidence and molecular mechanisms. Int. J. Mol. Sci. 2018, 19, 765. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Naraki, K.; Roohbakhsh, A.; Hayes, A.W.; Karimi, G. The protective effects of rutin on the liver, kidneys, and heart by counteracting organ toxicity caused by synthetic and natural compounds. Food Sci. Nutr. 2023, 11, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zhang, X.; Liu, Y.; Zhao, T.; Sun, Z.; Liu, P.; Xiang, Q.; Xiong, J.; Du, X.; Yang, X.; et al. Rutin alleviates EndMT by restoring autophagy through inhibiting HDAC1 via PI3K/AKT/mTOR pathway in diabetic kidney disease. Phytomedicine 2023, 112, 154700. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, X.; Kuang, W.; Wang, S.; Zhao, Y. Kaempferol improves acute kidney injury via inhibition of macrophage infiltration in septic mice. Biosci. Rep. 2023, 43, BSR20230873. [Google Scholar] [CrossRef] [PubMed]
- Alagal, R.I.; AlFaris, N.A.; Alshammari, G.M.; ALTamimi, J.Z.; AlMousa, L.A.; Yahya, M.A. Kaempferol attenuates doxorubicin-mediated nephropathy in rats by activating SIRT1 signaling. J. Funct. Foods 2022, 89, 104918. [Google Scholar] [CrossRef]
- Wu, Q.; Wong, J.P.C.; Kwok, H.F. Putting the brakes on tumorigenesis with natural products of plant origin: Insights into the molecular mechanisms of actions and immune targets for bladder cancer treatment. Cells 2020, 9, 1213. [Google Scholar] [CrossRef]
- Moradi, A.; Abolfathi, M.; Javadian, M.; Heidarian, E.; Roshanmehr, H.; Khaledi, M.; Nouri, A. Gallic Acid Exerts Nephroprotective, Anti-Oxidative Stress, and Anti-Inflammatory Effects Against Diclofenac-Induced Renal Injury in Malerats. Arch. Med. Res. 2021, 52, 380–388. [Google Scholar] [CrossRef]
- Salama, A.A.A.; Elgohary, R.; Fahmy, M.I. Protocatechuic acid ameliorates lipopolysaccharide-induced kidney damage in mice via downregulation of TLR-4-mediated IKBKB/NF-κB and MAPK/Erk signaling pathways. J. Appl. Toxicol. 2023, 43, 1119–1129. [Google Scholar] [CrossRef]
- Jiao, H.; Zhang, M.; Xu, W.; Pan, T.; Luan, J.; Zhao, Y.; Zhang, Z. Chlorogenic acid alleviate kidney fibrosis through regulating TLR4/NF-қB mediated oxidative stress and inflammation. J. Ethnopharmacol. 2024, 335, 118693. [Google Scholar] [CrossRef] [PubMed]
- Kamarauskaite, J.; Baniene, R.; Trumbeckas, D.; Strazdauskas, A.; Trumbeckaite, S. Caffeic acid phenethyl ester protects kidney mitochondria against ischemia/reperfusion induced injury in an in vivo rat model. Antioxidants 2021, 10, 747. [Google Scholar] [CrossRef]
- Sherkhane, B.; Yerra, V.G.; Sharma, A.; Kumar, A.K.; Chayanika, G.; Kumar, A.V.; Kumar, A. Nephroprotective potential of syringic acid in experimental diabetic nephropathy: Focus on oxidative stress and autophagy. Indian J. Pharmacol. 2018, 49, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Sancak, E.B.; Akbas, A.; Silan, C.; Cakir, D.U.; Turkon, H.; Ozkanli, S.S. Protective effect of syringic acid on kidney ischemia-reperfusion injury. Ren. Fail. 2016, 38, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kumar, A.; Singh, H.; Kaur, S.; Arora, S.; Singh, D.B. Protective effect of vanillic acid against diabetes and diabetic nephropathy by attenuating oxidative stress and upregulation of NF-κB, TNF-α and COX-2 proteins in rats. Phyther. Res. 2022, 36, 1338–1352. [Google Scholar] [CrossRef]
- Chowdhury, S.; Ghosh, S.; Das, A.K.; Sil, P.C. Ferulic acid protects hyperglycemia-induced kidney damage by regulating oxidative insult, inflammation and autophagy. Front. Pharmacol. 2019, 10, 27. [Google Scholar] [CrossRef]
- Alam, M.A.; Sernia, C.; Brown, L. Ferulic Acid Improves Cardiovascular and Kidney Structure and Function in Hypertensive Rats. J. Cardiovasc. Pharmacol. 2013, 61, 240–249. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Q.; Guo, J.; Gu, D.; Liu, J.; Luo, P.; Bai, Y.; Chen, J.; Zhang, X.; Nie, S.; et al. Single-cell transcriptomics reveals the ameliorative effect of rosmarinic acid on diabetic nephropathy-induced kidney injury by modulating oxidative stress and inflammation. Acta Pharm. Sin. B 2024, 14, 1661–1676. [Google Scholar] [CrossRef]
- Abdullah; Angelina; Yumna, M.; Arbianti, R.; Utami, T.S.; Hermansyah, H.; Ningsih, S. Flavonoid isolation and identification of mother-in-law’s tongue leaves (sansevieria trifasciata) and the inhibitory activities to xanthine oxidase enzyme. E3S Web Conf. 2018, 67, 03011. [Google Scholar] [CrossRef]
- Anlar, H.G.; Bacanli, M.; Çal, T.; Aydin, S.; Ari, N.; Ündeğer Bucurgat, Ü.; Başaran, A.A.; Başaran, A.N. Effects of cinnamic acid on complications of diabetes. Turkish J. Med. Sci. 2018, 48, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, Y.S.; Kathem, S.H. Protective effect of citronellol in rhabdomyolysis-induced acute kidney injury in mice. J. Med. Life 2023, 16, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.Z.; Kathem, S.H. Citronellol protects renal function by exerting anti-inflammatory and antiapoptotic effects against acute kidney injury induced by folic acid in mice. Naunyn. Schmiedebergs. Arch. Pharmacol. 2025, 398, 5927–5937. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, C.; Liu, T.; Zhang, L.; Wen, X.; Liu, X.; Fan, W. Identification of Markers for Diagnosis and Treatment of Diabetic Kidney Disease Based on the Ferroptosis and Immune. Oxid. Med. Cell. Longev. 2022, 2022, 9957172. [Google Scholar] [CrossRef]
- Pavlou, G.; Spitz, S.; Pramotton, F.M.; Tsai, A.; Li, B.M.; Wang, X.; Barr, O.M.; Ko, E.C.; Zhang, S.; Ashley, S.J.; et al. Engineered 3D human neurovascular model of Alzheimer’s disease to study vascular dysfunction. Biomaterials 2025, 314, 122864. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome p450 enzymes and drug metabolism in humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef]
- Durairaj, P.; Liu, Z.L. Brain Cytochrome P450: Navigating Neurological Health and Metabolic Regulation. J. Xenobiotics 2025, 15, 44. [Google Scholar] [CrossRef]

| Group | Name | Structure | Molecular Concentration (mg/100 g of Plant) | Biological Activity |
|---|---|---|---|---|
| Flavonoids | (2S)-3′,4′-methylenedioxy-5,7-dimethoxyflavone (FL1) | 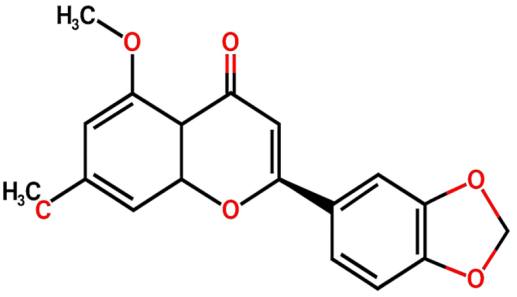 | Not quantified [29] | Antidiabetic activity [11]. |
| Chrysin (FL2) | 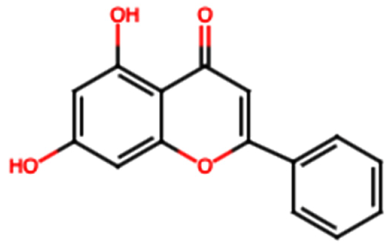 | Not quantified [30] | Nephroprotective properties against various nephrotoxic agents, such as cisplatin, doxorubicin, paracetamol, gentamicin, and streptozotocin. Protection against nephropathies [13,31]. | |
| Isoflavone (FL3) | 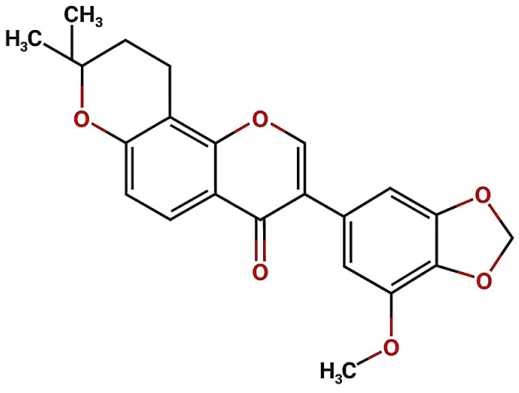 | Not quantified [32] | Antioxidant activity in diabetic nephropathy. Anti-inflammatory activity in diabetic nephropathy [33,34]. | |
| Rutin (FL4) |  | 0.07 [35] | Protection against nephropathies [31,36,37,38]. | |
| Quercetin (FL5) | 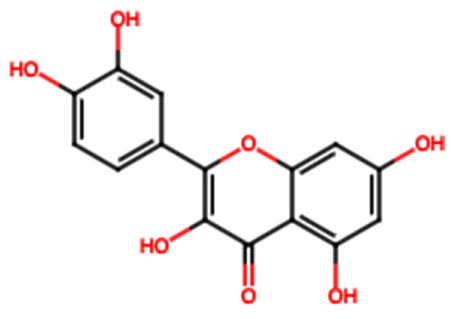 | 1.42 [35] | Suppresses renal toxicity, apoptosis, fibrosis, and inflammation in a variety of renal pathologies [15]. | |
| Kaempferol (FL6) | 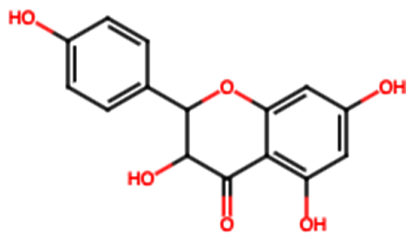 | 17.17 [35] | Nephroprotective effect in sepsis-induced acute kidney injury; attenuates doxorubicin-induced nephropathy [39,40]. | |
| Catechin (FL7) | 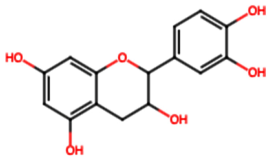 | 22.13 [35] | Urolithiasis prevention and anticancer activity [36,41]. | |
| Phenolic acids | Gallic acid (PA1) |  | 0.34 [35] | Ameliorates calcium oxalate crystal-induced renal injury and nephroprotective effect against diclofenac-induced Renal Injury [12,42]. |
| Protocatechuic acid (PA2) | 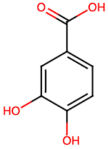 | 2.40 [35] | Improves lipopolysaccharide-induced kidney damage [43]. | |
| Chlorogenic acid (PA3) | 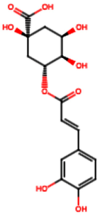 | 0.09 [35] | Renal antifibrotic activity [44]. | |
| Caffeic acid (PA4) | 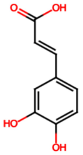 | 0.34 [35] | Protects the kidney against ischemia–reperfusion injury [45]. | |
| Syringic acid (PA5) |  | 1.42 [35] | Mitigates diabetic kidney disease and protects the kidney against ischemia–reperfusion injury [46,47]. | |
| Vanillic acid (PA6) | 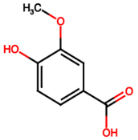 | 1.09 [35] | Mitigates diabetic kidney disease [14,48]. | |
| Ferulic acid (PA7) | 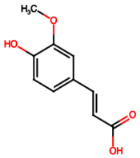 | 0.34 [35] | Protects against hyperglycemia-induced renal damage; improves kidney structure and function in hypertensive patients [49,50]. | |
| Rosmarinic acid (PA8) |  | 3.05 [35] | Attenuates renal tubular epithelial damage associated with diabetic nephropathy [51]. | |
| Cinnamic acid (PA9) | 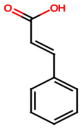 | Not quantified [35,52] | Mitigates diabetic kidney disease [53]. | |
| Terpenes | Citronellol (TP1) | 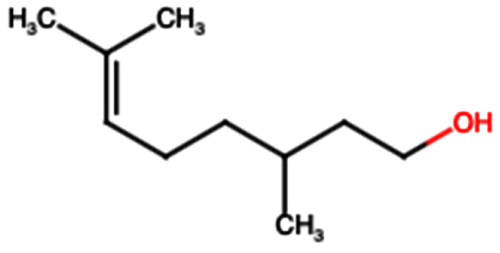 | Not quantified [35,52] | Nephroprotective effects against rhabdomyolysis-induced renal injury and demonstrates anti-apoptotic activity in folic acid-induced kidney injury [54,55]. |
| Phytocompounds | Pharmacokinetic Targets | |||||||
|---|---|---|---|---|---|---|---|---|
| GI Absorption | BBB Permeant | P-gp Substrate | CYP1A2 Inhibitor | CYP2C19 Inhibitor | CYP2C9 Inhibitor | CYP2D6 Inhibitor | CYP3A4 Inhibitor | |
| FL1 | High | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| FL2 | High | Yes | No | Yes | Yes | Yes | No | Yes |
| FL3 | High | No | No | No | No | Yes | No | Yes |
| FL4 | Low | No | Yes | No | No | No | No | No |
| FL5 | High | No | No | Yes | No | No | Yes | Yes |
| FL6 | High | No | No | Yes | No | No | Yes | Yes |
| FL7 | High | No | Yes | No | No | No | No | No |
| PA1 | High | No | No | No | No | No | No | YES |
| PA2 | High | No | No | No | No | No | No | YES |
| PA3 | Low | No | No | No | No | No | No | No |
| PA4 | High | No | No | No | No | No | No | No |
| PA5 | High | No | No | No | No | No | No | No |
| PA6 | High | No | No | No | No | No | No | No |
| PA7 | High | Yes | No | No | No | No | No | No |
| PA8 | Low | No | No | No | No | No | No | No |
| PA9 | High | Yes | No | No | No | No | No | No |
| TP1 | High | Yes | No | No | No | No | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos Islas, J.; Lomelí, M.L.-C.; González Martínez, B.E.; Ramos Islas, I.R.; López, M.G.; Tijerina-Sáenz, A.; Vázquez Rodríguez, J.A.; López, L.F.M.; Verde-Star, M.J.; García-Ponce, R.; et al. Nephroprotective Effect of Sansevieria trifasciata. Int. J. Mol. Sci. 2025, 26, 8619. https://doi.org/10.3390/ijms26178619
Ramos Islas J, Lomelí ML-C, González Martínez BE, Ramos Islas IR, López MG, Tijerina-Sáenz A, Vázquez Rodríguez JA, López LFM, Verde-Star MJ, García-Ponce R, et al. Nephroprotective Effect of Sansevieria trifasciata. International Journal of Molecular Sciences. 2025; 26(17):8619. https://doi.org/10.3390/ijms26178619
Chicago/Turabian StyleRamos Islas, Josue, Manuel López-Cabanillas Lomelí, Blanca Edelia González Martínez, Israel Ricardo Ramos Islas, Myriam Gutiérrez López, Alexandra Tijerina-Sáenz, Jesús Alberto Vázquez Rodríguez, Luis Fernando Méndez López, María Julia Verde-Star, Romario García-Ponce, and et al. 2025. "Nephroprotective Effect of Sansevieria trifasciata" International Journal of Molecular Sciences 26, no. 17: 8619. https://doi.org/10.3390/ijms26178619
APA StyleRamos Islas, J., Lomelí, M. L.-C., González Martínez, B. E., Ramos Islas, I. R., López, M. G., Tijerina-Sáenz, A., Vázquez Rodríguez, J. A., López, L. F. M., Verde-Star, M. J., García-Ponce, R., García-Hernández, D. G., & Heya, M. S. (2025). Nephroprotective Effect of Sansevieria trifasciata. International Journal of Molecular Sciences, 26(17), 8619. https://doi.org/10.3390/ijms26178619









