Immunomodulatory Effects of Traditional Korean Gochujang in Rats Immunosuppressed with Cyclophosphamide
Abstract
1. Introduction
2. Results
2.1. Component of GCJs
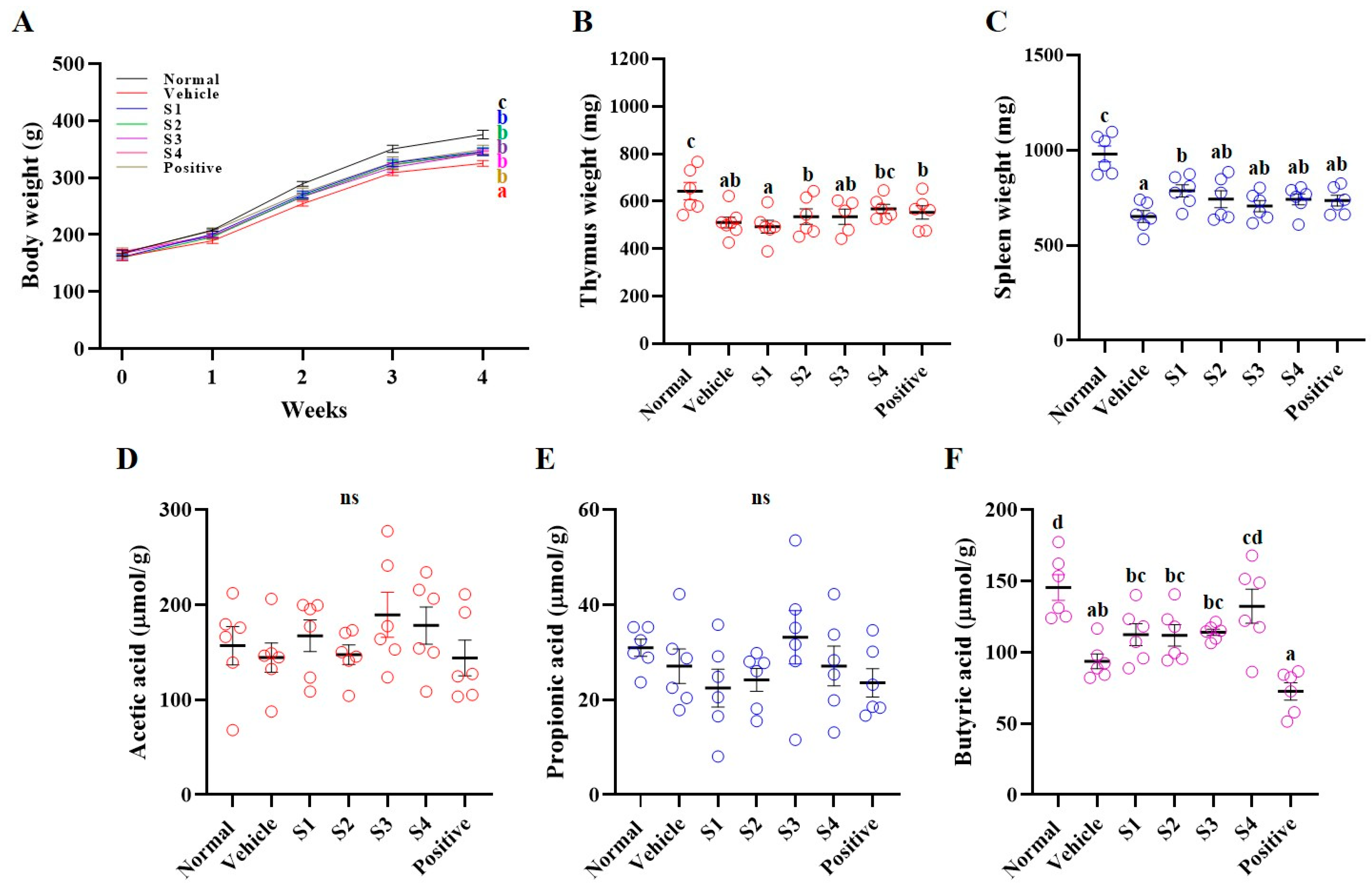
2.2. Animal Monitoring and SCFAs Analysis
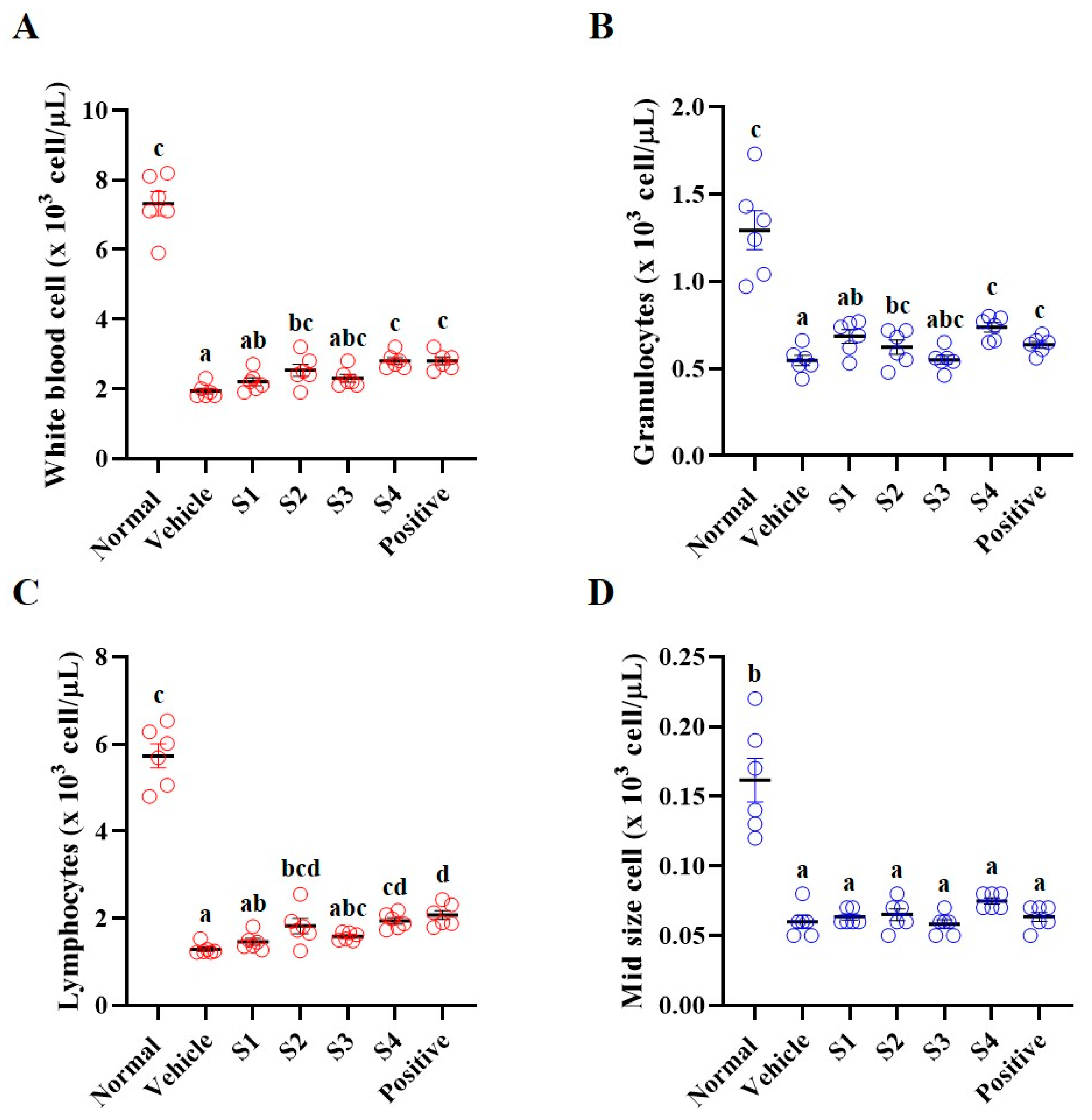
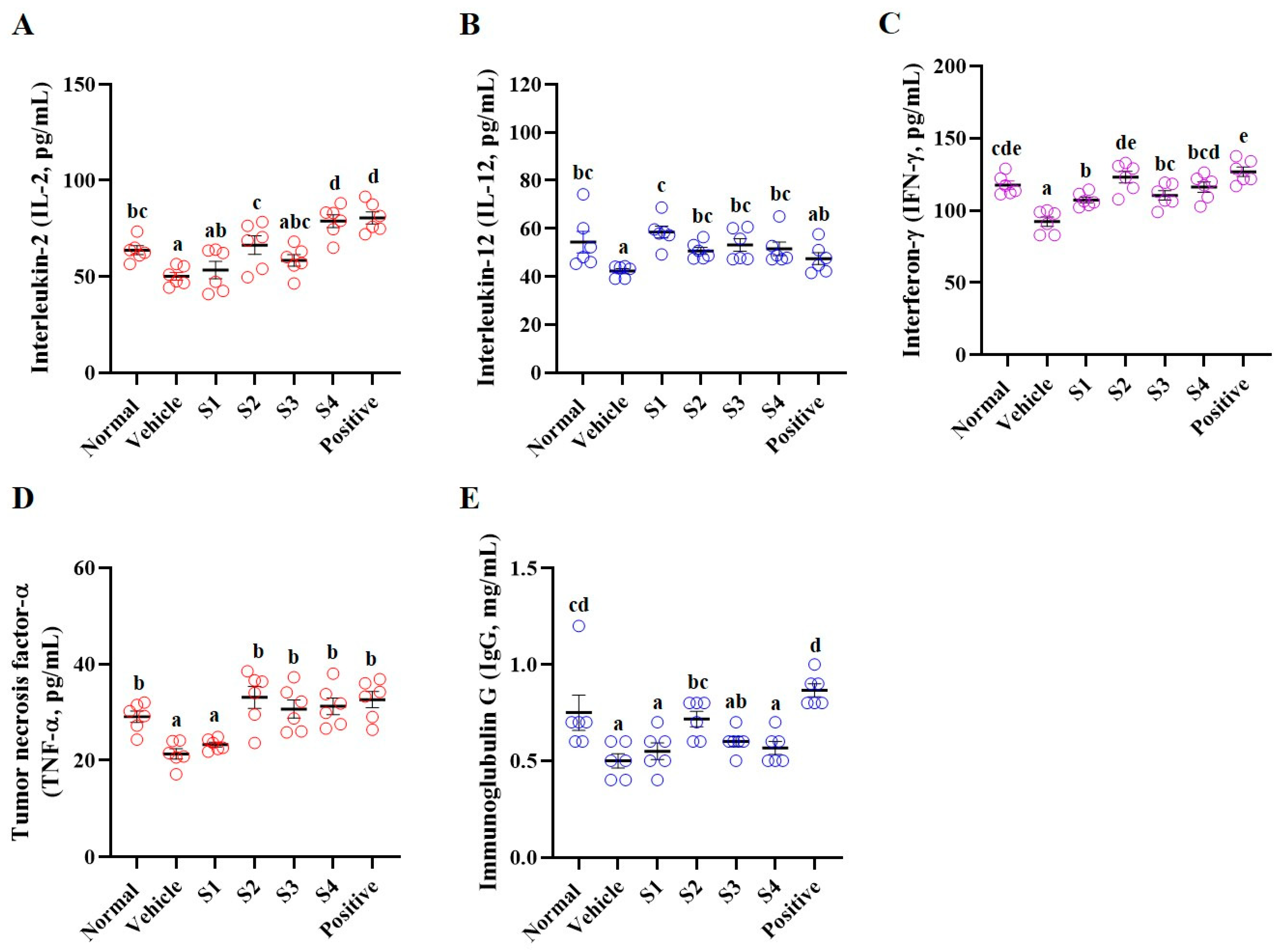
2.3. Blood and Serum Analyses
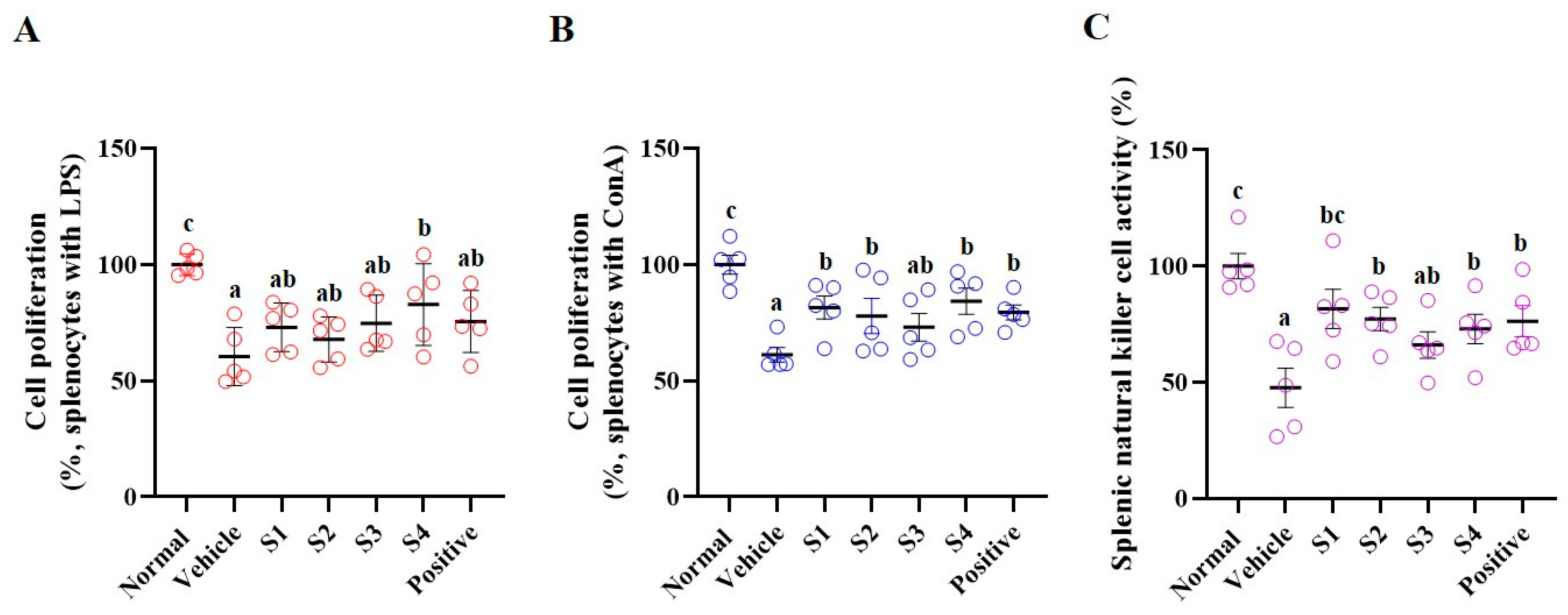
2.4. Splenocyte Proliferation and NK Cell Activity
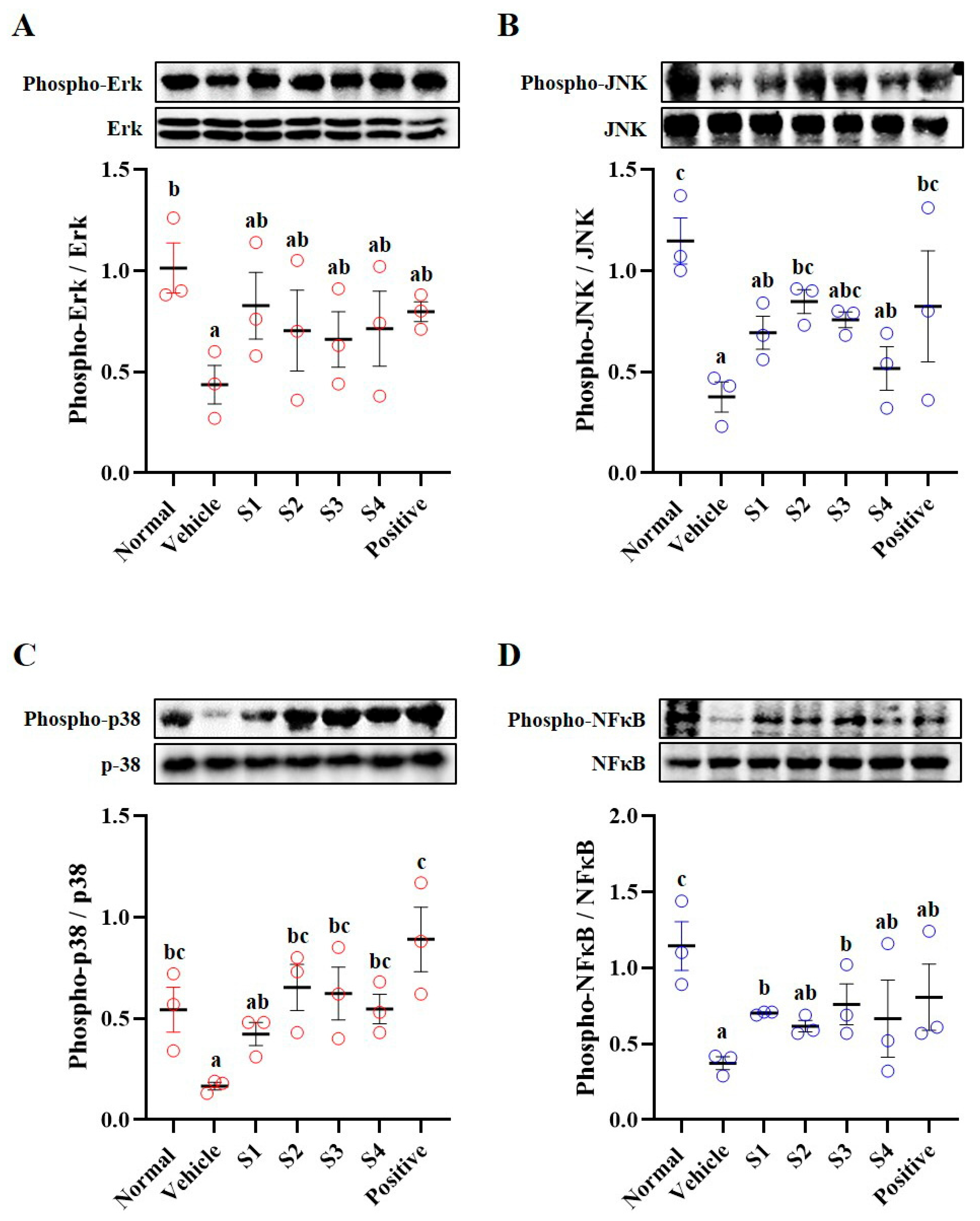
2.5. MAPKs/NFκB Pathway
2.6. Histological Analysis
3. Discussion
4. Materials and Methods
4.1. Preparation of GCJs
4.2. High Performance Liquid Chromatography of GCJs
4.3. Animals and Oral Administration
4.4. Short-Chain Fatty Acid Analysis
4.5. Complete Blood Cell (CBC) Count Analysis
4.6. Serum Levels of Cytokines and Immunoglobulin G (IgG)
4.7. Primary Cell Culture and Proliferation of Splenocytes
4.8. Splenic NK Cell Activity
4.9. Tissue Lysis and Western Blot Analysis
4.10. Histologic Analysis of the Spleen
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef]
- Rathee, P.; Chaudhary, H.; Rathee, S.; Rathee, D.; Kumar, V. Immunosuppressants: A review. Pharma Innov. 2013, 1, 90–101. [Google Scholar]
- Lee, H.Y.; Park, Y.M.; Shin, D.Y.; Hwang, H.M.; Jeong, H.N.; Park, H.Y.; Yang, H.J.; Ha, G.S.; Ryu, M.S.; Seo, J.W.; et al. Immune-enhancing effect of fermented soybean food, Cheonggukjang on cyclophosphamide-treated immunosuppressed rat. Heliyon 2024, 10, e37845. [Google Scholar] [CrossRef]
- Carnaud, C.; Lee, D.; Donnars, O.; Park, S.-H.; Beavis, A.; Koezuka, Y.; Bendelac, A. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J. Immunol. 1999, 163, 4647–4650. [Google Scholar] [CrossRef]
- Steinkraus, K.H. Classification of fermented foods: Worldwide review of household fermentation techniques. Food Control 1997, 8, 311–317. [Google Scholar] [CrossRef]
- Jung, S.-J.; Chae, S.-W.; Shin, D.-H. Fermented foods of Korea and their functionalities. Fermentation 2022, 8, 645. [Google Scholar] [CrossRef]
- Harahap, I.A.; Suliburska, J.; Karaca, A.C.; Capanoglu, E.; Esatbeyoglu, T. Fermented soy products: A review of bioactives for health from fermentation to functionality. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70080. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-Y.; Lee, H.J.; Shin, H.-C.; Lee, J.-M.; Park, K.-H.; Moon, J.-H. Behavior of flavonoid glycosides contained in Korean red pepper paste (Gochujang) during fermentation: Participation of a β-glucosidase inhibitor. Food Sci. Biotechnol. 2013, 22, 1–8. [Google Scholar] [CrossRef]
- Maharjan, A.; Vasamsetti, B.M.K.; Park, J.-H. A comprehensive review of capsaicin: Biosynthesis, industrial productions, processing to applications, and clinical uses. Heliyon 2024, 10, e39721. [Google Scholar] [CrossRef]
- Tang, J.; Luo, K.; Li, Y.; Chen, Q.; Tang, D.; Wang, D.; Xiao, J. Capsaicin attenuates LPS-induced inflammatory cytokine production by upregulation of LXRalpha. Int. Immunopharmacol. 2015, 28, 264–269. [Google Scholar] [CrossRef]
- Mace, T.A.; Ware, M.B.; King, S.A.; Loftus, S.; Farren, M.R.; McMichael, E.; Scoville, S.; Geraghty, C.; Young, G.; Carson, W.E., III. Soy isoflavones and their metabolites modulate cytokine-induced natural killer cell function. Sci. Rep. 2019, 9, 5068. [Google Scholar] [CrossRef]
- Choo, M.-K.; Park, E.-K.; Yoon, H.-K.; Kim, D.-H. Antithrombotic and antiallergic activities of daidzein, a metabolite of puerarin and daidzin produced by human intestinal microflora. Biol. Pharm. Bull. 2002, 25, 1328–1332. [Google Scholar] [CrossRef]
- Kaufman-Szymczyk, A.; Jalmuzna, J.; Lubecka-Gajewska, K. Soy-derived isoflavones as chemo-preventive agents targeting multiple signalling pathways for cancer prevention and therapy. Br. J. Pharmacol. 2025, 182, 2259–2286. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef]
- Rastogi, S.; Singh, A. Gut microbiome and human health: Exploring how the probiotic genus Lactobacillus modulate immune responses. Front. Pharmacol. 2022, 13, 1042189. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Park, Y.M.; Shin, D.Y.; Hwang, H.M.; Jeong, H.; Jeong, S.-J.; Yang, H.-J.; Ryu, M.S.; Seo, J.W.; Jeong, D.-Y. Gochujang, a traditional Korean fermented food, protects through suppressed inflammatory pathways and histological structure disruption in dextran sodium sulfate (DSS)-induced colitis mice. Heliyon 2024, 10, e27383. [Google Scholar] [CrossRef]
- Mahoro, P.; Moon, H.-J.; Yang, H.-J.; Kim, K.-A.; Cha, Y.-S. Protective effect of gochujang on inflammation in a DSS-induced colitis rat model. Foods 2021, 10, 1072. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Li, T.; Niu, M.; Zhang, H.; Wu, Y.; Wu, K.; Dai, Z. Targeting cytokine and chemokine signaling pathways for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 176. [Google Scholar] [CrossRef]
- Park, Y.M.; Lee, H.Y.; Shin, D.Y.; Jeong, H.N.; Hwang, H.M.; Park, H.Y.; Kim, S.H.; Kim, M.J.; Kang, H.J.; Kim, J.H.; et al. Immune-Enhancing Activity of Vitis coignetiae Extract via Increasing Cytokine and Natural Killer Cell Activity in Splenocytes and Cyclophosphamide-Induced Immunosuppressed Rats. J. Food Biochem. 2024, 2024, 5010095. [Google Scholar] [CrossRef]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef]
- Unsoeld, H.; Voehringer, D.; Krautwald, S.; Pircher, H. Constitutive expression of CCR7 directs effector CD8 T cells into the splenic white pulp and impairs functional activity. J. Immunol. 2004, 173, 3013–3019. [Google Scholar] [CrossRef]
- Kapasi, Z.F. The immune system and infectious diseases and disorders. In Acute Care Physical Therapy; Routledge: London, UK, 2024; pp. 149–176. [Google Scholar]
- Siddiqui, M.T.; Cresci, G.A. The immunomodulatory functions of butyrate. J. Inflamm. Res. 2021, 14, 6025–6041. [Google Scholar] [CrossRef]
- Corrêa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Yang, H.J.; Zhang, T.; Li, C.; Kim, M.J.; Kim, K.-N.; Park, S. Porcine Brain Enzyme Hydrolysate Enhances Immune Function and Antioxidant Defense via Modulation of Gut Microbiota in a Cyclophosphamide-Induced Immunodeficiency Model. Antioxidants 2024, 13, 476. [Google Scholar] [CrossRef] [PubMed]
- Konjević, G.M.; Vuletić, A.M.; Martinović, K.M.M.; Larsen, A.K.; Jurišić, V.B. The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine 2019, 117, 30–40. [Google Scholar] [CrossRef]
- Brendolan, A.; Rosado, M.M.; Carsetti, R.; Selleri, L.; Dear, T.N. Development and function of the mammalian spleen. Bioessays 2007, 29, 166–177. [Google Scholar] [CrossRef]
- Pulendran, B.; Palucka, K.; Banchereau, J. Sensing pathogens and tuning immune responses. Science 2001, 293, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shepherd, E.G.; Nelin, L.D. MAPK phosphatases—Regulating the immune response. Nat. Rev. Immunol. 2007, 7, 202–212. [Google Scholar] [CrossRef]
- Schulze-Osthoff, K.; Ferrari, D.; Riehemann, K.; Wesselborg, S. Regulation of NF-κB activation by MAP kinase cascades. Immunobiology 1997, 198, 35–49. [Google Scholar] [CrossRef]
- Duan, X.; Gao, S.; Li, J.; Wu, L.; Zhang, Y.; Li, W.; Zhao, L.; Chen, J.; Yang, S.; Sun, G. Acute arsenic exposure induces inflammatory responses and CD4+ T cell subpopulations differentiation in spleen and thymus with the involvement of MAPK, NF-kB, and Nrf2. Mol. Immunol. 2017, 81, 160–172. [Google Scholar] [CrossRef] [PubMed]

| GCJs Region in Korea | S1 Jeju-si | S2 Sunchang-gun | S3 Yangyang-gun | S4 Commercial |
|---|---|---|---|---|
| Total capsaicin (μg/kg) | 86.75 ± 1.78 | 139.21 ± 2.75 | 77.92 ± 1.38 | 13.67 ± 0.52 |
| Groups | Oral Administration for 4 Weeks |
|---|---|
| Normal group | Untreated group |
| Vehicle group | Only CP (5 mg/kg)-treated group |
| GCJs (S1–S4) treated groups | CP (5 mg/kg) with GCJs (S1–S4; 500 mg/kg) treated groups |
| Positive (HemoHim) treated group | CP (5 mg/kg) with HemoHim (1000 mg/kg) treated group |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.Y.; Park, Y.M.; Shin, D.Y.; Hwang, H.M.; Chun, S.H.; Lim, S.J.; Yang, H.-J.; Ha, G.S.; Ryu, M.S.; Seo, J.-W.; et al. Immunomodulatory Effects of Traditional Korean Gochujang in Rats Immunosuppressed with Cyclophosphamide. Int. J. Mol. Sci. 2025, 26, 8325. https://doi.org/10.3390/ijms26178325
Lee HY, Park YM, Shin DY, Hwang HM, Chun SH, Lim SJ, Yang H-J, Ha GS, Ryu MS, Seo J-W, et al. Immunomodulatory Effects of Traditional Korean Gochujang in Rats Immunosuppressed with Cyclophosphamide. International Journal of Molecular Sciences. 2025; 26(17):8325. https://doi.org/10.3390/ijms26178325
Chicago/Turabian StyleLee, Hak Yong, Young Mi Park, Dong Yeop Shin, Hai Min Hwang, Sung Hak Chun, Sang Jin Lim, Hee-Jong Yang, Gwang Su Ha, Myeong Seon Ryu, Ji-Won Seo, and et al. 2025. "Immunomodulatory Effects of Traditional Korean Gochujang in Rats Immunosuppressed with Cyclophosphamide" International Journal of Molecular Sciences 26, no. 17: 8325. https://doi.org/10.3390/ijms26178325
APA StyleLee, H. Y., Park, Y. M., Shin, D. Y., Hwang, H. M., Chun, S. H., Lim, S. J., Yang, H.-J., Ha, G. S., Ryu, M. S., Seo, J.-W., Jeong, D.-Y., Bae, J. S., & Kim, J. G. (2025). Immunomodulatory Effects of Traditional Korean Gochujang in Rats Immunosuppressed with Cyclophosphamide. International Journal of Molecular Sciences, 26(17), 8325. https://doi.org/10.3390/ijms26178325







